Abstract
Purpose.
A strong association between retinal degeneration and obesity has been shown in humans. However, the molecular basis of increased risk for retinal degeneration in obesity is unknown. Thus, an animal model with obesity and retinal degeneration would greatly aid the understanding of obesity-associated retinal degeneration. The retinal abnormalities in a novel rat model (WNIN-Ob) with spontaneously developed obesity are described.
Methods.
Histologic and immunohistochemical examination were performed on retinal sections of 2- to 12-month-old WNIN-Ob rats, and findings were compared with those of lean littermate controls. RNA from retinas of 12-month-old WNIN-Ob and lean littermate rats was used for microarray and qRT-PCR analysis.
Results.
The WNIN-Ob rats developed severe obesity, with an onset at approximately 35 days. Evaluation of retinal morphology in 2- to 12-month-old WNIN-Ob and age-matched lean littermate controls revealed progressive retinal degeneration, with an onset between 4 to 6 months of age. Immunohistochemical analysis with anti–rhodopsin, anti–cone opsin, and PSD-95 antibodies further confirmed retinal degeneration, particularly rod cell loss and thinner outer plexiform layer, in the obese rat retina. Gene expression by microarray analysis and qRT-PCR established activation of stress response, tissue remodeling, impaired phototransduction, and photoreceptor degeneration in WNIN-Ob rat retina.
Conclusions.
WNIN-Ob rats develop increased stress in retinal tissue and progressive retinal degeneration after the onset of severe obesity. The WNIN-Ob rat is the first rat model to develop retinal degeneration after the onset of obesity. This novel rat model may be a valuable tool for investigating retinal degeneration associated with obesity in humans.
The problems of excess weight and obesity have been recognized globally both in developed and in developing countries.1–5 Excess weight is the second leading modifiable risk factor for death in the United States,3,6 where 64% of adults age 20 years and older are overweight and 30% are obese. In the past 20 years, the rates of obesity have tripled in developing countries.6,7 Eye problems are probably the latest addition to the list of complications associated with obesity.1,8 The ocular complications of obesity include diabetic retinopathy, high intraocular pressure, cataracts, macular degeneration, floppy lid syndrome, and exophthalmos.1,8 Although these complications can have serious consequences, the effects of overweight and obesity on the eye are not well known to patients or to health care providers.
There is evidence of the increased risk for retinal degeneration and diabetic retinopathy in obese persons. Obese men are more than twice as likely to have dry macular degeneration than men with a normal basal metabolic index.9 An association between higher basal metabolic index and early macular degeneration has been reported.10 Similarly, abdominal obesity in patients with early or intermediate stages of macular degeneration increases the risk for progression to advanced macular degeneration.11 Reduction in the macular pigments, lutein, and zeaxanthin in obese persons further supports the association between obesity and retinal degeneration.12 Patients with Bardet-Biedl syndrome (BBS) develop retinal dystrophy, obesity, polydactyly, renal malformation, and learning disabilities.13,14 In several populations, obesity is one of the basic components of metabolic syndrome, which is implicated in microvascular changes in the retina.15
Retinal degeneration, including age-related macular degeneration (AMD) and diabetic retinopathy (DR), is a major cause of irreversible blindness in the developed and the developing world.16,17 The alarming increase in the prevalence of obesity further exacerbates the concern about retinal degeneration. In the past 20 years, considerable progress has been made in our understanding of the molecular basis of retinal dystrophies. However, there is no experimental evidence to establish the mechanism by which obesity increases the risk for retinal dystrophies. Although retinal abnormalities are found in tubby mouse and mouse models of BBS,18–20 obesity develops along with or much later than retinal changes. Therefore, an animal model that develops retinal degeneration after the onset of obesity, similar to obesity-associated retinopathy in patients, would immensely aid our basic understanding of the relationship between retinal degeneration and obesity.
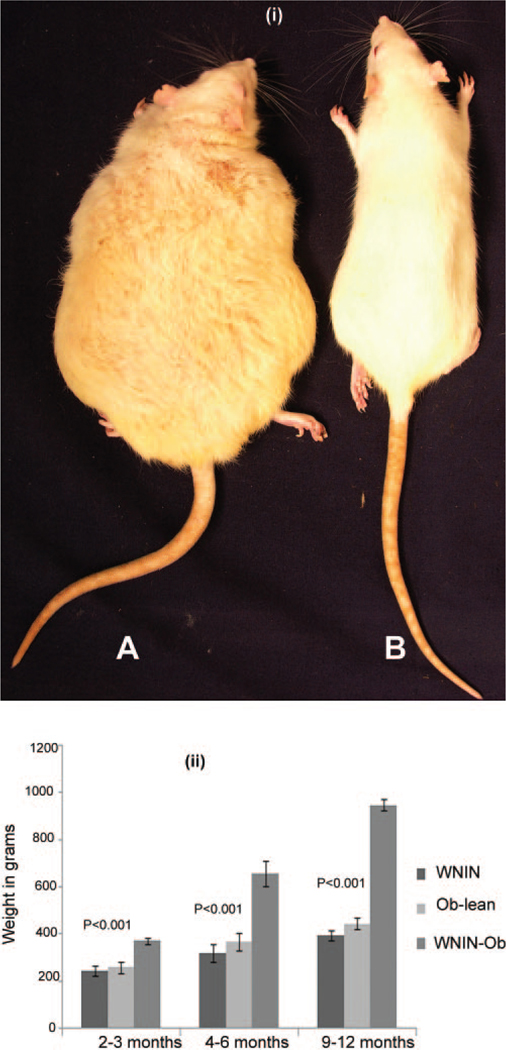
Wistar is the oldest strain of rats used in biomedical research. The National Institute of Nutrition (NIN) in India has an inbred stock of Wistar rats dating back to 1920 that is christened WNIN (Wistar maintained at NIN).21 A spontaneously developed obese rat was isolated from WNIN rats, and a colony of WNIN-Obese (WNIN-Ob) rats was generated by selective breeding. These rats (Fig. 1(i)) are maintained at the National Center for Laboratory Animal Sciences at NIN.21–23 The inheritance pattern and the biochemical and phenotypic characteristics of obesity in WNIN-Ob rats have been characterized in detail.21–23 Starting at 35 to 40 days of age, rats of the WNIN-Ob phenotype are different from their lean littermates in terms of body weight. Their body weight increased progressively until the age of 12 months (Fig. 1(ii)). By this age they weigh as much as 1.2 kg, in contrast to their lean littermates that weigh 500 to 600 g. WNIN-Ob rats demonstrate low fertility, and their average lifespan is 20 to 24 months. The colony is maintained by mating carriers (+/−), which, on crossing, gives three genotypes: lean (+/+), carrier (+/−), and WNINOb (−/−)—in a classical Mendelian ratio of 1:2:1, respectively.21–23 The present study describes retinal degeneration in this spontaneous obese rodent model.
