Abstract
The virulence factor database (VFDB, http://www.mgc.ac.cn/VFs/) is dedicated to presenting a comprehensive knowledge base and a versatile analysis platform for bacterial virulence factors (VFs). Recent developments in sequencing technologies have led to increasing demands to analyze potential VFs within microbiome data that always consist of many different bacteria. Nevertheless, the current classification of VFs from various pathogens is based on different schemes, which create a chaotic situation and form a barrier for the easy application of the VFDB dataset for future panbacterial metagenomic analyses. Therefore, based on extensive literature mining, we recently proposed a general category of bacterial VFs in the database and reorganized the VFDB dataset accordingly. Thus, all known bacterial VFs from 32 genera of common bacterial pathogens collected in the VFDB are well grouped into 14 basal categories along with over 100 subcategories in a hierarchical architecture. The new coherent and well-defined VFDB dataset will be feasible and applicable for future panbacterial analysis in terms of virulence factors. In addition, we introduced a redesigned JavaScript-independent web interface for the VFDB website to make the database readily accessible to all users with various client settings worldwide.
INTRODUCTION
Bacterial pathogens are usually only approximately one micrometer in size with a small genome of several mega base pairs but nonetheless are complex organisms that continuously threaten public health worldwide (1). In recent years, astonishing progress has been made to understand the abilities of bacterial pathogens to adhere, invade, survive and persist in various niches, and the characteristics that enable them to be so successful with a diverse repertoire of virulence factors (VFs). The virulence factor database (VFDB, http://www.mgc.ac.cn/VFs/) summarizes the current knowledge of bacterial VFs and provides significant insights aimed at exposing possible targets for the development of novel treatment and preventive strategies (2).
Boosted by the rapid development of next-generation sequencing (NGS) technologies, newly emerging human/environmental microbiome studies have become a hotspot of molecular microbiology in recent years (3,4). Traditional microbiological studies generally rely on laboratory bacterium isolation and cultivation, while recent NGS-based metagenomic analyses have revealed a large number of unculturable bacteria, the majority of which are yet uncharacterized (5). Thus, the follow-up in-depth mining of the panbacterial microbiome data in terms of pathogenesis requires a well-organized reference category of all established bacterial VFs from various known pathogens (6). However, the commonly used nomenclature of current bacterial VFs usually follows the historical conventions of the field. Although most of the VF nomenclatures are indicative and meaningful, some can be vague or even misleading. For example, Escherichia coli fimbriae K88 and K99 were given K denominations just because they were initially wrongly identified as capsular antigens (7). In addition, the independent naming of homologous VFs in different bacteria also leads to considerable confusion and hampers follow-up panbacterial analyses. The existing general classification schemes such as the Gene Ontology (GO) and the Clusters of Orthologous Genes (COGs) are designed to cover entire biological processes and molecular functions of various living organisms (8,9), so they have very few categories associated with bacterial pathogenesis. The recent established Victors database introduced an ontology of host-pathogen interactions (10), but no VF classification information is available yet. Therefore, a well-defined classification scheme with a unified nomenclature is essential for future efficient data mining of bacterial VFs.
To better organize and present bacterial VFs in the database, the VFDB proposed an individual simplified classification scheme for each bacterial genus based on field conventions since inception in 2004 (2). Nevertheless, the current VFDB database covers 32 genera of well-studied bacterial pathogens, and so has 32 different classification schemes (Supplementary Table 1). As traditional microbiologists usually focus on the pathogenesis of one or several related bacteria only, the 32 classification schemes are generally independent of each other, although they share certain similarities (11). The absence of a unified classification scheme for all bacterial VFs from various pathogens poses a barrier to future panbacterial metagenomic analyses in terms of pathogenesis. Therefore, we recently proposed a general VF category in the database and reorganized the VFDB dataset accordingly to make it readily applicable for future panbacterial data mining. In addition, we introduced a redesigned JavaScript-independent web interface in the database to keep it easily accessible to all users with various client settings worldwide.
DATABASE UPDATES
VF category: a general classification scheme for bacterial VFs
Because of the confusion caused by the aforementioned different classification schemes, we recently introduced a unified VF category applicable to various bacterial pathogens into the VFDB database. Since the majority of the current classifications of various bacterial VFs have proven very useful and durable for phylogenetic analyses, we have tried to fully follow the existing conventions based on extensive literature mining. Thus, the newly proposed general classification scheme should be instantly familiar to and readily acceptable by traditional bacteriologists. However, unlike the previous scheme proposed in the 2012 release (12), which contains only four major bacterial VF categories (i.e. adhesion and invasion, secretion system, toxin, and iron acquisition), the newly established general classification scheme was designed to be a comprehensive system capable of covering all known bacterial VFs.
