Abstract
Background
Dual checkpoint inhibitor therapy with anti-programmed cell death protein 1 and anti-cytotoxic T-lymphocyte-associated protein 4 therapy has shown promising results in patients with high-grade neuroendocrine neoplasms (NENs), demonstrating varying response rates of 9%-44%. More data are needed to evaluate the true response in a real-world cohort of patients.
Patients and methods
We conducted a retrospective study of all patients with high-grade NENs treated at the Moffitt Cancer Center and Mayo Clinic between September 2017 and July 2020 who received combination therapy with ipilimumab and nivolumab.
Results
Thirty-four patients met the eligibility criteria. Patients had received an average of two prior lines of therapy, including at least one cytotoxic chemotherapy regimen. Twenty-seven (79.4%) patients had poorly differentiated neuroendocrine carcinomas, and seven (20.6%) had well-differentiated high-grade neuroendocrine tumors. The most common primary site (10, 29.4%) was pancreas; other primary sites of disease included colon (n = 5), endometrium (n = 3), anorectum (n = 2), esophagus (n = 2), cervix (n = 1), stomach (n = 1), small intestine (n = 1), and unknown primary (n = 9). Five patients (14.7%) exhibited a best response of partial response as per RECIST 1.1 criteria, 9 (26.5%) stable disease, and 17 (50%) progressive disease: 3 patients did not have a follow-up scan as they discontinued treatment shortly after initiation due to clinical progression. The objective response rate was 14.7%, and disease control rate was 41.2%. Median progression-free survival was 1 month [95% confidence interval (CI), 0.54-1.46 months]; median overall survival (OS) from time of treatment initiation was 5.0 months (95% CI, 4.07-5.93 months), and median OS from diagnosis was 14.0 months (95% CI, 11.79-16.21 months). The median duration of treatment was 1 month (range 0-10 months). Twenty-eight patients discontinued treatment for progression, four patients for toxicity, and two remain on treatment at the time of data cut-off. Twelve patients (35%) experienced grade 3 and 4 treatment-emergent toxicities.
Conclusions
The ipilimumab and nivolumab regimen has modest activity in aggressive and heavily pretreated high-grade NENs who have progressed on prior cytotoxic chemotherapy.
Key words: neuroendocrine, ipilimumab, nivolumab, immunotherapy
Highlights
-
•
Dual checkpoint inhibitor therapy has shown promising results in high-grade NENs.
-
•
Thirty-four patients with progressive high-grade NENs were treated with ipilimumab and nivolumab.
-
•
The objective response rate was 14.7%; 35% of patients experienced grade 3-4 treatment-emergent toxicities.
-
•
The ipilimumab and nivolumab regimen has modest activity in aggressive and heavily pretreated high-grade NENs.
Introduction
Neuroendocrine neoplasms (NENs) are heterogeneous malignancies that are subdivided into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs).1 Low- and intermediate-grade NETs (G1 and G2) are often slow-growing, but high-grade (G3) NETs, defined by ki-67 proliferative index >20%, are usually aggressive.2 NETs are generally characterized by low tumor mutation burden (TMB), with identified mutations often involving chromatin remodeling genes such as DAXX/ATRX and MEN1.3,4 Microsatellite instability is not observed in low–intermediate-grade tumors.
Poorly differentiated NECs are aggressive cancers, usually characterized by ki-67 index >50% and subdivided into small-cell and large-cell carcinomas.5, 6, 7 They are characterized by a higher TMB than well-differentiated NETs, with mutations in common tumor suppressor and oncogenes including Rb1, p53, RAS, and RAF. Approximately 4% of NECs are microsatellite unstable.
Standard treatment options for NECs are limited to front-line platinum-based regimens such as cisplatin and etoposide. There are few data on treatment regimens for well-differentiated high-grade NETs, although temozolomide- and platinum-based chemotherapy regimens are often used. Novel treatments are urgently needed.
