Abstract
Background
Ovarian cancer is the most lethal gynecological cancer, causing over 200,000 deaths worldwide in 2020. Initial standard treatment for primary ovarian cancer is optimal cytoreductive surgery (CRS) preceded and/or followed by intravenous platinum-based chemotherapy. However, most women develop recurrence within the peritoneal cavity and die of disease. Results of the OVIHIPEC 1 trial (2018) showed improved survival of 34% when hyperthermic intraperitoneal chemotherapy (HIPEC) was given immediately following interval-CRS in women with stage III disease. However, it is unknown if the effect of HIPEC is due to hyperthermia, one extra cycle of intraperitoneal (IP) chemotherapy, or other factors. There is also concern that hyperthermia might be associated with an increase in adverse events (AEs) due to a heightened systemic inflammatory response. HyNOVA is a seamless, multi-stage randomized study that attempts to answer these questions by comparing HIPEC to normothermic intraperitoneal chemotherapy (NIPEC), focusing on safety (stage 1), then assessing activity (stage 2) and effectiveness (stage 3). In this initial study, we hypothesize that NIPEC will result in a lower rate of severe AEs compared to HIPEC.
Methods
This initial stage of HyNOVA is a phase II study of 80 women with International Federation of Gynaecology and Obstetrics stage III epithelial ovarian cancer, with at least stable disease following 3–4 cycles of neoadjuvant chemotherapy, achieving interval-CRS to <2.5 mm residual disease. Participants are randomized 1:1 to receive IP cisplatin 100 mg/m2 for 90 minutes either as HIPEC, heated to 42°C (41.5°C–42.5°C), or NIPEC, at 37°C (36.5°C–37.5°C). The primary outcome is the proportion of AEs ≥ grade 3 occurring within 90 days. Secondary outcomes are AE of interest, surgical morbidity, patient reported outcomes, resource allocation, feasibility, progression-free survival and overall survival. AEs are measured using both CTCAE v5.0 and Clavien-Dindo classification, particularly infection, pain, bowel dysfunction, and anemia. Tertiary outcomes are potential predictive biomarkers measured before and after HIPEC/NIPEC including circulating cell-free tumor DNA, tissue factors, and systemic inflammatory markers. There are 4 participating Australian sites with experience in CRS and HIPEC for peritoneal malignancy. HyNOVA is funded by an MRFF grant (APP1199155).
Trial Registration
ANZCTR Identifier: ACTRN12621000269831p
Keywords: Ovarian Cancer, Chemotherapy, Hyperthermia, Patient Reported Outcomes
INTRODUCTION
Ovarian cancer is the most lethal gynecological cancer worldwide, causing over 200,000 deaths in 2020 [1]. The majority of women (70%) present with International Federation of Gynaecology and Obstetrics (FIGO) stage III disease that has already spread to the peritoneal surfaces of the abdomen and pelvis [2]. The 5-year relative survival in this group of women is only 25%–30% [3]. The main reason for poor survival is the high rate of loco-regional tumor relapse within the peritoneal cavity.
The standard treatment for primary cancer of the ovary is a combination of optimal cytoreductive surgery (CRS) and intravenous (IV) platinum-based chemotherapy. Results of the OVIHIPEC 1 trial (2018) [4] showed improved survival of 34% following interval-CRS with hyperthermic intraperitoneal chemotherapy (HIPEC) compared to interval-CRS without HIPEC. However, uncertainty remains as to whether the positive effect of HIPEC found in this trial was due to the effect of hyperthermia, the effect of one extra cycle of intraperitoneal (IP) chemotherapy, or other factors associated with giving an infusion of intra-operative fluid directly into the peritoneal cavity at the time of surgery. There is also concern that hyperthermia might be associated with an increase in adverse events (AEs) due to heightened systemic inflammatory response.
