Abstract
Terminal cell differentiation entails definitive withdrawal from the cell cycle. Although most of the cells of an adult mammal are terminally differentiated, the molecular mechanisms preserving the postmitotic state are insufficiently understood. Terminally differentiated skeletal muscle cells, or myotubes, are a prototypic terminally differentiated system. We previously identified a mid-G1 block preventing myotubes from progressing beyond this point in the cell cycle. In this work, we set out to define the molecular basis of such a block. It is shown here that overexpression of highly active cyclin E and cdk2 in myotubes induces phosphorylation of pRb but cannot reactivate DNA synthesis, underscoring the tightness of cell cycle control in postmitotic cells. In contrast, forced expression of cyclin D1 and wild-type or dominant-negative cdk4 in myotubes restores physiological levels of cdk4 kinase activity, allowing progression through the cell cycle. Such reactivation occurs in myotubes derived from primary, as well as established, C2C12 myoblasts and is accompanied by impairment of muscle-specific gene expression. Other terminally differentiated systems as diverse as adipocytes and nerve cells are similarly reactivated. Thus, the present results indicate that the suppression of cyclin D1-associated kinase activity is of crucial importance for the maintenance of the postmitotic state in widely divergent terminally differentiated cell types.
The defining property of terminally differentiated (TD) cells is their physiologically irreversible growth arrest. Although TD cells constitute the majority in an adult mammal, the mechanisms ensuring the tight maintenance of their postmitotic state are incompletely understood. The inability of TD cells to proliferate generates a biological problem, since in some tissues they must live as long as the organism to which they belong, requiring long-term survival strategies. In addition, organs and systems devoid of stem cell compartments and whose parenchymas are composed exclusively of TD cells cannot resort to cell proliferation to renew their tissues. This makes them especially vulnerable to cell losses caused by injuries or diseases. The ability to induce proliferation of TD neurons, myocardiocytes, or endocrine cells might open new avenues to the therapy of ailments and traumas of such organs (44).
Skeletal muscle fibers are prototypic TD cells whose differentiation process can be recapitulated in vitro. Primary, as well as established, myoblasts can be propagated in culture in the presence of growth factors. Mitogen withdrawal triggers differentiation, which begins with an irreversible exit from the cell cycle. Postmitotic cells express muscle-specific genes and turn into mononucleated myocytes, which eventually fuse into multinucleated structures called myotubes (32).
The proliferative arrest of TD cells is qualitatively different from that of reversibly quiescent cells. The proliferation machinery of TD cells is so tightly controlled that they do not undergo DNA replication in response to growth factors or a number of otherwise powerful proliferation activators (46). The latter include combinations of transforming retroviral oncogenes and a number of key cellular promoters of proliferation. We and others (33, 34) have shown that TD myotubes from both C2C12 and primary mouse satellite cells are even resistant to the activity of E2F transcription factors, “master” regulators of the G1/S transition that can force S phase entry in a wide variety of non-TD cells. Although it has recently been reported that ectopic expression of the homeobox-containing msx1 gene can induce proliferation of C2C12 myotubes (31), the only established means by which to reactivate the cell cycle in TD mammalian muscle cells is expression of DNA tumor virus oncogenes, including those for the polyomavirus (9, 50) and simian virus 40 (4, 8) large T antigens and adenovirus E1A (6, 7).
We have shown that serum growth factor stimulation promotes myotube reentry into G1. However, serum-stimulated myotubes cannot progress beyond mid-G1 phase, leading us to suggest that one important block preventing DNA synthesis in muscle cells lies at this stage. To probe the molecular nature of this barrier, we forcibly activated the two major kinases responsible for G1 progression, cdk2 and cdk4.
Overexpression of cyclin E and cdk2 could not trigger DNA synthesis in myotubes, in spite of the considerable cyclin E-associated kinase activity obtained. In sharp contrast, reconstitution of physiological levels of cdk4 activity by simultaneous overexpression of cyclin D1 and cdk4 efficiently led myotubes through G1, S, and G2 phases. Most myotubes so reactivated arrested before entering mitosis, suggesting that a second block exists at the G2/M boundary. Cell cycle reactivation could be equally obtained in neurons and adipocytes, indicating that the suppression of the cyclin D-associated kinase is crucial to the maintenance of the postmitotic state in TD cells of different origins.
MATERIALS AND METHODS
Cells.
The murine C2C12 myoblast cell line (3) was cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Differentiation was induced by starving the cells in serum-free medium for 72 h (47). Unless otherwise stated, 1-β-d-arabinofuranosylcytosine (Ara-C; 50 μM) was added to serum-free medium in the first 48 h to eliminate undifferentiated cells and then removed between 48 and 72 h. Ara-C-purified myotubes contained more than 90% of the nuclei in the culture. Primary mouse satellite cells were isolated and cultured as previously described (35, 46). Primary quail myoblasts were isolated and cultured as previously described (45). Mouse 3T3-L1 preadipocytes (12) were cultured in DMEM supplemented with 15% FBS. Adipocyte differentiation was induced by treating confluent cells with 1 μM dexamethasone, 0.5 mM 1-methyl-3-isobutylxanthine, and insulin at 10 μg/ml. Two days later, the cells were switched to DMEM containing insulin at 1 μg/ml and 15% FBS. Differentiation was considered complete 7 days after the induction, when most adipocytes contained a single or a few large lipid droplets. P19 embryonal carcinoma cells (28) were cultured in minimun essential medium alpha (α-MEM) medium with 10% FBS. Differentiation was induced by culturing the cells in suspension in bacterial dishes in α-MEM supplemented with 1 μM retinoic acid. After 4 days, the embryoid bodies thus obtained were disaggregated with trypsin and replated onto poly-l-lysine-coated dishes without further retinoic acid treatment. Ara-C (1 μM) was added on the next day to prevent overgrowth of undifferentiated cells. Neuronal differentiation was complete by day 7 from the beginning of the suspension culture.
Adenoviruses.
The Ad-cdk2 and Ad-cycE recombinant adenoviruses have been described previously (23). The J-cdk4 adenovirus was generated by the method of Bett et al. (2). The Ad-cycD1, Ad-dncdk4, and Ad-Track viruses were generated by the method devised by He and colleagues (16). The mutant cdk4 gene inserted into the Ad-dncdk4 virus has been described previously (48). The dl520 virus is a deletion mutant of human adenovirus type 5 expressing 12S, but not 13S, E1A (14, 15). All of the recombinant adenoviruses used, except dl520, independently of the construction method, express their respective cDNAs under the control of the cytomegalovirus immediate-early promoter-enhancer. The viruses constructed by the method of He et al. (16) also express the green fluorescent protein under the control of a second copy of the cytomegalovirus promoter. All replication-defective, recombinant adenoviruses were grown and titrated in the permissive 293 cell line (17).
Transfection of quail myotubes.
Primary quail myoblasts were plated at 2.5 × 105/35-mm-diameter dish and induced to differentiate into myotubes in the presence of Ara-C. Myotubes were transfected with the pRc-cyclin D1 (J. Pines), pCMV-cdk4 (S. van den Heuvel), and, where indicated, pSV2Luc expression vectors, by using Lipofectamine Plus (Life Technologies) in accordance with the manufacturer's instructions. Some of the cultures were additionally infected with the empty recombinant adenovirus J-pCA13 immediately after transfection. The myotubes were then cultured in the presence of 5% FBS; 5-bromo-2′-deoxyuridine (BrdUrd) was added 16 h after the transfection. The cultures were fixed at 48 h postinfection (p.i.) and immunostained for either cyclin D1 or luciferase and BrdUrd. The number of double-positive cells in each culture was determined.
Immunofluorescence assay.
The following monoclonal antibodies (MAbs) or antisera were used for immunofluorescence assay: MAb Bu20a to BrdUrd (Dako), rabbit antiserum to muscle-specific myosin heavy chain (MyHC) (a kind gift of G. Cossu), rabbit M-20 antiserum to cyclin D1 (Santa Cruz), rabbit antiserum to Tau (Sigma), and rabbit antiserum to luciferase (Promega). MAbs were detected by fluorescein isothiocyanate-conjugated, affinity-purified, goat anti-mouse immunoglobulin G serum (Organon Teknika). Reaction of rabbit antisera was detected by tetramethyl rhodamine isocyanate-conjugated, affinity-purified goat anti-rabbit immunoglobulin G serum (Organon Teknika). After immunofluorescence treatments, nuclei were stained by a 3-min incubation in a 0.1-μg/ml solution of Hoechst 33258 dye in phosphate-buffered saline.
Western blot analysis.
