Abstract
Extracorporeal membrane oxygenation (ECMO) requires anticoagulation to prevent clotting when the patient’s blood contacts the circuit. Unfractionated heparin (UFH) usually prevents clotting but can cause life-threatening bleeding. An anticoagulant that selectively inhibits the contact activation (intrinsic) pathway while sparing the tissue factor (extrinsic) pathway of coagulation might prevent clotting triggered by the circuit while permitting physiologic coagulation at surgical sites. DTRI-178 is an RNA anticoagulant aptamer conjugated to polyethylene glycol that increases its half-life in circulation. This aptamer is based on a previously described molecule (9.3t) that inhibits intrinsic tenase activity by binding to factor IXa on an exosite. Using a piglet model of pediatric venoarterial (VA) ECMO, we compared thromboprevention and blood loss using a single dose of DTRI-178 versus UFH. In each of five experiments, we subjected two litter-matched piglets, one anticoagulated with DTRI-178 and the other with UFH, to simultaneous 12-h periods of VA ECMO. Both anticoagulants achieved satisfactory and comparable thromboprotection. However, UFH piglets had increased surgical site bleeding and required significantly greater blood transfusion volumes than piglets anticoagulated with DTRI-178. Our results indicate that DTRI-178, an aptamer against factor IXa, may be feasible, safer, and result in fewer transfusions and clinical bleeding events in ECMO.
Keywords: anticoagulation, extracorporeal membrane oxygenation, pediatric surgery, mechanical support, heart failure, aptamers, RNA therapeutics, contact activation, coagulation factor IX
Graphical Abstract

DTRI-178 is an RNA-based aptamer that inhibits coagulation factor IXa, part of blood’s contact activation system. DTRI-178 maintained piglets on extracorporeal membrane oxygenation for 12 h without clotting and reduced bleeding compared with standard treatment (heparin). Novel molecular strategies may exploit contact activation to prevent thrombosis but prevent bleeding.
Introduction
Venoarterial (VA) extracorporeal membrane oxygenation (ECMO) is an ex vivo temporary cardiopulmonary support procedure that may be used to support heart and lung functions for days, weeks, or months. Venous and arterial cannulae, polyvinylchloride (PVC) tubing segments, and a mechanical pump are used to channel central venous blood through a membrane oxygenator, through a heat exchanger, and then into the patient’s arterial system. This procedure is lifesaving for many infants with primary pulmonary hypertension, meconium aspiration, congenital diaphragmatic hernia, and other congenital anomalies; as well as for many adults and older children with acute respiratory distress syndrome consequent to critical illness, trauma, and viral infections. ECMO is also used to provide circulatory support following cardiac arrest in pediatric and adult patients.1,2
During ECMO, systemic anticoagulation is necessary to prevent coagulation that would otherwise be triggered when the patient’s blood contacts the surfaces of the ECMO circuit. Unfractionated heparin (UFH) is the standard anticoagulant used in ECMO and has numerous important drawbacks that arise from its origin, composition, structure, anticoagulation mechanisms, and immunogenicity. Extracted from porcine intestinal mucosa or bovine lung, UFH is a polydisperse mixture of sulfated polysaccharide chains. Only about one-third of UFH’s chains possess a specific pentasaccharide sequence that is necessary for antithrombin III (AT) binding.3 The ability of an individual UFH chain to inhibit factor (F)IXa, FXa, or thrombin depends not only on its ability to bind AT, but also on the number of monosaccharide units in the chain. Since AT is a suicide inhibitor, UFH’s principal anticoagulation mechanism leads to depletion of this cofactor.4 UFH infusion can be particularly problematic in newborns, in whom AT circulates at a concentration less than 50% of its concentration in healthy adults.5 Since AT exerts both anti-inflammatory and anticoagulant effects, the depletion of AT not only reduces UFH’s efficacy, but may also exacerbate disease-related and ECMO-induced inflammation.6 Another important complication of heparin therapy that is particularly prevalent among adults is development of heparin-induced thrombocytopenia (HIT), an immune response that develops in 1% to 3% of patients who receive prolonged UFH infusions. If unrecognized, HIT can cause end-organ damage and life-threatening thrombosis.7 In addition, UFH does not effectively inhibit thrombin generation during ECMO and cannot inactivate clot-bound thrombin. These limitations can result in the depletion of prothrombin and the activation of inflammation in this setting.8
The most overt, common, and perhaps important adverse effect of UFH infusion for ECMO is bleeding. Infants and children are at particularly high risk for hemorrhagic complications while on ECMO. Their smaller circulating blood volume necessitates near-daily blood transfusions due to small-volume but ongoing blood loss (typically from cannulation sites). Intracranial hemorrhage is also six times more common in neonates compared with adults.9,10 Last, the immaturity of the neonatal hemostatic system also makes effective monitoring much more complicated and potentially morbid.11,12 Strategies aimed at reducing heparin exposure and mitigating hemorrhagic risk, such as liberal platelet transfusions and improving biocompatibility of synthetic circuit components, do not obviate the need for systemic anticoagulation and associated effects of UFH.13,14
Aptamers are synthetic oligonucleotides that bind a target epitope with high specificity and avidity.15,16 Aptamers often act as protein inhibitors by binding and burying large patches on the surface of a target protein and thereby sterically interfering with the protein’s ability to form macromolecular interactions.17,18 As pathologic blood clotting remains a major driver of morbidity and mortality and is mediated through macromolecular interactions among coagulation factors, the concept of developing aptamers into anticoagulants has received considerable attention.16,19 In particular, recent studies have focused attention toward targeting the intrinsic or contact pathway factors (FXII, FXI, and FIX),20,21 which are activated when blood is passed over foreign surfaces, as occurs during ECMO.22 As ECMO is often performed for hours or days, it is important that aptamers can be chemically modified (e.g., conjugated to polyethylene glycol [“PEGylated”] to reduce renal clearance and increase circulating half-life) and manufactured at large scale by chemical synthesis. Finally, unlike the biological effects from monoclonal antibodies, aptamers also have the unique benefit of being rapidly and specifically reversible by administration of a second oligonucleotide composed of a complementary sequence. Through Watson-Crick base-pairing with the aptamer, the antidote oligonucleotide neutralizes the ability of the aptamer to bind its target epitope and rapidly reverses its anticoagulant activity,23, 24, 25, 26 an important safety benefit for an anticoagulant in the setting of ECMO, as these patients often walk a fine line between clotting and bleeding events.
FIXa is the enzymatic component of intrinsic tenase, the final procoagulant enzyme complex in the contact activation (intrinsic) coagulation pathway. The anticoagulant aptamer 9.3t is a 35-base RNA anticoagulant aptamer that binds a human FIXa exosite in a manner that inhibits the formation of intrinsic tenase.27 This molecule has also been shown to bind and inhibit porcine FIXa, and has been used to achieve short-term anticoagulation (<1 h) in a piglet model of cardiopulmonary bypass.28 Clinical investigations of DTRI-178, a 31-base PEGylated optimized version of 9.3t previously named pegnivacogin (RB006), showed that it achieved satisfactory anticoagulation in patients who were undergoing percutaneous coronary intervention.29, 30, 31 Therefore, DTRI-178 presents an attractive means of inhibiting the final step of the contact activation pathway while relatively sparing the tissue factor (TF; extrinsic) coagulation pathway. The sequence and secondary structure of DTRTI-178 have been solved and reported (Figure 1A).
Figure 1.

Schematics of DTRI-178 secondary structure and extracorporeal membrane oxygenation ECMO circuit
(A) Secondary structure of DTRI-178. (B) Venous blood is withdrawn from the superior cavoatrial junction via an 8-Fr cannula. A centrifugal pump (artificial heart) pumps blood through a polymethylpentene membrane oxygenator (artificial lung), where gas exchange and warming occurs. Arterialized blood is then returned via the arterial cannula terminating in the arch of the aorta. Resistance to flow across the membrane oxygenator is estimated by transduction of hydrostatic pressure immediately prior to (Ppre) and after (Ppost) the oxygenator.