Evidence regarding the role of immunotherapy in high-grade NETs and NECs is evolving. Single-agent programmed cell death protein 1 (PD-1) inhibitors appear to be relatively ineffective.8, 9, 10, 11 In one study of 29 high-grade patients treated with pembrolizumab, a PD-1 checkpoint inhibitor, only one patient (3%) with an esophageal NEC responded.10 Another anti-PD-1 antibody, spartalizumab, was evaluated in a cohort of 21 patients with NECs, also only yielding a single objective response (4.8%).12
Dual checkpoint inhibitor therapy, using anti-PD-1/programmed death-ligand 1 (PD-L1) and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies, has shown more promising results, although reported outcomes have varied substantially between different trials. In one basket, phase II study of nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) in rare cancers, a post hoc subset analysis of high-grade NENs in one of the two NEN cohorts reported that 8 of 18 high-grade patients of various primary sites (including lung) responded radiographically (44%), versus none of the patients with low- or intermediate-grade NETs.13 Another similar phase II basket trial of ipilimumab and nivolumab showed a response rate of 31% among 13 high-grade NENs enrolled.14 However, another trial of durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) in high-grade gastroenteropancreatic NENs reported only 3 responders out of 33 patients (9.1%).15
With such divergent results reported in clinical trials, there is an urgent need for real-world data with dual checkpoint inhibitor therapy, particularly given the limited treatment options available for platinum-resistant NECs. We, therefore, conducted a retrospective analysis of outcomes associated with ipilimumab/nivolumab (ipi/nivo) in patients with high-grade NENs treated at the Mayo Clinic and the Moffitt Cancer Center.
Patients and methods
We conducted a retrospective chart review of all patients with high-grade NENs treated at the Moffitt Cancer Center (Tampa, FL) and Mayo Clinic (Rochester, MN), between September 2017 and July 2020 who received combination therapy with ipilimumab and nivolumab. Neuroendocrine lung cancers, including small-cell lung cancer, and Merkel cell carcinomas were not included in this analysis given the biological differences and higher levels of prospective data on immunotherapy in those populations. Patients who received treatment as part of a clinical trial were excluded from this analysis. Patients were included if they had received at least one prior line of treatment consisting of cytotoxic chemotherapy. Patients who initiated immunotherapy treatment at outside institutions were included if complete records were available for review. Institutional review board approval was obtained from each center, and a waiver of consent was granted due to the study's retrospective nature.
Demographic and pathologic data were collected including age, sex, race, the primary site of disease, ki-67%, mitotic rate, differentiation, prior oncologic treatment history including surgical and locoregional therapies, post-immunotherapy oncologic treatment(s), date of treatment initiation, and date of last follow-up and death, if applicable. We collected data on outcomes [objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and disease control rate (DCR)], prescribed doses and dosing schedule, duration of treatment, dose interruptions or modifications, treatment-emergent toxicities, symptomatic response, and reasons for discontinuation. PFS was defined as the time from treatment initiation to either clinical or radiographic progression (whichever was shortest), or death due to any cause. The radiographic best response was determined based on response evaluation criteria in solid tumors (RECIST) 1.1 analysis conducted by the treating physicians and based on radiographic reports. OS was measured from the date of treatment initiation until death from any cause or last known follow-up. We also evaluated OS from initial diagnosis.
Data were analyzed using IBM (Armonk, New York, NY) SPSS® version 26. Survival curves were estimated using the Kaplan–Meier method, and categorical variables were analyzed using logistic regression or categorical response models. A P value set at 0.05 was used for Pearson correlations and chi-square analyses.
Results
Patient characteristics
Table 1 presents patient demographics and tumor characteristics. Thirty-four patients met the eligibility criteria for evaluation, including 17 (50%) males and 17 (50%) females, with a median age of 57.5 (range: 22-78) years. Twenty-seven (79.4%) patients had poorly differentiated NECs and seven (20.6%) had well-differentiated high-grade NETs. The most common primary site (10, 29.4%) was pancreas; other primary sites of disease included unknown primary (n = 9), colon (n = 5), endometrium (n = 3), anorectum (n = 2), esophagus (n = 2), cervix (n = 1), stomach (n = 1), and small intestine (n = 1). Ki-67% was unreported in four patients. For patients with well-differentiated grade 3 NETs, Ki-67% ranged from 34% to 90%.
Table 1.
