Highlights
-
•
This is the first randomised evaluation of the cost-effectiveness of a 0/1-hour high-sensitivity troponin protocol and has implications on clinical practice on a health system level.
-
•
The results demonstrate that the 0/1-hour hs-cTnT protocol is safe and does not incur excess resource compared to the conventional 0/3-hour protocol.
-
•
Whilst this cost-effectiveness analysis demonstrates superior ED efficiency and equivalent safety and resource associated with the 0/1-hour hs-cTnT protocol, further refinements in subsequent management is necessary to facilitate large-scale adaptation.
Keywords: Cost-effectiveness, High-sensitivity troponin, Acute coronary syndrome, Randomised trial, RAPID TnT
Abstract
Background
To understand the economic impact of an accelerated 0/1-hour high-sensitivity troponin-T (hs-cTnT) protocol.
Objective
To conduct a patient-level economic analysis of the RAPID-TnT randomised trial in patients presenting with suspected acute coronary syndrome (ACS).
Methods
An economic evaluation was conducted with 3265 patients randomised to either the 0/1-hour hs-cTnT protocol (n = 1634) or the conventional 0/3-hour standard-of-care protocol (n = 1631) with costs reported in Australian dollars. The primary clinical outcome was all-cause mortality or new/recurrent myocardial infarction.
Results
Over 12-months, mean per patient costs were numerically higher in the 0/1-hour arm compared to the conventional 0/3-hour arm (by $472.49/patient, 95% confidence interval [95 %CI]: $-1,380.15 to $2,325.13, P = 0.617) with no statistically significant difference in primary outcome (0/1-hour: 62/1634 [3.8%], 0/3-hour: 82/1631 [5.0%], HR: 1.32 [95 %CI: 0.95–1.83], P = 0.100). The mean emergency department (ED) length of stay (LOS) was significantly lower in the 0/1-hour arm (by 0.62 h/patient, 95 %CI: 0.85 to 0.39, P < 0.001), but the subsequent 12-month unplanned inpatient costs was numerically higher (by $891.22/patient, 95 %CI: $-96.07 to 1,878.50, P = 0.077). Restricting the analysis to patients with hs-cTnT concentrations ≤ 29 ng/L, mean per patient cost remained numerically higher in the 0/1-hour arm (by $152.44/patient, 95 %CI:$-1,793.11 to $2,097.99, P = 0.988), whilst the reduction in ED LOS was more pronounced (by 0.70 h/patient, 95 %CI: 0.45–0.95, P < 0.001).
Conclusions
There were no differences in resource utilization between the 0/1-hour hs-cTnT protocol versus the conventional 0/3-hour protocol for the assessment of suspected ACS, despite improved initial ED efficiency. Further refinements in strategies to improve clinical outcomes and subsequent management efficiency are needed.
1. Introduction
High-sensitivity cardiac troponin (hs-cTn)-based accelerated 0/1-hour and 0/2-hour algorithms are recommended by the 2016 and 2020 European Society of Cardiology (ESC) guidelines for the assessment of patients with suspected acute coronary syndrome (ACS) in preference to the conventional 0/3-hour algorithms [1], [2]. These recommendations are based on large multicentre observational studies of the accelerated protocols that have consistently demonstrated excellent diagnostic performance and equivalent safety [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. Our group previously reported the 30-day and the 12-month outcomes of the first patient-level randomised trial of a 0/1-hour hs-cTnT protocol, the Rapid Assessment of Possible ACS in the emergency Department with high sensitivity Troponin T trial (RAPID-TnT trial) [13] demonstrating equivalent safety and initial ED efficiency [14].
Given the expected increase in the use of hs-cTn following its approval by the United States Food and Drug administration (US FDA) in 2019 and ESC recommendations in 2020 [1], robust evaluation of accelerated hs-cTn-based algorithms and their translational benefit in terms of outcome as well as cost-effectiveness analyses is necessary. A prior randomised study of non-protocolised use of hs-cTnT by our group found fewer adverse cardiac events but with an incremental cost of $108,552 per adverse event avoided; raising cost as a potential barrier to implementation [15]. Other observational and modelling studies have also reported similar results [3], [16], [17], [18]. Several observational studies on accelerated hs-cTn algorithms have shown positive results with potential reductions in resource utilization [4], [6], [19]. Contemporary data from the US also reported similar findings with hs-cTn implementation being associated with fewer cardiac functional tests, invasive catheterisations, and no significant change in overall resource use [20], [21]. While these studies are informative, they are limited by a number of potential factors that may not accurately reflect real world costs, including non-randomised design, as well as relatively short follow-up period and imputed data in some studies [4], [6], [19], [20], [21]. No study to date has evaluated the cost-effectiveness of an accelerated 0/1-hour hs-cTn protocol in the setting of a randomised trial. In this study we conducted a formal patient-level economic analysis of the RAPID-TnT trial with a 12-month follow-up period in patients presenting to ED with suspected ACS.
2. Methods
2.1. Study design and patient population
The detailed methods and results of the RAPID-TnT trial have been described elsewhere [22]. In brief, this was a multicentre randomised trial that recruited patients presenting with clinical features of suspected ACS to four metropolitan public EDs in Adelaide (Australia) between August 2015 and April 2019. The main exclusion criteria were ST-elevation MI and need for permanent renal replacement therapy. Patient were randomised in a 1:1 ratio to either a ‘0/1-hour protocol’ using a hs-cTnT reporting format with guidance on subsequent care based on baseline hs-cTnT concentrations and change over 1 h (hereafter referred to as the ‘0/1-hour hs-cTnT protocol’) or a conventional standard-of-care ‘0/3-hour protocol’ with troponin T results masked equal to or below 29 ng/L and subsequent management at clinician discretion as per routine practice (hereafter referred to as the ‘conventional 0/3-hour protocol’). All troponin tests were performed using the Roche Diagnostics (Cobas) Elecsys 5th generation hs-cTnT assay (limit of detection [LOD]: 5 ng/L, 99th percentile: 14 ng/L). The Southern Adelaide Clinical Human Research Ethics Committee provided approval (207.15; Australian and New Zealand Clinical Trial Registry Number ACTRN12615001379505). Funding was provided by the National Health and Medical Research Council of Australia (GNT1124471) with supplementary support from an unrestricted grant from Roche Diagnostics International (Rotkreuz, Switzerland).