Assessing the efficacy of FIXa inhibition for thromboprevention during ECMO requires in vivo modeling with high clinical fidelity. Pigs (Sus scrofa) are commonly used as large animal models of human disease due to their similar cardiopulmonary physiology, cardiovascular anatomy, and hemostatic systems.32, 33, 34, 35 Young pigs and piglets have been used as models of pediatric ECMO in order to study ECMO-induced ischemic and inflammatory changes, and in a model of cardiopulmonary bypass to compare the efficacy of anticoagulants.36, 37, 38 Conventional coagulation parameters and individual factor activities in swine have been described in detail.39,40 Generally, pigs have higher activities of the same intrinsic coagulation factors established in humans (i.e., IX, XI, and XII). This high degree of conservation is inferred from both porcine enzymatic activity in assays using human substrates and structural analysis of clots ex vivo; as well as from overall interspecies amino acid sequence homology.41, 42, 43 Finally, we have previously described the use of piglets to model VA ECMO using UFH as the standard of care.44 Given the similarities between human and pig cardiovascular and coagulation systems, as well as prior establishment of models for extracorporeal support in piglets, a porcine model was selected to examine the effects of selective FIXa inhibition during VA ECMO.
We hypothesized that the FIXa aptamer DTRI-178 would provide improved hemostasis with equivalent thromboprevention compared with UFH infusion in a piglet model of VA ECMO.
Results
UFH and aptamer DTRI-178 both maintain circuit patency in piglet ECMO
Five pairs of piglets (five UFH, five aptamer) were cannulated for VA ECMO and supported through the study endpoint of 12 h. Each pair came from a single litter and were therefore identical in age (mean = 15.2 days, Figure S1). The mean weight was 5.03 kg for the UFH group and 4.84 kg for the aptamer group (p = 0.5714).
All animals achieved and maintained good ECMO flows throughout the experiment (Figure 2A). Mean flows ranged from 100 to 140 mL/kg/min and were stable throughout the procedure for both groups. Transmembrane oxygenator pressure gradient (ΔP), a measure of resistance to flow through the oxygenator and surrogate for oxygenator clot burden, was negligible for both groups at all but one time point in every experiment (Figure 2B). There was one outlying 17 mm Hg measurement at 11 h from an aptamer-treated animal, which normalized by the next hour’s data collection point (mean ΔP for all other measurements in all animals in all experiments ≤10 mm Hg). Oxygenation and decarbonation were adequate in both experimental groups throughout each run, although the partial pressure of carbon dioxide (PaCO2) tended to increase throughout the run in the case of heparinized animals (Figure S2).
Figure 2.
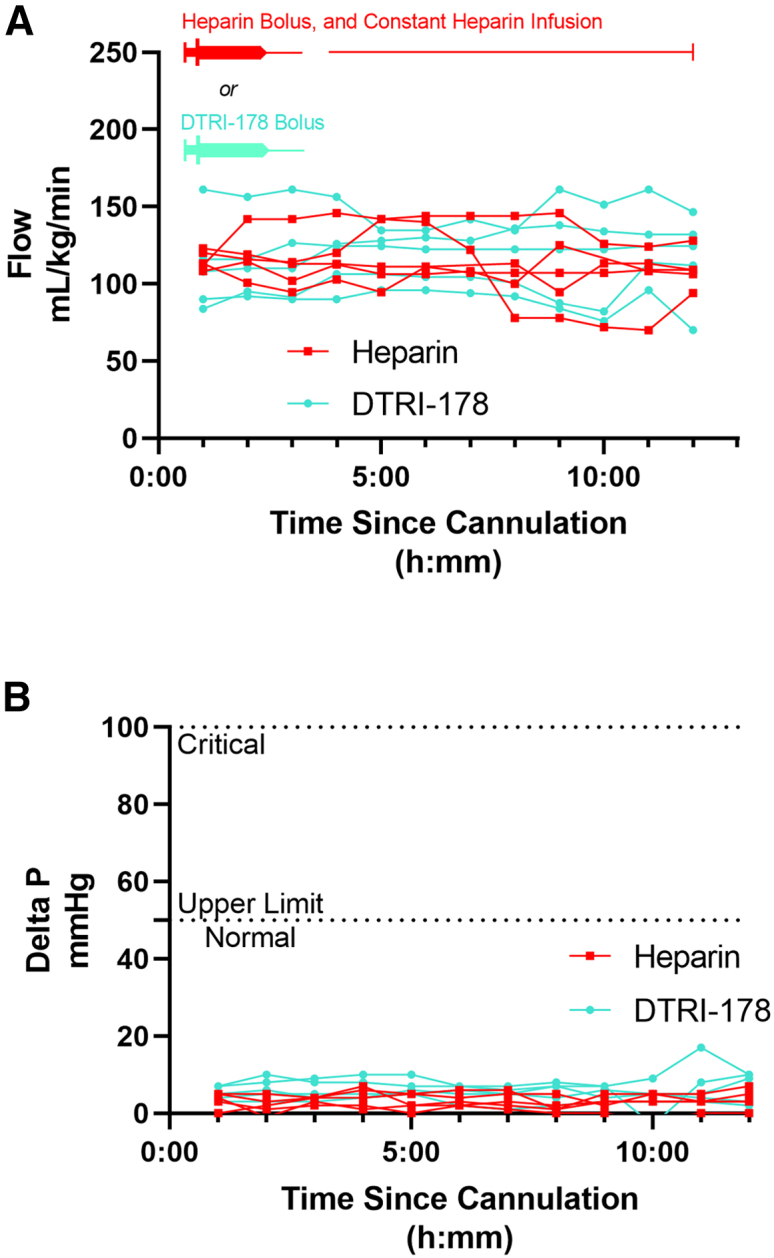
Blood flow and transmembrane oxygenator pressures through the extracorporeal membrane oxygenation ECMO circuit over time
(A) ECMO flow was measured hourly by a flow transducer immediately post-oxygenator. Each individual animal’s measurements are connected by line segments. (B) Transmembrane oxygenator pressure gradient (ΔP) was measured hourly by subtracting the hydrostatic pressure measured at the oxygenator outflow from the inflow. The clinically relevant normal and critical upper limit pressures are indicated with dashed lines. Each individual animal’s measurements are connected by line segments.
Hemostasis is superior with aptamer compared with heparin
Animals in the UFH group required significantly more blood transfusions in order to address hematocrit instability (Figure 3, 44.2 mL/kg versus 0.4 mL/kg, p = 0.0159). Furthermore, two of five animals in the aptamer group did not require any transfusions in order to maintain hematocrit stability despite repeated blood draws for laboratory studies and monitoring, while all animals in the UFH group required repeated transfusions. This corresponded with slow but persistent hemorrhage from cannulation and suprapubic bladder/femoral arterial catheterization sites for the duration of the run in five of five UFH animals. In contrast, three of five aptamer-treated animals had immediate hemostasis after the initial procedures, with the remainder achieving delayed hemostasis by the end of the experiment.
Figure 3.
Blood transfusion volumes and clinical bleeding events
(A–D) Clinical bleeding at the experiment completion (12 h total) at the sites of cannulation at neck (A and C) and instrumentation at groin (B and D). Representative photos of heparinized (top: A and B) and DTRI-178-treated (bottom: C and D) animals are provided. (E) Whole-blood transfusion volume. Individual points are transfusion totals in mL/kg. Bars represent the median transfusion total and error bars are interquartile ranges, and the difference is statistically significant (p = 0.0159). Striped boxes represent the total blood volume removed throughout the run for study sampling and monitoring.
Aptamer and heparin provide equivalent thromboprevention
After rinsing away residual blood with crystalloid, high-resolution photographs of all five oxygenators from each treatment group were examined to compare macroscopic clot burden (Figures 4A and 4B). In agreement with negligible ΔP throughout each run, clot burden was overall low in both groups (Figure 4C). There was no significant difference in the mean percentage of the oxygenator face occupied by clot on photography (0.72% for UFH versus 1.25% for aptamer, p = 0.0952).
Figure 4.