FIGURE 1.
(i) WNIN-Ob rat in comparison with its littermate lean control at the age of 12 months. (A) WNIN-Ob rat. (B) Lean control. (ii) Relative increase in body weights of WNIN-Ob rat compared with lean control and Wistar controls. Values represent mean ± SD of 12 animals in each group.
Materials and Methods
Reagents and Antibodies
The details of antibodies used for this study are as follows: rabbit-anti–opsin, red/green (1:250 dilution; Chemicon, Temecula CA), mouse monoclonal anti–rhodopsin (1:200 dilution; Chemicon), mouse postsynaptic density protein-95 (PSD-95; 1:250 dilution; Sigma-Aldrich, St. Louis, MO), anti–rabbit Alexa Fluor-555 (1:2500 dilution; Invitrogen-Molecular Probes, Carlsbad, CA), and anti–rabbit Alexa Fluor-488 (1:1000 dilution; Invitrogen-Molecular Probes).
Animals and Tissue Collection
All procedures involving rats were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Ethics Committee at the National Institute of Nutrition. Animals were kept in a 12-hour light/12-hour dark cycle with ambient light intensity and temperature at the National Center for Laboratory Animal Science, National Institute of Nutrition. Two- to 12-month-old WNIN-Ob rats and their lean littermates were fasted overnight before they were euthanatized at the end of the dark cycle. All studies described here were carried out by evaluating at least three animals.
Histology and Immunohistochemistry
Eyeballs were collected from 2- to 12-month-old animals and fixed in 4% paraformaldehyde in phosphate-buffer (pH 7.2), followed by embedding and sectioning for plastic and cryosections using standard protocols, as described previously.24–27 Three eyes were used for each age group. Plastic sections were stained with hematoxylin and eosin. Immunohistochemistry was performed on cryosections using respective primary antibody, as described previously.24–27 The slides were mounted in antifade reagent containing DAPI (ProlongGold; Invitrogen) and were observed under an epifluorescence microscope (E800; Nikon, Tokyo, Japan). Images were captured using appropriate filters. Higher magnification images were captured with a confocal microscope (Zeiss, Oberkochen, Germany).
RNA Preparation
Retinas dissected from the eyes of WNIN-Ob rats and age-matched littermate controls were collected in reagent (Trizol; Invitrogen) for RNA isolation. RNA was isolated according to the manufacturer’s instructions.
Global Gene Expression by Microarray Analysis
Five micrograms of total retinal RNA isolated from two pooled retinas was used to synthesize double-stranded cDNA with the use of first- and second-strand cDNA synthesis kits (Affymetrix, Santa Clara, CA) according to the manufacturer’s guidelines. Purification of ds-cDNA, labeling, hybridization, washing, and staining were carried out according to the Affymetrix protocol. The rat gene expression chip (230.20; Affymetrix) was used for hybridization. Three independent samples for each WNIN-Ob and control group were analyzed.
Gene chips were scanned (Microarray Suite 5.0 [Affymetrix] operated by GCOS software version 2.0), and expression data were normalized with the use of robust multiarray average.28 Data were based on three gene chips per each control and WNIN-Ob groups. Ratios of average signal intensity (log2-transformed data) were then calculated for the probe sets (WNIN-Ob relative to lean control) and were converted to fold change.28 The contrast between experimental and control was extracted selecting those genes with an adjusted P of 0.05 and a threefold difference.
Quantitative Real-Time PCR
To remove any trace of genomic DNA, RNA was treated with RNase-free DNase (RQ1; Promega, Madison, WI) according to the manufacturer’s protocol. Equal amounts of RNA (7 μg) were reverse transcribed for each sample (control and WNIN-Ob) using oligo-dT (12–18) primer to first-stand cDNA with reverse transcriptase (SS II; Invitrogen) according to the manufacturer’s recommendations. Quantitative realtime PCR was then performed on cDNA templates using gene specific primers, as described previously.24,27 Specificity of primers was confirmed by melt curve analysis and by gel electrophoresis. Primer sequences are available from the authors on request. PCR data were analyzed to estimate the relative expression of different genes based on the difference in threshold cycle (Ct) between control and WNIN-Ob groups after normalization to various housekeeping genes—hypoxanthine phosphoribosyltransferase (Hgprt), -actin, succinate dehydrogenase (SdhA), and ribosomal protein L19 (Rpl19)—as described.24–27 Mean (±SD) values of percentage expression over control (lean) calculated from at least three independent samples are presented.
