Abstract
Background
Patent Ductus Arteriosus (PDA remains a significant cause of mortality and morbidity in premature infants. Indomethacin is an effective treatment to close a PDA, and has been used for many years with several treatment regimes, including prophylactic use in all at risk premature infants. There are however concerns regarding adverse side effects of indomethacin. By targeting a group of infants with an asymptomatic PDA, rather than treating all VLBW infants prophylactically, indomethacin use would be restricted, limiting the possibility of significant side effects to those with greater chance of benefit.
Objectives
To assess whether in premature neonates with asymptomatic PDA, treatment with indomethacin improves short and long term outcomes; in particular: incidence of symptomatic PDA, mortality, chronic neonatal lung disease (CLD), intraventricular haemorrhage (IVH), retinopathy of prematurity (ROP), neurodevelopmental outcome, length of ventilation.
Search methods
Standard strategies of the Cochrane Neonatal Review Group were used. Searches were made of the Oxford Database of Perinatal Trials, MEDLINE and EMBASE from 1966 to September 2002, CINAHL from 1982 to September 2002, and the Cochrane Controlled Trials Register (CENTRAL/CCTR) in The Cochrane Library, Issue 3, 2002. Searches were also made of previous reviews including cross‐referencing, abstracts, and conference and symposia proceedings published in Pediatric Research.
Selection criteria
All randomised controlled trials of indomethacin compared with placebo or no intervention for the treatment of asymptomatic PDA in premature infants were eligible.
Data collection and analysis
Standard methods of the Cochrane Neonatal Review Group were used. Trials identified by the search strategy were independently reviewed by each author and assessed for eligibility and trial quality. Data were then extracted independently by each author and compared, with any differences resolved following discussion. Any additional information required was requested from trial authors. Only published data was available for review. Results are expressed as typical relative risk and typical risk difference for dichotomous outcomes, and weighted mean difference for continuous variables.
Main results
Three small trials involving a total of 97 infants were included. Meta analysis of combined data was possible for seven outcomes. Treatment of an asymptomatic PDA with indomethacin significantly reduced the incidence of symptomatic PDA (RR 0.36, 95% CI 0.19, 0.68) and duration of supplemental oxygen (WMD ‐12.5, 95% CI ‐23.8, ‐1.26). There was no evidence of effect on mortality (RR 1.32, 95% CI 0.45, 3.86), CLD (RR 0.91, 95% CI 0.62, 1.35), IVH (RR 1.21, 95% CI 0.62, 2.37), ROP (RR 0.68, 95% CI 0.26, 1.78), or length of ventilation (WMD ‐7.00 days, 95%CI ‐17.33, 3.34). Long term neurodevelopmental outcomes were not reported. One trial reported a significant reduction in the duration of supplemental oxygen following treatment with indomethacin in the subgroup of infants with birth weight less than 1000g.
Authors' conclusions
This review demonstrates a significant decrease in the incidence of symptomatic PDA following treatment of an asymptomatic PDA with indomethacin. There is also a small but statistically significant decrease in the duration of requirement for supplemental oxygen. There are no reported long term outcomes in the included trials, and so it is not possible to comment on possible long term effects. Further studies are required to determine the long term benefits or harms of closing a PDA prior to the onset of symptoms.
Plain language summary
Indomethacin for asymptomatic patent ductus arteriosus in preterm infants
Indomethacin for very preterm or small babies with signs of PDA but no symptoms can prevent PDA, but more research is needed on long‐term outcomes. A common complication for very preterm (premature) or very small babies is PDA (patent ductus arteriosus). PDA is an open channel between the lungs and heart which should have closed after birth, and can cause life‐threatening complications. Indomethacin is often given to all babies at risk to prevent PDA, but it can cause adverse effects. It can also be given only to those babies who have early signs of PDA, but who have not yet developed symptoms. The review of trials found that this selective use of indomethacin can prevent PDA and has short‐term benefits, but more research is needed on longer term outcomes.
Background
Patent ductus arteriosus (PDA) remains a significant cause of morbidity amongst premature infants, especially very‐low‐birth‐weight (VLBW <1500g)and extremely‐low‐birth‐weight (ELBW <1000g) infants.
The ductus arteriosus is patent at birth, closing within 72 hours in the majority of term infants as part of the adaptation to extrauterine life. This process may be delayed in premature infants, especially in the presence of significant lung disease. Cotton estimated that 44% of infants less than 1500g birth weight will develop breathlessness and congestive cardiac failure due to a PDA (Cotton 1978) while Evans more recently demonstrated that in preterm infants without respiratory distress syndrome, 90% of ductus arteriosus will be closed by 60 hours of life (Evans 1990).
A PDA is initially asymptomatic due to high pulmonary artery pressures immediately after birth limiting left to right shunting through the ductus. As the pulmonary artery pressure falls after birth the volume of blood flowing left to right through a PDA will increase, leading to a haemodynamically significant shunt. Once a significant shunt is present, it is possible that increased pulmonary blood flow causes damage to premature lungs (Brown 1979; Evans 1995). Thus, treating an asymptomatic (non haemodynamically significant) PDA, rather than delaying therapy until a haemodynamically significant PDA is clinically evident, may result in improved outcomes.