Macroscopic inspection and scanning electron microscopy analyses of clotting on membrane oxygenators
(A and B) Photograph of the median-clotted membrane oxygenator from (A) heparinized, and (B) DTRI-178-treated animals. (C) The percentage of total oxygenator surface area covered with clot, expressed as the median ± the interquartile range. Data points indicate value for all five animals in each group, and the difference is not statistically significant (p > 0.05, ns). (D and E) Scanning electron micrographs of oxygenator fibers from the median-clotted (D) heparin- and (E) DTRI-178-treated animals. Inlays are higher-magnification images of representative clots. Lower magnification = ×65, inlays = ×1,500 and ×2,500, respectively. Scale bars at lower magnification, 200 μm, inlays, 10 μm. (F) The percentage of the total oxygenator surface area covered with clot, expressed as the median ± the interquartile range. Data points indicate the separate average values for fibers obtained from the superficial and deep portions of the oxygenators for three animals in each group, and the difference is not statistically significant (p > 0.05, ns).
Ten regions from each of two layers (superficial, deep) from each of three oxygenators in each treatment group (UFH, DTRI-178 aptamer) were analyzed with scanning electron microscopy (Figures 4D and 4E). Blinded manual counting revealed no significant difference in the mean percent of area clotted among oxygenators from animals treated with UFH (1.55%) versus aptamer-treated animals (1.67%, p = 0.9372) using scanning electron microscopy (Figure 4F).
Aptamer anticoagulation is quantifiable and durable
Porcine UFH infusion was titrated to the activated clotting time (ACT) goal of 180 to 220 s, with a mean ACT across all time points for all animals on ECMO of 194 s. The aptamer dose required to double the baseline activated partial thromboplastin time (aPTT) was about 0.5 μM (Figure S3). Therefore, the five aptamer-treated piglets received only a single dose at the time of cannulation divided between the animal and circuit for a target plasma concentration of 0.50 μM. The mean dose received based on hematocrit, weight, and dose administered on the day of the procedure resulted in an actual plasma concentration of 0.49 μM. Administration of aptamer to the piglet immediately prior to cannulation resulted in a median ACT-low range (ACT-LR) increase of 23 s (Figure 5, p = 0.0159). This increase was durable across all five runs, with the median increase persisting at 12 h (108 s versus 132 s, p = 0.0079). The mean ACT-LR across all time points in all animals after aptamer treatment on ECMO was 127 s (normal = 103 s across all neonatal piglets in our experience).
Figure 5.
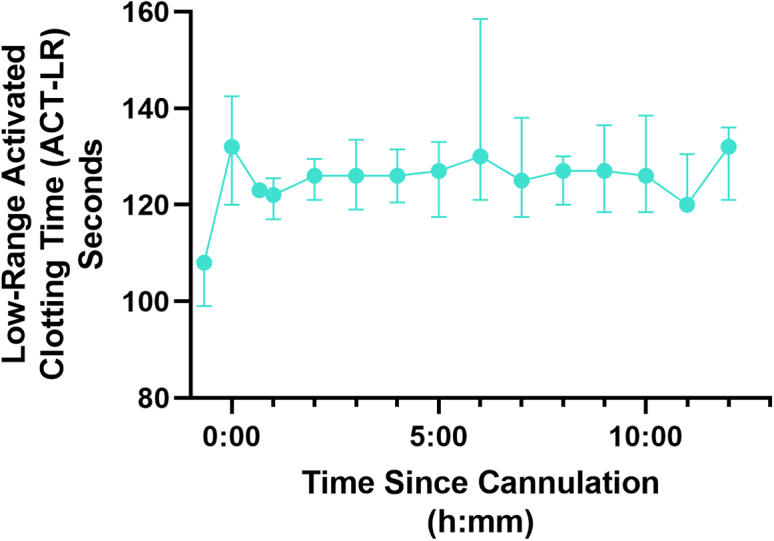
Low-range activated clotting time ACT-LR in DTRI-178-treated animals
Points represent the median ACT-LR and error bars are interquartile ranges. The first point (before time 0) is the baseline reading prior to DTRI-178 administration, the second point (at time 0) is immediately prior to starting ECMO, and the third point is immediately after commencing extracorporeal circulation.
Aptamer anticoagulation spares the TF pathway of coagulation
Rotational thromboelastometry (ROTEM) provides data similar to the older viscoelastic testing technology thromboelastography (TEG). It is a rapid whole-blood assay that quantifies clot formation kinetics and firmness in response to different activation agents. ROTEM samples taken throughout the run confirmed the specificity of the aptamer for the intrinsic pathway of coagulation. As expected, treatment with DTRI-178 resulted in a significant and reproducible increase in intrinsic pathway ROTEM (INTEM) coagulation time (CT), consistent with ACT-LR testing (Figure 6B). Specifically, the median INTEM CT increased from 160 s to 380 s after bolus administration of DTRI-178 but before starting ECMO (p = 0.0159). ROTEM demonstrated that the extrinsic pathway was spared by both UFH and aptamer, with extrinsic pathway ROTEM (EXTEM) CT unchanged after administration of each (54 s–56 s for UFH, p = 0.9268; and 57 s–56 s for DTRI-178; p = 0.3175; Figures 6A and 6C). Of note, INTEM testing was not performed for heparinized samples, as the heparin concentrations used in this experiment would result in no clot formation and no calculable CT.45 Instead, the ROTEM heparinase-treated HEPTEM protocol were used to determine if there was an underlying intrinsic coagulopathy. Finally, fibrin-only ROTEM (FIBTEM, which uses an extrinsic pathway activator after a cytochalasin D platelet inactivation step) revealed no differences in the trajectory of clot firmness over the 12-h runs between the two groups. Platelet counts and quantitative fibrinogen testing confirmed similarity between the two groups over each run (Figure S4). White blood cell count (WBC) was stable without clinically meaningful elevations or differences between treatment groups throughout the run. Although the WBC was statistically significantly less among DTRI-178 aptamer-treated piglets immediately after administration, the difference was not redemonstrated at other time points, and the magnitude of 1.0 × 103 cells/μL is not considered clinically significant (Figure S5).
Figure 6.
Rotational thromboelastometry ROTEM analyses of the extrinsic EXTEM, intrinsic INTEM or HEPTEM, and acellular fibrin-only FIBTEM pathways of coagulation
The first point (before time 0) is the baseline reading prior to DTRI-178 administration, the second point (at time 0) is immediately prior to starting ECMO, and the third point is immediately after commencing extracorporeal circulation. Points represent the median clotting time or firmness, and error bars are the interquartile range. (A) ROTEM EXTEM CT. This measure of clotting speed in response to a TF analog is similar to the conventional prothrombin time. As expected for agents that primarily or solely target the contact activation pathway, the CT of the extrinsic pathway is unaffected by the addition of either heparin (red) or DTRI-178 (turquoise). (B) ROTEM HEPTEM CT for heparin animals and INTEM CT for DTRI-178 animals. This measure of clotting speed in response to a contact activation agent (micronized silica) is analogous to the conventional aPTT. As expected, CT is prolonged among aptamer-treated animals immediately after administration (median 160 s at baseline compared with 380 s after aptamer, p = 0.0159). CT remains near normal among heparinized animal due to the effects of the HEPTEM test pre-treatment, which neutralizes heparin’s effects on the CT in order to demonstrate underlying coagulopathy. (C) ROTEM EXTEM maximum clot firmness (MCF). The overall clot firmness remains unchanged and similar between study groups. (D) ROTEM HEPTEM MCF for heparin animals and INTEM MCF for DTRI-178 animals. The overall clot firmness remains unchanged and similar between study groups. (E) ROTEM FIBTEM MCF. The acellular clot firmness (i.e., minus platelet contribution) is decreased after initiating ECMO, but is similar between groups and steady throughout the remainder of the experiment.