Results
Morphologic Evaluation
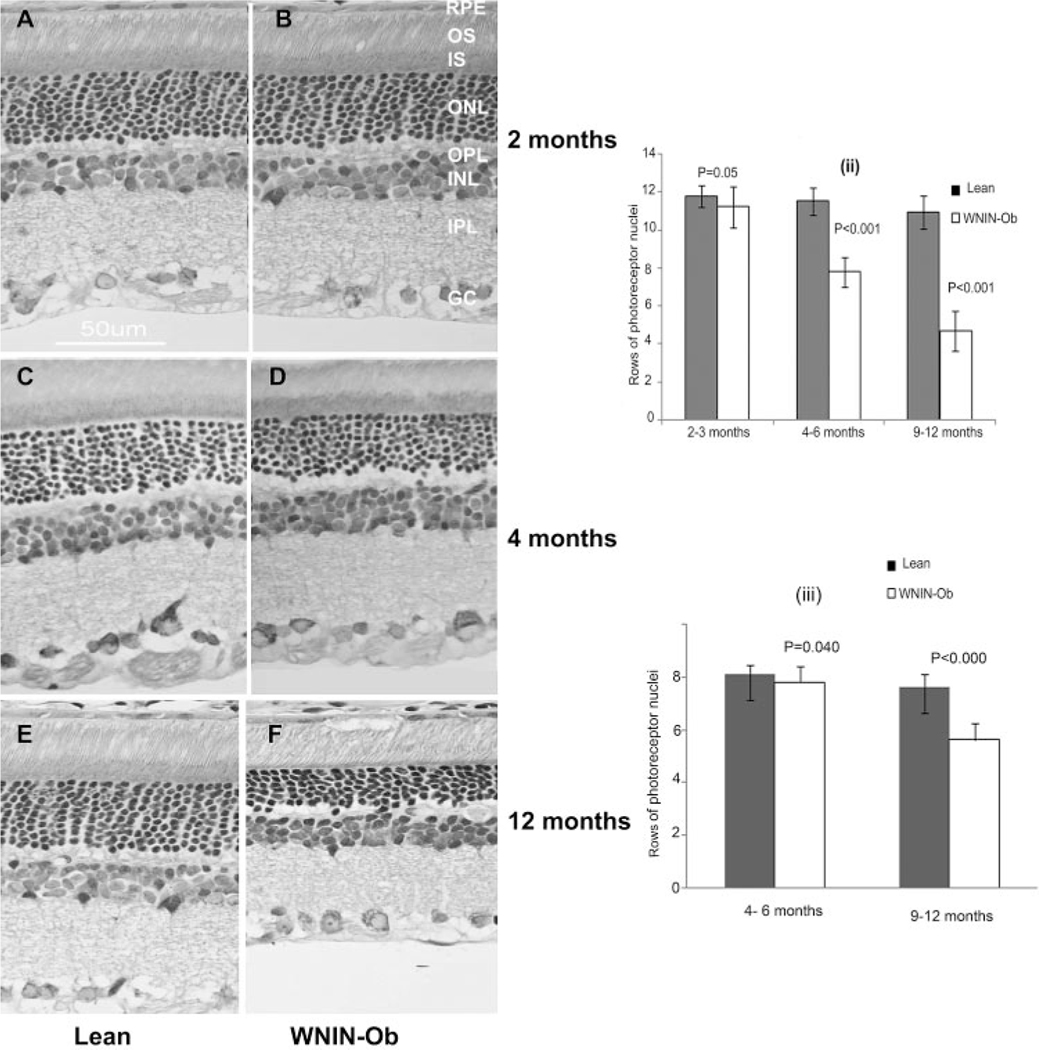
We have evaluated the retinal morphology of 2- to 12-monthold WNIN-Ob rats and compared our findings with those of lean littermate controls. No significant changes were observed in the morphology of 2-month-old WNIN-Ob animals compared with controls (Figs. 2(i), A, B), whereas the retinas of 4- to 12-month-old WNIN-Ob rats had significant alterations. At 4 months, the thickness of the outer nuclear layer (ONL) in the central retina of WNIN-Ob rats was reduced to approximately 7 to 8 nuclei compared with 10 to 12 nuclei in lean littermate controls (Figs. 2(i), C, D). The retinal changes found in WNIN-Ob rat at 6 months were not much different from the changes seen at 4 months age (data not shown). By 9 to 12 months, ONL thickness was reduced to 4 to 6 rows of nuclei in WNIN-Ob retinas compared with 10 to 12 nuclei in age-matched lean littermate controls (Figs. 2(i), E, F). Evaluation of retinal cell loss in the central and peripheral regions of 2- to 12-month-old WNIN-Ob and control rat retina demonstrated progressive degeneration of the photoreceptor cells in the central retina. In peripheral retina, a concomitant decrease occurred in the number of photoreceptor nuclei (Figs. 2(ii), (iii)).
FIGURE 2.
Representative histology of control rat retina (A, C, E) and WNIN-Ob rat retina (B, D, F) at various ages: (A) 2-month-old control; (B) 2-month-old WNIN-Ob; (C) 4-month-old control; (D) 4-monthold WNIN-Ob; (E) 12-month-old control; (F) 12-month-old WNIN-Ob. Histograms summarizing the progression of retinal degeneration in central (ii) and peripheral (iii) retina of WNIN-Ob rats compared with lean controls. Values represent mean ± SD of at least seven independent observations. OS, outer segments; IS, inner segments; INL, inner nuclear layer; IPL, inner plexiform layer; GC, ganglion cell layer.
Further histologic evaluation of retinas at age 9 to 12 months demonstrated abnormalities in the inner retinal layers. The gross structure of the inner retina did not demonstrate significant abnormalities at ages 4 to 6 months, but the thickness of the outer plexiform layer (OPL) and inner plexiform layer appeared to decrease in the 9- to 12-month-old WNIN-Ob rat retina compared with controls (Figs. 2(i), E, F). Concomitant with the decrease in the thickness of the OPL, a few photoreceptor nuclei appeared to migrate into this layer, suggesting disruption of its structure. By morphologic evaluation, the onset of retinal degeneration in the WNIN-Ob rat appeared to occur between 4 and 6 months age, and variation was observed in the severity of degeneration at a given age.
In addition to analyzing WNIN-Ob rats and lean littermate controls, we evaluated the retinal histology of 3- to 12-monthold WNIN parent strain rats. These rats did not show significant changes in retinal morphology (data not shown), suggesting that the retinal degeneration observed in the WNIN-Ob rat is neither sporadic nor a nonspecific observation in this strain of rats.
