Abstract
Sjögren's syndrome (SS) is a systemic autoimmune disease characterized by immune-mediated injury of exocrine glands. Extensive lymphocytic infiltrates may contribute to the destruction and loss of secretory function of glands. B-cell hyperactivity is a key feature of the disease resulting in the production of a diverse array of autoantibodies in these patients. Although not specific for SS, anti-Ro/SSA and anti-La/SSB antibodies have been useful biomarkers for disease classification and diagnosis. During recent years, novel autoantibodies have been discovered in SS. In this review, we summarize the historical role and clinical relevance that autoantibodies have played in the classification criteria of Sjögren's syndrome, discuss laboratory aspects in antibody detection and review the role of novel autoantibodies in predicting particular stages of the disease, clinical phenotypes and long-term complications.
Keywords: Sjögren's syndrome, Classification criteria, Autoantibodies
Highlights
-
•
Different classification criteria for SS have been developed over time with remarkable changes in the serological item.
-
•
Challenges in the harmonization of autoimmune diagnostics involvethe heterogeneity in autoantibodies and diagnostic methods.
-
•
The difficulties in standardization and harmonization in autoimmune diagnostics may have an impact on disease criteria.
-
•
Particular disease stages, clinical phenotypes and long-term complications may be associated with specific autoantibodies.
Abbreviations
- ACR
American College of Rheumatology
- AECG
American-European Consensus Group
- ALBIA
Addressable laser bead immunoassay
- ANA
Antinuclear antibodies
- APRIL
A proliferation-inducing ligand
- AUC
Area under the curve
- BAFF
B cell activating factor of the TNF family
- BCR
B-cell receptor
- CA6
Carbonic anhydrase VI
- CLIA
Chemiluminescence immunoassay
- ELISA
Enzyme-linked immunosorbent assay
- ENA
Extractable nuclear antigen
- ESSDAI
European Sjögren's syndrome disease activity index
- EULAR
European League Against Rheumatism
- FEIA
Fluorescence enzyme immunoassay
- GC
Germinal center
- IIF
Indirect immunofluorescence
- IFI16
Interferon-inducible protein-16
- ILD
Interstitial lung disease
- MALT
Mucosa-associated lymphoid tissue
- MDM2
Mouse double minute 2
- NA-14
Nuclear autoantigen 14
- NMOSD
Neuromyelitis optica spectrum disorder
- NPV
Negative predictive value
- PPV
Positive predictive value
- PSP
Parotid secretory protein
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- RGI2
Rho GDP-dissociation inhibitor 2
- RNA
Ribonucleic acid
- SGEC
Salivary gland epithelial cells
- SICCA
Sjögren's International Collaborative Clinical Alliance
- SLE
Systemic lupus erythematosus
- SP1
Salivary protein 1
- SS
Sjögren's syndrome
- SSc
Systemic sclerosis
- Tfh
T follicular helper cells
- TLR
Toll-like receptor
- TRIM
Tripartite motif
1. Introduction
Sjögren's syndrome (SS) is an autoimmune disease characterized by immune-mediated destruction of exocrine glands, mainly the lachrymal and salivary glands. Lymphocytic infiltration of the glands and the resultant autoimmune epithelitis impair lacrimal and salivary secretions. Primary SS (pSS) occurs in the absence of other autoimmune diseases, whereas secondary SS (sSS) is associated with other underlying autoimmune disorders like systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and systemic sclerosis (SSc). The prevalence of pSS varies widely and ranges from 0.01% to more than 3% of the general population. Moreover, pSS is characterized by a 9:1 female to male ratio of disease incidence with an increased risk during the post-menopausal stage. The aetiology of pSS is multifactorial with a complex interplay between genetic, hormonal, environmental, and immunological risk factors [[1], [2], [3], [4]]. In general, the diagnosis is based on oral and ocular sicca symptoms and their evaluation (e.g. Schirmer's test), a labial biopsy showing a focal lymphocytic infiltration as well as the presence and detection of autoantibodies (e.g. anti-Ro/SSA) in serum. This review highlights the historical role that autoantibodies have played in the classification criteria of Sjögren's syndrome. Moreover, it addresses the importance of novel autoantibodies in predicting particular disease manifestation and long-term complications.
2. B-cell dysregulation in Sjögren's syndrome
2.1. B-cell hyperactivity
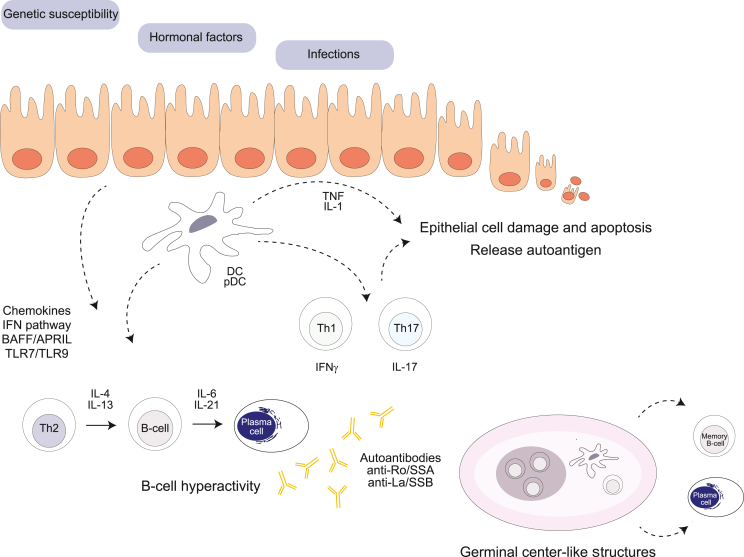
The pathogenesis of SS is a complex process (summarized in Fig. 1). Salivary gland epithelial cells (SGEC), the innate immune system, both arms of the adaptive immune system orchestrated by cytokines (e.g. interferons) and chemokines trigger and maintain autoimmune processes in a host with genetic susceptibility [[5], [6], [7]]. The salivary glands of SS patients show focal lymphocytic infiltration involving clusters of B cells. B cell hyperactivity is tightly linked to the pathogenesis of SS and is playing a critical role in the exacerbation and chronicity of inflammation [8,9]. Hypergammaglobulinemia and autoantibodies such as anti-Ro/SSA, anti-La/SSB and rheumatoid factor (RF) are common and important serological features in SS [7,10]. B cell infiltration in the salivary glands is caused by aberrant production of the B-cell chemoattractant CXCL13, expressed among others by SGEC [11,12]. Interestingly, elevated levels of the B-cell chemoattractant CXCL13 in either serum or saliva can be found in patients with SS and blockade of CXCL13 by a neutralizing monoclonal antibody reduces salivary gland inflammation in a murine model [13]. By attracting T follicular helper cells (Tfh) and B cells, CXCL13 and its receptor CXCR5 may contribute to the formation of ectopic germinal center (GC)-like structures in the exocrine glands [12,14]. Functional GC formation takes place in the salivary glands of a subset of patients with SS and correlates with higher focus scores, cell infiltration and autoantibody production [15]. The CXCL13-mediated migration of B cells is synergistically enhanced by B cell activating factor of the TNF family, so-called BAFF [16]. Once in the salivary glands, the local microenvironment promotes B-cell survival, activation and plasma cell differentiation. Notably, pSS is characterized by a hyperactivation of the type I IFN system resulting in elevated levels of BAFF and the proliferation-inducing ligand APRIL [17,18]. BAFF and APRIL contribute to the autoreactivity as both can promote survival and activation of B cells, plasma cell longevity and autoantibody production. Interestingly, the levels of BAFF correlate with antibody titers in sera of pSS patients and development of SS-like disease in BAFF-transgenic mice further emphasizes the role of BAFF in disease pathogenesis [19,20]. Moreover, serum levels of APRIL were increased in patients with pSS compared to healthy blood donors [21]. Thus, type I IFN contributes via the BAFF/APRIL axis to B-cell hyperreactivity in the salivary glands of pSS patients. Activation of B cells and generation of isotype-switched cells require stimulation of the B-cell receptor (BCR) and CD40 binding to CD40L in the presence of appropriate cytokines. Additionally, Toll-like receptor (TLR) engagement can fine-tune B cell responses [22]. Interestingly, B cells from patients with pSS exhibit altered TLR7 and TLR9 responsiveness and produce increased levels of cytokines after TLR stimulation [23]. Moreover, elevated levels of soluble CD40L can be found in sera of patients with pSS [24]. Various cytokines are involved in plasma cell differentiation of which IL-21 has been recognized as a potent cytokine in this process [25]. Patients with pSS exhibit higher serum levels of IL-21 than controls, and these levels positively correlate with serum IgG, the latter also correlating with anti-Ro/SSA antibody titer [26]. Moreover, IL21 transcript levels were increased in labial salivary glands of SS patients when compared to those from controls [27]. Altogether, the local inflamed salivary gland environment supports B-cell hyperactivity and long-term survival of plasma cells with autoreactive activity.
