Abstract
High altitude cerebral edema does not fall in routine definition of hypoxia and requires alternative therapeutic strategies. 12/15-Lipoxygenase (12/15 LOX), a key proinflammatory lipid peroxidative enzyme which site specifically inserts into cellular and subcellular membranes and plays an instrumental role in hypobaric hypoxia induced neuropathogenesis. Mitochondria, the master regulator organelles for oxygen consumption and ATP generation are sensitive to intracellular oxygen perturbations and are associated with activation of apoptosis based cell death cascades that seal the fate of the cell. The mechanistic involvement of 12/15 LOX in mitochondria mediated cell death in brain microenvironment during hypobaric hypoxia conditions can be an interesting preposition. In the present study, we have investigated underlying involvement of 12/15 LOX in hypobaric hypoxia (HH) induced disturbance in mitochondrial integrity and its relation with neuronal apoptosis. Male Balb/c mice subjected to simulated HH condition for three consecutive days showed robust increase in intra-hippocampal 12(S)HETE (12/15 LOX metabolite), which was significantly reduced following baicalein (12/15 LOX Inhibitor) treatment. The elevated level of 12(S)HETE following hypobaric hypoxia condition correlated with simultaneous increase in expression of 12/15 LOX in neurons and microglia lining the hippocampal CA3 region. Further, 12/15 LOX gets embedded onto the periphery of mitochondria following HH and a strong correlation has been observed with loss of mitochondrial integrity as evident from increased cytochrome-c in the cytosolic compartment and a subsequent upregulated activity of Caspase-3 and Caspase-9 as well as Bax/Bcl-2 expression ratio. The observed effects seen under HH were reversed upon treatment with baicalein suggesting a critical role of 12/15 LOX in HH induced mitochondrial damage Further, the hypobaric hypoxia-mediated increase in hippocampal pAKT and pmTOR protein expression were significantly ameliorated following 12/15 LOX inhibition, suggesting a mitochondrial involvement. We hereby demonstrate the contribution of 12/15 LOX in disorienting mitochondrial integrity with subsequent release of cytochrome-c in cytosol which drives the neuronal cells to intrinsic mode of cell death during hypobaric hypoxia. The protective role of baicalein by inhibition of 12/15 LOX dependent neuronal cell death and preservation of mitochondrial integrity suggests it to be a plausible therapeutic target in CNS related disorders.
Keywords: Hypobaric hypoxia, 12/15-Lipoxygenase, Mitochondrial integrity, Hippocampus, Neuroprotection
Graphical abstract
1. Introduction
Hypobaric hypoxia encountered at high altitude is known to cause neurodegeneration in the hippocampus region of the brain mediated through glutamate excitotoxicity, cholinergic hypofunction, oxidative/nitrosative stress, inflammation, apoptosis and mitochondrial dysfunction [[1], [2], [3], [4], [5]]. Under hypoxia condition, low availability of oxygen not only results in production of excessive free oxy-radicals but also weakens antioxidant defense mechanism which exert extreme oxidative stress in hippocampal brain region [3]. It has been documented that increased oxidative stress results in lipid peroxidation, DNA damage, organelle membrane damage, altered gene expression which consequently may command cells to die [4,6]. However, the precise mechanism behind hippocampal neurodegeneration during hypobaric hypoxia largely remains unknown.
Activation of 12/15 LOX mediated pathway results in progression of various CNS related disorders [[7], [8], [9]]. LOXs are non-heme iron containing dioxygenase enzymes that catalyse the insertion of molecular oxygen into free and esterified polyunsaturated fatty acids preferably arachidonic acid. Among LOX, 12/15-LOX is the predominant form of LOX that is present in brain and its presence has been well documented in neurons and glial cells throughout in cerebrum, basal ganglia as well as hippocampus [10]. 12/15-LOX expression and metabolic product levels were found to be increased in neuronal cells in an experimental model of brain ischemia-reperfusion injury [9]. Based on its pro-oxidant properties, 12/15-LOX enzyme has been considered as the crucial source of neuronal oxidative stress as its genetic absence leads to significant reduction of CNS oxidative stress in APOE-deficient mice [11]. 12/15-Lipoxygenase (LOX) and its metabolites previously have been reported to be prime propagators to exaggerate oxidative stress under hypoxic condition [9,12].
It has been documented that neuronal cells undergo caspase dependent Bax/Bcl-2 mediated apoptosis in hypobaric hypoxia paradigm [2,4,13]. Jain et al., 2015 also reported that hypobaric hypoxia stress severely altered the mitochondrial dynamics by decreasing the activities of mitochondria energy synthesizing complexes (complex I, II, III, IV) as well as interrupting mitochondrial functionality, therefore, distorting the major reservoir of cellular energy [2]. Several lines of evidences suggest that 12/15-LOX and its metabolites directly contribute to programmed cell death in Alzheimer's disease [14], myocardial ischemia/reperfusion, diabetics induced neuropathy [15]. Whereas inhibition of 12/15-LOX by baicalein prevents apoptosis via reducing caspase-3,9 activity, decreasing Bax/Bcl2 ratio and modulating pAkt pathway [16]. Pallast et al., 2009 observed upregulated 12/15 LOX in oxidative stress challenged neuronal HT22 cells which resulted in disturbances in mitochondrial membrane potential. The mitochondrial dysregulation was restored following 12/15 LOX inhibition. The damaging effect of 12/15-LOX is contributed by its unique property of direct attack on cell organelle membrane causing lipid peroxidation thereby altering cell organelle membrane structure as well as affecting its functions [17]. Circumstantial clue toward involvement of 12/15-LOX in mechanism of programmed organelle degradation during physiological process particularly in RBC maturation, bring forth 12/15-LOX on central stage of cellular damage [17,18]. Documented evidences revealed the damaging role of 12/15-LOX in neuronal degeneration in various CNS related pathological conditions [[7], [8], [9]]. Therefore, we hypothesized that activation of 12/15-LOX may lead to inevitable neuronal loss by modulating neuronal mitochondria dynamics consequently causing mitochondria mediated cell death during hypobaric hypoxia. Previously, we have reported that Baicalein, a flavonoid isolated from root of Scutellaria baicalensis and a selective inhibitor of 12/15 LOX, provide neuroprotection in animal model of hypobaric hypoxia [12] by reducing oxidative/nitrosative stress and altering cholinergic hypofunction. In the present study, the underlying involvement of 12/15-LOX in hypobaric hypoxia mediated neuronal damage and apoptosis have been studied and a possible mechanism for hypobaric hypoxia induced injury has been illustrated.
2. Material and methods
2.1. Experimental animals
All experiments were performed on adult male Balb/c mice (8–10 weeks old, 25–30 g) maintained in an animal research facility with 12 h dark/light cycle and 25 ± 2 °C temperatures at Dr. B. R. Ambedkar Center for Biomedical Research, University of Delhi, Delhi. The experimental protocols were approved by the Institutional animal ethics committee (IAEC) of Dr. B. R. Ambedkar Center for Biomedical Research, University of Delhi and ARRIVE guidelines were followed. The animal house is registered for experimentation on animals with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Animal Welfare Division, Government of India. Animals had free access to food and water ad libitum. All efforts were made to minimize animal suffering and to decrease the number of animals used.
2.2. Induction of hypobaric hypoxia
Male Balb/c mice were exposed to hypobaric hypoxia (corresponding to an altitude of 25,000 feet for 6 h daily) for 3 days in a specially designed decompression chamber (Model No.SS7001, Seven Stars, India) as described previously [5]. Fresh air was continuously flushed at the rate of 8 L/minute in the decompression chamber. Normoxia (N) and Normoxia treated with Baiclein (NBA) group of animals were kept at room air pressure. Mice had free access to food and water. The rate of ascent to the desired altitude and descent to sea level were at 700 ft/min. The chamber temperature was maintained at 26 ± 2 °C.
2.3. Experimental groups
Balb/c mice were randomly divided into four groups as follows: Normoxia (N), Normoxia treated with Baicalein (NBA, 10 mg/kg bow), Hypoxia (H) and Hypoxia treated with Baicalein (HBA, 10 mg/kg bw). NBA and HBA groups of animals were administered with baicalein intra-peritoneally for all three day 30 min before induction of hypobaric hypoxia. The dose regime of baicalein (10 mg/kg bow, i.p.) was based on our previously published study where we have reported that 10 mg/kg baicalein was effective in mitigating associative memory deficit along with oxidative stress evoked by hypobaric hypoxia [12].