Patient demographics and tumor characteristics
| n (%) | |
|---|---|
| Sex | |
| Male | 17 (50) |
| Female | 17 (50) |
| Age, years | |
| 20-29 | 3 (8.8) |
| 30-45 | 5 (14.7) |
| 46-60 | 11 (32.4) |
| 60-74 | 12 (35.3) |
| 75+ | 3 (8.8) |
| Primary site of disease | |
| Pancreas | 10 (29.4) |
| Unknown | 9 (26.5) |
| Colon | 5 (14.7) |
| Uterus | 3 (8.8) |
| Other (two or less per primary) | 7 (20.6) |
| Prior surgeries | |
| No | 27 (79.4) |
| Yes | 7 (20.6) |
| Prior locoregional therapy | |
| None | 20 (58.8) |
| Radiation | 11 (32.4) |
| Hepatic embolization | 2 (5.9) |
| Hepatic ablation | 1 (2.9) |
| Prior systemic therapies | |
| 1 line | 14 (41.2) |
| 2-3 lines | 12 (35.3) |
| 4-6 lines | 8 (23.5) |
All patients had received at least one prior line of treatment consisting of cytotoxic chemotherapy. Patients had received an average of two prior systemic therapies (range: 1-6). Prior systemic treatments included platinum (carboplatin or cisplatin)/etoposide, capecitabine and temozolomide, 5-fluorouracil and oxaliplatin (FOLFOX), 5-fluorouracil and irinotecan (FOLFIRI), 5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX), gemcitabine and docetaxel, cyclophosphamide, doxorubicin and vincristine, olaparib, dabrafenib and trametinib, sunitinib, everolimus, long-acting octreotide, topotecan, paclitaxel, and irinotecan. Two patients received prior atezolizumab with carboplatin/etoposide. No other patients received prior immunotherapy. Seven patients (20.6%) had undergone prior oncologic surgery, and 14 (41.2%) had received prior locoregional therapy (including 11 radiation treatments, 2 hepatic embolizations, and 1 hepatic ablation).
Sixteen patients initially responded to platinum/etoposide, and the median duration of response was 3 months (range: 0-18 months). Of the remaining patients, 11 progressed at the first restaging scan, 4 patients had stable disease (SD) on first restaging and progressed within 1-2 months of that scan, and the remaining patients did not have available response data.
Molecular data (FoundationOne®) were available in two of the five responders; however, no actionable mutations were identified in either patient: one patient had low TMB and another had intermediate. Table 2 provides further characterization of the responders.
Table 2.
Patients with PR as per RECIST v1.1
| Primary site | Histology | Prior therapies | Duration of response | Molecular data | |
|---|---|---|---|---|---|
| Patient 1 | Uterus | Poorly differentiated |
|
5 months |
|
| Patient 2 | Unknown | Poorly differentiated |
|
21 months | Unavailable |
| Patient 3 | Esophagus | Poorly differentiated |
|
5 months (ongoing) |
|
| Patient 4 | Pancreas | Poorly differentiated |
|
2 months | Unavailable |
| Patient 5 | Colon | Poorly differentiated |
|
1 month | Unavailable |
Mut/mb, mutations per megabase; PR, partial response; TMB, tumor mutation burden.
Treatment regimen
Patients were treated with combination ipilimumab and nivolumab at various schedules. Thirteen patients were treated with a flat dose of 240 mg nivolumab every 2 weeks and 1 mg/kg ipilimumab every 6 weeks. Of those, four patients were scheduled only to receive four doses of ipilimumab followed by nivolumab monotherapy. Eleven patients received 3 mg/kg nivolumab every 3 weeks and 1 mg/kg ipilimumab every 3 weeks; nine were scheduled only to receive four doses of ipilimumab followed by nivolumab monotherapy every 2 weeks. All patients continued on the same dose of nivolumab, except for one who transitioned to the flat dose of 480 mg every 28 days. Eight patients were treated with 1 mg/kg nivolumab every 3 weeks and 3 mg/kg ipilimumab every 3 weeks, for a total of four doses, and then maintained on 1 mg/kg nivolumab monotherapy every 2 weeks. Two patients completed treatment at outside facilities and treatment regimen was unknown.
The median duration on treatment was 1 month (range: 0-10 months). Seventeen patients (50%) did not complete the first four doses of ipilimumab and discontinued treatment after one to three doses of treatment, three for toxicity, and the remainder due to progressive disease (PD). Eight patients (23.5%) completed the first four doses of treatment and subsequently discontinued for progression. Seven patients completed the first four doses of treatment and continued on nivolumab monotherapy. The remaining two patients (both of whom have completed the first four doses) continue treatment at data cut-off (31 January 2021).