Discussion
The use of ECMO has steadily increased worldwide since 2006, with about 15,000 runs registered in 2018.46 The Extracorporeal Life Support Organization projects continued expansion of this lifesaving therapy worldwide, particularly as safer devices expand the indications for ECMO. Despite other advances in the field and well-established risks of hemorrhage and HIT, UFH infusion has remained the standard of care since the inception of ECMO in the 1970s. The present study demonstrates that in a piglet model of VA ECMO, animals anticoagulated with either an anti-FIXa aptamer or UFH have comparable thromboprevention, but that piglets anticoagulated with the aptamer have significantly better hemostasis.
Although FIX is activated to FIXa during TF-FVIIa complex-mediated coagulation, it is not required for FXa generation in the presence of TF.47 Therefore, thrombin can be generated with subsequent platelet aggregation and fibrin polymerization by TF-FVIIa complexes in the absence of FIXa. More recent studies have also described activation of FIX in a non-canonical pathway by the contact activation protease kallikrein, suggesting that foreign surfaces may be a very proximal stimulus for FIXa generation via kallikrein and independent of other mechanisms.48 Thus, FIXa is an attractive target to limit clotting during ECMO. Using the whole-blood ROTEM assay, we reported that aptamer DTRI-178 permits TF pathway-mediated FX activation, thereby preserving normal EXTEM parameters and hemostatic capacity in response to tissue trauma (i.e., surgical manipulation for the ECMO cannulation procedure). Simultaneously, DTRI-178 prevented undesired clot formation in and on the synthetic oxygenator.
These findings are a potentially significant advance in the development of a more rational systemic anticoagulation strategy for ECMO patients, and might reduce bleeding, decrease cost, and improve survival.49 Our findings are of particular interest in the treatment of patients with pre-existing coagulopathies who may be at the highest risk of hemorrhage and/or thrombosis. Patients undergoing a prolonged resuscitation, premature and early-term infants with increased intraventricular hemorrhage risk, those with disseminated intravascular coagulation, and those with severe COVID-19 pneumonia might stand to benefit most from more selective approaches to anticoagulation and improvement in hemostasis. In addition, the global COVID-19 pandemic highlights the potential for unprecedented and sudden increases in the need for ECMO, as well as attendant needs for safer anticoagulation in circumstances.
Our findings have significant implications not only for ECMO, but also for other applications in which blood-foreign surface contact necessitates ongoing anticoagulation. Ventricular assist devices (VADs) for heart failure, for example, typically use a centrifugal pump very similar to most ECMO systems. Patients with VADs routinely receive oral vitamin K antagonists, which carry considerable hemorrhagic risk and associated complications, including mortality.50
Furthermore, there is ongoing study of the specific mechanisms of hypercoagulability, thrombosis, and hemorrhage among children and adults while on extracorporeal circulation, and with a blood-device interface as a whole. Clinical observational studies of coagulation factor activities implicate the underlying disease process as well as acquired TF and contact activation coagulation deficiencies from extracorporeal circulation itself, and the cause of thrombosis and hemorrhage is ultimately probably multifactorial.51 To this end, a recent review on this subject in particular concluded that there is “a lack of data regarding the extent of TF-FVII as compared with contact activation mediated thrombin generation in ECMO.”52 Our findings directly implicate FIX/FIXa and intrinsic tenase in the development of clot associated with synthetic surfaces in a highly relevant clinical model using healthy animals, providing additional mechanistic insight that suggests a critical role for contact activation in the pathogenesis of device-related thrombosis. Overall, our observations should guide future studies and therapeutic targets in anticoagulation for extracorporeal circulation and synthetic devices as a whole, with special attention toward more hemostatic and safer anticoagulant strategies by exploiting the critical importance of the contact activation system.
Given the considerable risks of UFH, there has been recent interest in development of alternative anticoagulation strategies for a myriad of applications in which UFH is typically the standard of care, including ECMO.53 This study, therefore, accords with a rapidly growing body of studies investigating more selective approaches to anticoagulation. In particular, the past decade has seen a proliferation of experimental small molecule and antibody-based therapies targeting the contact activation coagulation system in extracorporeal circuits.54,55 Such strategies have the theoretical hemostatic benefit of sparing TF-mediated coagulation in the face of tissue trauma, while inhibiting enzymes specifically associated with foreign surface-induced clotting.22 For example, an anti-FXIIa antibody was sufficient to maintain ECMO circuit patency in four healthy adult rabbits cannulated via open abdominal approach for 6 h, with superior hemostasis compared with five adult rabbits treated with UFH. Proteins isolated from the midgut of blood-eating insects have been found to possess anti-FXIa and FXIIa activities that facilitate anticoagulation as measured by FeCl3-and foreign surface-induced clotting55,56 and aptamers have also been generated against FXI and FXII.20,21 All of these strategies offer the theoretical benefit of targeting enzyme effectors of coagulation that are most proximal to the procoagulant stimulus; i.e., foreign surface contact and FXIIa generation. None of these strategies have yet been integrated into routine clinical practice.
Our strategy targets a critical component of the intrinsic tenase complex that is dispensable for TF-FVIIa complex-mediated coagulation. Although it may theoretically present a greater hemorrhagic tendency compared with FXI and FXII inhibitors, we have demonstrated very favorable hemostasis in our model using FIXa inhibition while maintaining thromboprevention comparable to that achieved with UFH. The basis for this relative preservation of hemostasis may be multifactorial and complex. In two prior studies using the similar molecule 9.3t, it was demonstrated that this closely related anti-FIXa aptamer inhibited formation of intrinsic tenase.23,27 As intrinsic tenase activates FX orders of magnitude faster than does free FIXa; that effect alone likely provides potent anticoagulation. This is the basis for aPTT prolongation and likely the mechanism for thromboprevention during ECMO. Rusconi and colleagues additionally suggested that 9.3t disrupted the formation of pre-formed intrinsic tenase by binding FIXa; i.e., by “pulling” FIXa away from FVIIIa. In a separate report, Gopinath et al demonstrated that 9.3t, by binding FIX, also inhibited TF:FVIIa-mediated FIXa activation, but not FXIa-catalyzed cleavage of FIX.57 This ability to partially inhibit TF:FVIIa activation of FIX may be beneficial in the setting ECMO for patients who have suffered vascular injury and exposure of TF due to trauma, surgery, or infection such as has been observed in severe COVID-19.58 Unfortunately, inhibitors of FXII or FXI are not expected to be able to control clotting induced by the TF pathway in these settings, which may significantly compromise their utility.
Another intriguing potential explanation for adequate hemostasis despite FIXa inhibition is mounting evidence in multiple animal models of an extravascular FIX reservoir.59,60 Collagen-avid FIX may extravasate and provide a limited but significant reserve that is not immediately accessible by drugs administered intravenously such as aptamer DTRI-178. Such a reservoir might reach sufficient concentrations to promote hemostasis in injured tissue beds when intravascular FIX zymogen has been inactivated. More study is needed to investigate the clinical relevance of extravascular FIXa. In summary, we have demonstrated that an RNA aptamer against FIXa maintains circuit patency with superior hemostasis and equivalent thromboprevention compared with UFH in a high-fidelity porcine model of pediatric VA ECMO. This preclinical evidence demonstrates a role for FIXa inhibition in the management of children and adults on ECMO, particularly among those at highest risk for bleeding, clotting, or both (i.e., premature infants, adults with coagulopathy, COVID-19 pneumonia, and trauma victims). This rational approach to anticoagulation may reduce or obviate the need for frequent blood product transfusions, reduce devastating hemorrhagic complications, avoid the risk of HIT, and potentially make ECMO a safer therapy for additional populations of infants and adults for whom the risks of conventional anticoagulation have historically been unacceptably high, including those with severe COVID-19 pneumonia. As this aptamer has been evaluated in more than a thousand patients undergoing percutaneous coronary intervention and provided effective anticoagulation in these patients29, 30, 31 while proving safe except for rare individuals who contained high levels of pre-existing anti-PEG antibodies,61,62 we believe that DTRI-178 represents an anticoagulant agent that can be rapidly translated into the clinic by pre-screening individuals for anti-PEG antibodies through established immunoassays prior to administering the PEGylated aptamer and subjecting them to ECMO.