Fig. 1.
Pathogenesis of Sjögren's syndrome. The aetiology of pSS is complex and multifactorial with a strong interplay between genetic, hormonal, environmental, and immunological risk factors. Different pathways (BAFF/APRIL and interferons), immune cells (B and T cells, dendritic cells) and salivary gland epithelial cells are crucial players in the initiation and perpetuation of Sjögren's syndrome.
2.2. B-cell hyperactivity and clonal expansion
A potential risk of chronic antigenic stimulation is clonal expansion of B cells and lymphoma development. Increased clonal expansion of B cells has been observed in the salivary glands of pSS patients compared to non-pSS controls [28]. Patients with pSS are at increased risk of developing non-Hodgkin's lymphoma of which mucosa-associated lymphoid tissue (MALT) lymphomas are most frequent [29,30]. The presence of GC-like structures in exocrine gland biopsies of patients with pSS is a predisposing factor for lymphoma development [31,32]. Other predictors of lymphoma development in pSS involve the presence of both anti-Ro/SSA and anti-La/SSB, hypergammaglobulinemia, cryoglobulinemia, low C4 (and C3) levels and leukopenia [[33], [34], [35]].
3. Autoantibodies in Sjögren's syndrome
3.1. Autoantibodies precede disease
Hyperactivity of B cells and long-term survival of plasma cells give rise to a wide spectrum of autoantibodies. Autoantibodies are a key serological feature of pSS. As in other autoimmune diseases, many of these antibodies can precede the clinical manifestation of the disease [36]. Theander et al. [37] demonstrated that in patients with primary SS who showed autoantibody positivity after diagnosis, at least one autoantibody specificity was detected in 81% of the patients up to 18–20 years before diagnosis with a median of 4.3–5.1 years. The autoantibodies found most often were antinuclear antibodies (ANA), RF, anti-Ro/SSA and anti-La/SSB. Interestingly, patients diagnosed before age 40, showed a significantly higher prevalence of prediagnostic autoantibodies compared with those at the age of 40–60 years or >60 years at diagnosis. Early detection of these antibodies can be important, because especially anti-Ro/SSA antibodies in asymptomatic pregnant women may cause a passively acquired autoimmunity (e.g. neonatal lupus syndrome and congenital heart block) in the neonate due to the transplacental passage of antibodies [38,39].
3.2. Antibodies to nuclear antigen, anti-Ro/SSA, anti-La/SSB and rheumatoid factor
The prevalence of ANA in pSS is high (up to 80%) and their presence has been associated with extraglandular manifestation, hypergammaglobulinemia and a high frequency of anti-extractable nuclear antigen (ENA) antibodies [40,41]. The most common anti-ENA antibodies in SS patients are directed against Ro/SSA and La/SSB, with a frequency of 33%–74% and 23%–52%, respectively [42]. The Ro/SSA antigen is often described as consisting of two polypeptide components of 52 and 60 kDa. However, it has become clear that Ro52 and Ro60 are distinct subcellular proteins and therefore probably should not be grouped together as anti-SSA antibodies [43]. Since its molecular structure places Ro52 within the family of tripartite motif proteins (TRIM), Ro52 is also denoted as TRIM21 [44]. Ro52/TRIM21 is induced by IFN during viral infection and exhibits anti-proliferative and pro-apoptotic properties [45]. It has been shown to be a ubiquitin E3 ligase, thereby causing degradation of interferon regulatory factors and inhibition of the NFκB signaling pathway [[46], [47], [48]]. By inhibiting Bcl2 production, it can cause increased apoptosis which as a mechanism may lead to increased accessibility of autoantigens and eventually autoantibody formation [49]. Both Ro60/SSA and La/SSB are RNA binding proteins. Within cytoplasm, Ro60/SSA binds to small non-coding RNA called YRNA. Ro60/SSA is believed to play a role in repair mechanisms of intracellular damage following UV radiation [50]. A possible interaction at protein level may occur between Ro52/TRIM21 and Ro60/SSA since binding of Ro52/TRIM21 to YRNA requires the presence of Ro60/SSA [51]. It is still controversial whether Ro52/TRIM21 actually binds directly to RNA since it does not have an RNA binding site. La/SSB can interact with RNA polymerase III transcripts as well as a number of virus-encoded RNA and is thought to have antiviral capacities, although some controversy exists [[52], [53], [54]]. Although anti-Ro/SSA and anti-La/SSB antibodies are not specific for pSS, their presence is associated with an earlier disease onset in pSS patients, salivary gland lymphocytic infiltration and dysfunction, a higher prevalence of extra-glandular manifestations, hypergammaglobulinemia as well as other features of B-cell activation [42,55]. Serological positivity for anti-Ro/SSA and anti-La/SSB can be associated with RF in pSS patients. RF is detected in 36–74% of patients with pSS [42]. The presence of RF correlates with an early stage of the disease and an active serological profile characterized by hypergammaglobulinemia and hypocomplementemia, besides ANA and anti-ENA antibodies. Moreover, increased frequency of extraglandular manifestations such as arthritis, cutaneous vasculitis and interstitial lung disease (ILD) has been described for RF positive patients and specifically for IgA RF, an association with renal disease has been reported [41,42,[56], [57], [58], [59], [60]]. Of all the above autoantibodies, anti-Ro/SSA antibodies are considered as the best serological item in the current classification criteria for pSS.
4. Autoantibodies in the classification criteria: matter of debate
4.1. History of the classification criteria
Over the last few decades, many classification criteria have been developed to define pSS (Table 1). Before 2002, several classification and diagnostic criteria have been published but none have been approved by the American College of Rheumatology (ACR) or European League Against Rheumatism (EULAR). In the beginning of the 21st century, the criteria issued by the American-European Consensus Group (AECG) represented the gold standard in that time and appeared to be an important classification system used in clinical studies [61]. The AECG criteria include two autoantibodies, namely anti-Ro/SSA and/or anti-La/SSB. Since one-third of the patients with pSS do not have these antibodies, the Sjögren's International Collaborative Clinical Alliance (SICCA) developed new classification criteria, which were approved by the ACR in 2012 [62]. The 2012 ACR criteria differed substantially from the 2002 AECG criteria as rheumatoid factor (RF) positivity together with an antinuclear antibody (ANA) titer ≥1:320 was included as an alternative to anti-Ro/SSA and anti-La/SSB antibody positivity. Of particular interest, RF isotype and the in vitro diagnostic method to determine ANA titer were not specified in the classification criteria. The two different classification systems available at that time made it difficult to compare epidemiological studies and therapeutic trials since different methods were used to select cohorts of patients [63]. To overcome the non-favourable co-existence of two criteria and reach a final agreement, novel classification criteria for pSS combining features of the 2012 ACR and 2002 AECG classification system were developed and approved by ACR as well as EULAR committees in 2016 [64]. In the currently used 2016 ACR-EULAR criteria, anti-La/SSB positivity, high-titer ANA and positive RF are all removed as serological items and a weighed scoring system has been applied with 3 points for anti-Ro/SSA antibody positivity. The exclusion of anti-La/SSB positivity from the criteria was based on the finding that anti-La/SSB positive patients that showed negativity for anti-Ro/SSA in the SICCA cohort lacked phenotypic features of pSS [65]. The reason for excluding high-titer ANA and positive RF was due to the fact that only a very small number of individuals who met the ACR criteria were negative for anti-Ro/SSA and anti-La/SSB, but positive for ANA and RF [66].
Table 1.
