Abstract
Natural product (NP) has a long history in promoting modern drug discovery, which has derived or inspired a large number of currently prescribed drugs. Recently, the NPs have emerged as the ideal candidates to combine with other therapeutic strategies to deal with the persistent challenge of conventional therapy, and the molecular regulation mechanism underlying these combinations is crucial for the related communities. Thus, it is urgently demanded to comprehensively provide the disease-specific molecular regulation data for various NP-based drug combinations. However, no database has been developed yet to describe such valuable information. In this study, a newly developed database entitled ‘Natural Product-based Drug Combination and Its Disease-specific Molecular Regulation (NPCDR)’ was thus introduced. This database was unique in (a) providing the comprehensive information of NP-based drug combinations & describing their clinically or experimentally validated therapeutic effect, (b) giving the disease-specific molecular regulation data for a number of NP-based drug combinations, (c) fully referencing all NPs, drugs, regulated molecules/pathways by cross-linking them to the available databases describing their biological or pharmaceutical characteristics. Therefore, NPCDR is expected to have great implications for the future practice of network pharmacology, medical biochemistry, drug design, and medicinal chemistry. This database is now freely accessible without any login requirement at both official (https://idrblab.org/npcdr/) and mirror (http://npcdr.idrblab.net/) sites.
Graphical Abstract
Graphical Abstract.
NPCDR: natural product-based drug combination and its disease-specific molecular regulation.
INTRODUCTION
Compared with synthetic compounds, the natural products (NPs) show the unique advantages of metabolite-likeness (1), which makes them the main resource of marketed drugs (2). Recently, a variety of additional advantages of NPs have been identified, including good tolerability (3), low toxicity (4), poly-pharmacological modulation (5), etc. Due to these advantages, NP has emerged to be the ideal candidates to combine with other therapeutic agents for dealing with the persistent challenge of conventional therapies (6–8), which have then attracted tremendous research interest from worldwide scientists (9–11). Particularly, NP-based drug combinations are characterized by disease-specific molecular regulation (12,13), which make them able to achieve pharmacokinetic synergy by targeting multiple pathways or regulating the absorption, distribution, metabolism & excretion (ADME) profile of combined therapies (14,15), enhance the sensitivity of conventional therapy to disease cells or reversing drug resistance by acting in multi-specific manner (16), and reduce patients’ burden by lowering the effective dose of their accompanied therapies (6).
With the rapid advance of this research direction, many studies have been conducted, which has accumulated valuable data for the researchers in the diverse fields of: Network Pharmacology to uncover the molecular mechanisms (synergistic, potentiative or antagonistic (17)) underlying the traditional medicines of Africa, China, India, Mexico (18–20), Medical Biochemistry to identify disease marker (20), drug target (21) or target combination (22,23), and Medicinal Chemistry & Drug Design to discover new multitarget drug (24) or drug combination (25,26). To promote the development of these promising research directions, it is crucial to comprehensively collect the disease-specific molecular regulation data of NP-based drug combinations.
So far, many valuable databases have been constructed to provide the NP-related data. As shown in Table 1, some of them describe the traditional medicines around the world and their active or inactive ingredients (labeled by ‘TI’ in the second column of Table 1; e.g. HERB (27), SymMap (28), VIETHERB (29), BIOFACQUIM (30), ETCM (31), NANPDB (32), NuBBE (33), TCMID (34), etc.); some others provide the structural characteristics and biological activities of each NP (labeled by ‘SB’ in the second column of Table 1; e.g. NPASS (35), CMAUP (36), COCONUT (37), etc.); the remaining ones collect various NP data from certain species and their phylogenetic distributions (labeled by ‘PD’ in the second column of Table 1; e.g. StreptomeDB (38), CMNPD (39), etc.). Although these NP-related databases have their unique data coverage (the last column of Table 1), none of them contains the NP-based drug combinations. For the available databases offering drug combination information (e.g. DCDB (40), DrugCombDB (41), etc.), none of them specifies the identity of NP, let alone describes the NP-induced clinical effect on the accompanied conventional therapies (especially drugs; the seventh column of Table 1). Thus, it is essential to have a new database that describes the molecular regulations of NP-based drug combinations.
Table 1.
A variety of databases available for providing the data of natural product or drug combination (the first is the new database proposed in this study, and the remaining ones are those available databases in alphabetical order)
| Database | Data of natural product (NP) | Data of drug combination | Disease indication | Clinical status | Target or molecular regulation | NP’s effects on the efficacy of conventional therapy | Unique data contents provided in each database |
|---|---|---|---|---|---|---|---|
| NPCDR | ○ | ○ | ○ | ○ | ○ | ○ | NP-based drug combinations and their molecular regulations on targets |
| BIOFACQUIM | ○ | × | × | × | × | × | NPs isolated & characterized in Mexico and the structure-related data |
| CMAUP | ○ | × | ○ | × | ○ | × | Multi-target activities of functionally useful (e.g., food, medicinal) plants |
| CMNPD | ○ | × | × | × | ○ | × | Comprehensive data describing the various marine natural products |
| COCONUT | ○ | × | × | × | × | × | Aggregated data of the elucidated or predicted NPs from open sources |
| DCDB | × | ○ | ○ | ○ | ○ | × | The first database offering clinically important drug combinations |
| DrugCombDB | × | ○ | ○ | ○ | ○ | × | Dose responses of drug combinations found by high-throughput screening |
| ETCM | ○ | × | × | × | × | × | Ingredients, herbs, and formulas of traditional Chinese medicine (TCM) |
| HERB | ○ | × | ○ | × | ○ | × | High-throughput experimental and reference-guided TCM data |
| NANPDB | ○ | × | × | × | × | × | Natural products primarily collected from Northern African sources |
| NPASS | ○ | × | × | × | ○ | × | Experimental target activities and species origins of natural products |
| NuBBE | ○ | × | × | × | × | × | Chemical & biological diversities of the NPs originated from Brazil |
| StreptomeDB | ○ | × | × | × | × | × | Natural compounds isolated from the Streptomyces species |
| SymMap | ○ | × | ○ | × | ○ | × | Integrative data of TCM enhanced by symptom mapping strategy |
| TCMID | ○ | × | ○ | × | ○ | × | Ingredient, herb, disease, and target data and their relations in TCM |
| VIETHERB | ○ | × | ○ | × | × | × | NP, disease, morphology data of the Vietnamese herbal species |
The existence and non-existence of certain data type were indicated using ‘○’ and ‘×’, respectively. The unique contents covered by each database were briefly described in the last column.
