Abstract
LncACTdb 3.0 is a comprehensive database of experimentally supported interactions among competing endogenous RNA (ceRNA) and the corresponding personalized networks contributing to precision medicine. LncACTdb 3.0 is freely available at http://bio-bigdata.hrbmu.edu.cn/LncACTdb or http://www.bio-bigdata.net/LncACTdb. We have updated the LncACTdb 3.0 database with several new features, including (i) 5669 experimentally validated ceRNA interactions across 25 species and 537 diseases/phenotypes through manual curation of published literature, (ii) personalized ceRNA interactions and networks for 16 228 patients from 62 datasets in TCGA and GEO, (iii) sub-cellular and extracellular vesicle locations of ceRNA manually curated from literature and data sources, (iv) more than 10 000 experimentally supported long noncoding RNA (lncRNA) biomarkers associated with tumor diagnosis and therapy, and (v) lncRNA/mRNA/miRNA expression profiles with clinical and pathological information of thousands of cancer patients. A panel of improved tools has been developed to explore the effects of ceRNA on individuals with specific pathological backgrounds. For example, a network tool provides a comprehensive view of lncRNA-related, patient-specific, and custom-designed ceRNA networks. LncACTdb 3.0 will provide novel insights for further studies of complex diseases at the individual level and will facilitate the development of precision medicine to treat such diseases.
INTRODUCTION
MicroRNAs (miRNAs) are a type of noncoding RNA capable of regulating gene expression by suppressing protein production via the inhibition of translation or by destabilizing mRNAs by directing RNA-induced silencing complexes (RISCs) to miRNA response elements (MREs) (1–3). MREs are localized not only on mRNAs but also on noncoding transcripts, such as pseudogenes and long noncoding RNAs (lncRNAs) (4). Essentially, each miRNA has several RNA targets, while numerous RNA molecules have various MREs that can be repressed by different miRNAs. This multiplicity of targets leads to the hypothesis that different RNAs compete for the restriction pool of miRNAs (5,6), thereby acting as ceRNAs. The ceRNA hypothesis suggests that any RNA transcript harboring an MRE can sequester miRNAs from other targets sharing the same MRE, thus regulating their expression. Based on the ceRNA hypothesis, it has been proposed that a major function of noncoding RNAs (e.g. lncRNAs) may acting as decoys for endogenous miRNAs.
Accumulating evidence suggests that the function of many noncoding RNAs in tumorigenesis may be partly mediated by ceRNA cross-talk (7). For example, lncRNA HULC was upregulated in hepatocellular carcinoma, and miR-372 was experimentally shown to bind to both HULC and PRKACB in hepatocellular carcinoma cells. HULC and PRKACB may regulate each other's expression by competing for the binding of miR-372 in hepatocellular carcinoma (8). A recent study revealed that the upregulation of HOTAIR is associated with an unfavorable gastric cancer prognosis and that the regulation of HER2 levels through ceRNA interactions may be one of the mechanisms underlying the oncogenic role of HOTAIR in gastric cancer (9). Based on ceRNA regulatory patterns in cancer, researchers can develop new therapeutic strategies and discover cancer biomarkers.
As more ceRNAs have been proven to play roles in diseases and the advent of high-throughput and single-cell sequencing technologies, the number of experimentally confirmed ceRNA relationships has increased dramatically, necessitating the collection and organization of this information to facilitate extraction and utilization. Many databases have been established to collect lncRNA–target relationships, such as LncTarD (10), lnCeDB (11), starBase v2.0 (12), DIANA-LncBase v3 (13), miRSponge (14) and PceRBase (15). These databases provide valuable resources for studying lncRNA functions. However, they have a very limited collection of experimentally supported ceRNAs. We have developed a database for collecting, storing, and analyzing experimentally supported ceRNA interactions and comprehensive annotations: LncACTdb (16,17). Since its first release in 2015, more ceRNA interactions have been published, particularly experimentally validated ones in various disease states, and a public resource of high-quality curated experimentally validated ceRNA interactions and personalized ceRNA networks remain unavailable. Therefore, there is a great need to update LncACTdb with more resources and improved tools.
