Abstract
The global rate of cancer has increased in recent years, and cancer is still a threat to human health. Recent developments in cancer treatment have yielded the understanding that viruses have a high potential in cancer treatment. Using oncolytic viruses (OVs) is a promising approach in the treatment of malignant tumors. OVs can achieve their targeted treatment effects through selective cell death and induction of specific antitumor immunity. Targeting tumors and the mechanism for killing cancer cells are among the critical roles of OVs. Therefore, evaluating OVs and understanding their precise mechanisms of action can be beneficial in cancer therapy. This review study aimed to evaluate OVs and the mechanisms of their effects on cancer cells.
Keywords: oncolytic virus, cancer immunotherapy, cancer vaccine, targeted treatment, immune checkpoint
Background
Millions of individuals are affected by cancer annually. Cancer is considered the leading cause of death and the most important barrier to the increase in life expectancy in the twenty-first century. In 2018, 18.1 million new cancer cases (17.0 million cancer cases excluding non-melanoma skin cancers) were reported. The mortality due to cancer in 2018 was 9.6 million (9.5 million, excluding non-melanoma skin cancers) (1). Significant developments in cancer treatment started in 1900. The achievements of this progress include the development of diagnostic, surgery, chemotherapy, hormone therapy, gene therapy, and cell therapy methods. Regardless of these advancements, human is still incapable of combating cancer, as none of the identified treatment methods could be used in all stages of cancer (2). Many of cancer patients experience a relapse of disease progression regardless of the primary response to treatment.
Furthermore, complete resection of the tumor is difficult or impossible in many cases (3). Immunotherapy has evolved as a practical treatment choice against malignant diseases during the past decades. Studies in oncolytic virotherapy (OVT) developed in the early twentieth century as an observational science for the cases of spontaneous regression of tumors were reported due to infection with specific viruses (4).
Oncolytic viruses (OVs) include a group of viruses that selectively affect and kill malignant cells, leaving the surrounding healthy cells unaffected. OVs have direct cytotoxic effects on cancer cells and augment host immune reactions and result in the destruction of the remaining tumoral tissue and establish a sustained immunity (5). Indeed, OVs function in four ways against tumor cells, including oncolysis, antitumor immunity, transgene expression, and vascular collapse (6). Regarding the fact that cancer cells are developed to avoid detection and destruction by the host immune system and also to resist apoptosis, which are the critical responses of normal cells in limiting viral infections, OVs can kill cancer cells through a spectrum of actions ranging from direct cytotoxicity to induction of immune-mediated cytotoxicity. OVs can also indirectly destroy cancer cells by destroying tumor vasculature and mediating antitumor responses (7). Furthermore, in order to augment the therapeutic characteristics, modifications in OVs by genetic engineering such as insertions and deletions in the genome have been employed in many investigations; thus, additional antitumor molecules can be delivered to cancer cells and effectively bypass the widespread resistance of single-target anticancer drugs (8)
It should be noted that the use of OVs in cancer therapy was limited due to the pathogenicity and toxicity of these viruses in human cases. Recent advancements in genetic engineering have optimized the function of OVs through genetic modifications and therefore have become the issue of interest in OVT (9). Each virus tends to a specific tissue, and this tendency determines which host cells are affected by the virus and what type of disease will be generated. For instance, rabies, hepatitis B, human immunodeficiency virus (HIV), and influenza viruses affect neurons, hepatocytes, T lymphocytes, and respiratory tract epithelium, respectively. Several naturally occurring viruses have a preferential but not exclusive tendency towards cancer cells. This issue is more attributed to tumor cell biology compared to the biology of the virus.
OVs are generally categorized into two groups. One group is preferentially replicated in cancer cells and is not pathogenic for normal cells due to the increased sensitivity to the innate immune system’s antiviral signaling or dependence on the oncogenic signaling pathways. Autonomous parvovirus, myxoma virus (MYXV; poxvirus), Newcastle disease virus (NDV; paramyxovirus), reovirus, and Seneca valley virus (SVV; picornavirus) are categorized in this group. The second group of OVs includes viruses that are either genetically modified for purposes including vaccine vectors such as mumps virus (MV; paramyxovirus), poliovirus (PV; picornavirus), and vaccinia virus (VV; poxvirus), or genetically engineered through mutation/deletion of genes required for replication in normal cells, including adenovirus (Ad), Herpes simplex virus (HSV), VV, and vesicular stomatitis virus (VSV; rhabdovirus) (10).
Furthermore, the mutation in cancer cells, drug adaptation, resistance, and cell immortality were effective in the initiation and speed of viral dissemination. Today, researchers are trying to discover and identify a new generation of OVs to save more patients’ lives from cancer. Evaluation of OVs and identification of the exact mechanism of action of these viruses can be helpful in this way (11). This review study aimed to evaluate OVs and their mechanism of action against cancer cells.
Methodology
The key terms in the literature search included oncolytic virus, cancer, immunotherapy, innate immunity, adaptive immunity, virotherapy, viral therapy, oncolytic, and virus were searched in international databases, namely, Web of Science, PubMed, and Scopus from 2004 to 2021. The inclusion criterion was the evaluation of viruses using standard in vivo and in vitro laboratory methods. Exclusion criteria were lack of access to full text articles and incomplete description or assessment of diseases other than cancers.