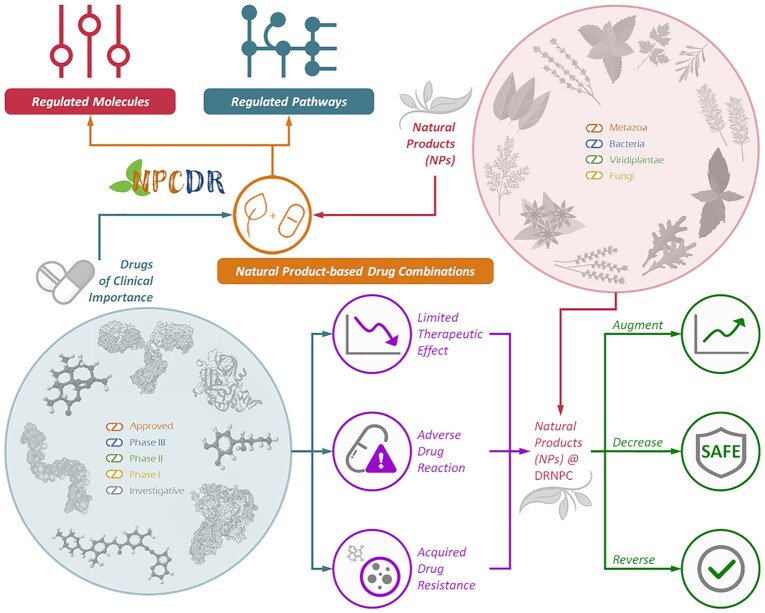
Herein, a newly constructed database, Natural Product-based Drug Combination and Its Disease-specific Molecular Regulation (NPCDR) was therefore introduced to provide the comprehensive molecular regulation data of NP-based drug combinations in various disease cell lines and model organisms. First, a number of clinically important drugs were collected from DrugBank (42) and TTD (43), and a systematic literature review on the NPs that were reported to combine with these drugs was conducted. As a result, the collected NP-based drug combinations (as shown in Figure 1) were found to (a) enhance drug efficacy by augmenting drug sensitivity (44,45) and achieving therapeutic synergy (46,47), (b) decrease the adverse drug reaction (48,49) and (c) reverse drug resistance (50,51). Second, the molecules or pathways regulated by these collected combinations were manually identified by additional literature review, and their regulation profiles (expression up/down-regulation, increased/decreased phosphorylation, etc.) were explicitly provided (shown in Figure 1). Finally, those data in NPCDR were fully cross-linked to well-established databases (UniProt (52), TTD (53), KEGG (54), NCBI Gene (55), VARIDT (56), BRENDA (57), INTEDE (58), TCDB (59), Pfam (60), Cellosaurus (61), miRbase (62), etc.) to facilitate the prediction of drug safety or sensitivity, the assessment of drug–drug interactions, and the discovery of detailed information for each NP or drug. Because of such unique characteristics and data provided online, NPCDR (https://idrblab.org/npcdr/) is expected to have great implications for the future practice of network pharmacology, medical biochemistry, medicinal chemistry and drug design.
Figure 1.
The unique contents and characteristics of NPCDR. The NPs were reported to enhance drug efficacy by augmenting drug sensitivity/achieve therapeutic synergy, decrease adverse drug reaction and reverse drug resistance. Thus, NPCDR is UNIQUE in providing the comprehensive NP-based drug combinations & describing their clinically/experimentally validated therapeutic effects, and describing the disease-specific regulations of molecules and pathways for a number of NP-based drug combinations.
FACTUAL CONTENT AND DATA RETRIEVAL
Collecting the regulation data for each combination
NP-based drug combinations together with their disease-specific molecular regulation data were collected using the sequentially steps shown below. First, a number of clinically important drugs were identified by retrieving from DrugBank (42) and TTD (43), which resulted in ∼2000 drugs approved by FDA, ∼9000 drugs in clinical trial, and ∼1000 preclinical or patented drugs. Second, 50 000 NPs were retrieved from existing NP-related databases: NPACT (63), HERB (27), ETCM (31), SANCDB (64), NANPDB (32), BIOFACQUIM (30), NuBBE DB (33) and VIETHERB (29). Third, NP-based drug combinations were collected by the literature review in PubMed (55) using such keyword combinations: ‘[NP name] + drug combination’, ‘[NP name] + combination’, ‘[NP name] + synergistic effects’, ‘[NP name] + synergy’, ‘natural product + [drug name]’, and so on. As a result, 1172 NP-based drug combinations between 425 NPs and 476 drugs were extensively identified and manually collected to the NPCDR database. Finally, the corresponding literatures of the newly collected NP-based drug combinations were carefully reviewed, and their regulating molecules and pathways (as illustrated in Figure 1) were recorded.