2.2. Primary adverse clinical outcome
The primary clinical outcome for the economic evaluation was the cumulative combined endpoint of all-cause mortality and new or recurrent MI up to 12 months. All re-presentations were assessed for cardiac investigations and the following were considered to be new or recurrent MI as per the Fourth Universal Definition of MI: (i) a rise and/or fall in hs-cTnT defined as a change > 20% with a rate of change of ≥ 3 ng/L/hr with at least one sample above 14 ng/L; (ii) clear evidence of ischemia by a typical clinical history (type 1 and 2 only) or ischemic electrocardiograph (ECG) changes, new pathological Q waves, new wall motion abnormalities on cardiac imaging, or angiographic findings; and (iii) MI diagnosis outside of the first 12 h since randomisation unless the patient was discharged and re-presented within 12 h (i.e. indicative of missed MI). Re-presentations to hospital or late troponin elevations without verifiable evidence of myocardial ischemia were reported as myocardial injury and not MI.
2.3. Cost-effectiveness evaluation
We conducted cost effectiveness analysis in line with best practice guidelines using an intention-to-treat (ITT) approach [23], [24], [25]. The perspective for the economic analysis was that of the Australian public health system. The economic evaluation assessed whether the 0/1-hour hs-cTnT protocol was cost- effective compared with the conventional 0/3-hour protocol. We used non-parametric bootstrapping [26] to derive 1000 paired estimates of mean differences in costs and events avoided from participant level data in order to account for uncertainty due to sampling variation, presented in the form of a cost-effectiveness plane (CEP) [27]. All costs are reported in 2020 Australian dollars (AUD). No discounting was required since the study duration was one year.
2.4. Service use and costs
Total number of ED presentations, hospital admissions, out-of-hospital services and prescription medication were presented as counts. Costs for each component of health service use were calculated as follows: Medicare costs for out-of-hospital services were estimated using the Medicare Benefits Schedule (MBS) public subsidy paid for each of these items. Medicare costs for out-of-hospital services included costs for tests (including hs-TnT and cTnT testing and reporting), treatments and investigations; medical consultations and primary care (including general practice visits). Publicly (Medicare) subsidised prescription drug costs were estimated using the Pharmaceutical Benefits Scheme (PBS) paid for each of the drugs dispensed. Healthcare costs for in-hospital care were based on the National Weighted Activity Units (NWAUs) associated with each in-patient hospital admission. NWAUs are the common unit of measurement of hospital activity used in Australia against which the National Efficient Price (NEP) is applied and funding provided to hospitals [28]. To calculate a total cost for each in-hospital admission, the NWAU for each admission was multiplied by the NEP to determine the cost to the government. Emergency Department (ED) costs were calculated based on the Urgency Related Groups (URG), a case mix classification based on type of visit, episode end status, triage and diagnosis. The national cost weights (as determined by the Independent Hospital Pricing Authority (IHPA)) for each URG was then applied to determine ED costs [28]. National hospital cost data collection cost report: Round 22 financial year 2017–2018). Data on PBS, hospital, and ED costs were provided by Service Australia. Total cost for each individual comprised the total cost of out of hospital medical (Medicare) services, prescription medications (PBS), ED presentations and in-patient admissions calculated appropriately for index presentation, 30-day and 12-months follow-up periods.
2.5. Quality of life outcomes
The secondary outcome was health-related quality of life (HRQoL) as measured by the EuroQol 5 dimensions 5 level (EQ-5D-5L) instrument [29], which was administered to participants at baseline, 30 days, 6 and 12 months. The EQ-5D-5L is a generic preference-based measure of HRQoL comprising of 5 dimensions [30]. The Australian scoring algorithm for the EQ-5D-5L was applied to convert the EQ-5D-5L health description into valuations ranging from negative 0.208 to 1, where the maximum score of 1 represents full health and a score of 0 represents the state of being dead. [31]. Missing EQ5D-5L data were imputed using imputation by iterative Markov chain Monte Carlo method based on a multivariate normal regression. It involved replacing each missing value with a set of 50 plausible values [32]. Each of the 50 resultant multiple imputed datasets were then analysed using standard complete-case procedures before combining the results using Rubin's rules [32]. Age, gender, randomisation group, recruitment date and mortality status were used in the imputation model. The quality adjusted life years (QALYs) for each participant over 12 months was estimated using the area under the curve method [33]. In accordance with best practice guidance, adjustment for differences in baseline EQ-5D-5L scores between the two groups was conducted, using regression analysis, to minimise bias [31].
2.6. Sensitivity analyses
To assess the robustness of the observed base-case results, we conducted the following sensitivity analyses: restricting analysis to patients with hs-cTnT below the reporting threshold of ≤ 29 ng/L, restricting analysis to patients who underwent subsequent functional testing, and assessing potential impact of very-high cost patients using trimmed analysis (removing most expensive 98th and 99th centile participants). A threshold analysis was also undertaken to determine how many inpatient admissions from the ED would need to be avoided for the 0/1-hour protocol for this strategy to be cost-effective (whilst keeping its effectiveness unchanged) [34]. This was conducted by reducing the proportion of inpatient admissions from the ED for the 0/1-hour hs-cTnT group and increasing the proportion not admitted and applying the estimated mean costs for admitted and non-admitted patients to the revised proportions, respectively.
2.7. Statistical analysis
The analyses used the intention-to-treat principle. Categorical data are reported as frequencies and were compared by use of the Fisher exact test. Continuous data are reported as mean ± standard deviation (SD) and were compared by 2-sample Student t tests for normally distributed variables or Wilcoxon rank-sum tests for non-normally distributed variables. For the primary clinical outcome, the time to first component of primary composite end point between the 2 randomised groups was compared using Cox proportional hazards models without covariate adjustment. To account for clustering between hospitals, shared frailty and robust variance estimators were used, with these methods demonstrating the smallest deviation in proportional hazards reported. A value of P < 0.05 (two-tailed) was considered statistically significant with no adjustment for multiple comparisons. All analyses were conducted within Stata version 16.1 (College Station, Texas, US).
3. Results
3.1. Participant characteristics
A total of 3378 participants were enrolled into the trial. Ninety participants withdrew initial consent and 18 participants withdrew consent during follow-up, leaving 1638 and 1632 participants in the 0/1-hour hs-cTnT arm and the conventional 0/3-hour hs-cTnT arm respectively. Medicare data was not available for five patients, leaving 1634 and 1631 participants in the 0/1-hour hs-cTnT arm and the 3-hour conventional hs-cTnT arm respectively for cost analysis. None of these 5 patients experienced a primary adverse clinical endpoint. There were no statistically significant differences in baseline characteristics between the groups (Table 1).
Table 1.