Immunohistochemistry
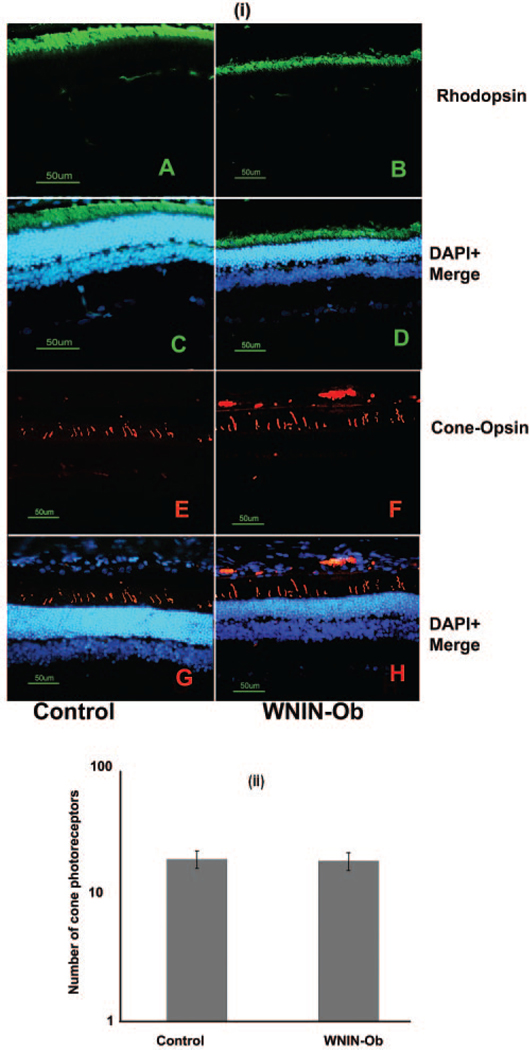
Immunohistochemical analysis of WNIN-Ob and lean littermate control rat retinas was carried out using rod and cone photoreceptor specific marker antibodies and anti–PSD-95 antibodies. Immunostaining with rhodopsin antibodies showed reduction in the brightness of the rhodopsin-specific signal in the WNIN-Ob rat retina compared with that of lean controls at 9 months (data not shown) and 12 months (Figs. 3A–D). Immunostaining with cone opsin (MW) antibodies indicated no significant alteration in the number of cones in the WNIN-Ob rat retina (Figs. 3E–H); staining the retinal sections with shorter wavelength cone opsin (SW) gave similar results (data not shown). The number of cones in the retinas of WNIN-Ob and lean control rats was found to be similar at ages 9 to 12 months (Fig. 3(ii)).
FIGURE 3.
Immunohistochemical evaluation of rod and cone photoreceptor markers in WNIN-Ob rat compared with controls at 12 months. (i) Retinal sections of (A, C, E, G) control and (B, D, F, H) WNIN-Ob rats were labeled with (A–D) rhodopsin and (E–H) cone opsin. Nuclei are labeled with DAPI. Scale bar, 50 μm. (ii) Histogram demonstrates the number of cones in WNIN-Ob and control rat retinas. Values represent mean ± SD of three independent observations (P = 0.2).
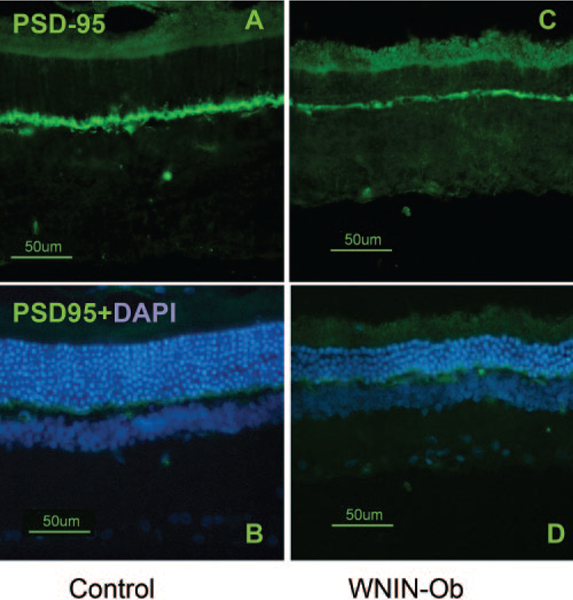
To further evaluate the decrease observed histologically in the thickness of OPL, we labeled the retinal sections from WNIN-Ob and control rats with postsynaptic density protein-95 (PSD-95) and compared the immunofluorescence (Fig. 4). In the 12-month-old WNIN-Ob rat retina, the immunofluorescence of PSD-95 was weaker (Figs. 4C, D) than the PSD-95 signal observed in age-matched lean littermates (Figs. 4A, B). This indicated a loss of synaptic terminals and further sup-ported the observed reduction in the thickness of OPL in WNIN-Ob rat retina.
FIGURE 4.
Immunofluorescence images of 12-month-old control (A, B) and WNIN-Ob rat (C, D) retinal sections labeled with PSD-95. Labeling of PSD-95 is prominent and intense in OPL layer of controls, whereas in the WNIN-Ob rat the fluorescence signal of PSD-95 is less intense.