The 2002 AECG, 2012 ACR and 2016 ACR/EULAR classification criteria for SS.
| 2002 AECG Classification Criteria |
2012 ACR Classification Criteria |
2016 ACR-EULAR Classification Criteria |
||||
|---|---|---|---|---|---|---|
| Item | Weight | Item | Weight | Item | Weight | |
| Dryness | Ocular dryness symptoms | Minor | ||||
| Oral dryness symptoms | Minor | |||||
| Ocular signs | Schirmer's test ≤5 mm/5 min OR van Bijsterveld score ≥4 | Minor | Keratoconjunctivitis sicca with ocular staining score ≥3 | 1 | Schirmer's test ≤5 mm/5 min | 1 |
| Ocular staining score ≥5 or van Bijsterveld score ≥4 | 1 | |||||
| Salivary gland | Focus score ≥1 focus/4 mm2 | Major | Focus score ≥1 focus/4 mm2 | 1 | Focus score ≥1 focus/4 mm2 | 3 |
| Unstimulated whole salivary flow ≤0.1 ml/min | Minor | Unstimulated whole salivary flow ≤0.1 ml/min | 1 | |||
| Autoantibodies | Anti-Ro/SSA or anti-La/SSB | Major | Anti-Ro/SSA or anti-La/SSB OR RF with ANA ≥1:320 | 1 | Anti-Ro/SSA | 3 |
| Rules for classification | 4 out of 6 with ≥1 major, or 3 out of 4 objective items | ≥2/3 criteria in a patient with suspected SS | Total score of ≥4 in a patient with sicca or ESSDAI ≥1 | |||
AECG = American-European Consensus Group; ACR = American College of Rheumatology; EULAR = European League Against Rheumatism; ESSDAI = European Sjögren's syndrome disease activity index; ANA = antinuclear antibodies.
4.2. Comparison of the three classification criteria sets for Sjögren's syndrome
Various studies have compared the three classification criteria in patient populations taking into account the different serological criterion. Cornec et al. [67] demonstrated that according the 2002 AECG criteria more patients were classified as having SS, while the 2012 ACR criteria appeared to include patients who did not have SS in the opinion of the physician. However, the latter did classify patients earlier in the disease course. The main reasons for these discrepancies were the absence of functional salivary gland testing in the 2012 ACR criteria and the differences between the ocular staining score (OSS) and Schirmer's test, which were not seen as two separate entities in the 2002 AECG criteria. The alternative serological item including high-titer ANA and RF positivity did not have added value to the classification potential of only anti-Ro/SSA and anti-La/SSB as seen in the 2002 AECG criteria. Only three out of 105 patients were negative for anti-Ro/SSA antibodies, but positive for RF and ANA titer ≥1:320, thereby fulfilling the ACR, but not the AECG serological criterion. However, these three patients all had abnormal OSS and focus score results.
In another study by Rasmussen et al. [66] a concordance rate of 0.81 (95% confidence interval = 0.77–0.86) was found for the 2012 ACR and the 2002 AECG classification criteria. The sensitivity of the ACR criteria was 87.5% with a specificity of 93.4%. In the overall cohort, 12.5% of the participants classified as SS according the 2002 AECG criteria only. Conversely, 8.9% of the participating subjects met only the 2012 ACR criteria. Seven of the participants that met the ACR criteria, but not the AECG criteria, did so by having positive RF and high-titer ANA. Although no formal expert testing was done, it has been postulated that these subjects were unlikely to be considered as having SS, especially without information about sicca symptoms. The two tests that performed the best across all comparison groups were the minor salivary gland biopsy as well as the anti-Ro/SSA and anti-La/SSB serology.
A comparison study by Le Goff et al. [68] revealed a sensitivity of 87.4% and a specificity of 95.4% for the current 2016 ACR-EULAR criteria when using the diagnosis of the physician as reference standard. In comparison, the 2002 AECG criteria showed a sensitivity and specificity of 82.2% and 98.1%, respectively. Most patients fulfilled the 2016 ACR-EULAR criteria, and the main reason for not also meeting the 2002 AECG criteria were absence of sicca symptoms, presence of either xerophthalmia or xerostomia but not both, and presence of only two other criteria including positive salivary gland biopsy or anti-Ro/SSA. In the overall cohort, two patients had anti-La/SSB, but not anti-Ro/SSA antibodies and therefore met the serological criterion of the 2002 AECG, but not the 2016 ACR-EULAR, classification system. However, these two patients did show typical features of pSS and fulfilled both AECG and ACR-EULAR criteria based on the other items.
4.3. How do the criteria sets affect the prevalence of secondary Sjögren's syndrome in RA patients?
One can argue that the addition of RF positivity and high-titer ANA as an alternative serological criterion might affect the frequency of SS detected in the context of RA as underlying autoimmune disease. The prevalence of secondary SS among RA patients has been studied over time (from 1987 to 2015) and appeared to be in the range of 3.6–55% [69]. The wide range of prevalence can be explained by the fact that different classification criteria have been used over time. In a recent study by Kim et al. [70] the prevalence of SS in RA patients has been investigated in the context of the different classification criteria. Among 827 RA patients, 72 patients (8.7%) were diagnosed with SS according to the physician and 7.3%, 6.3% and 6.8% fulfilled the 2002 AECG, 2012 ACR and 2016 ACR-EULAR classification criteria, respectively. Among the RF-positive patients, 9.0%, 8.3% and 8.1% were diagnosed with SS based on the 2002 AECG classification criteria, 2012 ACR and 2016 ACR-EULAR classification criteria, respectively. Taking ANA titers ≥1:80 into account, 13.9%, 12.7% and 13.6% met the 2002 AECG, 2012 ACR, and 2016 ACR-EULAR classification criteria for SS, respectively. Interestingly, among the anti-Ro/SSA antibody–positive RA patients, 57.4%, 54.4%, and 60.3% patients fulfilled the 2002 AECG, 2012 ACR, and 2016 ACR-EULAR classification criteria, respectively. In another study, RF positivity was found in approximately 40% of the patients with pSS, whereas almost none of the RA patients showed positivity for anti-Ro/SSA and anti-La/SSB antibodies. Patients with SS and RA showed a significantly higher percentage of ANA, anti-Ro/SSA and anti-La/SSB antibodies compared to RA patients, whereas no significant difference was observed between RA/SS and primary SS patients [58]. Altogether, applying different classification criteria does affect the prevalence of secondary SS in RA patients. Of all serological items included in the criteria sets, anti-La/SSB with anti-Ro/SSA antibodies appear to have the highest diagnostic specificity for SS.
4.4. Is it important to distinguish between anti-Ro52 and anti-Ro60?
The presence of anti-Ro/SSA antibody is a critical item in the current 2016 ACR-EULAR classification criteria [64]. Anti-Ro/SSA antibody, however, is not specific for SS and can be found in other systemic autoimmune diseases as well. A significant question would be whether it might be important to discriminate between anti-Ro52/TRIM21 and anti-Ro60/SSA antibodies. The positive predictive value (PPV) of prediagnostic anti-Ro60/SSA and to a lesser extent anti-Ro52/TRIM21 antibodies appear to be the highest among the most common antibodies found in pSS [37]. The diagnostic utility of separate detection of anti-Ro52/TRIM21 and anti-Ro60/SSA antibodies in autoimmune diseases has been investigated in various studies [43,[71], [72], [73], [74], [75], [76]]. Autoantibodies to Ro52/TRIM21 are more frequent, probably because the Ro52/TRIM21 antigen is more accessible and due to its ubiquitous nature. However, anti-Ro52/TRIM21 is not disease specific and prevalent in many systemic autoimmune diseases, and mostly shows no specific ANA immunofluorescence staining pattern on HEp2 cells [77,78]. Anti-Ro52/TRIM21 autoantibodies seem to be associated with myositis and to a lesser extent with SSc, whereas reactivity against both antigens and to a lesser extent against Ro60/SSA alone seems to associate with SS or SLE. Using the consensus of three different laboratory methods, the frequency of anti-Ro52/TRIM21 antibodies was similar to the frequency of anti-Ro60/SSA, except for the cohorts including patients with myositis (35.4% versus 0.0%) and systemic sclerosis (19.0% versus 6.0%) [79,80]. In a recent study, it was demonstrated that SLE was the most frequent diagnosis (48.5%) among patients showing anti-Ro60/SSA positivity and anti-Ro52/TRIM21 negativity [75]. This is supported by the fact that mice that lack the functional gene encoding Ro60/SSA develop a lupus-like syndrome with glomerulonephritis [81]. Primary SS is most likely in patients with both anti-Ro52/TRIM21 and anti-Ro60/SSA positivity [75]. Altogether, it can be concluded that specific testing for isolated anti-Ro52/TRIM21 antibodies during standard anti-ENA testing is of limited clinical value for SS. In contrast, separate anti-Ro52/TRIM21 and anti-Ro60/SSA determination may have added value for distinguishing between systemic autoimmune diseases.
