Abstract
Drug-drug interaction (DDI) can trigger many adverse effects in patients and has emerged as a threat to medicine and public health. Despite the continuous information accumulation of clinically significant DDIs, there are few open-access knowledge systems dedicated to the curation of DDI associations. To facilitate the clinicians to screen for dangerous drug combinations and improve health systems, we present DDInter, a curated DDI database with comprehensive data, practical medication guidance, intuitive function interface, and powerful visualization to the scientific community. Currently, DDInter contains about 0.24M DDI associations connecting 1833 approved drugs (1972 entities). Each drug is annotated with basic chemical and pharmacological information and its interaction network. For DDI associations, abundant and professional annotations are provided, including severity, mechanism description, strategies for managing potential side effects, alternative medications, etc. The drug entities and interaction entities are efficiently cross-linked. In addition to basic query and browsing, the prescription checking function is developed to facilitate clinicians to decide whether drugs combinations can be used safely. It can also be used for informatics-based DDI investigation and evaluation of other prediction frameworks. We hope that DDInter will prove useful in improving clinical decision-making and patient safety. DDInter is freely available, without registration, at http://ddinter.scbdd.com/.
Graphical Abstract
Graphical Abstract.
DDInter provides detailed annotations of each DDI association and enables users to conduct data query, data retrieval and prescription checking.
INTRODUCTION
Drug–drug interaction (DDI) is one of the most important concerns in clinical rational administration and post-marketing pharmacovigilance (1,2). When taking two or more drugs at the same time or in succession, the activity of one drug may be alerted significantly due to the presence of other drugs, which is described as DDI. DDIs can be roughly classified into two main types: pharmacokinetic (PK) and pharmacodynamic (PD). PK interactions occur when one drug alters the absorption, distribution, metabolism and/or excretion (ADME) of another drug (3,4), while PD interactions occur when one drug alters the pharmacological effects of another drug without affecting its pharmacokinetics (5).
With the continuous increase of new drugs, prescriptions containing multiple drugs have been common treatment options (6), especially for patients accompanied with many chronic diseases such as cancer, diabetes, and cardiovascular disorders. This tends to increase the risk of clinically relevant DDIs and create new challenges to therapeutic management. The occurrence of DDIs often triggers unexpected pharmacological effects (7). In a few cases, DDIs are beneficial and can be exploited as therapeutic strategies for improving efficiency, avoiding toxicity, or minimizing drug resistance, such as the combination of β-lactams and clavulanic acid (8). However, most DDIs are unpleasant and detrimental, which can make patients exposed to the risks of side effects and toxicity and even deteriorate their physical conditions (9). It has been reported that DDIs could be associated with up to 30% of all the reported adverse drug events, leading to increased hospitalizations and emergency department visits (10). A statistical analysis has revealed that ∼15% of older adults taking multiple medications were at risk for potential major DDIs (11). To minimize potential injury caused by unfavorable DDIs, physicians need to prescribe appropriate drugs to avoid risky drug combinations (12).
Databases containing rich DDI information are valuable tools for prescribing, which could warn physicians and pharmacists about potential risks timely. Currently, there are some commercial systems dedicated to providing exhaustive and professional DDI information as clinical decision support, such as Drug-Reax software from Micromedex® Healthcare Series and Lexi-Interact® software of Lexicomp. However, the subscription fees may prevent their broader access to medical practitioners, especially those in resource-limited areas. Although some free online tools, such as DrugBank (13), SuperDRUG2 (14), SuperCYPsPred (15) and Transformer (16), have slightly touched upon DDIs, the lack of sufficient mechanisms and management annotations make it difficult to give practical guidance to rational medication. Therefore, there is still an urgent need to develop comprehensive, professional, and open-access DDI databases to improve clinical decision-making and patient safety.
Here, we present DDInter, a comprehensive and practical DDI database, currently containing ∼0.24 M DDI pairs connecting 1833 approved drugs that have been reviewed and curated by a clinical pharmacist team. DDInter provides abundant information for each drug–drug pair, including interaction mechanism, severity level, strategy for managing potential risks, alternatives for drug replacement, literature citations, etc. Concretely, users could easily browse drug entries and interactions, retrieve the basic information and interaction networks of drugs, and carry out prescription checking with the help of the interaction checker module. To help users better understand and explore the searching results, several data visualization tools are embedded to display complex relationships dynamically. The web interface of DDInter is user-friendly and the entities are efficiently cross-linked. DDInter aims to provide a professional DDI knowledge base for the broad community. Physicians and pharmacists could gain practical guidance for dosage adjustment, drug replacement, as well as risk judgment and management. Meanwhile, data scientists could employ the database as the resource for the detection of potential DDIs and evaluation of other prediction tools.
