Abstract
Since completion of the Trastuzumab for Gastric Cancer study, trastuzumab with doublet chemotherapy (a fluoropyrimidine and a platinum) has been the gold-standard first-line therapy for patients with locally advanced unresectable or metastatic human epidermal growth factor receptor 2-positive (HER2+) gastroesophageal adenocarcinoma (GEA). The safety and efficacy of 23 studies of first-line trastuzumab plus doublet chemotherapy, without checkpoint inhibitors (n = 19) or with checkpoint inhibitors (n = 4), conducted in patients with locally advanced unresectable or metastatic HER2+ GEA, including phase II/III, prospective, and retrospective observational studies, were summarized. In studies without checkpoint inhibitors, the median duration of trastuzumab treatment ranged from 19.5 to 39.0 weeks and from 15.3 to 30.0 weeks for chemotherapy. In studies with checkpoint inhibitors, the median duration of pembrolizumab/trastuzumab/chemotherapy was 30 weeks, and 18 weeks for chemotherapy. In studies without checkpoint inhibitors, treatment-emergent adverse events (TEAEs) of grade ≥3 ranged from 32% to 84%. Serious adverse events (SAEs) ranged from 15% to 39%. Adverse events resulting in discontinuation ranged from 0% to 30%. Treatment-related deaths occurred in 0%-9% of patients. In studies with checkpoint inhibitors, TEAEs of grade ≥3 were 57%. SAEs ranged from 31% to 38%. Adverse events resulting in discontinuation ranged from 5% to 24%. Treatment-related deaths occurred in 0%-3% of patients. In studies without checkpoint inhibitors, objective response rate (ORR) ranged from 39% to 82%, median progression-free survival (PFS) from 5.7 to 11.6 months, and median overall survival (OS) from 11.2 to 27.6 months. In studies with checkpoint inhibitors, ORR ranged from 39% to 86%, median PFS from 8.0 to 13.0 months, and median OS from 19.3 to 27.3 months. This review provides a historical benchmark on safety and efficacy of available first-line chemotherapy-based standard of care for patients with locally advanced unresectable or metastatic HER2+ GEA.
Key words: first-line chemotherapy, gastroesophageal adenocarcinoma, human epidermal growth factor receptor 2, pembrolizumab, safety, trastuzumab
Highlights
-
•
A benchmark study on safety and efficacy of first-line standard of care in metastatic HER2+ GEA was conducted.
-
•
The safety and efficacy in 23 first-line trastuzumab + doublet chemotherapy ± checkpoint inhibitor studies were summarized.
-
•
Minus checkpoint inhibitors, grade ≥3 TEAEs ranged from 32% to 84%; SAEs ranged from 15% to 39%; ORR ranged from 39% to 82%.
-
•
With checkpoint inhibitors, grade ≥3 TEAEs were 57%; SAEs ranged from 31% to 38%; ORR ranged from 39% to 86%.
Introduction
For more than a decade, trastuzumab has been given in combination with chemotherapy as first-line therapy to patients with locally advanced and metastatic human epidermal growth factor receptor 2 (HER2+) gastroesophageal adenocarcinoma (GEA), defined as esophageal, gastroesophageal junction (GEJ), and gastric adenocarcinoma.1, 2, 3, 4, 5 The Trastuzumab for Gastric Cancer (ToGA) study established the standard-of-care (SOC) first-line therapy of trastuzumab plus doublet chemotherapy (a fluoropyrimidine and a platinum).6 On the basis of these findings, trastuzumab combined with a chemotherapy regimen consisting of capecitabine plus cisplatin or fluorouracil plus cisplatin is the standard option for patients with HER2+ advanced gastric or GEJ cancer. While technically ToGA and other studies did not include esophageal adenocarcinoma, practically these patients are treated with chemotherapy plus trastuzumab in the first-line setting.1,2 The doublet chemotherapy backbone used in the ToGA study (either capecitabine plus cisplatin or 5-fluorouracil [5-FU] plus cisplatin) was associated with a well-documented toxicity profile, including neutropenia, anemia, diarrhea, nausea, anorexia, and vomiting.6
Currently, there are several doublet chemotherapy backbones (fluoropyrimidine/platin) that can be used in combination with trastuzumab.1, 2, 3 The fluoropyrimidine component may include 5-FU, capecitabine,1,3 or the 5-FU prodrug S-1 (approved in Japan and Europe).7,8 S-1, also called TS-1, is a combination of the following three compounds: tegafur, gimeracil, and oteracil potassium. The platinum component may be either cisplatin or oxaliplatin. Oxaliplatin is generally preferred over cisplatin because of lower toxicity.9
Programmed cell death (PD) protein 1 (PD-1) and PD-ligand 1 (PD-L1) are key regulatory elements in the immune response and have an important role in tumor surveillance and evasion.10 PD-L1 positivity by combined positive score ≥1 is found in ∼60% of patients with gastric cancer and anti-PD-1/PD-L1 antibodies have shown encouraging clinical activity in advanced gastric or GEJ cancer.11,12 Moreover, trastuzumab has been reported to increase PD-L1 expression on syngeneic mouse tumor cells;13 therefore, the addition of the checkpoint inhibitor pembrolizumab to SOC trastuzumab/chemotherapy has been tested in clinical trials,14, 15, 16, 17 and recently led to the United States Food and Drug Administration approval of pembrolizumab in combination with trastuzumab, and fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic HER2+ gastric or GEJ adenocarcinoma,18 based on data from the phase III KEYNOTE-811 study (NCT03615326).14
Therefore, in this narrative review, we summarize safety and efficacy of 23 studies, 20 primary studies, and 3 subgroup analyses of first-line trastuzumab plus doublet chemotherapy, with or without checkpoint inhibitors, conducted in patients with locally advanced unresectable or metastatic HER2+ GEA, including phase II/III, prospective, and retrospective observational real-world evidence studies, published in PubMed over the last 11 years.
This analysis is meant to provide a historical benchmark on safety and efficacy of available first-line chemotherapy-based SOC for patients with locally advanced unresectable or metastatic HER2+ GEA.
Features of the studies analyzed
For the purposes of this narrative review, titles and abstracts were searched in PubMed from 2008 to 2021 for “trastuzumab” AND “chemotherapy” AND “gastric”; 285 hits were found. Articles were selected if they were conducted in patients with HER2+ metastatic GEA, including gastric adenocarcinoma, esophageal cancer, GEA/GEJ cancer, in the first-line setting. Prospective phase II, phase III, or observational studies, as well as retrospective studies involving trastuzumab with fluoropyrimidine plus platinum doublet therapy, were included. Meta-analyses and systematic reviews were excluded. Cut-off numbers for patients in studies were ≥35 patients. For studies on checkpoint inhibitors in combination with trastuzumab and chemotherapy, there was no cut-off. Key congresses were searched when considering treatment of patients with metastatic HER2+ GEA and first-line checkpoint inhibitors plus trastuzumab and chemotherapy.
The key details of each of the studies included in this analysis are provided in Table 1. The median age of patients across the studies examined ranged between 57 and 70 years. Most of the studies included both gastric cancer and GEJ cancer, with gastric cancer being the most common type (65%-96%). There was only one study where most of the patients (71%) had GEJ cancer.19 There were two studies where all patients enrolled had gastric cancer.20,21 Patients with esophageal cancer were included in two studies.15,22 Finally, the breakdown between gastric cancer and GEJ cancer was not provided in six studies.16,17,23, 24, 25, 26, 27 Most studies included only patients with HER2+ overexpression (defined as IHC3⁺ or IHC2⁺/FISH⁺), with IHC3⁺ being the most common biomarker subgroup (63%-91%). In three studies, the breakdown between IHC3⁺ and IHC2⁺/FISH⁺ was not provided;22,24,28 three studies included patients with IHC0-1 and/or IHC2⁺/FISH⁻ (16%-26%).6,15,29
Table 1.