4.5. Methods for serological detection of autoantibodies: important to take into consideration?
A variety of assays is available to detect anti-Ro/SSA antibodies, such as indirect immunofluorescence (IIF), precipitation assays (immunodiffusion), solid phase methods (ELISA, CLIA, FEIA, ALBIA) and immunoblot-based techniques (line immunoassay). The performance characteristics of available tests have been investigated in several studies and have yielded different results [[82], [83], [84], [85]]. Because of the low abundance of the Ro/SSA antigen, the classic HEp-2 substrate exhibits limited sensitivity for detection of isolated anti-Ro/SSA antibodies by indirect immunofluorescence. Therefore, HEp-2 cells transfected with Ro60/SSA cDNA have been developed, referred to as HEp-2000, yielding an increased sensitivity of 77% as defined by line immunoassay with recombinant Ro60/SSA and/or double immunodiffusion using natural Ro60/SSA [86]. In a study by Morozzi et al. the concordance rate for IIF using HEp-2000, immunodiffusion, ELISA and Western blot was in the range of 74–100% with respect to anti-Ro/SSA antibodies [82]. Although the overall performance appeared to be good, discrepancies were observed especially in terms of sensitivity. This can be explained, at least in part, by differences in the source of antigens used in the assays and possible conformational changes that occur during the assay resulting in loss of some antigen epitopes. Solid phase methods mostly contain an autoantigen mix including Ro52/TRIM21 and Ro60/SSA, either obtained by native purification or recombinant protein production, whereas line immunoassays more often allow individual detection of antibodies against Ro52/TRIM21 and Ro60/SSA. Recently, it has been described that especially the commercial and in-house immunoassays used for detection of anti-Ro52/TRIM21 differ in their sensitivity (48–79%). Only small differences in sensitivities were observed for anti-Ro60/SSA (69–77%) and anti-La/SSB (39–44%) immunoassays. Concordance rates of 65%, 79% and 73% for the anti-Ro52/TRIM21, anti-Ro60/SSA and anti-La/SSB assays were found, respectively. Higher sensitivities were found for commercial assays (line immunoassay, ELISA) when comparing to in-house assays (ELISA, peptide ELISA). Calculation of AUC revealed that FEIA, containing recombinant Ro52/TRIM21 and Ro60/SSA antigens showed the highest diagnostic accuracy of the assays tested [85]. To date, standardization and harmonization of autoantibody detection is a major challenge. Each patient produces a highly variable mixture of polyclonal antibodies that are unique in selectivity, affinity and avidity for the autoantigen. Besides, each in vitro diagnostic method has unique test characteristics [87,88]. Only limited attention has been paid to the impact of results obtained by different methods on for instance disease criteria. The diagnostic implications of especially the anti-Ro/SSA antibodies in for instance congenital heart block or neonatal lupus syndrome are very important and since separate anti-Ro52/TRIM21 and anti-Ro60/SSA determination can be helpful distinguishing features for systemic autoimmune diseases, it is recommended to use a combination of methods to maximize sensitivity/specificity in detecting anti-Ro/SSA antibodies and their subtypes anti-Ro52/TRIM21 and anti-Ro60/SSA.
5. Novel autoantibodies in Sjögren's syndrome
The detection of novel autoantibodies in SS has increased in the last years [89,90]. Many of the autoantibodies in SS, but also those in other connective tissue diseases, do not reach clinical diagnostics and the vast majority languish in the so-called autoantibody ‘death valley’ [91]. However, particular stages of disease, clinical phenotypes and long-term complications such as lymphoma may be associated with specific (combinations of) autoantibodies (Table 2). In a recent study by Martin-Nares et al. [90] novel antibodies have been evaluated for their usefulness as diagnostic tool, pathogenic role, identification of a clinical phenotype and as predictors of an overlap syndrome in SS patients. Three novel autoantibodies are considered to have added value to the diagnostics of SS patients, namely anti-salivary protein 1 (anti-SP1), anti-carbonic anhydrase VI (anti-CA6), and anti-parotid secretory protein (anti-PSP). In Il14a transgenic mice, an experimental model of Sjögren's disease, Shen et al. observed that these autoantibodies were present and could be detected earlier in the course of the disease than anti-Ro/SSA or anti-La/SSB antibodies. Moreover, these autoantibodies were more prevalent in SS patients negative for anti-Ro/SSA antibodies or in relatively newly diagnosed patients with sicca symptoms. Based on these data, anti-SP1, anti-CA6 and anti-PSP are considered as early biomarkers for SS [92]. Moreover, autoantibodies to nuclear autoantigen 14 (NA-14) are considered as a possible diagnostic marker useful for discriminating between primary versus secondary SS [93,94]. Of particular interest, autoantibodies against cofilin-1, alpha-enolase and Rho GDP-dissociation inhibitor 2 (RGI2) are considered as possible predictors of malignant lymphoma. The combination of these three autoantibodies led to an AUC value of 0.99 with a 95% sensitivity and 94% specificity in distinguishing pSS/MALT patients from healthy individuals and an AUC value of 0.86 with a 75% sensitivity and 94% specificity in distinguishing pSS/MALT patients from pSS patients [95]. Autoantibodies that have been predictors of an overlapping autoimmune condition include anti-centromere (Raynaud's phenomenon), anti-mitochondrial (liver involvement) and anti-aquaporine-4 (NMOSD). Moreover, anti-carbonic anhydrase 2 antibodies have been associated with renal tubular acidosis [40,42,[96], [97], [98], [99], [100]].
Table 2.
Traditional and novel autoantibodies in SS.
| Autoantibodies | Diagnostic characteristics and/or clinical association | References |
|---|---|---|
| Anti-Ro/SSA, anti-La/SSB | Earlier disease onset, salivary gland lymphocytic infiltration and dysfunction, extra-glandular manifestations, hypergammaglobulinemia, ANA, RF, neonatal lupus-congenital heart block | [39,42,55] |
| Antinuclear antibody | Early stage of the disease, hypergammaglobulinemia, anti-Ro/SSA, anti-La/SSB, RF, extraglandular manifestations | [40,42] |
| Rheumatoid factor | Early stage of the disease, hypergammaglobulinemia, anti-Ro/SSA, anti-La/SSB, ANA, hypocomplementemia, extra-glandular manifestations | [41,42,59,60] |
| Anti-SP-1, anti-CA6, anti-PSP | Early disease, anti-Ro/SSA and anti-La/SSB negative | [92] |
| Anti-NA14 | Primary SS » secondary SS, shorter disease duration | [93,94] |
| Anti-cofilin-1, anti-alpha-enolase, anti-RGI2 | MALT lymphoma | [95] |
| Anti-AQ4 | NMOSD | [98] |
| Anti-mitochondrial | Liver involvement | [40,42,97] |
| Anti-centromere | Higher mean age at disease onset, Raynaud's phenomenon, lower prevalence of anti-Ro/SAA, anti-La/SSB | [99,100] |
| Anti-carbonic anhydrase | Renal tubular acidosis | [96] |
| Anti-IFI16 | Severe disease, higher focus score, germinal center-like structures and high-titer ANA, abnormal Schirmer's test, hyperglobulinemia | [101] |
| Anti-MDM2 | Longer disease duration, higher disease activity index (ESSDAI), hyperglobulinemia | [103] |
| Anti-NR2 | Memory dysfunction and depression | [104] |
ANA = antinuclear antibodies; RF = rheumatoid factor; MALT = mucosa-associated lymphoid tissue; NMOSD = neuromyelitis optica spectrum disorder; ESSDAI = European Sjögren's syndrome disease activity index.
Autoantibodies that have not been reported as useful diagnostic tool yet, but may have added value for phenotype identification include autoantibodies against interferon-inducible protein-16 (IFI16), NR2 and mouse double minute 2 (MDM2). Anti-IFI16 antibodies have been associated with markers of a more severe disease such as higher focus score, germinal center-like structures and high-titer ANA [101]; however, controversy exists [102]. Moreover, pSS patients with anti-MDM2 antibodies were characterized by a longer disease duration and titers were positively associated with the European Sjögren's syndrome disease activity index (ESSDAI) and level of IgG in serum [103]. In a study by Lauvsnes et al., anti-NR2 antibodies have been detected in serum (20%) and cerebrospinal fluid (12%) of SS patients and appeared to be associated with memory dysfunction and depression in a subgroup of patients [104]. Altogether, insight in the plethora of autoantibodies detectable in pSS patients may be useful in diagnostics and future classification of disease subgroups as well as risk stratification.