HIPEC has been used for 30 years to treat cancers that spread to the peritoneum including pseudomyxoma peritonei, colorectal and appendiceal cancer [5]. Instilling chemotherapy directly into the peritoneal cavity utilizes the pharmacokinetic advantage of the peritoneal-plasma barrier, achieving a higher concentration gradient of chemotherapy than IV chemotherapy with less systemic side-effects [6]. This allows increased penetration of chemotherapy into tumor cells at a time-point when there is least tumor [7]. Heat results in a synergistic cytotoxic effect by enhancing penetration of chemotherapy, inhibiting DNA-repair, limiting angiogenesis, and accelerating apoptosis [8]. At the cellular level hyperthermia induces homologous recombination DNA-repair deficiency, sensitizing tumor cells to chemotherapy similar to the action of a poly-AD-ribose-polymerase inhibitor [9].
IP application of cisplatin achieves peritoneal-to-plasma concentration ratios of up to 20 times those achieved with IV administration.
As the penetration depth of cisplatin into tumor tissue lies between 1 and 3 mm [10], residual disease must be minimal to benefit most from platinum-based HIPEC. The majority (87%) of women in the OVHIPEC-1 study [4], which showed a survival benefit when HIPEC was given at the time of interval-CRS, had residual disease ≤2.5 mm.
The OVHIPEC 1 trial [4] showed that the application of HIPEC at the time of interval-CRS following neoadjuvant chemotherapy (NAC) for stage III ovarian cancer resulted in a significant survival benefit. In this study, 245 women were randomized to receive either HIPEC with cisplatin at a dose of 100 mg/m2 heated to 40°C circulated through the peritoneal cavity for 90 minutes at the completion of interval surgery, or no HIPEC. Results showed a significant reduction in recurrence and death at 4.7 years for those receiving HIPEC (hazard ratio=0.66; 95% confidence interval [CI]=0.50–0.87; p=0.003). The HIPEC group showed a benefit of 11.8 months in median overall survival (45.7 vs. 33.9 months).
The OVHIPEC 1 trial [4] reported no difference in grade 3–5 AEs between the CRS alone and CRS with HIPEC groups (27% vs. 25%, p=0.76, respectively), despite a longer hospital stay (10 vs. 8 days). There were also similar health-related quality of life (HRQOL) outcomes, despite a higher rate of stoma formation in the HIPEC group (72% vs. 43%, p=0.04). However, there are concerns that surgical complications and patient reported outcomes (PROs) were underreported in this study. AE data suggest that nausea, abdominal pain, fatigue, pain, and neuropathy were experienced at any grade by 63%, 60%, 37%, 33% and 31% of participants, respectively, in the surgery plus HIPEC group. These symptoms may impact on overall HRQOL as well as patient functioning.
The recently published PRODIGE 7 trial [11], a multicenter randomized controlled trial comparing CRS plus HIPEC (with oxaliplatin) to CRS alone in patients with peritoneal metastases from colorectal cancer, showed a significantly increased risk of grade 3–5 AEs at 60 days in the group receiving HIPEC compared to those not receiving HIPEC (34/131, 26% vs. 20/130, 15%, p=0.035, respectively). There was a higher rate of complications, particularly related to a slower return of bowel function, pulmonary complications, infections, and anemia. There was a significantly longer hospital stay in the HIPEC group (18 vs. 13 days, p<0.001).
The physiological effect of hyperthermia is to induce a significant systemic inflammatory response, similar to the systemic inflammatory response syndrome (SIRS) seen in sepsis [12]. There is a massive hemodynamic shift and release of inflammatory cytokines such as C-reactive protein (CRP), together with heat-shock proteins, into serum and peritoneal fluid following HIPEC [12]. The hypothesis is that a heat-induced SIRS may contribute to complications such as cardiorespiratory compromise, infection, and bowel-related injury. In turn, complications that cause a significant delay in the commencement of post-operative chemotherapy could compromise overall survival in women following CRS for stage III ovarian cancer [13]. The extent to which these toxicities are present for patients treated with normothermic intraperitoneal chemotherapy (NIPEC) is unknown, and further study is required to determine if NIPEC is a safer or more acceptable alternative to HIPEC.
The HyNOVA trial will aim to answer important questions not answered by the previous trials of HIPEC for ovarian cancer, including; 1) Does the application of hyperthermia result in excess toxicity/morbidity to patients undergoing maximal CRS? 2) Is there any benefit to survival achieved by inducing hyperthermia when administering IP chemotherapy following maximal CRS?