MATERIALS AND METHODS
Data collection and curation
DDI information is largely dispersed in scientific literature and labels of pharmaceutical products. The PubMed database was systematically searched to identify the studies that reported the effects of one drug upon another (as of March 2021). Literature pertaining to drug-food or drug–gene interactions and other irrelevant references were ruled out. Then, all the medication guides that had been approved by the US FDA for avoiding serious adverse events were downloaded from Drugs@FDA, with duplicates excluded. Finally, a total of 9460 scientific literature and the labels of pharmaceutical products were employed as the document library for interaction annotation. Most of these documents focused on specific drug classes that were inclined to interfere the activity of co-administered drugs, such as Ca-channel blockers and NSAIDs. About forty thousand DDIs were downloaded from the remarkable article published by a team from Stanford University in Sci Transl Med (17). These DDIs were collected from authoritative resources and had been confirmed significant after statistical correction of uncharacterized bias. Other DDIs were extracted from documents through semi-automated text-mining (18). For each drug pair, we recorded the names, the descriptions of mechanisms, and the strategies for managing potential risks and strictly followed the document contents without any further category-based deduction that had been used in other resources. When chemical or pharmacological classes were adopted to refer to a series of drugs that could exert influence on the paired drugs, we extracted the class-drug pairs and then translated them into multiple DDIs based on the drug lists reported in the documents. Certainly, it is common for some DDIs to be confronted with information missing although we have endeavored to collect more data. We used the drug names to query for standard names (generic names) and synonymies (chemical names, brand names, etc.) in DrugBank and Drugs@FDA. The standardization of drug names and merging of interactions were conducted based on the lexicon of drug synonymies. It should be noted that different administration routes of a drug were regarded as different records because their interaction networks may be distinct, especially when the route of administration of a drug might influence its pharmacological effects. In addition, we extracted the interaction descriptions of all the 236 834 DDIs and made further refinements, leading to 5560 distinct and high-quality mechanism descriptions. This suggested that many DDIs shared the same interactions. For each drug entry, a simple pharmacological summary, anatomical therapeutic chemical (ATC) codes, and some external links were collected to enable a better understanding of the specific drug. The small-molecule drugs in DDInter were also annotated with the basic chemical information, including molecular formula, molecular weight, IUPAC name, InChI, InChI key and canonical SMILES.
It is only the first step to identify the nature and the mode of action of DDIs. When many warnings are given, alert fatigue may undermine the usefulness of the interaction checking (19). Therefore, the registered pharmacist team from Xiangya hospital intensively reviewed these interactions and classified them into different risk levels (major, moderate, minor, and unknown) as suggested by DRUGDEX (20) and other similar resources. To achieve the maximum rationality, each of the interaction entries was reviewed by at least two pharmacists and the third person would be engaged in the annotation when conflicting opinions occurred. Generally, major interactions were highly clinically significant and the drug combinations should be strictly avoided; moderate interactions may result in exacerbation of the disease of the patient and/or change in therapy; minor interactions were minimally clinically significant and usually they do not require changes in therapy; unknown interactions meant that the interaction description was unavailable or incomplete. In addition to severity level, each of the interaction entries was also manually annotated with interaction mechanisms, including absorption, distribution, metabolism, excretion, synergy and antagonism. More details of the severity and mechanism annotations have been summarized in the website and Supplementary data.
Alternative medications
To allow for convenience and to facilitate clinical prescription, alternative medications were provided based on the ATC code (21), a widely accepted drug classification scheme in both academic and clinical practice. It categorized drugs into a hierarchy with five different levels based on their therapeutic, pharmacological, and chemical attributes. In DDInter, the third level of the ATC code was used to derive alternative medications, i.e. pharmacological subgroup. Taking atropine and cyproheptadine as an example, the alternatives of atropine share the same anatomical, therapeutic, and pharmacological subgroups with atropine, but have no interactions with cyproheptadine. For drugs with multiple ATC codes, such as aspirin and metformin, it is hard to distinguish which is responsible for the occurrence of DDIs, and therefore alternatives of all the ATC codes were displayed.