6. Conclusions
Autoantibodies are important biomarkers for diagnosis and classification of autoimmune diseases, including Sjögren's syndrome. Sjögren's syndrome is characterized by a variety of autoantibodies among which the anti-Ro/SSA is part of the current 2016 ACR/EULAR classification criteria. Although standardization and harmonization in autoantibody detection is still challenging and future large-scale validation studies are still needed for the novel antibodies present, it would be interesting to consider the development of subcriteria for pSS based on the extended serological antibody profiles as it would allow identification of subgroups of patients, thereby contributing to tailored treatment strategies for this heterogeneous disease.
Credit author statement
Sharon Veenbergen: Writing – original draft; Ana Kozmar: Writing - Reviewing and editing; Paul L.A. van Daele: Writing - Reviewing and editing; Marco W.J. Schreurs: Writing – original draft, Writing - Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Maślińska M., Przygodzka M., Kwiatkowska B., Sikorska-Siudek K. Sjögren's syndrome: still not fully understood disease. Rheumatol. Int. 2015;35:233–241. doi: 10.1007/s00296-014-3072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mavragani C.P., Moutsopoulos H.M. The geoepidemiology of Sjögren's syndrome. Autoimmun. Rev. 2010;9:A305–A310. doi: 10.1016/j.autrev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Both T., Dalm V.A., van Hagen P.M., van Daele P.L. Reviewing primary Sjögren's syndrome: beyond the dryness - from pathophysiology to diagnosis and treatment. Int. J. Med. Sci. 2017;14:191–200. doi: 10.7150/ijms.17718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narváez J., Sánchez-Fernández S., Seoane-Mato D., Díaz-González F., Bustabad S. Prevalence of Sjögren’s syndrome in the general adult population in Spain: estimating the proportion of undiagnosed cases. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-67462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lessard C.J., Li H., Adrianto I., Ice J.A., Rasmussen A., Grundahl K.M., Kelly J.A., Dozmorov M.G., Miceli-Richard C., Bowman S., Lester S., Eriksson P., Eloranta M.L., Brun J.G., Gøransson L.G., Harboe E., Guthridge J.M., Kaufman K.M., Kvarnström M., Jazebi H., Cunninghame Graham D.S., Grandits M.E., Nazmul-Hossain A.N., Patel K., Adler A.J., Maier-Moore J.S., Farris A.D., Brennan M.T., Lessard J.A., Chodosh J., Gopalakrishnan R., Hefner K.S., Houston G.D., Huang A.J., Hughes P.J., Lewis D.M., Radfar L., Rohrer M.D., Stone D.U., Wren J.D., Vyse T.J., Gaffney P.M., James J.A., Omdal R., Wahren-Herlenius M., Illei G.G., Witte T., Jonsson R., Rischmueller M., Rönnblom L., Nordmark G., Ng W.F., Registry U.K.P.S.s.S, Mariette X., Anaya J.M., Rhodus N.L., Segal B.M., Scofield R.H., Montgomery C.G., Harley J.B., Sivils K.L. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren's syndrome. Nat. Genet. 2013;45:1284–1292. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manoussakis M.N., Kapsogeorgou E.K. The role of epithelial cells in the pathogenesis of Sjögren's syndrome. Clin. Rev. Allergy Immunol. 2007;32:225–230. doi: 10.1007/s12016-007-8007-4. [DOI] [PubMed] [Google Scholar]
- 7.Nocturne G., Mariette X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat. Rev. Rheumatol. 2013;9:544–556. doi: 10.1038/nrrheum.2013.110. [DOI] [PubMed] [Google Scholar]
- 8.Kroese F.G., Abdulahad W.H., Haacke E., Bos N.A., Vissink A., Bootsma H. B-cell hyperactivity in primary Sjögren's syndrome. Expet Rev. Clin. Immunol. 2014;10:483–499. doi: 10.1586/1744666X.2014.891439. [DOI] [PubMed] [Google Scholar]
- 9.Mielle J., Tison A., Cornec D., Le Pottier L., Daien C., Pers J.O. B cells in Sjögren’s syndrome: from pathophysiology to therapeutic target. Rheumatology. 2021;60:2545–2560. doi: 10.1093/rheumatology/key332. [DOI] [PubMed] [Google Scholar]
- 10.Fox R.I. Sjögren's syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 11.Corsiero E., Bombardieri M., Manzo A., Bugatti S., Uguccioni M., Pitzalis C. Role of lymphoid chemokines in the development of functional ectopic lymphoid structures in rheumatic autoimmune diseases. Immunol. Lett. 2012;145:62–67. doi: 10.1016/j.imlet.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Salomonsson S., Larsson P., Tengnér P., Mellquist E., Hjelmström P., Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren's syndrome. Scand. J. Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 13.Kramer J.M., Klimatcheva E., Rothstein T.L. CXCL13 is elevated in Sjögren's syndrome in mice and humans and is implicated in disease pathogenesis. J. Leukoc. Biol. 2013;94:1079–1089. doi: 10.1189/jlb.0113036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen C.D., Ansel K.M., Low C., Lesley R., Tamamura H., Fujii N., Cyster J.G. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 15.Salomonsson S., Jonsson M.V., Skarstein K., Brokstad K.A., Hjelmström P., Wahren-Herlenius M., Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum. 2003;48:3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 16.Badr G., Borhis G., Lefevre E.A., Chaoul N., Deshayes F., Dessirier V., Lapree G., Tsapis A., Richard Y. BAFF enhances chemotaxis of primary human B cells: a particular synergy between BAFF and CXCL13 on memory B cells. Blood. 2008;111:2744–2754. doi: 10.1182/blood-2007-03-081232. [DOI] [PubMed] [Google Scholar]
- 17.Ittah M., Miceli-Richard C., Eric Gottenberg J., Lavie F., Lazure T., Ba N., Sellam J., Lepajolec C., Mariette X. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren's syndrome. Arthritis Res. Ther. 2006;8:R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay F., Schneider P. Cracking the BAFF code. Nat. Rev. Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 19.Mackay F., Woodcock S.A., Lawton P., Ambrose C., Baetscher M., Schneider P., Tschopp J., Browning J.L. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariette X., Roux S., Zhang J., Bengoufa D., Lavie F., Zhou T., Kimberly R. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjögren's syndrome. Ann. Rheum. Dis. 2003;62:168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonsson M.V., Szodoray P., Jellestad S., Jonsson R., Skarstein K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjögren's syndrome. J. Clin. Immunol. 2005;25:189–201. doi: 10.1007/s10875-005-4091-5. [DOI] [PubMed] [Google Scholar]
- 22.Rawlings D.J., Schwartz M.A., Jackson S.W., Meyer-Bahlburg A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat. Rev. Immunol. 2012;12:282–294. doi: 10.1038/nri3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsen M., Jonsson R., Brun J.G., Appel S., Hansen T. TLR-7 and -9 stimulation of peripheral blood B cells indicate altered TLR signalling in primary Sjögren's syndrome patients by increased secretion of cytokines. Scand. J. Immunol. 2015;82:523–531. doi: 10.1111/sji.12368. [DOI] [PubMed] [Google Scholar]
- 24.Goules A., Tzioufas A.G., Manousakis M.N., Kirou K.A., Crow M.K., Routsias J.G. Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. J. Autoimmun. 2006;26:165–171. doi: 10.1016/j.jaut.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger R., Sims G.P., Fairhurst A.M., Robbins R., da Silva Y.S., Spolski R., Leonard W.J., Lipsky P.E. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 26.Kang K.Y., Kim H.O., Kwok S.K., Ju J.H., Park K.S., Sun D.I., Jhun J.Y., Oh H.J., Park S.H., Kim H.Y. Impact of interleukin-21 in the pathogenesis of primary Sjögren's syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res. Ther. 2011;13:R179. doi: 10.1186/ar3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maehara T., Moriyama M., Hayashida J.N., Tanaka A., Shinozaki S., Kubo Y., Matsumura K., Nakamura S. Selective localization of T helper subsets in labial salivary glands from primary Sjögren's syndrome patients. Clin. Exp. Immunol. 2012;169:89–99. doi: 10.1111/j.1365-2249.2012.04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamza N., Bootsma H., Yuvaraj S., Spijkervet F.K., Haacke E.A., Pollard R.P., Visser A., Vissink A., Kallenberg C.G., Kroese F.G., Bos N.A. Persistence of immunoglobulin-producing cells in parotid salivary glands of patients with primary Sjögren's syndrome after B cell depletion therapy. Ann. Rheum. Dis. 2012;71:1881–1887. doi: 10.1136/annrheumdis-2011-201189. [DOI] [PubMed] [Google Scholar]