2.4. Hippocampus homogenate and plasma preparation
The total hippocampal homogenate was prepared for immunoblotting and biochemical analysis. All four groups of animals were sacrificed under euthanasia three days hypobaric hypoxia exposure and 24 h of reperfusion. The brains were harvested to dissect hippocampus [19], tissue was weighed immediately and homogenized in 10 vol of ice cold RIPA buffer (containing 1% IPEGAL CA 630, 10 mM Tris, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1X protease inhibitor cocktail). The homogenate was kept on ice for 10 min to enhance cell lysis, then centrifuged at 16,000 g for 20 min at 4 °C and the supernatant was stored at −80 °C until used.
The cytosolic extract from hippocampal tissue was used to estimate cytochrome-c by ELISA. The hippocampus was weighed and homogenized in 10 vol of ice cold PBS containing protease inhibitor cocktail. The homogenate was centrifuged for 5 min at 800 g and obtained supernatant was again centrifuged at 11,000 g for 25 min to get cytosolic extract. Protein concentration was estimated by the method of Bradford using a spectrophotometer (Spectramax M2e, Molecular devices, Sunnyvale, CA, USA) at 595 nm.
2.5. Quantitative measurement of 12 (S) HETE using LC-ESI MS
Previously reported sample preparation strategies were used as references to prepare samples from each group [20]. About 20 mg of accurately weighed hippocampal tissue was added to the tube containing 500 μL of ice-cold PBS-0.1% BHT. The samples were then homogenized using hand held Homogenizer. 1 ml of n-hexane/ethyl acetate (1:1, v/v) was added to the aqueous homogenate and vortex mixed for 2 min followed by centrifugation at 3500 rpm for 3 min. The upper organic layer was harvested and the extraction process was repeated two more times. Lower aqueous layer was used for protein assay as the amount of individual eicosanoids was normalized with respect to the total protein content. The organic phases obtained from three extractions were pooled and then evaporated to dryness under a stream of nitrogen at room temperature. Each sample was then reconstituted in 25 μL of methanol and used for LC-ESI MS analysis.
12(S) HETE profiles in standards and sample were performed on Dionex UltiMate 3000 system equipped with a C5-reverse phase column (Phenomenex) using a gradient of 0.1% formic acid in water and 0.1% formic acid in acetonitrile at a flow rate of 0.4 ml/min coupled to a AB SCIEX Triple TOF 5600 System mass spectrometer equipped with a Turbo Ion Spray electron spray ionization (ESI) source. Multiple reaction monitoring (MRM) experiments were performed using negative ESI mode. Data processing was performed using the Multi Quant software version 3.0. We identified 12(S) HETE peak at 3 min and 45 s which was confirmed by running 12 (S) HETE standards along with the samples. The area of the peaks were identified to deduce the concentration of 12(S) HETE. The relative quantification of 12 (S) HETE levels were performed in different groups and concentrations of respective groups were plotted as box graph.
2.6. Reverse transcriptase PCR amplification
Total RNA was isolated from hippocampus using TRI reagent (Ambion Inc.) and processed for reverse transcription PCR amplification (RT-PCR) as described earlier [21]. 5 μg of total RNA was used to perform reverse transcription PCR using first strand cDNA kit (Thermo Fisher Scientific Inc). For polymerase chain reaction, 1 μl of cDNA, 1 μl of primers (each 5 pmole), 2 μl of 10 × reaction buffer (Geneaid Biotech) and 1 unit of DNA polymerase (Geneaid Biotech) were added to 20 μl with DNase/RNase free water. PCR amplification was carried out by using a set of specific primers on thermo cycler (Eppendrof). PCR products were resolved on 2% agarose gel and densitometry analysis of PCR products was carried out using Image J.
2.7. Western blot analysis
Hippocampal homogenate equivalent to 30–40 μg of total protein was denatured with Laemmli loading buffer by heating at 95 °C for 5 min and the samples were resolved on 10–12.5% SDS-PAGE and electro-blotted to nitrocellulose membrane. Blots were blocked with 5% BSA in PBS overnight at 4 °C and then incubated with respective primary antibodies - rabbit polyclonal IgG anti-mouse pAkt (1: 300); rabbit polyclonal IgG anti-mouse pmTOR (1:100); rabbit polyclonal IgG anti-mouse Bcl-2, rabbit polyclonal IgG anti-mouse Bax (1∶800); rabbit anti-mouse GAPDH (1∶500) in 5% BSA in PBS for 2.5 h at room temperature followed by washing thrice with 0.05% PBST. The blots were incubated with HRP conjugated goat anti-rabbit secondary antibody (1: 8000) (Abcam, USA) in 5% PBS-BSA at room temperature for 2 h followed by washing with 0.05% PBST. The blots were developed utilizing luminol based enhanced chemiluminescence (ECL) detection method. The blot images were captured and band density analysis was performed using Image J. The values of the band density were normalized to the level of respective GAPDH intensity. All antibodies used in a study unless or otherwise stated were procured from Santacruz Biotech, Santacruz, CA, USA.
2.8. Immunofluorescence
The animals were anesthetized (thiopental sodium 80 mg/kg i.p.) and perfused intracardially with heparinized (10 U/ml) 0.1 M PBS (pH 7.4) followed by 4% paraformaldehyde in PBS. The whole brains were harvested and stored in a solution of 30% sucrose and 10% glycerol at −80 °C until used. Thin sections (5–8 μm) of the hippocampal region were prepared using cryotome (CM 1850 Leica, Heidelberg, Germany) as described previously and then processed for Immunofluorescence, double Immunofluorescence and TUNEL staining studies.
2.9. Localization of 12/15-LOX in hippocampus CA3 region by double Immunofluorescence method
Co localization of 12/15-LOX with neurons, activated Microglia cells and mitochondria in CA3 region of hippocampus was analyzed by double staining with an antibody to rabbit polyclonal IgG anti-mouse 12/15-LOX (Bioss Inc., USA 1:50); goat polyclonal IgG anti-mouse NeuN (1:50); goat polyclonal IgG anti-mouse IBA (1:50) and goat polyclonal IgG anti-mouse cytochrome b (1:50). Briefly, the sections were permeabilized with 0.1% IPEGAL CA 630 in PBS (pH 7.4) and blocked with 3% BSA in PBS for 1.5 h at 37 °C followed by overnight incubation with the cocktail of primary antibodies containing rabbit polyclonal IgG anti-mouse 12/15-LOX antibody with different cell and mitochondria specific markers in different experimental sets. The sections were then washed thrice with PBS, incubated with Cruz flour labeled respective secondary antibody for 1 h in a dark room and counterstained with Hoechst 33,342 (Sigma-Aldrich). The sections were mounted with Vectashield antifade mounting media and observed under 60 × objectives unless otherwise stated on Leica confocal microscope (TSC-SP5). The images acquired were analysis with Leica LAS AF Lite software version 2.6. The localization of 12/15-LOX with NeuN+ and IBA + cells was checked under 100X objective with Nikon Eclipse E600 microscope attached to the Evolution VF camera.
2.10. Detection of 12/15-LOX expression in hippocampus CA3 region
The expression profile of 12/15-LOX in CA3 hippocampus region during hypobaric hypoxia was determined by immunostaining of hippocampal section with an antibody to rabbit polyclonal IgG anti-mouse 12/15-LOX (1:50) followed by incubation with mouse anti-rabbit IgG-PE secondary antibody (1:300) and counterstained with Hoechst 33,342. The sections were observed with Leica confocal microscope (TSC-SP5) and images were acquired at a magnification of 600X followed by analysis with Leica LAS AF Lite software version 2.6.
2.11. TUNELassay for estimation of apoptotic neuronal death in hippocampus CA3 region
The apoptotic neuronal death in hippocampal CA3 region was deduced by terminal deoxynucleotidyl transferase mediated biotinylated dUTP nick end-labeling (TUNEL) staining was performed with the Apo-BrdU-IHC In Situ DNA Fragmentation Assay Kit as per the manufacturer's specifications (Novus biologicals., USA). After TUNEL reaction, the sections were counterstained with methyl green. The sections were mounted with mounting media (Himedia) and observed under 10 × objectives on Nikon Eclipse E600 microscope with an Evolution VF camera. The captured images were analyzed with Image-Pro Plus version 5.1.2 software (Media Cybernetics Bethesda, MD, USA). The counting of TUNEL positive cells was performed manually by the investigator blinded to experimental groups.