Baseline characteristics
| Study | Study design (study number) | Tx type | Region | Race | N | Male/female | Median age (range or IQR) | Tumor location | Stage of the disease | HER2+ expression status |
|---|---|---|---|---|---|---|---|---|---|---|
| Shitara Int J Clin Oncol 2020 (JACOB subgroup analysis)30 |
Phase IIIa (NCT01774786) |
Placebo + Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 80 mg/m2 IV Q3W + Cape 1000 mg/m2 PO BID for 28 doses (D1-15) Q3W |
Japan: 100% | Asian: 100% | 40 | 70%/ 30% |
70 (range, 53-82) |
Stomach: 90% GEJ: 10% |
Met: 100% 1-2 mets: 87.5% >2 mets: 12.5% |
IHC3⁺: 62.5% IHC2⁺/ISH⁺: 37.5% |
| Liu Cancer Commun 2019 (JACOB subgroup analysis)31 |
Phase IIIa (NCT01774786) |
Placebo + Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 80 mg/m2 IV Q3W + Cape 1000 mg/m2 PO BID for 28 doses (D1-15), Q3W OR 5-FU 800 mg/m2 IV every 24 h (D1-5) Q3W |
China: 100% | Asian: 100% | 81 Cape: 86.4% 5-FU: 16.0% |
87.7%/ 12.3% |
59 (range, 23-73) |
Stomach: 85.2% GEJ: 14.8% |
Met: 100% 1-2 mets: 89% >2 mets: 11% |
IHC3⁺: 79% IHC2⁺/ISH⁺: 21% |
| Tabernero Lancet Oncol 2018 (JACOB)32 |
Phase IIIa (NCT01774786) |
Placebo + Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 80 mg/m2 IV Q3W + Cape 1000 mg/m2 PO BID for 28 doses (D1-15), Q3W OR 5-FU 800 mg/m2 IV every 24 h (D1-5) Q3W |
Asia: 37% Japan: 10% North America, Western Europe, Australia: 34% South America, Eastern Europe: 19% |
Asian: 47.9% White: 47.7% Other: 3.8% |
392 | 82%/ 18% |
61 (IQR, 54-68) |
Stomach: 75% GEJ: 25% |
Met: 100% 1-2 mets: 77% >2 mets: 23% |
IHC3⁺: 67% IHC2⁺/ISH⁺: 33% |
| Shah J Clin Oncol 2017 (HELOISE)28 |
Phase IIIa (NCT01450696) |
Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 80 mg/m2 IV Q3W + Cape 800 mg/m2 PO BID for 28 doses over 14 days Q3W |
China: 25.8% ROW: 74.1% |
Asian: 29.8% White: 60.5% Other: 9.7% |
124 | 76.6%/ 23.4% |
60 (range, 26-83) |
Stomach: 76.6% GEJ: 23.4% |
Met: 100% 1-2 mets: 59.7% >2 mets: 40.3% |
IHC3⁺ or IHC2⁺/FISH⁺ (breakdown NR) |
| Sawaki Gastric Cancer 2012 (ToGA subgroup analysis)29 |
Phase IIIb (NCT01041404) |
Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 80 mg/m2 IV D1 Q3W + Cape 1000 mg/m2 PO BID for 28 doses (D1-14) Q3W |
Japan: 100% | Asian: 100% | 51 | 78.4%/ 21.6% |
63 (range, 29-76) |
Stomach: 96.1% GEJ: 3.9% |
Met: 100% 1-2 mets: 54.9% >2 mets: 45.1% |
IHC3⁺/FISH⁺: 31.4% IHC2⁺/FISH⁺: 35.3% IHC3⁺/FISH⁻: 2% IHC3⁺/FISHunk: 5.9% IHC1⁺/FISH⁺: 19.6% IHC0/FISH⁺: 5.9% |
| Bang Lancet 2010 (ToGA)6 |
Phase IIIb (NCT01041404) |
Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 80 mg/m2 IV D1 Q3W + Cape 1000 mg/m2 PO BID for 28 doses (D1-14) Q3W OR 5-FU 800 mg/m2 IV every 24 h (D1-5) Q3W |
Asia: 54.6% Europe: 32.5% Central or South America: 8.9% Other: 3.9% (N = 584) |
Asian: 51% White: 39% Black: <1% Other: 9% |
294 Cape: 87% 5-FU: 13% |
77%/ 23% |
Mean 59.4 (SD, 10.8) |
Stomach: 80% GEJ: 20% |
Met: 97% Locally advanced: 3% 1-2 mets: 52% >2 mets: 48% |
IHC3⁺/FISH⁺: 45% IHC2⁺/FISH⁺: 27% IHC3⁺/FISH⁻: 3% IHC3⁺/FISHunk: 3% IHC1⁺/FISH⁺: 13% IHC0/FISH⁺: 8% IHCunk/FISH⁺: 2% |
| Yuki Cancer Chemother Pharmacol 2020 (KSCC/HGCSG/CCOG/Perseus 1501B)27 |
Phase II (UMIN000017552) | Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Oxal 130 mg/m2 IV D1 Q3W + S-1 80 mg/m2 PO BID (D1-14) Q3W |
Japan: 100% | Asian: 100% | 39 | 79%/ 21% |
66.0 (range, 44-79) | Upper: 36% Middle: 28% Low: 36% |
Liver mets: 56% No liver mets: 44% |
IHC3⁺: 87% IHC2⁺/FISH⁺: 13% |
| Rivera Cancer Chemother Pharmacol 2019 (HERXO)33 |
Phase II (NCT01503983) | Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Oxal 130 mg/m2 IV D1 Q3W + Cape 1000 mg/m2 PO BID (D1-14) Q3W |
Spain: 100% | White: 100% | 45 | 82%/ 18% |
65 (range, 44-80) | Stomach: 69% GEJ: 31% |
Met: 82% Relapsed: 16% Unresectable locally advanced: 2% |
IHC3⁺: 73% IHC2⁺: 27% |
| Takahari Gastric Cancer 2019 (HIGHSOX)34 |
Phase II (UMIN000017602) |
Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Oxal 130 mg/m2 IV D1 Q3W + S-1 40-60 mg/m2 PO BID (D1-14) Q3W (40 mg for BSA <1.25 m2; 50 mg for BSA 1.25-1.5 m2; 60 mg for BSA >1.5 m2) |
Japan: 100% | Asian: 100% | 75 | 78.7%/ 21.3% | 64 (range, 21-75) | Stomach: 85.3% GEJ: 14.7% |
Advanced: 100% Mets, median (range): 1 (1-5) |
IHC3⁺: 73.3% IHC2⁺/ISH⁺: 26.7% |
| Miura Gastric Cancer 2018 (WJOG7212G/T-SPACE)35 |
Phase II (UMIN000008389) | Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 60 mg/m2 IV D8 Q5W + S-1 40-60 mg/m2 PO BID (D1-21) Q5W (40 mg for BSA <1.25 m2; 50 mg for BSA 1.25–1.5 m2; 60 mg for BSA >1.5 m2) |
Japan: 100% | Asian: 100% | 44 | 77.3%/ 22.7% |
64.5 (range, 31-77) | Stomach: 84.1% GEJ: 15.9% |
Met: 100% 0-1 mets: 54.5% ≥2 mets: 45.5% |
IHC3⁺: 72.7% IHC2⁺/FISH⁺: 27.3% |
| Ryu Eur J Cancer 201526 |
Phase II (NCT01396707) | Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Oxal 130 mg/m2 IV D1 Q3W + Cape 1000 mg/m2 PO BID (D1-14) Q3W |
South Korea: 100% | Asian: 100% | 55 | 66%/ 34% |
57 (range, 29-74) | Stomach or GEJ (breakdown NR) | Met: 82% Recurrent: 14% Inoperable locally advanced: 4% Met sites: Liver: 49% Peritoneum: 27% Lung: 20% Bone: 13% Lymph node: 76% |
IHC3⁺: 89% IHC2⁺/FISH⁺: 11% |
| Kurokawa Br J Cancer 2014 (HERBIS-1)23 |
Phase II (UMIN000005739) | Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 60 mg/m2 IV D1 Q3W + S-1 40-60 mg/m2 PO BID (D1-14) Q3W (40 mg for BSA <1.25 m2; 50 mg for BSA 1.25-1.5 m2; 60 mg for BSA ≥1.5 m2) |
Japan: 100% | Asian: 100% | 54 | 78%/ 22% |
66 (range, 34-75) | Stomach or GEJ (breakdown NR) | Unresectable: 94% Recurrent: 6% Met sites: Lymph nodes: 81% Liver: 59% Lung: 9% Peritoneum: 9% Bone: 4% Other: 2% |
IHC3⁺: 83% IHC2⁺/FISH⁺: 17% |
| Gong BMC Cancer 2016 (CGOG1001)36 |
Phase II (NCT01364493) | Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Oxal 130 mg/m2 IV D1 Q3W + Cape 1000 mg/m2 PO BID (D1-14) Q3W |
China: 100% | Asian: 100% | 51 | 71%/ 29% |
57 (range, 27-78) | Stomach: 65% GEJ: 35% |
Met: 86% Locally advanced: 14% |
IHC3⁺: 75% IHC2⁺/dual SISH⁺: 25% |
| Oh Cancer Chemother Pharmacol 201925 |
Retrospective | Trast IV on D1 (loading dose, 8 mg/kg, 90-min infusion; maintenance dose, 6 mg/kg, 30-min infusion) Q3W + Cis 60-100 mg/m2 IV D1 Q3W + Cape 1000 mg/m2 PO BID (D1-14) Q3W OR 5-FU 1000 mg/m2 IV every 24 h (D1-5) Q3W |
Korea: 100% | Asian: 100% | 128 Cape: 96% (3 pts received oxal vs cis) 5-FU: 3.1% |
80.5%/ 19.5% |
63 (range, 20-87) | Stomach or GEJ (breakdown NR) | Met: 100% 1 met: 60.2% 2 mets: 25.8% ≥3 mets: 14.0% |
IHC3⁺: 91.4% IHC2⁺/ISH⁺: 8.6% |
| Okita Tohoku J Exp Med 201837 |
Retrospective |
T-XP(cape + cis) (n= 28) Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cape 1000 mg/m2 PO BID (D1-14) Q3W + Cis 80 mg/m2 IV D1 Q3W T-SP(S-1 + cis) (n= 30) Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + S-1 40 mg/m2 PO BID (D1-14) Q3W + Cis 60 mg/m2 IV D1 Q3W |
Japan: 100% | Asian: 100% | 28 (T-XP) | 89.3%/10.7% | 68.5 (range, 44-81) | Stomach: 82.1% GEJ: 17.9% |
Inoperable advanced or recurrent: 100% | IHC3⁺: 82.1% IHC2⁺/ISH⁺: 14.3% Unk: 3.6% |
| 30 (T-SP) | 86.7%/ 13.3% |
63.5 (range, 38-74) | Stomach: 76.7% GEJ: 23.3% |
Inoperable advanced or recurrent: 100% | IHC3⁺: 83.3% IHC2⁺/ISH⁺: 16.7% |
|||||
| Soularue Bull Cancer 201519 | Retrospective | Trast IV on D1 (loading dose, 6 mg/kg; maintenance dose, 4 mg/kg) Q3W + mFOLFOX6 Oxal 85 mg/m2 IV D1 Q2W + Leucovorin 400 mg/m2 IV D1 Q2W + 5-FU bolus 400 mg/m2 IV D1 Q2W + 5-FU 2400 mg/m2 continuous IV D1 Q2W OR Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + XELOX Oxal 130 mg/m2 IV D1 Q3W + Cape 1000 mg/m2 PO BID (D1-14) Q3W |
France: 100% | NR | 34 | 79%/ 21% |
63 (range, 30-82) | GEJ: 71% Stomach: 29% |
Met: 100% Mets, median (range): 2 (1-4) 1 met: 64.7% 2 mets: 20.6% >2 mets: 14.7% |
IHC3⁺: 88% IHC2⁺/FISH⁺: 12% |
| Kim BMC Cancer 202120 |