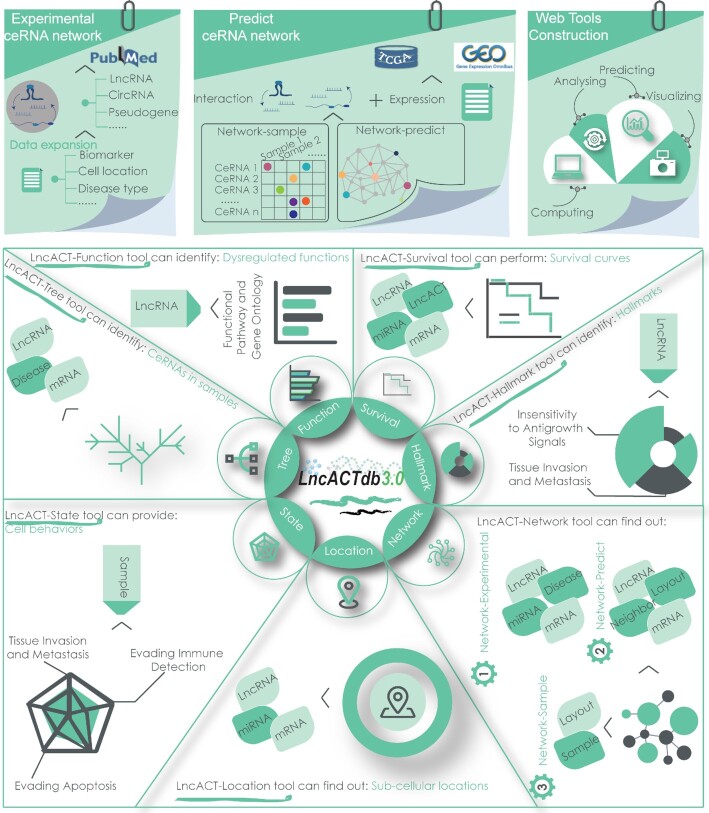
To meet these requirements, we have updated LncACTdb to version 3.0 (LncACTdb 3.0) with more data and newer features focusing on personalized ceRNA networks (Table 1). The current version of LncACTdb documents >5000 experimentally validated ceRNA interactions across 25 species and 537 diseases/phenotypes via manual curation of >6000 published literature. Newly identified personalized lncRNA-associated ceRNA interactions and networks for 16 228 patients from 62 datasets in The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov) and Gene Expression Omnibus (GEO) (18) have been integrated into LncACTdb 3.0 (Figure 1). To provide a more comprehensive annotation of lncRNAs, more than 10 000 experimentally supported lncRNA biomarkers associated with tumor metastasis (e.g. recurrence, prognosis, circulation and drug resistance) have been collected. LncACTdb 3.0 also provides detailed information of sub-cellular and extracellular locations by manual curation from literature and related data sources (19–23). By incorporating personalized ceRNA networks, the curation of lncRNA/mRNA/miRNA expression profiles and clinical information such as metastasis, recurrence, drug resistance, and prognosis from thousands of patients were integrated into LncACTdb 3.0 to perform individual pathology analysis. Furthermore, as an important supplement to the database, we set up several flexible tools to retrieve and analyze the data in LncACTdb 3.0. For example, the LncACT-Tree is a tool used to visualize ceRNA distributions and survival status across different patients, and the LncACT-Network is used to contribute a ceRNA network based on the dataset, individual, and molecular levels. Moreover, the identification of patient-specific gene regulation networks will help understanding the disease pathology at the individual level and further contribute to precision medicine. Collectively, this updated database could facilitate the identification of disease-associated ceRNAs and benefit the investigation of their roles in physiological and pathological processes.
Table 1.