A PDA can be closed pharmacologically with prostaglandin synthesis inhibitors, the most frequently used being indomethacin. Indomethacin inhibits prostaglandin synthesis, with the resultant vasoconstriction assisting ductal closure. However, the vasoconstriction is not selective and may also result in decreased blood flow to brain, gut and kidneys. Studies have demonstrated decreased urine output, increased serum creatinine, decreased serum sodium and decreased cerebral blood flow following indomethacin administration (Betkurer 1981; Kang 1999; Edwards 1990). Decreased gastrointestinal tract blood flow and gut perforation have also been shown to be associated with indomethacin administration (Dyess 1993; Shorter 1999).
The first randomised controlled trials of indomethacin for PDA were for treatment of symptomatic PDA and were performed early in the 1980s (Nestrud 1980; Yanagi 1981). The first multicentre trial of indomethacin for PDA was reported in 1983 (Gersony 1983), confirming its efficacy. Numerous trials have been performed using various criteria for inclusion and dosage regimens (Knight 2001).
Randomised controlled trials of prophylactic indomethacin in preterm infants were first published in the 1980s. These trials randomised infants to receive indomethacin or placebo before 24 hours of age in order to prevent intraventricular haemorrhage and PDA. Although a systematic review confirmed benefits of prophylactic indomethacin use in preventing these two conditions (Fowlie 2002), enough concern regarding potential side effects exists to have prevented universal implementation of the prophylaxis strategy. By targeting a group of infants with an asymptomatic PDA, rather than treating all VLBW infants prophylactically, indomethacin use would be restricted, limiting the possibility of significant side effects to those with greatest chance of benefit.
Ductal patency may be suspected on the basis of clinical signs and/or demonstrated by echocardiography (Hirsmaki 1990; Davis 1995). Ductal patency without haemodynamic significance may be defined as PDA without clinical signs of cardiac failure, or without echocardiographic signs such as increased LA:Ao ratio or positive Doppler flow which may indicate a haemodynamically significant left to right shunt (Mellander 1987).
This systematic review will answer the question: does the treatment of asymptomatic (non haemodynamically significant) PDA with indomethacin decrease mortality and morbidity in premature infants? In order to avoid inclusion of trials in which indomethacin was administered prophylactically, only trials in which treatment was commenced after 24 hours of age will be considered. A different Cochrane systematic review will address the issue of indomethacin treatment of a PDA which has become haemodynamically significant (Blakely 2001).
Objectives
The primary objective of this systematic review is to answer the question: Does the treatment of asymptomatic (non haemodynamically significant) PDA with indomethacin decrease mortality and morbidity in premature infants?
Subgroup analyses will be performed to determine whether the effects of indomethacin vary according to:
a) how asymptomatic PDA was diagnosed i) clinical diagnosis ii) echocardiographic diagnosis
b) how indomethacin was administered i) dose ii) duration of treatment iii) timing of treatment iv) route of administration
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials
Types of participants
Premature newborn infants (less than 37 weeks gestation) with asymptomatic PDA who received treatment after 24 hours of age. Infants with an asymptomatic PDA were defined as those who were known to have a PDA (identified clinically or by echocardiogram) without any clinical or radiologic evidence of heart failure.
Types of interventions
Indomethacin administered either enterally or parenterally, compared with either placebo or no treatment.
Types of outcome measures
One or more of the following outcomes must be reported:
Neonatal mortality
Mortality prior to hospital discharge
Chronic neonatal lung disease (oxygen requirement at 28 days)
Chronic neonatal lung disease (oxygen requirement at 36 weeks)
ROP (grades 3/4)
Intraventricular haemorrhage (all)
Intraventricular haemorrhage (grade III/IV)
Cystic intracerebral lesions
Symptomatic PDA confirmed on echocardiogram
PDA treated with indomethacin
PDA treated with surgery
NEC (all grades ‐ Bell's criteria)
Requirement for, and length of ventilatory support
Neurodevelopmental outcome (cerebral palsy, sensorineural hearing loss, visual impairment, developmental delay)
Length of hospitalisation
Bleeding complications, with or without requirement for transfusion
Gut perforation
Renal impairment
Search methods for identification of studies
Using MeSH search terms 'ductus arteriosus' and 'infant, newborn', and text search term 'indomethacin' searches were made of MEDLINE and EMBASE from 1966 to September 2002, CINAHL from 1982 to September 2002, and the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3, 2002). Previous reviews including cross references, abstracts, and conference and symposia proceedings published in Pediatric Research were also be searched. No limitations were placed on searches with respect to language.
Data collection and analysis
Criteria and methods used to assess the methodological quality of the trials:
Standard method of the Cochrane Collaboration (Clarke 2002) and its Neonatal Review Group was used. The three reviewers worked independently to search for and assess trials for inclusion and methodological quality. Studies were assessed using the following key criteria: blindness of randomisation, blindness of intervention, completeness of follow up and blinding of outcome measurement. Data were extracted independently by the reviewers. Differences were resolved by discussion and consensus of the reviewers. Where necessary, investigators were contacted for additional information or data.