Retrospective | Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + Cis 80 mg/m2 IV D1 Q3W + Cape 1000 mg/m2 PO BID (D1-14) Q3W OR 5-FU 800 mg/m2 IV every 24 h (D1-5) Q3W |
Korea: 100% | Asian: 100% | 47 | 81%/ 19% |
59 (range, 36-83) | Stomach: 100% | Met: 77% Recurrent: 23% Liver mets: 51% No liver mets: 49% |
IHC3⁺: 85% IHC2⁺/SISH⁺: 15% |
| Dijksterhuis Int J Cancer 202022 |
Retrospective | Trast + oxal + cape (n = 73) OR Trast + cis + cape (n = 65) OR Trast + other doublet CTX (n = 29) OR Trast + other CTX (n = 47) OR Trast monotherapy (n = 1) |
Netherlands: 100% | White: 100% | 215 | 79.5%/ 21.5% |
63 (IQR, 55-69) | Esophagus: 54% GEJ or cardiac: 19.1% Stomach: 27.0% |
Met: 100% 1-2 mets: 44% >2 mets: 56% |
IHC3⁺ or IHC2⁺/FISH⁺ (breakdown NR) |
| Li Clin Transl Oncol 201821 |
Prospective observational | Trast + platinum (cis, oxal) + fluoropyrimidine (5-FU, cape, S-1) (n = 75) Trast + taxane (doce, pacli) + fluoropyrimidine (5-FU, cape, S-1) (n = 20) Trast + monotherapy CTX (n = 10) Trast + iri + cis (n = 2) Trast IV (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W |
China: 100% | Asian: 100% | 107 (75 pts [70%] had fluoropyrimidine + platinum) |
77%/ 23% |
64 (range, 26-87) | Stomach: 100% | Advanced/met: 100% <2 mets: 35.7% >2 mets: 64.3% |
IHC3⁺: 65% IHC2⁺/FISH⁺: 35% |
| Janjigian Lancet Oncol 202015 |
Phase II (NCT02954536) | Pembro 200 mg IV D1 Q3W + Trast IV on D1 (loading dose, 8 mg/kg in C1; maintenance dose, 6 mg/kg in C2+) Q3W + Oxal 130 mg/m2 OR cis 80 mg/m2 IV on D1C2+ Q3W + Cape 850 mg/m2 PO BID (D1-14) C2+ OR 5-FU 800 mg/m2 IV (D1-5) Q2W |
USA: 100% | White: 86% Asian: 5% Black/ Hispanic/other: 8% |
37 Oxal: 97% Cis: 3% Cape: 65% 5-FU: 35% |
78%/22% | 60 (IQR, 21-84) | Esophagus: 38% GEJ: 32% Stomach: 30% |
Met: 100% |
IHC3⁺ or IHC2⁺/FISH⁺: 84% IHC2⁺/FISH⁻ or IHC1⁺ or IHC0: 16% PD-L1⁻ (CPS <1): 35% PD-L1⁺ (CPS ≥1): 38% PD-L1unkn: 27% |
| Lee AACR 2021, Abstr. CT174 (HCRN GI17-319)24 |
Phase II (NCT03783936) | Ave 800 mg IV D1 + Trast IV on D1 (loading dose, 8 mg/kg in C1; maintenance dose, 6 mg/kg in C2+) Q3W + mFOLFOX Oxal 85 mg/m2 IV D1 Q2W + Leucovorin 400 mg/m2 IV D1 Q2W + 5-FU bolus 400 mg/m2 IV D1 Q2W + 5-FU 2400 mg/m2 continuous IV D1 Q2W |
USA: 100% | NR | NR | NR | NR | Stomach or GEJ (breakdown NR) | Met: 100% | IHC3⁺ or IHC2⁺/FISH⁺ (breakdown NR) |
| Rha ASCO 2020, Abstr. 3031 Rha ASCO GI 2021, Abstr. 218 (PANTHERA)17 |
Phase Ib/II (NCT02901301) | Pembro 200 mg IV D1 Q3W + Trast biosimilar IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W (trastuzumab-pkrb) + Cape 1000 mg/m2 PO BID (D1-14) Q3W + Cis 80 mg/m2 IV D1 Q3W |
Korea: 100% | Asian: 100% | 43 | 77%/23% | 63 (range, 34-82) | Stomach or GEJ (breakdown NR) | Advanced: 100% | IHC3⁺: 70% IHC2⁺/SISH: 30% |
| Janjigian ASCO 2021, Abstr. 4013 (KEYNOTE-811)14 |
Phase III (NCT03615326) | Placebo + Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + FP 5-FU 800 mg/m2 IV (D1-5) Q3W + Cis 80 mg/m2 IV on D1 Q3W OR CAPOX Cape 1000 mg/m2 PO BID (D1-14) Q3W + Oxal 130 mg/m2 IV D1 Q3W OR SOX S-1 40-60 mg/m2 PO BID (D1-14) Q3W (40 mg for BSA <1.25 m2; 50 mg for BSA 1.25-1.5 m2; 60 mg for BSA ≥1.5 m2) + Oxal 130 mg/m2 IV D1 Q3W |
North America, Europe, Israel, Australia: 34% Asia: 30% ROW: 37% |
NR | 131 CAPOX: 88% FP: 12% |
79% 21% |
61 (range, 32-83) | Stomach: 68% GEJ: 32% |
Met: 100% | IHC3⁺: 79% IHC2⁺/ISH⁺: 21% |
| Pembro 200 mg IV D1 Q3W + Trast IV on D1 (loading dose, 8 mg/kg; maintenance dose, 6 mg/kg) Q3W + FP 5-FU 800 mg/m2 IV (D1-5) Q3W + Cis 80 mg/m2 IV on D1 Q3W OR CAPOX Cape 1000 mg/m2 PO BID (D1-14) Q3W + Oxal 130 mg/m2 IV D1 Q3W OR SOX S-1 40-60 mg/m2 PO BID (D1-14) Q3W (40 mg for BSA <1.25 m2; 50 mg for BSA 1.25-1.5 m2; 60 mg for BSA ≥1.5 m2) + Oxal 130 mg/m2 IV D1 Q3W |
North America, Europe, Israel, Australia: 31% Asia: 30% ROW: 39% |
NR | 133 CAPOX: 86% FP: 14% SOX: 0% |
84%/16% | 62 (range, 19-84) | Stomach: 72% GEJ: 28% |
Met: 100% | IHC3⁺: 82% IHC2⁺/ISH⁺: 18% |
5-FU, 5-fluorouracil; ave, avelumab; BID, twice daily; BSA, body surface area; C, cycle; cape, capecitabine; CAPOX, capecitabine plus oxaliplatin; cis, cisplatin; CPS, combined positive score; CTX, chemotherapy; D, day; doce, docetaxel; FP, 5-fluorouracil plus platinum; GEJ, gastroesophageal junction; HER2+, human epidermal growth factor receptor 2 positive; IHC, immunohistochemistry; IQR, interquartile range; iri, irinotecan; ISH, in situ hybridization; IV, intravenous; met, metastatic; mets, metastases; NR, not reported; oxal, oxaliplatin; pacli, paclitaxel; PD-L1, programmed death-ligand 1; PO, oral; pembro, pembrolizumab; pts, patients; Q2W, every 2 weeks; Q3W, every 3 weeks; Q5W, every 5 weeks; ROW, rest of the world; SD, standard deviation; SISH, silver-enhanced in situ hybridization; SOX, S-1 plus oxaliplatin; T-SP, trastuzumab plus S-1 plus cisplatin; ToGA, Trastuzumab for Gastric Cancer; trast, trastuzumab; tx, treatment; T-XP, trastuzumab plus capecitabine plus cisplatin; unk, unknown.
Only details on the control arm are reported in this table.
Only details on the experimental arm are reported in this table.
The studies that were analyzed include data from the ToGA study6 and three additional phase III studies (HELOISE,28 JACOB,32 and KEYNOTE-81114) conducted after the ToGA study, reporting safety and efficacy data of first-line trastuzumab plus chemotherapy in patients with metastatic gastric/GEJ cancer. Besides, the data that were reviewed included a subgroup analysis of ToGA, which focused on the population of Japan,29 and two subgroup analyses of the JACOB study (one investigating the population of China31 and the other Japan30). The patients treated in the phase III studies received trastuzumab plus cisplatin or oxaliplatin plus capecitabine or 5-FU.6,14,28,32
Seven phase II studies,23,26,27,36, 35, 33, 34 five retrospective studies,19,20,22,25,37 and a prospective observational study21 were also included. Four phase II studies used S-1, with two in combination with cisplatin23,35 and two with oxaliplatin.27,34 The other three phase II studies26,33,36 used capecitabine in combination with oxaliplatin. The remaining retrospective and prospective observational studies examined various regimens containing trastuzumab and fluoropyrimidine/platinum chemotherapy.19, 20, 21, 22,25,37 Three of the studies19,22,33 reporting data of first-line trastuzumab plus chemotherapy were conducted exclusively outside Asia.
Checkpoint inhibitor therapy was administered in combination with trastuzumab and chemotherapy in four studies.14, 15, 16, 17,24 Three of these studies investigated pembrolizumab (including the phase III KEYNOTE-81114 and the phase I/II PANTHERA16,17), and one tested avelumab.24
Overall, median follow-up across all studies ranged from 10 to 34 months (Table 2). Median follow-up for the Asian studies20,21,23,25, 26, 27,29, 32, 31,35, 33, 33, 34, 37 ranged from 14 to 34 months, while global studies6,14,28,32 ranged from 10 to 25 months, and European studies19,22,33 ranged from 14 to 15 months.
Table 2.
Overall safety
| Study | Exposure and follow-up | N | Tx-emergent AEsa |
Tx-related AEsa |
SAEs |
AEs resulting in tx modification or interruption | AEs resulting in tx discontinuation | AEs resulting in death | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All grades | Grade ≥3 | All grades | Grade ≥3 | Any | Tx related | ||||||
| Shitara Int J Clin Oncol 2020 (JACOB subgroup analysis)30 |
Median number of cycles (range) per pt: 8 (1-51) for trast 6 (1-51) for cape 6 (1-51) for cis Median follow-up: 34.0 mo |
40 | NR | 75.0% | NR | NR | 32.5% | NR | NR | 10.0% | 2.5% (1 multiple organ dysfunction syndrome) |
| Liu Cancer Commun 2019 (JACOB subgroup analysis)31 |
Average number of cycles ± SD per pt: 11.81 ± 8.35 (placebo + trast) NR for CTX Median follow-up: 18.0 mo |
80 | 97.5% | 65.0% | NR | NR | 15.0% | NR | NR | 6.3% | 7.5% (1 anemia, 1 septic shock, 1 respiratory failure, and 3 deaths) |