Results
The primary search yielded 1,450 articles. Finally, 47 articles were included in the review after eliminating irrelevant and duplicate studies. The characteristics of the 47 included articles are presented in Table 1 , performed from 2004 to 2021. The OV families assessed in the studies included Ad, MV, PV, NDV, SFV, HSV, VV Reovirus, and bovine herpesvirus (BHV). The most commonly assessed virus was adenovirus (Ad) (n = 15), followed by the herpesvirus (HSV) (n = 12) and measles virus (MV) (n = 7). The least assessed viruses were BHV, SFV, and Reovirus (n = 1).
Table 1.
The collective studies on OVs.
| Virus | Cancer | Model | Effects | Mechanism | References |
|---|---|---|---|---|---|
| Adenovirus | Head and neck squamous cell carcinoma | Murine | Ad-derived IL-12p70 prevents the destruction of HER2.CAR-expressing T cells at the tumor site. | Enhanced antitumor effects of HER2 CAR T cells by CAd12_PDL1 Controlling of primary tumor growth and metastasis. |
Shaw et al., 2017 (12) |
| Renal cell carcinoma | Murine | HRE-Ki67-Decorin suppressed tumor growth and induced decorin expression in the extracellular matrix (ECM) assembly. | An effective anticancer treatment strategy may be chimeric HRE-Ki67 promoter-regulated Ad carrying decorin. | Zhang et al., 2020 (13) | |
| Lung cancer stem cell (LCSC) | Murine | Tumor necrosis factor (ZD55-TRAIL) increased cytotoxicity and induced A549 sphere cells apoptosis through a mitochondrial pathway | Treatment of lung cancer is possible by targeting LCSCs with armed oncolytic adenovirus genes. | Yang et al., 2015 (14) | |
| Leukemia | Murine | Induction of autophagic cell death Enhanced cell killing in primary leukemic blasts |
Significant autophagic cell death | Tong et al., 2013 (15) | |
| Breast cancer | Murine | Tumor killing due to Sox2 and oct4 expression and Hoechst 33342 exclusion CD44+CD24−/low cells |
A positive effect against advanced orthotopic was that CD44+CD24−/low-derived tumors were observed. | Eriksson et al., 2007 (16) | |
| Breast cancer | Murine | Delta24 can replicate and help the E1-deleted adenovector replicate in cancer cells | Spontaneous liver metastasis with Delta 24 virus therapy alone was less reduced than in combination with TRAIL gene therapy. | Guo et al., 2006 (17) | |
| Liver cancer stem-like cells | Murine | Significant apoptosis Inhibition angiogenesis in xenograft tumor tissues Inhibition of the propagation of cells occurred due to GD55 |
GD55 had a higher effect in suppressing tumor growth than oncolytic adenovirus ZD55. | Zhang et al., 2016 (18) | |
| B16F10 | Murine | Infiltration of effector CD4+ and CD8+ T cells Increasing secretion of TNF-α and IFN-γ |
Activation the immune system Creating a proinflammatory environment |
Wei et al., 2020 (19) | |
| αvβ6-positive tumor cell lines of pancreatic and breast cancer | Murine | Cells expressing high levels of αvβ6 (BxPc, PANC0403, Suit2) were killed more efficiently by oncolytic Ad5NULL-A20 than by oncolytic Ad5 | Ad5NULL-A20-based virotherapies efficiently target αvβ6-integrin-positive tumors | Davies et al., 2021 (20) | |
| Advanced metastatic tumors | Murine | Increase in CD8+ T cells Reduction of IFN-γ secretion |
Specific immunity against tumor | Cerullo et al., 2010 (21) | |
| Breast cancer | Murine | Inflammation and neutrophil infiltration due to oncolytic adenovirus-GM-CSF. | Ad5/3-D24-GMCSF, combined with low-dose CP showed efficacy and antitumor activity | Bramante et al., 2016 (22) | |
| Solid tumors | Murine | CD8 cytotoxicity viruses efficiently lysed tumors | Significantly prolonged survival | Gürlevik et al., 2010 (23) | |
| Metastatic ductal breast cancer | Murine | Each virus featured 5/3 chimerism of a promoter controlling the expression of E1A and fiber, which was also deleted in the Rb binding domain for additional tumor selectivity | These viruses completely eradicated CD44+ low CD24−/cells in vitro
Significant antitumor activity in CD44+ CD24−/low-derived tumors in vivo |
Bauerschmitz et al., 2008 (24) | |
| Metastatic melanoma | In vitro | Activation and an increased costimulatory capacity of monocyte-derived antigen-presenting cells | A valuable immunotherapeutic agent for melanoma is ORCA-010 | González et al., 2020 (25) | |
| Gastric cancer MKN45 and MKN7 cells |
Murine | Cell death in stem cells such as CD133 resident cancer by stimulating cell-cycle-related proteins | Killing cancer cells | Yano et al., 2013 (26) | |
| Herpesvirus | Bearing M3-9-M tumors | Murine | Increasing the incidence of CD4+ and CD8+ T cells and no correlation with the CD4+CD25+Foxp3+ regulatory T-cell populations in the tumor | An efficient therapy strategy for soft tissue sarcoma in childhood | Chen et al., 2017 (27) |
| Breast cancer | Murine | Regulation of CD8+ T cell activation markers in the tumor microenvironment Inhibition of tumor angiogenesis |
Tumor regression Anticancer immune response |