2.12. Caspase assay
The Caspase-3 and 9 activity assays were performed using Caspase Fluorometric Assay kit (BioVision Inc., USA). The assay is based on detection of the intensity of fluorescence produced by cleavage of specific sequence (substrate) coupled with a fluorogenic compound, 7-amino-4-trifluoromethyl coumarin (AFC), which serves as a substrate. The tissue homogenate equivalent to 100 μg of total protein was diluted with 2 × reaction buffer containing 10 mM DTT. 5 μl of the respective substrate (Caspase-3: DEVD-AFC; Caspase-9: LEHD-AFC) were added to the reaction mixture in micro-plate and incubated at 37 °C for 1–2 h. The fluorescence intensity was measured using a fluorescence microplate reader (Spectramax M2e, Molecular devices, Sunnyvale, CA, USA).
2.13. Cytochrome-c assay in hippocampal cytosol
Cytochrome-c is small mitochondrial residing protein which gets released in cytosol from mitochondria upon apoptotic stimuli and triggers the mitochondria mediated caspase dependent programmed cell death. The cytochrome-c in hippocampal cytosol was determined according to the manufacturer's protocol (Wuhan USCN Co., USA). Hippocampal homogenate corresponding to 20 μg was used to deduce the concentration of cytochrome-c into the cytosol in various groups through sandwich ELISA. The concentration of cytochrome-c was determined by plotting a standard curve for cytochrome-c and the result was expressed in ng/20 μg protein.
2.14. Immunoelectron microscopy
The double immunostaining results suggested that most of 12/15-LOX colocalised with mitochondrial marker during hypobaric hypoxia. We further confirm the sub cellular localization of the 12/15-LOX in-vivo by the immunogold labeling method. The animals were anesthetized (pentobarbital-sodium 50 mg/kg i.p.) and perfused intracardially with heparinized (10 U/ml) 0.1 M PBS (pH 7.4) followed by 4% paraformaldehyde in PBS, the whole brains were harvested to dissect hippocampi further CA3 region was carefully isolated from hippocampus and fixed in a fixative solution (4% glutaraldehye + 2% paraformaldehyde) overnight at 4 °C. After washing in 0.1 M PBS, the tissue was dehydrated in a graded ethanol series and afterwards embedded in LR White. Ultrathin sections were mounted on nickel grids and were incubated for 2 h with the rabbit anti mouse 12/15 LOX antibody at 20 °C. After washing, the sections were incubated with a gold (particle diameter 20 μm) conjugated anti-rabbit IgG antibody. The sections were then dried and stained with uranyl acetate/lead citrate and stained section was examined on TECNAI 200 kV TEM (Fei, Electron Optics).
2.15. Statistical analysis
Data were analyzed using Graph Pad Prism 5.0. All results were expressed as mean ± S.E.M of groups (I-IV) of 5–6 animals measured individually. Data were analyzed by one way analysis of variance (ANOVA) followed by post hoc comparison using Tukey's multiple comparison test. The p value < 0.05 was considered statistically significant.
3. Results
3.1. Hypobaric hypoxia induces alteration in 12 (S) -HETE in hippocampal homogenate
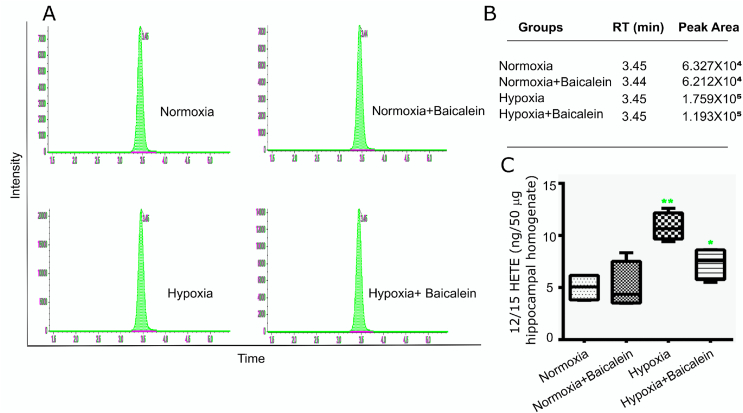
12(S)HETE (A product of 12/15 Lipoxygenase activity) levels were estimated in hippocampal homogenates of mice from all the groups using a sensitive method of ESI-MS. The samples were subjected to ESI-MS and 12(S)HETE peak was observed at 3 min and 45 s. The concentration of 12(S)HETE was deduced from the standards run along with the sample (Fig. 1C). We observed significantly increase in 12(S)HETE concentration in hippocampus homogenate of hypoxia (H) group as compared with Normoxia (N), Normoxia treated baicalein (NBA) group (p < 0.01). Further, it was also revealed that baicalein administration significantly lowered the 12(S)HETE concentrations in hippocampus (p < 0.05) of hypoxia treated Baicalein (HBA) group as compared with sham treated hypoxia exposed group (p < 0.05) (Fig. 1C).
Fig. 1.
Quantitative measurement of 12(S) HETE in hippocampal homogenate. Level of 12(S) HETE was measured by LC-ESI MS among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA).The data for which is shown as histogram [A] and its analysis as Table [B] and representation of relative conc. of 12(S) HETE as box plot [C]. Statistical analysis among groups is shown by p.value represented as **p < 0.01 (H v/s N), *p < 0.05 (HBA v/s H).
3.2. Hypobaric hypoxia increases 12/15-LOX expression in the hippocampus
Immunostaining of 12/15-LOX in CA3 sector of the hippocampus revealed that 12/15-LOX protein expression was significantly increased in Hypoxia group when compared with Normoxia (N) and Normoxia treated Baicalein groups (NBA) (p < 0.001) that robustly confirm our previous findings that hypobaric hypoxia resulted in significant increase in 12/15-LOX mRNA and protein expression [12]. Further, baicalein administration upon hypobaric hypoxia exposure partially decreased 12/15-LOX immunoreactivity in the CA3 region of the hippocampus (p < 0.05) (Fig. 2).
Fig. 2.
12/15 LOX expression in CA3 region of the Hippocampus. 12/15 LOX expression was evaluated by Immunoflouroscence microscopy among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA). Data are represented as microscopic images at 40X [A] and a bar graph of the relative fluorescence intensity [B]. Statistical analysis among groups is shown by p.value represented as **p < 0.01 (N v/s H, NBA, HBA), ***p < 0.01 (N v/s H, NBA, HBA).
3.3. 12/15-LOX widely expressed in neurons and microglia cells in CA3 hippocampus region
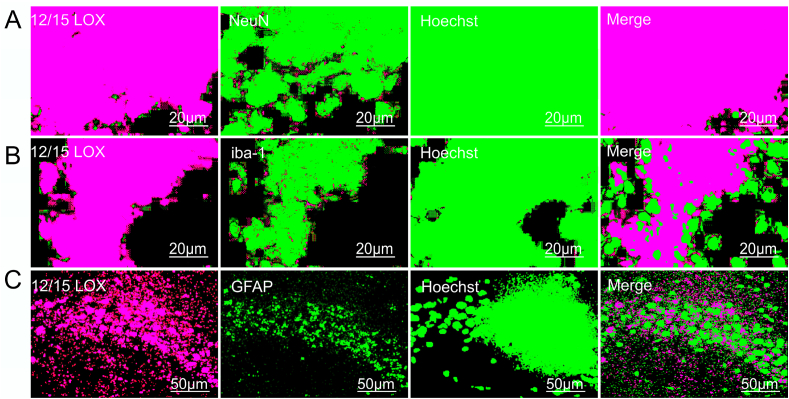
To confirm which cell type in CA3 region of the hippocampus expresses increased immounoreactivity of 12/15 LOX, we performed double labeling of 12/15 LOX in combination with an antibody directed against cell specific markers viz. NeuN (Neuronal cell), IBA (Activated Microglial cells), GFAP (Astrocytes). Our results revealed robust expression of 12/15-LOX in activated Neurons (Fig. 3A) as well as activated Microglia cells (Fig. 3B). The colocalization as assessed by Pearson's correlation and overlapping coefficient indicated significant expression of 12/15-LOX in NeuN+ (r2 = 0.8819, R = 0.981) and Iba+ cells (r2 = 0.75957, R = 0.964) following hypobaric hypoxia injury, whereas GFAP+ cells (Fig. 3C) did not show significant expression of 12/15-LOX in the same group of samples, indicating 12/15-LOX did not express in Astrocytes in response to hypobaric hypoxia insult (Fig. 3C).
Fig. 3.
Colocalization analysis of 12/15 LOX with marker of Neuron (NeuN), Microglia (Iba) and Astrocytes (GFAP) in CA3 region of the Hippocampus. 12/15 LOX Colocalization was assessed by confocal microscopy among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA) using antibodies (Ab) targeting 12/15 LOX in either of the combination of Ab targeting NeuN, Iba, GFAP which were further counter stained with Hoechst 33,342. Data are represented as photomicrographs at 600X (A,B) and 400X (C) magnification.