Weighted mean differences (WMD) are reported for continuous variables such as duration of oxygen therapy. For categorical outcomes such as mortality, the relative risks (RR) and 95% confidence intervals are reported. For significant findings, the risk difference (RD) and number need to treat (NNT) are also reported. The fixed effects model has been used for meta‐analysis.
Results
Description of studies
See Characteristics of Included Studies table.
To answer the question posed by the review, it was important to identify studies in which infants had an identified PDA (determined clinically or echocardiographically) who were deemed to be asymptomatic at the time of study entry.
The search identified eight studies. Three were included, and five excluded.
Included studies randomised infants less than 1750g birthweight with PDA diagnosed clinically (Mahony 1982) or by contrast echocardiogram (Weesner 1987; Hammerman 1987). None had signs of a haemodynamically significant left to right shunt. Each study excluded infants for a number of reasons including signs of cardiac failure, active bleeding, renal impairment, in utero growth restriction and necrotising enterocolitis. Each of the included studies utilised 3 doses of indomethacin or placebo given intravenously, with doses of 0.1‐0.3 mg/kg/dose, at intervals of 12 to 24 hours.
One study was excluded in which no attempt was made to identify a PDA at the time of randomisation (Vogtmann 1988). Also excluded were those studies in which infants had documented symptoms consistent with a haemodynamically significant left to right shunt (Kaapa 1983; Merritt 1981; Mullett 1982; Van Overmeire 2001). In order to avoid the inclusion of studies of prophylactic indomethacin, the study which administered indomethacin prior to 24 hours of age was excluded (Kaapa 1983).
One study stratified randomisation into birth weight groups of more than and less than 1000g (Mahony 1982). Where dichotomous outcomes were analysed, the data for the groups were amalgamated. Where continuous data were analysed, the author was contacted to provide original data for the whole group. This was not available, and the subgroup results (mean and standard deviation) for the outcomes recording continuous data were combined using the methods described in Armitage 1994.
Risk of bias in included studies
See Additional Table 1 ‐ Methodological quality of included studies.
1. Methodological quality of included studies.
| Study | Randomisation | Allocation | Intervention | Follow up | Outcome assessment | |
| Hammerman 1987 | Double blind | By statistician | Indomethacin or placebo prepared by pharmacologist, unidentifiable by clinicians | 2 post randomisation exclusions ‐1 died day 1 (control group), 1 removed at request of clinician (exp group) | Follow up of 92% of enrolled infants | |
| Mahony 1982 | Double blind, Stratified according to birth weight (<1000g and >1000g) | By uninvolved physician | Indomethacin or placebo supplied in unmarked vials | 2 post randomisation exclusions due to IVH (placebo group) ‐ included in statistical analyses for IVH | Follow up of 96% of enrolled infants | |
| Weesner 1987 | Double blind | By hospital pharmacy ‐ sealed numbered envelopes | Indomethacin or placebo, unidentifiable to clinicians | 2 post randomisation exclusions ‐ 1 no PDA evident, 1 received both placebo and indomethacin in error | Follow up of 93% of enrolled infants |
All included studies were double blind randomised controlled trials of intravenous indomethacin versus placebo, in premature infants with identified patent ductus arteriosus. Randomisation was performed independently and allocation was concealed from treating clinicians in all studies. Placebo was utilised in all studies, in equal volumes to the volume of indomethacin, in order to blind intervention.
Follow up was greater than 90%, but not complete, for all of the studies. All studies had post randomisation exclusions. Where outcomes for excluded infants were apparent from the text of the paper, these outcomes were included in the statistical analyses. This resulted in the inclusion of two infants for the outcome of IVH from one study (Mahony 1982).
Mahony 1982 Assessment of PDA: Clinical Blinding of randomisation: Yes Blinding of intervention: Yes Complete follow up: No Blinding of outcome assessment: Can't tell
Hammerman 1987 Assessment of PDA: Contrast echocardiogram Blinding of randomisation: Yes Blinding of intervention: Yes Complete follow up: No Blinding of outcome assessment: Can't tell
Weesner 1987 Assessment of PDA: Contrast echocardiogram Blinding of randomisation: Yes Blinding of intervention: Yes Complete follow up: No Blinding of outcome assessment: Can't tell
Effects of interventions
Three small studies contributed data to this review (Mahony 1982; Hammerman 1987; Weesner 1987). All were well conducted randomised controlled trials.
The following outcomes were reported in at least two of the included studies ‐ mortality, chronic lung disease, IVH (all grades), ROP (all grades), incidence of symptomatic PDA, PDA ligation, length of ventilation, duration of supplemental oxygen. Necrotising enterocolitis and length of hospital stay were each reported in one study only. No long term outcomes such as neurodevelopmental disability were reported in any of the included studies.
Mortality Two studies reported this outcome (Weesner 1987; Mahony 1982). Neither study found a significant difference between treatment and control groups, and there was no significant difference found in the meta‐analysis (typical RR 1.32, 95% CI 0.45, 3.86)
Chronic lung disease Two studies reported the outcome of chronic neonatal lung disease as oxygen requirement at 28 days of life (Weesner 1987; Hammerman 1987). There was no evidence of effect in either of the individual trials or in the meta‐analysis (typical RR 0.91, 95% CI 0.62, 1.35).