Ghouse et al., 2020 (28) | |
| Colon carcinoma | Murine | Decreased inhibitory immune cells Increased positive immune cells in the spleen. |
Generate tumor-specific immunity Elimination of primary tumors Developing immune memory to inhibit tumor recurrence and metastasis. |
Zhang et al., 2020 (29) | |
| Ovarian carcinoma | Murine | DC maturation and tumor infiltration of INF-γ+ CTL | The antitumor immune responses are facilitated | Benencia et al. 2008 (30) |
|
| Tumor | Murine | T-cell responses against primary or metastatic tumors | Antitumor immune response Prevention of tumor growth |
Li et al., 2007 (31) | |
| STING low-metastatic melanoma | Murine | Release of DAMP factors Release of IL-1β and inflammatory cytokines Induction of host antitumor immunity |
Induction of immunogenic cell death (ICD) Recruitment of viral and tumor-antigen-specific CD8+ T cells STING expression as a predictive biomarker of T-Vec Response |
Bommareddy et al., 2019 (32) | |
| Osteosarcoma cells | Murine | Antitumor efficacy in vivo
Inducing antitumor immunity |
The in vitro cytolytic properties of OVs are poor prognostic indicators of effective cancer virotherapy and in vivo antitumor activity | Sobol et al., 2011 (33) | |
| HCT8 human colon cancer cells | Murine | Cytotoxicity, viral replication, and Akt1 expression | Therapy of TIC-induced tumors with NV1066 slowed tumor growth and yielded tumor regression | Warner et al., 2016 (34) | |
| Glioblastoma-derived cancer stem-like cells (GBM-SC) | Murine | Infection with HSV G47Delta killed GBM-SCs and inhibited their self-renewal and the inability of viable cells to form secondary tumor spheres | Significant anti-tumor effect against xenografts in mice and effective killing of CSCs in vitro | Wakimoto et al., 2009 (35) | |
| Solid tumors | Human | The induction of adaptive antitumor immune responses | All patients were seropositive. No local recurrence was observed in patients and disease-specific survival was 82.4% | Harrington et al., 2010 (36) | |
| Breast, head and neck, and gastrointestinal cancers, and malignant melanoma | Human | Induction of adaptive anti-tumor immune responses | Biopsies contained residual tumor was observed in 19 patients after treatment that 14 of them showed tumor necrosis (extensive, or apoptosis) | Hu et al., 2006 (37) | |
| Metastatic melanoma | Human | ICP47 deletion increases US11 expression and enhances virus growth and replication in tumor cells | Overall survival at 12 and 24 months were 58% and 52%, respectively. | Senzer et al., 2009 (38) | |
| Measles virus | Solid tumor | Murine | GOS/MV-Edm significantly increases viral replication in tumor mass | Increased survival in passive antiserum immunized tumor-bearing mice | Xia et al., 2019 (39) |
| Orthotopic glioma tumor spheres and primary colon cancer | Murine | Overexpression of the CD133 target receptor or increased kinetics of proliferation through tumor cells | CD133-targeted measles viruses selectively removed CD133þ cells from tumor tissue | Bach et al., 2013 (40) | |
| Mesothelioma | Murine | Infiltration of CD68+ cells innate immune cells. | Oncolytic MVs is versatile and potent agents for the treatment of human mesothelioma. | Li et al., 2010 (41) | |
| Multiple myeloma | Murine | Induction of adaptive anti-tumor immune responses | Virus-infected T cells may induce systemic measles virus therapy in the presence of ABS antivirus. | Ong et al., 2007 (42) | |
| Breast cancer | In vitro | Inducing apoptosis | Induction of cell death leads to infection of breast cancer cells with rMV-BNiP | Lal and Rajala et al., 2019 (43) | |
| Breast cancer | In vitro | Increased percentage of apoptotic cells in infected MCF-7 cells | Significant apoptosis in breast cancer cell lines. | Abdullah et al., 2020 (44) | |
| T-cell lymphomas (CTCLs) | Human | An increase in the IFN-γ/CD4 and IFN-γ/CD8 mRNA ratio and a reduced CD4/CD8 ratio | MV can affect CTCL treatment. | Heinzerling et al., 2005 (45) | |
| Newcastle disease virus | Lung cancer | Murine | Caspase-dependent apoptosis associated with increased caspase-3 processing and ADP-ribose polymerase cleavage. | A potential strategy for targeting lung CSCs | Hu et al., 2015 (46) |
| B16 melanoma | Murine | Treatment with systemic CTLA-4 blockade was due to long-term survival and tumor rejection | Distant tumors are prone to systemic therapy with immunomodulatory antibodies using localized therapy with oncolytic NDV | Zamarin et al., 2014 (47) | |
| Lung cancer | Murine | DAMP release Autophagy induction |
Inhibited tumor growth Trigger ICD |
Ye et al., 2018 (48) | |
| GBM | Murine | GBM susceptibility to NDV is dependent on the loss of the type I IFN | Trigger the activation of immune cells against the tumor and show oncolytic effect | García-Romero et al., 2020 (49) | |
| Vaccinia virus | Melanoma | Murine | PD-L1 inhibition Neoantigen presentation |