MATERIALS AND METHODS
1. Objectives
The primary objective of the study is to determine the proportion of participants experiencing AEs ≥ grade 3 occurring within 90 days post-surgery. Secondary objectives of are to determine the frequency of AEs ≥ grade 3 within 90 days, surgical morbidity, PROs, resource allocation, feasibility of NIPEC, progression-free survival and overall survival. Tertiary objectives are to explore potential predictive biomarkers in blood samples and tumor before and after NIPEC/HIPEC.
2. Endpoints
Primary endpoint
The primary endpoint of the study is toxicity of treatment as measured by the proportion of patients in experiencing one or more grade 3 or 4 AEs as reported up to 90 days using the NCI-CTCAE version 5.0 and Clavien-Dindo classification for surgical AEs.
Secondary endpoint
Secondary endpoints are as follows;
1. Frequency of AEs ≥ grade 3. Of particular interest are AEs related to anemia, ileus and bowel dysfunction, renal impairment, infection, wound complications, stoma complications, cardiorespiratory, and metabolic derangements.
2. Surgical morbidity, including length of intensive care unit (ICU) stay, length of hospital stay, readmission to ICU, length of vasopressor use, intra- and post-operative blood transfusion, return to theatre and reason why, readmission to hospital and reason why, bowel function measures including time to first bowel motion, reinsertion of nasogastric tube, duration of total parenteral nutrition (TPN), use of stoma, type and reason.
3. PROs of bowel functioning, pain, fatigue/lack of energy, abdominal symptoms and burden of disease and treatment will be assessed using the EORTC QLQ-C30, QLQ-0V28, bowel scales of the QLQ-CR29, MOST v2 and EQ-5D-5L questionnaires [14,15,16,17,18,19]. The proportion of participants reporting “quite a bit” or “very much” will be reported for each group, at pre-cycle 1 and 6 months. These time points were selected because the acute effects of HIPEC or NIPEC should be reflected at pre-cycle 1, and any long-term issues should be present by 6 months. Methodological comparisons will be undertaken to determine which PRO measures are most efficient and will be taken forward to a phase III trial, as well as the most informative time points for PRO assessment. Health utility scores according to the QLU-C10D (cancer-specific health utility) and EQ-5D-5L (generic health utility) will be calculated and compared.
4. Resource utilization: Major components of resource utilization will be recorded, including days in ICU and days in hospital, return to operating theatre, emergency room visits, and the number of days of TPN.
5. Feasibility: The proportion of participants that correctly received their randomized treatment and the proportion of participants that received all 6 cycles of chemotherapy. The total number of chemotherapy cycles received and dose reductions or change in chemotherapy schedule will be collected.
6. Progression-free survival (PFS) is defined as the time from randomization until the date of first evidence of disease progression, as determined by RECISTv1.1 criteria or death due to any cause, whichever comes first. Progression-free survival according to CA125 measurements as per Gynecological Cancer InterGroup criteria for PFS will also be assessed.
7. Overall survival is defined as the time from randomization until death from any cause.
Tertiary endpoints
The exploratory endpoints of this study are to measure potential predictive biomarkers before and following NIPEC/HIPEC. This will include the removal of selected tumor nodules, of approximate size 1 cm, before and after application of NIPEC/HIPEC for assessment of cytotoxic effect, measuring circulating cell-free tumor DNA in plasma before and at different timepoints after treatment, and genetic profiling of tumor. A surrogate marker of toxicity will be the measurement of the systemic inflammatory response by following CRP levels during and after treatment.