Whole-cell extracts were obtained by disrupting cells in lysis buffer (50 mM Tris-HCl [pH 8] 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 1 mM EDTA, leupeptin at 5 μg/ml, aprotinin at 5 μg/ml, pepstatin at 5 μg/ml). To extract pRb, a high-salt buffer was used (50 mM Tris-HCl, 250 mM NaCl, 5 mM EDTA, 0.1% Triton X-100, 50 mM NaF, 0.1 mM sodium orthovanadate, and the same protease inhibitors as above). Protein extracts were separated by SDS-polyacrylamide gel electrophoresis and electroblotted onto a nitrocellulose membrane (Bio-Rad). Proteins were analyzed with the following antibodies: rabbit antiserum M-20 to cyclin D1 (Santa Cruz), MAb Ab-1 to cdk4 (NeoMarkers), rabbit antiserum to p21 (a kind gift of C. Schneider), MAb clone 57 to p27 (Transduction Laboratories), rabbit M-20 antiserum to cyclin E (Santa Cruz), rabbit antiserum C-19 to cyclin A (Santa Cruz), rabbit antiserum M2 to cdk2 (Santa Cruz), MAb clone G3-245 to pRb (Pharmingen), and rabbit antiserum to cdc2 (a kind gift of G. Draetta). Immunoreactions were detected with peroxidase-conjugated secondary antibodies and a chemiluminescent substrate (Pierce). Samples in all Western blots and immunoprecipitations were normalized so that lysates of cells possessing the same total number of nuclei were analyzed, to compensate for the higher protein content of myotubes than myoblasts (46). Repeated measurements under various conditions consistently showed that myotubes contain twice as much protein as myoblasts, based on equal numbers of nuclei.
Immunoprecipitation and kinase activity assays.
To evaluate cyclin D1-associated kinase activity, cells were lysed for 30 min at 4°C in 0.5 ml of lysis buffer containing 50 mM (HEPES) (pH 7.5), 10 mM MgCl2, 150 mM NaCl, 0.1% Tween 20, 1 mM dithiothreitol, leupeptin at 5 μg/ml, aprotinin at 5 μg/ml, pepstatin at 5 μg/ml, and 25 μM ATP. As myotubes are hard to disrupt, cell lysates were then sonicated and freeze-thawed twice. Protein extracts (2.5 mg per sample), were precleared by two 1-h incubations with 20 μl of a protein G agarose bead suspension (Pierce). Precleared lysates were then subjected to immunoprecipitation with a mixture of antibodies to cyclin D1 (MAb 72-13G to mouse protein [Santa Cruz] and MAb Ab-2 to human protein [NeoMarkers]; 1 μg of each antibody per mg of protein extract) bound to protein G agarose beads. After extensive washes, immunoprecipitates were resuspended in kinase buffer (50 mM HEPES [pH 8.0], 10 mM MgCl2, 2.5 mM EGTA, 1 mM dithiothreitol) supplemented with 50 μM unlabeled ATP, 2.5 μg of glutathione S-transferase–Rb protein as substrate (Santa Cruz) and 3.7 × 105 Bq of [γ-32P]ATP per sample and incubated for 20 min at 30°C. The reactions were terminated by the addition of 3× sample buffer; labeled proteins were resolved on an SDS–12.5% polyacrylamide gel and detected by autoradiography. Cdk4 kinase activity was precipitated as described above, from 0.5 mg of cell lysates, by using a mixture of MAb Ab-1 to human protein and rabbit polyclonal Ab-5 to mouse protein (NeoMarkers). Cyclin E-associated kinase activity was determined as previously described (33) by using the M-20 rabbit antiserum or the HE11 MAb to mouse or human cyclin E, respectively (Santa Cruz).
Cytofluorimetry.
For cell cycle analysis, Ara-C-purified mouse satellite cell-derived myocytes were incubated overnight at 4°C in phosphate-buffered saline containing propidium iodide at 100 μg/ml, RNase at 200 μg/ml, and 0.2% Triton X-100 and analyzed with an EPICS XL cytofluorimeter (Coulter).
Northern blot analysis.
For extraction of total cellular RNA 6 × 106 C2C12 cells were plated into 150-mm-diameter collagen-coated dishes, induced to differentiate, and infected as described above. Samples of 15 to 20 μg were run on formaldehyde gels, transferred, blotted, and hybridized in accordance with standard protocols (38). Full-length cDNAs were used as probes for muscle creatine kinase (MCK), myoD, myogenin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and 28S rRNA. As a probe for myosin light chain 1 (MLC-1), we used the first exon of the gene, and for myosin heavy chain (MyHC), we used an internal cDNA fragment.
RESULTS
Forced cyclin E-cdk2 activation fails to elicit DNA replication in myotubes.
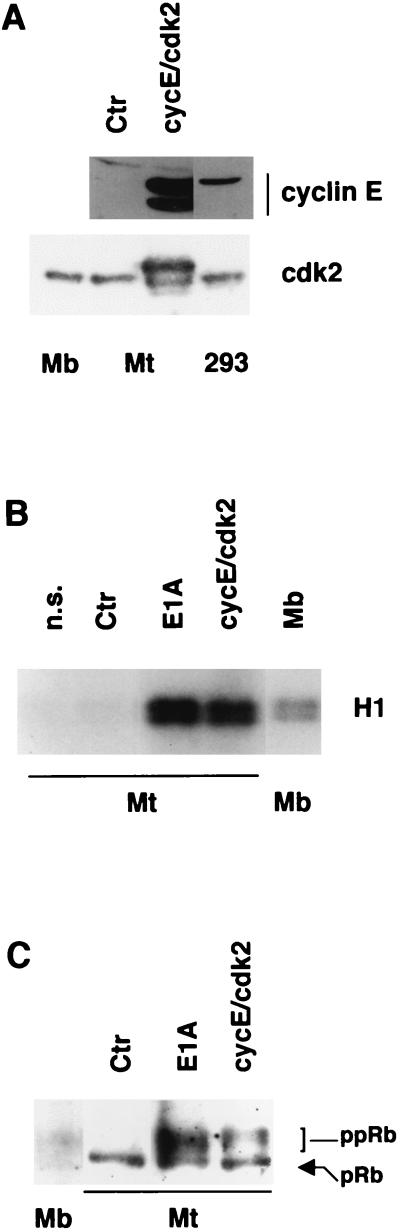
In initial attempts to trigger DNA synthesis in TD skeletal muscle cells with cellular genes, we infected C2C12 myotubes with recombinant adenoviruses carrying human cyclin E (Ad-cycE) and cdk2 (Ad-cdk2). The myotubes were infected with either virus or both up to the highest feasible multiplicities of infection (MOIs). Accumulation of the exogenous proteins was confirmed by Western blot analysis. Figure 1A shows that the expression levels of cyclin E and cdk2 in Ad-cycE–Ad-cdk2-infected myotubes were much higher than those found in proliferating myoblasts and/or human 293 cells. The corresponding cyclin E-associated kinase activity was far higher than that of proliferating myoblasts and comparable to the strong activity elicited by the cell cycle-reactivating E1A oncogene (Fig. 1B). In agreement, the endogenous pRb was evidently phosphorylated in Ad-cycE–Ad-cdk2-infected myotubes (Fig. 1C). To determine whether cdk2 activation induced DNA synthesis in the infected myotubes, they were subjected to immunofluorescence analysis of BrdUrd incorporation in the 3 days following the infection. Although thousands of myotubes transduced with the Ad-cycE and/or Ad-cdk2 viruses at different MOIs were scored, BrdUrd-positive myotubes were never found (data not shown). Thus, forcing TD myotubes to re-express cdk2 activity does not bring them back into the cell cycle, in spite of manifest pRb phosphorylation, in keeping with a recent report (27).
FIG. 1.
Exogenous cyclin E and cdk2 expression. (A) Western blot analysis of C2C12 myotubes (Mt) infected with the Ad-Track control virus (Ctr; MOI, 300) or with the Ad-cycE and Ad-cdk2 viruses (cycE/cdk2; MOIs, 200 and 350, respectively) at 48 h p.i. Proliferating C2C12 myoblasts (Mb) and/or human 293 cells, expressing high levels of endogenous cyclin E, are shown for comparison. 293 cells are shown because human cyclin E levels in myotubes could not be compared with those physiologically expressed in mouse C2C12 myoblasts, since an antibody reacting equally with murine and human cyclin E was not available. (B) Cyclin E-associated kinase activity immunoprecipitated from myotubes infected with Ad-cycE and Ad-cdk2, the E1A-expressing virus dl520 (for reference), or a control virus. Myoblasts are shown for comparison. A nonspecific (n.s.) antibody was used as a control. The precipitates were assayed by using histone H1 (H1) as the substrate. (C) Western blot analysis of pRb in myotubes infected with the Ad-cycE and Ad-cdk2, dl520, and control viruses and myoblasts. The slow-migrating, hyperphosphorylated (ppRb) and the hypophosphorylated (pRb) forms of Rb are indicated.
Reconstitution of cdk4 kinase activity in myotubes.