Potential metabolism interactions
Cytochrome P450 (CYP) enzymes are responsible for the metabolism of approximately two-thirds of the clinical drugs and therefore play an important role in many metabolic DDIs (22,23). It is necessary to record the CYPs-mediated metabolic profile as additional information to make better elucidation and understanding of potential metabolic interactions. For each small-molecule drug, the two activity modes (substrate and inhibitor) of five major CYP isoenzymes (1A2, 3A4, 2C9, 2C19 and 2D6) were predicted using ADMETlab 2.0 (24), an integrated online platform for ADMET property evaluation. According to the original paper, the molecules with prediction probability values higher than 0.7 are more likely to be substrates or inhibitors. Metabolic interactions will take place when both of the interacted drugs are inhibitors or substrates of one particular enzyme, including substrate–substrate interaction, inhibitor–inhibitor interaction and inhibitor–substrate interaction.
Online database implementation
DDinter was built based on the Python web framework of Django 3.0 and Bootstrap 4.3.1. The web interface was developed using HTML5, CSS and JavaScript. All the data was stored and managed using MySQL. For molecule visualization, RDKit (25) was applied to generate 2D images, and 3Dmol.js (26) was used to display the 3D structures of drugs. All the online data visualizations, including the relation graph and sunburst chart of drug entries, and the bar chart of potential metabolism interactions, were supported by ECharts 4.0 (27), an open-sourced JavaScript library for the rapid construction of interactive visualization. The website has been tested thoroughly to ensure the functionality across multiple operating systems and web browsers.
DATABASE CONTENT AND USAGE
Data summary and analysis
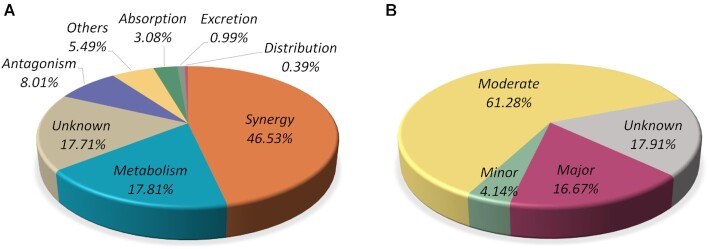
DDInter is a comprehensive database that annotates a total of 236 834 clinically relevant DDI associations connecting 1833 US FDA-approved drugs (1972 drug entities). About 82% of the DDI entities were annotated with mechanism categories and severity risks, and the distributions have been described in Figure 1. Synergy subclass accounts for the largest proportion reaching 46.53%, followed by the interactions mediated via metabolism actions (17.81%) and antagonism actions (8.01%). The most frequently appeared synergy DDI is QTc-interval prolongation (28), which is a rhythm disturbance and can be caused by an extensive list of medications, such as antiarrhythmic agents, azole antifungal, antipsychotic and antidepressant agents. The concurrent use of these medications should be avoided unless the benefits overweight the risk. And frequent cardiac monitoring is recommended for high-risk populations. Regarding severity levels, 16.67% of the DDIs fall under the category major, 61.28% under the category moderate, and 4.14% under the category minor. It is easy to observe that moderate DDIs take the largest share, which typically need simple interventions to minimize adverse effects such as dosage adjustment and symptoms monitoring. Although moderate DDIs are not life-threatening, clinicians are bound to handle these co-administrations seriously and make patients informed of possible side effects. Here, the proportion of minor DDIs may be grossly underestimated because minor DDIs that do not have serious influence on the quality of life could be scarcely reported (29).
Figure 1.
The statistics of the DDI associations in DDInter based on mechanisms (A) and risk levels (B). Associations with unavailable or incomplete description of interaction details were labeled as ‘Unknown’.
To gain insight into the DDI associations in the disease area, the degree (number of DDIs involved) of each drug in the DDInter network was calculated and linked to the corresponding ATC codes. The number of drugs and average degrees per therapeutic area have been summarized in Table 1. Note that drugs with multiple ATC codes were counted once in all the therapeutic areas. On average, the degree of drugs in our database is ∼240. The high degrees of drugs targeting the nervous system or cardiovascular system are clear, exceeding 400 and 350, respectively. A closer analysis demonstrated that 64% and 56% of the drugs targeting these two systems appear in >400 DDI associations. The ten most connected drugs in DDInter are shown in Table 2, of which six drugs fall under the nervous system. Interestingly, the drugs related to the cardiovascular system are not in the list although their average degree is second only to the nervous system, which may be explained by the even distribution of degrees within the system. The second connected drug is dexamethasone, which has 16 ATC codes covering multiple therapeutic areas like endocrine, rheumatic, allergic, respiratory, etc. The character of multiple pharmacological activities suggests that dexamethasone can influence various therapeutic targets or signaling pathways, which is an important precipitating cause of DDIs. Besides, phenytoin and warfarin are special, whose high degrees are largely contributed by their narrow therapeutic index (30,31). The non-linear pharmacokinetic character of these drugs makes them sensitive to the effects exerted by other drugs. Collectively, the drugs targeting the cardiovascular and nervous systems have high DDI risks and more caution should be given to patients treated by drugs with multiple pharmacological activities or narrow therapeutic index.