3. Trial design and patients
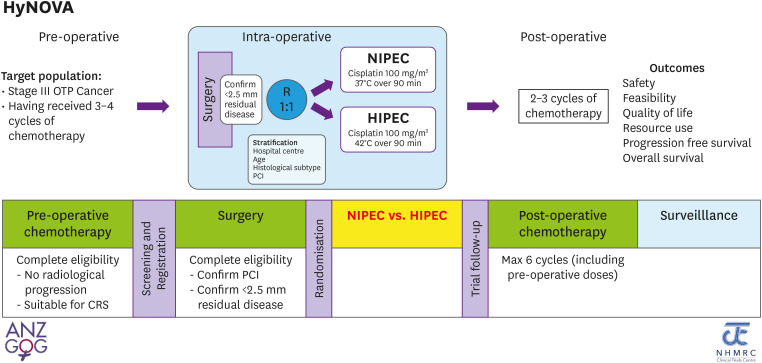
The HyNOVA trial is a seamless, multi-stage, randomized study of NIPEC vs. HIPEC given at the time of interval-CRS to minimal residual disease (<2.5 mm) following NAC for stage III epithelial ovarian, fallopian tube and peritoneal cancer. The study will focus on safety in stage 1, with a view to assessing activity in stage 2, and effectiveness in stage 3. In this first stage, we hypothesize that NIPEC will result in a lower rate of severe AEs in comparison with HIPEC.
This stage of HyNOVA is a phase II trial focusing on safety and activity of NIPEC versus HIPEC. It will include 80 participants with primary stage III ovarian, fallopian tube or peritoneal cancer being treated in 4 Australian centers with experience in extended CRS and HIPEC. Participants will have received 3–4 cycles of platinum-based NAC, with at least stable disease, and will undergo interval-CRS to residual disease <2.5 mm in largest size. The participant will then be randomized 1:1 intra-operatively to receive cisplatin 100 mg/m2 given to the IP cavity for 90 minutes either as HIPEC, heated to 42°C (41.5°C–42.5°C), or NIPEC, given at normal body temperature 37°C (36.5°C–37.5°C). The 2 treatment arms are shown in Table 1.
Table 1. The 2 arms of the intra-operative drug treatment.
| Arm | Participants will be randomised to one of the following 2 arms: | |||
|---|---|---|---|---|
| Agent | Dose | Temperature | Route | |
| NIPEC | Cisplatin | 100 mg/m2 | 37 (36.5–37.5)°C | IP |
| Q90mins | ||||
| HIPEC | Cisplatin | 100 mg/m2 | 42 (41.5–42.5)°C | IP |
| Q90mins | ||||
HIPEC, hyperthermic intraperitoneal chemotherapy; IP, intraperitoneal; NIPEC, normothermic intraperitoneal chemotherapy.
Participants will then receive their final 2–3 cycles (to total 6 cycles) of platinum-based chemotherapy post-operatively. The study schema is shown in Fig. 1.
Fig. 1. The HyNOVA study schema.
CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; NIPEC, normothermic intraperitoneal chemotherapy; OTP, orthopedia homeobox protein; PCI, peritoneal cancer index.
4. Eligibility criteria
Eligible Patients
Participants with histologically or cytologically confirmed stage III epithelial ovarian, fallopian tube or peritoneal cancer following platinum-based NAC with at least stable disease undergoing interval-CRS with residual disease of <2.5 mm.
Inclusion criteria
1. Age 18–75 years.
2. Primary FIGO stage III epithelial ovarian, fallopian tube or peritoneal cancer with disease that is limited to the abdominal cavity. This includes retroperitoneal lymph node involvement, superficial/subcapsular liver lesions, splenic disease, abdominal wall disease, and full thickness bowel wall involvement, that can be completely resected.
3. Histopathology high-grade serous, endometroid (grade 2/3), clear cell, or mixed high-grade histology.
4. Have 3–4 cycles of pre-operative platinum-based chemotherapy. The decision for NAC is to be made by the referring clinical team and includes the following indications; predicted inability to achieve minimal residual disease at up-front surgery (based on imaging and/or laparoscopic assessment), high volume abdominal disease, or a poor candidate for surgery without pre-habilitation.
5. No progression of disease on radiological imaging and/or CA125 during NAC.
6. Eastern Cooperative Oncology Group performance status 0 or 1.
7. Fit for surgery as determined by study surgical team.
8. Surgery should be performed at least 21 days after cycle 3 or 4 day 1 but before day 42.
9. Adequate bone, liver and renal function.
10. Willing and able to comply with all study requirements.
11. Signed, written informed consent.
Exclusion criteria
1. Participants with extra-abdominal disease.
2. Participants with intrahepatic or other visceral metastasis detected on radiological imaging which is not surgically resectable at diagnosis and/or after pre-operative chemotherapy treatment.