However refractory they are to replicating DNA, myotubes do respond to serum growth factors by leaving G0 and proceeding to mid-G1 phase (46). The initial progression of serum-stimulated myotubes is indistinguishable from that of reactivated myoblasts. Yet, no cell cycle events have been observed in the former beyond upregulation of the gene for cyclin D1 in mid-G1, suggesting that an important barrier lies in close proximity to this point. We asked whether this postulated block could be ascribed to the absence of cyclin D1-associated kinase activity and whether reconstitution of this activity would be sufficient to allow progression of TD cells through the cell cycle. To determine whether TD myotubes can activate cyclin D1-associated kinases in response to growth factors, we stimulated myotubes derived from the C2C12 myoblast line with serum for up to 48 h. Although cyclin D1 protein accumulated significantly (Fig. 2A), no cyclin D1-associated or cdk4 kinase activity could be detected in myotubes under these conditions (Fig. 2B), with cdk4 levels comparable to those measured in proliferating myoblasts (reference 47, and Fig. 2A and D). With the aim of forcing the expression of significant cdk4 activity in TD muscle cells, human cyclin D1 and cdk4 were transduced into C2C12 myotubes by infection with recombinant adenoviruses carrying the two cDNAs (Ad-cycD1 and J-cdk4, respectively). Figure 2D shows that simultaneous infection with the two viruses resulted in significant overexpression of both proteins. Subsequent time course studies showed that expression of both cyclin D1 and cdk4 reached a plateau at 24 h p.i. and remained essentially constant up to at least 48 h p.i. (data not shown). Next, the kinase activity associated with cyclin D1 was measured in myotubes infected with the two viruses and stimulated with serum. The activity obtained in myotubes thus treated was comparable to that found in proliferating myoblasts (Fig. 2E). No activity beyond the background was measurable in control myotubes infected with the empty control virus.
FIG. 2.
Endogenous and exogenous expression of cyclin D1 and cdk4 in myotubes. (A) Western blot (WB) analysis of endogenous cyclin D1 expression in TD C2C12 myotubes treated with serum for the indicated times. (B) Kinase activities precipitated by anti-cyclin D1 or anti-cdk4 antibodies from C2C12 myoblasts or TD myotubes treated with serum for 0 or 48 h. Nonspecific immunoprecipitation (IP) was done as described in the legend to Fig. 1. The kinase activities were assayed by using a glutathione S–transferase Rb (Rb) fusion protein as the substrate. (C) Western blot analysis of cyclin D1 in the immunoprecipitates whose kinase activity is shown in panel B. (D) Western blot analysis of cyclin D1 and cdk4 proteins in myotubes infected with the control virus (MOI, 830) or coinfected with the recombinant adenoviruses Ad-cycD1 and J-cdk4 (MOIs, 60 and 770, respectively) and switched to 5% FBS at 12 h p.i.; whole-cell lysates were prepared 24 h p.i.; proliferating myoblasts are included for comparison. (E) Anti-cyclin D1- and anti-cdk4-precipitated kinase activities were measured in proliferating C2C12 myoblasts and C2C12 myotubes infected as described above with the control virus (20 h p.i.) or coinfected with the Ad-cycD1 and J-cdk4 viruses, stimulated with 5% serum from 12 h p.i., and analyzed at 20 h p.i. Protein extracts (2.5 mg per sample) were immunoprecipitated by using a nonspecific antibody, a mixture of two distinct mouse MAbs to cyclin D1, or a mixture of two different anti-cdk4 antibodies (see Materials and Methods).
Reactivation of the cell cycle in cyclin D1-cdk4-expressing myotubes.
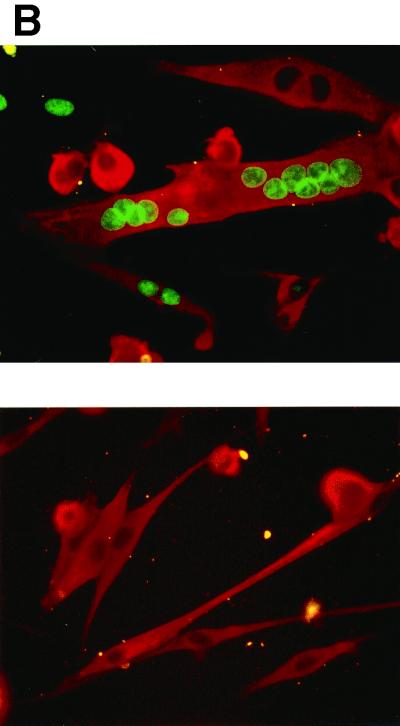
Having demonstrated that cyclin D1 and cdk4 can accumulate and function in myotubes, we assessed whether expression of these regulators would reactivate DNA synthesis in these cells. C2C12 myotubes were infected with the Ad-cycD1 and J-cdk4 viruses, cultured in the presence of 5% serum, and incubated with BrdUrd. In this experiment (Fig. 3A), 72% of the myotubes were induced to synthesize DNA between 24 and 48 h p.i., as determined by double-immunofluorescence assay for BrdUrd and MyHC, a marker of muscle differentiation. The percentage of reactivated myotubes varied somewhat among different experiments, depending on the viral batches used and the reciprocal infection efficiency of the two viruses. However, in a large number of experiments, S phase always began at around 30 h p.i. Myotubes derived from primary mouse (Fig. 3B) and human (data not shown) satellite cells were reactivated with efficiency similar to that of C2C12 myotubes, demonstrating that cyclin D1-cdk4-mediated reactivation is not confined to immortal cells and applies to human TD myotubes as well.
FIG. 3.
Cyclin D1 and cdk4 expression induces DNA synthesis in myotubes. (A) C2C12 myotubes were coinfected with the Ad-cycD1 and J-cdk4 viruses as described in the legend to Fig. 2 and cultured, from 12 h p.i., in 5% FBS and 20 μM BrdUrd. Cells were fixed at the indicated time points p.i. and subjected to double immunofluorescence staining for MyHC and BrdUrd. The graph shows the percentages of BrdUrd+ myotubes (BrdUrd+ MyHC+ cells). (B) Primary murine satellite cell-derived myotubes were infected with the Ad-cycD1 and J-cdk4 viruses (top) or the control virus (bottom) as described in the legend to Fig. 2 and cultured in 5% FBS. The cells were fixed at 48 h p.i. and immunostained for MyHC (red) and BrdUrd (green). The upper image shows examples of BrdUrd+ MyHC+ cells, while none of the cells in the lower image incorporated BrdUrd.
To determine whether the simultaneous expression of both cyclin D1 and cdk4 is required to reactivate myotubes, we subjected C2C12 myotubes to infection with different ratios of the two viruses. For comparison, C3H-10T1/2 mouse fibroblasts (37) rendered quiescent by serum starvation were similarly infected. Table 1 shows that expression of both proteins, but of neither alone, can reactivate DNA synthesis in myotubes in a dose-dependent fashion. In contrast, serum-starved fibroblasts were efficiently reactivated by Ad-cycD1 infection alone and J-cdk4 superinfection contributed negligibly to the increase in the percentage of fibroblasts undergoing S phase.
TABLE 1.
Dependence of myotube and fibroblast reactivation on both cyclin D1 and cdk4
| J-cdk4 virus MOI | Ad-cycD1 virus MOIa
|
||||
|---|---|---|---|---|---|
| 60 | 30 | 15 | 7.5 | 0 | |
| 770 | 72 | 44 | 10 | 21 | 0 |
| 255 | 30 | 8 | 12 | 1 | 0 |
| 85 | 1 | 0 | 1 | 1 | 0 |
| 28 | 1 | 0.5 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 |
C2C12 myotubes were infected with the indicated viruses at the MOIs shown. Results are expressed as percentages of BrdUrd+ myotubes at 48 h p.i. Serum-starved, quiescent C3H10T1/2 fibroblasts were infected with Ad-Track (MOI, 800) or Ad-cycD1 (MOI, 35) alone or together with J-cdk4 (MOI, 750). BrdUrd+ fibroblasts at 48 h p.i. were 19, 53, and 60%, respectively.
These results show that DNA synthesis can be triggered in myotubes by the concurrent expression of cyclin D1 and cdk4. At variance with quiescent fibroblasts, reactivation of myotubes absolutely requires overexpression of cdk4 in addition to cyclin D1, confirming that TD muscle cells are subject to a different and tighter control of growth arrest.
It is known that accumulation and activation of cyclin D1 are both modulated by serum through multiple pathways. Thus, we looked at the influence of serum on myotube reactivation. C2C12 myotubes were infected with the Ad-cycD1 and J-cdk4 viruses in serum-free medium or medium containing 5 or 10% FBS. At 48 h p.i., the percentages of BrdUrd-positive myotubes were 16, 72, and 78%, respectively. Even at 72 h p.i., there was no further increase in the percentage of reactivated myotubes under any of these conditions, indicating that the absence of serum not only slows down the rate of cell cycle reentry but reduces altogether the recruitment of TD cells into S phase. In conclusion, as described in non-TD systems (20, 40), serum plays a major role in promoting the formation of active cyclin D1-cdk4 complexes, as reflected by the number of myotubes driven into the cell cycle.