Retinopathy of prematurity (all grades) / intraventricular haemorrhage (all grades) Both studies which reported these outcomes (Weesner 1987; Mahony 1982) found no evidence of effect on either retinopathy of prematurity or intraventricular haemorrhage. The meta‐analysis did not support an effect on either ROP (typical RR 0.68, 95% CI 0.26,1.78) or IVH (typical RR 1.21, 95% CI 0.62, 2.37).
Necrotising enterocolitis One trial reported the incidence of necrotising enterocolitis (Mahony 1982). There was no evidence of effect on the incidence of necrotising enterocolitis (typical RR 0.41, 95% CI 0.05, 3.68).
Symptomatic PDA / PDA ligation All three included studies reported the incidence of a symptomatic PDA. A significant reduction in the incidence of symptomatic PDA was observed after treatment with indomethacin compared with control in all studies. These results were confirmed by the meta‐analysis (typical RR 0.36, 95% CI 0.19, 0.68, typical RD ‐0.35, 95% CI 0.52, ‐0.17). This resulted in NNT of three infants treated with indomethacin in order to prevent one symptomatic PDA. When the need for PDA ligation was considered, two studies reported this outcome (Mahony 1982; Weesner 1987). While the rates of PDA ligation in each separate study were very different, no significant difference between treatment and control groups was seen. One study found a significant decrease in the need for PDA ligation in the indomethacin treated group in the subgroup of infants less than 1000g birth weight (2/10 vs 8/12, p<0.05) (Mahony 1982).
Length of ventilation There were no significant differences in the length of ventilation between the treatment and control groups for any of the studies who reported this outcome (Hammerman 1987; Weesner 1987; Mahony 1982). The meta‐analysis found no evidence of effect (WMD ‐7.00 days, 95% CI ‐17.33, 3.34) .
Length of hospital stay This outcome was reported in one study (Weesner 1987). No evidence of effect of treatment with indomethacin was found (MD ‐11.00 days, 95% CI ‐45.21, 23.21).
Duration of supplemental oxygen There were no significant differences in the duration of supplemental oxygen between the treatment and control groups for either study which reported these data for the group as a whole (Hammerman 1987; Weesner 1987). In the study where infants were stratified into birth weight groups (Mahony 1982) data were amalgamated using the methods described in Armitage 1994. This study showed a small but significant reduction in duration of supplemental oxygen (MD ‐14.7 days, 95% CI ‐28.4, ‐0.98) following the use of indomethacin. Combining all three trials in the meta‐analysis showed a significant reduction in the duration of supplemental oxygen (WMD ‐12.53 days, 95% CI ‐23.81, ‐1.26)
Neurodevelopmental outcome/ Cystic intracerebral lesions/ Gut perforation/ Renal impairment/ Bleeding complications None of the included studies reported data for any of these outcomes.
Subgroup Analyses Pre‐planned subgroup analyses with respect to how the PDA had been diagnosed (clinically or echocardiographically) and how indomethacin was administered were not performed because of insufficient data for each subgroup.
Discussion
The strict criteria for inclusion in this review meant that few trials were identified which fulfilled those criteria. All included trials were small, resulting in overall small numbers of infants for inclusion in the meta‐analysis. The quality of the three included studies was good, and follow up was greater than 90% for the short term outcomes considered. The findings of this review reflect those of other reviews with more liberal entry criteria and randomised controlled trials in both the treatment of asymptomatic and symptomatic PDA (Knight 1992; Knight 2001). The short term benefits of ductal closure and reduction in the duration of supplemental oxygen are seen, but no long term outcomes are reported which would permit determination of whether this confers any long term benefits. All three included studies found similar results for the incidence of symptomatic PDA and requirement for supplemental oxygen, showing consistent direction of effect. The reduction in risk due to indomethacin treatment of asymptomatic PDA means that to prevent one symptomatic PDA, three babies would need to be treated. With respect to the rates of PDA ligation, the marked differences observed between the two studies probably reflects institutional differences in threshold for PDA ligation.
Previous reviews including Cochrane review of indomethacin given prophylactically have demonstrated short term benefits of both decreased severe IVH (grades III and IV) and decreased incidence of PDA (Knight 2001; Knight 1992; Fowlie 2002). The finding of decreased IVH was not supported by the data in this review and it is likely that the number of infants included in this review was not large enough to demonstrate any significant difference in incidence of IVH between the treatment groups.
None of the included studies reported any measures of long term neurological outcome. Many studies (including those in this review) fail to consider long term outcomes, and instead use proxy measures of neurodevelopmental outcome such as significant intraventricular haemorrhage. A large, recently published trial of prophylactic indomethacin included neurodevelopmental outcomes at eighteen months of age; although grade III and IV IVH were significantly decreased in the indomethacin group, the long term neurological outcomes did not differ significantly (Schmidt 2001). This highlights the difficulties in using proxy measures of neurological outcome, and suggests that indomethacin has the potential to do harm, possibly by altering cerebral blood flow. By targeting a group of infants with proven PDA, the number of infants exposed to potential side effects and adverse long term neurological outcomes would be limited to those who would benefit from the treatment.