3. Any contraindications to receiving IP cisplatin chemotherapy as per the treating medical oncologist such as drug allergy.
4. Had received bevacizumab in combination with NAC treatment.
5. Serious medical or psychiatric conditions that, despite adequate treatment, may pose an unacceptable risk of morbidity or prevent compliance with the trial.
6. History of another malignancy within 5 years prior to registration (excluding non-melanomatous skin cancer).
7. Concurrent illness, including active intra-abdominal sepsis, that may jeopardize the ability of the participant to undergo the procedures outlined in this protocol.
5. Screening and registration of participants
Following 2–3 cycles of NAC, participants will be assessed as to their medical and surgical suitability for the trial. Following written informed consent, all screening investigations will be performed. Once screening investigations are reviewed, including tumor response to NAC (by clinical assessment, Ca 125 response, and RECIST criteria on computed tomography [CT] imaging, and can include assessment of positron emission tomography [PET]/CT and magnetic resonance imaging [MRI], or diagnostic laparoscopy), participants that show no progression of disease can undergo registration. The registration process must be completed at least 24 hours prior to surgery.
6. Randomization
Participants are randomized intra-operatively. Surgery and recording of peritoneal cancer index (PCI) will be performed as per principles outlined by Sugarbaker [20]. If the largest tumor nodule <2.5 mm is achieved, the participant is eligible and randomization to either HIPEC or NIPEC (1:1) will occur by electronic means. If <2.5 mm residual is not achieved, the participant is ineligible for the trial and will continue care as per standard treatment. The method of randomization is a stratified dynamic allocation approach, randomly alternating between minimization (80%) and complete randomization (20%).
Stratification
Stratification will occur according to hospital center, age (≤70 years vs. >70 years), histological type (serous vs. non-serous vs. undetermined), and PCI at the time of surgery (≤15 vs. >15).
7. Treatment
i) Neoadjuvant chemotherapy
Pre-operative chemotherapy is given as per standard of care. Current standard of care for stage III participants includes combination treatment with carboplatin (AUC=5 or 6) and paclitaxel (175 mg/m2). However, changes to the above regimen at the discretion of the treating medical oncologist is permitted and recorded on the electronic case report form (eCRF). Bevacizumab is not permitted in the pre-operative chemotherapy regime in this study.
ii) CRS
Surgery should be performed at least 21 days after cycle 3 or 4 day 1 (C3D22 or C4D22) but before day 42 (C3D42 or C4D42). The aim of surgery is to remove all areas of tumor so that no macroscopic residual disease remains. Principles of the surgery are outlined by Sugarbaker [20]. Following a full midline laparotomy, the 9 important areas in the abdomen and pelvis will be thoroughly evaluated and a PCI score according to Sugarbaker criteria is recorded on the eCRF. The highest score possible is a PCI of 39. If there are areas of peritoneal carcinomatosis with multiple (>10) tumor nodules in a region this is also recorded as carcinomatosis. Any macroscopic enlarged retroperitoneal lymph nodes are noted on the surgery scoring form in the eCRF. Surgery to remove all disease consists of parietal peritonectomy and, if necessary, a visceral peritonectomy including splenectomy, liver resection, cholecystectomy, small or large bowel resection, appendicectomy, hysterectomy, bilateral salpingo-oophorectomy, omentectomy and diaphragm resections.