Data content and improved functions of LncACTdb 3.0
| Datasets and features | LncACTdb 2.0 | LncACTdb 3.0 | Fold increase | |
|---|---|---|---|---|
| Experimental dataset | CeRNA interactions | 2663 | 5669 | 2.13 |
| LncRNAs | 312 | 913 | 2.92 | |
| MiRNAs | 479 | 1048 | 2.19 | |
| CircRNAs | 59 | 337 | 5.71 | |
| MRNAs | 131 | 1723 | 13.15 | |
| Pseudogenes | 16 | 19 | 1.19 | |
| Species | 23 | 25 | 1.09 | |
| Diseases/Phenotypes | 213 | 537 | 2.52 | |
| Predicted dataset | CeRNA interactions | 47 673 | 93 762 | 1.97 |
| LncRNAs | 1191 | 2679 | 2.25 | |
| MiRNAs | 502 | 806 | 1.61 | |
| MRNAs | 2792 | 9198 | 3.29 | |
| Datasets | 33 | 62 | 1.98 | |
| Patients | 10 141 | 16 228 | 1.60 | |
| Functional annotations | Diagnosis and therapy Biomarkers | – | 10 084 | New |
| Sub-cellular locations | – | 21 751 | New | |
| Extra-cellular locations | – | 89 925 | New | |
| Cancer cell states | – | 14 states, 1574 genes | New | |
| LncRNA-ceRNA networks | Yes | Yes | New | |
| Patient-specific ceRNA networks | – | Yes | New | |
| Custome-designed | – | Yes | New | |
| Clinical and pathological information | – | Stage, grade, metastasis, recurrence, drug-resistance, survival status, etc. | New | |
Figure 1.
Data expansion and features of LncACTdb 3.0. The upper panel is the database content which includes experimentally validated ceRNAs and predicted patient-specific networks, and web tools construction. The lower panel is the user interface of LncACTdb 3.0, which provides flexible ways to explore effects of ceRNA on patients with complex diseases and specific backgrounds.
IMPROVED EXPANSION AND NEW FEATURES
Expansion of experimentally supported ceRNAs
LncACTdb 3.0 was updated to host an increased number and types of ceRNA associations (Figure 1). In this update, we reviewed published literature from the PubMed database using keywords related to ceRNA interactions and found >15 000 relevant articles (before September 2021, Supplementary Methods). From 2018 to 2021, there was a great increase in ceRNA-related studies (∼10 000 articles), indicating an urgent need to update the LncACTdb database (Supplementary Figure S1). Based on these articles, we manually curated experimentally supported ceRNA interactions according to our previously defined criteria (17,24,25). Only ceRNA interactions validated by high-confidence experiments such as luciferase reporter assay, PCR, western blot, or other reliable experiments were considered as candidates. A candidate ceRNA interaction was confirmed by at least two researchers and catalogued into the curation. Detailed information on the data curation pipeline and principles are provided in the Supplementary Methods. The current version of LncACTdb documents 5669 experimentally supported ceRNA interactions, including 913 lncRNAs, 1723 mRNAs, 337 circRNAs and 19 pseudogenes. The scope of LncACTdb 3.0 has been expanded to 25 species and 537 diseases/phenotypes.
Manual curation of experimentally supported biomarkers
Emerging evidence suggests that lncRNAs play an important role in ceRNA regulation (26). The upstream lncRNA can dynamically buffer target expression and further affect different physiological and pathological processes (27). Some well-known lncRNAs, such as MALAT1, have been identified as biomarkers for metastasis, recurrence, and potential drug targets (28). In LncACTdb 3.0, we performed manual curation of experimentally supported lncRNA biomarkers to provide new insights into tumor diagnosis and therapy. According to our previous studies (24,29), the biological processes associated with these lncRNA biomarkers were classified as autophagy, apoptosis, cell growth, circulation, drug resistance, epithelial-mesenchymal transition (EMT), immunity, metastasis, recurrence, and survival (Supplementary Figure S2). Only lncRNAs involved in these processes were collected by high-confidence experiments (Supplementary Methods). LncACTdb 3.0 currently documents a total of 10 084 experimentally supported lncRNA biomarkers.
Manual curation of functional annotations
In the previous version (LncACTdb 2.0), a ‘guilt-by-association’ strategy was used for functional annotation of ceRNAs (Supplementary Methods, Supplementary Figure S3) (16,17). To provide a more comprehensive annotation background, LncACTdb 3.0 also collected thousands of pathways including KEGG (21), Reactome (22) and PID (23). For Gene Ontology (GO) annotation (25), thousands of gene sets representing functional terms were integrated. Furthermore, we manually curated gene sets of 10 classic cancer hallmark processes (30) and 14 functional states of tumor cells (31) into LncACTdb 3.0, to explore the regulatory mechanisms of ceRNAs at the individual level (Supplementary Methods, Supplementary Figure S4). Moreover, 111 676 entries illustrating sub-cellular and extracellular vesicle locations of lncRNAs, miRNAs and mRNAs were collected by manual curation from related data sources and literature (Supplementary Figure S5).