Baseline characteristics.
| 0/3-hour masked protocol (n = 1631) | 0/1-hour unmasked protocol (n = 1634) | p-value | |
|---|---|---|---|
| Age (median, IQR) | 58.6 (48.8, 71.2) | 58.7 (48.6, 69.4) | 0.25 |
| Female (n, %) | 762 (46.7%) | 767 (46.8%) | 0.94 |
| Hypertension | 334 (20.5%) | 322 (19.7%) | 0.56 |
| Diabetes | 285 (17.5%) | 257 (15.7%) | 0.17 |
| Dyslipidaemia | 719 (44.1%) | 711 (43.4%) | 0.71 |
| Current smoker | 582 (35.7%) | 566 (34.6%) | 0.51 |
| Family history of CAD | 951 (59.4%) | 987 (61.2%) | 0.28 |
| Known CAD | 473 (29.0%) | 456 (27.8%) | 0.47 |
| Prior AMI | 161 (9.9%) | 170 (10.4%) | 0.63 |
| Prior heart failure | 92 (5.6%) | 77 (4.7%) | 0.23 |
| Prior atrial fibrillation | 153 (9.4%) | 134 (8.2%) | 0.23 |
| Prior COPD | 73 (4.5%) | 77 (4.7%) | 0.76 |
| Prior CVA | 51 (3.1%) | 53 (3.2%) | 0.86 |
| Prior CABG | 45 (2.8%) | 49 (3.0%) | 0.69 |
| Prior PCI | 138 (8.5%) | 171 (10.4%) | 0.053 |
| GFR (median, IQR) | 86.0 (71.4, 98.0) | 86.2 (71.6, 98.2) | 0.65 |
| GRACE Score (median, IQR) | 75.0 (56.1, 100.7) | 74.1 (55.3, 97.2) | 0.16 |
Abbreviations: AMI, Acute myocardial infarction; CABG, Coronary artery bypass graft; CAD, Coronary artery disease; COPD, Chronic obstructive pulmonary disease; CVA, Cerebral vascular accident; GFR, Glomerular filtration rate; GRACE, Global Registry of Acute Coronary Events; IQR, Interquartile range; n, number; PCI, percutaneous coronary intervention.
3.2. Adverse primary clinical outcomes
Within the 12-months follow-up period, 611/3265 (18.69%) participants re-presented to hospital at least once and 144/3265 (4.4%) participants experienced the primary clinical endpoint of all-cause mortality or new/recurrent MI (Table 2). There was no statistically significant difference in the primary clinic endpoint between the two study arms with a trend toward higher event rate in the 0/1-hour hs-cTnT arm (0/1-hour arm = 82/1634 [5.0%]; 0/3-hour arm = 62/1632 [3.8%], hazard ratio = 1.32, 95 %CI = 0.95–1.83, P = 0.010).
Table 2.
Mean outcomes per patient.
| 0/3-hour masked protocol (per pt, SE, n = 1631) | 0/1-hour unmasked protocol (per pt, SE, n = 1634) | Difference, bootstrapped p-value | |
|---|---|---|---|
| Number of adverse clinical outcomes | |||
| Primary outcome (death or MI) | 0.038 (0.006) | 0.050 (0.009) | 0.0120, 0.093 |
| MI | 0.0349 (0.005) | 0.0041 (0.007) | 0.0064, 0.117 |
| Quality of life outcomes | |||
| EQ-5D 5L at baseline | 0.760 (0.006) | 0.768 (0.006) | 0.008, 0.344 |
| EQ-5D 5L at 30 days | 0.748 (0.010) | 0.757 (0.009) | 0.009, 0.398 |
| EQ-5D 5L at 6 months | 0.772 (0.010) | 0.773 (0.008) | 0.0004, 0.973 |
| EQ-5D 5L at 12 months | 0.706 (0.011) | 0.715 (0.010) | 0.009, 0.545 |
| Overall QALY (unadjusted) | 0.749 (0.006) | 0.754 (0.006) | 0.007, 0.559 |
| QALY (baseline adjusted) | 0.400 (0.013) | 0.401 (0.020) | 0.001, 0.866 |
Abbreviations: MI, Myocardial infarction; QALY, Quality adjusted life year; SE, Standard error.
3.3. Service use, costs, quality of life and economic evaluation
Table 3 and Supplementary Table 2 provide detailed breakdown of mean resource use and healthcare costs per participant over a 12-month follow-up. By 12-months, the mean total costs per patient did not differ significantly between the study arms, with a trend towards higher costs in patients randomised to the 0/1-hour hs-cTnT protocol ($19,088.76 vs. $1,8616.27; difference= $472.49 [95 %CI= $-1,380.15 to $2,325.13], P = 0.617). The index ED LOS was significantly shorter in the 0/1-hour hs-cTnT arm (5.6 vs. 6.2 h, difference = 0.62 h [95 %CI = 0.39 to 0.85], P < 0.001). Over the 12-month follow-up period, the total unplanned inpatient LOS and mean number of invasive coronary angiograms were numerically higher for those in the 0/1-hour hs-cTnT arm, resulting in numerically higher unplanned inpatient costs ($6,275.13 vs $5,383.91, difference= $891.22 [95 %CI: $-96.07 to $1,878.50], P = 0.077). In comparison, the planned hospital LOS and associated costs were marginally lower for patients in the 0/1-hour hs-cTnT arm ($2,930.03 vs. $3,020.02, P = 0.808). There was no significant difference in diagnostic imaging, therapeutic procedures, diagnostic procedure, outpatient attendance and medication-related costs between the group. Non-invasive functional testing was significantly lower amongst participants in the 0/1-hour hs-cTnT arm (0/1-hour: 122/1638 [7.4%] versus 0/3-hour: 178/1632 [10.9%], P < 0.001).
Table 3.
Mean resource use and cost per participant over 12 months in Australian 2019/2020 dollars.