The VF category was constructed in a tree-like hierarchical architecture with 14 basal categories and >100 subcategories thus far (Table 1). The current basal categories not only fully cover the four major categories previously proposed but also refine all of them to make each category well defined and independent of each other. Adherence is usually the primary step in bacterial pathogenesis (13). Though the adhesion process is always followed by bacterial invasion into host cells by some intracellular pathogens, it is not necessary for many noninvasive bacteria. Hence, for clarity, the current scheme separates adherence and invasion into two independent categories, which consist of 1885 and 391 known bacterial VFs, respectively, in the current VFDB database (Table 1). Bacterial secretion systems are membrane-anchored nanomachines that enable the bacteria to transport various effector proteins either out of the cell into the surrounding niche or directly into the cytoplasm of eukaryotic/prokaryotic cells. A variety of different bacterial secretion systems (e.g. type III, type IV, type VI and type VII secretion systems) have been identified to play a critical role in bacterial pathogenesis (14). Interestingly, recent studies have revealed that the widespread extracellular contractile injection systems are capable of delivering effectors in a contact-independent manner and highly resemble type VI secretion systems (T6SSs) in the headless phage-like overall structure (15). The original category of secretion systems was therefore renamed effector delivery systems to improve their commonality to accommodate additional related bacterial VFs in the future. Generally, bacterial toxins include both exotoxins and endotoxins (16). However, in most contexts, they specifically refer to only exotoxins, which are proteins synthesized inside bacterial cells and then released to outside medium or target cells to produce virulence. In contrast, endotoxins are membrane-associated lipopolysaccharides (LPS) produced by only gram-negative bacteria that can induce a variety of host immune disorders. Given the distinct mechanisms of exotoxins and endotoxins, the previous category of toxins is now replaced by the more specific term ‘exotoxin’, whereas endotoxins are now grouped into an additional category of immune modulation (Table 1). Besides the aforementioned LPS, pathogenic bacteria have developed various mechanisms to control and modulate the host immune system to benefit their survival, such as anti-phagocytosis, disrupting and depleting the complement system, and interfering with the inflammatory signaling pathway (17). Iron is an essential nutrient for the proliferation and pathogenicity of bacterial pathogens. The well-characterized host defense strategy of iron sequestration highlights the crucial role of iron acquisition systems in bacterial pathogenesis. Nevertheless, recent studies show that many other nutritional requirements or metabolic adaptation strategies, such as other metal ions (e.g. zinc and magnesium), certain carbon, nitrogen, and sulfur sources, can also contribute to bacterial virulence, particularly for intracellular pathogens (18). Thus, we proposed a new category of nutritional/metabolic factors to cover and extend the original category of iron acquisition (Table 1).
Table 1.
The newly proposed VF category and the current statistics of known bacterial VFs from 32 genera in the VFDB database (as of October 2021)
| Category | Subcategorya | Number of VFs |
|---|---|---|
| Adherence | 1885 | |
| Fimbrial adhesin | 608 | |
| Non-fimbrial adhesin | 1273 | |
| Invasion | 391 | |
| Effector delivery system | 1242 | |
| Type II secretion system | 41 | |
| Type III secretion system | 228 | |
| Type IV secretion system | 141 | |
| Type V secretion system | 391 | |
| Type VI secretion system | 171 | |
| Type VII secretion system | 270 | |
| Motility | 189 | |
| Flagella-mediated motility | 91 | |
| Intracellular motility | 98 | |
| Exotoxin | 1101 | |
| Membrane-acting toxin | 893 | |
| Intracellularly active toxin | 188 | |
| Exoenzyme | 522 | |
| Hyaluronidase | 33 | |
| Kinase | 24 | |
| Coagulase | 15 | |
| Lipase | 19 | |
| Protease | 244 | |
| Nuclease | 43 | |
| Immune modulation | 1540 | |
| Antiphagocytosis | 180 | |
| Complement evasion/Serum resistance | 201 | |
| Immunoglobulin | 29 | |
| Antigen variation | 20 | |
| Apoptosis | 80 | |
| Inflammatory signaling pathway | 237 | |
| Biofilm | 297 | |
| Biofilm formation | 222 | |
| Quorum sensing | 75 | |
| Nutritional/Metabolic factor | 1912 | |
| Metal uptake | 900 | |
| Metabolic adaptation | 708 | |
| Stress survival | 492 | |
| Post-translational modification | 308 | |
| Antimicrobial activity/Competitive advantage | 86 | |
| Regulation | 1140 | |
| Others | 427 | |
aFor brevity, only the top level subcategories are listed. Full VF category is available from the VFDB database website (http://www.mgc.ac.cn/VFs/VFcategory.htm).