NP-based drug combinations and therapeutic effects
Among those newly identified 1172 NP-based drug combinations, the vast majority (93.5%) of them were between one NP and one drug, and the remaining ones (6.5%) were the combinations among >2 NPs/drugs (with at least one NP in each combination). Such newly collected NP-based drug combinations were reported to treat the disease indications of 218 classes as defined by the latest International Classification of Diseases (65) released by World Health Organization. These indication classes belonged to the extremely diverse super-classes, which could be classified to: infections (e.g. influenza, malaria, hepatitis virus, etc.), neoplasms (e.g. melanoma, breast cancer, leukemia, thymoma, etc.), metabolic disorders (e.g. hypoandrogenism, hyperlipidemia, diabetes, etc.), metal disorders (e.g. depression, schizophrenia, anxiety, etc.), nervous system diseases (e.g. Parkinson, Alzheimer, etc.), visual system disorders (e.g. retinal vein occlusion, glaucoma, optic nerve contusion, etc.), circulatory system diseases (e.g. arrhythmias, atherosclerosis, myocardial infarct, etc.), respiratory disorders (e.g. COPD, pulmonary fibrosis, etc.), digestive diseases (e.g. diverticulosis, ulcerative colitis, gastric ulcer, etc.), musculoskeletal diseases (e.g. osteomyelitis, rheumatoid arthritis, etc.), genitourinary diseases (e.g. nephropathy, etc.), and so on. Furthermore, the clinical developmental statuses of the NPs, drugs and drug combinations that were collected from ClinicalTrials.gov (66), and TTD (43), were all provided in the NPCDR database.
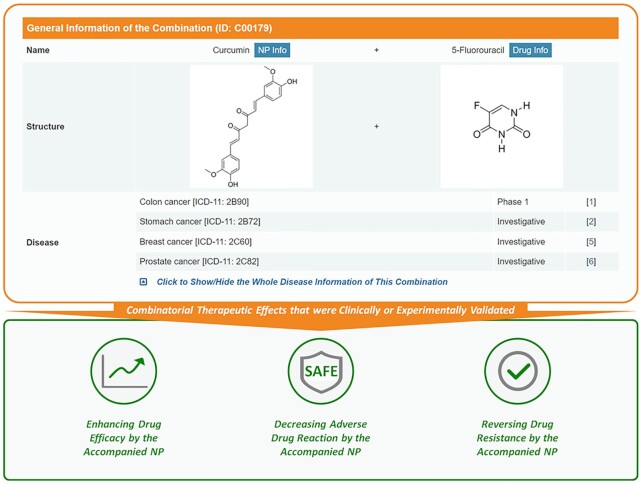
The administration of drugs was reported to be significantly restricted by their limited therapeutic effect (67), adverse drug reaction (68), acquired drug resistance (69) and so on. Natural products were thus reported capable of (a) enhancing drug efficacy via augmenting its sensitivity (44,45) or achieving therapeutic synergy (46,47), (b) decreasing adverse drug reactions (48,49) and (c) reversing drug resistance (50,51). To have such valuable data about NP-based regulations in this database, the improved therapeutic effects of NP on their corresponding drug were reviewed and explicitly described in NPCDR. Particularly, 58 NPs were reported to augment the sensitivity of 66 drugs in 184 combinations for the treatment of 38 diseases; 370 NPs were found to achieve therapeutic synergies with 430 drugs in 921 combinations for treating 184 diseases; 64 NPs were reported to decrease the adverse reaction of 57 drugs in 84 combinations for the treatment of 44 diseases; 57 NPs were discovered to reverse the resistances of 33 drugs in 93 combinations for the treatment of 27 diseases. As shown in Figure 2, the therapeutic effect of each NP-based drug combination was described, and the corresponding experiments for clinically or experimentally validating such therapeutic effects were shown in NPCDR. All in all, NPCDR covered a number of NP-based drug combinations, and was the first source describing the therapeutic effects of NP on enhancing drug efficacy, decreasing adverse drug reactions or reversing drug resistance.
Figure 2.
Combinatorial therapeutic effects that were clinically or experimentally validated. The NPs were reported able to enhance the drug efficacy by augmenting its sensitivity and achieving therapeutic synergy, decrease adverse drug reaction, and reverse drug resistance. The therapeutic effects of each NP-based drug combination were thus described in the bottom panel (highlighted using the green frame) of this figure.
Disease-specific regulation of molecules and pathways
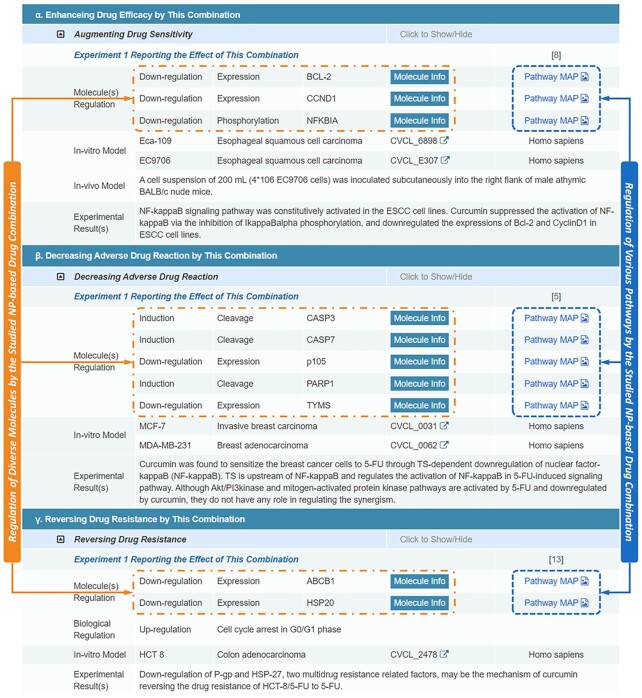
Disease-specific regulations of molecules and pathways by the collected drug combinations were carefully identified by literature review. Particularly, 518 molecules (primarily, protein and RNA) and 217 pathways (physiological or pathological) that were regulated by these drug combinations were provided in NPCDR. These regulated molecules were from 71 biochemical classes such as GPCR, peptidase, transcription factor, microRNA, kinase, ABC transporter and so on. As shown in Figure 3, the mechanisms of molecular regulations were explicitly described, which included the induction of protein degradation, the up/down-regulation of molecule's expression, cleavage, activity, phosphorylation or ubiquitination, and so on. Apart from these molecular regulation data, the biological regulation data of some drug combinations had also been reported, which included the induction of cell cycle arrest, inhibition of metabolites biosynthesis, accumulation of reactive oxygen species, extension of clotting time, induction of DNA damage, and so on. All in all, such data of molecular & biological regulation were essential for the understanding of the mechanisms underlying the NPs’ therapeutic effects on a particular drug to enhance its efficacy, decrease its adverse reaction, or reverse its acquired resistance.