Several studies have investigated alternative non‐steroidal anti‐inflammatory drugs such as ibuprofen which are effective in closing the ductus, without a large impact on cerebral blood flow (Romagnoli 2000; Patel 2000). Ibuprofen has also been shown to be effective when compared with indomethacin in closing a PDA but again no data exist with respect to long term neurodevelopmental outcome (Van Overmeire 2000).
There appears to be no benefit in closing a PDA prior to the onset of symptoms with respect to the short term outcomes addressed by this review other than that of preventing symptomatic PDA. In a recent study in which infants with PDA were randomised to receive early or late indomethacin irrespective of the degree of left to right shunt, there was no significant difference in the numbers of haemodynamically significant PDAs requiring ligation, but significantly fewer courses of indomethacin were administered to the late treatment group, limiting the number of infants exposed to potential adverse side effects (Van Overmeire 2001).
Authors' conclusions
Implications for practice.
The use of indomethacin in the treatment of asymptomatic PDA in preterm infants has the short term benefit of prevention of symptomatic PDA. Long term outcomes were not reported in any of the included studies, so it was not possible to determine whether there are long term benefits or harms of the use of indomethacin in this population.
Implications for research.
Further studies are required to determine the long term benefits or harms of closing a PDA prior to the onset of symptoms.
What's new
| Date | Event | Description |
|---|---|---|
| 13 February 2009 | Amended | Updated contact details |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 21 May 2008 | Amended | Converted to new review format. |
Data and analyses
Comparison 1. Indomethacin vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.45, 3.86] |
| 2 Chronic lung disease in survivors | 2 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.35] |
| 3 Intraventricular haemorrhage ‐ all grades | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.62, 2.37] |
| 4 Necrotising enterocolitis | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.05, 3.68] |
| 5 Retinopathy of prematurity ‐ all grades (infants examined) | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.26, 1.78] |
| 6 Symptomatic PDA | 3 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.19, 0.68] |
| 7 PDA ligation | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.21] |
| 8 Length of ventilation ‐ days | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐5.00 [‐17.33, 3.34] |
| 9 Length of hospital stay ‐ Days | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐45.21, 23.21] |
| 10 Duration of supplemental oxygen ‐ days | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐12.53 [‐23.80, ‐1.26] |
1.1. Analysis.
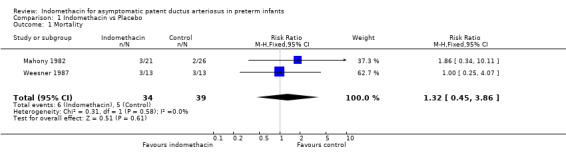
Comparison 1 Indomethacin vs Placebo, Outcome 1 Mortality.
1.2. Analysis.
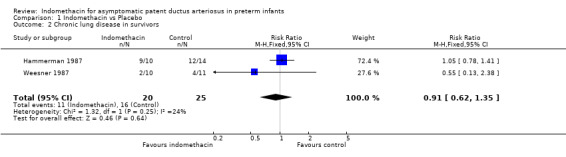
Comparison 1 Indomethacin vs Placebo, Outcome 2 Chronic lung disease in survivors.
1.3. Analysis.
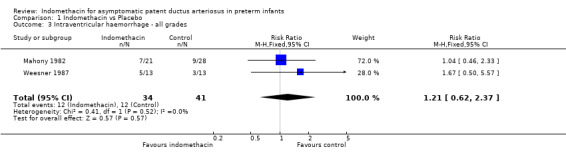
Comparison 1 Indomethacin vs Placebo, Outcome 3 Intraventricular haemorrhage ‐ all grades.
1.4. Analysis.
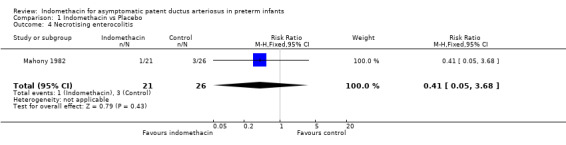
Comparison 1 Indomethacin vs Placebo, Outcome 4 Necrotising enterocolitis.
1.5. Analysis.
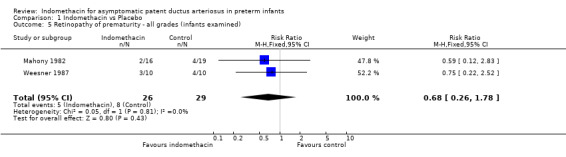
Comparison 1 Indomethacin vs Placebo, Outcome 5 Retinopathy of prematurity ‐ all grades (infants examined).
1.6. Analysis.
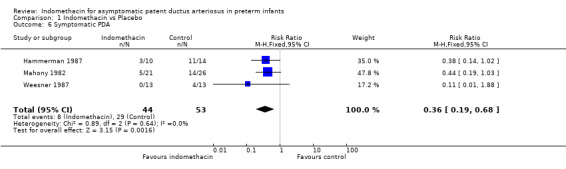
Comparison 1 Indomethacin vs Placebo, Outcome 6 Symptomatic PDA.