| Tabernero Lancet Oncol 2018 (JACOB)32 |
Mean number of cycles ± SD per pt: 11.2 ± 10.0 for trast 5.1 ± 2.7 for cis 7.4 ± 7.6 for cape 5.2 ± 3.5 for 5-FU Median follow-up: 25.0 mo |
388 | 99% | 73% | NR | NR | 39% (5% diarrhea) | 10% | 54% for cape 28% for 5-FU 19% for cis |
12% (disc of placebo and trast) | 8% (2% tx related: 1 multiple organ failure, 1 pulmonary embolism, 1 hemodynamic instability, 1 unexplained death, and 3 septic shock) |
| Shah J Clin Oncol 2017 (HELOISE)28 |
Median number of cycles (range) per pt: 6.5 (1-36) for trast 6 (1-7) for cape 6 (1-7) for cis |
124 | 91.1% | 59.7% | 88.7% (38.7% related to trast) | NR | 24.2% (all grade ≥3) | 18.5% | NR | 7.3% (any tx disc) 2.4% (trast disc) |
5.6% |
| Sawaki Gastric Cancer 2012 (ToGA subgroup analysis)29 |
Median number of cycles (range) per pt: 8 (1-24) for trast NR for cape NR for cis Median follow-up: 18.6 mo |
51 | 100% | 84% | 2% | NR | NR | NR | NR | 2% | 3.9% (1 cardiac failure & unstable angina likely related to trast, and 1 gastrointestinal perforation) |
| Bang Lancet 2010 (ToGA)6 |
Median number of cycles (range) per pt: 8 (1-49) for trast 6 (1-14) for cis 6 (1-20) for cape 6 (1-6) for 5-FU Median follow-up: 18.6 mo |
294 | 99% | 68% | NR | NR | 32% | NR | 84% | NR | 3% (tx related) |
| Yuki Cancer Chemother Pharmacol 2020 (KSCC/HGCSG/CCOG/Perseus 1501B)27 |
Median number of cycles (range) per pt: 8 (1-33) Median follow-up: 22.4 mo |
39 | NR | NR | NR | NR | NR | NR | NR | 16% | 0% (tx related) |
| Rivera Cancer Chemother Pharmacol 2019 (HERXO)33 |
Median follow-up: 13.7 mo | 45 | 93% | 44% | NR | NR | NR | NR | Tx modif: 38% for oxal 36% for cape 18% for trast |
NR | NR |
| Takahari Gastric Cancer 2019 (HIGHSOX)34 |
Median number of cycles (range) per pt: 8 (1-36+) Median follow-up: 15.6 mo |
75 | NR | NR | 98.6% | 45.3% | NR | NR | Tx modif: 54.6% for S-1 50.6% for oxal 10.6% for trast Tx interr: 50.6% for S-1 46.6% for oxal 40.0% for trast |
18.6% (disc of S-1) 29.3% (disc of oxal) 0% (disc of trast) |
0% (tx related) |
| Miura Gastric Cancer 2018 (WJOG7212G/T-SPACE)35 |
Median number of cycles (range) per pt: 5.0 (1.0-17.0) for S-1 5.0 (0-8.0) for cis 8.5 (1.0-29.0) for trast Median follow-up: 19.3 mo |
44 | NR | NR | NR | NR | NR | NR | NR | 15.9% | 2% (tx related) |
| Ryu Eur J Cancer 201526 |
Median number of cycles (range) per pt: 10 (1-30) for cape 8 (1-30) for oxal 10 (1-30) for trast Median follow-up: 13.8 mo |
55 | NR | NR | NR | NR | NR | NR | NR | 2% | 2% (tx-related diarrhea and sepsis) |
| Kurokawa Br J Cancer 2014 (HERBIS-1)23 |
Median number of cycles (range) per pt: 6 (1-27) Median follow-up: 13.5 mo |
53 | NR | NR | NR | NR | NR | NR | NR | 30% | 2% (tx-related myelosuppression) |
| Gong BMC Cancer 2016 (CGOG1001)36 |
Median number of cycles (range) per pt: 8 (1-32) Median follow-up: 28.6 mo |
51 | NR | NR | NR | NR | 16% | NR | Tx modif: 24% for oxal 33% for cape Tx interr: 4% for trast |
4% | 2% (septic shock) |
| Oh Cancer Chemother Pharmacol 201925 |
Median number of cycles (range) per pt: 6 (1-17) for trast + CTX 3 (0-61) for trast single-agent maintenance |
123 | NR | NR | NR | NR | NR | NR | NR | 0% | NR |
| Okita Tohoku J Exp Med 201837 |
Mean delivered dose intensity (cis): 14.8 mg/m2/week Relative dose intensity (cis): 55.6% |
28 | 92.9% | 60.7% | NR | NR | NR | NR | NR | NR | NR |
| Okita Tohoku J Exp Med 201837 |
Mean delivered dose intensity (cis): 10.5 mg/m2/week Relative dose intensity (cis): 52.6% |
30 | 93.3% | 56.7% | NR | NR | NR | NR | NR | NR | NR |
| Soularue Bull Cancer 201519 |
Median number of cycles (range) per pt: 13 (3-38) for trast 8 (2-12) for oxal Median follow-up: 14.7 mo |
34 | NR | 32% | NR | NR | NR | NR | NR | NR | 0% (tx related) |
| Kim BMC Cancer 202120 |
Median number of cycles (range) per pt: 8 (1-56) for trast 6 (1-15) for cis 7 (1-56) for cape 6 (1-8) for 5-FU Median follow-up: 18.8 mo |
47 | NR | NR | NR | NR | NR | NR | NR | 17% (disc of cis) | 8.5% (tx-related pneumonia and sepsis, heart failure, cerebral infarction) |
| Dijksterhuis Int J Cancer 202022 |
NR | 71 | 45.1% | 40.8% | NR | NR | NR | NR | NR | NR | 4% |
| Li Clin Transl Oncol 201821 |
Median number of cycles (range) per pt: 9 (1-44) Median follow-up: 14.0 mo |
107 | NR | NR | NR | NR | NR | NR | NR | 1% (disc of trast for ↓ LVEF) | 0% (tx related) |
| Janjigian Lancet Oncol 202015 |
Median number of cycles (IQR) per pt: 6 (5-8) for oxal 10 (7-17) for pembro/trast/ fluoropyrimidine combined Median follow-up: 13 mo |
37 | NR | NR | 97% | 57% | 5% | 5% | 97% (tx modif) | 5% (tx related) | 0% (tx related) |
| Lee AACR 2021, Abstr. CT174 (HCRN GI17-319)24 |
NR | 18 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Rha ASCO 2020, Abstr. 3031 Rha ASCO GI 2021, Abstr. 218 (PANTHERA)17 |
Median follow-up: 18.2 mo | 43 | NR | NR | 98% | 81% | NR | NR | NR | NR | NR |
| Janjigian ASCO 2021, Abstr. 4013 (KEYNOTE-811)14 |
Median follow-up for safety: 9.9 mo Median follow-up for efficacy: 12.0 mo |
216 (placebo) 217 (pembro) |
98% 97% |
57% 57% |
NR NR |
NR NR |
38% 31% |
NR NR |
NR NR |
26% 24% |
5% 3% |
5-FU, 5-fluorouracil; AE, adverse event; cape, capecitabine; cis, cisplatin; CTX, chemotherapy; disc, discontinuation; IQR, interquartile range; LVEF, left ventricular ejection fraction; modif, modification of dosage; NR, not reported; oxal, oxaliplatin; pembro, pembrolizumab; pt, patient; SAE, serious adverse event; SD, standard deviation; ToGA, Trastuzumab for Gastric Cancer; trast, trastuzumab; tx, treatment.
While treatment emergent AEs refers to adverse events that occur only once treatment has started, regardless of causality, treatment-related AEs refer to adverse events that are considered by the investigator as related to treatment.
Considerations on safety
Overall safety profile of trastuzumab plus fluoropyrimidine plus platin
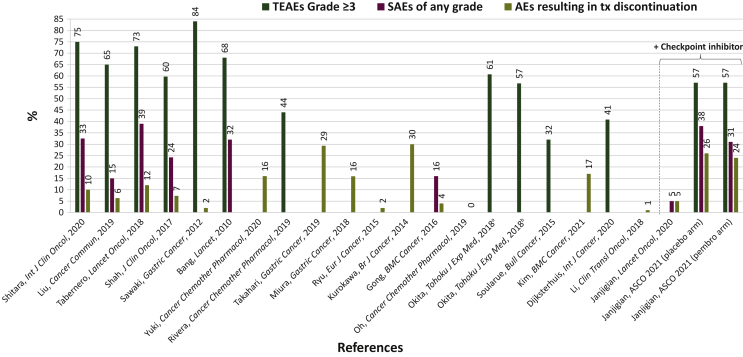
The overall safety profile was assessed among the 20 studies6,14,19, 20, 21, 22, 23,25, 26, 27, 28, 29, 32, 31, 30, 36, 35, 33, 34, 37 of various regimens containing trastuzumab, fluoropyrimidine, and platinum (Table 2, Figure 1). The median number of cycles administered per patient ranged from 6.5 to 13.0 for trastuzumab (corresponding to 19.5-39.0 weeks of treatment), and 5 to 10 for chemotherapy (corresponding to 15.3-30.0 weeks of treatment). The median follow-up time ranged from 10 to 34 months. Treatment-emergent adverse events (TEAEs) of all grades ranged from 45% to 100%. TEAEs are adverse events (AEs) that emerge during treatment, regardless of causality. Grade ≥3 TEAEs ranged from 32% to 84%. Serious adverse events (SAEs) of all grades ranged from 15% to 39%. SAEs are life-threatening AEs that may require hospitalization, may result in significant disability, or in birth defects of the offspring. AEs resulting in treatment discontinuation ranged from 0% to 30%. Treatment-related deaths occurred in 0%-9% of patients.
Figure 1.
Safety comparison across the trials analyzed. See also 6, 15, 17, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 32, 31, 30, 36, 35, 33, 34, 37
AEs, adverse events; SAEs, serious adverse events; TEAEs, treatment-emergent adverse events; Tx, treatment.
aTrastuzumab + capecitabine + cisplatin.
bTrastuzumab + S-1 + cisplatin.
The TEAEs of all grades occurring in ≥70% of patients in at least two studies were peripheral neuropathy (19%-97%), anemia (32%-97%), thrombocytopenia (28%-95%), nausea (29%-86%), anorexia (40%-84%), fatigue (20%-75%), leukopenia (43%-74%), and neutropenia (25%-66%; Table 3). Grade ≥3 TEAEs occurring in ≥15% of patients in at least two studies were neutropenia (7%-36%), anorexia (4%-25%), anemia (3%-25%), thrombocytopenia (4%-22%), and leukopenia (4%-17%; Table 3).
Table 3.