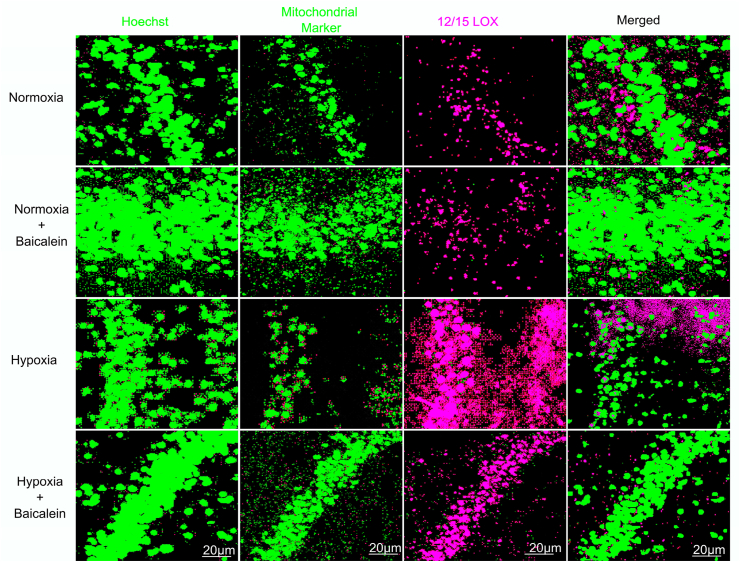
3.4. 12/15-LOX gets localized in mitochondria following hypobaric hypoxia
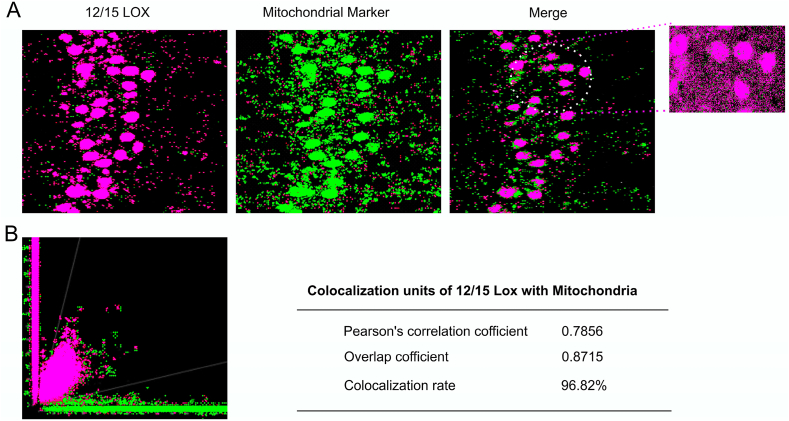
Previous studies reported mitochondria as vulnerable targets for 12/15-LOX and its known propensity for binding to organelle membranes, prompting us to test whether 12/15 LOX immune reactivity directly associates with mitochondria after hypobaric hypoxia. We performed double immunostaining of 12/15-LOX with mitochondrial protein cytochrome b (a typical mitochondrial marker) (Fig. 4, Fig. 5). We found that 12/15-LOX (red) and Mitochondrial marker (green) showed Colocalization (yellow) in hypoxia group and other groups exhibit little amount of 12/15 LOX immunoreactivity. The quantitative analysis was conducted using Leica LAS AF Lite software version 2.6 (Fig. 5). The green (mitochondria) and the red (12/15 LOX) colors were designated as ‘channel 1’ and ‘channel 2’, respectively. The merged ICA plots of channel 1 (CH1) and channel 2 (CH2) were generated where we observed increased yellow pixels (overlapped pixel) which represent greater level of colocalization. Statistical analysis of the data demonstrated that Pearson's correlation coefficient and overlapping coefficient (overlap of green and red pixel) is 0.7856 as well as 0.8715 respectively signifying hypobaric hypoxia promoted 12/15- LOX to mitochondrial translocation (Fig. 5 B).
Fig. 4.
Co-expression of 12/15 LOX with mitochondrial marker in CA3 region of the Hippocampus. 12/15 LOX Colocalization among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA) was assessed by confocal microscopy using antibodies (Ab) targeting 12/15 LOX in combination with Cytochrome b (Mitochondrial Marker) and further counter stained with Hoechst 33,342. Data are represented as photomicrographs at 600X magnification.
Fig. 5.
Colocalization analysis of 12/15 LOX with mitochondria in CA3 region of the Hippocampus. 12/15 LOX Colocalization was assessed by confocal microscopy using antibodies (Ab) targeting 12/15 LOX in combination with Cytochrome b (Mitochondrial Marker) and counter stained with Hoechst 33,342. Data are represented as photomicrographs (600X) and an ICA plot (B) which was generated by LAS AF Leica software showing red pixels(12/15 LOX), greens pixels (mito. marker) and yellow (merge) alongside a table showing Colocalization indices (Pearson's correlation coefficient, Overlap coefficient, Colocalization rate). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
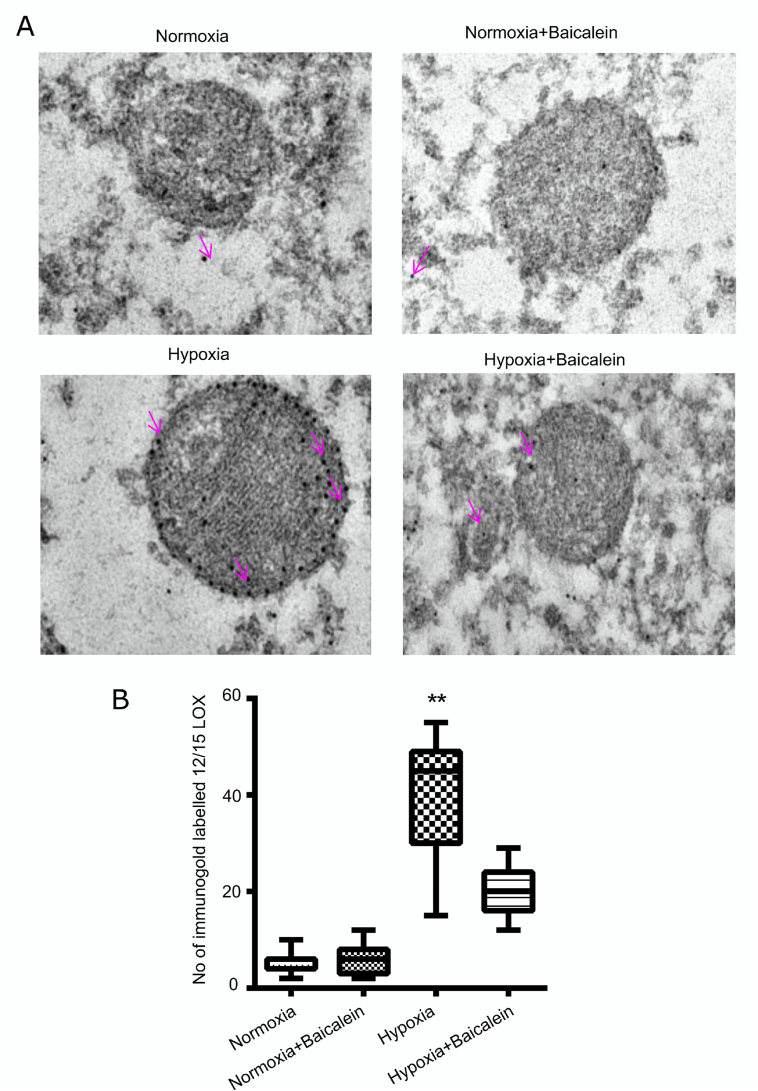
3.5. Immunogold localization of 12/15-LOX to mitochondria in CA3 region of hippocampus after exposure to hypobaric hypoxia
To validate our observation obtained during the double Immunofluorescence experiment, we performed immunogold labeling of 12/15-LOX in the CA3 region of hippocampi for various groups of mice. In hypobaric hypoxia group, we observed significantly increased number of 12/15-LOX antibodies tagged with gold particles, particularly on mitochondria (p < 0.01). We found significantly less 12/15-LOX stained gold particles, mostly in the cytoplasm of cells in Normoxia (N), Normoxia treated with Baicalein (NBA) and hypoxia treated with Baicalein (HBA) group as compared to hypobaric hypoxia (H) group (Fig. 6 A, B). Above observation.
Fig. 6.