Differential Expression of Genes in WNIN-Ob Retina
Having observed retinal degeneration in the WNIN-Ob rat, microarray analysis was performed on the WNIN-Ob rat retina at age 12 months to assess the global gene expression profile of the WNIN-Ob rat retina under degenerative conditions. The rat gene expression chip (230.2.0; Affymetrix) used in this experiment contains 30,000 probes representing 26,000 genes. Based on absent and present calls, approximately 60% of the probe sets were reported as present. We have calculated the expression values (log2 transformed) for each gene using robust multiarray average. Principal component analysis confirmed that biological replicate samples were grouped together. After gene filtering, 13,570 genes were identified by removing any that did not appear to be differentially expressed in any of the three independent samples in each group. We then extracted the contrast of experimental versus control, selecting those genes with an adjusted P of 0.05 and a threefold difference. This resulted in 423 genes, of which 369 showed lower levels of expression, whereas the expression of 54 genes was found to be significantly higher than in controls. Most downregulated genes were involved in phototransduction pathways or related to retinal structural proteins, carrier proteins, or transcription factors (Supplementary Table S1; both Supplementary Tables are online at http://www.iovs.org/cgi/content/full/50/7/3456/DC1). Most upregulated genes were related to apoptotic stress response and inflammatory mechanisms (Supplementary Table S2).
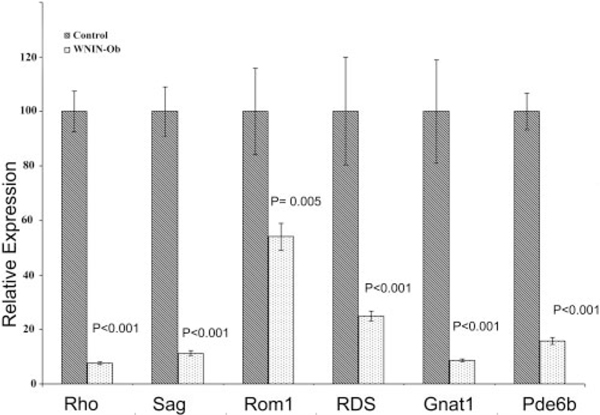
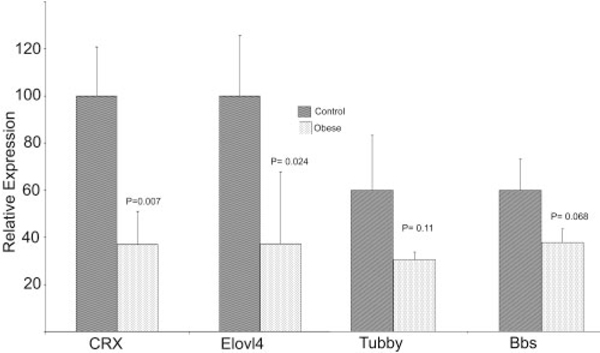
Quantitative real-time PCR analysis was carried out to validate the observations by microarray analysis and to verify the changes observed by histologic and immunohistochemical examination by quantifying the expression of selected retinal genes in 12-month-old lean and WNIN-Ob rats. Expression of rod-specific genes such as rhodopsin (Rho), rod transducin (Gnat1), rod arrestin (Sag), peripherin (Rds), retinal outer segment membrane protein (Rom1), and cGMP-dependent phosphodiesterase 6B (Pde6B) was found to be significantly lower in WNIN-Ob rat retina than in lean controls (Fig. 5). Levels of expression of the retinal transcription factor, conerod homeobox gene (Crx), and elongation of very long chain fatty acids 4 (Elovl4) genes expressed in rods and cones also decreased significantly in 12-month-old WNIN-Ob rats (Fig. 6). Obesity-associated genes tubby and Bbs7 also showed a lower level of expression in the retinas of WNIN-Ob rats than in those of littermate controls (Fig. 6), but the decrease was not significant.
FIGURE 5.
Quantitative expression of rod-specific genes in 12-monthold WNIN-Ob rats and their lean littermate controls. Expression values were determined by qRT-PCR and presented on an arbitrary scale (y-axis) after normalization with rat Hgprt. Data represent the mean (±SD) on an arbitrary scale and were calculated from at least three independent observations.
FIGURE 6.
Expression of Crx, Elovl4, and obesity-related genes in the retinas of 12-month-old WNIN-Ob rats and their lean littermate controls. Expression values were determined by qRT-PCR and presented on an arbitrary scale (y-axis) after normalization with rat Hgprt. Data represent the mean (±SD) on an arbitrary scale and were calculated from at least three independent observations.
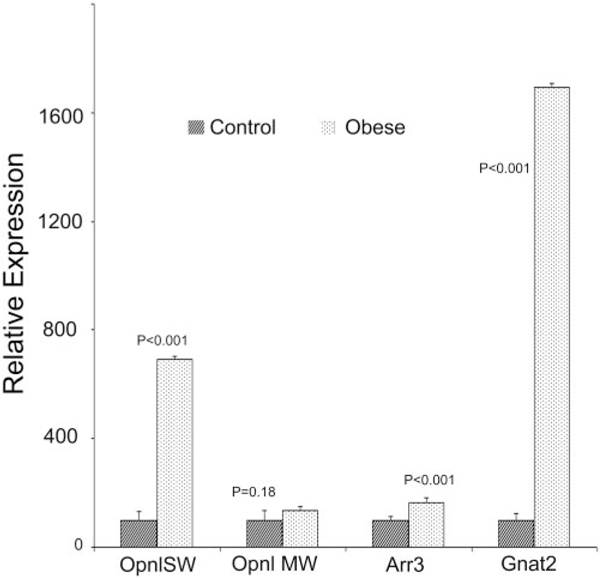
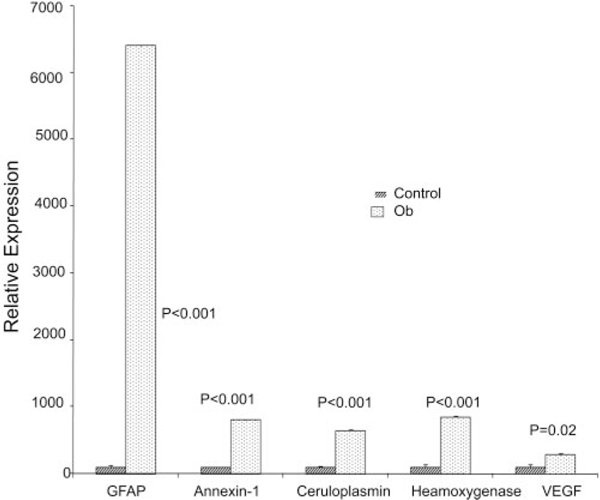
Interestingly, expression of cone cell marker genes, SW cone opsin (Opn1SW), MW cone opsin (Opn1MW), cone arrestin (Arr3), and cone transducin (Gnat2) was unaltered or significantly increased (Fig. 7). Expression of stress response genes, glial fibrillary acidic protein (Gfap), annexin-1, ceruloplasmin (Cp), hemeoxygenase-1 (Ho-1), and vascular endothelial growth factor (Vegf) were significantly higher in 12-monthold WNIN-Ob rat retinas than in those of lean controls (Fig. 8). These results indicate the preservation of cone cells, degener-ation of rod photoreceptors, activation of stress response, and remodeling of tissue pathways in the WNIN-Ob rat retina at 12 months.