There were no differences in survival outcomes, the incidence of grade 3/4 toxicities, or duration on treatment between the various dosing regimens. Fifteen patients went on to receive additional anticancer therapy after discontinuing ipi/nivo. Of those, three remain alive at the time of data cut-off.
Adverse events
Treatment-emergent adverse events were graded as per Common Terminology Criteria for Adverse Events v5.0. Twenty-two patients (65%) experienced at least one treatment-related toxicity, 12 of whom experienced grade 3 and 4 toxicities. Eleven patients required dose delays or adjustments due to toxicity. Three patients discontinued treatment after one dose of treatment due to grade 3 or 4 toxicities: one for grade 3 elevated transaminitis, one for grade 3 arthralgia and lower-extremity edema, and one for grade 3 myocarditis, grade 4 acute kidney injury, and rhabdomyolysis.
Efficacy
Radiographic responses were assessed as per RECIST 1.1 by review of radiology reports. Five patients (14.7%) exhibited a best response of partial response (PR) as per RECIST 1.1 criteria, 9 (26.5%) had SD, and 17 (50%) had PD. Response was not assessable for three patients due to discontinuing treatment shortly after initiation due to clinical progression. The five patients with a best response of PR were all poorly differentiated NECs with primary tumors in the colon, esophagus, pancreas, cervix, and unknown. Of the five patients with PR, one received only one dose of treatment and discontinued due to grade 3 myalgias and lower-extremity edema; however, she experienced a prolonged response of 23 months before progression. This patient previously received adjuvant therapy with carboplatin/etoposide for 6 months, and progressed in the lungs and kidneys bilaterally 3 months after completing therapy. She remains in partial remission of most of her disease, however progressed in the brain at 23 months. She received radiation to the brain lesion and remains stable since then. No next-generation sequencing data are available on this patient; tumor is mismatch repair proficient, p16 positive, and p53 negative. Ki-67% was 50%, mitotic count was 42 per 10 high-power field. Three of the remaining patients' responses lasted 2, 3, and 6 months, and one patient remains on treatment.