Electron Microscopy to identify the localization of 12/15 LOX on mitochondrial membrane. Transmission electron microscopy (TEM) was performed for showing presence of 12/15 LOX onto mitochondrial compartment of cells among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA). Data are represented as photomicrographs [A] and its analysis as bar graph [B]. **p < 0.01H v/s N.
confirmed that hypobaric hypoxia exposure resulted in the 12/15-LOX attachment to mitochondria membrane further resulting in perturbation of the mitochondrial membrane.
3.6. 12/15-LOX inhibition ameliorates hypobaric hypoxia mediated apoptosis in hippocampus
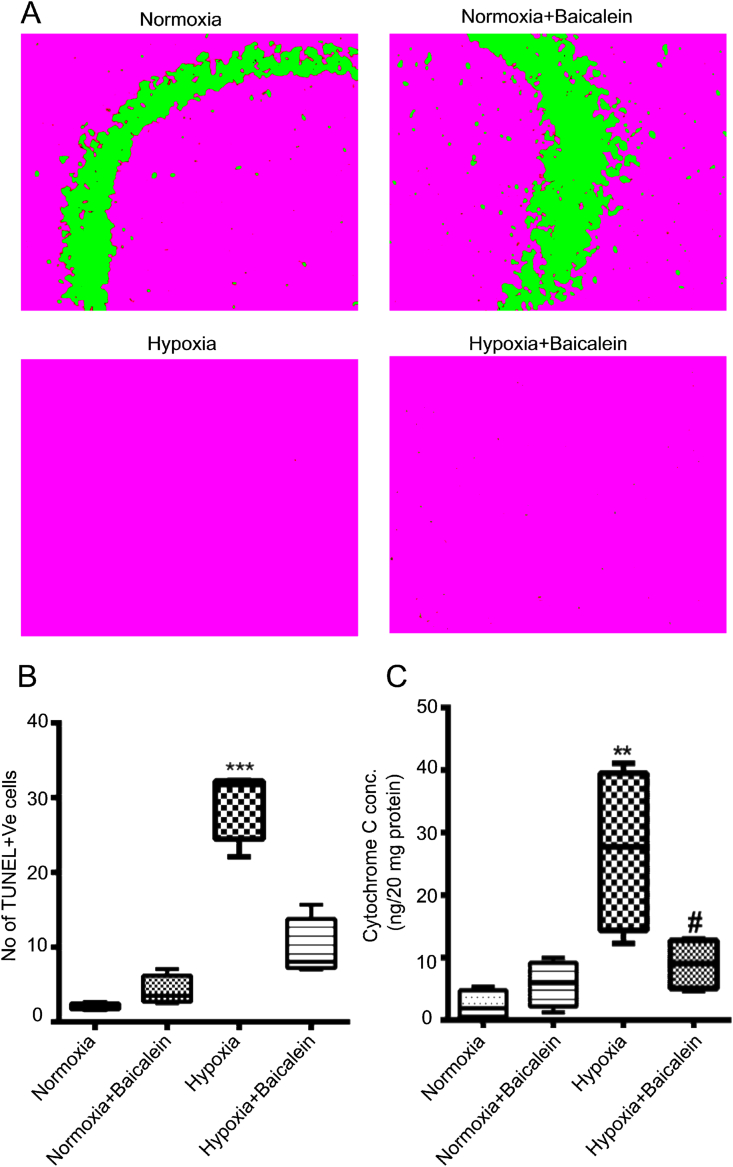
The results of the present study demonstrated that hypobaric hypoxia exposure resulted in consistent and significant apoptosis (TUNEL positive cells) in the CA3 region of the hippocampus (p < 0.001) as compared to Normoxia group. TUNEL positive cells were not detected in sections of Normoxia, Normoxia treated by Baicalein group. However, it was found that 12/15-LOXinhibition by baicalein significantly decreased TUNEL positive cells (p < 0.001) in hypobaric hypoxia paradigm (Fig. 7 A, B).
Fig. 7.
12/15 LOX influences the apoptotic status of cells in CA3 region of the Hippocampus following hypobaric hypoxia. The extent of apoptosis was analyzed by TUNEL analysis and Cytochrome C release into cytosol among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA). Data are represented as photomicrographs [A] and its analysis as bar graph [B]. The cytosolic concentration of Cyctochrome C was analyzed by ELISA and is shown as bar graph (C). Statistical analysis was done among groups and is shown by p.value represented as **p < 0.01 (N v/s H), ***p < 0.001 (N v/s H), #p < 0.01 (NBA v/s HBA).
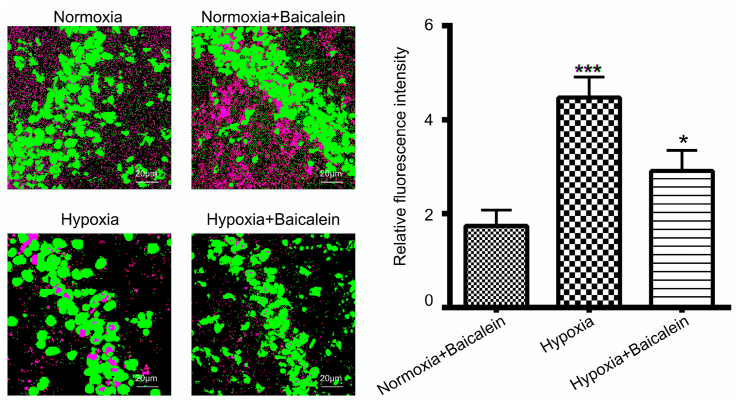
3.7. 12/15-LOX inhibitor baicalein decreases cytosolic cytochrome-c in hippocampus following hypobaric hypoxia
Recently, it has been reported that subsequent increase in 12/15-LOX during oxidative stress results in disturbance in mitochondria membrane potential which ultimately leads to release of apoptotic molecule cytochrome-c. We measured the amount of cytochrome-c in the hippocampal cytosol of various groups of mice. We observed significantly increased concentration of cytochrome-c in the cytosolic compartment of the hypobaric hypoxia group as compared to Normoxia, Normoxia treated with Baicalein (p < 0.01). Further, Baicalein administration significantly reduced its concentration (p < 0.05) in the hippocampal cytosol (Fig. 7C).
3.7.1. 12/15-LOX inhibition protects hippocampal neurons from the mitochondria mediated intrinsic pathway
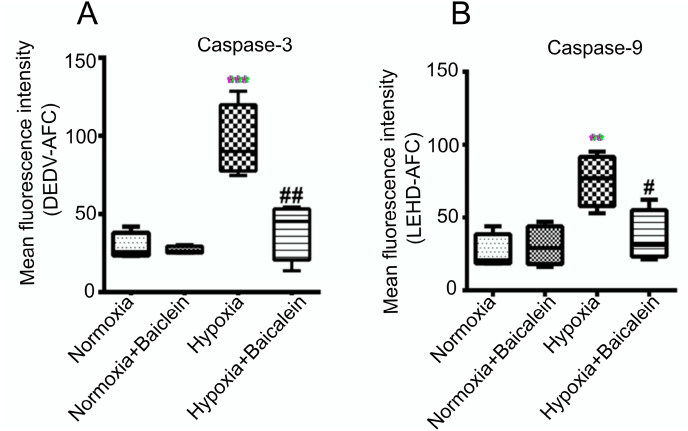
Cytochrome-c release from mitochondria to cytosol has been considered as commitment step of mitochondria mediated cell death pathway. Caspases are the main executors of apoptosis. Moreover, Caspase-9 is an initiator caspase of mitochondria mediated pathway and Caspase-3 acts as executor caspase. We observed a significant increase in the activity of caspase-9 (p < 0.01) as well as Caspase-3 (p < 0.001) during Hypobaric Hypoxia when compared with Normoxia, Normoxia treated with Baicalein and hypoxia treated Baicalein groups showed significant reduction in activity of caspase-9 (p < 0.05) and caspase-3 (p < 0.01) (Fig. 8 A&B).
Fig. 8.
Caspase activity Level in CA3 region of the Hippocampus.The level of Caspase 3 and Caspase-9 was estimated by fluorometric assay among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA). Data are represented as bar graphs [A, B]. Statistical analysis was done among groups and is shown by p.value represented as **p < 0.01 (N v/s H), ***p < 0.001 (N v/s H), #p < 0.05 (NBA v/s HBA), ##p < 0.01 (NBA v/s HBA).