| 0/3-hour protocol (mean, SE; n = 1631) | 0/1-hour protocol (mean, SE; n = 1634) | Difference, bootstrapped p-value (95 %CI) | Total (mean, SE; n = 3265) | |
|---|---|---|---|---|
| Number of unplanned admissions | 1.26 (0.04) | 1.17 (0.05) | −0.09, 0.115 (−0.21 to 0.02) | 1.21 (0.03) |
| Number of planned admissions | 0.70 (0.16) | 0.57 (0.05) | −0.13, 0.391 (−0.43 to 0.17) | 0.63 (0.08) |
| Number of specialist Attendances | 2.81 (0.15) | 2.76 (0.15) | −0.04, 0.831 (−0.43 to 0.35) | 2.78 (0.10) |
| Number of general practitioner attendances | 10.83 (0.26) | 10.99 (0.27) | 0.16, 0.677 (−0.58 to 0.89) | 10.91 (0.19) |
| Number of other attendances | 0.90 (0.05) | 0.79 (0.04) | −0.11, 0.087 (−0.23 to 0.02) | 0.84 (0.03) |
| Number of diagnostic imaging | 2.51 (0.09) | 2.53 (0.08) | 0.03, 0.825 (−0.23 to 0.29) | 2.52 (0.06) |
| Number of therapeutic procedures | 1.82 (0.15) | 1.75 (0.14) | −0.07, 0.760 (−0.50 to 0.36) | 1.78 (0.10) |
| Number of diagnostic procedures | 1.31 (0.05) | 1.40 (0.05) | 0.09, 0.211 (−0.05 to 0.22) | 1.36 (0.03) |
| Number of coronary angiography 30 days | 0.07 (0.01) | 0.08 (0.01) | −0.01, 0.45 (−0.01 to 0.02) | 0.07 (0.00) |
| Rate of direct discharge from ED during index presentation | 528 (32.37%) | 737 (45.10%) | 1264 (0.39) | |
| Index ED LOS (hours, median, Q1−Q3) | 6.24 (5.6, 4–7.1) | 5.63 (4.6, 3.4–6.4) | −0.62, <0.001 (−0.85 to −0.39) | 5.93 (5.1) |
| Index episode total LOS(hours, median, Q1−Q3) | 26.75 (6.5, 4.95–24.2) | 24.14 (5.3, 3.7–23.7) | −2.61, 0.298 (−7.53 to 2.31) | 25.44 (6.0) |
| Total ED LOS excluding index presentation (days, median, Q1-Q3) | 0.47 (0.27, 0.16–0.52) | 0.49(0.24, 0.15–0.50) | 0.02, 0.415 (−0.03 to 0.08) | 0.48 (0.26) |
| Total inpatient LOS(days, median, Q1-Q3) | 3.58 (0.37, 0.08–2.68) | 4.14 (0.32, 0–2.30) | 0.55, 0.158 (−0.22 to 1.33) | 3.86 (0.35) |
| Costs based on time | ||||
| Index ED costs | 1030.26 (6.87) | 1003.60 (5.88) | −26.67, 0.002 (−43.26 to −10.07) | 1016.92 (4.53) |
| Index total episode cost | 3560.57 (118.54) | 3905.64 (160.13) | −345.07, 0.066 (–22.88 to 713.02) | 3733.32 (99.68) |
| Index total episode cost if admitted | N = 1103/16314042.34 (151.04) | N = 897/16344734.63 (253.41) | −692.29, 0.024 (91.77 to 1292.81) | 4353.02 (141.11) |
| Total ED cost excluding index presentation | 1090.62 (61.68) | 1161.97 (75.15) | −71.35, 0.46 (−119.76 to 262.46) | 1126.33 (48.62) |
| Total ED costs within 30 days | 1248.23 (14.56) | 1236.98(16.48) | −11.25, 0.578 (−50.85 to 28.36) | 1242.6 (11.00) |
| Total ED costs beyond 30 days | 872.65 (56.52) | 928.58 (67.57) | −55.93, 0.540 (−123.16 to 235.01) | 900.64 (44.05) |
| Total inpatient costs within 30 days | 3102.65 (175.45) | 3279.34 (210.54) | 176.69, 0.501 (−338.14 to 691.52) | 3191.08 (137.04) |
| Total inpatient costs beyond 30 days | 5301.27 (397.22) | 5925.82 (422.81) | 624.55, 0.363 (−720.23 to 1969.32) | 5613.83 (290.09) |
| Total unplanned inpatient costs within 30 days | 2731.11 (156.78) | 2959.50 (204.57) | 228.39, 0.402 (−305.31 to 762.09) | 2845,41 (128.89) |
| Total unplanned inpatient costs beyond 30 days | 2652.79 (240.36) | 3315.62 (336.83) | 662.83, 0.119 (−170.23 to 1495.89) | 2984.51 (207.01) |
| Total planned inpatient costs within 30 days | 371.54 (74.06) | 319.84 (50.70) | 51.70, 0.574 (–232.13 to 128.73) | 345.66 (44.86) |
| Total planned inpatient costs beyond 30 days | 2648.48 (295.44) | 2610.20 (223.35) | − 38.28, 0.924 (−824.86 to 748.30) | 2629.32 (185.11) |
| In−hospital costs | ||||
| Total unplanned inpatient costs | 5383.91 (299.46) | 6275.13 (427.89) | 891.22, 0.077 (−96.07 to 1878.50) | 5829.93 (261.29) |
| Total planned inpatient costs | 3020.02 (316.83) | 2930.03 (232.92) | −89.98, 0.808 (−814.97 to 635.01) | 2974.98 (196.53) |
| Unplanned inpatient costs excluding index episode | 3152.99 (263.77) | 3861.06 (369.23) | 708.07, 0.196 (−365.48 to 1781.63) | 3507.35 (227.00) |
| Planned admission inpatient costs excluding index episode | 2721.16 (307.28) | 2442.05 (227.55) | −279.12, 0.452 (−1006.43 to 448.19) | 2581.48 (191.12) |
| Other costs | ||||
| Pathology | 567.62 (22.77) | 556.36 (21.46) | −11.26, 0.745 (−79.23 to 56.72) | 561.99 (15.64) |
| GP attendances | 1515.47 (43.36) | 1526.45 (44.64) | 10.97, 0.863 (−114.00 to 135.95) | 1520.96 (31.11) |
| Specialist attendances | 584.16 (32.78) | 556.03 (27.37) | −28.13, 0.476 (−105.43 to 49.16) | 570.08 (21.35) |
| Other attendances | 96.01 (4.84) | 87.11 (4.15) | −8.91, 0.186 (–22.12 to 4.30) | 91.56 (3.19) |
| Diagnostic imaging | 1129.03 (45.22) | 1098.15 (40.65) | −30.88, 0.609 (−149.06 to 87.29) | 1113.58 (30.40) |
| Therapeutic procedures | 796.73 (70.54) | 764.80 (69.04) | −31.93, 0.742 (–222.29 to 158.44) | 780.75 (49.35) |
| Diagnostic procedures | 250.77 (13.68) | 250.53 (13.26) | −0.24, 0.990 (−36.51406 to 36.0288) | 250.65 (9.52) |
| Total attendance | 2169.72 (64.55) | 2131.57 (60.17) | −38.15, 0.672 (−214.51 to 138.21) | 2150.62 (44.11) |
| Total MBS items (excluding inpatient costs) | 5176.62 (165.56) | 5043.07 (158.34) | −133.55, 0.571 (−596.04 to 328.94) | 5109.78 (114.53) |
| Total medication costs | 2914.86 (372.11) | 2674.98 (272.09) | −239.88, 0.594 (−1121.54 to 641.78) | 2794.81 (230.40) |
| Total costs | 18616.27 (722.82) | 19088.76 (743.23) | 472.49, 0.617 (−1380.15 to 2325.13) | 18852.74 (518.33) |
Abbreviations: CI, Confidence interval; ED, Emergency department; GP, General Practitioner; LOS, Length of stay; MBS, Medicare Benefits Schedule; SE, Standard error.