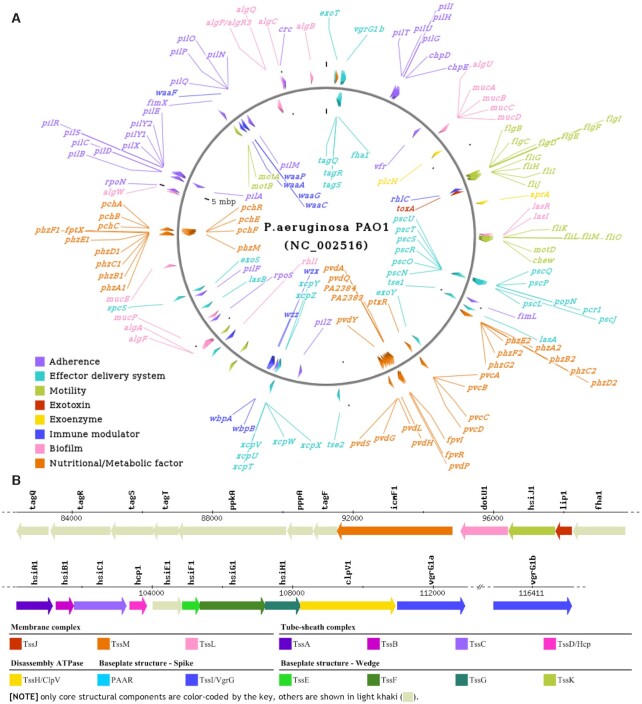
In addition to the aforementioned VF categories associated with the previous scheme, some additional categories were further introduced into the current scheme to represent the entire VF repertoires of pathogenic bacteria (Table 1). Exoenzymes are a group of special enzymes functioning outside of the producing cells to degrade macromolecules to small soluble molecules. In contrast to exotoxins that directly target host cells, some bacterial exoenzymes (e.g. hyaluronidase) contribute to virulence by facilitating the intracellular/intercellular spread of pathogens (19). Bacterial motility is an important capability for a successful pathogen to avoid hostile environments and discover useful resources for survival (20). Many pathogenic bacteria employ surface appendages such as flagella and type IV pili to drive motility. In addition, recent studies revealed that some bacterial pathogens are able to form a biofilm, which largely improves their resistance to antimicrobials and host immunities to contribute to the survival, dispersion and colonization of the bacteria (21). Bacterial defenses against various severe host conditions (e.g. acidic, oxidative and nitrosative stresses) are indispensable for successful colonization and disease pathogenesis (22). Further, some host-derived molecules such as antimicrobial peptides are critical components of the host defense system to protect against microorganisms. As a consequence, pathogenic bacteria have evolved diverse systems (e.g. AcrAB of Klebsiella and MtrCDE of Neisseria) to provide resistance to hostile molecules, so as to provide competitive advantages and contribute to bacterial virulence (23). Post-translational modification of some VFs has been demonstrated to be pivotal to activate virulence. For instance, both O-glycosylation of the flagellin structural protein and N-glycosylation of many membrane-associated proteins in Campylobacter jejuni are essential for bacterial adherence, invasion and colonization (24). Moreover, it has been established that for a successful pathogen the tight and fine regulation of each VF is as important as the possession of the VF itself (25). Table 1 lists the 14 basal categories along with the top level of subcategories for brevity, while a complete list of the current VF category is available from the database website (http://www.mgc.ac.cn/VFs/VFcategory.htm). The full enrollment of all known bacterial VFs into the new classification scheme enables easy visualization of the bacterial genome from the perspective of virulence categories. An example pathogenomic map of Pseudomonas aeruginosa available from the VFDB website is shown in Figure 1A, which is more informative than previous illustration using general classification scheme (Supplementary Figure 1A). Since the database presents a dynamic circular map created by the CGView package (26) for each of the 32 genera, it offers an intuitive overview of the molecular diversity of bacterial VFs from various pathogens.
Figure 1.