Cell cycle progression in reactivated myotubes.
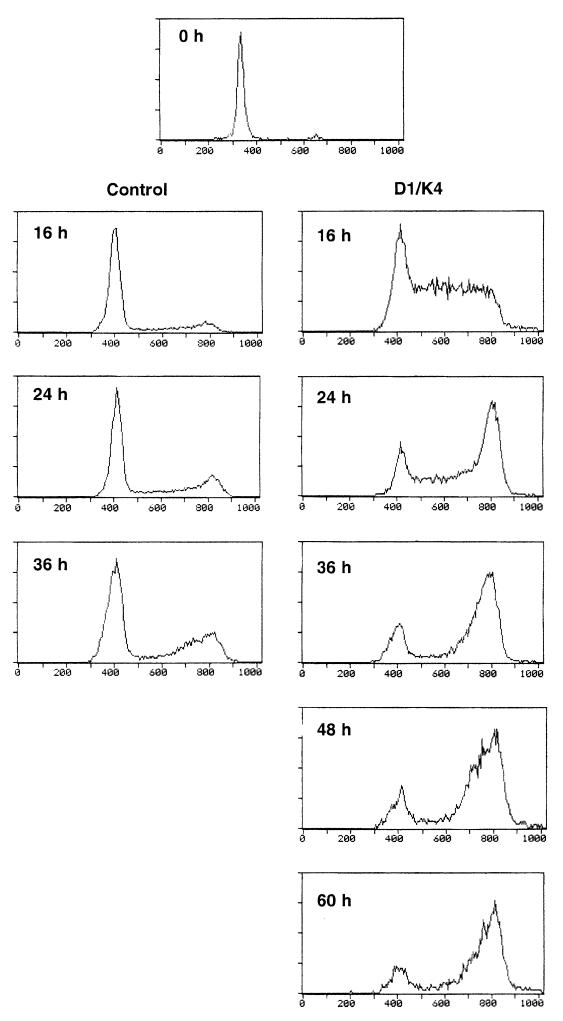
We wished to confirm that the observed BrdUrd incorporation was due to replicative, rather than reparative, DNA synthesis. TD, mononucleated myocytes derived from primary murine satellite cells were infected with the Ad-cycD1 and J-cdk4 or control viruses, cultured in the presence of 5% FBS, and subjected to cytofluorimetric analysis at successive time points. Control virus-infected myocytes showed progression through the cell cycle of only a modest fraction of the total cell population, corresponding to contaminating undifferentiated cells (Fig. 4). In sharp contrast, about 90% of the cells infected with the Ad-cycD1 and J-cdk4 viruses traversed S phase in a synchronous manner at around 16 h p.i. and accumulated in G2 at later times (Fig. 4). Most myocytes never entered M phase during the observation period, although rare mitoses were observed in cells double stained in an immunofluorescence assay for MyHC and BrdUrd (data not shown). These results indicate that the myocytes and myotubes reactivated by expression of cyclin D1 and cdk4 can successfully go through G1 and S phases but meet a block that prevents them from entering mitosis.
FIG. 4.
Cyclin D1 and cdk4 expression allows myotube progression through G1 and S phases, but reactivated muscle cells meet a block in G2. Mononucleated myocytes derived from primary murine satellite cells were infected with the Ad-cycD1 and J-cdk4 viruses or the control Ad-Track virus as described in the legend to Fig. 2 and subjected to cytofluorimetric analysis at the indicated time points. To maximize recruitment into the cell cycle, 5% FBS was added immediately after infection.
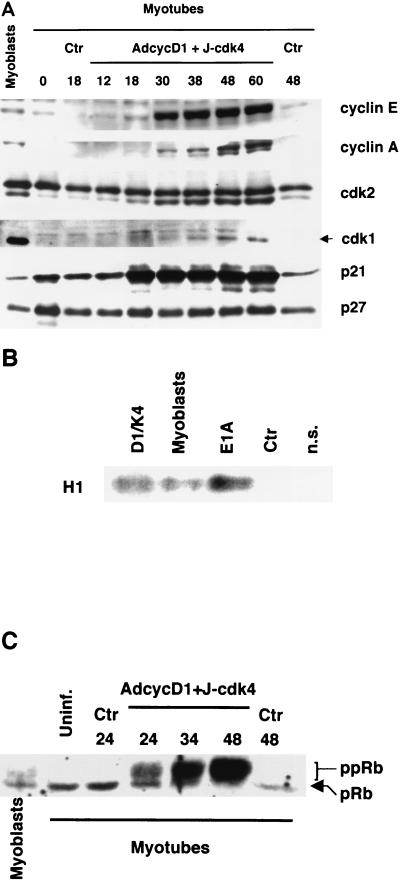
To determine whether the cell cycle reactivated by cyclin D1-cdk4 in myotubes progresses in the same orderly fashion as that of physiologically proliferating cells, virus-infected C2C12 myotubes were analyzed by Western blotting at various time points after infection; for comparison, proliferating, undifferentiated myoblasts were also analyzed. Figure 5 shows that the timing of important cell cycle events was consistent with the kinetics of entry into S phase shown in Fig. 3A. Cyclin E began to accumulate between 18 and 30 h p.i. and reached levels significantly higher than those found in proliferating myoblasts; cyclin A followed with some delay (Fig. 5A). Consistent with the rise in cyclin E, the faster-migrating, active form of cdk2 began to increase at 18 h p.i. Approximately coinciding with entry into S phase, exogenous cyclin D1, initially located mostly in the nucleus as assessed by immunofluorescence, started to translocate into the cytoplasm and became mostly cytoplasmic by 36 h p.i. (data not shown). The kinase inhibitor p21 levels increased substantially at 18 h and remained high until at least 60 h p.i. (Fig. 5A), consistent with similar findings obtained with cyclin D1-overexpressing fibroblasts and gliomas (17); expression of another inhibitor, p27, was essentially unaltered. The cdk1 kinase, which controls the G2/M transition, was upregulated in reactivated myotubes starting at 30 h p.i. but never reached the levels detected in proliferating myoblasts. Cyclin E-associated kinase activity was measured in the reactivated myotubes at 24 h p.i. and found to be comparable to that of myoblasts (Fig. 5B). Accordingly, the key regulatory protein pRb, detected as a single, hypophosphorylated band in uninfected and control virus-infected myotubes, showed progressively increasing amounts of slow-migrating, hyperphosphorylated forms, starting from 24 h p.i. (Fig. 5C). Thus, the impulse provided by cyclin D1 and cdk4 is sufficient to activate the physiological cascade of events that regulates progression of the cell cycle to the G2 phase.
FIG. 5.
Cell cycle-related events in myotubes reactivated by cyclin D1 and cdk4. (A) TD C2C12 myotubes were infected with the Ad-cycD1 and J-cdk4 viruses or a control virus as described in the legend to Fig. 2 and treated with 5% FBS from 12 h p.i.; total protein extracts were prepared at the indicated time points (h) after infection. Proliferating myoblasts and uninfected myotubes (0 h) were included for comparison. The indicated gene products were analyzed by Western blotting. (B) Cyclin E-associated kinase activity was measured in myotubes infected as described above. Myoblasts synchronized in late G1 (peak kinase activity) were included for comparison. A precipitation performed with a nonspecific (n.s.) antibody on a myoblast extract was also included. Total protein extracts were prepared at 24 h p.i. Precipitations were performed with an anti-cyclin E antibody from 2 mg of protein extract from control (Ctr) virus or Ad-cycD1–J-cdk4 virus-infected myotubes or 1 mg of protein extract from myoblasts (see Materials and Methods); as a positive control, a similar kinase assay was also performed on 1 mg of protein extract from myotubes infected with the 12S E1A-expressing dl520 virus. (C) Western blot analysis of the phosphorylation status of pRb. Proteins were extracted from myoblasts, uninfected (Uninf.) myotubes, or myotubes infected with the Ad-cycD1 and J-cdk4 viruses or a control virus at the indicated time points p.i. (hours).