Tumor neoantigen-specific T-cell responses | Wang et al., 2020 (50) |
| Solid tumors | Murine | Activated the inflammatory immune status | Complete tumor regression long-term tumor-specific immune memory |
Nakao et al., 2020 (51) | |
| Solid cancer | Murine | Replication was activated by EGFR/Ras pathway signaling, cellular TK levels, and cancer cell resistance to IFNs | Selectively cell lysis and stimulation of antitumoral immunity | Parato et al., 2012 (52) | |
| M1 virus | Melanoma | Murine | CD8+ T-cell-dependent therapeutic effects long-term antitumor immune memory Upregulating the expression of PD-L1 |
Immunogenic tumor cell death Restores the ability of dendritic cells to prime antitumor T cells |
Yang Liu et al., 2020 (11) |
| Bladder tumor | Murine | Inhibition of CCDC6 improve viral replication and then induced endoplasmic reticulum stress to facilitate M1 virus oncolytic effects. | CCDC6 inhibition resulted in better antitumor activity | Liu et al., 2021 (53) | |
| Poxvirus | MC-38 colon adenocarcinoma tumors | Murine | Elicited TILs with lower quantities of exhausted PD-1hiTim-3+ CD8+ T cells and regulatory T cells | Tumor regression and improved survival | Mathilde et al., 2020 (54) |
| Poliovirus | Breast cancer | Murine | Primary oncolytic viral receptors are highly expressed in tumor cells and transmitted among cells. | Oncolytic PV recombinants may affect tumor cells by viral receptor CD155 | Ochiai et al., 2004 (55) |
| Reovirus | Solid tumor | Murine | Induction of Golgi fragmentation and accumulation of oncogenic Ras in the Golgi body | Initiating apoptotic signaling events required for virus release and spread. | Garant et al., 2016 (56) |
| Adenovirus (Ad), Semliki Forest virus (SFV) and Vaccinia virus (VV) | Osteosarcoma | Murine | Activates immunogenic apoptosis Triggering phagocytosis and maturation of DCs Th1-cytokine release by DCs and antigen-specific T-cell activation. |
Induction of T-cell-mediated antitumor immune responses. Increased cell death processes |
Jing Ma et al., 2020 (57) |
PD-L1, programmed death-ligand 1; Ad, adenovirus; MV, measles virus; GBM, glioblastoma; NDV, Newcastle disease virus; VV, Vaccina virus; Th, T helper; ICD, immunogenic cell death; EGFR, epidermal growth factor receptor; TK, thymidine kinase; IFN-I, type-I interferon; HSV, herpes simplex viruses; TIL, tumor infiltration lymphocyte; DC, dendritic cells; BHV, bovine herpesvirus; DAMP, damage-associated molecular pattern; Trail, TNF-related apoptosis-inducing ligand; GD-55, GOLPH2-regulated oncolytic adenovirus; GOS, graphene oxide arms PV, polio virus; LAPV, Israeli acute paralysis virus; CP, cisplatin; GM-CSF, granulocyte–macrophage colony-stimulating factor.
According to Table 1 , OVs may employ multifunction against tumor cells; however, the most antitumor actions of OVs were related to cytolysis activity and inducing antitumor immunity (n = 26) in which adenovirus (n = 11) and HSV (n = 9) were the most responsible OVs in their categories, respectively. However, the last action was associated with vascular collapse. The collective data in Table 2 exhibited a summary of clinical trials of OVs implicated in malignancies highlighting the most considerable focus on engineered VV by TKdel GMCSF exp (JX-594) on solid tumors supported by Jennerex Biotherapeutics Company. The majority of studies under clinical trials involve a transgene virus encoding an immune-stimulatory or proapoptic gene to boost the oncolytic features of the virus. As Table 2 reveals, granulocyte–macrophage colony-stimulating factor (GM-CSF) and pro-drug-converting enzymes are the most popular transgenes, although many OVs encoding novel therapeutic cargos are in clinical development. Streby et al., in phase I clinical trial, examined the effects of HSV1716 on relapsed/refractory solid tumors. Despite the fact that none of the patients exhibited objective responses, virus replication and inflammatory reactions were seen in patients (58). In another clinical trial, Desjardins et al. reported a higher survival rate in grade IV malignant glioma patients who received recombinant nonpathogenic polio–rhinovirus chimera (59). In a phase I clinical trial, Rocio Garcia-Carbonero et al. discovered that enadenotucirev IV infusion was associated with high local CD8+ cell infiltration in 80% of tumor samples evaluated, indicating a possible enadenotucirev-driven immune response (60). TG4023, a modified vaccinia Ankara viral vector carrying the FCU1 suicide gene, was used in a phase I trial to convert the non-cytotoxic prodrug flucytosine (5-FC) into 5-fluorouracil (5-FU) in the intratumor. Finally, 16 patients with liver tumors were successfully injected; the MTD was not achieved, and a high therapeutic index was demonstrated (61). Dispenzieri et al. examined MV-NIS effects in patients with relapsed, refractory myeloma and reported satisfactory primary results (62).
Table 2.
