Abstract
We present the case of an 18-year-old woman who suffered from complications of Ehlers-Danlos syndrome (EDS). Her pain was poorly controlled despite being on a myriad of analgesic medications at the time. On initiating cannabinoid-based treatment, her pain was drastically reduced, immediately enhancing the patient’s quality of life. As the patient continued to self-administer, she was able to eliminate her opioid requirement. Considering the recent legalisation, we underline the need for physicians to be educated regarding the use of cannabinoids. In this case, specifically for chronic pain stemming from hypermobile EDS. Furthermore, we review the various impediments preventing ease of access to this potentially beneficial treatment.
Keywords: health economics, connective tissue disease, musculoskeletal syndromes, physiotherapy (rehabilitation), disability
Background
An estimated 28 million people in the UK suffer with chronic pain.1 Among this population are those with hypermobile Ehlers-Danlos syndrome (EDS).
This is a multisystem syndrome that spans a plethora of symptoms, such as joint hypermobility, joint pain, visceral and autonomic dysfunction, as well as significant psychosocial elements.
The condition is underdiagnosed, hence patients endure a protracted diagnostic process.2 This relatively young cohort of patients present late, with several complications and already having exhausted the WHO pain ladder. Considering the pain relief provided by cannabinoid-based medication is a step towards providing better care for such patients.
Case presentation
We highlight here the case of a patient who was diagnosed with hypermobile EDS after a long, painful clinical path and her struggle to find the right pain management therapy.
Since the age of 10 years, she had experienced a constellation of symptoms which included finger, dual hip and shoulder dislocations triggered by even the slightest of movements. A reduced sense of proprioception worsened agility further. Autonomic dysfunction was a separate, but noteworthy facet in the presentation. The patient was receiving parenteral feeding overnight due to gastroparesis. She experienced postural hypotension. She used self-catheterisation for bladder spasms, which resulted in recurrent cystitis. The patient also required a microdiscectomy.
The reality of these events was debilitating; the patient had left school aged 15 years as pain control became increasingly difficult even with codeine and tramadol.
The patient was finally diagnosed with hypermobile EDS in 2017 when she was aged 17 years. The diagnosis and care did not prevent the worsening of her condition and she presented with repeated temporomandibular joint dislocation.
Investigations
Routine bloods showed no abnormalities at any point in the clinical path to diagnosis.
Treatment
The patient had been prescribed various lines of treatment.
Her pharmacological treatment of pain consisted of fentanyl transdermal patches 25 μg/hour, buprenorphine 400 μg four times a day, a diazepam suppository once a day and morphine sulfate for breakthrough pain. This accumulated a minimum morphine equivalent daily dose of 220 mg. Given the stress of the condition and the persistent pain, the patient’s mental health had deteriorated. To manage this, she had been prescribed fluoxetine.
Psychotherapy and physiotherapy formed other separate elements of her pain management, but the patient reported having minimal benefit from these because the mental burden of her pain prevented her from fully engaging in her care.
As surgical treatment, she underwent intermaxillary fixation for refractory jaw dislocation, but this was not effective. Invasive interventions, such as Botox injections, failed to treat the muscle spasms in her face as well and dislocation continued whenever the patient yawned or vomited.
Failing this, the patient was unable to move her mouth entirely due to the pain and the newly licensed cannabis-based medical product nabiximols was discussed for the spasticity. Despite being legal, the prescription was rejected by her local National Health Service trust on the grounds of lack of published evidence of efficacy in her indication.
Having had little benefit from standard analgesia in the past and being unable to obtain nabiximols, the patient decided to try cannabinoid-based treatment independently and self-administer illegal cannabis flower and oil.
Outcome and follow-up
Within days of self-medicating with vaporised cannabis flower, the pain levels considerably subsided. The patient reported reduction of muscular spasms as well.