Table 1.
DDI involvement of drugs categorized by the therapeutic area
| ATC category | Therapeutic area | Number of drugs | Average degree |
|---|---|---|---|
| A | Alimentary tract and metabolism system | 219 | 254.47 |
| B | Blood and blood forming organs | 99 | 157.98 |
| C | Cardiovascular system | 177 | 356.35 |
| D | Dermatologicals | 117 | 202.60 |
| G | Genito-urinary system | 127 | 266.28 |
| H | Hormonal preparations | 42 | 258.90 |
| J | Antiinfectives for systemic use | 202 | 227.16 |
| L | Antineoplastic and immunomodulating agents | 268 | 274.51 |
| M | Musculo-skeletal system | 78 | 285.08 |
| N | Nervous system | 269 | 403.25 |
| P | Antiparasitic products, insecticides and repellents | 36 | 151.42 |
| R | Respiratory system | 111 | 267.98 |
| S | Sensory organs | 125 | 241.13 |
| V | Various | 95 | 121.93 |
| U | Unclassified | 243 | 143.42 |
*The statistic was based on the approved drugs recorded in the DDInter database only. The ATC code of each drug was extracted from DrugBank or DrugCentral, and the drugs that could not be mapped to the ATC codes were labelled ‘Unclassified’. ATC, WHO Anatomical Therapeutic Chemical Classification System.
Table 2.
Information of the most connected ten drugs in the DDInter database
| Rank | ID | Drug name | Degree | ATC code(s) |
|---|---|---|---|---|
| 1 | DDInter388 | Citalopram | 905 | N06AB04 |
| 2 | DDInter513 | Dexamethasone | 894 | R01AD53; D07XB05; R01AD03; D10AA03; S01CB01; S02CA06; S03CA01; C05AA09; S01CA01; D07CB04; H02AB02; S01BA01; A01AC02; S03BA01; D07AB19; S02BA06 |
| 3 | DDInter293 | Carbamazepine | 872 | N03AF01 |
| 4 | DDInter419 | Clozapine | 868 | N05AH02 |
| 5 | DDInter1460 | Phenytoin | 854 | N03AB02; N03AB52 |
| 6 | DDInter1951 | Warfarin | 845 | B01AA03 |
| 7 | DDInter1662 | Sertraline | 845 | N06AB06 |
| 8 | DDInter1735 | Tacrolimus | 844 | D11AH01; L04AD02 |
| 9 | DDInter1927 | Venlafaxine | 837 | N06AX16 |
| 10 | DDInter384 | Ciprofloxacin | 832 | J01RA11; S03AA07; J01MA02; S02AA15; J01RA10; J01RA12; S01AE03 |
Web design and interface
DDInter offers an effective and user-friendly web interface to make full advantage of the wealthy data, accessible at http://ddinter.scbdd.com/. The search bar in the home page allows users to query the interaction networks of drugs by entering drug names. The ‘Browse’ module provides an overview of the data content, while the ‘Interaction checker’ is designed for prescribing checking. The ‘Download’ page provides links to download DDIs of different therapeutic areas. Some explanation of the annotations, statistic information, detailed tutorials, and user terms and conditions are readily accessible in the ‘About’ page.
Data browsing
Two types of entries, drugs and interactions, could be browsed. All the drugs entities are assigned with unique identifiers and shown in the form of molecular structures. The interactive filter located on the left side of the page allows users to explore a subset of the original data, such as small-molecule drugs, biotech drugs, and drugs targeting different therapeutic areas. Clicking on a specific drug ID will open the drug information page that displays affluent interaction contents as well as chemical and pharmacological descriptions. For interaction browsing, severity level and mechanism filters are both provided to guide users to find the entries of interest. These interaction entities are shown in the tabular format with basic information. When clicking on the ‘view’ button of specific interaction, a list of drug–drug pairs corresponding to this entry would be accessed, where users could obtain concrete interaction content from the provided hyperlinks. By default, drug entities and interaction entities are presented in order of identifier and risk level, respectively.