Figure 3.
Regulation of molecules and pathways by NP-based drug combinations. Mechanisms of molecular regulation were explicitly described (including the induction of protein degradation, the up or down-regulation of molecule's cleavage, activity, phosphorylation, ubiquitination, and expression). The biological regulation of drug combinations was also provided (e.g. the induction of cell cycle arrest, inhibition of metabolite biosynthesis, etc.). These regulation data were linked to their in-vitro or in-vivo disease model, and an extended description on each regulated molecule could be accessed by clicking the ‘Molecule Info’ buttons.
As shown in Figure 3, all molecular & biological regulation data were described in NPCDR and linked to their in-vitro and in-vivo disease models (Figure 3), which made all the regulation data disease-specific and experimentally-verified (the disease names were identified according to the models applied in corresponding experiment, including different cell lines and model organisms). In total, 715 cell lines of a variety of disease & species origins together with 23 model organisms (including mouse, rat, rabbit, zebrafish, etc.) were collected in NPCDR to describe the regulation data of each drug combination. Moreover, a variety of experimental techniques that were applied to identify the molecular and biological regulations were also recorded, which included shRNA, siRNA, western-blot, qPCR, etc., and the analytical results of various experiments were recorded to give comprehensive information for each combination, and the extended descriptions on each regulated molecule can be accessed by clicking the ‘Molecule Info’ buttons given in Figure 3. Additionally, the pathways altered by the particular drug combination were also identified by the literature review. These identified pathways were then manually linked to available pathway data, such as KEGG (54), Reactome (70), Biocyc (71), SIGNOR (72) & Pathway Commons (73). All the regulated molecules were finally highlighted on their corresponding pathway maps (both the physiological and the pathological pathway maps).
Descriptions of the NP and drug in each combination
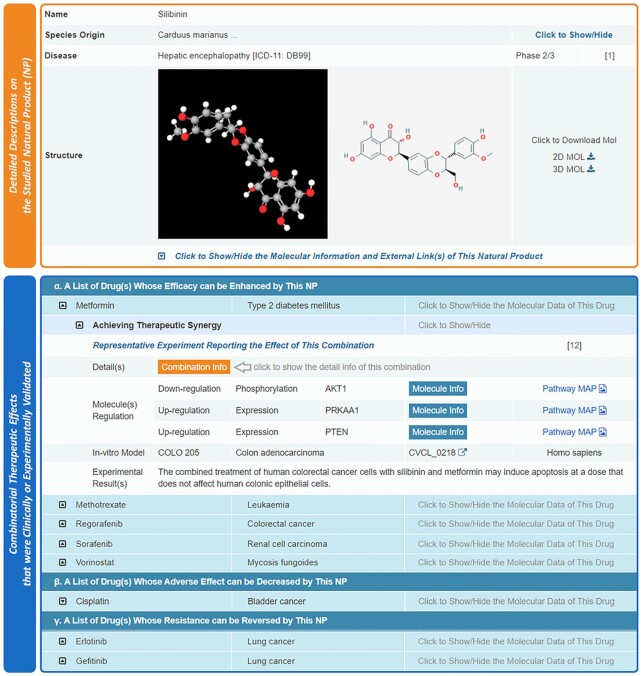
For each natural product (NP), the detailed descriptions on its general information were provided in NPCDR. As illustrated in Figure 4, the descriptions included NP name, NP synonyms, species origin(s), applied disease indication(s), 3D and 2D molecular structures in various formats (MOL and PNG, both could be directly downloaded), and other molecular information associated with the external links to: PubChem (55), TTD (43), HERB (27), ETMC (31), SymMap (28), TCMSP (74), and so on. Meanwhile, the combinatorial therapeutic effects of a particular NP on a list of drugs that were clinically/experimentally validated, were also described (as shown in Figure 4). These accompanied drugs were grouped based on three types of NP’s combinatorial effects: (a) a list of drugs whose efficacy can be enhanced by this NP, (b) a list of drugs whose adverse effect can be decreased by this NP and (c) a list of drugs whose resistance can be reversed by this NP. Under each therapeutic effect, the regulated molecules and pathways, in-vivo and in-vitro models, together with the results of experimental validations were demonstrated (illustrated in Figure 4). Based on the information provided on the NP page of NPCDR, the users could readily retrieve a list of drugs whose therapeutic effects were improved by this particular NP.
Figure 4.
The natural product (NP) page of this database. The general information (upper orange panel) and the combinatorial therapeutic effects of this NP (lower blue panel) were provided in NPCDR. Particularly, the combinatorial therapeutic effects of this NP on a list of drugs that were clinically/experimentally validated were shown. These accompanied drugs were grouped based on three types of combinatorial effects of NP: (a) a list of drugs whose efficacy can be enhanced by this NP, (b) a list of drugs whose adverse effects can be decreased by this NP and (c) a list of drugs whose resistance can be reversed by this NP.
Similar to the NP page, the drug page of NPCDR also provided the general information of certain drug. Such general information included drug name, drug synonyms, molecular type, the applied disease indication(s), 3D and 2D drug structures in various formats (MOL and PNG, both formats were directly downloadable), and other molecular information associated with the external links to ChEBI (75), GDSC (76), DrugBank (42), TTD (43) and PubChem (55). In the meantime, the combinatorial therapeutic effects of a drug on a list of NPs that were clinically or experimentally validated, were described. These accompanied NPs were grouped by three combinatorial effects of a drug: (a) a list of NPs capable of enhancing the efficacy of this drug, (b) a list of NPs capable of decreasing the adverse reactions of this drug and (c) a list of NPs able to reverse the resistance of this drug. Under each therapeutic effect, the regulated molecules and pathways, and validating experimental models (in-vivo/in-vitro, various cell lines/model organisms & experimental details) were fully collected and described. Based on the information provided on the NPCDR drug page, the audiences could readily retrieve a list of natural products that were capable of improving the therapeutic effects (enhancing drug efficacy, decreasing adverse drug reactions, or reversing drug resistance) of the corresponding drug described on that particular drug page.