Costs incurred stratified by time since index presentation is shown in Table 3, with unplanned inpatient costs incurred beyond 30 days contributing most to the cost difference between the two randomisation groups. Conversely, the difference ED and planned inpatient costs did not differ significantly within or beyond 30 days of index presentation between the two groups. Supp Table 2 provide a breakdown of event rate and costs based on stratification status.
Results from the sensitivity analyses are presented in Supplementary Tables 1A-C and 3. Firstly, restricting the analysis to 2988/3265 participants with hs-cTnT ≤ 29 ng/L, the mean total costs per patient did not differ between 2 groups ($17,061.53 vs. $16,909.09; difference=$152.44 [95 %CI= $-1,793.11 to $2,097.99], P = 0.878). Secondly, among the 300/3270 participants who underwent non-invasive functional test after randomisation, the mean total cost per patient remained similar between the groups ($22,641.49 vs. $18,865.14; difference=$3,776.35 [95 %CI= $-2,972.04 to $10,524.74], P = 0.273). Third, trimmed analyses excluding most expensive participant did not alter the direction of the cost estimates. Fourth, QoL outcomes showed that the mean adjusted QALY were similar between the two arms (0.754 vs. 0.749, P = 0.866). Lastly, the threshold analysis estimates that approximately 57.4% of patients need to be directly discharged from ED in the 0/1-hour hs-cTnT arm for the strategy to be cost-neutral to the conventional 0/3-hour protocol.
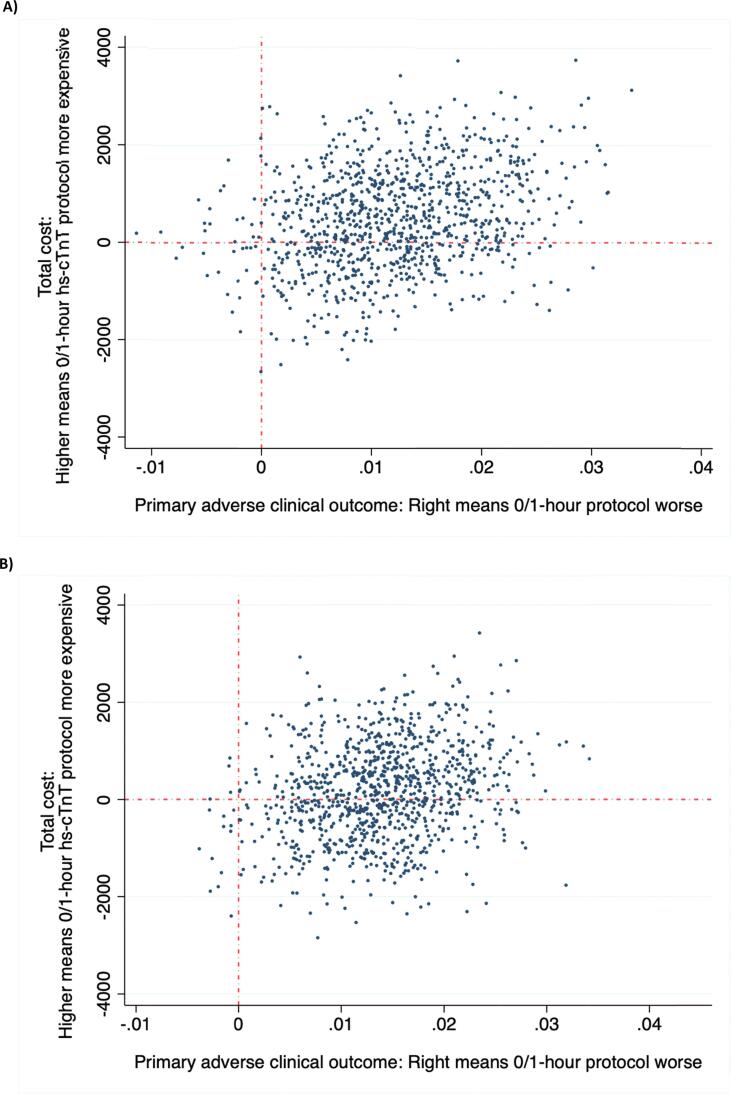
Uncertainty surrounding the estimates of total costs and primary adverse clinical outcome avoided are presented by the CEP for the primary analysis (Fig. 1A). The bootstrapped estimates appear in all four quadrants indicating a degree of variability surrounding the presence and magnitude of cost saving and effectiveness. No strategy dominates. The CEP of the subgroup with hs-cTnT concentration of ≤ 29 ng/L is presented in Fig. 1B.
Fig. 1.
Cost-effectiveness plane (CEP) of the incremental cost and adverse clinical outcomes avoided of 0/1-hour high-sensitivity troponin-T protocol compared to 0/3-hour conventional protocol in the (A) total cohort and (B) patients with hs-cTnT concentration of ≤ 29 ng/L.
4. Discussion
This analysis is the first to evaluate the cost-effectiveness of a 0/1-hour hs-cTnT protocol versus the conventional 0/3-hour standard-of-care protocol using patient-level data from a randomised trial for the clinical assessment of suspected ACS from the Australian healthcare system perspective. We report four main findings. First, there was no statistically significant differences in total costs and adverse clinical outcomes between the two groups. Second, ED LOS was significantly shorter amongst patients randomised to the 0/1-hour hs-cTnT protocol. Third, the overall unplanned inpatient costs were higher in the 0/1-hour hs-cTnT arm. Fourth, there was no statistically significant differences in total costs when analysis was confined to the subgroup with hs-cTnT concentration of ≤ 29 ng/L and those who have undergone subsequent functional testing.