Identification of patient-specific ceRNAs and networks
Under the complex microenvironment of tumors, patients with the same disease may exhibit different behaviors driven by the fine-tuning of ceRNA regulation (29). Thus, the identification of patient-specific ceRNA and networks will deepen the understanding of disease pathology and contribute to the development of precision medicine. ceRNA regulations of a total of 108 668 candidates were downloaded from starBase (v2.0) (12) and LncACTdb (v2.0) (17). In this study, we used a published method (32), which was developed based on probability theory, to identify patient-specific ceRNAs and networks across different datasets (Supplementary Methods, Supplementary Figure S6). This strategy was utilized in our previous study to construct ceRNA networks at single-cell resolution (29). Briefly, the association of a ceRNA interaction (lncRNA–mRNA) within a specific sample was estimated by testing the statistical independence of lncRNA and mRNA expression values in these samples. After screening all samples whose ceRNAs were not statistically independently expressed, we performed Pearson correlation tests for lncRNA and mRNA expression in these samples. ceRNA relationships in these samples were retained only when the correlation coefficient was positive, and the P-value was less than 0.05. We collected 62 high-throughput expression profiles for 16 228 patients across 33 cancer types from the TCGA and GEO public datasets (Supplementary Figure S7). Detailed information of the data selection criteria and analysis pipeline is provided in the Supplementary Methods. We identified 93 307 patient-specific ceRNA regulations with a false discovery rate (FDR) <0.05. Furthermore, we purified ceRNA pairs in a cancer-specific manner (Supplementary Methods). The specificity of the ceRNAs was characterized quantitatively by calculating the specificity score using a previously described method (16,33,34). To explore the ceRNA regulatory mechanisms behind different individual phenotypes, we collected detailed clinical and pathological information such as tumor metastasis, recurrence, drug resistance and survival status for each patient. The incorporation of personalized ceRNA networks, clinical and pathological characteristics, and functional backgrounds will provide insights into individual pathology analysis.
DATABASE CONSTRUCTION AND IMPROVED USER INTERFACE
LncACTdb 3.0, performed data management using MySQL software (v 5.5). The web pages were developed using Java server pages and deployed on the Tomcat web server (v6). Several Java script plugins such as jQuery (v1.11.3), Datatable (1.10.10), and ECharts (V4.0) were used for data table creation and visualization. All statistical analyses were performed using R framework (v3.6.3). The LncACTdb 3.0 database is freely available at http://bio-bigdata.hrbmu.edu.cn/LncACTdb or http://www.bio-bigdata.net/LncACTdb. The version 2.0 of LncACTdb, also remains available. To access LncACTdb 2.0, users can visit links from the LncACTdb 3.0, homepage (Supplementary Figure S8), or directly at http://www.bio-bigdata.net/LncACTdb2.0.
LncACTdb 3.0 provides a user-friendly web interface that enables users to search, browse, analyze, and download data in a few easy steps (Figure 2). On the ‘HOME’ page, a fast search engine is available for users to directly investigate data or perform analyses (Supplementary Figures S8 and S9). We used the well-known lncRNA MALAT1 as an example to explore (i) related experimentally supported ceRNAs, (ii) patient-specific ceRNAs, (iii) clinical information of samples and (iv) biomarkers for diagnosis and therapy (Figure 2A). All possible records are displayed on the search results page (Figure 2B and Supplementary Figure S9A). To obtain interesting records, users can flexibly reorder the results table by clicking on the headers of different columns. The first and last columns will lead users to the detailed information page indicating the related disease, number of competing miRNAs, dataset information, experimental validation methods, and number/percentage of patients in which this ceRNA can be found (Figure 2C). LncACTdb 3.0 also provides a browse page to access the dataset based on different classifications (Figure 2D). Furthermore, a panel of online tools for studying the regulatory mechanisms of ceRNAs has been developed (Figure 2E–K). Using the function tool, users can explore lncRNA functions based on GO terms and biological pathways (Figure 2E). For a ceRNA, the Location tool provides all possible sub-cellular and extracellular vesicle locations of lncRNAs, miRNAs and mRNAs (Figure 2F). Survival analysis was performed and Kaplan–Meier survival curves were generated for a ceRNA interaction or a single gene (lncRNA, mRNA, miRNA or pseudogene) across 62 cancer datasets (Figure 2G). The hallmark and state tools allow users to study the ceRNA effects on 10 classic cancer hallmark processes and 14 tumor cell states at the individual level (Figure 2H–I). For a certain disease, the Tree tool provides a ceRNA distribution across different samples, which allows users to explore ceRNA effects on the clinical and pathological status of different groups (Figure 2J). The network tool provides a global view of (i) all possible related ceRNA interactions of input lncRNA, (ii) patient-specific, and (iii) customized ceRNA networks in which the diseases and nodes were selected by users (Figure 2K). Users can adjust the network with different layouts, such as force-direct or circular. Moreover, to provide a summary of the present research situations of ceRNAs, a hot-point page illustrating statistics of the most visited items was developed for LncACTdb 3.0 (Supplementary Figure S9B).