Standardization and customized retrieval of NPCDR data
To make the access and analysis of NPCDR data convenient to all readers, the collected raw data were carefully cleaned up and then systematically standardized. These standardizations included: (a) all NPCDR diseases were standardized using the latest version of International Classification of Disease that was officially released by the World Health Organization (65); (b) all NPs, drugs, proteins, RNAs, pathways, cell lines, species and disease indications in this database were fully cross-linked to a number of well-established databases (UniProt (52), BRENDA (57), TTD (53), Pfam (60), KEGG (54), VARIDT (56), NCBI Gene (55), Cellosaurus (61), TCDB (59), INTEDE (58), miRbase (62), etc.), which could facilitate the prediction of drug safety or sensitivity, drug-drug interactions, and so on. These databases could also help to discover the detailed information for each molecule in this database. All NP-based drug combination data can be viewed, assessed, and downloaded from the NPCDR website, which is freely assessable without login requirement by all users at its official (https://idrblab.org/npcdr/) and mirror (http://npcdr.idrblab.net/) sites.
CONCLUSION
NP-based drug combinations have attracted broad interests from worldwide scientists, since they have great benefits in treating complex disease by regulating multiple targets/signaling pathways, enhancing the sensitivity of conventional therapy, and reversing drug resistance. Therefore, their valuable data (such as the clinically/experimentally-validated molecular regulations of target and pathway, disease indications, improved therapeutic effects and so on) provided in NPCDR could have great impacts on promoting the identification of NP-based drug, the investigation of disease mechanism, and the development of new computational method/software tool that facilitates the researches in network pharmacology, medical biochemistry, medicinal chemistry & drug design, etc. Those literature-supported and clinically-tested drug combinations collected in NPCDR are reported to be much more credible than the predicted/simulated data, which can thus serve as the gold standards for the construction of novel in-silico tools. Moreover, disease-specific molecular regulation data could help to clarify the elusive biological process underlying each combination, and inspire new therapeutic potential of the combinations in other disease indications.
Contributor Information
Xueni Sun, School of Pharmacy and Department of Medical Oncology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou 311121, China.
Yintao Zhang, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China.
Ying Zhou, State Key Laboratory for Diagnosis and Treatment of Infectious Disease, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang Provincial Key Laboratory for Drug Clinical Research and Evaluation, The First Affiliated Hospital, Zhejiang University, 79 QingChun Road, Hangzhou, Zhejiang 310000, China.
Xichen Lian, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China.
Lili Yan, School of Pharmacy and Department of Medical Oncology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou 311121, China.
Ting Pan, School of Pharmacy and Department of Medical Oncology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou 311121, China.
Ting Jin, School of Pharmacy and Department of Medical Oncology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou 311121, China.
Han Xie, School of Pharmacy and Department of Medical Oncology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou 311121, China.
Zimao Liang, School of Pharmacy and Department of Medical Oncology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou 311121, China.
Wenqi Qiu, Department of Surgery, HKU-SZH & Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
Jianxin Wang, School of Computer Science and Engineering, Central South University, Changsha 410083, China.
Zhaorong Li, Innovation Institute for Artificial Intelligence in Medicine of Zhejiang University, Alibaba-Zhejiang University Joint Research Center of Future Digital Healthcare, Hangzhou 330110, China.
Feng Zhu, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, China; Innovation Institute for Artificial Intelligence in Medicine of Zhejiang University, Alibaba-Zhejiang University Joint Research Center of Future Digital Healthcare, Hangzhou 330110, China.
Xinbing Sui, School of Pharmacy and Department of Medical Oncology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou Normal University, Hangzhou 311121, China.
FUNDING
National Natural Science Foundation of China [U1909208, 81872798, 82022075, 81874380, 82104207]; Natural Science Foundation of Zhejiang Province [LR21H300001, LR18H160001]; Leading Talent of ‘Ten Thousand Plan’ – National High-Level Talents Special Support Plan of China; Fundamental Research Fund for Central University [2018QNA7023]; Key R&D Program of Zhejiang Province [2020C03010]; Double Top-Class University Project [181201*194232101]; Alibaba-Zhejiang University Joint Research Center of Future Digital Healthcare; Alibaba Cloud; Information Technology Center of Zhejiang University. Funding for open access charge: National Natural Science Foundation of China [82022075].
Conflict of interest statement. None declared.