- 29.Voulgarelis M., Dafni U.G., Isenberg D.A., Moutsopoulos H.M. Malignant lymphoma in primary Sjögren's syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren's Syndrome. Arthritis Rheum. 1999;42:1765–1772. doi: 10.1002/1529-0131(199908)42:8<1765::AID-ANR28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Zintzaras E., Voulgarelis M., Moutsopoulos H.M. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch. Intern. Med. 2005;165:2337–2344. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 31.Theander E., Vasaitis L., Baecklund E., Nordmark G., Warfvinge G., Liedholm R., Brokstad K., Jonsson R., Jonsson M.V. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren's syndrome. Ann. Rheum. Dis. 2011;70:1363–1368. doi: 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Risselada A.P., Looije M.F., Kruize A.A., Bijlsma J.W., van Roon J.A. The role of ectopic germinal centers in the immunopathology of primary Sjögren's syndrome: a systematic review. Semin. Arthritis Rheum. 2013;42:368–376. doi: 10.1016/j.semarthrit.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Quartuccio L., Isola M., Baldini C., Priori R., Bartoloni Bocci E., Carubbi F., Maset M., Gregoraci G., Della Mea V., Salvin S., De Marchi G., Luciano N., Colafrancesco S., Alunno A., Giacomelli R., Gerli R., Valesini G., Bombardieri S., De Vita S. Biomarkers of lymphoma in Sjögren's syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J. Autoimmun. 2014;51:75–80. doi: 10.1016/j.jaut.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Casals M., Brito-Zerón P., Yagüe J., Akasbi M., Bautista R., Ruano M., Claver G., Gil V., Font J. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren's syndrome. Rheumatology. 2005;44:89–94. doi: 10.1093/rheumatology/keh407. [DOI] [PubMed] [Google Scholar]
- 35.Solans-Laqué R., López-Hernandez A., Bosch-Gil J.A., Palacios A., Campillo M., Vilardell-Tarres M. Risk, predictors, and clinical characteristics of lymphoma development in primary Sjögren's syndrome. Semin. Arthritis Rheum. 2011;41:415–423. doi: 10.1016/j.semarthrit.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Scofield R.H. Autoantibodies as predictors of disease. Lancet. 2004;363:1544–1546. doi: 10.1016/S0140-6736(04)16154-0. [DOI] [PubMed] [Google Scholar]
- 37.Theander E., Jonsson R., Sjöström B., Brokstad K., Olsson P., Henriksson G. Prediction of Sjögren's syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling. Arthritis Rheumatol. 2015;67:2427–2436. doi: 10.1002/art.39214. [DOI] [PubMed] [Google Scholar]
- 38.Popescu M.R., Dudu A., Jurcut C., Ciobanu A.M., Zagrean A.M., Panaitescu A.M. A broader perspective on anti-ro antibodies and their fetal consequences-A case report and literature review. Diagnostics. 2020;10 doi: 10.3390/diagnostics10070478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambrosi A., Wahren-Herlenius M. Congenital heart block: evidence for a pathogenic role of maternal autoantibodies. Arthritis Res. Ther. 2012;14:208. doi: 10.1186/ar3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nardi N., Brito-Zerón P., Ramos-Casals M., Aguiló S., Cervera R., Ingelmo M., Font J. Circulating auto-antibodies against nuclear and non-nuclear antigens in primary Sjögren's syndrome: prevalence and clinical significance in 335 patients. Clin. Rheumatol. 2006;25:341–346. doi: 10.1007/s10067-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 41.Huo A.P., Lin K.C., Chou C.T. Predictive and prognostic value of antinuclear antibodies and rheumatoid factor in primary Sjogren's syndrome. Int J Rheum Dis. 2010;13:39–47. doi: 10.1111/j.1756-185X.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 42.Bournia V.K., Vlachoyiannopoulos P.G. Subgroups of Sjögren syndrome patients according to serological profiles. J. Autoimmun. 2012;39:15–26. doi: 10.1016/j.jaut.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Lee A.Y.S., Reed J.H., Gordon T.P. Anti-Ro60 and anti-Ro52/TRIM21: two distinct autoantibodies in systemic autoimmune diseases. J. Autoimmun. 2021;124:102724. doi: 10.1016/j.jaut.2021.102724. [DOI] [PubMed] [Google Scholar]
- 44.Oke V., Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J. Autoimmun. 2012;39:77–82. doi: 10.1016/j.jaut.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Espinosa A., Zhou W., Ek M., Hedlund M., Brauner S., Popovic K., Horvath L., Wallerskog T., Oukka M., Nyberg F., Kuchroo V.K., Wahren-Herlenius M. The Sjogren's syndrome-associated autoantigen Ro52 is an E3 ligase that regulates proliferation and cell death. J. Immunol. 2006;176:6277–6285. doi: 10.4049/jimmunol.176.10.6277. [DOI] [PubMed] [Google Scholar]
- 46.Strandberg L., Ambrosi A., Espinosa A., Ottosson L., Eloranta M.L., Zhou W., Elfving A., Greenfield E., Kuchroo V.K., Wahren-Herlenius M. Interferon-alpha induces up-regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52-specific monoclonal antibodies. J. Clin. Immunol. 2008;28:220–231. doi: 10.1007/s10875-007-9157-0. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimi R., Chang T.H., Wang H., Atsumi T., Morse H.C., 3rd, Ozato K. Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts. J. Immunol. 2009;182:7527–7538. doi: 10.4049/jimmunol.0804121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgs R., Lazzari E., Wynne C., N G J., Espinosa A., Wahren-Herlenius M., Jefferies C.A. Self protection from anti-viral responses-Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral Toll-Like receptors. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jauharoh S.N., Saegusa J., Sugimoto T., Ardianto B., Kasagi S., Sugiyama D., Kurimoto C., Tokuno O., Nakamachi Y., Kumagai S., Kawano S. SS-A/Ro52 promotes apoptosis by regulating Bcl-2 production. Biochem. Biophys. Res. Commun. 2012;417:582–587. doi: 10.1016/j.bbrc.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Chen X., Smith J.D., Shi H., Yang D.D., Flavell R.A., Wolin S.L. The Ro autoantigen binds misfolded U2 small nuclear RNAs and assists mammalian cell survival after UV irradiation. Curr. Biol. 2003;13:2206–2211. doi: 10.1016/j.cub.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 51.Slobbe R.L., Pluk W., van Venrooij W.J., Pruijn G.J. Ro ribonucleoprotein assembly in vitro. Identification of RNA-protein and protein-protein interactions. J. Mol. Biol. 1992;227:361–366. doi: 10.1016/0022-2836(92)90890-v. [DOI] [PubMed] [Google Scholar]