Within 3 months of self-administering, she was able to cease all opioid medications, no longer needed to self-catheterise and did not experience any new recurrent bladder infections. In the 3 months leading up to commencing the cannabinoid treatment, she had five accident and emergency (A&E) admissions related to her condition. This fell to zero A&E admissions in the 3 months following treatment. In the year prior to starting treatment, the patient received 54 days of inpatient treatment, which dropped to just 5 days of inpatient care in the year following treatment. The patient reported spending £500 per month on illegal cannabis at the time.
Eventually, the patient was able to obtain cannabis flowers as a legal unlicensed cannabis-based medical product prescribed at a private medical cannabis clinic in the UK at a cost of £1450/month. She was using two types of cannabis dried flower (first flower: 22% tetrahydrocannabinol (THC) and <1% cannabidiol (CBD); and second flower: 14% THC and <1% CBD; Bedrocan Cannabis Corp).
Since May 2020, the patient’s current medication is CBD oil (CBD 50 mg/mL, full-spectrum extract, BOD Pharmaceuticals), CBD:THC oil (THC 10 mg/mL and CBD 10 mg/mL, Cellen Biotech Limited) and dried cannabis flower (20% THC and 4% CBD, BOL Pharma). The oil is used for the baseline treatment given its slow and long bioavailability. The vaporised flower is used primarily for acute breakthrough pain, given its fast and short bioavailability. As part of the UK registry study Project Twenty21, the patient was prescribed unlicensed cannabinoid-based medication at a subsidised cost of £450 per month.3
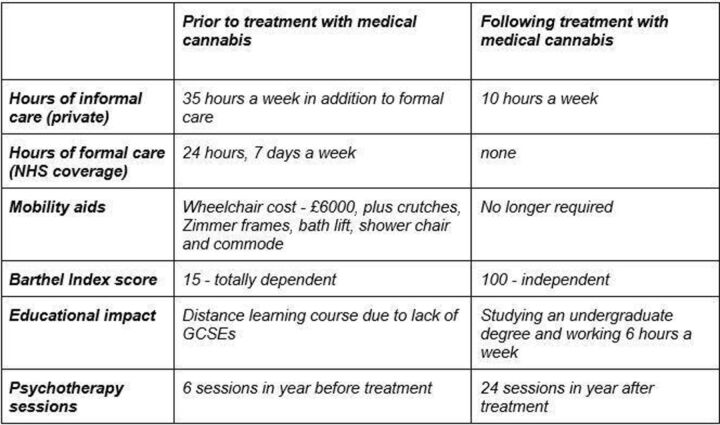
Initiating treatment with medical cannabis improved her quality of life. She was able to partake in physiotherapy more meaningfully and regain muscle strength, whereas previously, she was simply unable to complete the exercises recommended for her. The patient also reports better pain management, facilitating increased participation in psychotherapy. Over the following months, the frequency and extent of joint dislocation declined. This meant the patient was able to, at first, manoeuvre her wheelchair with greater ease for longer distances and, in time, relearn how to walk. Additional socioeconomic health benefits are outlined in figure 1 below.
Figure 1.
Socioeconomic health variables associated with treatment. GCSE, General Certificate of Secondary Education; NHS, National Health Service.
Nonetheless, medical cannabis came with its own host of adverse effects. During the titration period of the medication, the patient had trouble concentrating, nausea, sedation and highs. She thought that the nausea may have been induced by the carrier oil rather than the active ingredient. She contacted Cellen Biotech Limited to manufacture a personalised cannabis-based medical product manufactured with coconut oil which resulted in the disappearance of the side effect, thereby making the treatment more tolerable.