Limitations of our study include the 12-h duration and sample size employed. Children on ECMO may require days to wean support or reach stability for destination therapy. In one retrospective multicenter observational study, the median pediatric ECMO run length was 4 days.63 Therefore, the ideal study would similarly observe animals maintained on ECMO for a minimum of several days, regardless of resources required. Despite this apparent limitation, it is worthwhile to note that our study used ECMO for longer than similar such studies using ECMO in swine and rabbits, and animals rapidly reached and maintained a steady state of anticoagulation and flow as demonstrated in Figures 5, 6, and 2; respectively.38,54,64 Although the number of animals studied could be expanded to strengthen our conclusions, piglets were selected from the same litters for each ECMO run in order to decrease the chance that genetic heterogeneity could affect anticoagulation efficacy and bleeding phenotype. Particularly with respect to blood loss and transfusions required, the magnitude and reproducibility of our findings were sufficient to draw conclusions about the effects of DTRI-178. Future studies with additional animals might study additional doses of DTRI-178 or its cognate reversal agent, which has been previously described and employed to good effect in human subjects.65 However, with the benefit of such human studies as well as our rational approach to dose selection based on studies of aPTT prolongation in vitro, we believe that the current dosing regimen represents a reasonable empiric strategy for this application and may guide future studies.
Materials and methods
The animal experiments were reviewed, approved, and monitored by the Duke Institutional Animal Care and Use Committee.
Ten litter-matched, approximately 2-week-old male Yorkshire farm piglets, 4.5 to 5.5 kg, were randomly assigned to control (UFH) or aptamer (DTRI-178) study groups. Ten juvenile female Yorkshire pigs (3–4 months of age, 30–55 kg) were used for circuit priming and blood transfusions. All piglets and donor animals were confirmed to be blood type O with EldonCard (Eldon Biologicals, Gentofte, Denmark) kits as previously described.66 Animals were only included if they met size criteria and were blood type O as described above.
DTRI-178 anticoagulation
The in vivo and in vitro activities of DTRI-178 in human blood have been previously described.30,67 In order to confirm species specificity and estimate dosing in porcine models, normal plasma was obtained from 10 healthy adult pigs, pooled, and subjected to aPTT tests with graded dilutions of DTRI-178. Briefly, 10-fold dilutions of DTRI-178 were added to pooled normal porcine plasma for final reaction mixture concentrations of 5 × 10−3 μM–5 μM in 20 mM HEPES buffer, pH 7.4. The plasma-aptamer mixture was incubated for 5 min at 37°C before addition of the silica-based SynthasIL aPTT activation reagent (Instrumentation Laboratory). After 2 min of incubation with the activation reagent, calcium chloride 0.2 M was added to commence coagulation (Instrumentation Laboratory), and a BBL fibrometer (BD, Franklin Lakes, NJ) was used to measure the time until coagulation began. All tests were performed in triplicate, and the aptamer concentration at which the aPTT was doubled was selected for further experimentation in vivo.
The half-life of DTRI-178 is dose-dependent and has been previously studied in humans. In healthy adult subjects receiving sufficient aptamer to double the aPTT in vivo, the duration of activity was about 30 h.30 In subjects receiving aptamer to increase the aPTT 1.3-fold (i.e., the approximate ACT-LR increase in our animal studies), the duration of effect was 20 to 24 h, well over the duration of the study.
Donor blood collection procedures
After overnight fasting, two donor animals were sedated with intramuscular ketamine (22 mg/kg) and acepromazine (1.1 mg/kg), and then anesthetized with isoflurane (1%–5%), intubated, and mechanically ventilated. Following infiltration with 1% bupivacaine, the right carotid artery was exposed and cannulated for whole blood collection. The first 5 mL of blood was discarded, and then donor collection bags containing sterile anticoagulant-citrate-dextrose A (ACD-A; Fenwal Inc., Lake Zurich, IL) were filled with donor blood in a 1:8 ratio. When sluggish flow was encountered at the end of the collection or in the case of incompletely filled bags, the blood was discarded. After exsanguination, donor animals were killed humanely under deep anesthesia with approved methods, and the piglet ECMO cannulation procedure commenced immediately.
Donor blood was stored at 4°C, and individual bags were introduced into each of the two ECMO circuits in each experiment in the same order (in order to maintain similar donor-recipient dynamics). Blood was transfused through the heated ECMO circuit to prevent hypothermia. All blood was used within 24 h of collection.
Piglet cannulation procedures and ECMO circuit description
After 8 h of fasting, two litter-matched piglets were sedated with isoflurane (1%–5%) and intravenous propofol infusion (4–10 mg/kg/h). After infiltration of bupivacaine, a femoral arterial catheter and suprapubic urinary bladder catheter were placed for monitoring. The first piglet’s assignment (UFH versus aptamer) was alternated for each of the study days. The right common carotid artery and external jugular vein were exposed with blunt and sharp dissection, and both vessels were controlled with monofilament suture. Immediately prior to carotid ligation and arteriotomy, an anticoagulant bolus (heparin for goal ACT 180–200 s or target plasma aptamer concentration 0.50 μm) was administered. Finally, the common carotid artery and external jugular vein were cannulated under direct vision with 8-Fr venous and 8-Fr arterial cannulae (Biomedicus Medtronic, Minneapolis, MN). The arterial and venous cannulae were advanced 2 to 3 and 7 cm, respectively, and their tip positions were confirmed at necropsy to be in the transverse arch of the aorta and cavoatrial junction, respectively.
Simultaneously, the ECMO circuit was prepared in a manner consistent with our clinical practice (Figure 1B). The circuit employed uncoated PVC tubing and a centrifugal magnetic levitation pump from a commercially available perfusion pack (Custom Revolution Pump Base Pack and Tubing Pack, Sorin Group Liva Nova, London, United Kingdom). An adult membrane oxygenator was used (Quadrox-iD; Maquet Getinge Group, Wayne, NJ). The circuit was clear-primed in anticipation of cannulation with the calcium-free balanced saline-containing crystalloid Plasmalyte-A (Baxter International, Deerfield, IL). Immediately prior to commencing ECMO, the circuit was primed with whole citrated donor blood (∼450 mL), followed by anticoagulation (300 U heparin or target plasma aptamer concentration 0.50 μm), recalcification with 500 mg CaCl2, and 3 to 5 mEq sodium bicarbonate for a target pH of 7.4.
ECMO settings, monitoring, and lab draws
The target ECMO flow was 120 mL/kg/min (range: 80–160 mL/kg/min) and the pump speed was the dependent variable. The sweep gas was initially set to 1/32 L/min of flow and titrated to achieve a PaCO2 40 to 60 mm Hg. The pressure gradient across the membrane oxygenator (Ppre – Ppost = ΔP) was calculated as a real-time surrogate of oxygenator clot burden. All settings were recorded hourly. Vital signs including rectal temperature, heart rate, SpO2, respiratory rate, end-tidal CO2, and femoral arterial blood pressure were recorded every 15 min for the first 4 hours and then every 30 min thereafter.
Piglets underwent arterial blood gas (ABG) sampling via femoral catheter every hour using a GEM Premier 3000 analyzer with fresh whole blood (Instrumentation Laboratory, Bedford, MA). Hematocrit was measured every hour and a 10 mL/kg transfusion was delivered if ABG hematocrit was <21%. In order to exclude variability incurred during cannulation or at baseline, only transfusions after 4 h were compared. Based on previously established ECMO modeling in piglets as well as clinical guidelines for humans, a UFH anticoagulation target using ACT 180 to 220 s was established a priori.38,68 Hourly MAX-ACT with an Actalyte Mini II analyzer (Helena Laboratories, Beaumont, TX) was performed with fresh whole blood to monitor heparin anticoagulation hourly. Heparin infusion rate adjustments were made after any MAX-ACT value outside of the 180- to 220-s range. The MAX-ACT was repeated 5 min after subtherapeutic (<180 s) or very supratherapeutic (>250 s) readings until a therapeutic range reading was obtained. For aptamer-treated piglets, ACT-LR (Instrumentation Laboratory) was performed hourly with a HemoChron Jr Signature analyzer (Instrumentation Laboratory) designed to monitor patients receiving less potent anticoagulation. The heparin strategy (bolus and then constant infusion) was ultimately based on typical clinical practice.