Data retrieval
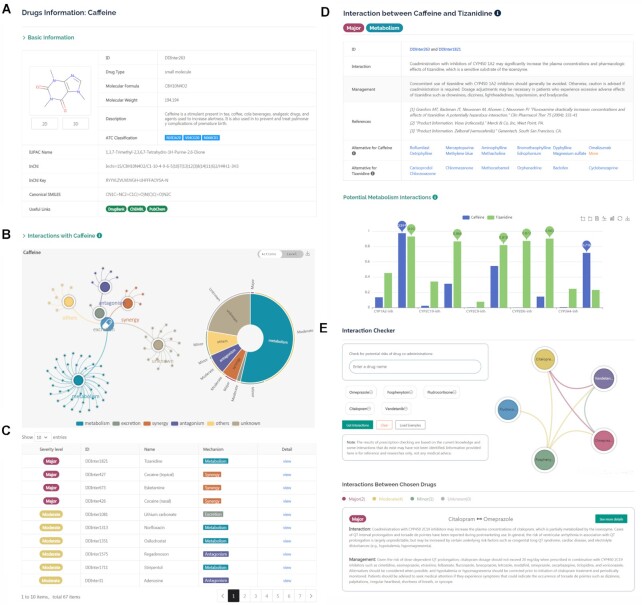
The quick search of the DDI information of drugs is available in the middle of the homepage. The text-based search enables users to enter the generic name or synonyms of a drug. Clicking on the search icon will jump to a new page where all the DDI associations of this drug are summarized. If the typed terms could match with multiple drug entities in the database, a list of suitable suggestions will be provided, with hyperlinks to the corresponding full pages. The queried drug information page consists of two major parts: basic chemical and pharmacological annotations, and drug interaction network. As shown in Figure 2A, the ‘Basic Information’ field describes drug structures and other characteristics, including DDInter identifier, drug type, molecular formula and weight, pharmacological summary, ATC codes, useful external links, and some chemical representations. To facilitate the understanding of the DDI associations, a relation graph and a sunburst chart are provided to demonstrate the distributions of all the involved DDIs intuitively (Figure 2B). Both mechanism-based and risk-based distribution schemes are provided and the conversion is achieved through the switch on the top right corner of the graph. In the DDI relation graph, the secondary nodes represent action mechanisms or risk levels, and interacted drugs are linked to these nodes closely. Users can hover over a connection line to learn the specific interaction information and click on the legend entities to filter out those nodes of less interest. The sunburst chart depicts different hierarchies of DDI associations, where each section can be further expanded by simple clicking. These graphs can be downloaded from the website in PNG format. Below the distribution graphs, all the DDI entries involved are presented in a table (Figure 2C), containing severity level, identifiers and names of the associated drugs, mechanism of action, and hyperlinks to pages of drug–drug pairs. Specific DDI information can be accessed from these hyperlinks. In addition, a filtering tool is provided to help users to refine the results.
Figure 2.
The web interface of DDInter. (A) Basic chemical and pharmacological information of the queried drug (caffeine, DDInter263). (B) Visualization of DDI network. The relation graph displays the distributions of all the involved DDIs and the sunburst chart shows different hierarchies of DDI associations. (C) A list of drugs interacting with the query drug. (D) The page of specific drug–drug pair shows extensive information of interactions. (E) The interaction checker module helps physicians screen for risks in prescriptions.
Interaction information of drug–drug pairs is mainly displayed in the tabular format in the DDI page (Figure 2D). The ‘Interaction’ field shows expanded descriptions mechanisms, including pharmacologic conflicts, synergetic toxicity, metabolic enzymes competition, etc. The ‘Management’ field displays the strategies for managing potential side effects, including avoiding combinations, monitoring potential toxicity, adjusting the dosage of drugs, changing time of administration, etc. These annotations are valuable for clinical decision-making, from which physicians could judge whether the DDI risks are tolerated and receive guidance on dose adjustment and therapeutic monitor. If available, the source from which the information is extracted is also provided to allow users to trace back to the original documents. Clinically, replacing a drug by another drug with similar efficacy but lower interaction risks is frequently used in DDI management. Therefore, DDInter provides the alternatives of each drug in DDI associations based on the third level (pharmacological subgroup) of the ATC codes. The metabolic profiles targeting the most important five CYP isoenzymes are presented in a bar chart at the bottom of the page, which can be used to investigate the potential metabolic interactions that have been not detected or summarized. Prediction values higher than 0.7 mean that corresponding drugs are inclined to be substrates or inhibitors of specific enzymes and lead to the occurrence of DDI events. Users can browse the concrete predicted values, switch the chart types and scaling, and download the full chart by using the operation broad in the upper-right corner.