Discussion
There is emerging evidence for the role of cannabinoid-based medicines in the treatment of non-cancer chronic pain.4–6
Unlicensed cannabinoid-based medicinal products were reclassified from schedule 1 to schedule 2 in 2018 in the UK, thus allowing doctors on the specialist register of the General Medical Council to legally issue prescriptions. However, in spite of recent legal reforms, treating chronic pain with medical cannabis remains unpopular. A lack of physician knowledge on the topic and education on medical cannabis use have been listed as the main reasons behind this.7 Alongside restrictive guidelines and fears of adverse effects (psychosis and/or dependence), the British Journal of Psychopharmacology lists cost and supply issues as additional barriers to access.8
Poorly treated chronic pain experienced by patients with hypermobile EDS is at risk of deconditioning and opioid dependence. As discussed by this case, the nature of the condition lends itself to substantial polypharmacy. In addition, due to a prolonged lack of coordinated care and underfunded social care services, patients often face complex psychosocial challenges that compound the burden of pain.9
Resorting to illegal therapies is not uncommon. In fact, a recent survey of 500 patients with EDS in the USA found that 37% used cannabis therapeutically and that cannabis use was highest in those experiencing moderate-to-severe pain.10 There is also growing evidence for this in the UK.11 Evidently, self-medication with medical cannabis is a hidden, but frequent choice in patients with EDS. This case report hopes to shed more light on this subject in order to support a better understanding of the improved clinical outcome in such patients. Ideally, this could lead to further research or adjustment in clinical practices for patients with EDS.
Research on medical cannabis is slowed by sizeable ethical and legal implications. Cannabinoids remain an attractive candidate for pain management, given their efficacy and promising results in animal models, combined with their safety, non-euphoric and non-psychoactive properties.12 Furthermore, there are numerous individual patient reports documenting the therapeutic value of cannabinoid-based medications.13 However, these are often summarily dismissed due to the scarcity of randomised controlled trials.
This interplay of physician-related and practical barriers makes it difficult for patients to benefit from such treatment. Continuing to neglect this possibility means patients become responsible for sourcing and tailoring their own cannabinoid intake, sometimes illicitly and using non-medical grade cannabis. Inevitably, this leads to undershooting or overshooting in terms of the optimum concentration and poses the risk of contamination with unwanted substances. For patients with EDS, analgesia is often required long term and additional amounts are needed for pain flare-ups. Therefore, having a reliable and high quality supply is essential. Currently, patients are often left to their own devices, forced to travel abroad to stock up on vital medication and absorb the financial pressure of private prescriptions themselves. Such a costly burden is unsustainable for many.
Moving forward, doctors, pharmacists and nurses need to be provided with sufficient information and trained to better support patients with chronic pain who could benefit from cannabinoid-based medication, especially those who have failed standard therapies and have an unmet clinical need. This is likely to include a host of patients with EDS.
Finally, the body of evidence on the impact of medical cannabis on the prescription of opioids and other medications is growing and cost implications should be studied.14 15 This becomes even more essential in the context of treating those with chronic pain stemming from multisystem conditions like EDS. Thus, to formally assess the macroeconomic benefits of prescribing medical cannabis, health economics analyses should be encouraged.14 16 17
Increased cognizance of this therapeutic option for patients with severe hypermobile EDS, as well as other chronic pain sufferers, has the possibility to improve their quality of life. In accordance with this, we call for the expansion of clinical trials seeking to strengthen the link between chronic pain and cannabinoid-based medication so that patients can receive the best pain management available. Adopting a patient-centred approach highlighting observational research and pharmacoepidemiology will allow the use of vast swathes of reports illustrating the therapeutic benefits of medical cannabis.17 Indeed, there is a critical need for impartial clinical data collection to elevate the debate around medical cannabis above the socioeconomic and political issues in which it is held currently.
Patient’s perspective.
Unrelenting severe pain, increasing doses of opioids and frequent hospitalisations are what I recall most about my adolescence. From requiring full-time care, mobilising using a wheelchair and being unable to focus enough to continue my education, being prescribed cannabis-based medicines has enabled me to live independently, walk, undertake regular exercise and study for an undergraduate degree. However, I have been unable to obtain funding for an National Health Service prescription, so the cost burden to myself remains significant.
Learning points.
Support clinical expertise in the prescription of medical cannabis.
Enhance access to prescribed medication by addressing barriers to access.