Examples of visualization of the newly proposed VF category available from the VFDB website. (A) A circular pathogenomic map of Pseudomonas aeruginosa. Each VF-related gene is denoted by a directional triangle and color-coded by the corresponding VF category (key). Gene name is indicated inside/outside of the circle with a hyperlink to the web page of gene details. (B) A linear illustration of the genomic locus encoding T6SS in P. aeruginosa. Each gene is represented by a horizontal arrow in the direction of its coding strand (to scale) and color-coded by the designated core structural component (key). For comparison, the counterparts of the circular and linear illustrations available from the previous VFDB website without VF category are shown in Supplementary Figure 1.
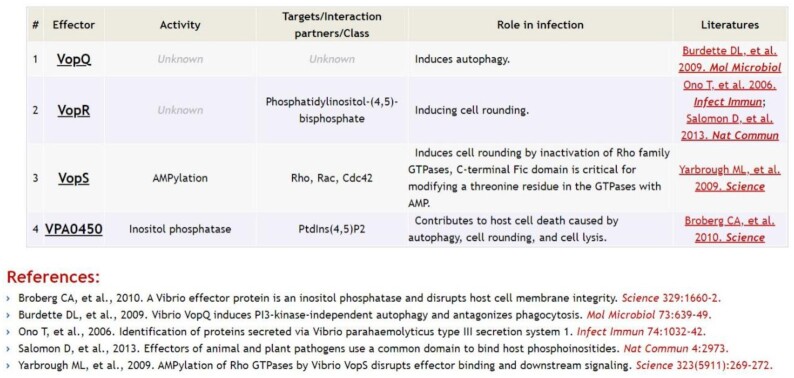
Many bacterial VFs comprise multiple structural components that need to be subtly assembled into a complex nanomachine for function, such as secretion systems and flagella. Accurate recognition of each structural component is critical for sequence homology-based identification of such complex bacterial VFs (15,27). Further, one recent alignment-independent machine-learning-based bacterial VF classification attempt also relied on a well-curated dataset of orthologous components to achieve high accuracy (28). Therefore, we further provided a unified nomenclature of the core structural components for these complex bacterial VFs to avoid confusion caused by independent naming in different bacteria. Figure 1B is an example of a linear map of the T6SS from P. aeruginosa that is color-coded by the designated core structural components, including the membrane complex (TssJ, TssM and TssL), baseplate structure spike (PAAR and TssI/VgrG) and wedge (TssE, TssF, TssG and TssK), tube-sheath complex (TssA, TssB, TssC and TssD/Hcp) and disassembly ATPase (TssH/ClpV). Since the unified nomenclature is specialized for these VF-related genes, it provides more valuable information across bacterial species than the previous version categorized by the COGs (Supplementary Figure 1B). In addition, we created a tabular list of all characterized effectors (where available) of each secretion system to highlight the activity, targets or interaction partners, role in infection and related literature of each effector (Figure 2). In contrast to the core structural components that are generally conserved among various pathogens, diverse secreted effectors are the most fascinating constituent of secretion systems that actually enable miscellaneous functions, such as host cell invasion, immune evasion, nutrients acquisition and growth competition. Thus, the detailed information in our table of effectors will benefit further biological interpretation of associated sequence analyses.
Figure 2.
The tabular list of detailed information on known effectors of the type III secretion system from Vibrio cholerae. Related publication(s) of each effector are shown after the table.
Redesigned basic web interface
Since the previous release in 2019, an enhanced alternative JavaScript-rich web interface was introduced into the VFDB to provide users with improved experiences through a set of interactive online analysis tools (29). To ensure that the database is easily accessible by all users worldwide, including those who have very limited computer/network settings, VFDB continues to maintain the traditional JavaScript-independent web pages as default. Nevertheless, the basic web pages of the VFDB were originally designed in 2004, which have a fixed page width setting of 800 pixels to make all contents visible in most of the former client monitors. This obsolete style severely limits the effective presentation of page contents for modern computers. Therefore, we redesigned the basic web interface with new styles to use percentage-based relative sizing rather than absolute width in pixels, so as to make the contents of each page automatically fit to the screen size of different users to maximize web page usage of various client settings.
DISCUSSION
One of the cutting-edge areas of current molecular microbiology is studies on microbial communities at the ecosystem level (e.g. microbiome). However, NGS-based panbacterial metagenomic data analyses require high-quality and well-curated reference datasets to produce meaningful results. The VFDB database is dedicated to providing the scientific community with a comprehensive knowledge base as well as a versatile analysis platform for bacterial VFs (2,29). The newly introduced general VF classification scheme will facilitate future mining of panbacterial metagenomic data from the aspect of bacterial pathogenesis, since explicit and detailed labelling of the reference dataset will allow both homology-based and machine-learning-based algorithms to efficiently classify the data and make fine-grained statistics or predictions. Moreover, the general VF category with unified nomenclature is valuable to the identification of potential novel VFs from uncultured bacterial genomes, which may in turn contribute to future discovery of unknown bacterial VFs.