Finally, ROTEM tests of the extrinsic, intrinsic, and fibrin-only coagulation pathways were performed at baseline, after anticoagulation but pre-ECMO, 5 min after starting ECMO, and then at 4, 8, and 12 h after starting ECMO (Instrumentation Laboratory). In order to account for the effects of heparin (which arrests INTEM intrinsic coagulation entirely at the concentrations used in this experiment) and probe for underlying coagulopathy, all heparin-containing specimens were pre-treated with heparinase as per the manufacturer HEPTEM protocol. Citrated whole blood and commercially available reagents from Instrumentation Laboratory were used for ROTEM testing on a ROTEM delta analyzer according to manufacturer instructions, and reactions were allowed to proceed for at least 30 min.
Additional laboratory testing included complete blood count obtained with an automated hematology analyzer (Model KX-21N; Sysmex, Lincolnshire, IL) at baseline, and 0, 4, 8, and 12 h; and quantitative fibrinogen levels (IDEXX BioAnalytics, North Grafton, MA) at 0, 4, and 12 h.
Membrane oxygenator analyses
At the completion of each run, membrane oxygenators were gently rinsed with isotonic normal saline to remove blood, and then the face of each oxygenator was photographed for clot quantification. Oxygenators were then processed for scanning electron microscopy. Briefly, a 3 × 3-cm section of the outermost superficial layer of the oxygenator membrane and a similar-sized section of deep interior layer were cut and then dehydrated in graded series of aqueous ethanol (50%, 70%, 90%, and then three times in 100%). A final dehydration step was completed in hexamethyldisilazane (EM Sciences, Hatfield, PA) before specimens were placed in a dehydration chamber overnight. Samples were sputter-coated with gold in a Desk V unit (Denton Vacuum, Moorestown, NJ). Finally, 1 × 1-cm sections from both shallow and deep portions of the oxygenator were mounted onto aluminum stubs. Ten random images were acquired from both depths on each of six oxygenators (three UFH, three aptamer) at ×200,000 magnification using an Apreo S scanning electron microscope (ThermoFisher Scientific, Hillsboro, OR).
For photography analysis and comparison, lossless TIF photographs of all 10 oxygenators were analyzed in Fiji by two blinded authors.69 Both authors quantified the number of macroscopic clots as well as the overall percent of the oxygenator face that was clotted. The number of clots and percent of oxygenator face clotted for both experimental groups were ultimately expressed as the mean and SEM of the duplicate means calculated for each oxygenator.
For scanning electron microscopy analysis, all 20 images (10 superficial, 10 deep) for each of the three oxygenators per study group were analyzed for combined fibrin and cellular clot burden by the same blinded authors. In a manner similar to that described above for photographs, Fiji was used to count all clots ≥5 μM in any dimension, and then to estimate the percent area clotted.
Statistical analysis
Data were analyzed and graphed using GraphPad Prism 9 (GraphPad Software, San Diego, CA). Continuous data were described using the median and interquartile range. Given the limited sample size, nonparametric testing was selected for inferential statistics. Point comparisons of continuous variables between study groups (i.e., weight) were made using the Mann-Whitney U test. For all tests, p < 0.05 was interpreted as statistically significant. No correction was made for multiple comparisons.
Acknowledgments
The authors acknowledge Ianthia Parker, Mike Lowe, and Christie Holmes for their veterinary technical assistance. The authors also acknowledge Carl Stanfield and Meredith Achey for their technical assistance with ECMO and animal experiments. This work was funded by NIH grant P01-HL139420 (Sullenger) and the American Pediatric Surgical Association Foundation Research Award (Tracy).
Author contributions
C.R., D.B., J.O., G.A., B.S., and E.T. designed the animal experiments and previously established the piglet ECMO model. J.F., J.L., C.C., and B.S. characterized DTRI-178 in human and porcine blood and wrote protocols for its application in these animal experiments. C.R., D.B., G.M., J.O., and E.T. performed all animal experiments. C.R. acquired photographs and micrographs for analysis. G.M. and J.O. analyzed all photographs and micrographs. C.R. and M.K. performed statistical analysis. C.R., J.O., G.A., and E.T. interpreted data. C.R. wrote the manuscript. D.B., C.M., J.O., M.K., J.L., G.A., C.C., J.F., B.S., and E.T. provided critical input on the manuscript.
Declaration of interests
Duke University has issued patents on the anti-FIXa aptamer used in the manuscript, DTRI-178. Dr. Sullenger is listed as an inventor on these patents. Duke University has applied for a use patent for DTRI-178 in extracorporeal circulation. Drs. Reed, Tracy, and Sullenger are listed as inventors on this application. None of the authors have otherwise commercialized or monetized this experimental oligonucleotide.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2021.12.011.
Supplemental information
References
- 1.Barbaro R.P., Paden M.L., Guner Y.S., Raman L., Ryerson L.M., Alexander P., Nasr V.G., Bembea M.M., Rycus P.T., Thiagarajan R.R., et al. Pediatric Extracorporeal Life Support Organization Registry International report 2016. ASAIO J. 2017;63:456–463. doi: 10.1097/MAT.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makdisi G., Wang I.W. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J. Thorac. Dis. 2015;7:E166–E176. doi: 10.3978/j.issn.2072-1439.2015.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nugent M.A. Heparin sequencing brings structure to the function of complex oligosaccharides. Proc. Natl. Acad. Sci. U S A. 2000;97:10301–10303. doi: 10.1073/pnas.97.19.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker C.P., Royston D. Thrombin generation and its inhibition: a review of the scientific basis and mechanism of action of anticoagulant therapies. Br. J. Anaesth. 2002;88:848–863. doi: 10.1093/bja/88.6.848. [DOI] [PubMed] [Google Scholar]
- 5.Toulon P., Berruyer M., Brionne-Francois M., Grand F., Lasne D., Telion C., Arcizet J., Giacomello R., De Pooter N. Age dependency for coagulation parameters in paediatric populations. Results of a multicentre study aimed at defining the age-specific reference ranges. Thromb. Haemost. 2016;116:9–16. doi: 10.1160/TH15-12-0964. [DOI] [PubMed] [Google Scholar]
- 6.Levy J.H., Sniecinski R.M., Welsby I.J., Levi M. Antithrombin: anti-inflammatory properties and clinical applications. Thromb. Haemost. 2016;115:712–728. doi: 10.1160/TH15-08-0687. [DOI] [PubMed] [Google Scholar]
- 7.Arepally G.M. Heparin-induced thrombocytopenia. Blood. 2017;129:2864–2872. doi: 10.1182/blood-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunaratne R., Kumar S., Frederiksen J.W., Stayrook S., Lohrmann J.L., Perry K., Bompiani K.M., Chabata C.V., Thalji N.K., Ho M.D., et al. Combination of aptamer and drug for reversible anticoagulation in cardiopulmonary bypass. Nat. Biotechnol. 2018;36:606–613. doi: 10.1038/nbt.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werho D.K., Pasquali S.K., Yu S., Donohue J., Annich G.M., Thiagarajan R.R., Hirsch-Romano J.C., Gaies M.G., Extracorporeal Life Support Organization Hemorrhagic complications in pediatric cardiac patients on extracorporeal membrane oxygenation: an analysis of the Extracorporeal Life Support Organization Registry. Pediatr. Crit. Care Med. 2015;16:276–288. doi: 10.1097/PCC.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paden M.L., Conrad S.A., Rycus P.T., Thiagarajan R.R., Registry E. Extracorporeal life support Organization Registry report 2012. ASAIO J. 2013;59:202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 11.Kuhle S., Eulmesekian P., Kavanagh B., Massicotte P., Vegh P., Lau A., Mitchell L.G. Lack of correlation between heparin dose and standard clinical monitoring tests in treatment with unfractionated heparin in critically ill children. Haematologica. 2007;92:554–557. doi: 10.3324/haematol.10696. [DOI] [PubMed] [Google Scholar]
- 12.Bateman S.T., Lacroix J., Boven K., Forbes P., Barton R., Thomas N.J., Jacobs B., Markovitz B., Goldstein B., Hanson J.H., et al. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am. J. Respir. Crit. Care Med. 2008;178:26–33. doi: 10.1164/rccm.200711-1637OC. [DOI] [PubMed] [Google Scholar]