1.7. Analysis.
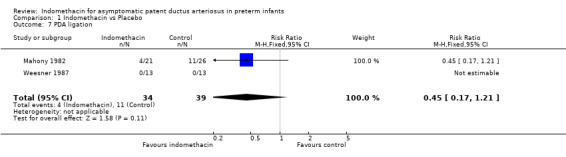
Comparison 1 Indomethacin vs Placebo, Outcome 7 PDA ligation.
1.8. Analysis.
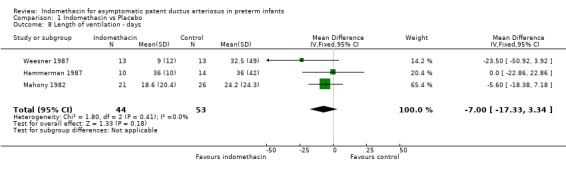
Comparison 1 Indomethacin vs Placebo, Outcome 8 Length of ventilation ‐ days.
1.9. Analysis.
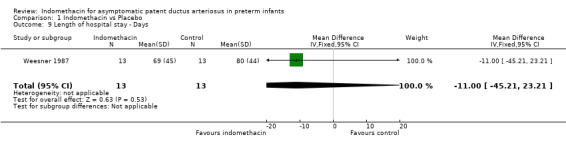
Comparison 1 Indomethacin vs Placebo, Outcome 9 Length of hospital stay ‐ Days.
1.10. Analysis.
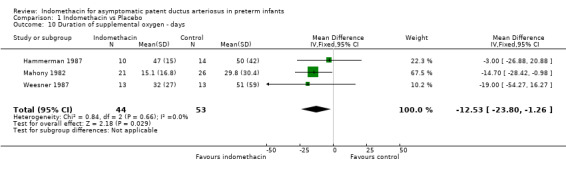
Comparison 1 Indomethacin vs Placebo, Outcome 10 Duration of supplemental oxygen ‐ days.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hammerman 1987.
| Methods | Double blind RCT of indomethacin vs placebo for infants with asymptomatic PDA Blinding of randomisation: Yes Blinding of intervention: Yes Complete follow up: No Blinding of outcome assessment: can't tell | |
| Participants | Infants <1000g birth weight with contrast echocardiogram evidence of PDA with left to right shunt at day 3 of life, in the absence of symptoms of left to right shunt. Excluded if congenital cyanotic heart disease, PPHN, or no UAC indomethacin n= 11 placebo n= 15 | |
| Interventions | Indomethacin or placebo 0.2mg/kg for 3 doses, 12 hours apart | |
| Outcomes | Chronic Lung disease, Symptomatic PDA, Treated PDA, Length of ventilation, Days of supplemental oxygen | |
| Notes | 2 post randomisation exclusions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mahony 1982.
| Methods | Double blind RCT of indomethacin vs placebo for infants with asymptomatic PDA Blinding of randomisation: Yes Blinding of intervention: Yes Complete follow up: No Blinding of outcome assessment: can't tell | |
| Participants | Infants <1700g birth weight with typical PDA murmur, and absence of signs of left to right shunt (active praecordium, bounding pulses, increased pulmonary vascular markings), without structural heart disease as demonstrated on echocardiogram. indomethacin n= 21 placebo n=28 | |
| Interventions | Indomethacin or placebo 0.2mg/kg at 0 hours, 0.1mg/kg at 12 hours & 0.1mg/kg at 36 hours | |
| Outcomes | Mortality, IVH, NEC, ROP, Symptomatic PDA, PDA ligation, Length of ventilation, Length of hospital stay, Days of supplemental oxygen | |
| Notes | 2 post randomisation exclusions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Weesner 1987.
| Methods | Double blind RCT of indomethacin vs placebo for infants with asymptomatic PDA Blinding of randomisation: Yes Blinding of intervention: Yes Complete follow up: No Blinding of outcome assessment: can't tell | |
| Participants | Infants <1750g birth weight ventilated with UAC in situ. Documented PDA on contrast echocardiogram, without clinical symptoms of left to right shunt indomethacin n=13 placebo n=14 | |
| Interventions | Indomethacin or placebo 0.3mg/kg repeated at 24, 48 & 72 hours if echo remains positive | |
| Outcomes | Mortality,Chronic lung disease, IVH, ROP, Symptomatic PDA, PDA ligation, Length of ventilation, Length of hospital stay, Days of supplemental oxygen | |
| Notes | 1 post randomisation exclusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kaapa 1983 | RCT of indomethacin for PDA ‐ symptomatic infants were not excluded. The median timing of first dose of indomethacin was less than 24 hours of age |
| Merritt 1981 | Not a trial of asymptomatic PDA. All randomised infants had clinical evidence of a left to right shunt and were therefore symptomatic |
| Mullett 1982 | No determination made of symptoms related to PDA. Infants included with both asymptomatic and symptomatic PDA |
| Van Overmeire 2001 | A randomised controlled trial of early indomethacin treatment. Infants noted to have one of three levels of left to right shunt at randomisation ‐ minor, moderate or severe; i.e. not all infants were asymptomatic |
| Vogtmann 1988 | No attempt made to identify infants with PDA ‐ all VLBW infants eligible with or without PDA. Indomethacin administered after 24 hours of age, and therefore unable to determine easily whether truly a trial of prophylactic indomethacin or treatment for asymptomatic PDA |
Contributions of authors
The primary author has been responsible for writing the background, methods, results and discussion sections, all of which have been reviewed by the other authors prior to submission. Trials identified by the search stategy were independently reviewed by each author and assessed for eligibility and trial quality. Data were extracted separately by the authors, and differences resolved by discussion. Data entry and analysis was performed by the primary author (LC)
Sources of support
Internal sources
Centre for Clinical Studies ‐ Women's and Children's Health, Mater Mothers' Hospital, South Brisbane, Queensland, Australia.