However, we would like to stress that the current VF category is a preliminary and tentative scheme rather than a complete solution, since the classification of bacterial VFs is indeed complicated. On the one hand, as mentioned above, the current VF category has tried to be consistent with the existing community conventions of bacterial pathogenesis. However, the original researchers might begin to name and classify VFs based on different criteria, such as morphology, serology or functional characteristics. For instance, colonization factor antigens were named after their functions, whereas bundle-forming pili were designated based on their characteristic appearance. As a consequence, the current proposed VF category is actually based on the combination of structural and functional characteristics rather than a simple clear criterion. On the other hand, bacterial pathogens have evolved a multitude of strategies against prokaryotic competitors and eukaryotic hosts to colonize, invade and overcome the host immune response. Empirical evidence has demonstrated that many bacterial VFs are multifaceted proteins with various functions. For example, listeriolysin O (LLO), which is a major virulence factor of the facultative intracellular pathogen Listeria monocytogenes, is involved in multiple stages of the intracellular lifecycle of the bacterium and displays unique characteristics (30). It has long been known that following bacterial internalization into host cells, LLO disrupts the primary vacuole, enabling the bacterium to replicate into the host cell cytosol. LLO is also required for disruption of the newly formed secondary vacuole to release the bacterium into the cytosol, where it repeats its intracellular lifecycle. In addition, LLO can act as an invasin that stimulates L. monocytogenes internalization and affects the transcriptional activity of infected cells. Therefore, the structure of an ideal classification scheme of bacterial VFs might be net-like rather than tree-like.
In the future, the VFDB database will continue to integrate up-to-date knowledge of additional bacterial pathogens with medical importance to fulfill the demands of all users worldwide. Moreover, to circumvent the aforementioned dilemma behind the current classification scheme, an ontology-based computer-interpretable system tailored for bacterial VFs might be necessary to better depict the functional and/or structural features and the possible multiple roles in virulence for each characterized VF.
Supplementary Material
Contributor Information
Bo Liu, NHC Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, P. R. China.
Dandan Zheng, NHC Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, P. R. China.
Siyu Zhou, NHC Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, P. R. China.
Lihong Chen, NHC Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, P. R. China.
Jian Yang, NHC Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, P. R. China.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Natural Science Foundation of China [31970635]; CAMS Innovation Fund for Medical Sciences [2017-I2M-3-017]; National Scientific Data Sharing Platform for Population and Health. Funding for open access charge: National Natural Science Foundation of China.
Conflict of interest statement. None declared.
REFERENCES
- 1. van Oosten M., Hahn M., Crane L.M., Pleijhuis R.G., Francis K.P., van Dijl J.M., van Dam G.M.. Targeted imaging of bacterial infections: advances, hurdles and hopes. FEMS Microbiol. Rev. 2015; 39:892–916. [DOI] [PubMed] [Google Scholar]
- 2. Chen L., Yang J., Yu J., Yao Z., Sun L., Shen Y., Jin Q.. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res. 2005; 33:D325–D328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert J.A., Blaser M.J., Caporaso J.G., Jansson J.K., Lynch S.V., Knight R.. Current understanding of the human microbiome. Nat. Med. 2018; 24:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jansson J.K., Hofmockel K.S.. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020; 18:35–46. [DOI] [PubMed] [Google Scholar]
- 5. Human Microbiome Project C. A framework for human microbiome research. Nature. 2012; 486:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen L., Zheng D., Liu B., Yang J., Jin Q.. VFDB 2016: hierarchical and refined dataset for big data analysis–10 years on. Nucleic Acids Res. 2016; 44:D694–D697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bakker D., Vader C.E., Roosendaal B., Mooi F.R., Oudega B., de Graaf F.K.. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol. Microbiol. 1991; 5:875–886. [DOI] [PubMed] [Google Scholar]