Interaction checker
Prescribing multiple drugs to treat the disorders of patients is common in clinical practice. To facilitate the screening of potential DDIs in prescriptions and improve patient safety, the ‘interaction checker’ module was designed and developed, accessible from the top navigation bar. This module is based on the complete DDI database and allows for the checking of no more than five drugs at once. The detected DDI associations are shown in separate report cards, with information on risk levels, interaction mechanisms, and management (Figure 2E). A relation chart is provided to visualize the DDI network of the chosen drugs. Simple counting statistics of DDI risks are carried out and the risk status of each DDI association is color-coded. If users are interested in a specific DDI entry, they can click on the ‘see more details’ button to jump to the pages of drug–drug pairs where complete information is summarized.
Downloads and updates
All the DDI associations can be downloaded from the website without login or registration. To facilitate the physicians to focus the DDIs of specific therapeutic areas, the dataset was split into multiple sub-datasets according to the ATC codes. Meanwhile, we hope these datasets could help bioinformaticians to detect potential DDIs and to evaluate other DDI prediction methods. In the future, we will continue to maintain and update the database. The data in DDInter will be updated every 6 months based on the evolving scientific literature and we will integrate more reliable data from other resources to present the most comprehensive landscape of knowledge of clinical-related DDIs.
DISCUSSION AND CONCLUSION
The understanding and management of DDI events is a real challenge in clinical pharmacy. Some clinical groups and researchers have proposed some strategies to address this confusing area. For instance, the American Geriatrics Society (AGS) has published the Beers Criteria (32), containing lists of potentially inappropriate medications that should be avoided in elderly people. Pharmacists have also summarized some characters of drugs with high DDI risks, including narrow therapeutic indexes, inhibitors or inducers of CYP450 enzymes, treatment for chronic diseases, etc. (33). Some data scientists proposed various methods for drug–drug-interaction predictions (34,35). However, these empirical rules cover only a small part of DDI associations and more clinical-related DDIs reported in scientific documents have not been summarized and provided to physicians. And the reliability of predicted DDIs may be influenced by the accuracy of methods. Hence, it is urgent to develop a DDI knowledge system to screen for potential DDI risks proactively. In this work, we proposed DDInter to help physicians and pharmacists detect inappropriate medications and manage clinical outcomes. The manually curated platform contains about 0.24M DDI associations connecting 1833 approved drugs by bringing together the DDI information scattering in literature and product labels. It supplies detailed and professional information of each DDI association, including severity level, mechanism description, management of concurrence, alternative medications, etc. Additionally, it integrates various functions including data browsing, retrieval, and interaction checker to support clinical decision-making. Some data visualization tools are embedded to help users to understand and explore the searching results. The comparison of DDInter and other excellent public medicine resources have been summarized in Supplementary Table S1 for interested readers. To our knowledge, DDInter is the only open-access database that provides professional DDI information to support clinical medication.
Nevertheless, several limitations in the DDInter framework should be elaborated. First, it lacks a ‘gold standard’ to define the clinical significance of DDIs. Since the practical experience of pharmacists is subjective and biased, the definition of severity levels may not be perfectly suitable for all the circumstances worldwide. It would be necessary for users to learn about the annotation standards of our database before using it in clinical practices. The second is data incompleteness. Although we have endeavored to collect information from multiple resources, it is impossible to claim that all the DDIs identified are included. Moreover, certain DDI associations may be concealed by complex disease status and have never been reported yet. Thirdly, this work merely focuses on interactions between two drugs, while three or more drug interactions are not included. In the future, we plan to integrate more data from other resources into DDInter. In summary, the management of DDI events is a complex and important issue and multidisciplinary collaborations are required to address the challenges of DDIs. We hope that our database will prove useful in improving clinical decision-making and patient safety.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the pharmacist team from Xiangya hospital for their professional technical support in the data curation and valuable feedback about clinical demands. The studies meet with the approval of the university's review board.
Contributor Information
Guoli Xiong, Department of Pharmacy, Xiangya Hospital, Central South University, Changsha 410013, Hunan, China; Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, Hunan, China; Hunan Key laboratory of Diagnostic and Therapeutic Drug Research for Chronic Diseases, Central South University, Changsha 410013, Hunan, China.
Zhijiang Yang, Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, Hunan, China; Hunan Key laboratory of Diagnostic and Therapeutic Drug Research for Chronic Diseases, Central South University, Changsha 410013, Hunan, China.
Jiacai Yi, College of Computer, National University of Defense Technology, Changsha 410073, Hunan, China.
Ningning Wang, Department of Pharmacy, Xiangya Hospital, Central South University, Changsha 410013, Hunan, China.