- 13.Ontaneda A., Annich G.M. Novel surfaces in extracorporeal membrane oxygenation circuits. Front. Med. 2018;5:321. doi: 10.3389/fmed.2018.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cashen K., Dalton H., Reeder R.W., Saini A., Zuppa A.F., Shanley T.P., Newth C.J.L., Pollack M.M., Wessel D., Carcillo J., et al. Platelet transfusion practice and related outcomes in pediatric extracorporeal membrane oxygenation. Pediatr. Crit. Care Med. 2020;21:178–185. doi: 10.1097/PCC.0000000000002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nimjee S.M., White R.R., Becker R.C., Sullenger B.A. Aptamers as therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017;57:61–79. doi: 10.1146/annurev-pharmtox-010716-104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chabata C.V., Frederiksen J.W., Sullenger B.A., Gunaratne R. Emerging applications of aptamers for anticoagulation and hemostasis. Curr. Opin. Hematol. 2018;25:382–388. doi: 10.1097/MOH.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 17.Long S.B., Long M.B., White R.R., Sullenger B.A. Crystal structure of an RNA aptamer bound to thrombin. RNA. 2008;14:2504–2512. doi: 10.1261/rna.1239308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelinas A.D., Davies D.R., Janjic N. Embracing proteins: structural themes in aptamer-protein complexes. Curr. Opin. Struct. Biol. 2016;36:122–132. doi: 10.1016/j.sbi.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Becker R.C., Povsic T., Cohen M.G., Rusconi C.P., Sullenger B. Nucleic acid aptamers as antithrombotic agents: opportunities in extracellular therapeutics. Thromb. Haemost. 2010;103:586–595. doi: 10.1160/TH09-10-0716. [DOI] [PubMed] [Google Scholar]
- 20.Woodruff R.S., Xu Y., Layzer J., Wu W., Ogletree M.L., Sullenger B.A. Inhibiting the intrinsic pathway of coagulation with a factor XII-targeting RNA aptamer. J. Thromb. Haemost. 2013;11:1364–1373. doi: 10.1111/jth.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodruff R.S., Ivanov I., Verhamme I.M., Sun M.F., Gailani D., Sullenger B.A. Generation and characterization of aptamers targeting factor XIa. Thromb. Res. 2017;156:134–141. doi: 10.1016/j.thromres.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodruff R.S., Sullenger B., Becker R.C. The many faces of the contact pathway and their role in thrombosis. J. Thromb. Thrombolysis. 2011;32:9–20. doi: 10.1007/s11239-011-0578-5. [DOI] [PubMed] [Google Scholar]
- 23.Rusconi C.P., Scardino E., Layzer J., Pitoc G.A., Ortel T.L., Monroe D., Sullenger B.A. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature. 2002;419:90–94. doi: 10.1038/nature00963. [DOI] [PubMed] [Google Scholar]
- 24.MacLaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245–1246. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- 25.Nimjee S.M., Dornbos D., 3rd, Pitoc G.A., Wheeler D.G., Layzer J.M., Venetos N., Huttinger A., Talentino S.E., Musgrave N.J., Moody H., et al. Preclinical development of a vWF aptamer to limit thrombosis and Engender arterial Recanalization of Occluded vessels. Mol. Ther. 2019;27:1228–1241. doi: 10.1016/j.ymthe.2019.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oney S., Lam R.T., Bompiani K.M., Blake C.M., Quick G., Heidel J.D., Liu J.Y., Mack B.C., Davis M.E., Leong K.W., et al. Development of universal antidotes to control aptamer activity. Nat. Med. 2009;15:1224–1228. doi: 10.1038/nm.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullenger B., Woodruff R., Monroe D.M. Potent anticoagulant aptamer directed against factor IXa blocks macromolecular substrate interaction. J. Biol. Chem. 2012;287:12779–12786. doi: 10.1074/jbc.M111.300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nimjee S.M., Keys J.R., Pitoc G.A., Quick G., Rusconi C.P., Sullenger B.A. A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery. Mol. Ther. 2006;14:408–415. doi: 10.1016/j.ymthe.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Nimjee S.M., Povsic T.J., Sullenger B.A., Becker R.C. Translation and clinical development of antithrombotic aptamers. Nucleic Acid Ther. 2016;26:147–155. doi: 10.1089/nat.2015.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyke C.K., Steinhubl S.R., Kleiman N.S., Cannon R.O., Aberle L.G., Lin M., Myles S.K., Melloni C., Harrington R.A., Alexander J.H., et al. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation. 2006;114:2490–2497. doi: 10.1161/CIRCULATIONAHA.106.668434. [DOI] [PubMed] [Google Scholar]
- 31.Cohen M.G., Purdy D.A., Rossi J.S., Grinfeld L.R., Myles S.K., Aberle L.H., Greenbaum A.B., Fry E., Chan M.Y., Tonkens R.M., et al. First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation. 2010;122:614–622. doi: 10.1161/CIRCULATIONAHA.109.927756. [DOI] [PubMed] [Google Scholar]
- 32.Lunney J.K. Advances in swine biomedical model genomics. Int. J. Biol. Sci. 2007;3:179–184. doi: 10.7150/ijbs.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lelovas P.P., Kostomitsopoulos N.G., Xanthos T.T. A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 2014;53:432–438. [PMC free article] [PubMed] [Google Scholar]
- 34.Xanthos T., Bassiakou E., Koudouna E., Tsirikos-Karapanos N., Lelovas P., Papadimitriou D., Dontas I., Papadimitriou L. Baseline hemodynamics in anesthetized landrace-large white swine: reference values for research in cardiac arrest and cardiopulmonary resuscitation models. J. Am. Assoc. Lab. Anim. Sci. 2007;46:21–25. [PubMed] [Google Scholar]
- 35.Massicotte P., Mitchell L., Andrew M. A comparative study of coagulation systems in newborn animals. Pediatr. Res. 1986;20:961–965. doi: 10.1203/00006450-198610000-00014. [DOI] [PubMed] [Google Scholar]
- 36.MohanKumar K., Killingsworth C.R., McIlwain R.B., Timpa J.G., Jagadeeswaran R., Namachivayam K., Kurundkar A.R., Kelly D.R., Garzon S.A., Maheshwari A. Intestinal epithelial apoptosis initiates gut mucosal injury during extracorporeal membrane oxygenation in the newborn piglet. Lab. Invest. 2014;94:150–160. doi: 10.1038/labinvest.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagge L., Wahlberg T., Holmer E., Tyden H., Nystrom S.O., Malm T. Low-molecular-weight heparin (Fragmin) versus heparin for anticoagulation during cardiopulmonary bypass in open heart surgery, using a pig model. Blood Coagul. Fibrinolysis. 1994;5:265–272. doi: 10.1097/00001721-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Kurundkar A.R., Killingsworth C.R., McIlwain R.B., Timpa J.G., Hartman Y.E., He D., Karnatak R.K., Neel M.L., Clancy J.P., Anantharamaiah G.M., et al. Extracorporeal membrane oxygenation causes loss of intestinal epithelial barrier in the newborn piglet. Pediatr. Res. 2010;68:128–133. doi: 10.1203/PDR.0b013e3181e4c9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kostering H., Mast W.P., Kaethner T., Nebendahl K., Holtz W.H. Blood coagulation studies in domestic pigs (Hanover breed) and minipigs (Goettingen breed) Lab. Anim. 1983;17:346–349. doi: 10.1258/002367783781062262. [DOI] [PubMed] [Google Scholar]
- 40.Olsen A., Hansen A., Jespersen J., Marckmann P., Bladbjerg E. The pig as a model in blood coagulation and fibrinolysis research. Scand. J. Lab. Anim. Sci. 1999;26:10. [Google Scholar]
- 41.Munster A.M., Olsen A.K., Bladbjerg E.M. Usefulness of human coagulation and fibrinolysis assays in domestic pigs. Comp. Med. 2002;52:39–43. [PubMed] [Google Scholar]
- 42.Groenen M.A., Archibald A.L., Uenishi H., Tuggle C.K., Takeuchi Y., Rothschild M.F., Rogel-Gaillard C., Park C., Milan D., Megens H.J., et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. 2012;491:393–398. doi: 10.1038/nature11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nellenbach K.A., Nandi S., Kyu A., Sivadanam S., Guzzetta N.A., Brown A.C. Comparison of neonatal and adult fibrin clot properties between porcine and human plasma. Anesthesiology. 2020;132:1091–1101. doi: 10.1097/ALN.0000000000003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed C.R., Bonadonna D., Everitt J., Robinson V., Otto J., Tracy E.T. Coagulopathy characterized by rotational thromboelastometry in a porcine pediatric ECMO model. J. Extra Corpor. Technol. 2020;52:203–211. doi: 10.1182/ject-2000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittermayr M., Margreiter J., Velik-Salchner C., Klingler A., Streif W., Fries D., Innerhofer P. Effects of protamine and heparin can be detected and easily differentiated by modified thrombelastography (Rotem): an in vitro study. Br. J. Anaesth. 2005;95:310–316. doi: 10.1093/bja/aei197. [DOI] [PubMed] [Google Scholar]
- 46.ELSO. ECLS Registry Report & International Summary of Statistics. https://www.elso.org/Registry/InternationalSummary.aspx. 2020.