- 8. Galperin M.Y., Wolf Y.I., Makarova K.S., Vera Alvarez R., Landsman D., Koonin E.V.. COG database update: focus on microbial diversity, model organisms, and widespread pathogens. Nucleic Acids Res. 2021; 49:D274–D281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gene Ontology, C. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021; 49:D325–D334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sayers S., Li L., Ong E., Deng S., Fu G., Lin Y., Yang B., Zhang S., Fa Z., Zhao B.et al.. Victors: a web-based knowledge base of virulence factors in human and animal pathogens. Nucleic Acids Res. 2019; 47:D693–D700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang J., Chen L., Sun L., Yu J., Jin Q.. VFDB 2008 release: an enhanced web-based resource for comparative pathogenomics. Nucleic Acids Res. 2008; 36:D539–D542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L., Xiong Z., Sun L., Yang J., Jin Q.. VFDB 2012 update: toward the genetic diversity and molecular evolution of bacterial virulence factors. NucleicAcids Res. 2012; 40:D641–D645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hori K., Matsumoto S.. Bacterial adhesion: from mechanism to control. Biochem. Eng. J. 2010; 48:424–434. [Google Scholar]
- 14. Green E.R., Mecsas J.. Bacterial secretion systems: an overview. Microbiol. Spectrum. 2016; 4: 10.1128/microbiolspec.vmbf-0012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen L., Song N., Liu B., Zhang N., Alikhan N.F., Zhou Z., Zhou Y., Zhou S., Zheng D., Chen M.et al.. Genome-wide identification and characterization of a superfamily of bacterial extracellular contractile injection systems. Cell Rep. 2019; 29:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cavaillon J.M. Exotoxins and endotoxins: inducers of inflammatory cytokines. Toxicon. 2018; 149:45–53. [DOI] [PubMed] [Google Scholar]
- 17. Ruter C., Hardwidge P.R.. Drugs from bugs’: bacterial effector proteins as promising biological (immune-) therapeutics. FEMS Microbiol. Lett. 2014; 351:126–132. [DOI] [PubMed] [Google Scholar]
- 18. Best A., Abu Kwaik Y.. Nutrition and bipartite metabolism of intracellular pathogens. Trends Microbiol. 2019; 27:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hynes W.L., Walton S.L.. Hyaluronidases of Gram-positive bacteria. FEMS Microbiol. Lett. 2000; 183:201–207. [DOI] [PubMed] [Google Scholar]
- 20. Erhardt M. Strategies to block bacterial pathogenesis by interference with motility and chemotaxis. Curr. Top. Microbiol. Immunol. 2016; 398:185–205. [DOI] [PubMed] [Google Scholar]
- 21. Solano C., Echeverz M., Lasa I.. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014; 18:96–104. [DOI] [PubMed] [Google Scholar]
- 22. Flint A., Butcher J., Stintzi A.. Stress responses, adaptation, and virulence of bacterial pathogens during host gastrointestinal colonization. Microbiol. Spectrum. 2016; 4: 10.1128/microbiolspec.vmbf-0007-2015. [DOI] [PubMed] [Google Scholar]
- 23. Beceiro A., Tomas M., Bou G.. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world?. Clin. Microbiol. Rev. 2013; 26:185–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cain J.A., Dale A.L., Sumer-Bayraktar Z., Solis N., Cordwell S.J.. Identifying the targets and functions of N-linked protein glycosylation in Campylobacter jejuni. Molecular omics. 2020; 16:287–304. [DOI] [PubMed] [Google Scholar]
- 25. Clements M., Eriksson S., Tezcan-Merdol D., Hinton J.C., Rhen M.. Virulence gene regulation in Salmonella enterica. Ann. Med. 2001; 33:178–185. [DOI] [PubMed] [Google Scholar]
- 26. Stothard P., Wishart D.S.. Circular genome visualization and exploration using CGView. Bioinformatics. 2005; 21:537–539. [DOI] [PubMed] [Google Scholar]
- 27. Song N., Chen L., Zhou Z., Ren X., Liu B., Zhou S., Wang C., Wu Y., Waterfield N.R., Yang J.et al.. Genome-wide dissection reveals diverse pathogenic roles of bacterial Tc toxins. PLoS Pathog. 2021; 17:e1009102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng D., Pang G., Liu B., Chen L., Yang J.. Learning transferable deep convolutional neural networks for the classification of bacterial virulence factors. Bioinformatics. 2020; 36:3693–3702. [DOI] [PubMed] [Google Scholar]
- 29. Liu B., Zheng D., Jin Q., Chen L., Yang J.. VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019; 47:D687–D692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen B.N., Peterson B.N., Portnoy D.A.. Listeriolysin O: a phagosome-specific cytolysin revisited. Cell. Microbiol. 2019; 21:e12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.