Figure 2.
Case study and workflow for LncACTdb 3.0. (A) LncRNA MATLAT1 was used as an example in the search interface. (B) Search results of MALAT1, including both predicted and experimentally supported ceRNA interactions and biomarker annotations. (C) Detailed information of ceRNAs. (D) The browse interface of LncACTdb 3.0. (E) CeRNA functions based on GO terms and biological pathways. (F) Possible sub-cellular and extracellular vesicle locations of ceRNAs. (G) Survival tool performs survival analysis and provides Kaplan–Meier survival curves for a ceRNA interaction. (H–I) Exploring the effects of ceRNA on hallmark processes for cancer and on tumor cell states. (J) Exploring how ceRNA affects the clinical and pathological status of different groups of patients. (K) The lncRNA-related, patient-specific, and user-designed ceRNA networks of LncACTdb 3.0.
CONCLUSIONS AND FUTURE DEVELOPMENT
In the previous version of the LncACTdb database (version 1.0 and 2.0), only a limited number of ceRNA interactions were documented. With the gradual improvement of high-throughput technology and experimental methods, an increasing number of ceRNAs have been recently found, particularly from 2019 to 2021 (Supplementary Figure S1). Rapid emergence of related studies necessitates the collection of corresponding datasets into a new version of the LncACTdb database. Together with the development of precision medicine, the identification of patient-specific ceRNAs and networks will deepen the understanding of disease pathology and contribute to individual diagnosis and therapy. Thus, we developed LncACTdb 3.0, with more data and new features focusing on personalized ceRNA networks. The current version of LncACTdb documents 5669 experimentally validated ceRNA interactions across 25 species and more than 537 diseases/phenotypes through manual curation of >6000 published manuscripts. Newly identified personalized lncRNA-associated ceRNA interactions and networks for 16 228 patients from 62 datasets were integrated into LncACTdb 3.0. To provide a more comprehensive annotation, thousands of diagnostic and therapeutic biomarkers, biological terms, pathway gene sets, sub-cellular and extracellular vesicle locations, and clinical and pathological information of patients were collected. As an upgrade, a panel of flexible tools to retrieve and analyze the data was developed. In LncACTdb 3.0, the identification of patient-specific gene regulation networks aids in understanding disease pathology at the individual level and further contribute to precision medicine. A rapid and continued increase in the number of high-confidential ceRNA datasets identified from experiments is expected in the future, and we will continually maintain and update the LncACTdb database with more datasets and improved services, thereby improving our understanding of lncRNAs and contributing to personalized cancer diagnosis and treatment.
DATA AVAILABILITY
All the data could be downloaded from http://bio-bigdata.hrbmu.edu.cn/LncACTdb/.
Supplementary Material
Contributor Information
Peng Wang, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Qiuyan Guo, Department of Gynecology, the First Affiliated Hospital of Harbin Medical University, Harbin 150081, China.