Detailed safety and efficacy
| Study | Safety |
Efficacy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Tx-emergent AEs |
Tx-related AEs |
|||||||||
| All grades | Grade ≥3 | All grades |
Grade ≥3 |
N | Median OS | Median PFS | ORR | ||||
| Trast related | CTX related | Trast related | CTX related | ||||||||
| Shitara Int J Clin Oncol 2020 (JACOB subgroup analysis)30 |
40 | Occurring in ≥50% of pts
|
Occurring in ≥10% of pts
|
NR | NR | NR | NR | 40 | 15.6 mo (primary endpoint) | 6.3 mo | 40.5% |
| Liu Cancer Commun 2019 (JACOB subgroup analysis)31 |
80 | Occurring in ≥45% of pts
|
Occurring in ≥7% of pts
|
NR | NR | NR | NR | 81 | 16.1 mo (primary endpoint) | 8.6 mo | 55.7% |
| Tabernero Lancet Oncol 2018 (JACOB)32 |
388 | Occurring in ≥35% of pts
|
Occurring in ≥6% of pts
|
NR | NR | NR | NR | 392 | 14.2 mo (primary endpoint) | 7.0 mo | 48.3% |
| Shah J Clin Oncol 2017 (HELOISE)28 |
124 | Occurring in ≥20% of pts
|
NR | NR | NR | NR | NR | 124 | 12.5 mo (primary endpoint) | 5.7 mo | 58.9% |
| Sawaki Gastric Cancer 2012 (ToGA subgroup analysis)29 |
51 | Occurring in ≥50% of pts
|
Occurring in ≥10% of pts
|
2% | NR | NR | NR | 51 | 15.9 mo (primary endpoint) | 6.2 mo | 64.4% |
| Bang Lancet 2010 (ToGA)6 |
294 | Occurring in ≥30% of pts
|
Occurring in ≥6% of pts
|
NR | NR | NR | NR | 294 | 13.8 mo (primary endpoint) | 6.7 mo | 47% |
| Yuki Cancer Chemother Pharmacol 2020 (KSCC/HGCSG/CCOG/Perseus 1501B)27 |
39 | Occurring in ≥75% of pts
|
Occurring in ≥6% of pts
|
NR | NR | NR | NR | 39 | 27.6 mo | 7.0 mo | 82.1% (primary endpoint) |
| Rivera Cancer Chemother Pharmacol 2019 (HERXO)33 |
45 | NR | NR | Occurring in ≥30% of pts
|
Occurring in ≥10% of pts
|
45 | 13.8 mo | 7.1 mo | 46.7% (primary endpoint) | ||
| Takahari Gastric Cancer 2019 (HIGHSOX)34 |
75 | NR | NR | Occurring in ≥60% of pts
|
Occurring in ≥5% of pts
|
75 | 18.1 mo | 8.8 mo | 70.7% (primary endpoint) | ||
| Miura Gastric Cancer 2018 (WJOG7212G/T-SPACE)35 |
44 | Occurring in ≥50% of pts
|
Occurring in ≥10% of pts
|
NR | NR | NR | NR | 44 | 16.5 mo | 5.9 mo | 61.4% (primary endpoint) |
| Ryu Eur J Cancer 201526 |
55 | Occurring in ≥50% of pts
|
Occurring in ≥4% of pts
|
NR | NR | NR | NR | 55 | 21.0 mo | 9.8 mo | 67% (primary endpoint) |
| Kurokawa Br J Cancer 2014 (HERBIS-1)23 |
53 | Occurring in ≥50% of pts
|
Occurring in ≥8% of pts
|
NR | NR | NR | NR | 53 | 16.0 mo | 7.8 mo | 67.9% (primary endpoint) |
| Gong BMC Cancer 2016 (CGOG1001)36 |
51 | Occurring in ≥30% of pts
|
Occurring in ≥4% of pts
|
|
NR | NR |
|
51 | 19.5 mo | 9.2 mo | 66.7% (primary endpoint) |
| Oh Cancer Chemother Pharmacol 201925 |
123 (excludes 5 pts who received induction tx only) | NR | NR | Occurring in ≥10% of pts
|
Occurring in ≥1 pts
|
128 | 14.8 mo | 11.6 mo | 62.5% | ||
| Okita Tohoku J Exp Med 201837 |
28 | Occurring in ≥40% of pts
|
Occurring in ≥10% of pts
|
NR | NR | 28 | 20.0 mo | 7.9 mo | 39.3% | ||
| Okita Tohoku J Exp Med 201837 |
30 | Occurring in ≥40% of pts
|
Occurring in ≥10% of pts
|
NR | NR | 30 | 16.7 mo | 6.9 mo | 50.0% | ||
| Soularue Bull Cancer 201519 |
34 | Occurring in ≥30% of pts
|
Occurring in ≥3% of pts
|
NR | NR | 34 | 17.3 mo (primary endpoint) | 9.0 mo | 41% | ||
| Kim BMC Cancer 202120 |
47 | NR | NR | NR | NR | 47 | 12.8 mo | 6.9 mo | 53% | ||
| Dijksterhuis Int J Cancer 202022 |
215 | NR | NR | NR | NR | 215 | 11.2 mo | NR | NR | ||
| Li Clin Transl Oncol 201821 |
75 | Occurring in ≥25% of pts
|
Occurring in ≥4% of pts
|
NR | NR | 107 | 16.0 mo | 7.7 mo | 58.9% | ||
| Janjigian Lancet Oncol 202015 |
37 | NR | NR | Occurring in ≥50% of pts
|
Occurring in ≥5% of pts
|
37 | 27.3 mo | 13.0 mo | 86.4% | ||
| Lee AACR 2021, Abstr. CT174 (HCRN GI17-319)24 |
18 | NR | NR | NR | NR |
|
18 | NR | 8.0 mo | 61% at 24 weeks (primary endpoint) 39% confirmed RR |
|
| Rha ASCO 2020, Abstr. 3031 Rha ASCO GI 2021, Abstr. 218 (PANTHERA)17 |
43 | NR | NR | Occurring in ≥20% of pts
|
Occurring in ≥5% of pts
|
43 | 19.3 mo | 8.6 mo | 76.7% (primary endpoint) | ||
| Janjigian ASCO 2021, Abstr. 4013 (KEYNOTE-811)14 |
216 (placebo) | Occurring in ≥20% of pts
|
|
NR | NR | NR | NR | 131 | NR (primary endpoint) | NR (primary endpoint) | 51.9% |
| 217 (pembro) | Occurring in ≥20% of pts
|
|
NR | NR | NR | NR | 133 | NR (primary endpoint) | NR (primary endpoint) | 74.4% | |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTX, chemotherapy; HFS, hand-foot syndrome; LVEF, left ventricular ejection fraction; mo, months; NR, not reported; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PPE, palmar-plantar erythrodysesthesia; pts, patients; RR, response rate; ToGA, Trastuzumab for Gastric Cancer; trast, trastuzumab; tx, treatment.
Analysis of a select type of TEAEs across the 20 studies6,14,19, 20, 21, 22, 23,25, 26, 27, 28, 29, 32, 31, 30, 36, 35, 33, 34, 37 of regimens containing trastuzumab and chemotherapy reveals the following ranges (Table 4): fatigue (all grades: 10%-75%; grade ≥3: 0%-14%); nausea (all grades: 19%-86%; grade ≥3: 2%-20%); diarrhea (all grades: 13%-53%; grade ≥3: 0%-27%); neutropenia (all grades: 22%-79%; grade ≥3: 7%-36%); anemia (all grades: 3%-97%; grade ≥3: 0%-25%); peripheral neuropathy (all grades: 11%-97%; grade ≥3: 0%-18%); pneumonitis (all grades: 1%; grade ≥3: 0%); stomatitis (all grades: 5%-57%; grade ≥3: 0%-5%); and palmar-plantar erythrodysesthesia (PPE)/hand-foot syndrome (HFS; all grades: 10%-63%; grade ≥3: 1%-14%).
Table 4.
Focus on specific AEs
| Study | N | Fatigue/general weakness |
Nausea |
Diarrhea |
Neutropenia |
Anemia |
Peripheral neuropathy/neurotoxicity |
Pneumonitis |
Stomatitis/mucositis/mucosal inflammation |
PPE/HFS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All gr | Gr ≥3 | All gr | Gr ≥3 | All gr | Gr ≥3 | All gr | Gr ≥3 | All gr | Gr ≥3 | All gr | Gr ≥3 | All gr | Gr ≥3 | All gr | Gr ≥3 | All gr | Gr ≥3 | ||
| Shitara Int J Clin Oncol 2020 (JACOB subgroup analysis)30 |
40 | 35% | 2.5% | 60% | 10% | 37.5% | 5% | 52.5% | 30% | NR | NR | 17.5% | 0% | NR | NR | 50% | 2.5% | 62.5% | 0% |
| Liu Cancer Commun 2019 (JACOB subgroup analysis)31 |
80 | 20% | 1.3% | 61.3% | 2.5% | 16.3% (10% tx related) | 1.3% | 65% | 30% | 53.8% | 22.5% | NR | NR | NR | NR | 8.8% | 1.3% | 20% | 7.5% |
| Tabernero Lancet Oncol 2018 (JACOB)32 |
388 | 31% | 4% | 57% | 5% | 35% | 6% | 52% | 28% | 39% | 17% | 7% | 0% | 4% | 2% | 18% | 2% | 25% | 3% |
| Shah J Clin Oncol 2017 (HELOISE)28 |
124 | 15.3% | NR | 41.9% | NR | 16.9% | NR | 46.8% | NR | 32.3% | NR | NR | NR | NR | NR | NR | NR | 9.7% | NR |
| Sawaki Gastric Cancer 2012 (ToGA subgroup analysis)29 |
51 | 61% | 8% | 86% | 14% | 45% | 8% | 59% | 35% | 29% | 25% | 31% | 2% | NR | NR | 57% | 0% | 41% | 0% |
| Bang Lancet6 2010 (ToGA) |
294 | 35% | 4% | 67% | 7% | 37% | 9% | 53% | 27% | 28% | 12% | NR | NR | NR | NR | 24% (stomatitis) 13% (mucosal inflammation) |
1% (stomatitis) 2% (mucosal inflammation) |
26% | 1% |
| Yuki Cancer Chemother Pharmacol 2020 (KSCC/HGCSG/CCOG/Perseus 1501B)27 |
39 | 62% | 5% | 59% | 8% | 51% | 8% | 74% | 10% | 97% | 13% | 82% | 5% | 3% | 3% | 49% | 0% | 41% | 0% |
| Rivera Cancer Chemother Pharmacol 2019 (HERXO)33 |
45 | 73% (tx related) | 16% (tx related) | 47% (tx related) | 20% (tx related) | 53% (tx related) | 27% (tx related) | 22% (tx related) | 2% (tx related) | 38% (tx related) | 2% (tx related) | 78% (tx related) | 2% (tx related) | NR | NR | 13% (tx related) | 2% (tx related) | 13% (tx related) | 2% (tx related) |
| Takahari Gastric Cancer 2019 (HIGHSOX)34 |
75 | 57% (tx related) | 3% (tx related) | 65% (tx related) | 4% (tx related) | 52% (tx related) | 7% (tx related) | 79% (tx related) | 11% (tx related) | 96% (tx related) | 7% (tx related) | 84% (tx related) | 16% (tx related) | NR | NR | 25% (tx related) | 1% (tx related) | NR | NR |
| Miura Gastric Cancer 2018 (WJOG7212G/T-SPACE)35 |
44 | 75% | 14% | 61% | 11% | 50% | 11% | 66% | 30% | 86% | 18% | NR | NR | NR | NR | 46% | 5% | 18% | 0% |
| Ryu Eur J Cancer 201526 |
55 | 55% | 5% | 55% | 2% | 38% | 2% | 56% | 18% | 96% | 11% | 71% | 11% | NR | NR | 22% | 2% | 33% | 2% |
| Kurokawa Br J Cancer 2014 (HERBIS-1)23 |
53 | 64% | 4% | 62% | 2% | 40% | 8% | 60% | 36% | 66% | 15% | 11% | 0% | NR | NR | 32% | 2% | NR | NR |
| Gong BMC Cancer 2016 (CGOG1001)36 |
51 | 10% | 2% | 55%a | 4%a | 22% | 4% | 65% | 14% | 49% | 6% | 14% | 4% | NR | NR | NR | NR | 37% | 4% |
| Oh Cancer Chemother Pharmacol 201925 |
123 | 11% | 0% | 19% | 3% | NR | NR | NR | NR | 3% | 0% | 11% | 1% | NR | NR | 11% | 1% | 11% | 0% |
| Okita Tohoku J Exp Med 201837 |
28 | 42.9% | 14.3% | 32.1% | 3.6% | 17.9% | 3.6% | 57.1% | 28.6% | 75% | 21.4% | 14.3% | 3.6% | NR | NR | 21.4% | 0% | 32.1% | 14.3% |
| Okita Tohoku J Exp Med 201837 |
30 | 43.3% | 10% | 36.7% | 10% | 26.7% | 6.7% | 56.7% | 26.7% | 86.7% | 23.3% | 13.3% | 0% | NR | NR | 16.7% | 0% | 23.3% | 0% |
| Soularue Bull Cancer 201519 |
34 | NR | NR | 47% | 3% | 32% | 0% | 29% | 9% | 59% | 3% | 97% | 18% | NR | NR | 32% | 0% | 32% | 3% |
| Kim BMC Cancer 202120 |
47 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Dijksterhuis Int J Cancer 202022 |
71 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Li Clin Transl Oncol 201821 |
75 | 27% | 1% | 29% | 5% | 13% | 1% | 60% | 15% | 24% | 4% | 27% | 3% | NR | NR | 5% | 1% | NR | NR |
| Janjigian Lancet Oncol 202015 |
37 | 86% | 0% | 84% | 5% | 73% | 3% | 19% | 0% | 81% | 11% | 97% | 0% | NR | NR | 38% | 5% | 22% | 0% |
| Lee AACR 2021, Abstr. CT174 (HCRN GI17-319)24 |
18 | NR | NR | NR | NR | NR | NR | NR | 28% | NR | 11% | NR | NR | NR | NR | NR | NR | NR | NR |
| Rha ASCO 2020, Abstr. 303117 Rha ASCO GI 2021, Abstr. 218 (PANTHERA)16 |
43 | 18.6% | 2.3% | 32.6% | 0% | 30.2% | 2.3% | 46.5% | 41.8% | 32.6% | 16.3% | 18.6% | 0% | NR | NR | 23.3% | 2.3% | 23.3% | 0% |
| Janjigian ASCO 2021, Abstr. 4013 (KEYNOTE-811)14 |
216 (placebo) | 20% | 3% | 44% | 6% | 44% | 8% | 25% | 7% | 44% | 9% | 19% | 1% | 1% | 0% | NR | NR | NR | NR |
| 217 (pembro) | 24% | 4% | 49% | 5% | 53% | 7% | 24% | 7% | 41% | 9% | 23% | 3% | 5% | 1% | NR | NR | NR | NR | |
AE, adverse event; gr, grade; HFS, hand-foot syndrome; NR, not reported; pembro, pembrolizumab; PPE, palmar-plantar erythrodysesthesia; ToGA, Trastuzumab for Gastric Cancer; tx, treatment.