The summary of clinical trials for oncolytic viruses.
| Phase | Virus | Tumor | Interventions | Trial code | Country | Company |
|---|---|---|---|---|---|---|
| Phase I | JX-594 | Refractory solid tumors | Intratumoral injection | NCT01169584 | USA | Jennerex Biotherapeutics |
| JX-594 | Refractory solid tumors | Intravenous infusion | NCT00625456 | Canada | Jennerex Biotherapeutics | |
| HSV-1, TBI-1401 (HF10) | Solid tumor with superficial lesions | Intratumoral administration | NCT02428036 | Japan | Takara Bio Inc. | |
| Recombinant measles virus | Ovarian cancer Primary peritoneal cavity cancer |
Intraperitoneal administration | NCT00408590 | USA | Mayo Clinic | |
| GM-CSF-Adenovirus CGTG-102 | Malignant solid tumor | In combination with low dose cyclophosphamide | NCT01598129 | Finland | Targovax Oy | |
| Adenovirus VCN-01 | Solid tumor | Intravenous administration with or without gemcitabine | NCT02045602 | Spain | VCN Biosciences, S.L. | |
| REOLYSIN® | KRAS mutant metastatic colorectal Cancer | Intravenous administration with Irinotecan/Fluorouracil/Leucovorin and Bevacizumab | NCT01274624 | USA | Oncolytics Biotech | |
| Adenovirus VCN-01 | Pancreatic cancer | Intratumoral injections with intravenous Gemcitabine and Abraxane® | NCT02045589 | Spain | VCN Biosciences, S.L. | |
| JX-594 | Hepatic carcinoma | Transdermal injection | NCT00629759 | Korea | Jennerex Biotherapeutics | |
| Attenuated Vaccinia Virus, GL-ONC1 | Solid organ cancers | Intravenous administration | NCT00794131 | United Kingdom | Genelux Corporation | |
| Coxsackievirus Type A21 | Melanoma | Intratumoural injection | NCT00438009 | Australia | Viralytics | |
| REOLYSIN® | Pancreatic adenocarcinoma | Pembrolizumab (KEYTRUDA®) | NCT02620423 | USA | Oncolytics Biotech | |
| Vaccinia Virus (GL-ONC1) | Head and neck carcinoma | With concurrent Cisplatin and radiotherapy | NCT01584284 | USA | Genelux Corporation | |
| Phase II | TBI-1401(HF10) | Melanoma | In combination with Ipilimumab | NCT03153085 | Japan | Takara Bio Inc. |
| HF10 | Malignant melanoma | With Ipilimumab | NCT02272855 | USA | Takara Bio Inc. | |
| OncoVEX^GM-CSF | Melanoma | Intratumoral injection | NCT00289016 | United Kingdom | – | |
| Edmonston strain of Measles Virus Expressing NIS | Refractory multiple myeloma | Systemic Administration with cyclophosphamide | NCT02192775 | USA | University of Arkansas | |
| Reovirus Serotype 3 REOLYSIN® | Non-small cell lung cancer | Intravenous administration with paclitaxel and carboplatin | NCT00861627 | USA | Oncolytics Biotech | |
| JX-594 | Hepatocellular carcinoma | Intratumoral injection | NCT00554372 | USA | Jennerex Biotherapeutics | |
| CG0070 | Non-muscle invasive bladder carcinoma | – | NCT02365818 | USA | CG Oncology, Inc. | |
| Wild-type Reovirus REOLYSIN® | Bone and soft tissue sarcomas | Intravenous injection | NCT00503295 | USA | Oncolytics Biotech | |
| Phase I/II | Vaccinia Virus JX-594 | Melanoma | Intratumoral injection | NCT00429312 | USA | Jennerex Biotherapeutics |
| Parvovirus H-1 | Glioblastoma multiforme | Intratumoral/Intracerebral injection | NCT01301430 | Germany | Oryx GmbH & Co. KG | |
| HSV1716 | Malignant pleural mesothelioma | Intrapleural injection | NCT01721018 | United Kingdom | Virttu Biologics Limited | |
| Ad-MAGEA3 | Metastatic non-small cell lung cancer | With pembrolizumab | NCT02879760 | Canada | Turnstone Biologics, Corp. | |
| REOLYSIN® | Recurrent malignant gliomas | Intralesional administration | NCT00528684 | USA | Oncolytics Biotech | |
| JX 594 | Colorectal carcinoma | Multiple intravenous with Irinotecan | NCT01394939 | USA | Jennerex Biotherapeutics | |
| Vaccinia Virus GL-ONC1 | Peritoneal Carcinomatosis | Intraperitoneal administration | NCT01443260 | Germany | Genelux GmbH |
Cohn et al., in phase II clinical trial, evaluated the effects of oncolytic reovirus (Reolysin®) plus weekly paclitaxel in women with recurrent or persistent ovarian, tubal, or primary peritoneal cancer. The results did not show any improvement in the patient status (63), although Mahalingam et al. showed that REOLYSIN®, plus carboplatin and paclitaxel, is an effective treatment in advanced malignant melanoma (64). Packiam et al. showed that CG0070 (GM-CSF expressing adenovirus) has a 47% CR rate at 6 months for all patients and 50% for patients with carcinoma-in situ (65).
Geletneky et al. evaluated H-1 parvovirus (H-1PV) effects in recurrent glioblastoma patients and reported microglia/macrophage activation and cytotoxic T-cell infiltration in the infected tumors, proposing initiation of the immunogenic response (66).
Andtbacka et al., in a phase III study, evaluated Talimogene laherparepvec (T-VEC) in stage IIIc and stage IV malignant melanoma. T-VEC was the first approved OVs against melanoma in a phase III clinical trial. This virus compared with GM-CSF showed a higher durable response rate and overall survival (67). In another newest phase III study, Talimogene laherparepvec was approved by the Food and Drug Administration (FDA) in the USA, European Union, and Australia (68).