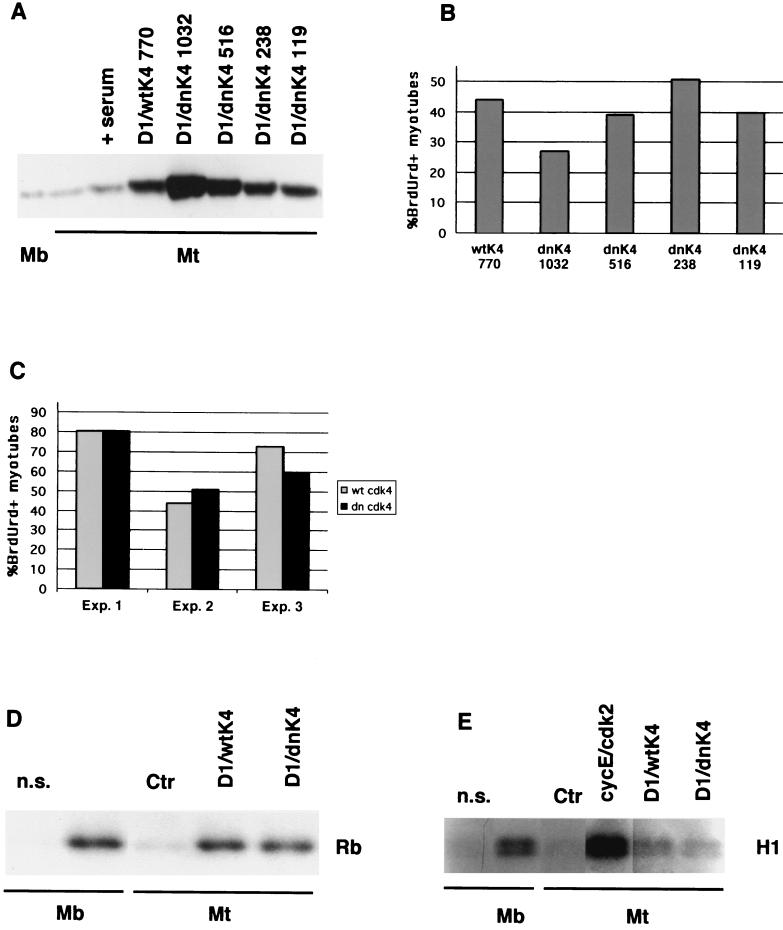
Activity of wild-type cdk4 versus that of dominant-negative cdk4.
In our system, overexpression of cdk4 might be needed either to achieve supraphysiological levels of kinase activity or, alternatively, to titrate cdk inhibitors that prevent endogenous kinases from functioning. The first possibility can be ruled out, since reactivated myotubes show cyclin D1-associated kinase activity levels comparable to those of proliferating cells (Fig. 2E). The second hypothesis predicts that, in synergism with exogenous cyclin D1, even kinase-inactive mutant forms of cdk4 able to bind cdk inhibitors should be capable of reactivating myotubes expressing endogenous cdk4. Such mutant forms have already been shown to be proficient in cell cycle reactivation (22) and transformation (13) assays. To test this prediction, we generated a recombinant adenovirus carrying dominant-negative mutant cdk4 (48) devoid of kinase activity (Ad-dncdk4). We performed infections of myotubes with Ad-cycD1 (MOI, 60) and with various amounts of Ad-dncdk4 virus to identify the MOI yielding the same cdk4 protein levels as the optimal MOI for J-cdk4 (770; Table 1). This MOI was found to be 238 (Fig. 6A), and as shown in Fig. 6B, it induced cell cycle reentry more efficiently than higher or lower MOIs. Moreover, despite some variability, the capacity of the mutant cdk4 to reactivate myotubes was constantly comparable to that of the wild-type protein within the same experiment (Fig. 6B and C). Similar to J-cdk4, the Ad-dncdk4 virus alone induced no BrdUrd incorporation at any of the MOIs tested (data not shown). Altogether, these results confirm our interpretation of the role of exogenous cdk4 as a titrating agent for kinase inhibitors (see Discussion).
FIG. 6.
Expression and activity of dncdk4. (A) Western blot comparing expression of exogenous wild-type (wtK4) and dominant-negative cdk4 (dnK4) in myotubes infected at the indicated MOIs with the corresponding viruses along with Ad-cycD1 (MOI, 60). (B) Percentages of reactivated myotubes infected as described above. (C) Percentages of reactivated myotubes infected with Ad-cycD1 (MOI, 60) and either J-cdk4 (wt cdk4; MOI, 770) or Ad-dncdk4 (dn cdk4; MOI, 238). Three independent experiments are shown. (D) Anti-cdk4-immunoprecipitated kinase activity from myotubes infected as described for panel C. Myoblasts were included for comparison. Kinase assays were done as described in the legend to Fig. 2. (E) Cyclin E-associated kinase activity immunoprecipitated from myotubes infected with the control virus, Ad-cycE plus Ad-cdk2, Ad-cycD1 plus J-cdk4, or Ad-cycD1 plus-Ad-dncdk4. n.s., nonspecific antibody.
To investigate the mechanism of cell cycle reactivation by Ad-dncdk4–Ad-cycD1, the cdk4 kinase activity in the doubly infected myotubes was measured. The Ad-dncdk4 virus did elicit a cdk4 activity that was comparable to that obtained with the virus carrying the wild-type protein (Fig. 6D). In both cases, the infected myotubes expressed a cdk4 enzymatic activity no higher than that found in proliferating myoblasts and a cyclin E-associated kinase activity much lower than that produced by exogenous cyclin E-cdk2 expression (Fig. 6E). We conclude that in our system, in the presence of high levels of exogenous cyclin D1, wild-type and mutant cdk4 proteins are equally effective in activating the cdk4 kinase. Moreover, their main mechanism of action is not titration of kinase inhibitors from the endogenous cyclin E complexes (see Discussion).
Transfection experiments were performed in which C2C12 or primary mouse myotubes were transduced with plasmids carrying cyclin D1 and either wild-type cdk4 or wild-type cdk6. The two combinations induced BrdUrd incorporation in myotubes with similar efficiencies (A.S., M.T., and M.C., unpublished observations), demonstrating that exogenous cdk4 and cdk6 function similarly in this system and ruling out essential contributions to reactivation by adenovirus genes. However, it was still possible that adenovirus infection might synergize with cyclin D1 and cdk4, enhancing their reactivation capabilities. To rule out this possibility, we resorted to primary quail myotubes, which can be transfected with higher efficiencies than murine muscle cells. Table 2 shows the results of two independent experiments in which 80 to 90% of the transfected myotubes were reactivated by cotransfection of cyclin D1 and cdk4, irrespective of the simultaneous infection with an empty control adenovirus. These experiments show that the sole expression of the two cell cycle-regulatory proteins suffices to efficiently induce DNA synthesis in TD myotubes and that any potential contribution by adenovirus proteins produces no discernible effect.
TABLE 2.
Effectiveness of transfection-mediated expression of cyclin D1 and cdk4 in myotubesa
| Condition | % BrdUrd+ transfected myotubes |
|---|---|
| Expt 1 | |
| cyclin D1 + cdk4 | 90.9 |
| cyclin D1 + cdk4 + J-pCA13 | 88.9 |
| Expt 2 | |
| cyclin D1 + cdk4 | 82.0 |
| cyclin D1 + cdk4 + J-pCA13 | 88.4 |
Primary quail myotubes were cotransfected with cyclin D1 and cdk4 expression vectors, infected or not with the empty J-pCA13 adenovirus (MOI, 770), and cultured in the presence of serum. BrdUrd was added 16 h later, and its incorporation was assessed by immunofluorescence assay 48 h after the transfection. The transfected myotubes were identified by immunostaining for cyclin D1 (experiment 1) or cotransfected luciferase (experiment 2). In these experiments, transfection efficiency was approximately 40%, as determined by evaluating either marker.
Differentiation.
In skeletal muscle cells, proliferation is generally incompatible with expression of the differentiation program. To assess whether cyclin D1-cdk4-mediated reactivation of myotubes interferes with tissue-specific gene expression, we analyzed the mRNAs of representative muscle-specific structural genes, including those for MCK, MyHC, and MLC-1. Myotubes infected with the Ad-cycD1 and J-cdk4 or control Ad-Track viruses and cultured in 5% FBS were harvested at different times after infection, and total RNA was prepared. Northern analysis showed that the levels of mRNA of the three muscle genes were markedly reduced by 48 h p.i. (Fig. 7). Thus, cyclin D1-cdk4 expression in myotubes induces generalized downregulation of muscle-specific genes.
FIG. 7.
Cyclin D1-cdk4-dependent cell cycle reactivation in myotubes impairs tissue-specific gene expression. C2C12 myotubes infected with a control virus or the Ad-cycD1 and J-cdk4 viruses as described in the legend to Fig. 2 and immediately treated with 5% FBS were lysed at the indicated times p.i. (hours), total RNA was hybridized with labeled probes to MCK, MyHC, and MLC-1. The hybridizations were performed onto two different blots. For technical reasons, normalization was carried out by hybridizing one filter with a GAPDH probe and the other with a 28S rRNA probe. Each blot should be compared with its respective normalizing hybridization, indicated by the letters in parentheses that follow the names of the genes (G, GAPDH; r, 28S rRNA). Ctr, control.
Cell cycle reactivation in adipocytes and neurons.
We wondered whether cyclin D1-mediated reactivation is specific to skeletal muscle cells or also applies to other TD cellular systems. To address this question, we attempted to reactivate two other TD cell types, adipocytes and neurons.