REFERENCES
- 1. Harvey A.L., Edrada-Ebel R., Quinn R.J.. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015; 14:111–129. [DOI] [PubMed] [Google Scholar]
- 2. Newman D.J., Cragg G.M.. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020; 83:770–803. [DOI] [PubMed] [Google Scholar]
- 3. Laskar Y.B., Mazumder P.B.. Insight into the molecular evidence supporting the remarkable chemotherapeutic potential of Hibiscus sabdariffa L. Biomed. Pharmacother. 2020; 127:110153. [DOI] [PubMed] [Google Scholar]
- 4. Zhang B., Jiang J., Wu P., Zou J., Le J., Lin J., Li C., Luo B., Zhang Y., Huang R.et al.. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm. Sin. B. 2021; 11:246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mok S.W., Wong V.K., Lo H.H., de Seabra Rodrigues Dias I.R., Leung E.L., Law B.Y., Liu L.. Natural products-based polypharmacological modulation of the peripheral immune system for the treatment of neuropsychiatric disorders. Pharmacol. Ther. 2020; 208:107480. [DOI] [PubMed] [Google Scholar]
- 6. Rejhová A., Opattová A., Čumová A., Slíva D., Vodička P.. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018; 144:582–594. [DOI] [PubMed] [Google Scholar]
- 7. Yuan R., Hou Y., Sun W., Yu J., Liu X., Niu Y., Lu J.J., Chen X.. Natural products to prevent drug resistance in cancer chemotherapy: a review. Ann. N. Y. Acad. Sci. 2017; 1401:19–27. [DOI] [PubMed] [Google Scholar]
- 8. Sauter E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020; 13:265–285. [DOI] [PubMed] [Google Scholar]
- 9. Lin S.R., Chang C.H., Hsu C.F., Tsai M.J., Cheng H., Leong M.K., Sung P.J., Chen J.C., Weng C.F.. Natural compounds as potential adjuvants to cancer therapy: preclinical evidence. Br. J. Pharmacol. 2020; 177:1409–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H.S., Qi S.H., Shen J.G.. One-compound-multi-target: combination prospect of natural compounds with thrombolytic therapy in acute ischemic stroke. Curr. Neuropharmacol. 2017; 15:134–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi J., Li J., Li J., Li R., Wu X., Gao F., Zou L., Mak W.W.S., Fu C., Zhang J.et al.. Synergistic breast cancer suppression efficacy of doxorubicin by combination with glycyrrhetinic acid as an angiogenesis inhibitor. Phytomedicine. 2021; 81:153408. [DOI] [PubMed] [Google Scholar]
- 12. Goossens J.F., Bailly C.. Ursodeoxycholic acid and cancer: from chemoprevention to chemotherapy. Pharmacol. Ther. 2019; 203:107396. [DOI] [PubMed] [Google Scholar]
- 13. Qureshi M.Z., Attar R., Romero M.A., Sabitaliyevich U.Y., Nurmurzayevich S.B., Ozturk O., Wakim L.H., Lin X., Ozbey U., Yelekenova A.B.et al.. Regulation of signaling pathways by beta-elemene in cancer progression and metastasis. J. Cell. Biochem. 2019; 120:12091–12100. [DOI] [PubMed] [Google Scholar]
- 14. Hemaiswarya S., Kruthiventi A.K., Doble M.. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008; 15:639–652. [DOI] [PubMed] [Google Scholar]
- 15. Afrin S., Giampieri F., Gasparrini M., Forbes-Hernandez T.Y., Cianciosi D., Reboredo-Rodriguez P., Zhang J., Manna P.P., Daglia M., Atanasov A.G.et al.. Dietary phytochemicals in colorectal cancer prevention and treatment: a focus on the molecular mechanisms involved. Biotechnol. Adv. 2020; 38:107322. [DOI] [PubMed] [Google Scholar]
- 16. Efferth T., Saeed M.E.M., Kadioglu O., Seo E.J., Shirooie S., Mbaveng A.T., Nabavi S.M., Kuete V.. Collateral sensitivity of natural products in drug-resistant cancer cells. Biotechnol. Adv. 2020; 38:107342. [DOI] [PubMed] [Google Scholar]
- 17. Jia J., Zhu F., Ma X., Cao Z., Cao Z.W., Li Y., Li Y.X., Chen Y.Z.. Mechanisms of drug combinations: interaction and network perspectives. Nat. Rev. Drug Discov. 2009; 8:111–128. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Z., Yang L., Hou J., Tian S., Liu Y.. Molecular mechanisms underlying the anticancer activities of licorice flavonoids. J. Ethnopharmacol. 2021; 267:113635. [DOI] [PubMed] [Google Scholar]
- 19. Yang Z., Zhang Q., Yu L., Zhu J., Cao Y., Gao X.. The signaling pathways and targets of traditional chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 2021; 264:113249. [DOI] [PubMed] [Google Scholar]
- 20. Ganguly A., Frank D., Kumar N., Cheng Y.C., Chu E.. Cancer biomarkers for integrative oncology. Curr. Oncol. Rep. 2019; 21:32. [DOI] [PubMed] [Google Scholar]
- 21. Nayak D., Katoch A., Sharma D., Faheem M.M., Chakraborty S., Sahu P.K., Chikan N.A., Amin H., Gupta A.P., Gandhi S.G.et al.. Indolylkojyl methane analogue IKM5 potentially inhibits invasion of breast cancer cells via attenuation of GRP78. Breast Cancer Res. Treat. 2019; 177:307–323. [DOI] [PubMed] [Google Scholar]
- 22. Isgut M., Rao M., Yang C., Subrahmanyam V., Rida P.C.G., Aneja R.. Application of combination high-throughput phenotypic screening and target identification methods for the discovery of natural product-based combination drugs. Med. Res. Rev. 2018; 38:504–524. [DOI] [PubMed] [Google Scholar]
- 23. Chamberlin S.R., Blucher A., Wu G., Shinto L., Choonoo G., Kulesz-Martin M., McWeeney S.. Natural product target network reveals potential for cancer combination therapies. Front. Pharmacol. 2019; 10:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alfei S., Turrini F., Catena S., Zunin P., Grilli M., Pittaluga A.M., Boggia R.. Ellagic acid a multi-target bioactive compound for drug discovery in CNS. Eur. J. Med. Chem. 2019; 183:111724. [DOI] [PubMed] [Google Scholar]
- 25. Mujumdar P., Kopecka J., Bua S., Supuran C.T., Riganti C., Poulsen S.A.. Carbonic anhydrase XII inhibitors overcome temozolomide resistance in glioblastoma. J. Med. Chem. 2019; 62:4174–4192. [DOI] [PubMed] [Google Scholar]
- 26. Wei G., Sun J., Luan W., Hou Z., Wang S., Cui S., Cheng M., Liu Y.. Natural product albiziabioside A conjugated with pyruvate dehydrogenase kinase inhibitor dichloroacetate to induce apoptosis-ferroptosis-M2-TAMs polarization for combined cancer therapy. J. Med. Chem. 2019; 62:8760–8772. [DOI] [PubMed] [Google Scholar]