- 52.Mahony R., Broadbent L., Maier-Moore J.S., Power U.F., Jefferies C.A. The RNA binding protein La/SS-B promotes RIG-I-mediated type I and type III IFN responses following Sendai viral infection. Sci. Rep. 2017;7:14537. doi: 10.1038/s41598-017-15197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y., Tan H., Tian H., Liang C., Chen S., Liu Q. Autoantigen La promotes efficient RNAi, antiviral response, and transposon silencing by facilitating multiple-turnover RISC catalysis. Mol. Cell. 2011;44:502–508. doi: 10.1016/j.molcel.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bitko V., Musiyenko A., Bayfield M.A., Maraia R.J., Barik S. Cellular La protein shields nonsegmented negative-strand RNA viral leader RNA from RIG-I and enhances virus growth by diverse mechanisms. J. Virol. 2008;82:7977–7987. doi: 10.1128/JVI.02762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernández-Molina G., Leal-Alegre G., Michel-Peregrina M. The meaning of anti-Ro and anti-La antibodies in primary Sjögren's syndrome. Autoimmun. Rev. 2011;10:123–125. doi: 10.1016/j.autrev.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Peen E., Mellbye O.J., Haga H.J. IgA rheumatoid factor in primary Sjogren's syndrome. Scand. J. Rheumatol. 2009;38:46–49. doi: 10.1080/03009740802366043. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y., Hou Z., Qiu M., Ye Q. Risk factors for primary Sjögren syndrome-associated interstitial lung disease. J. Thorac. Dis. 2018;10:2108–2117. doi: 10.21037/jtd.2018.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang H., Bian S., Chen H., Wang L., Zhao L., Zhang X., Zhao Y., Zeng X., Zhang F. Clinical characteristics and risk factors for overlapping rheumatoid arthritis and Sjögren's syndrome. Sci. Rep. 2018;8:6180. doi: 10.1038/s41598-018-24279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martel C., Gondran G., Launay D., Lalloué F., Palat S., Lambert M., Ly K., Loustaud-Ratti V., Bezanahary H., Hachulla E., Jauberteau M.O., Vidal E., Hatron P.Y., Fauchais A.L. Active immunological profile is associated with systemic Sjögren's syndrome. J. Clin. Immunol. 2011;31:840–847. doi: 10.1007/s10875-011-9553-3. [DOI] [PubMed] [Google Scholar]
- 60.Ramos-Casals M., Solans R., Rosas J., Camps M.T., Gil A., Del Pino-Montes J., Calvo-Alen J., Jiménez-Alonso J., Micó M.L., Beltrán J., Belenguer R., Pallarés L., Group G.S. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltim.) 2008;87:210–219. doi: 10.1097/MD.0b013e318181e6af. [DOI] [PubMed] [Google Scholar]
- 61.Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H.M., Alexander E.L., Carsons S.E., Daniels T.E., Fox P.C., Fox R.I., Kassan S.S., Pillemer S.R., Talal N., Weisman M.H., European Study S. Group on Classification Criteria for Sjögren's, Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiboski S.C., Shiboski C.H., Criswell L., Baer A., Challacombe S., Lanfranchi H., Schiødt M., Umehara H., Vivino F., Zhao Y., Dong Y., Greenspan D., Heidenreich A.M., Helin P., Kirkham B., Kitagawa K., Larkin G., Li M., Lietman T., Lindegaard J., McNamara N., Sack K., Shirlaw P., Sugai S., Vollenweider C., Whitcher J., Wu A., Zhang S., Zhang W., Greenspan J., Daniels T., Sjögren's International Collaborative Clinical Alliance Research G. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort. Arthritis Care Res. 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vitali C., Bootsma H., Bowman S.J., Dorner T., Gottenberg J.E., Mariette X., Ramos-Casals M., Ravaud P., Seror R., Theander E., Tzioufas A.G. Classification criteria for Sjogren's syndrome: we actually need to definitively resolve the long debate on the issue. Ann. Rheum. Dis. 2013;72:476–478. doi: 10.1136/annrheumdis-2012-202565. [DOI] [PubMed] [Google Scholar]
- 64.Shiboski C.H., Shiboski S.C., Seror R., Criswell L.A., Labetoulle M., Lietman T.M., Rasmussen A., Scofield H., Vitali C., Bowman S.J., Mariette X. G. International Sjögren's syndrome criteria working, 2016 American College of Rheumatology/European League against rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69:35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baer A.N., McAdams DeMarco M., Shiboski S.C., Lam M.Y., Challacombe S., Daniels T.E., Dong Y., Greenspan J.S., Kirkham B.W., Lanfranchi H.E., Schiødt M., Srinivasan M., Umehara H., Vivino F.B., Vollenweider C.F., Zhao Y., Criswell L.A., Shiboski C.H., Sjögren's International Collaborative Clinical Alliance Research G. The SSB-positive/SSA-negative antibody profile is not associated with key phenotypic features of Sjögren's syndrome. Ann. Rheum. Dis. 2015;74:1557–1561. doi: 10.1136/annrheumdis-2014-206683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasmussen A., Ice J.A., Li H., Grundahl K., Kelly J.A., Radfar L., Stone D.U., Hefner K.S., Anaya J.M., Rohrer M., Gopalakrishnan R., Houston G.D., Lewis D.M., Chodosh J., Harley J.B., Hughes P., Maier-Moore J.S., Montgomery C.G., Rhodus N.L., Farris A.D., Segal B.M., Jonsson R., Lessard C.J., Scofield R.H., Sivils K.L. Comparison of the American-European Consensus Group Sjogren's syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann. Rheum. Dis. 2014;73:31–38. doi: 10.1136/annrheumdis-2013-203845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cornec D., Saraux A., Cochener B., Pers J.O., Jousse-Joulin S., Renaudineau Y., Marhadour T., Devauchelle-Pensec V. Level of agreement between 2002 American-European Consensus Group and 2012 American College of Rheumatology classification criteria for Sjögren's syndrome and reasons for discrepancies. Arthritis Res. Ther. 2014;16:R74. doi: 10.1186/ar4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le Goff M., Cornec D., Jousse-Joulin S., Guellec D., Costa S., Marhadour T., Le Berre R., Genestet S., Cochener B., Boisrame-Gastrin S., Renaudineau Y., Pers J.O., Saraux A., Devauchelle-Pensec V. Comparison of 2002 AECG and 2016 ACR/EULAR classification criteria and added value of salivary gland ultrasonography in a patient cohort with suspected primary Sjögren's syndrome. Arthritis Res. Ther. 2017;19:269. doi: 10.1186/s13075-017-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alani H., Henty J.R., Thompson N.L., Jury E., Ciurtin C. Systematic review and meta-analysis of the epidemiology of polyautoimmunity in Sjögren's syndrome (secondary Sjögren's syndrome) focusing on autoimmune rheumatic diseases. Scand. J. Rheumatol. 2018;47:141–154. doi: 10.1080/03009742.2017.1324909. [DOI] [PubMed] [Google Scholar]
- 70.Kim H., Cho S.K., Kim H.W., Han J., Kim Y., Hwang K.G., Sung Y.K. The prevalence of Sjögren's syndrome in rheumatoid arthritis patients and their clinical features. J. Kor. Med. Sci. 2020;35:e369. doi: 10.3346/jkms.2020.35.e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Defendenti C., Atzeni F., Spina M.F., Grosso S., Cereda A., Guercilena G., Bollani S., Saibeni S., Puttini P.S. Clinical and laboratory aspects of Ro/SSA-52 autoantibodies. Autoimmun. Rev. 2011;10:150–154. doi: 10.1016/j.autrev.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Dugar M., Cox S., Limaye V., Gordon T.P., Roberts-Thomson P.J. Diagnostic utility of anti-Ro52 detection in systemic autoimmunity. Postgrad. Med. 2010;86:79–82. doi: 10.1136/pgmj.2009.089656. [DOI] [PubMed] [Google Scholar]
- 73.Ghillani P., André C., Toly C., Rouquette A.M., Bengoufa D., Nicaise P., Goulvestre C., Gleizes A., Dragon-Durey M.A., Alyanakian M.A., Chretien P., Chollet-Martin S., Musset L., Weill B., Johanet C. Clinical significance of anti-Ro52 (TRIM21) antibodies non-associated with anti-SSA 60kDa antibodies: results of a multicentric study. Autoimmun. Rev. 2011;10:509–513. doi: 10.1016/j.autrev.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Menéndez A., Gómez J., Escanlar E., Caminal-Montero L., Mozo L. Clinical associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies: diagnostic utility of their separate detection. Autoimmunity. 2013;46:32–39. doi: 10.3109/08916934.2012.732131. [DOI] [PubMed] [Google Scholar]
- 75.Robbins A., Hentzien M., Toquet S., Didier K., Servettaz A., Pham B.N., Giusti D. Diagnostic utility of separate anti-ro60 and anti-ro52/TRIM21 antibody detection in autoimmune diseases. Front. Immunol. 2019;10:444. doi: 10.3389/fimmu.2019.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakamura H., Morimoto S., Shimizu T., Takatani A., Nishihata S.Y., Kawakami A. Clinical manifestations in anti-Ro52/SS-A antibody-seropositive patients with Sjögren's syndrome. Immunol Med. 2021:1–11. doi: 10.1080/25785826.2021.1919342. [DOI] [PubMed] [Google Scholar]