Preferred term is nausea/vomiting.
Overall safety profile of checkpoint inhibitors plus trastuzumab plus fluoropyrimidine plus platin
The overall safety profile was determined among the four studies14, 15, 16, 17,24 of various regimens containing checkpoint inhibitors (pembrolizumab or avelumab), trastuzumab, fluoropyrimidine, and platinum (Table 2, Figure 1). The median number of cycles administered per patient was 10 (30 weeks) for pembrolizumab/trastuzumab/chemotherapy combined and 6 (18 weeks) for chemotherapy.15 The median follow-up time ranged from 9.9 to 18.2 months for safety and from 12.0 to 18.2 months for efficacy. TEAE of all grades ranged from 97% to 98%. Grade ≥3 TEAEs were 57%. SAEs of all grades ranged from 31% to 38%. AEs resulting in treatment discontinuation ranged from 5% to 24%. Treatment-related deaths occurred in 0%-3% of patients.
The TEAEs of all grades occurring in ≥30% of patients in the pembrolizumab arm of one phase III study14 were diarrhea (53%), nausea (49%), anemia (41%), decreased appetite (31%), and vomiting (31%; Table 3). Grade ≥3 TEAEs occurring in ≥5% of patients in the pembrolizumab arm of one phase III study14 were anemia (9%), thrombocytopenia (8%), neutropenia (7%), diarrhea (7%), nausea (5%), and vomiting (5%; Table 3). The treatment-related AEs of all grades occurring in ≥30% of patients in at least two studies were nausea (33%-84%), anemia (33%-81%), and diarrhea (30%-73%; Table 3).15,17 Grade ≥3 treatment-related AEs occurring in ≥10% of patients in at least two studies were neutropenia (28%-42%), anemia (11%-16%), and thrombocytopenia (7%-11%; Table 3).15,17,24
Analysis of a select type of TEAEs across the four studies14, 15, 16, 17,24 of regimens containing checkpoint inhibitors, trastuzumab, and chemotherapy reveals the following ranges (Table 4): fatigue (all grades: 24%-86%; grade ≥3: 0%-4%); nausea (all grades: 49%-84%; grade ≥3: 0%-5%); diarrhea (all grades: 53%-73%; grade ≥3: 2%-7%); neutropenia (all grades: 19%-47%; grade ≥3: 0%-42%); anemia (all grades: 33%-81%; grade ≥3: 9%-16%); peripheral neuropathy (all grades: 19%-97%; grade ≥3: 0%-3%); pneumonitis (all grades: 5%; grade ≥3: 1%); stomatitis (all grades: 23%-38%; grade ≥3: 2%-5%); and PPE/HFS (all grades: 22%-23%; grade ≥3: 0%).
Considerations by type of platin backbone
Of the 11 studies6, 20,23,25,28, 29, 32, 31, 30,35,37 with cisplatin plus capecitabine or S-1 or 5-FU as the chemotherapy regimen used in combination with trastuzumab, the range of grade ≥3 AEs was 57%-84% (Tables 1 and 2). Four20,23,25,35 of these studies did not report grade ≥3 AEs. Of the seven studies14,19,26,27,33,34,36 with oxaliplatin plus capecitabine or S-1 or 5-FU as the chemotherapy regimen used in combination with trastuzumab, the range of grade ≥3 AEs was 32%-57% (Tables 1 and 2). Four26,27,34,36 of these studies did not report grade ≥3 AEs.
Considerations by type of fluoropyrimidine backbone
Of the eight studies14,26,28,29,30,33,36,37 in which capecitabine was used as the fluoropyrimidine backbone in combination with trastuzumab, the range of grade ≥3 AEs was 44%-84% (Tables 1 and 2). Two26,36 of these studies did not report grade ≥3 AEs. Of the five studies23,27,34,35,37 in which S-1 was used as the fluoropyrimidine backbone in combination with trastuzumab, grade ≥3 AEs occurred in 57%37 (Tables 1 and 2). Four23,27,34,35 of these studies did not report grade ≥3 AEs. Because there were no studies in which 5-FU was the only choice as fluoropyrimidine backbone, a safety comparison between 5-FU and the other fluoropyrimidine backbones was not possible.
Considerations by geographical region
Studies conducted in Asia
Geographical location was considered among the 13 studies20,21,23,25, 26, 27,29, 32, 31,35, 33, 34, 37 conducted exclusively in Asia testing various regimens containing trastuzumab, fluoropyrimidine, and platinum (Table 2). TEAEs of all grades ranged from 93% to 100%. Grade ≥3 TEAEs ranged from 57% to 84%. SAEs of all grades ranged from 15% to 33%. AEs resulting in treatment discontinuation ranged from 0% to 30%. Treatment-related deaths occurred in 0% to 9% of patients. Analysis of a select type of TEAEs, among 12 Asian studies21,23,25, 26, 27,29, 32, 31,35, 33, 34, 37 testing various regimens containing trastuzumab, fluoropyrimidine, and platinum, revealed the following ranges (Table 4): fatigue (all grades: 10%-75%; grade ≥3: 0%-14%); nausea (all grades: 19%-86%; grade ≥3: 2%-14%); diarrhea (all grades: 13%-52%; grade ≥3: 1%-11%); neutropenia (all grades: 53%-79%; grade ≥3: 10%-36%); anemia (all grades: 3%-97%; grade ≥3: 0%-25%); peripheral neuropathy (all grades: 11%-84%; grade ≥3: 0%-16%); pneumonitis (all grades: 3%; grade ≥3: 3%); stomatitis (all grades: 5%-57%; grade ≥3: 0%-5%); and PPE/HFS (all grades: 11%-63%; grade ≥3: 0%-14%).
Studies conducted globally
Four studies6,14,28,32 conducted in various locations worldwide and testing various regimens containing trastuzumab, fluoropyrimidine, and platinum were reviewed (Table 2). TEAEs of all grades ranged from 91% to 99%. Grade ≥3 TEAEs ranged from 57% to 73%. SAEs of all grades ranged from 24% to 39%. AEs resulting in treatment discontinuation ranged from 7% to 26%. Treatment-related deaths occurred in 2%-3% of patients. Analysis of a select type of AEs, among four studies6,14,28,32 conducted in various locations worldwide, testing various regimens containing trastuzumab, fluoropyrimidine, and platinum, revealed the following ranges (Table 4): fatigue (all grades: 15%-35%; grade ≥3: 3%-4%); nausea (all grades: 42%-67%; grade ≥3: 5%-7%); diarrhea (all grades: 17%-44%; grade ≥3: 6%-9%); neutropenia (all grades: 25%-53%; grade ≥3: 7%-28%); anemia (all grades: 28%-44%; grades ≥3: 9%-17%); peripheral neuropathy (all grades: 19%; grade ≥3: 1%); pneumonitis (all grades: 1%-4%; grade ≥3: 0%-2%); stomatitis (all grades: 13%-24%; grade ≥3: 1%-2%); and PPE/HFS (all grades: 10%-26%; grade ≥3: 1%-3%).
Studies conducted in Europe
Three studies19,22,33 conducted exclusively in Europe testing various regimens containing trastuzumab, fluoropyrimidine, and platinum were reviewed (Table 2). TEAEs of all grades ranged from 45% to 93%. TEAEs of grade ≥3 ranged from 32% to 44%. SAEs of all grades were not reported. AEs resulting in treatment discontinuation were not reported. Treatment-related deaths did not occur. Analysis of a select type of AEs, among two studies19,33 conducted exclusively in Europe, testing various regimens containing trastuzumab, fluoropyrimidine, and platinum, revealed the following ranges (Table 4): fatigue (all grades: 73%; grade ≥3: 16%); nausea (all grades: 47%; grade ≥3: 3%-20%); diarrhea (all grades: 32%-53%; grade ≥3: 0%-27%); neutropenia (all grades: 22%-29%; grade ≥3: 2%-9%); anemia (all grades: 38%-59%; grades ≥3: 2%-3%); peripheral neuropathy (all grades: 78%-97%; grade ≥3: 2%-18%); pneumonitis was not reported; stomatitis (all grades: 13%-32%; grade ≥3: 0%-2%); and PPE/HFS (all grades: 13%-32%; grade ≥3: 2%-3%).
Considerations by tumor location among studies of trastuzumab plus fluoropyrimidine plus platin
Among 12 studies6,14,28, 29, 32, 31, 30, 36, 35, 33, 34, 37 in which gastric cancer was the most common, grade ≥3 AEs ranged from 44% to 84% (Tables 1 and 2). Grade ≥3 AEs occurred at 32% in the study19 where GEJ was the predominant type and 41% in the study22 where esophageal cancer was the predominant type (Tables 1 and 2).