- 27. Fang S., Dong L., Liu L., Guo J., Zhao L., Zhang J., Bu D., Liu X., Huo P., Cao W.et al.. HERB: a high-throughput experiment- and reference-guided database of traditional chinese medicine. Nucleic Acids Res. 2021; 49:D1197–D1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu Y., Zhang F., Yang K., Fang S., Bu D., Li H., Sun L., Hu H., Gao K., Wang W.et al.. SymMap: an integrative database of traditional chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019; 47:D1110–D1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen-Vo T.H., Le T., Pham D., Nguyen T., Le P., Nguyen A., Nguyen T., Nguyen T.N., Nguyen V., Do H.et al.. VIETHERB: a database for vietnamese herbal species. J. Chem. Inf. Model. 2019; 59:1–9. [DOI] [PubMed] [Google Scholar]
- 30. Pilón-Jiménez B.A., Saldívar-González F.I., Díaz-Eufracio B.I., Medina-Franco J.L.. BIOFACQUIM: a mexican compound database of natural products. Biomolecules. 2019; 9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu H.Y., Zhang Y.Q., Liu Z.M., Chen T., Lv C.Y., Tang S.H., Zhang X.B., Zhang W., Li Z.Y., Zhou R.R.et al.. ETCM: an encyclopaedia of traditional chinese medicine. Nucleic Acids Res. 2019; 47:D976–D982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ntie-Kang F., Telukunta K.K., Döring K., Simoben C.V., AF A.M., Malange Y.I., Njume L.E., Yong J.N., Sippl W., Günther S.. NANPDB: a resource for natural products from northern african sources. J. Nat. Prod. 2017; 80:2067–2076. [DOI] [PubMed] [Google Scholar]
- 33. Pilon A.C., Valli M., Dametto A.C., Pinto M.E.F., Freire R.T., Castro-Gamboa I., Andricopulo A.D., Bolzani V.S.. NuBBE: an updated database to uncover chemical and biological information from Brazilian biodiversity. Sci. Rep. 2017; 7:7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang L., Xie D., Yu Y., Liu H., Shi Y., Shi T., Wen C.. TCMID 2.0: a comprehensive resource for TCM. Nucleic Acids Res. 2018; 46:D1117–D1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeng X., Zhang P., He W., Qin C., Chen S., Tao L., Wang Y., Tan Y., Gao D., Wang B.et al.. NPASS: natural product activity and species source database for natural product research, discovery and tool development. Nucleic Acids Res. 2018; 46:D1217–D1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zeng X., Zhang P., Wang Y., Qin C., Chen S., He W., Tao L., Tan Y., Gao D., Wang B.et al.. CMAUP: a database of collective molecular activities of useful plants. Nucleic Acids Res. 2019; 47:D1118–D1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sorokina M., Merseburger P., Rajan K., Yirik M.A., Steinbeck C.. COCONUT online: collection of open natural products database. J. Cheminform. 2021; 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moumbock A.F.A., Gao M., Qaseem A., Li J., Kirchner P.A., Ndingkokhar B., Bekono B.D., Simoben C.V., Babiaka S.B., Malange Y.I.et al.. StreptomeDB 3.0: an updated compendium of streptomycetes natural products. Nucleic Acids Res. 2021; 49:D600–D604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lyu C., Chen T., Qiang B., Liu N., Wang H., Zhang L., Liu Z.. CMNPD: a comprehensive marine natural products database towards facilitating drug discovery from the ocean. Nucleic Acids Res. 2021; 49:D509–D515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y., Wei Q., Yu G., Gai W., Li Y., Chen X.. DCDB 2.0: a major update of the drug combination database. Database. 2014; 2014:bau124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu H., Zhang W., Zou B., Wang J., Deng Y., Deng L.. DrugCombDB: a comprehensive database of drug combinations toward the discovery of combinatorial therapy. Nucleic Acids Res. 2020; 48:D871–D881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Sajed T., Johnson D., Li C., Sayeeda Z.et al.. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018; 46:D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Y., Zhang S., Li F., Zhou Y., Zhang Y., Wang Z., Zhang R., Zhu J., Ren Y., Tan Y.et al.. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020; 48:D1031–D1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang W., Shi H., Chen C., Ren K., Xu Y., Liu X., He L.. Curcumin enhances cisplatin sensitivity of human NSCLC cell lines through influencing Cu-Sp1-CTR1 regulatory loop. Phytomedicine. 2018; 48:51–61. [DOI] [PubMed] [Google Scholar]
- 45. Lin J.H., Chen S.Y., Lu C.C., Lin J.A., Yen G.C.. Ursolic acid promotes apoptosis, autophagy, and chemosensitivity in gemcitabine-resistant human pancreatic cancer cells. Phytother. Res. 2020; 34:2053–2066. [DOI] [PubMed] [Google Scholar]
- 46. Hu X., Zhang Z.Y., Wu L.W., Zeng L.H., Chen H., Zhu H.J., Zhang J.K., Shao J., Zhang C., Li Y.L.et al.. A natural anthraquinone derivative shikonin synergizes with AZD9291 against wtEGFR NSCLC cells through reactive oxygen species-mediated endoplasmic reticulum stress. Phytomedicine. 2020; 68:153189. [DOI] [PubMed] [Google Scholar]
- 47. Zhang P., Lai Z.L., Chen H.F., Zhang M., Wang A., Jia T., Sun W.Q., Zhu X.M., Chen X.F., Zhao Z.et al.. Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice. J. Exp. Clin. Cancer Res. 2017; 36:190. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48. Baptista Moreno Martin A.C., Tomasin R., Luna-Dulcey L., Graminha A.E., Araujo Naves M., Teles R.H.G., da Silva V.D., da Silva J.A., Vieira P.C., Annabi B.et al.. [10]-gingerol improves doxorubicin anticancer activity and decreases its side effects in triple negative breast cancer models. Cell. Oncol. 2020; 43:915–929. [DOI] [PubMed] [Google Scholar]
- 49. Qu X., Li Q., Zhang X., Wang Z., Wang S., Zhou Z.. Amentoflavone protects the hematopoietic system of mice against gamma-irradiation. Arch. Pharm. Res. 2019; 42:1021–1029. [DOI] [PubMed] [Google Scholar]