To place this economic analysis in context, it is useful to consider our results relative to other studies that evaluated the resource implications of accelerated hs-cTn algorithms in the setting of ED ACS assessment. Whilst the diagnostic accuracy and safety of accelerated 0/1 and 0/2-hour hs-cTn algorithms have now been well-established in numerous randomised and observational studies with class 1B recommendation by the 2020 ESC guidelines (1, 4, 6, 7, 13, 19, [35], [36]), only five observational studies have reported their resource implications. A recently published US-based observation study reported that, except for coronary angiography rates which increased, implementation of hs-cTn assays did not increase overall resource utilization. Of note, the authors reported increased ED discharges, reduced functional testing in patients without elevated troponin [20]. Another US-based study also reported a reduction in stress testing, coronary catheterisation and hospital admissions, despite an increase in net upfront tests and no overall net change in spending. [21]. In a 2016 observational study, Twerenbold et al. reported findings from the APACE registry, which compared three European centres that transitioned to hs-cTnT (n = 1089) and three European centres that did not (n = 1455) [4]. The authors reported similar coronary angiography rate, less cardiac stress testing, shorter ED LOS with similar overall costs. In another study, Ambavane et al. estimated the potential cost saving when the 0/1-hour protocol is applied to 1282 real-world patients. The authors reported an estimated 46% reduction in cost and 33% reduction in LOS [6]. However, this study was based on the assumption that the 0/1-hour algorithm was applied absolutely and projected the potential cost saving, whilst the patients were in fact managed as per standard of care. Furthermore, the authors assumed all ‘ruled-out’ patients underwent outpatient stress testing, which is likely unreflective of real-world practice. Lastly, in the RAPID-CPU study, Stoyanov et al. compared resources utilization before and after implementation of the 0/1-hour hs-cTnT protocol in a German centre [19]. The investigators found that the overall LOS was shorter following implementation (3.2 vs. 5.3 h) with no significant increase in invasive and non-invasive testing.
A key driver of higher costs in 0/1-hour hs-cTnT protocol was the higher subsequent unplanned inpatient stay, with most of the cost difference occurring beyond 30 days after index presentation. In comparison, ED LOS, planned inpatient costs and subsequent diagnostic-related costs were marginally lower in the 0/1-hour hs-cTnT group. This may be related to the marginally higher rate of late adverse clinical outcome observed in the 0/1-hour hs-cTnT arm. Our observation is largely consistent with other observational studies [4], [6], [19]. However, the subsequent unplanned inpatient costs were higher in the 0/1-hour hs-cTnT group when considering late health system impacts.
In the RAPID-TnT study, we observed a low overall functional test rate of 9.2% (300/3265) within 30 days of assessment with a very low computed tomography coronary angiography (CTCA) utilization rate of 0.3% (9/3288). This pattern of practice is comparable to other observational studies of accelerated protocols with RAPID-CPU reporting a CTCA utilization of 0.6% [19]. Results from the PROMISE and SCOT-HEART trials provide evidence supporting the use of non-invasive anatomical testing in the workup of stable CAD in the ambulatory setting. However, it is unclear whether an increase in non-invasive testing leads to better outcomes in patients evaluated for suspected ACS in an acute setting. A population study of patients discharged from ED for chest pain suggested that non-invasive testing may be beneficial in the assessment of patients at high risk for CAD but not for low-to-intermediate risk patients [37]. Similarly, another study reported that non-invasive testing was associated with a small decrease of subsequent adverse cardiovascular events [38]. However, the absolute risk reduction was small with number needed to treat of approximately 250 to avoid 1 MI or cardiovascular death. Furthermore, patients who underwent non-invasive testing had higher rates of invasive testing. From a resource perspective, our subgroup analysis on patients who had functional testing did not show any significant difference in mean total cost between the two strategies. Whether or not the use of non-invasive anatomical testing in the setting of the 0/1-hour hs-cTnT protocol will significantly affect subsequent costs and clinical outcomes remain to be investigated.
To further characterise the potential cost effectiveness of patients in a low-risk population, we restricted our analysis to participants with hs-cTnT levels ≤ 29 ng/L. Here, we observed a shorter ED LOS for participants randomised to the 0/1-hour hs-cTnT study arm without significant difference in total mean cost between the two study groups. This result is consistent with prior data showing an increase in ED discharges and reduction in LOS among patients without an increase in hs-cTnT assay [20]. Our threshold analysis indicates that approximately 57.4% of patients need to be directly discharged from ED in the 0/1-hour hs-cTnT protocol to be cost-neutral when compared to the conventional 0/3-hour protocol. In comparison, the direct ED discharge rate observed in our study was 45.1%. While increasing the ED discharge rate from ED using accelerated hs-cTnT algorithms is possible, further refinements in downstream investigation, particularly in patient with low-range hs-cTnT elevation is clearly required.
4.1. Limitations of our study
Although no data were missing on the primary outcome (adverse clinical outcomes), there was some missing data relating to costs (∼1.5%) and QALY outcomes (∼60%). However, robust imputation methods were employed to account for these missing data. Secondly, patients who re-presented to ED were not subsequently re-randomised and thus received standard of care troponin reporting. This may dilute the power of the study and bias the estimate of treatment effect and cost-effectiveness outcomes towards the null. Thirdly, given that the hs-cTnT assay was already being used by pathology providers at these hospitals in South Australia with values less than or equal to 29 ng/L masked (i.e. unavailable) to the treating clinician in routine care, no additional costs were incurred from the transition to reporting hs-cTnT results. Therefore, a cost unaccounted for in our analysis is the additional costs associated with changing to the newer generation hs-cTnT assays in healthcare systems that still utilise standard cTnT assays.
5. Conclusions
The results of this economic evaluation suggest that there were no differences in costs and outcomes between the 0/1-hour hs-cTnT protocol and the conventional 0/3-hour standard-of-care protocol for the assessment of patients presenting with suspected ACS at 12 months. Our findings also support previous findings that the 0/1-hour hs-cTnT protocol improves ED efficiency. However, the associated cost savings were offset by the higher late unplanned inpatient costs. As the burden of suspected and confirmed cardiac conditions increases, further refinements in downstream management strategies to reduce late clinical events whilst efficiently utilising healthcare resources is needed.