- 77.Peene I., Meheus L., De Keyser S., Humbel R., Veys E.M., De Keyser F. Anti-Ro52 reactivity is an independent and additional serum marker in connective tissue disease. Ann. Rheum. Dis. 2002;61:929–933. doi: 10.1136/ard.61.10.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Langguth D.M., Morris S., Clifford L., Wilson R.J., Neil J., Hogan P.G., Wong R.C. Specific testing for "isolated" anti-52 kDa SSA/Ro antibodies during standard anti-extractable nuclear antigen testing is of limited clinical value. J. Clin. Pathol. 2007;60:670–673. doi: 10.1136/jcp.2006.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rutjes S.A., Vree Egberts W.T., Jongen P., Van Den Hoogen F., Pruijn G.J., Van Venrooij W.J. Anti-Ro52 antibodies frequently co-occur with anti-Jo-1 antibodies in sera from patients with idiopathic inflammatory myopathy. Clin. Exp. Immunol. 1997;109:32–40. doi: 10.1046/j.1365-2249.1997.4081308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schulte-Pelkum J., Fritzler M., Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun. Rev. 2009;8:632–637. doi: 10.1016/j.autrev.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 81.Xue D., Shi H., Smith J.D., Chen X., Noe D.A., Cedervall T., Yang D.D., Eynon E., Brash D.E., Kashgarian M., Flavell R.A., Wolin S.L. A lupus-like syndrome develops in mice lacking the Ro 60-kDa protein, a major lupus autoantigen. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7503–7508. doi: 10.1073/pnas.0832411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morozzi G., Bellisai F., Simpatico A., Pucci G., Bacarelli M.R., Campanella V., Marcolongo R., Galeazzi M. Comparison of different methods for the detection of anti-Ro/SSA antibodies in connective tissue diseases. Clin. Exp. Rheumatol. 2000;18:729–731. [PubMed] [Google Scholar]
- 83.Manoussakis M.N., Kistis K.G., Liu X., Aidinis V., Guialis A., Moutsopoulos H.M. Detection of anti-Ro(SSA) antibodies in autoimmune diseases: comparison of five methods. Br. J. Rheumatol. 1993;32:449–455. doi: 10.1093/rheumatology/32.6.449. [DOI] [PubMed] [Google Scholar]
- 84.Burbelo P.D., Ching K.H., Issa A.T., Loftus C.M., Li Y., Satoh M., Reeves W.H., Iadarola M.J. Rapid serological detection of autoantibodies associated with Sjögren's syndrome. J. Transl. Med. 2009;7:83. doi: 10.1186/1479-5876-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trier N.H., Nielsen I., Friis T., Houen G., Theander E. Comparison of antibody assays for detection of autoantibodies to Ro 52, Ro 60 and La associated with primary Sjögren's syndrome. J. Immunol. Methods. 2016;433:44–50. doi: 10.1016/j.jim.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Peene I., Van Ael W., Vandenbossche M., Vervaet T., Veys E., De Keyser F. Sensitivity of the HEp-2000 substrate for the detection of anti-SSA/Ro60 antibodies. Clin. Rheumatol. 2000;19:291–295. doi: 10.1007/s100670070048. [DOI] [PubMed] [Google Scholar]
- 87.Jacobs J.F.M., Bossuyt X. Standardization and harmonization of autoimmune diagnostics. Clin. Chem. Lab. Med. 2018;56:1563–1567. doi: 10.1515/cclm-2018-0807. [DOI] [PubMed] [Google Scholar]
- 88.Rönnelid J. The choice of laboratory methodology influences autoantibody test results. Front. Immunol. 2015;6:392. doi: 10.3389/fimmu.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fayyaz A., Kurien B.T., Scofield R.H. Autoantibodies in Sjögren's syndrome. Rheum. Dis. Clin. N. Am. 2016;42:419–434. doi: 10.1016/j.rdc.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martín-Nares E., Hernández-Molina G. Novel autoantibodies in Sjögren's syndrome: a comprehensive review. Autoimmun. Rev. 2019;18:192–198. doi: 10.1016/j.autrev.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 91.Fritzler M.J., Choi M.Y., Satoh M., Mahler M. Autoantibody discovery, assay development and adoption: death Valley, the sea of survival and beyond. Front. Immunol. 2021;12:679613. doi: 10.3389/fimmu.2021.679613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen L., Suresh L., Lindemann M., Xuan J., Kowal P., Malyavantham K., Ambrus J.L., Jr. Novel autoantibodies in Sjogren's syndrome. Clin. Immunol. 2012;145:251–255. doi: 10.1016/j.clim.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 93.Nozawa K., Ikeda K., Satoh M., Reeves W.H., Stewart C.M., Li Y.C., Yen T.J., Rios R.M., Takamori K., Ogawa H., Sekigawa I., Takasaki Y., Chan E.K. Autoantibody to NA14 is an independent marker primarily for Sjogren's syndrome. Front Biosci (Landmark Ed) 2009;14:3733–3739. doi: 10.2741/3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uomori K., Nozawa K., Ikeda K., Doe K., Yamada Y., Yamaguchi A., Fujishiro M., Kawasaki M., Morimoto S., Takamori K., Sekigawa I., Chan E.K., Takasaki Y. A re-evaluation of anti-NA-14 antibodies in patients with primary Sjögren's syndrome: significant role of interferon-γ in the production of autoantibodies against NA-14. Autoimmunity. 2016;49:347–356. doi: 10.1080/08916934.2016.1196676. [DOI] [PubMed] [Google Scholar]
- 95.Cui L., Elzakra N., Xu S., Xiao G.G., Yang Y., Hu S. Investigation of three potential autoantibodies in Sjogren's syndrome and associated MALT lymphoma. Oncotarget. 2017;8:30039–30049. doi: 10.18632/oncotarget.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takemoto F., Hoshino J., Sawa N., Tamura Y., Tagami T., Yokota M., Katori H., Yokoyama K., Ubara Y., Hara S., Takaichi K., Yamada A., Uchida S. Autoantibodies against carbonic anhydrase II are increased in renal tubular acidosis associated with Sjogren syndrome. Am. J. Med. 2005;118:181–184. doi: 10.1016/j.amjmed.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 97.Hatzis G.S., Fragoulis G.E., Karatzaferis A., Delladetsima I., Barbatis C., Moutsopoulos H.M. Prevalence and longterm course of primary biliary cirrhosis in primary Sjögren's syndrome. J. Rheumatol. 2008;35:2012–2016. [PubMed] [Google Scholar]
- 98.Birnbaum J., Atri N.M., Baer A.N., Cimbro R., Montagne J., Casciola-Rosen L. Relationship between neuromyelitis optica spectrum disorder and Sjögren's syndrome: central nervous system extraglandular disease or unrelated, Co-occurring autoimmunity? Arthritis Care Res. 2017;69:1069–1075. doi: 10.1002/acr.23107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salliot C., Gottenberg J.E., Bengoufa D., Desmoulins F., Miceli-Richard C., Mariette X. Anticentromere antibodies identify patients with Sjögren's syndrome and autoimmune overlap syndrome. J. Rheumatol. 2007;34:2253–2258. [PubMed] [Google Scholar]
- 100.Katano K., Kawano M., Koni I., Sugai S., Muro Y. Clinical and laboratory features of anticentromere antibody positive primary Sjögren's syndrome. J. Rheumatol. 2001;28:2238–2244. [PubMed] [Google Scholar]
- 101.Baer A.N., Petri M., Sohn J., Rosen A., Casciola-Rosen L. Association of antibodies to interferon-inducible protein-16 with markers of more severe disease in primary Sjögren's syndrome. Arthritis Care Res. 2016;68:254–260. doi: 10.1002/acr.22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alunno A., Caneparo V., Carubbi F., Bistoni O., Caterbi S., Bartoloni E., Giacomelli R., Gariglio M., Landolfo S., Gerli R. Interferon gamma-inducible protein 16 in primary Sjögren's syndrome: a novel player in disease pathogenesis? Arthritis Res. Ther. 2015;17:208. doi: 10.1186/s13075-015-0722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Y., Liao X., Wang Y., Chen S., Sun Y., Lin Q., Shi G. Autoantibody to MDM2: a potential serological marker of primary Sjogren's syndrome. Oncotarget. 2017;8:14306–14313. doi: 10.18632/oncotarget.14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lauvsnes M.B., Maroni S.S., Appenzeller S., Beyer M.K., Greve O.J., Kvaløy J.T., Harboe E., Gøransson L.G., Tjensvoll A.B., Omdal R. Memory dysfunction in primary Sjögren's syndrome is associated with anti-NR2 antibodies. Arthritis Rheum. 2013;65:3209–3217. doi: 10.1002/art.38127. [DOI] [PubMed] [Google Scholar]