Expansion of clinical data collection regarding the therapeutic and wider economic benefits of medical cannabis.
Footnotes
Twitter: @sabeera_dar
Contributors: SD is responsible for the conception and analysis of data. SD is grateful for the contributions of Patient-Led Engagement for Access for helping in the acquisition of data and reviewing drafts of the original manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Fayaz A, Croft P, Langford RM, et al. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016;6:e010364. 10.1136/bmjopen-2015-010364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castori M, Syndrome E-D. Ehlers-Danlos syndrome, hypermobility type: an underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol 2012;2012:1–22. 10.5402/2012/751768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Project Twenty21 | drugscience.org.uk [Internet]. drugscience.org.uk, 2021. Available: https://www.drugscience.org.uk/project-twenty21/
- 4.Boyaji S, Merkow J, Elman RNM, et al. The role of cannabidiol (CBD) in chronic pain management: an assessment of current evidence. Curr Pain Headache Rep 2020;24:4. 10.1007/s11916-020-0835-4 [DOI] [PubMed] [Google Scholar]
- 5.Haroutounian S, Ratz Y, Ginosar Y, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain 2016;32:1036–43. 10.1097/AJP.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 6.Sharon H, Brill S. Cannabis-based medicines for chronic pain management. Curr Opin Anaesthesiol 2019;32:623–8. 10.1097/ACO.0000000000000775 [DOI] [PubMed] [Google Scholar]
- 7.NHS England . Barriers to accessing cannabis-based products for medicinal use on NHS prescription [Internet], 2019. England.nhs.uk. Available: https://www.england.nhs.uk/publication/barriers-to-accessing-cannabis-based-products-for-medicinal-use-on-nhs-prescription/
- 8.Schlag AK, Baldwin DS, Barnes M, et al. Medical cannabis in the UK: from principle to practice. J Psychopharmacol 2020;34:931–7. 10.1177/0269881120926677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett SE, Walsh N, Moss T, et al. Understanding the psychosocial impact of joint hypermobility syndrome and Ehlers-Danlos syndrome hypermobility type: a qualitative interview study. Disabil Rehabil 2021;43:795–804. 10.1080/09638288.2019.1641848 [DOI] [PubMed] [Google Scholar]
- 10.Demes JS, McNair B, Taylor MRG. Use of complementary therapies for chronic pain management in patients with reported Ehlers-Danlos syndrome or hypermobility spectrum disorders. Am J Med Genet A 2020;182:2611–23. 10.1002/ajmg.a.61837 [DOI] [PubMed] [Google Scholar]
- 11.The Lancet Rheumatology . Medical cannabis: bridging the evidence gap. Lancet Rheumatol 2019;1:e195. 10.1016/S2665-9913(19)30099-2 [DOI] [PubMed] [Google Scholar]
- 12.Babalonis S, Haney M, Malcolm RJ, et al. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend 2017;172:9–13. 10.1016/j.drugalcdep.2016.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medical Cannabis Programme Data [Internet], 2021. Available: https://www.health.state.mn.us/people/cannabis/data/index.html
- 14.McMichael BJ, Van Horn RL, Viscusi WK. The impact of cannabis access laws on opioid prescribing. J Health Econ 2020;69:102273. 10.1016/j.jhealeco.2019.102273 [DOI] [PubMed] [Google Scholar]
- 15.Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain 2016;17:739–44. 10.1016/j.jpain.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 16.Bellnier T, Brown GW, Ortega TR. Preliminary evaluation of the efficacy, safety, and costs associated with the treatment of chronic pain with medical cannabis. Ment Health Clin 2018;8:110–5. 10.9740/mhc.2018.05.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nutt D, Bazire S, Phillips LD, et al. So near yet so far: why won't the UK prescribe medical cannabis? BMJ Open 2020;10:e038687. 10.1136/bmjopen-2020-038687 [DOI] [PMC free article] [PubMed] [Google Scholar]