External sources
Department of Health and Ageing, Commonwealth Government, Canberra ACT ‐ Supporting the Centre for Clinical Studies, Mater Hospital, Australia.
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Hammerman 1987 {published and unpublished data}
- Hammerman C, Strates E, Komar K, Kim‐chi B. Failure of prophylactic indomethacin to improve the outcome of the very low birth weight infant. Developmental Pharmacology and Therapeutics 1987;10:393‐404. [DOI] [PubMed] [Google Scholar]
- Hammerman C, Strates E, Vailates S. The silent ductus: Its precursors and its aftermath. Pediatric Cardiology 1986;7:121‐7. [DOI] [PubMed] [Google Scholar]
Mahony 1982 {published data only}
- Mahony L, Carnero V, Brett C, Heymann M, Clyman R. Prophylactic indomethacin therapy for patent ductus arteriosus in very‐low‐birthweight infants. New England Journal of Medicine 1982;306:506‐10. [DOI] [PubMed] [Google Scholar]
- Mahony L, Heymann M, Carnero V, Brett C, Clymann R. When to treat the patent ductus arteriosus with indomethacin in very‐low‐birth‐weight infants. Advances in Prostaglandin, Thromboxane, and Leukotriene Research 1983;12:491‐4. [PubMed] [Google Scholar]
Weesner 1987 {published data only}
- Weesner K, Dillard R, Boyle R, Block S. Prophlactic treatment of asymptomatic patent ductus arteriosus in premature infants with respiratory distress syndrome. Southern Medical Journal 1987;80:706‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Kaapa 1983 {published data only}
- Kaapa P, Lanning P, Koivisto M. Early closure of patent ductus arteriosus with indomethacin in preterm infants with idiopathic respiratory distress syndrome. Acta Paediatrica Scandinavica 1983;72:179‐84. [DOI] [PubMed] [Google Scholar]
Merritt 1981 {published data only}
- Merritt T, Harris J, Roghmann K, Wood B, Campanella V, Alexson C, Manning J, Shapiro D. Early closure of the patent ductus arteriosus in very low birth weight infants: A controlled trial. Journal of Pediatrics 1981;99:281‐6. [DOI] [PubMed] [Google Scholar]
Mullett 1982 {published data only}
- Mullett M, Croghan T, Myerberg D, Krall J, Neal W. Indomethacin for closure of patent ductus arteriosus in prematures. Clinical Pediatrics 1982;21:217‐20. [DOI] [PubMed] [Google Scholar]
Van Overmeire 2001 {published data only}
- Overmeire B, Broek H, LAer P, Weyler J, Vanbaesebrouck P. Early versus late indomethacin treatment for patent ductus arteriosus in premature babies with respiratory distress syndrome. Journal of Pediatrics 2001;138:205‐11. [DOI] [PubMed] [Google Scholar]
Vogtmann 1988 {published data only}
- Vogtmann C, Grubbe G, Ruckhaberle K‐E, Bottcher H, Ockert C. The effects of an early indomethacin treatment on the manifestation of a patent ductus arteriosus in very low birth weight infants [Auswirkungen einer Fruhtherapie mit Indomethazin auf die Manifestation eines persistierenden Ductus arteriosus bei extrem untergewichtigen Fruhgeborenen]. Monatsschr Kinderheilkd 1988;136:636‐639. [PubMed] [Google Scholar]
Additional references
Armitage 1994
- Armitage PA, Berry G. Statistical Methods in Medical Research. 3rd Edition. Oxford: Blackwell, 1994:207‐11. [Google Scholar]
Betkurer 1981
- Betkurer MV, Yeh TF, Miller K, Glasser RJ, Pildes RS. Indomethacin and its effect on renal function and urinary kallikrein excretion in premature infants with patent ductus arteriosus. Pediatrics 1981;68:99‐102. [PubMed] [Google Scholar]
Blakely 2001
- Blakely ML, Kennedy KA, Lally KP, Tyson JE. Intravenous indomethacin for symptomatic patent ductus arterious in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003479] [DOI] [Google Scholar]
Brown 1979
- Brown ER. Increased risk of bronchopulmonary dysplasia in infants with patent ductus arteriosus. Journal of Pediatrics 1979;95:865‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1.5 [Updated April 2002] Updated quarterly. Cochrane Database of Systematic Reviews 2002, Issue 3. [Google Scholar]
Cotton 1978
- Cotton RB, Stahlman MT, Bender HW, Graham TP, Catterton WZ, Kovar I. Randomized trial of early closure of symptomatic patent ductus arteriosus in small preterm infants. Journal of Pediatr 1978;93:647‐651. [DOI] [PubMed] [Google Scholar]
Davis 1995