FIGURE 7.
Quantitative expression of cone-specific genes in 12month-old WNIN-Ob rats and their lean littermate controls. Expression values were determined by qRT-PCR and presented on an arbitrary scale (y-axis) after normalization with rat Hgprt. Data represent the mean (±SD) on an arbitrary scale and were calculated from at least three independent observations.
FIGURE 8.
Quantitative expression of selected stress-related genes in retinas of 12-month-old WNIN-Ob rats and their lean littermate controls. Expression values were determined by qRT-PCR and presented on arbitrary scale (y-axis) after normalization with rat Hgprt. Data represent the mean (±SD) on an arbitrary scale and were calculated from at least three independent observations.
Discussion
Retinal degeneration, including age-related macular degeneration (AMD) and diabetic retinopathy (DR), is a major cause of blindness in the developed and the developing world. The molecular events that contribute to retinal degeneration are highly complex because of the involvement of different retinal cell types. Mutations in approximately 150 genes have been implicated in retinal degeneration (RetNet, www.sph.uth.tmc.edu/Retnet/home.htm). Despite extensive knowledge about the disease-causing genes,29–32 the molecular basis underlying many forms of retinal degeneration are not understood. The interplay between multiple genetic and environmental factors could add to the complexity for the pathogenesis of retinal dystrophies. A strong association found between retinal degeneration and obesity further adds to this complexity. The alarming increase in the prevalence of obesity in Southeast Asian countries6,7,33,34 further exacerbates concern about retinal degeneration globally. Therefore, identifying a suitable animal model will aid in investigating the underlying mechanism for the association between retinal degeneration, obesity, and metabolic syndrome.
Evaluation of the morphology, photoreceptor-specific protein, and gene expression profile of the WNIN-Ob rat retina indicated significant and progressive rod photoreceptor degeneration with preservation of cone cells. The preservation of cone photoreceptors is remarkable in WNIN-Ob rats even at 12 months, when ONL thickness is reduced to 50% to 60% of controls. Survival of cones beyond the age of 12 months in these rats is yet to be investigated. In WNIN-Ob rats, preservation of the number of cone cells is intriguing. In several types of retinal degeneration, in patients and in animal models, rod cell death precedes cone loss.35,36 It is likely that the molecular defect underlying retinal degeneration in these rats is associated with a biological pathway critical for rod cell survival. Cone viability is believed to depend on diffusible or contact-mediated survival factors that are synthesized by rods.37–39 Therefore, this rat model with unaltered cone cell number, despite significant rod cell loss, may aid in understanding possible means to preserve cones in the absence of rods.
Retinal abnormalities were observed in a few rodent models with obesity. The tubby mice develop neurosensory abnormalities and obesity, but neurosensory abnormalities develop much earlier than the onset of obesity. The obesity of tubby mice is relatively mild, has late onset, and progresses slowly.19,20 Mouse models for Bardet-Biedl syndrome develop obesity and retinal abnormalities in addition to a spectrum of other phenotypic features.40,41 Among other obese rodent models, ocular complications, particularly pronounced retinal changes, were found in diabetic obese (fa/fa) rats when they were fed a sucrose diet for 68 weeks.42 Mice lacking functional tubby-like protein-1 (TULP-1) develop retinal degeneration but not obesity.43 The phenotype observed in WNIN-Ob rats is unique because of the early age of onset and the severity of obesity and retinal degeneration.
The pattern of gene expression in the WNIN-Ob rat retina supports photoreceptor degeneration. Downregulated genes appear to be involved predominantly in transcriptional regulation (Crx, neural fold homeobox, high mobility group box, zinc finger proteins) and visual transduction (Gnb, Gnat, Cng, Pde6B, Rho, phosphatases, retinol binding protein). In addition, rod cell structural proteins (Rho, Rom, peripherin) were also observed to be significantly decreased. A number of these downregulated genes are also implicated in various forms of inherited retinal degeneration (RetNet, www.sph.uth.tmc.edu/Retnet/home.htm). Lower levels of expression of rod cell specific genes may reflect the loss of photoreceptors rather than specific downregulation of these genes.
The upregulated genes are mostly related to apoptotic, stress response, tissue remodeling pathways (annexin-1, cathepsin, glutathione S-transferase, Cp, Gfap) indicating activation of the stress response in the WNIN-Ob rat retina compared with the lean littermate control rat retina. The expression pattern of some genes observed in the WNIN-Ob rat retina is similar to the gene expression changes observed in rd1 mice and injured rat retina.44,45 Levels of expression of obesity-related genes, tubby-like, and Bbs7 were found to be lower on microarray and qRT-PCR analysis, indicating a possible direct role for these obesity genes in maintaining normal retinal physiology.41,43,46 Unlike many studies involving microarray analysis, we found that only a limited number of genes are differentially expressed in the Ob-rat retina compared with lean littermate controls. Most of the genes showing altered expression are likely to have a significant role in retinal physiology. The expression profile of genes observed in the WINN-Ob rat retina is similar to the findings reported in Bbs4 knockout mice.41 These observations suggest a direct association between obesity and retinal degeneration in these rats.