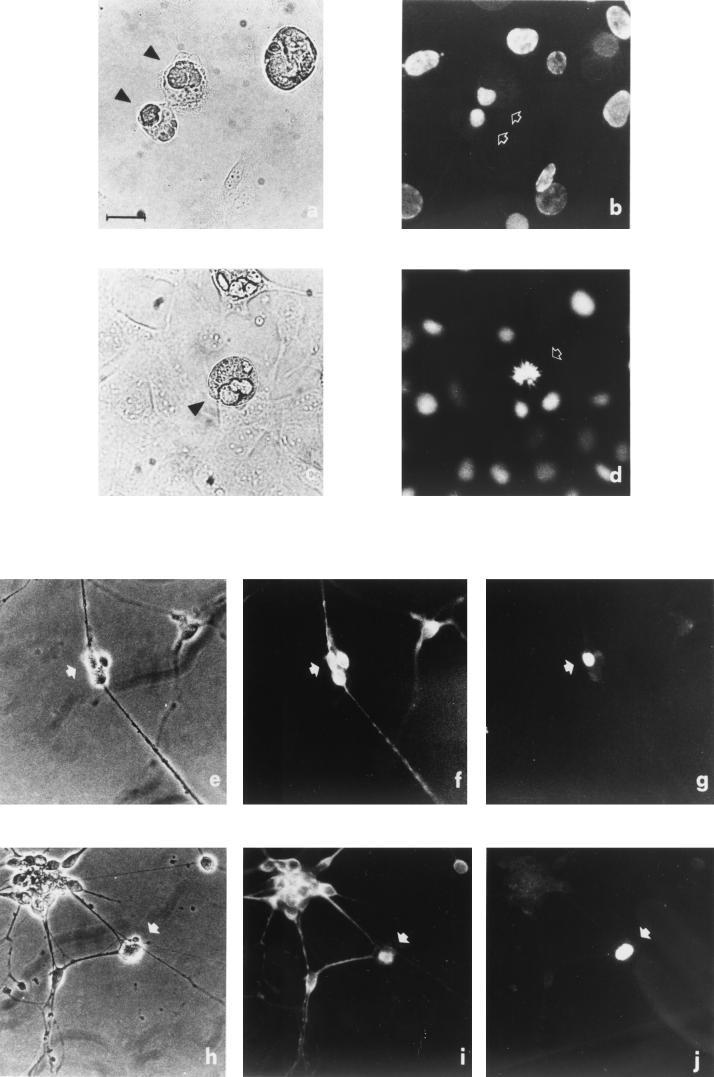
3T3-L1 is a preadipocytic cell line widely used as an in vitro model of adipogenesis. These cells differentiate into TD, fat-laden adipocytes upon reaching confluence in mitogen-rich medium and after treatment with inducing chemicals (see Materials and Methods). Fully mature adipocytes were infected with the Ad-cycD1 and J-cdk4 viruses or the control Ad-Track virus. As expected, Ad-Track virus infection did not elicit DNA synthesis in adipocytes. In contrast, in three independent experiments, about 50% of the Ad-cycD1–J-cdk4-infected adipocytes incorporated BrdUrd in the 48 h following infection (Fig. 8A and B) and frequent mitotic figures were noticed (Fig. 8C and D).
FIG. 8.
Cyclin D1 and cdk4 reactivate TD adipocytes and neurons. TD 3T3-L1 adipocytes were infected with the Ad-cycD1 and J-cdk4 viruses and, 48 h later, subjected to BrdUrd immunofluorescence. Two microscopic fields (a, b and c, d) are shown. Two adipocytes (arrowheads in panel a) are BrdUrd+ (arrows in panel b) and show condensed chromatin, suggestive of a late telophase. The adipocyte photographed in phase contrast (arrowhead in panel c) shows a metaphase plate by Hoechst 33258 staining (arrow in panel d). P19-derived neurons were infected with the Ad-cycD1 and J-cdk4 viruses and doubly stained for the neuron-specific marker Tau and BrdUrd. Two examples of reactivated neurons (arrows) are shown in phase contrast (e and h); the same two fields are shown stained with anti-Tau (f and i) and anti-BrdUrd (g and j). Bar, 100 μm.
P19 embryonal carcinoma cells were chosen as a neurogenic model. These are undifferentiated embryo cells that form embryoid bodies in suspension culture. Cells transiently treated with retinoic acid in suspension and replated onto a suitable substrate differentiate within a few days into TD neurons expressing a variety of neurotransmitters and neuron-specific markers (1, 43). Completeness of differentiation is shown by the ability of P19-derived neurons to integrate in the central nervous system in vivo (29). P19-derived neurons were infected with the Ad-cycD1 and J-cdk4 viruses and doubly stained for the neuron-specific marker Tau and BrdUrd. Although these cells are relatively resistant to adenovirus infection, as determined by expression of the green fluorescent protein carried by Ad-cycD1 (data not shown), roughly 5% of the neurons in the culture incorporated BrdUrd within 36 h of infection (Fig. 8E to J). As expected, mock- and Ad-Track-infected neurons were uniformly BrdUrd negative (data not shown).
Together, these results allow us to conclude that forced expression of cyclin D1 and cdk4 has the ability to reactivate the cell cycle in a wide variety of TD cells, suggesting that the maintenance of the postmitotic state absolutely requires tight control of these two molecules.
DISCUSSION
Reactivation of TD cells by expression of cellular regulators.
In this paper, we demonstrate cell cycle progression in TD skeletal muscle cells by forced expression of cyclin D1 and cdk4. Reactivation of TD skeletal muscle cells by expression of cellular genes has been exceedingly difficult to achieve. Indeed, in the present work, gross overexpression of cyclin E and cdk2 resulted in no reactivation of myotubes, although the sole overexpression of cyclin E has been shown to drive p16-arrested fibroblasts into S phase even in the absence of E2F activity or in the presence of a phosphorylation-deficient mutant form of Rb (25). Thus, reliable reactivation of TD skeletal muscle cells has been obtained so far only by making use of DNA tumor virus oncogenes (4, 7, 8, 9, 50). Although successful, this strategy is unsatisfactory, since viral oncogene-mediated reactivation sheds little light on the mechanisms preventing cell cycle reentry by these cells. In the case of E1A, we have previously shown that, by acting directly at the G1/S transition (47), it simply bypasses the inability of TD cells to progress beyond mid-G1 (Fig. 9). In this work, we have attempted to remove directly the mid-G1 block, assuming that it is embodied by the absence of cyclin D1-dependent kinase activity. Indeed, reconstitution of such activity drives myotubes across this barrier and allows progression through the cell cycle. Importantly, the same strategy succeeded in reactivating two other widely different types of TD cells, adipocytes and neurons, indicating that our conclusions may be extended to a variety of TD cells.
FIG. 9.
Working model of mitotic cycle regulation in TD cells. In this schematic, the first half of the cell cycle is represented in a linear fashion (thick arrow); boundaries between cell cycle phases are marked by thin vertical lines. The approximate points where transcription of some cell cycle regulatory genes begins are marked. Growth factor or retroviral-oncogene stimulation induces G0-arrested TD cells to enter G1 and progress to the mid-G1 block (thick vertical line) but not beyond. The adenovirus oncogene E1A bypasses the mid-G1 block by acting directly at the G1/S boundary, in part by freeing E2F from the control of pRb family proteins. In turn, E2F promotes the transcription of genes directly involved in S phase transition and DNA replication, among which are those for cyclin E and cyclin A. In contrast, simultaneous expression of cyclin D1 and cdk4 reconstitutes cdk4 activity and allows progression beyond the G1 cell cycle block initiated by serum stimulation. A second block, represented by a thin double line close to the G1/S boundary prevents cyclin E (this work)- or E2F (references 30 and 31)-mediated reactivation of myotubes.
It has been reported that transgenic mice expressing cyclin D1 in the myocardium (42) or cyclin D1 and cdk4 in the lens (11) show deranged control of the postmitotic state in the respective tissues. The results obtained with these animals suggest that cyclin D1 and cdk4 have the potential to interfere with the postmitotic state. However, in these transgenic mice, it is impossible to distinguish impaired entry into terminal differentiation from true reactivation of postmitotic cells, since in both cases the tissue-specific promoters used to drive the transgenes are already active before and during the establishment of terminal differentiation.
Mechanisms of cell cycle reactivation.