iii) HIPEC/NIPEC
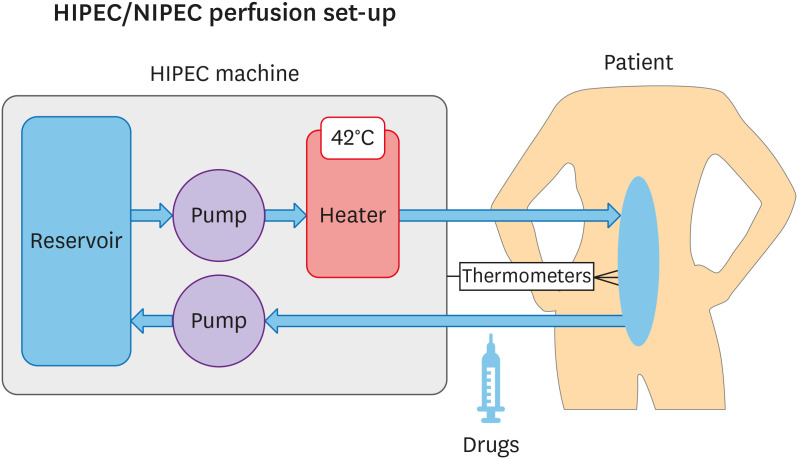
The set-up and process of drug administration for either HIPEC or NIPEC is identical, the only difference being the infusion temperature. The system for administration of the IP study treatment consists of a roller pump, a heat exchanger, and a reservoir with filter. The method of application may be an open or closed technique according to the usual institutional procedure. Inflow catheters are placed in the abdomen, and a central outflow catheter is connected to the reservoir. The inflow and outflow catheters with thermometers are placed so that there is maximal perfusion of all structures in all regions of the abdominal cavity and that the temperature is maintained in the specified range for that subject. After filling the abdomen with 0.9% sodium chloride, and once the abdominal fluid is heated to the specified temperature, the cisplatin (pre-prepared by pharmacy using 100 mg/m2 of cisplatin to 1 L of normal saline) is added to the perfusion system and circulated for 90 minutes. The inflow temperature of the perfusate is kept at 42 (41.5–42.5)°C for HIPEC and 37 (36.5–37.5)°C for NIPEC.
The HIPEC/NIPEC infusion set-up is shown in Fig. 2.
Fig. 2. The HIPEC/NIPEC infusion system set-up.
HIPEC, hyperthermic intraperitoneal chemotherapy; NIPEC, normothermic intraperitoneal chemotherapy.
Nephrotoxicity is prevented by administration of sodium thiosulphate in accordance with the OVHIPEC 1 [4] trial protocol. Urine production will be closely monitored during and after the procedure and should be not less than 0.5 mL/kg/hr.
At completion of the procedure, the abdomen is washed out with 2 L of sterile water and hemostasis achieved. Any bowel anastomosis are then made to reconstruct continuity where possible. The reason for not restoring continuity of the bowel and performing a stoma will be registered. Following completion of surgery, the abdomen is closed according to the standard technique.
iv) Post-operative chemotherapy
Post-operative chemotherapy following CRS with HIPEC/NIPEC will be IV platinum-based chemotherapy as given in the pre-operative setting. This will consist of 2–3 cycles to a total of 6 cycles after inclusion of the cycles prior to surgery. Participants will be assessed as to fitness to commence chemotherapy according to local protocol, ideally within 28 days after surgery (21–42 days).
Following completion of post-operative chemotherapy, there is no restriction on subsequent anti-cancer treatment.
8. Schedule of assessments
Daily clinical assessments and blood tests are performed post-operatively until discharge. Clinical assessment and blood tests occur 30 days and 90 days post-surgery to determine any AEs. CT scan of the chest/abdomen/pelvis will be performed at 90 days, 6 months, 12 months, and 24 months after surgery, or if clinically indicated. Clinical assessments, serum CA125 levels, and PROs assessments will continue every 3 months for 2 years or until evidence of disease progression (RECISTv1.1) or death (whichever occurs first).
Important aspects of this trial in regard to schedule of assessments are;
• Tumor diagnosis will preferably be made by tissue biopsy, but if cytology only has been performed, an intra-operative frozen section will be performed.
• Germline testing for BRCA 1 and 2 will be performed on all patients, and somatic testing for BRCA 1 and 2 where possible. Results will be included in the analysis.
• Radiological evaluation with a CT chest/abdomen/pelvis (with or without PET/CT scan or diffusion weighted MRI) must be performed after 2–4 cycles of chemotherapy and within 4 weeks of surgery with recording of (radiological) PCI.
• If laparoscopic assessment is required pre-CRS with HIPEC/NIPEC, the PCI score will be recorded and compared to PCI scored on imaging and at surgery.
• Blood will be collected for assessment of cell-free tumor DNA at surgery and compared to day 3–7 post-surgery and 30 days post-surgery (±7 days).