Further studies are needed to determine the causal relationship between obesity and retinal degeneration in the WNIN-Ob rat and to determine whether obesity, either in itself or through its metabolic consequences, alters retinal structure and function in these rats. No reports relate metabolic changes (similar to those seen in this model, such as hyperphagia, hypertriglyceridemia, hypercholesterolemia) to retinal changes, particularly photoreceptor degeneration, in a temporal manner. Nevertheless, studies are under way in our laboratories wherein we are inducing these metabolic changes in the parent strain of rat, which are not obese, by manipulating the diet. The effect of these metabolic changes without overt obesity might help us to address their relationship with retinal degeneration. This animal model may aid in investigating the association between obesity and retinal degeneration and the possibility of modifying obesity-associated risk by altering diet. The WNIN-Ob rat may thus be explored in greater detail for its usefulness as a model to study obesity-associated retinal degeneration. Evaluation of retinal vasculature and lipofuscin content in WNIN-Ob rats may provide valuable information. Given the impact of retinal dystrophies on blindness and the increased risk for retinal dystrophy caused by obesity, it is highly desirable that we understand the molecular basis of obesity-associated retinal degeneration. In this context, the observations made in this study provided such an opportunity.
Although obesity may be a risk factor for many ocular conditions, including retinal degeneration, the present literature is inadequate in establishing any convincing association. Nevertheless, the reduced levels of macular pigments in obese persons12,47 support the association of increased risk for retinal degeneration in this population. Reductions in macular lutein and zeaxanthin may be caused by decreased dietary intake in obese persons or by competition between retina and adipose tissue for the uptake of lutein and zeaxanthin.12,47 However, whether weight loss, by physical activities and dietary manipulations, reduces the risk for eye diseases remains unresolved. Because of the potential public health impact of obesity, there is a greater need to understand its ocular effects. The WNIN-Ob rat may aid investigators to address these issues.
Supplementary Material
Acknowledgments
The authors thank Boindla Sesikeran (National Institute of Nutrition) for assistance in examining the histology slides, Krishnakumar Sarada and Pasupuleti Lopamudra (National Institute of Nutrition) for assistance in preparing histologic specimens, Motha Satyavani (National Institute of Nutrition) for providing animal maintenance and technical assistance, Austra Liepa (University of Michigan) for assistance in manuscript preparation, and Mitchell Gillet (University of Michigan) for histologic tissue preparation.
Supported in part by the Indian Council of Medical Research and Department of Biotechnology, New Delhi; National Institutes of Health Grants EY13198 (RA) and EY015208 (MMJ); National Eye Institute Core Grants EY013080 (University of Tennessee Health Science Center), P30EY007003 (Department of Ophthalmology, University of Michigan), and P30EY07060 (Department of Vision Research, University of Michigan); an unrestricted grant from Research to Prevent Blindness, Inc. (Department of Ophthalmology, University of Tennessee Health Science Center); Foundation Fighting Blindness (RA); and Research to Prevent Blindness Inc. (RA, GBR). GBR is the recipient of an International Fellowship from the Indian Council of Medical Research and an Overseas Associateship (Niche Areas) from the Department of Biotechnology, New Delhi.
Footnotes
Disclosure: G.B. Reddy, None; V. Vasireddy, None; M.N.A. Mandal, None; M. Tiruvalluru, None; X.F. Wang, None; M.M. Jablonski, None; G. Nappanveettil, None; R. Ayyagari, None
Geereddy Bhanuprakash Reddy, National Institute of Nutrition, Hyderabad 500007, India; geereddy@yahoo.com.
References
- 1.Bohlman H. Communicating the ocular and systemic complications of obesity to patients. Optometry. 2005;76:701–712. [DOI] [PubMed] [Google Scholar]
- 2.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States: 2000. JAMA. 2004;291:1238–1245. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States: 1999–2004. JAMA. 2006;295:1549–1555. [DOI] [PubMed] [Google Scholar]
- 5.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. [DOI] [PubMed] [Google Scholar]
- 6.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007;356: 213–215. [DOI] [PubMed] [Google Scholar]
- 7.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35:93–99. [DOI] [PubMed] [Google Scholar]
- 8.Cheung N, Wong TY. Obesity and eye diseases. Surv Ophthalmol. 2007;52:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaumberg DA, Christen WG, Hankinson SE, Glynn RJ. Body mass index and the incidence of visually significant age-related maculopathy in men. Arch Ophthalmol. 2001;119:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith W, Mitchell P, Leeder SR, Wang JJ. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 1998;116:583–587. [DOI] [PubMed] [Google Scholar]
- 11.Seddon JM, Cote J, Davis N, Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol. 2003;121:785–792. [DOI] [PubMed] [Google Scholar]
- 12.Johnson EJ. Obesity, lutein metabolism, and age-related macular degeneration: a web of connections. Nutr Rev. 2005;63:9–15. [DOI] [PubMed] [Google Scholar]
- 13.Green JS, Parfrey PS, Harnett JD, et al. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med. 1989;321:1002–1009. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura DY, Fath M, Mullins RF, et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A. 2004;101:16588–16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong TY, Duncan BB, Golden SH, et al. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk in Communities Study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137: 486–495. [DOI] [PubMed] [Google Scholar]