Discussion
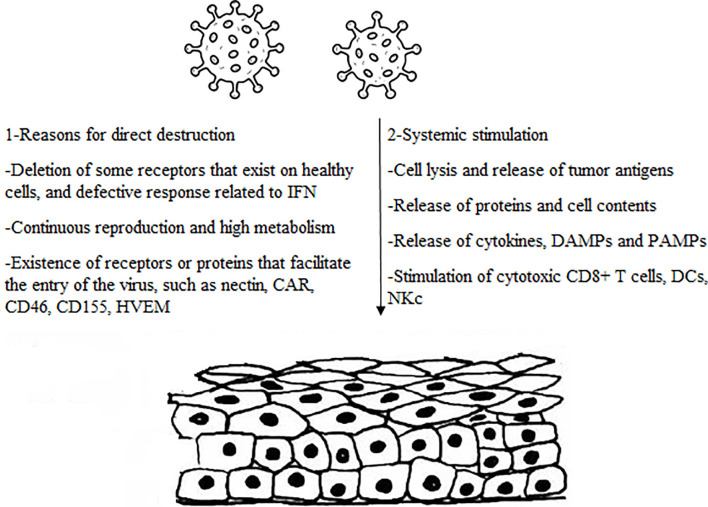
As a challenge in cancer therapy approaches (1), the exclusive features of oncolytic viruses have attracted plenty of researchers in recent years. OVs have the dramatic capability to selectively infect tumor cells leading to direct or indirect cancer cell death without harming normal cells (7). This study focused on some mechanisms employed by OVs against tumor cells, which are exactly various from virus to virus ( Figure 1 ).
Figure 1.
The main mechanism involved by oncolytic viruses.
According to most studies, OVs can target cancer cells and benefit from tumor conditions in favor of replication in infected cells, eventually leading to oncolysis. Indeed, tumor cells tend to resist apoptosis and translational suppression, which are both compatible with the growth of several viruses (7). One of the main actions of OVs is to take advantage of immune-evading properties of cancer cells to escape from recognition and destruction by the immune system. Antiviral processes in normal cells are associated with the interferon pathway in which the secretion of type I interferon (IFN) cytokine can trigger an antiviral response and induce ISGs to block viral replication (69). This subsequently leads to cell apoptosis, as it is known that the IFN-I signaling regulates the expression of proapoptotic genes such as tumor necrosis factor alpha (TNF-α), FAS ligand, and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (70).
Regarding the IFN-I signaling is defective in most tumor cells, it makes tumor cells susceptible to being infected by some OVs including NDV, VSV, MYXV, and raccoon pox virus (71–73). García-Romero et al. showed that NDV was able to replicate in glioblastoma (GBM) cancer stem cells (CSCs) due to type I IFN gene loss occurring in more than 50% of patients. Infection of GBM with NDV represents oncolytic and immunostimulatory properties through the production of type I IFN in non-tumor cells such as tumor infiltrated macrophages and DC or other cells present at the tumor microenvironment (49). NDV therapy also declines CSCs self-renewing capacity to improve their differentiation ability and facilitate cancer therapy (49, 74). OVs can also benefit from the abnormal expression of the proto-oncogene RAS which generally occurs in normal cells but actives in tumor cells (75). OV infection outcomes can be affected by up-regulation of RAS in tumoral cells and further down-regulation of interferon-inducible genes due to activation of RAS/MEK signaling pathway that reduces viral response in tumoral cells (76). On the contrary with this attempt, Garant et al. demonstrated that reovirus could translocate and accumulate RAS into Golgi apparatus to increase apoptotic signaling events required for virus release (56). This highlighted that the outcomes of OVT are exclusively associated with the characteristics and type of OVs.
High expression of some viral receptors by cancer cells permits higher viral uptake in cancer cells than in normal ones. Some receptors such as CAR (77), laminin (78), CD155 (79), and CD46 (80) are overexpressed in various cancer cells which result in increased uptake of Ad (81), Sindbis virus (82), PV (83), and MV (84) respectively. Interestingly, some viral proteins are poisonous for neoplastic cells and can directly kill cells before viral replication. This was evidenced by the E3 death protein and E4orf4 proteins encoded by Ads and are toxic for cells that end in cytolysis at the time of virus exposure (3). However, deletion in specific viral genes can be another mechanism for the action of the OVs. These genes are necessary for the longevity of viruses in normal cells but not essential for viral activity in cancer cells. Thymidine kinase (TK) is an indispensable enzyme for nucleic acid metabolism encoded in infection with wild type vaccinia virus and enables the replicating of the virus in normal cells. Lister strain virus with TK gene deletion as a type of VV has shown a beneficial antitumor potency and cancer-selective replication in vivo since tumoral cells have a high TK content, which enables the virus to replicate in cancer cells regardless of the deletion in viral TK gene (85). In parallel with this study, Parato et al. analyzed the mechanism of cancer-selectivity by an engineered vaccina virus with TK deletion and epidermal growth factor (EGFR) and lac-Z transgenes observing the replication in tumor cells was related to activation of EGFR/RAS signaling, high cellular TK level and tumor cell resistance to IFN-I (52). These results displayed noticeably the beneficial implication of OVs with inherent and engineered mechanistic properties in cancer therapy approaches.