• Serum CRP will be measured at beginning of surgery, then just before and just after IP chemotherapy, and post-operatively.
• Intra-operative tumor tissue will be collected for freezing (genomic analysis, including somatic BRCA 1/2 testing), and if possible, a 0.5 cm–1 cm tumor nodule will be collected before and after HIPEC/NIPEC for translational studies.
9. Statistical analysis
Sample size is calculated based on an estimated grade 3–5 rate of AEs at 90 days with HIPEC of 30% and NIPEC of 15%. Based upon an exact binomial test, 37 participants will have 80% power (10% 1-sided alpha) to detect a decrease in the grade 3 or higher AEs lower than 30%, if the true rate is 15%. Thus, we will randomize 40 participants per arm to allow for up to 10% dropouts, with a 2-year accrual. To meet the primary endpoint, there must be 7 or fewer participants experiencing a grade 3 or higher AE by 90 days in the first 37 participants in the NIPEC arm. This analysis is not powered to formally compare the NIPEC and HIPEC arms, however we will be able to report estimates of the proportion with AE ≥ grade 3 and 95% CI for each treatment arm. Given that the primary outcome will be known after 90 days, the trial will be analyzed within 12 months of completion (i.e., at 3 years).
Secondary outcomes will be estimated in each treatment arm by either proportions and 95% CI or means and 95% CI, as appropriate.
10. Ethics and dissemination
The HyNOVA trial has ethics approval from Sydney Local Health District X20-0519 and 2020/ETH03005.
Dissemination will be by peer reviewed journals, presentations at national ANZGOG and international presentations, and patient representative groups.
11. Patient and public involvement
HyNOVA has been reviewed and endorsed by members of the ANZGOG consumer review panel and is funded by an NHMRC MRFF grant following support by an external consumer review process.
DISCUSSION
There remains considerable uncertainty around the use of HIPEC for the treatment of ovarian cancer despite the results of the first randomized trial of HIPEC for primary ovarian cancer, the OVHIPEC 1 trial [4], reporting improved survival without increased morbidity. Whether the effect of HIPEC is due to the effect of hyperthermia, the IP application of chemotherapy, or some other factor, remains unanswered. There are also concerns around increased toxicity, as shown in the recent PRODIGE 7 trial [11] of HIPEC for treatment of colorectal peritoneal metastases. The HyNOVA trial will attempt to answer some of these questions by comparing the application of NIPEC to HIPEC. This initial stage of HyNOVA is a phase II study randomizing women to receive NIPEC or HIPEC at the time of interval-CRS for stage III ovarian cancer and will focus on safety and activity of NIPEC/HIPEC. There will be a strong focus on measuring AEs by using both NCI-CTCAE v 5.0 and Clavien-Dindo classification, and reporting important PROs. Effectiveness of NIPEC/HIPEC will be determined in a later stage. HyNOVA will therefore expand on existing knowledge and produce new high-quality evidence by answering important questions about HIPEC not answered in previous trials.
ACKNOWLEDGEMENTS
We wish to acknowledge the following members of the protocol steering committee; Brand A, Perrin L, Barry S, Chetty N, Nascimento M, McNally O, Harrison M, Ananda S, Koh C, Ansari N, Coward J, Yip S, Ford C, Warton K, Richards A, Beale P, Gebski V, Moran I, Beach R, Gorzeman M, Alkhateeb A, Diamante K.
This investigator-initiated trial is conducted as a collaboration between the Australian New Zealand Gynecological Oncology Group (ANZGOG) and the NHMRC Clinical Trials Centre, University of Sydney.
Footnotes
Study sites and trial registration: HyNOVA will recruit participants in 4 Australian centers with experience in cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of peritoneal malignancy. The study protocol has ethics approval by Sydney Local Health District. The trial protocol is registered at ANZCTR - ACTRN12621000269831p.
Funding: HyNOVA is funded by an NHMRC MRFF Grant App1199155 and which is administered by University of Sydney, Australia.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: F.R., P.S.
- Data curation: F.R.
- Formal analysis: F.R., R.K., M.R., S.M.