Lei Wang, Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, Hunan, China; Hunan Key laboratory of Diagnostic and Therapeutic Drug Research for Chronic Diseases, Central South University, Changsha 410013, Hunan, China.
Huimin Zhu, Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, Hunan, China; Hunan Key laboratory of Diagnostic and Therapeutic Drug Research for Chronic Diseases, Central South University, Changsha 410013, Hunan, China.
Chengkun Wu, College of Computer, National University of Defense Technology, Changsha 410073, Hunan, China.
Aiping Lu, Institute for Advancing Translational Medicine in Bone and Joint Diseases, School of Chinese Medicine, Hong Kong Baptist University, Hong Kong SAR, China.
Xiang Chen, Department of Dermatology, Hunan Engineering Research Center of Skin Health and Disease, Hunan Key Laboratory of Skin Cancer and Psoriasis, Xiangya Hospital, Central South University, Changsha 410008, Hunan, China.
Shao Liu, Department of Pharmacy, Xiangya Hospital, Central South University, Changsha 410013, Hunan, China.
Tingjun Hou, Innovation Institute for Artificial Intelligence in Medicine of Zhejiang University, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, Zhejiang, China.
Dongsheng Cao, Department of Pharmacy, Xiangya Hospital, Central South University, Changsha 410013, Hunan, China; Xiangya School of Pharmaceutical Sciences, Central South University, Changsha 410013, Hunan, China; Hunan Key laboratory of Diagnostic and Therapeutic Drug Research for Chronic Diseases, Central South University, Changsha 410013, Hunan, China; Institute for Advancing Translational Medicine in Bone and Joint Diseases, School of Chinese Medicine, Hong Kong Baptist University, Hong Kong SAR, China.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Science Foundation of China [2217030451, 21575128, 81773632]; Changsha Municipal Natural Science Foundation [kq2014144]; Changsha Science and Technology Bureau project [kq2001034]; Zhejiang Provincial Natural Science Foundation of China [LZ19H300001]; Key R&D Program of Zhejiang Province [2020C03010]; Leading Talent of ‘Ten Thousand Plan’-National High-Level Talents Special Support Plan; HKBU Strategic Development Fund project [SDF19-0402-P02]; Key Research Project of Ningxia Hui Autonomous Region in 2021 (Major Project) [2021BEG01001]. Funding for open access charge: HKBU Strategic Development Fund project [SDF19-0402-P02].
Conflict of interest statement. None declared.
REFERENCES
- 1. Control, C.f.D. National Hospital Ambulatory Medical Care Survey 2009, Centers for Disease Control. National Center for Health Statistics. 2010; [Google Scholar]
- 2. Wienkers L.C., Heath T.G.. Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Discov. 2005; 4:825–833. [DOI] [PubMed] [Google Scholar]
- 3. Nebert D.W., Russell D.W.. Clinical importance of the cytochromes P450. Lancet. 2002; 360:1155–1162. [DOI] [PubMed] [Google Scholar]
- 4. Nigam S.K. What do drug transporters really do?. Nat. Rev. Drug Discov. 2015; 14:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niu J., Straubinger R.M., Mager D.E.. Pharmacodynamic drug-drug interactions. Clin. Pharmacol. Ther. 2019; 105:1395–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onder G., Marengoni A.. Polypharmacy. JAMA. 2017; 318:1728. [DOI] [PubMed] [Google Scholar]
- 7. Ryu J.Y., Kim H.U., Lee S.Y.. Deep learning improves prediction of drug–drug and drug-food interactions. Proc. Natl. Acad. Sci. U.S.A. 2018; 115:E4304–E4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guieu B., Jourdan J.P., Dreneau A., Willand N., Rochais C., Dallemagne P.. Desirable drug–drug interactions or when a matter of concern becomes a renewed therapeutic strategy. Drug Discov. Today. 2021; 26:315–328. [DOI] [PubMed] [Google Scholar]
- 9. Vilar S., Friedman C., Hripcsak G.. Detection of drug–drug interactions through data mining studies using clinical sources, scientific literature and social media. Brief. Bioinform. 2018; 19:863–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pirmohamed M., Orme M.. Drug interactions of clinical importance. Davies's Textbook of Adverse Drug Reactions. 1998; 888–912. [Google Scholar]
- 11. Qato D.M., Wilder J., Schumm L.P., Gillet V., Alexander G.C.. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern. Med. 2016; 176:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qiu Y., Zhang Y., Deng Y., Liu S., Zhang W.. A comprehensive review of computational methods for drug-drug interaction detection. IEEE/ACM Trans. Comput. Biol. Bioinf. 2021; 10.1109/TCBB.2021.3081268. [DOI] [PubMed] [Google Scholar]