Oncolytic viruses may interfere with normal physiological process of tumor cells to induce the secretion of pro-inflammatory mediators or even lead to the exposure of tumor-associated antigens (TAA), pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) following apoptosis or oncolysis. These responses can also result in a change in tumor status from immune desert to inflamed status and further recruit a collection of immune cells such as cytotoxic T lymphocytes, dendritic cells, natural killer cells and phagocytic cells to induce immune cell death along with antiviral responses (86, 87).
Remarkably, most viruses continue their infection by expressing genes responsible for escaping the immune system and disseminating in host cells (88). Mutation in these genes can probably improve immune induction and thus increase the anti-tumoral responses regardless these mutations may reduce virus replication further (10). Thus, oncolytic viruses are often engineered to express various genes aided in the overall anti-tumor efficacy of the virus. Transgenes mostly include ranging from immune-stimulatory (IL-2, IL-4, IL-12 and GM-CSF) to pro-apoptotic (tumor necrosis factor alpha, p53 and TRAIL genes inserted into oncolytic viruses (87, 89–94). Interestingly, bystander effects of OVs through local release of cytokines can potentially cause immune response against nearby tumor cells even without direct antigen expression (95).
Furthermore, OVs can destroy tumor vasculature and impede sufficient intratumoral blood reserve, which is essential for tumor progression and metastasis (96). Breitbach et al. demonstrated that intravenous injection of JX-594, an engineered vaccine virus with TK deletion and overexpression of human granulocyte-monocyte colony-stimulating factor (hGM-CSF), led to replication of the virus in endothelial cells of the nearby tumor and disrupted tumor blood flow, which ultimately ended in intensive tumor necrosis within 5 days. Consistently, patients with advanced hepatocellular carcinoma, hypervascular and VEGFhigh tumor type, treated by JX-594 in phase II clinical trials confirmed the efficiency of the JX-594 OV in tumor vasculature disruption without toxicity to normal blood vessels in which inhibition of angiogenesis can passively result in tumor regression (97). This evidence may open promising technologies toward cancer therapy in a way tumor cells are targeted selectively and bypass the side effects of conventional approaches.
Recently, conditionally replication-competent adenoviruses (CRCAs) have been introduced as a successful method for cancer therapy. Sarkar et al. showed that Ad.PEG-E1A-mda-7, a cancer terminator virus (CTV), selectively replicated in cancer cells, inhibits their growth and induces apoptosis (98).
Qian et al. showed that ZD55 expressing melanoma differentiation-associated gene-7/interleukin-24 (ZD55-IL-24) affects B-lymphoblastic leukemia/lymphoma through upregulation of RNA-dependent protein kinase R, enhance phosphorylation of p38 mitogen-activated protein kinase, and induce of endoplasmic reticulum (ER) stress (99).
Azab et al. showed that Ad.5/3-CTV potently suppressed in vivo tumor growth in mouse (100).
Bhoopathi showed that Ad.5/3-CTV induces apoptosis through apoptosis-inducing factor (AIF) translocation into the nucleus, independent of the caspase-3/caspase-9 pathway (101).
In an interesting study, Bhoopathi et al. introduced a novel tripartite CTV “theranostic” adenovirus (TCTV) that targets virus replication, cytokine production, and imaging capabilities uniquely in cancer cells. This TCTV permits targeted treatment of tumors while monitoring tumor regression, with the potential to simultaneously detect metastasis due to the cancer-selective activity of reporter gene expression (102).
Greco et al. showed that ultrasound (US) contrast agents guided MB/Ad.mda-7 complexes to DU-145 cells successfully and eradicated not only targeted DU-145/Bcl-xL-therapy-resistant tumors but also nontargeted distant tumors (103).
T-VEC, adenovirus, and vaccinia virus are the most popular OVs in clinical trials. Approving T-VEC by FDA for the first time could pave the way for other OVs in the clinic. Oncolytic viruses have a broad therapeutic method; hence, their clinical development requires a multidisciplinary view. It is necessary to understand viral generation and viability in infected cells. To improve clinical trials, important factors such as viral entrance, replication, dissemination, oncolysis, and immune activation should be controlled. These factors can vary between tumor types and OVs. It is also critical to understand the immune composition of diverse cancers and the immunological repercussions of viro-immunotherapy.
Conclusion and Future Direction
Cancer is among the most important causes of mortality worldwide, and many chemotherapies and radiotherapy approaches do not have a specific effect on cancer cells and are sometimes accompanied by side effects. Today, a biological war has evolved against cancer by genetically modifying natural pathogens to activate them against neoplastic cells. OVT is a promising therapeutic option in cancer therapy. The mechanisms of action of OVs differ entirely from the mechanism of action of chemotherapy, radiotherapy, surgery, and embolization. They can result in success in the treatment of cancers that are resistant to other therapeutic modalities. Better understanding and acquiring comprehensive information regarding OV therapy and the biology of cancer is an essential step in assessing and controlling cancer programs.
Author Contributions
Conceptualization, WK and HE. Methodology, MF and RD. Validation, BJ. Data curation, MB. Writing—original draft preparation, HE and WK. Writing—review and editing, all. All authors have read and agreed to the published version of the manuscript.