- Davis P, Turner GS, Cunningham K, Way C, Roberts R, Schmidt B. Precision and accuracy of clinical and radiological signs in premature infants at risk of patent ductus arteriosus. Archives of Pediatrics and Adolescent Medicine 1995;149:1136‐41. [DOI] [PubMed] [Google Scholar]
Dyess 1993
- Dyess DL, Peeples GL, Ardell JL, Tacchi EJ, Roberts WS, Ferrara JJ, et al. Indomethacin‐induced blood flow distribution in premature and full term piglets. Journal of Pediatric Surgery 1993;28:1396‐400. [DOI] [PubMed] [Google Scholar]
Edwards 1990
- Edwards AD, Wyatt JS, Richardson C, Potter A, Cope M, Delpy DT, et al. Effects of indomethacin on cerebral haemodynamics in very preterm infants. Lancet 1990;335:1491‐5. [DOI] [PubMed] [Google Scholar]
Evans 1990
- Evans N, Archer LN. Post natal circulatory adaptation in healthy term and preterm neonates. Archives of Disease in Childhood 1990;65:24‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1995
- Evans N, Iyer P. Longitudinal changes in the diameter of the ductus arteriosus in ventilated preterm infants: correlation with respiratory outcomes. Archives of Disease in Childhood. Fetal and Neonatal Edition 1995;72:F156‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fowlie 2002
- Fowlie PW. Intravenous indomethacin for preventing mortality and morbidity in very low birth weight infants (Cochrane Review). Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000174] [DOI] [PubMed] [Google Scholar]
Gersony 1983
- Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. Journal of Pediatrics 1983;102:895‐906. [DOI] [PubMed] [Google Scholar]
Hirsmaki 1990
- Hirsmaki H, Kero P, Wanne O. Doppler ultrasound and clinical evaluation in detection and grading of patent ductus arteriosus in neonates. Critical Care Medicine 1990;18:490‐3. [DOI] [PubMed] [Google Scholar]
Kang 1999
- Kang NS, Yoo KH, Cheon H, Choi BM, Hong YS, Lee JW, et al. Indomethacin treatment decreases renal blood flow velocity in human neonates. Biology of the Neonate 1999;76:261‐5. [DOI] [PubMed] [Google Scholar]
Knight 1992
- Knight DB. Patent ductus arteriosus: how important to which babies?. Early Human Development 1992;29:287‐92. [DOI] [PubMed] [Google Scholar]
Knight 2001
- Knight DB. The treatment of patent ductus arteriosus in preterm infants. A review and overview of randomised trials. Seminars in Neonatology 2001;6:63‐73. [DOI] [PubMed] [Google Scholar]
Mellander 1987
- Mellander M, Larsson LE, Ekstrom‐Jodal B, Sabel KG. Prediction of symptomatic patent ductus arteriosus in preterm infants using Doppler and m‐mode echocardiography. Acta Paediatrica Scandinavica 1987;76:553‐9. [DOI] [PubMed] [Google Scholar]
Nestrud 1980
- Nestrud RM, Hill DE, Arrington RW, Beard AG, Dungan WT, Lau PY, et al. Indomethacin treatment in patent ductus arteriosus. A double blind study using indomethacin plasma levels. Developmental Pharmacology and Therapeutics 1980;1:125‐36. [PubMed] [Google Scholar]
Patel 2000
- Patel J, Roberts I, Azzopardi D, Hamilton P, Ewards AD. Randomized Double‐Blind Controlled trial Comparing the effects of ibuprofen with indomethacin on Cerebral haemodynamics in preterm infants with patent ductus arteriosus. Pediatric Research 2000;47:36‐42. [DOI] [PubMed] [Google Scholar]
Romagnoli 2000
- Romagnoli C, Carolis MP, Papacci P, Polimeni V, Luciano R, Piersigilli F, Delogu AB, Tortorolo G. Effects of prophylactic ibuprofen on cerebral and renal haemodynamics in very preterm neonates. Clinical Pharmacology and Therapeutics 2000;67:676‐83. [DOI] [PubMed] [Google Scholar]
Schmidt 2001
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, Solimano A, Vincer M, Wright LL. Long‐term effects of indomethacin prophylaxis in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344:1966‐72. [DOI] [PubMed] [Google Scholar]
Shorter 1999
- Shorter NA, Liu JY, Mooney DP, Harmon BJ. Indomethacin associated bowel perforations: a study of possible risk factors. Journal of Pediatric Surgery 1999;34:442‐4. [DOI] [PubMed] [Google Scholar]
Van Overmeire 2000
- Overmeire B, Smets K, Lecoutere D, Broek H, Weyler J, Degroote K, Langhendries JP. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. New England Journal of Medicine 2000;343:674‐81. [DOI] [PubMed] [Google Scholar]
Yanagi 1981
- Yanagi RM, Wilson A, Newfeld EA, Aziz KU, Hunt CE. Indomethacin treatment for symptomatic patent ductus arteriosus: a double‐blind control study. Pediatrics 1981;67:647‐52. [PubMed] [Google Scholar]