Different from fibroblasts, myotube reactivation, in addition to cyclin D1, requires exogenous cdk4, although significant levels of this protein are normally present in these cells (41). Since a number of kinase inhibitors of the INK4 and Kip families are highly expressed in myotubes, overexpression of both kinase subunits might be required to titrate them. Indeed, our results strongly indicate this to be the case. Similar levels of cdk4 kinase activity were measured in myotubes forced to express cyclin D1 and either wild-type or kinase-dead cdk4 (Fig. 6D). In the latter case, the activity must be expressed by the endogenous kinase, suggesting that the main role of dncdk4 and, by extension, of the wild-type protein is to sequester inhibitors of the endogenous kinase. The apparent paradox of a cdk4 activity in cells overexpressing a dominant-negative kinase might be explained by the simultaneous presence of excess cyclin D1. While most dncdk4 protein would serve to titrate endogenous inhibitors, any residual amounts of such a molecule would form inactive complexes with cyclin D1. Enough endogenous and/or exogenous cyclin D1 would still be present in the myotube to form active complexes with the endogenous cdk4, now in the functional absence of inhibitors. The fact that very similar amounts of wild-type and mutant cdk4 attain similar peak reactivation efficiencies (Fig. 6A and B) indicates that optimal myotube reactivation is achieved only by a defined amount of dominant-negative cdk4. Too little of this protein would not suffice to titrate the inhibitors, while too much would block all available cyclin D1.
In principle, overexpression of cyclin D1 and cdk4 might subtract Kip-type inhibitors from endogenous cyclin E-cdk2 complexes. That this is not the main mechanism through which myotube reactivation is achieved is demonstrated by the low levels of cyclin E-associated kinase activity in cyclin D1-cdk4-overexpressing myotubes (Fig. 6E). These levels are far lower than those attained by direct overexpression of cyclin E and cdk2, which nonetheless fails to trigger DNA synthesis. Forced expression of cyclin E and cdk2 in myotubes is able to induce substantial pRb phosphorylation (Fig. 1C) but not DNA replication. These finding are only apparently contradictory. On one side, expression of cyclin E under the control of the cyclin D1 promoter rescues the phenotype of cyclin D1 nullizygous mice (10), suggesting that early activation of the cyclin E-associated kinase is sufficient to drive the cell cycle. On the other side, a substantial body of work has shown that cyclin D1- and cyclin E-dependent kinases phosphorylate partly different pRb residues (10, 19, 51). In addition, it has been shown that the two kinases are not equally able to inactivate pRb (5). Finally, at least in SaOS-2 cells, phosphorylation and inactivation of pRb by the cyclin E-associated kinase clearly requires previous phosphorylation by cyclin D-regulated kinases (26). Thus, most biochemical and cellular data converge on the conclusion that the two kinases are both required for full pRb inactivation. Accordingly, it has been recently demonstrated that cyclin E kinase reactivation by mutant E1A in C2C12 myotubes is insufficient to drive these cells into S phase (27). However, it is formally possible that other activities of cyclin D1 and cdk4 might be crucial, alone or along with pRb phosphorylation, for myotube reactivation.
Multiple mechanisms might underlie the ability of serum to greatly increase the percentage of cyclin D1-cdk4-infected myotubes reentering S phase. Serum upregulates the endogenous cyclin D1-encoding gene and increases the levels of exogenous cyclin D1 about twofold (data not shown). In addition, serum promotes cyclin D-cdk4 complex formation, activation, and nuclear localization through diverse pathways (40). Finally, since serum allows TD muscle cells to traverse G1 up to the point where the cdk4 kinase is required (46), it might facilitate the response to cdk4 activity by inducing preliminary, early G1 events.
In myotubes infected with the Ad-cycD1 and J-cdk4 viruses, cell cycle reactivation is accompanied by downregulation of late differentiation markers. Suppression of muscle structural genes, also observed during viral oncogene-mediated myotube reactivation (8, 47), might be a consequence of cell cycle reentry, since the muscle differentiation program is generally incompatible with proliferation. In addition, both cyclin D1 and cdk4 have been shown to interfere with the myoD transacting function, possibly in multiple ways (36, 41, 52, 53).
Cell cycle progression and G2 block.
Some aspects of cell cycle reactivation in myotubes are worth discussing. Cyclin E is expressed at very high levels, similarly to E1A-stimulated myotubes (47). In the latter case, however, the corresponding kinase activity is also very high (33). On the contrary, cyclin D1-cdk4-reactivated myotubes only show cyclin E-associated kinase activity comparable to that of myoblasts (Fig. 5B), suggesting that the high cyclin E levels are partly neutralized by the increased p21 levels (Fig. 5A). Cdk1 is first clearly detectable after the onset of DNA synthesis but never accumulates to the levels found in proliferating myoblasts, consistent with a premitotic block. The almost complete absence of mitoses in reactivated muscle cells and the cytofluorimetric analysis of Ad-cycD1–J-cdk4-infected myocytes show that these cells undergo a G2 arrest. A number of hypotheses can be made as to the causes of such an arrest, which is not observed in TD muscle cells reactivated by E1A (7, 21). The more “physiological” cell cycle reentry promoted by cyclin D1-cdk4 expression might uncover in TD cells a second G2 block that is overridden and thus made inconspicuous by E1A. Hints that such a block exists can be found in TD myocardiocytes that, even when reactivated by E1A, accumulate in G2 (18, 24). Another suggestion of the presence of a G2 block in TD cells comes from skeletal myoblasts derived from Rb knockout mice. These cells never definitively withdraw from the cell cycle during differentiation (30, 39). However, when induced to reenter the cell cycle by serum stimulation, they rarely undergo mitosis (30). Alternatively, these observations and ours might be explained by the activation of the G2 checkpoint by DNA damage consequent to forced cell cycle reentry. In addition, in the case of cyclin D1-cdk4-induced reactivation, the persistent, deregulated overexpression of cyclin D1 might derange the control of later stages of the cell cycle. We cannot rule out the possibility that the ability of recombinant adenovirus to modify cell cycle progression (49) contributes to the observed G2 block. In any case, it should be stressed that the failure to enter mitosis does not appear to be a universal feature of all TD cells, as at least adipocytes frequently undergo M phase in response to cyclin D1-cdk4 expression (Fig. 8C and D).
The current working model.
The present results contribute to our understanding of the postmitotic state. As schematized in Fig. 9, growth factors cannot promote cell cycle reentry in TD cells because of their inability to activate the cdk4 and cdk6 kinases. Retroviral oncogenes, largely mimicking the presence of growth factors, hit the same barrier. Reconstitution of cyclin D1-associated kinase activity removes the obstacle and allows TD cells to continue their initial response to mitogens, passing the G1/S transition. This feat cannot be accomplished by acting directly at the G1/S boundary through expression of the downstream regulator E2F or cyclin E-cdk2, which in turn suggests that additional controls act in late G1 in myotubes to negate DNA synthesis.
ACKNOWLEDGMENTS
We are grateful to J. Cook and J. Nevins for generously donating unpublished viruses. Our thanks go to T.-C. He and B. Vogelstein for the adenovirus construction system. We also thank S. van den Heuvel, G. Cossu, C. Schneider, G. Draetta, and J. Pines, who donated reagents that allowed us to perform the present work. We thank F. Tató for critically reading the manuscript.
A.S., D.P., and A.F. are recipients of FIRC fellowships. This work was supported by the Comitato Telethon Fondazione Onlus, the Associazione Italiana per la Ricerca sul Cancro, and the Italian Ministry of Health.
The first two authors contributed equally to this work.