Yue Qi, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Yangyang Hao, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Yue Gao, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Hui Zhi, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Yuanfu Zhang, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Yue Sun, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Yakun Zhang, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Mengyu Xin, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Yunpeng Zhang, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Shangwei Ning, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
Xia Li, College of Bioinformatics Science and Technology, Harbin Medical University, Harbin 150081, China.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Key R&D Program of China [2018YFC2000100]; National Natural Science Foundation of China [32070673, 32070622, 32070672, 61873075, 62172131, 81902646]; Heilongjiang Touyan Innovation Team Program; Heilongjiang Provincial Natural Science Foundation [YQ2021C026]; Postdoctoral Science Foundation of China [2020M670922]; Postdoctoral Foundation of Heilongjiang Province [LBH-Z19077, LBH-Q20047]. Funding for open access charge: National Key R&D Program of China [2018YFC2000100]; National Natural Science Foundation of China [32070673, 32070622, 32070672, 61873075, 62172131, 81902646]; Heilongjiang Touyan Innovation Team Program; Heilongjiang Provincial Natural Science Foundation [YQ2021C026]; Postdoctoral Science Foundation of China [2020M670922]; Postdoctoral Foundation of Heilongjiang Province [LBH-Z19077, LBH-Q20047].
Conflict of interest statement. None declared.
REFERENCES
- 1. Guo H., Ingolia N.T., Weissman J.S., Bartel D.P.. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010; 466:835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hendrickson D.G., Hogan D.J., McCullough H.L., Myers J.W., Herschlag D., Ferrell J.E., Brown P.O.. Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol. 2009; 7:e1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kozomara A., Birgaoanu M., Griffiths-Jones S.. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019; 47:D155–D162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paraskevopoulou M.D., Georgakilas G., Kostoulas N., Reczko M., Maragkakis M., Dalamagas T.M., Hatzigeorgiou A.G.. DIANA-LncBase: experimentally verified and computationally predicted microRNA targets on long non-coding RNAs. Nucleic Acids Res. 2013; 41:D239–D245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P.. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language?. Cell. 2011; 146:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seitz H. Redefining microRNA targets. Curr. Biol. 2009; 19:870–873. [DOI] [PubMed] [Google Scholar]
- 7. Qi X., Zhang D.H., Wu N., Xiao J.H., Wang X., Ma W.. ceRNA in cancer: possible functions and clinical implications. J. Med. Genet. 2015; 52:710–718. [DOI] [PubMed] [Google Scholar]
- 8. Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N.. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008; 455:58–63. [DOI] [PubMed] [Google Scholar]
- 9. Liu X.H., Sun M., Nie F.Q., Ge Y.B., Zhang E.B., Yin D.D., Kong R., Xia R., Lu K.H., Li J.H.et al.. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol. Cancer. 2014; 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao H., Shi J., Zhang Y., Xie A., Yu L., Zhang C., Lei J., Xu H., Leng Z., Li T.et al.. LncTarD: a manually-curated database of experimentally-supported functional lncRNA-target regulations in human diseases. Nucleic Acids Res. 2020; 48:D118–D126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Das S., Ghosal S., Sen R., Chakrabarti J.. lnCeDB: database of human long noncoding RNA acting as competing endogenous RNA. PLoS One. 2014; 9:e98965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J.H., Liu S., Zhou H., Qu L.H., Yang J.H.. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014; 42:D92–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karagkouni D., Paraskevopoulou M.D., Tastsoglou S., Skoufos G., Karavangeli A., Pierros V., Zacharopoulou E., Hatzigeorgiou A.G.. DIANA-LncBase v3: indexing experimentally supported miRNA targets on non-coding transcripts. Nucleic Acids Res. 2020; 48:D101–D110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang P., Zhi H., Zhang Y., Liu Y., Zhang J., Gao Y., Guo M., Ning S., Li X.. miRSponge: a manually curated database for experimentally supported miRNA sponges and ceRNAs. Database. 