- 47.Hoffman M., Monroe D.M., Oliver J.A., Roberts H.R. Factors IXa and Xa play distinct roles in tissue factor-dependent initiation of coagulation. Blood. 1995;86:1794–1801. [PubMed] [Google Scholar]
- 48.Noubouossie D.F., Henderson M.W., Mooberry M., Ilich A., Ellsworth P., Piegore M., Skinner S.C., Pawlinski R., Welsby I., Renne T., et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood. 2020;135:755–765. doi: 10.1182/blood.2019001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sy E., Sklar M.C., Lequier L., Fan E., Kanji H.D. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: a systematic review and meta-analysis. J. Crit. Care. 2017;39:87–96. doi: 10.1016/j.jcrc.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Slaughter M.S., Pagani F.D., McGee E.C., Birks E.J., Cotts W.G., Gregoric I., Howard Frazier O., Icenogle T., Najjar S.S., Boyce S.W., et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J. Heart Lung Transpl. 2013;32:675–683. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 51.McManus M.L., Kevy S.V., Bower L.K., Hickey P.R. Coagulation factor deficiencies during initiation of extracorporeal membrane oxygenation. J. Pediatr. 1995;126:900–904. doi: 10.1016/s0022-3476(95)70205-9. [DOI] [PubMed] [Google Scholar]
- 52.Doyle A.J., Hunt B.J. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front. Med. 2018;5:352. doi: 10.3389/fmed.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanfilippo F., Asmussen S., Maybauer D.M., Santonocito C., Fraser J.F., Erdoes G., Maybauer M.O. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J. Intensive Care Med. 2017;32:312–319. doi: 10.1177/0885066616656333. [DOI] [PubMed] [Google Scholar]
- 54.Larsson M., Rayzman V., Nolte M.W., Nickel K.F., Bjorkqvist J., Jamsa A., Hardy M.P., Fries M., Schmidbauer S., Hedenqvist P., et al. A factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. Sci. Transl. Med. 2014;6:222ra217. doi: 10.1126/scitranslmed.3006804. [DOI] [PubMed] [Google Scholar]
- 55.Pireaux V., Tassignon J., Demoulin S., Derochette S., Borenstein N., Ente A., Fiette L., Douxfils J., Lancellotti P., Guyaux M., et al. Anticoagulation with an inhibitor of factors XIa and XIIa during cardiopulmonary bypass. J. Am. Coll. Cardiol. 2019;74:2178–2189. doi: 10.1016/j.jacc.2019.08.1028. [DOI] [PubMed] [Google Scholar]
- 56.May F., Krupka J., Fries M., Thielmann I., Pragst I., Weimer T., Panousis C., Nieswandt B., Stoll G., Dickneite G., et al. FXIIa inhibitor rHA-Infestin-4: safe thromboprotection in experimental venous, arterial and foreign surface-induced thrombosis. Br. J. Haematol. 2016;173:769–778. doi: 10.1111/bjh.13990. [DOI] [PubMed] [Google Scholar]
- 57.Gopinath S.C., Shikamoto Y., Mizuno H., Kumar P.K. A potent anti-coagulant RNA aptamer inhibits blood coagulation by specifically blocking the extrinsic clotting pathway. Thromb. Haemost. 2006;95:767–771. [PubMed] [Google Scholar]
- 58.Guervilly C., Bonifay A., Burtey S., Sabatier F., Cauchois R., Abdili E., Arnaud L., Lano G., Pietri L., Robert T., et al. Dissemination of extreme levels of extracellular vesicles: tissue factor activity in patients with severe COVID-19. Blood Adv. 2021;5:628–634. doi: 10.1182/bloodadvances.2020003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stafford D.W. Extravascular FIX and coagulation. Thromb. J. 2016;14:35. doi: 10.1186/s12959-016-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stern D.M., Knitter G., Kisiel W., Nawroth P.P. In vivo evidence of intravascular binding sites for coagulation factor IX. Br. J. Haematol. 1987;66:227–232. doi: 10.1111/j.1365-2141.1987.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 61.Ganson N.J., Povsic T.J., Sullenger B.A., Alexander J.H., Zelenkofske S.L., Sailstad J.M., Rusconi C.P., Hershfield M.S. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016;137:1610–1613.e1617. doi: 10.1016/j.jaci.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Povsic T.J., Lawrence M.G., Lincoff A.M., Mehran R., Rusconi C.P., Zelenkofske S.L., Huang Z., Sailstad J., Armstrong P.W., Steg P.G., et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J. Allergy Clin. Immunol. 2016;138:1712–1715. doi: 10.1016/j.jaci.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 63.Gupta P., Robertson M.J., Beam B., Gossett J.M., Schmitz M.L., Carroll C.L., Edwards J.D., Fortenberry J.D., Butt W. Relationship of ECMO duration with outcomes after pediatric cardiac surgery: a multi-institutional analysis. Minerva Anestesiol. 2015;81:619–627. [PubMed] [Google Scholar]
- 64.Batts S.G., Mu T.S., Uyehara-Lock J.H., Murata L.A., Uyehara C.F. ECMO maintains cerebral blood flow during endotoxic shock in piglets. ASAIO J. 2016;62:732–736. doi: 10.1097/MAT.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Povsic T.J., Vavalle J.P., Aberle L.H., Kasprzak J.D., Cohen M.G., Mehran R., Bode C., Buller C.E., Montalescot G., Cornel J.H., et al. A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial. Eur. Heart J. 2013;34:2481–2489. doi: 10.1093/eurheartj/ehs232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walchak A.C., Zarow G.J., Gamble C.S., Conley S.P., Hampshire V. Simple blood typing and cross matching techniques in swine. Lab. Anim. 2016;45:366–368. doi: 10.1038/laban.1120. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka K.A., Szlam F., Rusconi C.P., Levy J.H. In-vitro evaluation of anti-factor IXa aptamer on thrombin generation, clotting time, and viscoelastometry. Thromb. Haemost. 2009;101:827–833. [PubMed] [Google Scholar]
- 68.Lequier L., Annich G., Al-Ibrahim O., Bembea M.M., Brodie D., Brogan T., Buckvold S., Chicoine L., Conrad S., Cooper D., et al. ELSO Anticoagulation Guideline. 2014. https://www.elso.org/portals/0/files/elsoanticoagulationguideline8-2014-table-contents.pdf
- 69.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