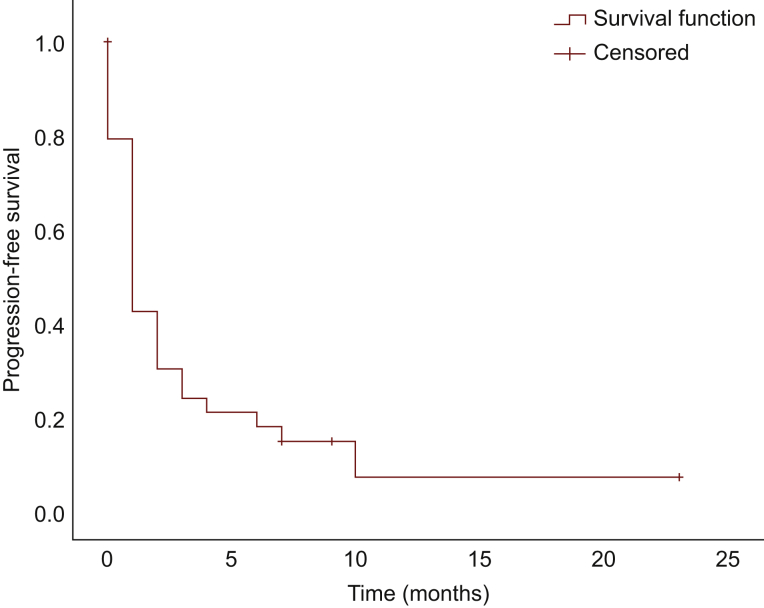
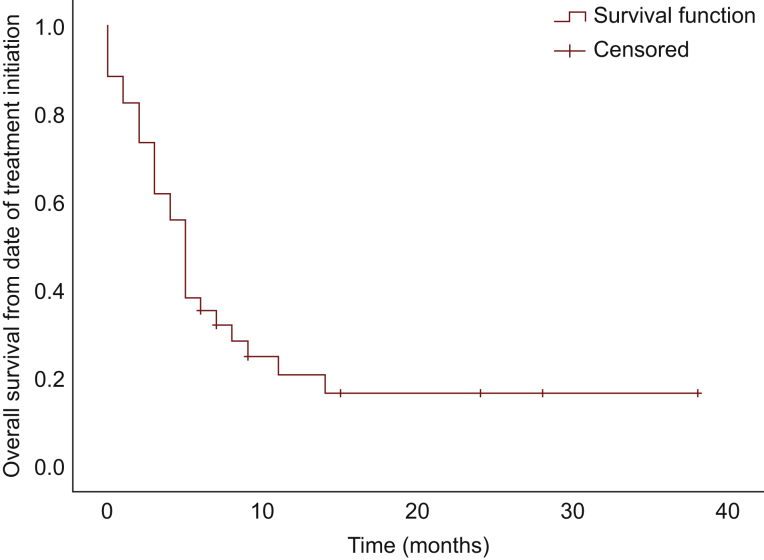
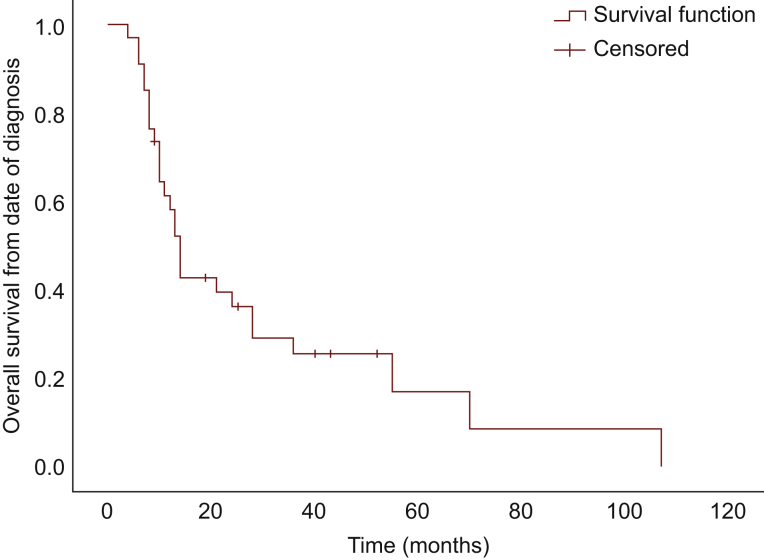
Median PFS was 1 month [95% confidence interval (CI), 0.54-1.46 months]; median OS from time of treatment initiation was 5.0 months (95% CI, 4.07-5.93 months), and median OS from diagnosis was 14.0 months (95% CI, 11.79-16.21 months) (Figure 1, Figure 2, Figure 3, respectively). There was no significant difference in PFS and OS from treatment initiation, or OS from diagnosis among patients with well- and poorly differentiated tumors (P = 0.40, P = 0.66, and P = 0.09, respectively). Twenty-eight patients discontinued treatment due to PD, four for toxicity, and two remain on treatment at the time of data cut-off. Of note, seven patients experienced symptomatic improvement of pre-treatment disease-related symptoms, three of those with PR as best response, and four of those with SD.
Figure 1.
Progression-free survival.
Figure 2.
Overall survival from date of treatmentinitiation.
Figure 3.
Overall survival from date of diagnosis.
Discussion
Our analysis of dual checkpoint inhibitor therapy with ipilimumab and nivolumab describes real-world outcomes in a rare patient population where prospective data are limited. Response rates in our cohort of patients were modestly higher than those reported in the durvalumab/tremelimumab clinical trial; however, they were substantially lower than those reported in the subgroup analyses of both ipi/nivo basket trials, the DART S1609 and CA209-538 trials. Our data show no significant difference in PFS or OS from the time of immunotherapy treatment initiation between grade 3 NETs and NECs, possibly due to the fact that grade 3 NETs were heavily pretreated and biologically aggressive. Of note, however, all five responders were poorly differentiated NECs.
Due to this study's retrospective nature, only a limited number of patients had available molecular data for review. Of the responding patients, only two patients had molecular data and no favorable predictive biomarkers were noted. Both patients were microsatellite stable; one responder had an intermediate TMB and RB1 mutation, and another had a TP53 mutation and low TMB.