- 17.Krishnaiah S, Das T, Nirmalan PK, et al. Risk factors for age-related macular degeneration: findings from the Andhra Pradesh Eye Disease Study in South India. Invest Ophthalmol Vis Sci. 2005;46: 4442–4449. [DOI] [PubMed] [Google Scholar]
- 18.Blacque OE, Leroux MR. Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci. 2006; 63:2145–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagstrom SA, North MA, Nishina PL, Berson EL, Dryja TP. Recessive mutations in the gene encoding the tubby-like protein TULP1 in patients with retinitis pigmentosa. Nat Genet. 1998;18:174–176. [DOI] [PubMed] [Google Scholar]
- 20.Ohlemiller KK, Hughes RM, Lett JM, et al. Progression of cochlear and retinal degeneration in the tubby (rd5) mouse. Audiol Neurootol. 1997;2:175–185. [DOI] [PubMed] [Google Scholar]
- 21.Giridharan NV. Animal models of obesity and their usefulness in molecular approach to obesity. Indian J Med Res. 1998;108:225–242. [PubMed] [Google Scholar]
- 22.Jayaraman KS. ‘Sumo’ rats set researchers on hunt for obesity genes. Nat Med. 2005;11:108. [DOI] [PubMed] [Google Scholar]
- 23.Girdharan NV, Harishankar N, Satyavani M. A new rat model for the study of obesity. Scand J Lab Anim Sci. 1996;23:131. [Google Scholar]
- 24.Mandal MN, Ambasudhan R, Wong PW, Gage PJ, Sieving PA, Ayyagari R. Characterization of mouse orthologue of ELOVL4: genomic organization and spatial and temporal expression. Genomics. 2004;83:626–635. [DOI] [PubMed] [Google Scholar]
- 25.Mandal MN, Vasireddy V, Jablonski MM, et al. Spatial and temporal expression of MFRP and its interaction with CTRP5. Invest Ophthalmol Vis Sci. 2006;47:5514–5521. [DOI] [PubMed] [Google Scholar]
- 26.Mandal MN, Vasireddy V, Reddy GB, et al. CTRP5 is a membrane associated and secretory protein in the RPE and ciliary body and the S163R mutation of CTRP5 impairs its secretion. Invest Ophthalmol Vis Sci. 2006;47:5505–5513. [DOI] [PubMed] [Google Scholar]
- 27.Vasireddy V, Jablonski MM, Mandal MN, et al. Elovl4 5-bp-deletionknock-in mice develop progressive photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2006;47:4558–4568. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida S, Mears AJ, Friedman JS, et al. Expression profiling of the developing and mature Nrl/ mouse retina: identification of retinal disease candidates and transcriptional regulatory targets of Nrl. Hum Mol Genet. 2004;13:1487–1503. [DOI] [PubMed] [Google Scholar]
- 29.Mutch DM, Clement K. Unraveling the genetics of human obesity. PLoS Genet. 2006;2:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. [DOI] [PubMed] [Google Scholar]
- 31.Rattner A, Sun H, Nathans J. Molecular genetics of human retinal disease. Annu Rev Genet. 1999;33:89–131. [DOI] [PubMed] [Google Scholar]
- 32.Rivolta C, Sharon D, DeAngelis MM, Dryja TP. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet. 2002;11:1219–1227. [DOI] [PubMed] [Google Scholar]
- 33.Misra A, Pandey RM, Devi JR, Sharma R, Vikram NK, Khanna N. High prevalence of diabetes, obesity and dyslipidaemia in urban slum population in northern India. Int J Obes Relat Metab Disord. 2001;25:1722–1729. [DOI] [PubMed] [Google Scholar]
- 34.Yajnik CS. Obesity epidemic in India: intrauterine origins? Proc Nutr Soc. 2004;63:387–396. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez AJ, Garcia-Fernandez JM, Gonzalez B, Foster RG. The spatio-temporal pattern of photoreceptor degeneration in the aged rd/rd mouse retina. Cell Tissue Res. 1996;284:193–202. [DOI] [PubMed] [Google Scholar]
- 36.Ogilvie JM, Tenkova T, Lett JM, Speck J, Landgraf M, Silverman MS. Age-related distribution of cones and ON-bipolar cells in the rd mouse retina. Curr Eye Res. 1997;16:244–251. [DOI] [PubMed] [Google Scholar]
- 37.LaVail MM, Yasumura D, Matthes MT, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 38.Mohand-Said S, Deudon-Combe A, Hicks D, et al. Normal retina releases a diffusible factor stimulating cone survival in the retinal degeneration mouse. Proc Natl Acad Sci U S A. 1998;95:8357–8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohand-Said S, Hicks D, Dreyfus H, Sahel JA. Selective transplantation of rods delays cone loss in a retinitis pigmentosa model. Arch Ophthalmol. 2000;118:807–811. [DOI] [PubMed] [Google Scholar]
- 40.Davis RE, Swiderski RE, Rahmouni K, et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104:19422–19427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swiderski RE, Nishimura DY, Mullins RF, et al. Gene expression analysis of photoreceptor cell loss in bbs4-knockout mice reveals an early stress gene response and photoreceptor cell damage. Invest Ophthalmol Vis Sci. 2007;48:3329–3340. [DOI] [PubMed] [Google Scholar]
- 42.Dosso A, Rungger-Brandle E, Rohner-Jeanrenaud F, et al. Ocular complications in the old and glucose-intolerant genetically obese (fa/fa) rat. Diabetologia. 1990;33:137–144. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda S, Shiva N, Ikeda A, et al. Retinal degeneration but not obesity is observed in null mutants of the tubby-like protein 1 gene. Hum Mol Genet. 2000;9:155–163. [DOI] [PubMed] [Google Scholar]
- 44.Hackam AS, Strom R, Liu D, et al. Identification of gene expression changes associated with the progression of retinal degeneration in the rd1 mouse. Invest Ophthalmol Vis Sci. 2004;45:2929–2942. [DOI] [PubMed] [Google Scholar]
- 45.Vazquez-Chona F, Song BK, Geisert EE Jr. Temporal changes in gene expression after injury in the rat retina. Invest Ophthalmol Vis Sci. 2004;45:2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim LS, Fishman GA, Seiple WH, Szlyk JP, Stone EM. Retinaldys function in carriers of Bardet-Biedl syndrome. Ophthalmic Genet. 2007;28:163–168. [DOI] [PubMed] [Google Scholar]
- 47.Hammond BR Jr, Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci. 2002;43:47–50. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.