- 13. Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Sajed T., Johnson D., Li C., Sayeeda Z.et al.. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018; 46:D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siramshetty V.B., Eckert O.A., Gohlke B.O., Goede A., Chen Q., Devarakonda P., Preissner S., Preissner R.. SuperDRUG2: a one stop resource for approved/marketed drugs. Nucleic Acids Res. 2018; 46:D1137–D1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banerjee P., Dunkel M., Kemmler E., Preissner R.. SuperCYPsPred-a web server for the prediction of cytochrome activity. Nucleic Acids Res. 2020; 48:W580–W585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hoffmann M.F., Preissner S.C., Nickel J., Dunkel M., Preissner R., Preissner S.. The transformer database: biotransformation of xenobiotics. Nucleic Acids Res. 2014; 42:D1113–D1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tatonetti N.P., Ye P.P., Daneshjou R., Altman R.B.. Data-driven prediction of drug effects and interactions. Sci. Transl. Med. 2012; 4:125ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W., Yang X., Yang C., Guo X., Zhang X., Wu C.. Dependency-based long short term memory network for drug–drug interaction extraction. BMC Bioinformatics. 2017; 18:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenberg M., Ridgely M.S.. Clinical decision support and malpractice risk. JAMA. 2011; 306:90–91. [DOI] [PubMed] [Google Scholar]
- 20. Klasco R. DRUGDEX® system (electronic version). Thomson MICROMEDEX. Greenwood Village, Colorado, USA. 2009; 1:06–09. [Google Scholar]
- 21. MacDonald K., Potvin K.. Interprovincial variation in access to publicly funded pharmaceuticals: a review based on the WHO anatomical therapeutic chemical classification system. Can. Pharm. J. 2004; 137:29–34. [Google Scholar]
- 22. Sikka R., Magauran B., Ulrich A., Shannon M.. Bench to bedside: pharmacogenomics, adverse drug interactions, and the cytochrome P450 system. Acad. Emerg. Med. 2005; 12:1227–1235. [DOI] [PubMed] [Google Scholar]
- 23. Lynch T., Price A.. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician. 2007; 76:391–396. [PubMed] [Google Scholar]
- 24. Xiong G., Wu Z., Yi J., Fu L., Yang Z., Hsieh C., Yin M., Zeng X., Wu C., Lu A.et al.. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021; 49:W5–W14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landrum G. RDKit: open-source cheminformatics. 2016; [Google Scholar]
- 26. Rego N., Koes D. 3Dmol.js: molecular visualization with WebGL. Bioinformatics. 2015; 31:1322–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li D., Mei H., Shen Y., Su S., Zhang W., Wang J., Zu M., Chen W.. ECharts: a declarative framework for rapid construction of web-based visualization. Visual Informatics. 2018; 2:136–146. [Google Scholar]
- 28. Sheikh-Taha M., Asmar M.. Polypharmacy and severe potential drug–drug interactions among older adults with cardiovascular disease in the United States. BMC Geriatr. 2021; 21:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Triplitt C. Drug interactions of medications commonly used in diabetes. Diabetes Spectrum. 2006; 19:202–211. [Google Scholar]
- 30. Leite P.M., Martins M.A.P., Castilho R.O.. Review on mechanisms and interactions in concomitant use of herbs and warfarin therapy. Biomed. Pharmacother. 2016; 83:14–21. [DOI] [PubMed] [Google Scholar]
- 31. Walia K.S., Khan E.A., Ko D.H., Raza S.S., Khan Y.N.. Side effects of antiepileptics–a review. Pain Pract. 2004; 4:194–203. [DOI] [PubMed] [Google Scholar]
- 32. By the American Geriatrics Society Beers Criteria Update Expert, P. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2015; 63:2227–2246. [DOI] [PubMed] [Google Scholar]
- 33. Mallet L., Spinewine A., Huang A.. The challenge of managing drug interactions in elderly people. Lancet. 2007; 370:185–191. [DOI] [PubMed] [Google Scholar]
- 34. Zheng Y., Peng H., Zhang X., Zhao Z., Gao X., Li J.. DDI-PULearn: a positive-unlabeled learning method for large-scale prediction of drug–drug interactions. BMC Bioinformatics. 2019; 20:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nyamabo A.K., Yu H., Shi J.Y.. SSI-DDI: substructure-substructure interactions for drug–drug interaction prediction. Brief. Bioinform. 2021; bbab133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.