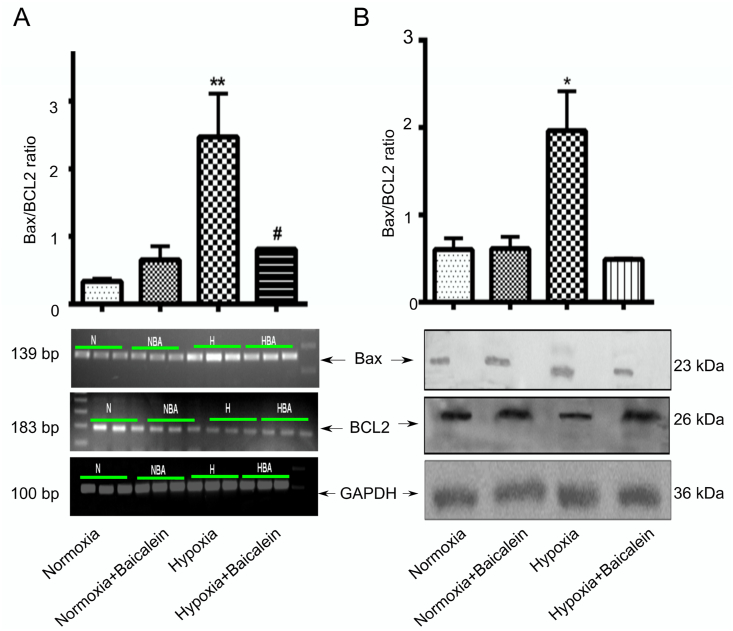
In addition, we also observed that hypobaric hypoxia showed significant (mRNA expression p < 0.01; protein expression p < 0.05) increase in the relative Bax/Bcl-2 expression as compared with the Normoxia and Normoxia treated baicalein groups in the hippocampus (Fig. 9-A, B). Baicalein treated group showed a significant reduction (mRNA expression p < 0.05; protein expression p < 0.05) in the relative Bax/Bcl-2 expression when compared with the hypoxic group.
Fig. 9.
Alteration in Bax/Bcl2 ratio in CA3 region of the Hippocampus. Shift in Bax/Bcl2 equilibrium was studied by RT-PCR and Western blot analysis among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA). Data for them are represented as photomicrographs for RT-PCR [A] and Western blot [B] along with their analysis as bar graph. Statistical analysis was done among groups and is shown by p.value represented as *p < 0.05 (N v/s H), **p < 0.01 (N v/s H), #p < 0.05 (NBA v/s HBA).
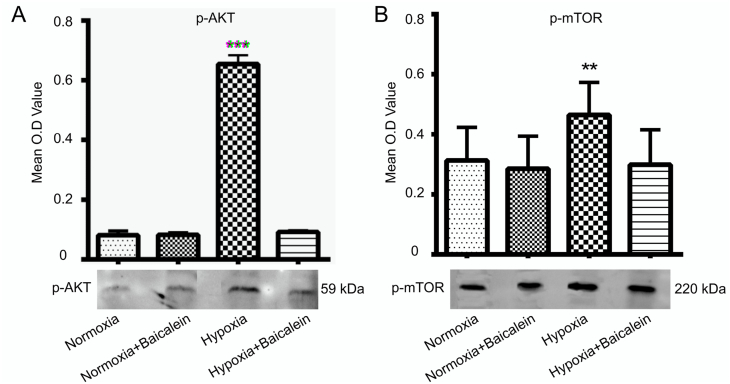
3.8. Expression of p-AKT during hypobaric hypoxia in presence of baicalein
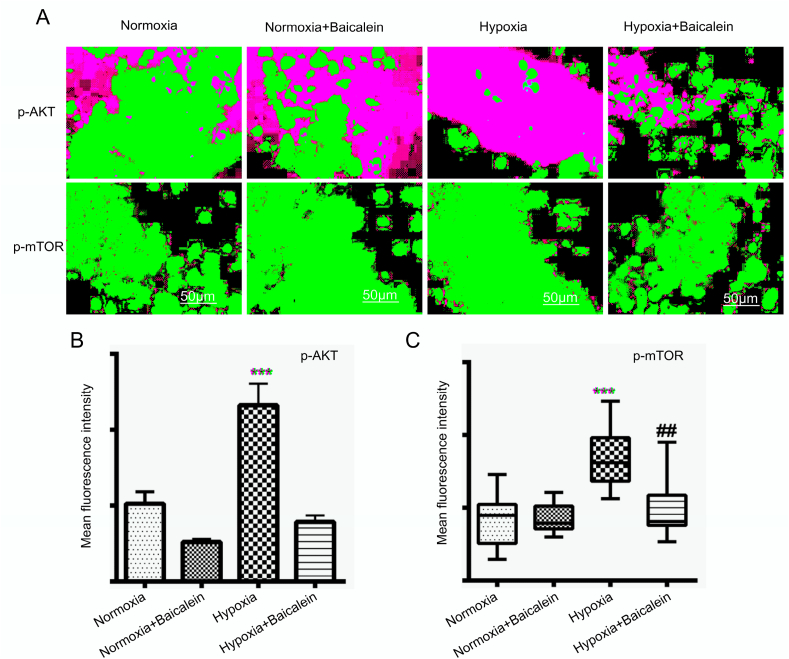
Western blot analysis revealed that hypobaric hypoxia significantly increased protein expression of the p-AKT (p < 0.001). Further, baicalein administration downregulated p-AKT protein expression (p < 0.001) during hypobaric hypoxia (Fig. 10-A). Immunofluorescence study also corroborated the finding, as pAkt expression was significantly increased in the CA3 region of hippocampus from hypoxia group animals (p < 0.001) as compared with Normoxia, Normoxia treated Baicalein group. Inhibition of 12/15-LOX by Baicalein significantly downregulated its expression (p < 0.001) (Fig. 11-A, B).
Fig. 10.
p-AKT and p-mTOR level in CA3 region of the Hippocampus. The level of p-AKT and p-mTOR was assessed by Western blot analysis among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA). Their data are shown as photomicrographs and their analysis as bar graph for p-AKT [A] and p-mTOR. Statistical analysis was done among groups and is shown by p.value represented as **p < 0.01 (N v/s H), **p < 0.001 (N v/s H).
Fig. 11.
p-AKT and p-mTOR localization in CA3 region of the Hippocampus. The localization of p-AKT and p-mTOR was assessed with fluorescence microscopy among various groups: Normoxia (N), Hypoxia (H), Normoxia + Baicalein treated (NBA) and Hypoxia + Baicalein Treated (HBA). Data for which are shown as photomicrographs [A] and the quantification data as bar graph for p-AKT [B] and p-mTOR [C] respectively. Statistical analysis among groups is shown by p.value represented as **p < 0.01 (N v/s H), **p < 0.001 (N v/s H), ##p < 0.01 (NBA v/s HBA).
3.9. Expression of pmTOR during hypobaric hypoxia in presence of baicalein
Western blot analysis suggested that hypobaric hypoxia significantly upregulated the protein expression of pmTOR (p < 0.01) and baicalein administration downregulated pmTOR protein expression (p < 0.01) (Fig. 10-B) during hypobaric hypoxia. Immunofluorescence study also confirmed that pmTOR expression was significantly increased in hypoxia group animals (p < 0. 001) as compared with normoxia, normoxia treated baicalein group (Fig. 11 A, C). Further, Baicalein treatment significantly downregulated its protein expression when compared with a hypoxia group (p < 0.01).
4. Discussion
The current study demonstrates upregulated expression of 12/15 LOX with concomitant increase in brain 12(S) HETE levels following intermittent hypobaric hypoxia/reperfusion injury. The condition results in corresponding increase in oxidative and nirosative stress in CA3 sector of hippocampus [12]. The presence of cerebral 12/15 LOX and its products has been associated with Alzheimer's disease [8,22], diabetes induced neuropathy [23], subarachnoid hemorrhage [24] and traumatic brain injury [25]. We further observed a marked increase in 12(S)-HETE in the hippocampus which coincides with the observed increase in 12/15-LOX expression. Baicalein administration resulted in significant decline in 12/15-LOX immunoreactivity as well as 12(S)–HETE synthesis in hippocampal homogenate from mice subjected to hypobaric hypoxia contemplating its critical involvement in neuronal injury in this condition. Cui et al., 2010, suggested similar 12/15 LOX dependent neuroprotective effect of baicalein in focal cerebral ischemia. The reduction in infarct size and edema formation following baicalein administration in focal cerebral ischemia model was comparable to 12/15-LOX gene knockouts [9,26] envisaging a similar role of 12/15 LOX in HH induced neuropathology.