Funding
Dr Chuang received scholarship funding from the Royal Australasian College of Physicians (Sydney, New South Wales, Australia). Dr Chew received grants from National Health and Medical Research Council of Australia (GNT1124471; Canberra, ACT, Australia) and Roche Diagnostics (Rotkreuz, Switzerland). There are no other funding to disclose relevant to this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100933.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Collet J.-P., Thiele H., Barbato E., Barthélémy O., Bauersachs J., Bhatt D.L., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2020 [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev. Esp. Cardiol. (Engl. Ed.) 2016;69(12):1167. doi: 10.1016/j.rec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Westwood M., van Asselt T., Ramaekers B., Whiting P., Thokala P., Joore M., Armstrong N., Ross J., Severens J., Kleijnen J. High-sensitivity troponin assays for the early rule-out or diagnosis of acute myocardial infarction in people with acute chest pain: a systematic review and cost-effectiveness analysis. Health Technol. Assess. 2015;19(44):1–234. doi: 10.3310/hta19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Twerenbold R., Jaeger C., Rubini Gimenez M., Wildi K., Reichlin T., Nestelberger T., Boeddinghaus J., Grimm K., Puelacher C., Moehring B., Pretre G., Schaerli N., Campodarve I., Rentsch K., Steuer S., Osswald S., Mueller C. Impact of high-sensitivity cardiac troponin on use of coronary angiography, cardiac stress testing, and time to discharge in suspected acute myocardial infarction. Eur. Heart J. 2016;37(44):3324–3332. doi: 10.1093/eurheartj/ehw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambavane A., Lindahl B., Giannitsis E., Roiz J., Mendivil J., Frankenstein L., Body R., Christ M., Bingisser R., Alquezar A., Mueller C. Correction: Economic evaluation of the one-hour rule-out and rule-in algorithm for acute myocardial infarction using the high-sensitivity cardiac troponin T assay in the emergency department. PLoS ONE. 2018;13(1):e0191348. doi: 10.1371/journal.pone.0191348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambavane A., Lindahl B., Giannitis E., Roiz J., Mendivil J., Frankenstein L., Body R., Christ M., Bingisser R., Alquezar A., Mueller C., Zeller T. Economic evaluation of the one-hour rule-out and rule-in algorithm for acute myocardial infarction using the high-sensitivity cardiac troponin T assay in the emergency department. PLoS ONE. 2017;12(11):e0187662. doi: 10.1371/journal.pone.0187662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang C.-H., Chiang C.-H., Lee G.H., Gi W.-T., Wu Y.-K., Huang S.-S., Yeo Y.H., Giannitsis E., Lee C.-C. Safety and efficacy of the European Society of Cardiology 0/1-hour algorithm for diagnosis of myocardial infarction: systematic review and meta-analysis. Heart. 2020;106(13):985–991. doi: 10.1136/heartjnl-2019-316343. [DOI] [PubMed] [Google Scholar]
- 8.Chapman A.R., Sandeman D., Ferry A.V., Stewart S., Strachan F.E., Wereski R., Bularga A., Anand A., Shah A.S.V., Mills N.L. Risk stratification using high-sensitivity cardiac troponin T in patients with suspected acute coronary syndrome. J. Am. Coll. Cardiol. 2020;75(8):985–987. doi: 10.1016/j.jacc.2019.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twerenbold R., Costabel J.P., Nestelberger T., Campos R., Wussler D., Arbucci R., Cortes M., Boeddinghaus J., Baumgartner B., Nickel C.H., Bingisser R., Badertscher P., Puelacher C., du Fay de Lavallaz J., Wildi K., Rubini Giménez M., Walter J., Meier M., Hafner B., Lopez Ayala P., Lohrmann J., Troester V., Koechlin L., Zimmermann T., Gualandro D.M., Reichlin T., Lambardi F., Resi S., Alves de Lima A., Trivi M., Mueller C. Outcome of Applying the ESC 0/1-hour Algorithm in Patients With Suspected Myocardial Infarction. J. Am. Coll. Cardiol. 2019;74(4):483–494. doi: 10.1016/j.jacc.2019.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Chapman A.R., Anand A., Boeddinghaus J., Ferry A.V., Sandeman D., Adamson P.D., Andrews J., Tan S., Cheng S.F., D’Souza M., Orme K., Strachan F.E., Nestelberger T., Twerenbold R., Badertscher P., Reichlin T., Gray A., Shah A.S.V., Mueller C., Newby D.E., Mills N.L. Comparison of the efficacy and safety of early rule-out pathways for acute myocardial infarction. Circulation. 2017;135(17):1586–1596. doi: 10.1161/CIRCULATIONAHA.116.025021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Than M.P., Pickering J.W., Aldous S.J., Cullen L., Frampton C.M., Peacock W.F., et al. Effectiveness of EDACS versus ADAPT accelerated diagnostic pathways for chest pain: a pragmatic randomised controlled trial embedded within practice. Ann. Emerg. Med. 2016;68(1):93–102.e1. doi: 10.1016/j.annemergmed.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Mahler S.A., Riley R.F., Hiestand B.C., Russell G.B., Hoekstra J.W., Lefebvre C.W., et al. The HEART Pathway randomised trial: identifying emergency department patients with acute chest pain for early discharge. Circ. Cardiovasc. Qual. Outcomes. 2015;8(2):195–203. doi: 10.1161/CIRCOUTCOMES.114.001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chew D.P., Lambrakis K., Blyth A., Seshadri A., Edmonds M.J.R., Briffa T., et al. A randomised trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: the rapid assessment of possible acute coronary syndrome in the emergency department with high-sensitivity troponin T study (RAPID-TnT) Circulation. 2019;140(19):1543–1556. doi: 10.1161/CIRCULATIONAHA.119.042891. [DOI] [PubMed] [Google Scholar]
- 14.Lambrakis K., Papendick C., French J.K., Quinn S., Blyth A., Seshadri A., et al. Late outcomes of the RAPID-TnT randomised controlled trial: 0/1-hour high-sensitivity troponin T protocol in suspected ACS. Circulation. 2021;144(2):113–125. doi: 10.1161/CIRCULATIONAHA.121.055009. [DOI] [PubMed] [Google Scholar]
- 15.Kaambwa B., Ratcliffe J., Horsfall M., Astley C., Karnon J., Coates P., Arstall M., Zeitz C., Worthley M., Beltrame J., Chew D.P. Cost effectiveness of high-sensitivity troponin compared to conventional troponin among patients presenting with undifferentiated chest pain: A trial based analysis. Int. J. Cardiol. 2017;238:144–150. doi: 10.1016/j.ijcard.2017.02.141. [DOI] [PubMed] [Google Scholar]