2015; 2015:bav098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yuan C., Meng X., Li X., Illing N., Ingle R.A., Wang J., Chen M.. PceRBase: a database of plant competing endogenous RNA. Nucleic Acids Res. 2017; 45:D1009–D1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang P., Ning S., Zhang Y., Li R., Ye J., Zhao Z., Zhi H., Wang T., Guo Z., Li X.. Identification of lncRNA-associated competing triplets reveals global patterns and prognostic markers for cancer. Nucleic Acids Res. 2015; 43:3478–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang P., Li X., Gao Y., Guo Q., Wang Y., Fang Y., Ma X., Zhi H., Zhou D., Shen W.et al.. LncACTdb 2.0: an updated database of experimentally supported ceRNA interactions curated from low- and high-throughput experiments. Nucleic Acids Res. 2019; 47:D121–D127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrett T., Troup D.B., Wilhite S.E., Ledoux P., Rudnev D., Evangelista C., Kim I.F., Soboleva A., Tomashevsky M., Edgar R.. NCBI GEO: mining tens of millions of expression profiles–database and tools update. Nucleic Acids Res. 2007; 35:D760–D765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li S., Li Y., Chen B., Zhao J., Yu S., Tang Y., Zheng Q., Li Y., Wang P., He X.et al.. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018; 46:D106–D112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu T., Zhang Q., Zhang J., Li C., Miao Y.R., Lei Q., Li Q., Guo A.Y.. EVmiRNA: a database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019; 47:D89–D93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mas-Ponte D., Carlevaro-Fita J., Palumbo E., Hermoso Pulido T., Guigo R., Johnson R.. LncATLAS database for subcellular localization of long noncoding RNAs. RNA. 2017; 23:1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quek X.C., Thomson D.W., Maag J.L., Bartonicek N., Signal B., Clark M.B., Gloss B.S., Dinger M.E.. lncRNAdb v2.0: expanding the reference database for functional long noncoding RNAs. Nucleic Acids Res. 2015; 43:D168–D173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia S., Feng J., Chen K., Ma Y., Gong J., Cai F., Jin Y., Gao Y., Xia L., Chang H.et al.. CSCD: a database for cancer-specific circular RNAs. Nucleic Acids Res. 2018; 46:D925–D929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao Y., Shang S., Guo S., Li X., Zhou H., Liu H., Sun Y., Wang J., Wang P., Zhi H.et al.. Lnc2Cancer 3.0: an updated resource for experimentally supported lncRNA/circRNA cancer associations and web tools based on RNA-seq and scRNA-seq data. Nucleic Acids Res. 2021; 49:D1251–D1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang P., Li X., Gao Y., Guo Q., Ning S., Zhang Y., Shang S., Wang J., Wang Y., Zhi H.et al.. LnCeVar: a comprehensive database of genomic variations that disturb ceRNA network regulation. Nucleic Acids Res. 2020; 48:D111–D117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tay Y., Rinn J., Pandolfi P.P.. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014; 505:344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao H., Liu X., Yu L., Lin S., Zhang C., Xu H., Leng Z., Huang W., Lei J., Li T.et al.. Comprehensive landscape of epigenetic-dysregulated lncRNAs reveals a profound role of enhancers in carcinogenesis in BC subtypes. Mol. Ther. Nucleic Acids. 2021; 23:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim S.S., Harford J.B., Moghe M., Rait A., Pirollo K.F., Chang E.H.. Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide. Nucleic Acids Res. 2018; 46:1424–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang P., Guo Q., Hao Y., Liu Q., Gao Y., Zhi H., Li X., Shang S., Guo S., Zhang Y.et al.. LnCeCell: a comprehensive database of predicted lncRNA-associated ceRNA networks at single-cell resolution. Nucleic Acids Res. 2021; 49:D125–D133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanahan D., Weinberg R.A.. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674. [DOI] [PubMed] [Google Scholar]
- 31. Yuan H., Yan M., Zhang G., Liu W., Deng C., Liao G., Xu L., Luo T., Yan H., Long Z.et al.. CancerSEA: a cancer single-cell state atlas. Nucleic Acids Res. 2019; 47:D900–D908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dai H., Li L., Zeng T., Chen L.. Cell-specific network constructed by single-cell RNA sequencing data. Nucleic Acids Res. 2019; 47:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yanai I., Benjamin H., Shmoish M., Chalifa-Caspi V., Shklar M., Ophir R., Bar-Even A., Horn-Saban S., Safran M., Domany E.et al.. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 2005; 21:650–659. [DOI] [PubMed] [Google Scholar]
- 34. Guo Q., Wang J., Gao Y., Li X., Hao Y., Ning S., Wang P.. Dynamic TF-lncRNA regulatory networks revealed prognostic signatures in the development of ovarian cancer. Front. Bioeng. Biotechnol. 2020; 8:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data could be downloaded from http://bio-bigdata.hrbmu.edu.cn/LncACTdb/.