The neuroinflammatory sequalae in brain regions is a close interplay of neurons, microglia and astrocytes. Microglia once activated produce many toxic molecules like nitric oxide, superoxide and contribute magnificently towards neuroinflammation. Colocalization and robust expression of 12/15-LOX with NeuN (Neuronal cell marker) and IBA (activated microglial cell marker) in the CA3 region of the hippocampus following hypobaric hypoxia suggest its detrimental role in HH related neuropathogenesis. However, 12/15 LOX immunolocalisation didn't coincide with activated astrocytes (GFAP+) in the CA3 region of the hippocampus. The study signifies the unique participation of 12/15 LOX in modulation of neuronal and microglial functional architecture following hypobaric hypoxia, sparing the involvement of astrocytes in instigating inflammation through this pathway.
It has been demonstrated in-vitro that 12/15 LOX is involved in neuronal cell death cascades [27], which is in line with our observations on increased activity of 12/15 LOX in neurons and its relation to neuronal cell death program in CA3 region of hippocampus post hypobaric hypoxia. Further, Baicalein treatment suppressed apoptosis in CA3 region, suggesting the strong involvement of 12/15-LOX in hypobaric hypoxia evoked apoptosis. The key hallmarks of neuronal apoptosis is excessive generation of proinflammatory mediators such as ROS and RNS etc. by neighbouring microglial cells and loss of mitochondrial potential. 12/15-LOX inhibition by baicalein prevents excessive ROS generation and restores mitochondria membrane potential in HT22 cell subjected to oxidative stress. 12/15-LOX immunostaining are found to be aggregated around the periphery of the nucleus upon glutamate challenge in vivo, this pernicious area is occupied by cell organelle like mitochondria. One of the most interesting aspect of 12/15-LOX is that they directly attack the membrane of cell organelle and initiate enzymatic reactions which induce structural or metabolic damage in cell/organelle membranes by causing peroxidation reaction. Pallast et al., 2010 demonstrated that incubation of mitochondria isolated from HTT22 cells with minimal amount of 12/15-LOX resulted in increased oxidative index (up to threefold over the control) and culminated in disturbance of mitochondria membrane potential (up to eightfold over the control), both indicative of the disruption of membrane integrity [28]. They also observed that incubation of mitochondria with LOX protein trigger the release of cytochrome c (up to fivefold over the control), a critical mediator of mitochondria mediated apoptosis cell death. They confirmed that 12/15 LOX directly attacks on mitochondria and interaction of 12/15 LOX with mitochondria is the major source of ROS in HT22 cells. These observations prompted us to deduce subcellular localization of 12/15 LOX with mitochondria specific antibody in hypobaric hypoxia paradigm. We observed 12/15 LOX localization within mitochondria following hypoxia reperfusion injury in CA3 region of hippocampus, which indicates the association of 12/15 LOX with mitochondria in hippocampus during hypobaric hypoxia. The data was further substantiated by TEM analysis clearly confirming that 12/15 LOX directly attack the mitochondrial membrane and induces structural and functional changes during hypobaric hypoxia. We speculate that this major event caused peroxidation of mitochondrial membrane, hence altering membrane fluidity and disturbing mitochondrial membrane potential. The downstream effect of dysregulated mitochondrial potential i.e. accumulation of cytochrome-c in cytosolic fraction of hippocampal homogenate was also noticed in HH group and baicalein administration decrease concentration of cytochrome-c release in cytosolic compartment, which signifies that 12/15 LOX involvement in mitochondria dependent neuronal apoptosis Concurrent release of cytochrome-c from mitochondria and activation of caspase-9 are instrumental in intrinsic pathway of programmed cell death. The previous report from our lab suggested that caspase-9 and 3 activities were significantly elevated during hypobaric hypoxia [4] substantiated by current findings. Further, we demonstrate that administration of baicalein resulted in significant decrease in caspase-9 and 3 activities strengthening the involvement of intrinsic pathway in 12/15 LOX dependent apoptosis. It has been previously documented that cerebral hypoxia leads to increase in activity of caspase-9 and caspase-3 in the cerebral cortex of new born piglets [29,30]. It has been recently reported that 12/15-LOX activation during I/R injury resulted in cardiomyocyte apoptosis with subsequent increase in caspase 3 activity [16] which strongly supports our findings.
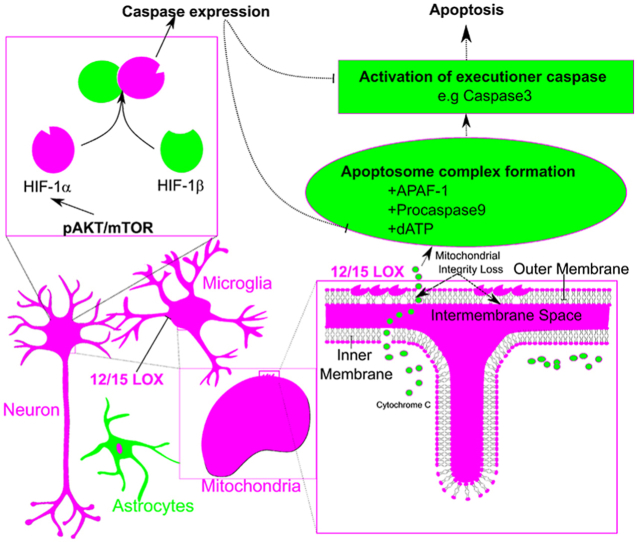
The upregulation of HIF-1α, an oxygen sensing universal transcription factor has been associated with induction of Bax mediated intrinsic apoptosis through pAkt-pmTOR signaling mechanisms [31]. Previously, we have also documented that 12/15 LOX inhibition could effectively suppress the HIF-1α protein accumulation in hypobaric hypoxia paradigm [12]. In the current study, we observed that marked increase in apoptotic neuronal death observed in CA3 sector of hippocampus is due to 12/15 LOX dependent HIF-1α protein accumulation and downstream pronounced expression of Caspase-9 of the intrinsic pathway of apoptosis. Baicalein effectively inhibited hypoxia induced HIF-1α activation via. inhibition of the PI3K/pAkt/pmTOR pathway which ultimately attenuated the expression of HIF-1α targeted apoptotic genes after three days of hypoxia exposure. Similar to our observation, it has been reported that hypoxia-ischemia brain damage of neonatal rats activates PI3K/Akt pathway and involved in regulation of HIF-1α following hypoxic ischemic brain damage [32,33]. Further, Baicalein treatment inhibited hyperactivation of PI3K/Akt pathway strongly suggesting that 12/15 LOX may regulate HIF-1α through PI3K/Akt pathway.
Moreover, it is known that Bcl-2 family proteins play a prominent anti-apoptotic role by acting upstream of Caspase activation. Interestingly, in our study, we observed that the Bax/Bcl-2 ratio was increased in hypoxia group, suggesting Bax as well as Bcl-2 mediated apoptosis. Reduction in Bax/Bcl-2 ratio and neuronal apoptosis, as observed in Baicalein treated group substantiates the possible involvement of 12/15 LOX in cytochrome-c release from mitochondria, there by downstream caspase activation by modulating the expression of Bcl-2 family proteins involving pro-apoptotic Bax or anti-apoptotic Bcl-2 during hypoxic condition in hippocampus region. It was reported that 12/15-lipoxygenase metabolite induced apoptosis in cancer cells by altering the Bax/Bcl-2 expression ratio which further strengthen our observation.
In conclusion, the results from the current study suggest the detrimental role of 12/15-LOX in neuronal damage via mitochondria mediated cell death concomitant with release of cytochrome-c in cytosol followed by Caspase-9 & 3 activation in-vivo under hypoxic injury Moreover, baicalein treatment inhibited hyperactivation of PI3K/Akt pathway during hypobaric hypoxia. These findings suggest inhibition of 12/15-LOX through baicalein rescues neuronal cells from the intrinsic mode of apoptosis induced by hypobaric hypoxia. Our finding suggested a novel pathway of 12/15-LOX mediated neuronal damage in hypobaric hypoxia paradigm.
Declaration of interest
None.
Authors contribution
Anju Katyal: Supervision, Conceptualization, Methodology, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Project administration, Funding acquisition.
Richa Choudhary: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing.
Mukesh Kumar: Writing - Review & Editing, Graphical designing.