REFERENCES
- 1.Bain G, Gottlieb D I. Neural cells derived by in vitro differentiation of P19 and embryonic stem cells. Perspect Dev Neurobiol. 1998;5:175–178. [PubMed] [Google Scholar]
- 2.Bett A J, Haddara W, Prevec L, Graham F L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blau H M, Pavlath G K, Hardeman E C, Chiu C-P, Silberstein L, Webster S G, Miller S C, Webster C. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso M C, Leonhardt H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- 5.Connell-Crowley L, Harper J W, Goodrich D W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation Mol. Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crescenzi M, Soddu S, Sacchi A, Tató F. Adenovirus infection induces reentry into the cell cycle of terminally differentiated skeletal muscle cells. Ann N Y Acad Sci. 1995;752:9–18. doi: 10.1111/j.1749-6632.1995.tb17402.x. [DOI] [PubMed] [Google Scholar]
- 7.Crescenzi M, Soddu S, Tató F. Mitotic cycle reactivation in terminally differentiated cells by adenovirus infection. J Cell Physiol. 1995;162:26–35. doi: 10.1002/jcp.1041620105. [DOI] [PubMed] [Google Scholar]
- 8.Endo T, Nadal-Ginard B. SV40 large T antigen induces reentry of terminally differentiated myotubes into the cell cycle. In: Kedes L H, Stockdale F E, editors. Cellular and molecular biology of muscle development. New York, N.Y: Alan R. Liss, Inc.; 1989. pp. 95–104. [Google Scholar]
- 9.Fogel M, Defendi V. Infection of muscle cultures from various species with oncogenic DNA viruses (SV40 and polyoma) Proc Natl Acad Sci USA. 1967;58:967–973. doi: 10.1073/pnas.58.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng Y, Whoriskey W, Park M Y, Bronson R T, Medema R H, Li T, Weinberg R A, Sicinski P. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell. 1999;97:767–777. doi: 10.1016/s0092-8674(00)80788-6. [DOI] [PubMed] [Google Scholar]
- 11.Gomez Lahoz E, Liegeois N J, Zhang P, Engelman J A, Horner J, Silverman A, Burde R, Roussel M F, Sherr C J, Elledge S J, DePinho R A. Cyclin D- and E-dependent kinases and the p57(KIP2) inhibitor: cooperative interactions in vivo. Mol Cell Biol. 1999;19:353–363. doi: 10.1128/mcb.19.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 13.Haas K, Staller P, Geisen C, Bartek J, Eilers M, Moroy T. Mutual requirement of CDK4 and Myc in malignant transformation: evidence for cyclin D1/CDK4 and p16INK4A as upstream regulators of Myc. Oncogene. 1997;15:179–192. doi: 10.1038/sj.onc.1201171. [DOI] [PubMed] [Google Scholar]
- 14.Haley K P, Overhauser J, Babiss L E, Ginsberg H S, Jones N C. Transformation properties of type 5 adenovirus mutants that differentially express the E1A gene products. Proc Natl Acad Sci USA. 1984;81:5734–5738. doi: 10.1073/pnas.81.18.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison T, Graham F L, Williams J. Host-range mutant of adenovirus type 5 defective for growth in HeLa cells. Virology. 1977;77:319–329. doi: 10.1016/0042-6822(77)90428-7. [DOI] [PubMed] [Google Scholar]
- 16.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiyama H, Iavarone A, LaBaer J, Reeves S. Regulated ectopic expression of cyclin D1 induces transcriptional activation of the cdk inhibitor p21 gene without altering cell cycle progression. Oncogene. 1997;14:2533–2542. doi: 10.1038/sj.onc.1201080. [DOI] [PubMed] [Google Scholar]
- 18.Kirshenbaum L A, Schneider M D. Adenovirus E1A represses cardiac gene transcription and reactivates DNA synthesis in ventricular myocytes, via alternative pocket protein- and p300-binding domains. J Biol Chem. 1995;270:7791–7794. doi: 10.1074/jbc.270.14.7791. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa M, Higashi H, Jung H K, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, Taya Y. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 20.Ladha M H, Lee K Y, Upton T M, Reed M F, Ewen M E. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol Cell Biol. 1998;18:6605–6615. doi: 10.1128/mcb.18.11.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latella L, Sacchi A, Crescenzi M. Long-term fate of terminally differentiated skeletal muscle cells following E1A-initiated cell cycle reactivation. Cell Death Differ. 2000;7:145–154. doi: 10.1038/sj.cdd.4400592. [DOI] [PubMed] [Google Scholar]
- 22.Latham K M, Eastman S W, Wong A, Hinds P W. Inhibition of p53-mediated growth arrest by overexpression of cyclin-dependent kinases. Mol Cell Biol. 1996;16:4445–4455. doi: 10.1128/mcb.16.8.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone G, DeGregori J, Jakoi L, Cook J G, Nevins J R. Collaborative role of E2F transcriptional activity and G1 cyclin-dependent kinase activity in the induction of S phase. Proc Natl Acad Sci USA. 1999;96:6626–6631. doi: 10.1073/pnas.96.12.6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Kitsis R N. Induction of DNA synthesis and apoptosis in cardiac myocytes by E1A oncoprotein. J Cell Biol. 1996;133:325–334. doi: 10.1083/jcb.133.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 26.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mal A, Chattopadhyay D, Ghosh M K, Poon R Y, Hunter T, Harter M L. p21 and Retinoblastoma protein control the absence of DNA replication in terminally differentiated muscle cells. J Cell Biol. 2000;149:281–292. doi: 10.1083/jcb.149.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBurney M W. P19 embryonal carcinoma cells. Int J Dev Biol. 1993;37:135–140. [PubMed] [Google Scholar]
- 29.Morassutti D J, Staines W A, Magnuson D S, Marshall K C, McBurney M W. Murine embryonal carcinoma-derived neurons survive and mature following transplantation into adult rat striatum. Neuroscience. 1994;58:753–763. doi: 10.1016/0306-4522(94)90452-9. [DOI] [PubMed] [Google Scholar]
- 30.Novitch B G, Mulligan G J, Jacks T, Lassar A B. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odelberg S J, Kollhoff A, Keating M T. Dedifferentiation of mammalian myotubes induced by msx1. Cell. 2000;103:1099–1109. doi: 10.1016/s0092-8674(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki K, Holtzer H. Myogenesis: fusion, myosin synthesis, and the mitotic cycle. Proc Natl Acad Sci USA. 1966;56:1484–1490. doi: 10.1073/pnas.56.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajalunga D, Tognozzi D, Tiainen M, D'Angelo M, Ferrantelli F, Helin K, Sacchi A, Crescenzi M. E2F activates late-G1 events but cannot replace E1A in inducing S phase in terminally differentiated skeletal muscle cells. Oncogene. 1999;18:5054–5062. doi: 10.1038/sj.onc.1202897. [DOI] [PubMed] [Google Scholar]
- 34.Puri P L, Cimino L, Fulco M, Zimmerman C, La Thangue N B, Giordano A, Graessmann A, Levrero M. Regulation of E2F4 mitogenic activity during terminal differentiation by its heterodimerization partners for nuclear translocation. Cancer Res. 1998;58:1325–1331. [PubMed] [Google Scholar]
- 35.Rando T A, Blau H M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao S S, Chu C, Kohtz S D. Ectopic expression of cyclin D1 prevents activation of gene transcription by myogenic basic helix-loop-helix regulators. Mol Cell Biol. 1994;14:5259–5267. doi: 10.1128/mcb.14.8.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reznikoff C A, Brankow D W, Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973;33:3231–3238. [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Schneider J W, Gu W, Zhu L, Mahdavi V, Nadal-Ginard B. Reversal of terminal differentiation mediated by p107 in Rb−/− muscle cells. Science. 1994;264:1467–1471. doi: 10.1126/science.8197461. [DOI] [PubMed] [Google Scholar]
- 40.Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 41.Skapek S S, Rhee J, Spicer D B, Lassar A B. Inhibition of myogenic differentiation in proliferating myoblasts by cyclin D1-dependent kinase. Science. 1995;267:1022–1024. doi: 10.1126/science.7863328. [DOI] [PubMed] [Google Scholar]
- 42.Soonpaa M H, Koh G Y, Pajak L, Jing S, Wang H, Franklin M T, Kim K K, Field L J. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Investig. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staines W A, Morassutti D J, Reuhl K R, Ally A I, McBurney M W. Neurons derived from P19 embryonal carcinoma cells have varied morphologies and neurotransmitters. Neuroscience. 1994;58:735–751. doi: 10.1016/0306-4522(94)90451-0. [DOI] [PubMed] [Google Scholar]
- 44.Stocum D L. New tissues from old. Science. 1997;276:15. doi: 10.1126/science.276.5309.15. [DOI] [PubMed] [Google Scholar]
- 45.Tató F, Alema S, Dlugosz A, Boettiger D, Holtzer H, Cossu G, Pacifici M. Development of ‘revertant’ myotubes in cultures of Rous sarcoma virus transformed avian myogenic cells. Differentiation. 1983;24:131–139. doi: 10.1111/j.1432-0436.1983.tb01312.x. [DOI] [PubMed] [Google Scholar]
- 46.Tiainen M, Pajalunga D, Ferrantelli F, Soddu S, Salvatori G, Sacchi A, Crescenzi M. Terminally differentiated skeletal myotubes are not confined in G0, but can enter G1 upon growth factor stimulation. Cell Growth Differ. 1996;7:1039–1050. [PubMed] [Google Scholar]
- 47.Tiainen M, Spitkovsky D, Jansen-Dürr P, Sacchi A, Crescenzi M. Expression of E1A in terminally differentiated muscle cells reactivates the cell cycle and suppresses tissue-specific genes by separable mechanisms. Mol Cell Biol. 1996;16:5302–5312. doi: 10.1128/mcb.16.10.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 49.Wersto R P, Rosenthal E R, Seth P K, Eissa N T, Donahue R E. Recombinant, replication-defective adenovirus gene transfer vectors induce cell cycle dysregulation and inappropriate expression of cyclin proteins. J Virol. 1998;72:9491–9502. doi: 10.1128/jvi.72.12.9491-9502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaffe D, Gershon D. Multinucleated muscle fibres: induction of DNA synthesis and mitosis by polyoma virus infection. Nature. 1967;215:421–424. doi: 10.1038/215421a0. [DOI] [PubMed] [Google Scholar]
- 51.Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J M, Wei Q, Zhao X, Paterson B M. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J. 1999;18:926–933. doi: 10.1093/emboj/18.4.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J M, Zhao X, Wei Q, Paterson B M. Direct inhibition of G1 cdk kinase activity by MyoD promotes myoblast cell cycle withdrawal and terminal differentiation. EMBO J. 1999;18:6983–6993. doi: 10.1093/emboj/18.24.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]