By comparing the global ToGA and the JACOB studies with their respective subgroup analyses in Asian patients, we observed that the prevalence of GEJ cancer, known to have high HER2 positivity,38 was lower among Asian patients (ranging from 3.9% to 14.8%),29,30,31 compared with the overall study populations (ranging from 20% to 25%; Table 1).6,32 Moreover, the prevalence of HER2 IHC 3+ was lower among Japanese patients (ranging from 31.4% to 62.5%),29,30 compared with the overall study population (ranging from 45% to 67%; Table 1).6,32
Considerations on efficacy
Trastuzumab plus fluoropyrimidine plus platinum
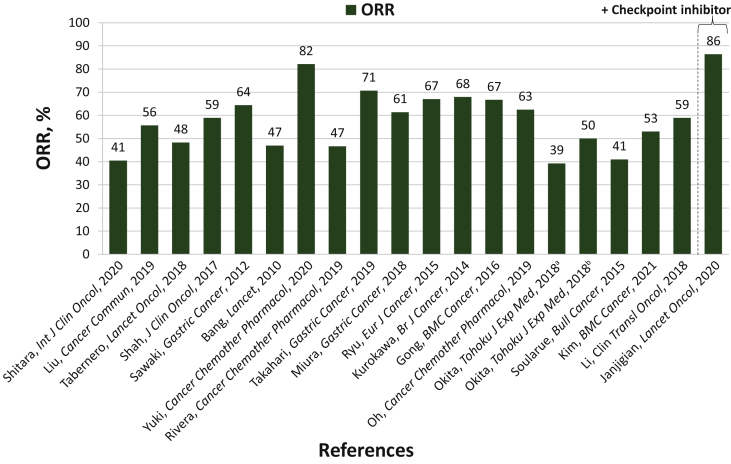
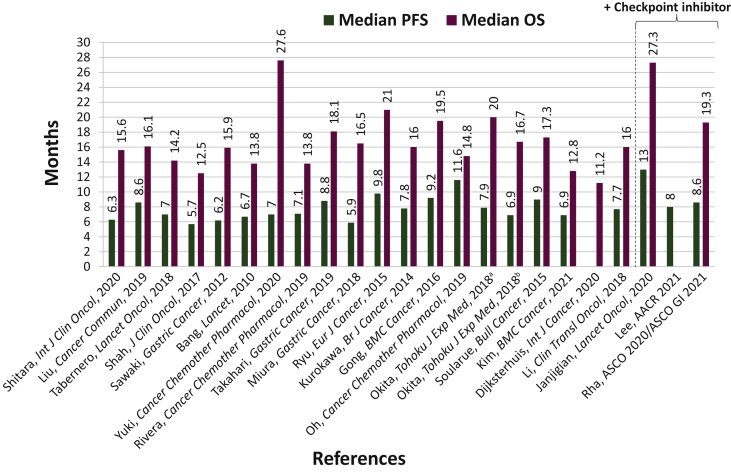
Objective response rate (ORR)6,14,19, 20, 21,23,25, 26, 27, 28, 29, 32, 31, 30, 36, 35, 33, 34, 37 ranged from 39% to 82%, median progression-free survival (PFS)6,19, 20, 21,23,25, 26, 27, 28, 29, 32, 31, 30, 36, 35, 33, 34, 37 from 5.7 to 11.6 months, and median overall survival (OS)6,19, 20, 21,23,25, 26, 27, 28, 29, 32, 31, 30, 36, 35, 33, 34, 37 from 11.2 to 27.6 months (Table 3, Figures 2 and 3). ORR ranged from 39% to 82% in the Asian studies;20,21,23,25, 26, 27,29, 32, 31,35, 33, 34, 37 in the global studies, 47% to 59%;6,14,28,32 and in the European studies, 41% to 47%.19,33 The median PFS ranged from 5.9 to 11.6 months in the Asian studies;20,21,23,25, 26, 27,29, 32, 31,35, 33, 34, 37 in the global studies, 5.7 to 7.0 months;6,28,32 and in the European studies, 7.1 to 9.0 months.19,33 The median OS ranged from 12.8 to 27.6 months in the Asian studies;20,21,23,25, 26, 27,29, 32, 31,35, 33, 34, 37 in the global studies, 12.5 to 14.2 months;6,28,32 and in the European studies, 11.2 to 17.3 months.19,22,33
Figure 2.
ORR comparison across the trials analyzed. See also 6, 15, 19, 20, 21, 23, 25, 26, 27, 28, 29, 32, 31, 30, 36, 35, 33, 34, 37
ORR, objective response rate.
aTrastuzumab + capecitabine + cisplatin.
bTrastuzumab + S-1 + cisplatin.
Figure 3.
Median PFS and OS comparison across the trials analyzed. See also 6, 15, 17, 19, 20, 21, 22, 23, 25, 26, 27, 28, 29, 32, 31, 30, 36, 35, 33, 34, 37
OS, overall survival; PFS, progression-free survival.
aTrastuzumab + capecitabine + cisplatin.
bTrastuzumab + S-1 + cisplatin.
Checkpoint inhibitors plus trastuzumab plus fluoropyrimidine plus platin
ORR14, 15, 16, 17,24 ranged from 39% to 86%, median PFS15, 16, 17,24 from 8.0 to 13.0 months, and median OS15, 16, 17 from 19.3 to 27.3 months (Table 3).
Discussion
The trastuzumab plus fluoropyrimidine plus platin regimen has considerable toxicity in patients with HER2+ GEA; therefore, less toxic regimens while maintaining clinical benefit are critical in these patients. TEAEs of all grades ranged from 45% to 100% and grade ≥3 TEAEs ranged from 32% to 84%. Efforts to improve safety and efficacy are essential. To this end, safety and efficacy study endpoints of checkpoint inhibitors combined with trastuzumab plus fluoropyrimidine and platin have begun to read out.14, 15, 16, 17,24
Regimens using oxaliplatin rather than cisplatin have potentially reduced toxicity.9 Although a direct comparison cannot be made in this review, regimens with a cisplatin backbone had higher rates of grade ≥3 AEs (57%-84%) compared with regimens with an oxaliplatin backbone (32%-57%).
Oral capecitabine and S-1, the oral form of 5-FU, have some logistical advantages over intravenously administered 5-FU. Given the few studies using S-1 as a fluoropyrimidine backbone reporting grade ≥3 AEs, and the fact that there were no studies in which 5-FU was used exclusively, it is not possible to appropriately compare safety across the three fluoropyrimidine backbones that are available. In general, patients with GEJ cancer have more dysphagia from partial obstruction, which causes more nausea and vomiting than antral or other body locations, and many of these patients prefer intravenous over oral agents.
When considering the trastuzumab plus fluoropyrimidine plus platinum regimen by geographic region, we did not observe striking differences in terms of safety between the Asian population and the rest of the world across the studies analyzed. Grade ≥3 TEAEs ranged from 57% to 84% in studies conducted in Asia20,21,23,25, 26, 27,29, 32, 31,35, 33, 34, 37 and from 57% to 73% in the studies conducted globally;6,14,28,32 similarly, SAEs of all grades ranged from 15% to 33% in studies conducted in Asia20,21,23,25, 26, 27,29, 32, 31,35, 33, 34, 37 and from 24% to 39% in the studies conducted globally.6,14,28,32 If we consider the specific example of the JACOB study and its Asian subgroup analyses, we confirmed a similar safety profile, as Grade ≥3 TEAEs were 73% in the total population32 and ranged from 65% to 75% in the subgroup analyses with Asian patients.31,30 Similarly, SAEs of all grades were 39% in the JACOB overall population32 and ranged from 15% to 33% in the JACOB subgroup analyses with Asian patients.31,30
The trastuzumab plus fluoropyrimidine plus platinum regimen has demonstrated clinical efficacy in patients with GEA, with ORR reaching up to 82%, median PFS up to 11.6 months, and median OS up to 27.6 months. In the Asian studies,20,21,23,25, 26, 27,29, 32, 31,35, 33, 34, 37 the median OS and median PFS were up to 27.6 and 11.6 months, respectively; in the European studies,19,22,33 they were up to 17.3 and 9.0 months, respectively; and in the global studies,6,28,32 they were up to 14.2 and 7.0 months, respectively. In the ToGA study, the median OS that the Japanese population achieved with trastuzumab plus chemotherapy was longer (15.9 months), compared with the total population (13.8 months).6,29 However, there were some confounding factors including that all Japanese patients in ToGA received capecitabine, while 16% of the non-Japanese population in ToGA received 5-FU, and that no ECOG 2 Japanese patients enrolled, while 12% of the non-Japanese population in ToGA were ECOG 2.29 In the JACOB study, the median OS that the Japanese population achieved with trastuzumab plus chemotherapy was slightly longer (15.6 months), compared with the total population (14.2 months).30,32 The median OS in the Chinese population of JACOB was longer (16.1 months) than the total population (14.2 months).31,32 In general, the follow-up period was longer in the Asian studies (up to 34 months30 when including the subgroup analyses of JACOB/ToGA; up to 29 months36 when these subgroup analyses are excluded), compared with the global (up to 25 months)32 and European studies (up to 15 months)19 because of longer median PFS and median OS. Among the few studies of checkpoint inhibitors plus trastuzumab plus chemotherapy, the follow-up time for efficacy analyses was similar, ranging from 12.0 to 18.2 months, and because there were only 3 studies providing follow-up times, one US-based, one Asian, and one global, it is not possible to assess potential correlations between clinical efficacy and follow-up period. The addition of checkpoint inhibitors further increased the ORR up to 86% and extended the median PFS up to 13 months, while maintaining the median OS at ∼27 months. It is possible that to detect a longer OS advantage with the addition of checkpoint inhibitors, a longer follow-up time may be necessary. One limitation to consider is that several of the studies analyzed in this review, especially those related to immunotherapy, have quite short follow-up times; therefore, the survival outcomes may change in the future as new analyses at longer follow-up become available.
Over a decade has passed since the ToGA study investigators published their seminal findings about HER2+ metastatic gastric cancer, and trastuzumab plus fluoropyrimidine and platinum has been the mainstay of therapy in those patients. Novel chemotherapy-free regimens may have potential benefit, especially in patients who cannot tolerate chemotherapy. Recently, the chemotherapy-free combination of anti-HER2+ monoclonal antibodies margetuximab and pembrolizumab was studied in a phase I/II study (NCT02689284) in the second-line treatment of patients with metastatic HER2+, PD-L1-unselected gastric and GEJ cancer refractory to trastuzumab. The study provided an initial proof of concept of clinically meaningful activity with a manageable safety profile with the combination of an anti-HER2+ agent along with an anti-PD-1 checkpoint blockade.39 It would be desirable to develop novel anti-HER2+-based combination strategies without chemotherapy as first-line treatment for appropriate patients with locally advanced unresectable or metastatic HER2+ GEA. Of note, 25 patients in a first-line study received an initial induction cycle of 200 mg flat dose pembrolizumab and an 8 mg/kg loading dose of trastuzumab, followed by pembrolizumab, trastuzumab, and chemotherapy beginning with cycle 2.15 Among these 25 patients who received one induction cycle of pembrolizumab and trastuzumab, 2 achieved partial responses (ORR of 8%) before initiation of chemotherapy.15 The phase II/III MAHOGANY study (NCT04082364) is ongoing to investigate the safety and efficacy of margetuximab plus checkpoint inhibitors with or without chemotherapy in the first-line setting for patients with HER2+ GEA.40 An initial report of MAHOGANY Cohort A Part 1 observed a grade ≥3 treatment-related AE rate of 18.6%, and an ORR of 52.5% in 40 patients with HER2 IHC3+ and PD-L1 CPS≥1 tumors, providing support for further evaluation of this first-line chemotherapy-free concept in appropriately selected patients.41
Acknowledgements
Medical writing and/or editorial assistance was provided by Emily Cullinan, PhD, CMPP, and Francesca Balordi, PhD, of The Lockwood Group (Stamford, CT, USA), in accordance with Good Publication Practice (GPP3) guidelines, funded by MacroGenics, Inc.
Funding
Funding for this review was provided by MacroGenics, Inc.