Acknowledgement
Authors acknowledge Indian Council of Medical Research, India and University of Delhi for partial funding of project. Richa Chaudhary received Junior Research Fellowship of CSIR. We thank IGIB,CSIR to allow us to use the facility of LC-MS and Dr.Amitesh to carry out the experiment. We declare that the above mentioned agencies have no role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1.Hota S.K., Barhwal K., Baitharu I., Prasad D., Singh S.B., Ilavazhagan G. Bacopa monniera leaf extract ameliorates hypobaric hypoxia induced spatial memory impairment. Neurobiol. Dis. 2009;34:23–39. doi: 10.1016/j.nbd.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Jain K., Prasad D., Singh S.B., Kohli E. Hypobaric hypoxia imbalances mitochondrial dynamics in rat brain Hippocampus. Neurol Res Int. 2015:742059. doi: 10.1155/2015/742059. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiti P., Singh S.B., Sharma A.K., Muthuraju S., Banerjee P.K., Ilavazhagan G. Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem. Int. 2006;49:709–716. doi: 10.1016/j.neuint.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Malairaman U., Dandapani K., Katyal A. Effect of Ca2EDTA on zinc mediated inflammation and neuronal apoptosis in hippocampus of an in vivo mouse model of hypobaric hypoxia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udayabanu M., Kumaran D., Katyal A. Free chelatable zinc modulates the cholinergic function during hypobaric hypoxia-induced neuronal damage: an in vivo study. Neuroscience. 2012;202:434–445. doi: 10.1016/j.neuroscience.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Sinha S., Dutta A., Singh S.N., Ray U.S. Protein nitration, lipid peroxidation and DNA damage at high altitude in acclimatized lowlanders and native highlanders: relation with oxygen consumption. Respir. Physiol. Neurobiol. 2010;171:115–121. doi: 10.1016/j.resp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Cui L., Zhang X., Yang R., Liu L., Wang L., Li M., Du W. Baicalein is neuroprotective in rat MCAO model: role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacol. Biochem. Behav. 2010;96:469–475. doi: 10.1016/j.pbb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Praticò D., Zhukareva V., Yao Y., Uryu K., Funk C.D., Lawson J.A., Trojanowski J.Q., Lee V.M.-Y. 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. Am. J. Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Leyen K., Kim H.Y., Lee S.-R., Jin G., Arai K., Lo E.H. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama M., Watanabe T., Ueda N., Tsukamoto H., Watanabe K. Arachidonate 12-lipoxygenase is localized in neurons, glial cells, and endothelial cells of the canine brain. J. Histochem. Cytochem. 1993;41:111–117. doi: 10.1177/41.1.8417106. [DOI] [PubMed] [Google Scholar]
- 11.Chinnici C.M., Yao Y., Ding T., Funk C.D., Praticò D. Absence of 12/15 lipoxygenase reduces brain oxidative stress in apolipoprotein E-deficient mice. Am. J. Pathol. 2005;167:1371–1377. doi: 10.1016/S0002-9440(10)61224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choudhary R., Malairaman U., Katyal A. Inhibition of 12/15 LOX ameliorates cognitive and cholinergic dysfunction in mouse model of hypobaric hypoxia via. attenuation of oxidative/nitrosative stress. Neuroscience. 2017;359:308–324. doi: 10.1016/j.neuroscience.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Khurana P., Ashraf Q.M., Mishra O.P., Delivoria-Papadopoulos M. Effect of hypoxia on caspase-3, -8, and -9 activity and expression in the cerebral cortex of newborn piglets. Neurochem. Res. 2002;27:931–938. doi: 10.1023/a:1020347732741. [DOI] [PubMed] [Google Scholar]
- 14.Lebeau A., Terro F., Rostene W., Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ. 2004;11:875–884. doi: 10.1038/sj.cdd.4401395. [DOI] [PubMed] [Google Scholar]
- 15.Song L., Yang H., Wang H.-X., Tian C., Liu Y., Zeng X.-J., Gao E., Kang Y.-M., Du J., Li H.-H. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis. 2014;19:567–580. doi: 10.1007/s10495-013-0946-z. [DOI] [PubMed] [Google Scholar]
- 16.Song L., Yang H., Wang H.-X., Tian C., Liu Y., Zeng X.-J., Gao E., Kang Y.-M., Du J., Li H.-H. Inhibition of 12/15 lipoxygenase by baicalein reduces myocardial ischemia/reperfusion injury via modulation of multiple signaling pathways. Apoptosis. 2014;19:567–580. doi: 10.1007/s10495-013-0946-z. [DOI] [PubMed] [Google Scholar]
- 17.van Leyen K., Duvoisin R.M., Engelhardt H., Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport S.M., Schewe T. The maturational breakdown of mitochondria in reticulocytes. Biochim. Biophys. Acta. 1986;864:471–495. doi: 10.1016/0304-4157(86)90006-7. [DOI] [PubMed] [Google Scholar]
- 19.Glowinski J., Iversen L.L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J. Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- 20.Mal M., Koh P.K., Cheah P.Y., Chan E.C.Y. Ultra-pressure liquid chromatography/tandem mass spectrometry targeted profiling of arachidonic acid and eicosanoids in human colorectal cancer. Rapid Commun. Mass Spectrom. 2011;25:755–764. doi: 10.1002/rcm.4926. [DOI] [PubMed] [Google Scholar]
- 21.Kumaran D., Udayabanu M., Kumar M., Aneja R., Katyal A. Involvement of angiotensin converting enzyme in cerebral hypoperfusion induced anterograde memory impairment and cholinergic dysfunction in rats. Neuroscience. 2008;155:626–639. doi: 10.1016/j.neuroscience.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Yao Y., Clark C.M., Trojanowski J.Q., Lee V.M.-Y., Praticò D. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann. Neurol. 2005;58:623–626. doi: 10.1002/ana.20558. [DOI] [PubMed] [Google Scholar]
- 23.Dobrian A.D., Morris M.A., Taylor-Fishwick D.A., Holman T.R., Imai Y., Mirmira R.G., Nadler J.L. Role of the 12-lipoxygenase pathway in diabetes pathogenesis and complications. Pharmacol. Ther. 2019;195:100–110. doi: 10.1016/j.pharmthera.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poloyac S.M., Reynolds R.B., Yonas H., Kerr M.E. Identification and quantification of the hydroxyeicosatetraenoic acids, 20-HETE and 12-HETE, in the cerebrospinal fluid after subarachnoid hemorrhage. J. Neurosci. Methods. 2005;144:257–263. doi: 10.1016/j.jneumeth.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 25.Farias S.E., Heidenreich K.A., Wohlauer M.V., Murphy R.C., Moore E.E. Lipid mediators in cerebral spinal fluid of traumatic brain injured patients. J. Trauma. 2011;71:1211–1218. doi: 10.1097/TA.0b013e3182092c62. [DOI] [PubMed] [Google Scholar]
- 26.Jin G., Arai K., Murata Y., Wang S., Stins M.F., Lo E.H., van Leyen K. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Wang H., Li J., Jimenez D.A., Levitan E.S., Aizenman E., Rosenberg P.A. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J. Neurosci. 2004;24:10616–10627. doi: 10.1523/JNEUROSCI.2469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pallast S., Arai K., Wang X., Lo E.H., van Leyen K. 12/15-Lipoxygenase targets neuronal mitochondria under oxidative stress. J. Neurochem. 2009;111:882–889. doi: 10.1111/j.1471-4159.2009.06379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khurana P., Ashraf Q.M., Mishra O.P., Delivoria-Papadopoulos M. Effect of hypoxia on caspase-3, -8, and -9 activity and expression in the cerebral cortex of newborn piglets. Neurochem. Res. 2002;27:931–938. doi: 10.1023/a:1020347732741. [DOI] [PubMed] [Google Scholar]
- 30.Mishra O.P., Delivoria-Papadopoulos M. Effect of neuronal nitric oxide synthase inhibition on caspase-9 activity during hypoxia in the cerebral cortex of newborn piglets. Neurosci. Lett. 2006;401:81–85. doi: 10.1016/j.neulet.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Zhou C., Calvert J.W., Colohan A.R.T., Zhang J.H. Multiple effects of hyperbaric oxygen on the expression of HIF-1 alpha and apoptotic genes in a global ischemia-hypotension rat model. Exp. Neurol. 2005;191:198–210. doi: 10.1016/j.expneurol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Li L., Qu Y., Mao M., Xiong Y., Mu D. The involvement of phosphoinositid 3-kinase/Akt pathway in the activation of hypoxia-inducible factor-1alpha in the developing rat brain after hypoxia-ischemia. Brain Res. 2008;1197:152–158. doi: 10.1016/j.brainres.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Xiong Y., Qu Y., Mao M., Mu W., Wang H., Mu D. The requirement of extracellular signal-related protein kinase pathway in the activation of hypoxia inducible factor 1 alpha in the developing rat brain after hypoxia-ischemia. Acta Neuropathol. 2008;115:297–303. doi: 10.1007/s00401-008-0339-5. [DOI] [PubMed] [Google Scholar]