- Funding acquisition: F.R., S.M.
- Investigation: F.R., B.M., S.M.
- Methodology: F.R., B.M., L.Y.C., P.S., R.K., M.R., S.M.
- Project administration: F.R., B.M., L.Y.C., R.K., S.M.
- Resources: F.R., B.M., R.K., M.R., S.M.
- Supervision: F.R., B.M., S.M.
- Validation: F.R.
- Visualization: F.R.
- Writing - original draft: F.R., B.M., R.K., M.R., S.M.
- Writing - review & editing: F.R., B.M., L.Y.C., P.S., R.K., M.R., S.M.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2.Prat J FIGO Committee on Gynecologic Oncology. FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol. 2015;26:87–89. doi: 10.3802/jgo.2015.26.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anuradha S, Webb PM, Blomfield P, Brand AH, Friedlander M, Leung Y, et al. Survival of Australian women with invasive epithelial ovarian cancer: a population-based study. Med J Aust. 2014;201:283–288. doi: 10.5694/mja14.00132. [DOI] [PubMed] [Google Scholar]
- 4.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HW, Hermans RH, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 5.Ansari N, Chandrakumaran K, Dayal S, Mohamed F, Cecil TD, Moran BJ. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur J Surg Oncol. 2016;42:1035–1041. doi: 10.1016/j.ejso.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Van der Speeten K, Stuart OA, Sugarbaker PH. Pharmacokinetics and pharmacodynamics of perioperative cancer chemotherapy in peritoneal surface malignancy. Cancer J. 2009;15:216–224. doi: 10.1097/PPO.0b013e3181a58d95. [DOI] [PubMed] [Google Scholar]
- 7.El-Kareh AW, Secomb TW. A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia. 2004;6:117–127. doi: 10.1593/neo.03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW, et al. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148–154. doi: 10.1016/s0959-8049(97)00370-5. [DOI] [PubMed] [Google Scholar]
- 9.Oei AL, Vriend LE, Crezee J, Franken NA, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol. 2015;10:165. doi: 10.1186/s13014-015-0462-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol. 1990;25:389–394. doi: 10.1007/BF00686048. [DOI] [PubMed] [Google Scholar]
- 11.Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:256–266. doi: 10.1016/S1470-2045(20)30599-4. [DOI] [PubMed] [Google Scholar]
- 12.Coccolini F, Corbella D, Finazzi P, Brambillasca P, Benigni A, Prussiani V, et al. Time course of cytokines, hemodynamic and metabolic parameters during hyperthermic intraperitoneal chemotherapy. Minerva Anestesiol. 2016;82:310–319. [PubMed] [Google Scholar]
- 13.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 14.King MT, Stockler MR, O'Connell RL, Buizen L, Joly F, Lanceley A, et al. Measuring what matters MOST: validation of the Measure of Ovarian Symptoms and Treatment, a patient-reported outcome measure of symptom burden and impact of chemotherapy in recurrent ovarian cancer. Qual Life Res. 2018;27:59–74. doi: 10.1007/s11136-017-1729-8. [DOI] [PubMed] [Google Scholar]
- 15.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. The EORTC QLQ-C30 scoring manual. Brussels: European Organisation for Research and Treatment of Cancer (EORTC); 2001. [Google Scholar]
- 16.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 17.Greimel E, Bottomley A, Cull A, Waldenstrom AC, Arraras J, Chauvenet L, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-OV28) in assessing the quality of life of patients with ovarian cancer. Eur J Cancer. 2003;39:1402–1408. doi: 10.1016/s0959-8049(03)00307-1. [DOI] [PubMed] [Google Scholar]
- 18.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King MT, Viney R, Simon Pickard A, Rowen D, Aaronson NK, Brazier JE, et al. Australian utility weights for the EORTC QLU-C10D, a multi-attribute utility instrument derived from the cancer-specific quality of life questionnaire, EORTC QLQ-C30. Pharmacoeconomics. 2018;36:225–238. doi: 10.1007/s40273-017-0582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugarbaker PH. Peritoneal carcinomatosis: principles of management. Boston, MA: Kluwer; 1996. [Google Scholar]