Disclosure
DVTC reports personal fees from Archer, Astellas Pharma, Bristol-Myers Squibb, Daiichi Sankyo, Five Prime, Foundation Medicine, Guardant Health, Genentech/Roche, Gritstone Oncology, Lilly, Merck, Natera, Pieris Pharmaceuticals, QED Therapeutics, Seattle Genetics, Taiho Pharmaceutical, Tempus Labs, and Zymeworks; HCC has received consultation fees from Taiho Pharmaceutical, Celltrion, Merck Sharpe & Dohme, Eli Lilly, Bristol Myers Squibb, Merck-Serono, Gloria Pharmaceuticals, BeiGene, Amgen, and Zymeworks; grants to institution for clinical trial from Eli Lilly, GlaxoSmithKline, Merck Sharpe & Dohme, Merck-Serono, Bristol Myers Squibb/Ono Pharmaceutical, Taiho Pharmaceutical, Amgen, BeiGene, and Incyte; and honoraria from Merck-Serono and Eli Lilly; LS reports grants from Beijing Xiantong Biomedical Technology, Beihai Kangcheng (Beijing) Medical Technology, Boehringer Ingelheim, Jacobio Pharmaceuticals, Qilu Pharmaceutical, and Zaiding Pharmaceutical (Shanghai); and consulting fees from Harbour BioMed and Merck; MM reports grants and nonfinancial support from Arbeitsgemeinschaft Internistische Onkologie, German Ministry of Education and Research, the European Organisation for Research and Treatment of Cancer, and German Cancer Aid; personal fees from Amgen, Bristol-Myers Squibb, Falk Foundation, Lilly, MCI Group, Merck Serono, Merck Sharp & Dohme Corp., Pfizer and Roche; grants to the university from Amgen, Bristol-Myers Squibb, Merck Serono, Merck Sharp & Dohme Corp., and Pfizer; and nonfinancial support from Amgen and Bristol-Myers Squibb; HHY reports honoraria for advisory board and steering committee from MacroGenics, honoraria for advisory boards from Bristol-Myers Squibb and Zymeworks, and honoraria for steering committees from BeiGene and Merck; Y-KK reports consulting fees from ALX Oncology, Amgen, Astellas Pharma, Bristol-Myers Squibb, Daehwa Pharmaceutical, MacroGenics, Merck, Novartis, Ono Pharmaceutical, Surface Oncology, Taiho Pharmaceutical, and Zymeworks; MKR is a full-time employee of MacroGenics.
Data Sharing
Not applicable.
Ethical approval
Not applicable.
References
- 1.Bartley A.N., Washington M.K., Colasacco C., et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:446–464. doi: 10.1200/JCO.2016.69.4836. [DOI] [PubMed] [Google Scholar]
- 2.Lordick F., Mariette C., Haustermans K., Obermannova R., Arnold D., ESMO Guidelines Committee Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 3.Smyth E.C., Verheij M., Allum W., Cunningham D., Cervantes A., Arnold D., ESMO Guidelines Committee Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
- 4.Wagner A.D., Moehler M. Development of targeted therapies in advanced gastric cancer: promising exploratory steps in a new era. Curr Opin Oncol. 2009;21:381–385. doi: 10.1097/CCO.0b013e32832c42e0. [DOI] [PubMed] [Google Scholar]
- 5.Wagner A.D., Syn N.L., Moehler M., et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang Y.J., van Cutsem E., Feyereislova A., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency Teysuno (tegafur/gimeracil/oteracil) https://www.ema.europa.eu/en/medicines/human/EPAR/teysuno Available at.
- 8.Kobayakawa M., Kojima Y. Tegafur/gimeracil/oteracil (S-1) approved for the treatment of advanced gastric cancer in adults when given in combination with cisplatin: a review comparing it with other fluoropyrimidine-based therapies. Onco Targets Ther. 2011;4:193–201. doi: 10.2147/OTT.S19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montagnani F., Turrisi G., Marinozzi C., Aliberti C., Fiorentini G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2011;14:50–55. doi: 10.1007/s10120-011-0007-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J., Dang F., Ren J., Wei W. Biochemical aspects of PD-L1 regulation in cancer immunotherapy. Trends Biochem Sci. 2018;43:1014–1032. doi: 10.1016/j.tibs.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park Y., Koh J., Na H.Y., et al. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res Treat. 2020;52:661–670. doi: 10.4143/crt.2019.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taieb J., Moehler M., Boku N., et al. Evolution of checkpoint inhibitors for the treatment of metastatic gastric cancers: current status and future perspectives. Cancer Treat Rev. 2018;66:104–113. doi: 10.1016/j.ctrv.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Chaganty B.K.R., Qiu S., Gest A., et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett. 2018;430:47–56. doi: 10.1016/j.canlet.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janjigian Y.Y., Kawazoe A., Yanez P.E., et al. on behalf of the KEYNOTE-811 investigators Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: initial findings of the global phase 3 KEYNOTE-811 study. J Clin Oncol. 2021;39:4013. [Google Scholar]
- 15.Janjigian Y.Y., Maron S.B., Chatila W.K., et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821–831. doi: 10.1016/S1470-2045(20)30169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rha S.Y., Lee C.-K., Kim H.S., et al. A multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric and gastroesophageal junction cancer (PANTHERA Trial): molecular profiling and clinical update. J Clin Oncol. 2021;39:218. [Google Scholar]
- 17.Rha S.Y., Lee C.-K., Kim S., et al. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: a multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC) J Clin Oncol. 2020;38:3081. [Google Scholar]
- 18.Keytruda (pembrolizumab): US prescribing information. Merck & Co., Inc; Whitehouse Station, NJ: 2021. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf Available at. [Google Scholar]
- 19.Soularue E., Cohen R., Tournigand C., et al. Efficacy and safety of trastuzumab in combination with oxaliplatin and fluorouracil-based chemotherapy for patients with HER2-positive metastatic gastric and gastro-oesophageal junction adenocarcinoma patients: a retrospective study. Bull Cancer. 2015;102:324–331. doi: 10.1016/j.bulcan.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Kim T.H., Do Cho H., Choi Y.W., et al. Trastuzumab-based palliative chemotherapy for HER2-positive gastric cancer: a single-center real-world data. BMC Cancer. 2021;21:325. doi: 10.1186/s12885-021-08058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q., Li H., Jiang H., et al. Predictive factors of trastuzumab-based chemotherapy in HER2 positive advanced gastric cancer: a single-center prospective observational study. Clin Transl Oncol. 2018;20:695–702. doi: 10.1007/s12094-017-1772-5. [DOI] [PubMed] [Google Scholar]
- 22.Dijksterhuis W.P.M., Verhoeven R.H.A., Slingerland M., et al. Heterogeneity of first-line palliative systemic treatment in synchronous metastatic esophagogastric cancer patients: a real-world evidence study. Int J Cancer. 2020;146:1889–1901. doi: 10.1002/ijc.32580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurokawa Y., Sugimoto N., Miwa H., et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1) Br J Cancer. 2014;110:1163–1168. doi: 10.1038/bjc.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M.S., Chao J., Mulcahy M.F., et al. AACR; Philadelphia (PA): 2021. Phase II study of avelumab and trastuzumab with FOLFOX chemotherapy in previously untreated HER2-amplified metastatic gastroesophageal adenocarcinoma. Poster presented at: Proceedings of the 112th Annual Meeting of the American Association for Cancer Research. April 10-15, 2021. Abstract CT174. [Google Scholar]
- 25.Oh S.Y., Lee S., Huh S.J., et al. Safety and efficacy of trastuzumab administered as a 30-min infusion in patients with HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol. 2019;83:501–508. doi: 10.1007/s00280-018-3753-y. [DOI] [PubMed] [Google Scholar]
- 26.Ryu M.H., Yoo C., Kim J.G., et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer. 2015;51:482–488. doi: 10.1016/j.ejca.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Yuki S., Shinozaki K., Kashiwada T., et al. Multicenter phase II study of SOX plus trastuzumab for patients with HER2(+) metastatic or recurrent gastric cancer: KSCC/HGCSG/CCOG/PerSeUS 1501B. Cancer Chemother Pharmacol. 2020;85:217–223. doi: 10.1007/s00280-019-03991-3. [DOI] [PubMed] [Google Scholar]
- 28.Shah M.A., Xu R.H., Bang Y.J., et al. HELOISE: Phase IIIb randomized multicenter study comparing standard-of-care and higher-dose trastuzumab regimens combined with chemotherapy as first-line therapy in patients with human epidermal growth factor receptor 2-positive metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2017;35:2558–2567. doi: 10.1200/JCO.2016.71.6852. [DOI] [PubMed] [Google Scholar]
- 29.Sawaki A., Ohashi Y., Omuro Y., et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. 2012;15:313–322. doi: 10.1007/s10120-011-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shitara K., Hara H., Yoshikawa T., et al. Pertuzumab plus trastuzumab and chemotherapy for Japanese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subgroup analysis of the JACOB trial. Int J Clin Oncol. 2020;25:301–311. doi: 10.1007/s10147-019-01558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T., Qin Y., Li J., et al. Pertuzumab in combination with trastuzumab and chemotherapy for Chinese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subpopulation analysis of the JACOB trial. Cancer Commun (Lond) 2019;39:38. doi: 10.1186/s40880-019-0384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabernero J., Hoff P.M., Shen L., et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–1384. doi: 10.1016/S1470-2045(18)30481-9. [DOI] [PubMed] [Google Scholar]
- 33.Rivera F., Romero C., Jimenez-Fonseca P., et al. Phase II study to evaluate the efficacy of trastuzumab in combination with capecitabine and oxaliplatin in first-line treatment of HER2-positive advanced gastric cancer: HERXO trial. Cancer Chemother Pharmacol. 2019;83:1175–1181. doi: 10.1007/s00280-019-03820-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahari D., Chin K., Ishizuka N., et al. Multicenter phase II study of trastuzumab with S-1 plus oxaliplatin for chemotherapy-naïve, HER2-positive advanced gastric cancer. Gastric Cancer. 2019;22:1238–1246. doi: 10.1007/s10120-019-00973-5. [DOI] [PubMed] [Google Scholar]
- 35.Miura Y., Sukawa Y., Hironaka S., et al. Five-weekly S-1 plus cisplatin therapy combined with trastuzumab therapy in HER2-positive gastric cancer: a phase II trial and biomarker study (WJOG7212G) Gastric Cancer. 2018;21:84–95. doi: 10.1007/s10120-017-0725-6. [DOI] [PubMed] [Google Scholar]
- 36.Gong J., Liu T., Fan Q., et al. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68. doi: 10.1186/s12885-016-2092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okita A., Imai H., Takahashi M., et al. Efficacy and safety of trastuzumab in combination with S-1 and cisplatin therapy for Japanese patients with HER2-positive advanced gastric cancer: retrospective analysis. Tohoku J Exp Med. 2018;245:123–129. doi: 10.1620/tjem.245.123. [DOI] [PubMed] [Google Scholar]
- 38.van Cutsem E., Bang Y.J., Feng-Yi F., et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catenacci D.V.T., Kang Y.K., Park H., et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21:1066–1076. doi: 10.1016/S1470-2045(20)30326-0. [DOI] [PubMed] [Google Scholar]
- 40.Catenacci D.V., Rosales M., Chung H.C., et al. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17:1155–1164. doi: 10.2217/fon-2020-1007. [DOI] [PubMed] [Google Scholar]
- 41.Catenacci D.V., Park H., Shim B.Y., et al. Margetuximab with retifanlimab in HER2+, PD-L1+ 1st-line unresectable/metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. Ann Oncol. 2021;32:S1040–S1075. doi: 10.1016/j.esmoop.2022.100563. [DOI] [PMC free article] [PubMed] [Google Scholar]