- 50. Chen P., Huang H.P., Wang Y., Jin J., Long W.G., Chen K., Zhao X.H., Chen C.G., Li J.. Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J. Exp. Clin. Cancer Res. 2019; 38:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu J., Liu D., Niu H., Zhu G., Xu Y., Ye D., Li J., Zhang Q.. Resveratrol reverses doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017; 36:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. UniProt, C. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021; 49:D480–D489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li Y.H., Yu C.Y., Li X.X., Zhang P., Tang J., Yang Q., Fu T., Zhang X., Cui X., Tu G.et al.. Therapeutic target database update 2018: enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Res. 2018; 46:D1121–D1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K.. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017; 45:D353–D361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sayers E.W., Beck J., Bolton E.E., Bourexis D., Brister J.R., Canese K., Comeau D.C., Funk K., Kim S., Klimke W.et al.. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2021; 49:D10–D17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yin J., Sun W., Li F., Hong J., Li X., Zhou Y., Lu Y., Liu M., Zhang X., Chen N.et al.. VARIDT 1.0: variability of drug transporter database. Nucleic Acids Res. 2020; 48:D1042–D1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jeske L., Placzek S., Schomburg I., Chang A., Schomburg D.. BRENDA in 2019: a european ELIXIR core data resource. Nucleic Acids Res. 2019; 47:D542–D549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yin J., Li F., Zhou Y., Mou M., Lu Y., Chen K., Xue J., Luo Y., Fu J., He X.et al.. INTEDE: interactome of drug-metabolizing enzymes. Nucleic Acids Res. 2021; 49:D1233–D1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saier M.H., Reddy V.S., Moreno-Hagelsieb G., Hendargo K.J., Zhang Y., Iddamsetty V., Lam K.J.K., Tian N., Russum S., Wang J.et al.. The transporter classification database (TCDB): 2021 update. Nucleic Acids Res. 2021; 49:D461–D467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mistry J., Chuguransky S., Williams L., Qureshi M., Salazar G.A., Sonnhammer E.L.L., Tosatto S.C.E., Paladin L., Raj S., Richardson L.J.et al.. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021; 49:D412–D419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bairoch A. The cellosaurus, a cell-line knowledge resource. J. Biomol. Tech. 2018; 29:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kozomara A., Birgaoanu M., Griffiths-Jones S.. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019; 47:D155–D162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mangal M., Sagar P., Singh H., Raghava G.P.S., Agarwal S.M.. NPACT: naturally occurring plant-based anti-cancer compound-activity-target database. Nucleic Acids Res. 2013; 41:D1124–D1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hatherley R., Brown D.K., Musyoka T.M., Penkler D.L., Faya N., Lobb K.A., Bishop O.T.. SANCDB: a south african natural compound database. J. Cheminform. 2015; 7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lancet T. ICD-11: a brave attempt at classifying a new world. Lancet. 2018; 391:2476. [DOI] [PubMed] [Google Scholar]
- 66. DeVito N.J., Bacon S., Goldacre B.. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study. Lancet. 2020; 395:361–369. [DOI] [PubMed] [Google Scholar]
- 67. Vodenkova S., Buchler T., Cervena K., Veskrnova V., Vodicka P., Vymetalkova V.. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol. Ther. 2020; 206:107447. [DOI] [PubMed] [Google Scholar]
- 68. Martins F., Sofiya L., Sykiotis G.P., Lamine F., Maillard M., Fraga M., Shabafrouz K., Ribi C., Cairoli A., Guex-Crosier Y.et al.. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019; 16:563–580. [DOI] [PubMed] [Google Scholar]
- 69. Mullard A. Stemming the tide of drug resistance in cancer. Nat. Rev. Drug Discov. 2020; 19:221–223. [DOI] [PubMed] [Google Scholar]
- 70. Jassal B., Matthews L., Viteri G., Gong C., Lorente P., Fabregat A., Sidiropoulos K., Cook J., Gillespie M., Haw R.et al.. The reactome pathway knowledgebase. Nucleic Acids Res. 2020; 48:D498–D503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Karp P.D., Billington R., Caspi R., Fulcher C.A., Latendresse M., Kothari A., Keseler I.M., Krummenacker M., Midford P.E., Ong Q.et al.. The BioCyc collection of microbial genomes and metabolic pathways. Brief. Bioinform. 2019; 20:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Licata L., Lo Surdo P., Iannuccelli M., Palma A., Micarelli E., Perfetto L., Peluso D., Calderone A., Castagnoli L., Cesareni G.. SIGNOR 2.0, the signaling network open resource 2.0: 2019 update. Nucleic Acids Res. 2020; 48:D504–D510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rodchenkov I., Babur O., Luna A., Aksoy B.A., Wong J.V., Fong D., Franz M., Siper M.C., Cheung M., Wrana M.et al.. Pathway Commons 2019 Update: integration, analysis and exploration of pathway data. Nucleic Acids Res. 2020; 48:D489–D497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ru J., Li P., Wang J., Zhou W., Li B., Huang C., Li P., Guo Z., Tao W., Yang Y.et al.. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014; 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hastings J., Owen G., Dekker A., Ennis M., Kale N., Muthukrishnan V., Turner S., Swainston N., Mendes P., Steinbeck C.. ChEBI in 2016: improved services and an expanding collection of metabolites. Nucleic Acids Res. 2016; 44:D1214–D1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Iorio F., Knijnenburg T.A., Vis D.J., Bignell G.R., Menden M.P., Schubert M., Aben N., Goncalves E., Barthorpe S., Lightfoot H.et al.. A landscape of pharmacogenomic interactions in cancer. Cell. 2016; 166:740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]