Adverse events of any grade are typically reported to occur in 30%-40% of patients receiving checkpoint inhibition therapy, while grade 3 and 4 events are reported to occur in ∼10%.16, 17, 18, 19 Our data show a higher incidence of treatment-emergent toxicities, although this may be due to our population of patients having aggressive disease. Our data show that the varying dosing regimens did not significantly associate with the incidence of grade 3/4 toxicities.
Our study's limitations include its retrospective nature, varying initial dosing regimens, limited biomarker data, and relatively small sample size for data of this nature. It is important to note that while the sample size presented here is small, it is the largest cohort of NENs treated with combination checkpoint inhibitor therapy outside of a clinical trial, to our knowledge. The response rate of 14.7% is similar to that seen in other cancers. It supports the need for further evaluation of this regimen in this rare patient population of high-grade NENs, with rigorous stratification of patients according to primary site, differentiation, and analysis of potential predictive biomarkers such as TMB.
Conclusion
Dual checkpoint inhibitor therapy with ipi/nivo has modest activity in patients with high-grade NENs progressing on or after prior cytotoxic chemotherapy. Clinically significant treatment-emergent toxicities are risks. Predictive biomarkers specific to this population of patients have not been established. In the absence of alternative treatment options, particularly for platinum-refractory NECs, use of this regimen should be considered.
Acknowledgments
Funding
None declared.
Disclosure
JS: Novartis (Consult), Ipsen and Lexicon (Speaker Bureau). TH: Curium, Lexicon, Ipsen, Advanced Accelerator Applications (Consult). All other authors have declared no conflicts of interest.
References
- 1.Dasari A., Shen C., Halperin D., et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cives M., Strosberg J.R. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2018;68(6):471–487. doi: 10.3322/caac.21493. [DOI] [PubMed] [Google Scholar]
- 3.Scarpa A., Chang D.K., Nones K., et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543(7643):65–71. doi: 10.1038/nature21063. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Cuesta L., Peifer M., Lu X., et al. Frequent mutations in chromatin-remodelling genes in pulmonary carcinoids. Nat Commun. 2014;5:3518. doi: 10.1038/ncomms4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cives M., Pelle E., Quaresmini D., Rizzo F.M., Tucci M., Silvestris F. The tumor microenvironment in neuroendocrine tumors: biology and therapeutic implications. Neuroendocrinology. 2019;109(2):83–99. doi: 10.1159/000497355. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence M.S., Stojanov P., Polak P., et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijayvergia N., Boland P.M., Handorf E., et al. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: a Fox Chase Cancer Center Pilot Study. Br J Cancer. 2016;115(5):564–570. doi: 10.1038/bjc.2016.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehnert JM, Rugo HS, O'Neil BH, et al. Pembrolizumab for patients with PD-L1–positive advanced carcinoid or pancreatic neuroendocrine tumors: results from the KEYNOTE-028 study. Paper presented at ESMO 2017 Congress. 2017; Barcelona, Spain.
- 9.Mulvey C., Raj N.P., Chan J.A., et al. Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: results of Part A (pembrolizumab alone) J Clin Oncol. 2019;37(suppl 4):363. [Google Scholar]
- 10.Vijayvergia N., Dasari A., Deng M., et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer. 2020;122:1309–1314. doi: 10.1038/s41416-020-0775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strosberg J., Mizuno N., Doi T., et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the phase II KEYNOTE-158 study. Clin Cancer Res. 2020;26(9):2124–2130. doi: 10.1158/1078-0432.CCR-19-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao J.C., Strosberg J., Fazio N., et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx) Ann Oncol. 2018;29(suppl 8):VIII467–VIII468. [Google Scholar]
- 13.Patel S.P., Othus M., Chae Y.K., et al. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res. 2020;26(10):2290–2296. doi: 10.1158/1078-0432.CCR-19-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein O., Kee D., Markman B., et al. Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: a subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res. 2020;26(17):4454–4459. doi: 10.1158/1078-0432.CCR-20-0621. [DOI] [PubMed] [Google Scholar]
- 15.Capdevila J, Teule A, Lopez C, al. e. A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: the DUNE trial (GETNE 1601). Paper presented at ESMO Virtual Conference. September 19-21, 2020; Virtual.
- 16.Hodi F.S., O'Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok J.D., Neyns B., Linette G., et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 18.Naidoo J., Page D.B., Li B.T., et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian S.L., Sznol M., McDermott D.F., et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]