- 16.Jülicher P., Greenslade J.H., Parsonage W.A., Cullen L. The organisational value of diagnostic strategies using high-sensitivity troponin for patients with possible acute coronary syndromes: a trial-based cost-effectiveness analysis. BMJ Open. 2017;7(6):e013653. doi: 10.1136/bmjopen-2016-013653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Q., Greenslade J.H., Parsonage W.A., Barnett A.G., Merollini K., Graves N., Peacock W.F., Cullen L. Change to costs and lengths of stay in the emergency department and the Brisbane protocol: an observational study. BMJ Open. 2016;6(2):e009746. doi: 10.1136/bmjopen-2015-009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaidya A., Severens J.L., Bongaerts B.WC., Cleutjens K.B., Nelemans P.J., Hofstra L., van Dieijen-Visser M., Biessen E.AL. High-sensitive troponin T assay for the diagnosis of acute myocardial infarction: an economic evaluation. BMC Cardiovasc. Disord. 2014;14(1) doi: 10.1186/1471-2261-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoyanov K.M., Hund H., Biener M., Gandowitz J., Riedle C., Löhr J., Mueller-Hennessen M., Vafaie M., Katus H.A., Giannitsis E. RAPID-CPU: a prospective study on implementation of the ESC 0/1-hour algorithm and safety of discharge after rule-out of myocardial infarction. Eur. Heart J. Acute Cardiovascular Care. 2020;9(1):39–51. doi: 10.1177/2048872619861911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ola O., Akula A., De Michieli L., Dworak M., Crockford E., Lobo R., et al. Clinical impact of high-sensitivity cardiac troponin T implementation in the community. J. Am. Coll. Cardiol. 2021;77(25):3160–3170. doi: 10.1016/S0735-1097(21)04515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguli I., Cui J., Thakore N., Orav E.J., Januzzi J.L., Baugh C.W., et al. Downstream cascades of care following high-sensitivity troponin test implementation. J. Am. Coll. Cardiol. 2021;77(25):3171–3179. doi: 10.1016/S0735-1097(21)04526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papendick C., Blyth A., Seshadri A., Edmonds M.J.R., Briffa T., Cullen L., et al. A randomised trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: Design of the Rapid Assessment of Possible ACS In the emergency Department with high sensitivity Troponin T (RAPID-TnT) study. Am. Heart J. 2017;190:25–33. doi: 10.1016/j.ahj.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey S.D., Willke R.J., Glick H., Reed S.D., Augustovski F., Jonsson B., Briggs A., Sullivan S.D. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–172. doi: 10.1016/j.jval.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Anderson J.L., Heidenreich P.A., Barnett P.G., Creager M.A., Fonarow G.C., Gibbons R.J., Halperin J.L., Hlatky M.A., Jacobs A.K., Mark D.B., Masoudi F.A., Peterson E.D., Shaw L.J. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures. Circulation. 2014;129(22):2329–2345. doi: 10.1161/CIR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 25.Husereau D., Drummond M., Petrou S., Carswell C., Moher D., Greenberg D., Augustovski F., Briggs A.H., Mauskopf J., Loder E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Barber J.A., Thompson S.G. Analysis and interpretation of cost data in randomised controlled trials: review of published studies. BMJ (Clinical Research Ed) 1998;317(7167):1195–1200. doi: 10.1136/bmj.317.7167.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black W.C. The CE plane: a graphic representation of cost-effectiveness. Med. Decis. Making. 1990;10(3):212–214. doi: 10.1177/0272989X9001000308. [DOI] [PubMed] [Google Scholar]
- 28.The Independent Hospital Pricing Authority (IHPA). Understanding the NEP and NEC 2021–22. 2021.
- 29.Herdman M., Gudex C., Lloyd A., Janssen M.F., Kind P., Parkin D., Bonsel G., Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.K. Cheung, M. Oemar, M. Oppe, R. Rabin, EQ-5D user guide:basic information on how to use EQ-5D (Ver 2.0), 2009.
- 31.Devlin N.J., Shah K.K., Feng Y., Mulhern B., van Hout B. Valuing health-related quality of life: An EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22. doi: 10.1002/hec.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.L. Knorr-Held, Analysis of Incomplete Multivariate Data, J.L. Schafer, Chapman & Hall, London, 1997. No. of pages: xiv+430. Price: £39.95. ISBN 0-412-04061-1. Statistics in Medicine. 2000;19(7):1006-8.
- 33.Brazier J., Ratcliffe J., Salomon J., Tsuchiya A. Oxford University Press; 2007. Measuring and Valuing Health Benefits for Economic Evaluation. [Google Scholar]
- 34.Harris A.H., Hill S.R., Chin G., Li J.J., Walkom E. The role of value for money in public insurance coverage decisions for drugs in Australia: a retrospective analysis 1994–2004. Med. Decis. Making. 2008;28(5):713–722. doi: 10.1177/0272989X08315247. [DOI] [PubMed] [Google Scholar]
- 35.Shah A.S.V., Anand A., Strachan F.E., Ferry A.V., Lee K.K., Chapman A.R., Sandeman D., Stables C.L., Adamson P.D., Andrews J.P.M., Anwar M.S., Hung J., Moss A.J., O'Brien R., Berry C., Findlay I., Walker S., Cruickshank A., Reid A., Gray A., Collinson P.O., Apple F.S., McAllister D.A., Maguire D., Fox K.A.A., Newby D.E., Tuck C., Harkess R., Parker R.A., Keerie C., Weir C.J., Mills N.L., Marshall L., Stewart S.D., Fujisawa T., Vallejos C.A., Tsanas A., Hautvast M., McPherson J., McKinlay L., Malo J., Fischbacher C.M., Croal B.L., Leslie S.J., Walker A., Wackett T., Armstrong R., Stirling L., MacDonald C., Sadat I., Finlay F., Charles H., Linksted P., Young S., Alexander B., Duncan C. High-sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped-wedge, cluster-randomised controlled trial. Lancet. 2018;392(10151):919–928. doi: 10.1016/S0140-6736(18)31923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wildi K., Boeddinghaus J., Nestelberger T., Twerenbold R., Badertscher P., Wussler D., Giménez M.R., Puelacher C., du Fay de Lavallaz J., Dietsche S., Walter J., Kozhuharov N., Morawiec B., Miró Ò., Javier Martin-Sanchez F., Subramaniam S., Geigy N., Keller D.I., Reichlin T., Mueller C., Mueller D., Sazgary L., Marbot S., Sabti Z., Flores D., Meissner K., Kulangara C., Freese M., Osswald S., Stelzig C., Bingisser R., López B., Agüero M.M., Nowalany-Kozielska E., Kawecki D., Parenica J., Ganovská E., Lohrmann J., Buser A., Flores D., Grimm K., Hartmann B., Muzyk P., Rentsch K., von Eckardstein A. Comparison of fourteen rule-out strategies for acute myocardial infarction. Int. J. Cardiol. 2019;283:41–47. doi: 10.1016/j.ijcard.2018.11.140. [DOI] [PubMed] [Google Scholar]
- 37.Roifman I., Han L.u., Koh M., Wijeysundera H.C., Austin P.C., Douglas P.S., Ko D.T. Clinical effectiveness of cardiac noninvasive diagnostic testing in patients discharged from the emergency department for chest pain. J. Am. Heart Assoc. 2019;8(21) doi: 10.1161/JAHA.119.013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foy A.J., Liu G., Davidson W.R., Jr., Sciamanna C., Leslie D.L. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: an analysis of downstream testing, interventions, and outcomes. JAMA Intern. Med. 2015;175(3):428–436. doi: 10.1001/jamainternmed.2014.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.