Funding
This study was fully sponsored by Applied Virology Research Center; Baqiyatallah University of Medical Science; Tehran; Iran.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Authors wish to thank all the staff of Applied Virology Research Center; Baqiyatallah University of Medical Science; Tehran, Iran, for their cooperation in implementing procedures.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Davis J, Fang B. Oncolytic Virotherapy for Cancer Treatment: Challenges and Solutions. J Gene Med (2005) 7(11):1380–9. doi: 10.1002/jgm.800 [DOI] [PubMed] [Google Scholar]
- 3. Chaurasiya S, Chen NG, Warner SG. Oncolytic Virotherapy Versus Cancer Stem Cells: A Review of Approaches and Mechanisms. Cancers (Basel) (2018) 10(4):124. doi: 10.3390/cancers10040124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell J, McFadden G. Viruses for Tumor Therapy. Cell Host Microbe (2014) 15(3):260–5. doi: 10.1016/j.chom.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly E, Russell SJ. History of Oncolytic Viruses: Genesis to Genetic Engineering. Mol Ther (2007) 15(4):651–9. doi: 10.1038/sj.mt.6300108 [DOI] [PubMed] [Google Scholar]
- 6. Gujar S, Bell J, Diallo J-S. Snapshot: Cancer Immunotherapy With Oncolytic Viruses. Cell (2019) 176(5):1240–1240.e1. doi: 10.1016/j.cell.2019.01.051 [DOI] [PubMed] [Google Scholar]
- 7. Russell SJ, Peng K-W, Bell JC. Oncolytic Virotherapy. Nat Biotechnol (2012) 30(7):658. doi: 10.1038/nbt.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat Rev Drug Discov (2015) 14(9):642–62. doi: 10.1038/nrd4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filley AC, Dey M. Immune System, Friend or Foe of Oncolytic Virotherapy? Front Oncol (2017) 7:106. doi: 10.3389/fonc.2017.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiocca EA, Rabkin SD. Oncolytic Viruses and Their Application to Cancer Immunotherapy. Cancer Immunol Res (2014) 2(4):295–300. doi: 10.1158/2326-6066.CIR-14-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Cai J, Liu W, Lin Y, Guo L, Liu X, et al. Intravenous Injection of the Oncolytic Virus M1 Awakens Antitumor T Cells and Overcomes Resistance to Checkpoint Blockade. Cell Death Dis (2020) 11(12):1–13. doi: 10.1038/s41419-020-03285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosewell Shaw A, Porter CE, Watanabe N, Tanoue K, Sikora A, Gottschalk S, et al. Adenovirotherapy Delivering Cytokine and Checkpoint Inhibitor Augments CAR T Cells Against Metastatic Head and Neck Cancer. Mol Ther (2017) 25(11):2440–51. doi: 10.1016/j.ymthe.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang W, Zhang C, Tian W, Qin J, Chen J, Zhang Q, et al. Efficacy of an Oncolytic Adenovirus Driven by a Chimeric Promoter and Armed With Decorin Against Renal Cell Carcinoma. Hum Gene Ther (2020) 31(11-12):651–63. doi: 10.1089/hum.2019.352 [DOI] [PubMed] [Google Scholar]
- 14. Yang Y, Xu H, Huang W, Ding M, Xiao J, Yang D, et al. Targeting Lung Cancer Stem-Like Cells With TRAIL Gene Armed Oncolytic Adenovirus. J Cell Mol Med (2015) 19(5):915–23. doi: 10.1111/jcmm.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tong Y, You L, Liu H, Li L, Meng H, Qian Q, et al. Potent Antitumor Activity of Oncolytic Adenovirus Expressing Beclin-1 via Induction of Autophagic Cell Death in Leukemia. Oncotarget (2013) 4(6):860–74. doi: 10.18632/oncotarget.1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eriksson M, Guse K, Bauerschmitz G, Virkkunen P, Tarkkanen M, Tanner M, et al. Oncolytic Adenoviruses Kill Breast Cancer Initiating CD44+CD24-/Low Cells. Mol Ther (2007) 15(12):2088–93. doi: 10.1038/sj.mt.6300300 [DOI] [PubMed] [Google Scholar]
- 17. Guo W, Zhu H, Zhang L, Davis J, Teraishi F, Roth JA, et al. Combination Effect of Oncolytic Adenovirotherapy and TRAIL Gene Therapy in Syngeneic Murine Breast Cancer Models. Cancer Gene Ther (2006) 13(1):82–90. doi: 10.1038/sj.cgt.7700863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang X, Meng S, Zhang R, Ma B, Liu T, Yang Y, et al. GP73-Regulated Oncolytic Adenoviruses Possess Potent Killing Effect on Human Liver Cancer Stem-Like Cells. Oncotarget (2016) 7(20):29346–58. doi: 10.18632/oncotarget.8830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Wang X, Li X, Xi D, Mao R, Wu X, et al. Potential Contribution of Increased Soluble IL-2R to Lymphopenia in COVID-19 Patients. Cell Mol Immunol (2020) 17(8):878–80. doi: 10.1038/s41423-020-0484-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies JA, Marlow G, Uusi-Kerttula HK, Seaton G, Piggott L, Badder LM, et al. Efficient Intravenous Tumor Targeting Using the αvβ6 Integrin-Selective Precision Virotherapy Ad5(Null)-A20. Viruses (2021) 13(5):864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, et al. Oncolytic Adenovirus Coding for Granulocyte Macrophage Colony-Stimulating Factor Induces Antitumoral Immunity in Cancer Patients. Cancer Res (2010) 70(11):4297–309. doi: 10.1158/0008-5472.CAN-09-3567 [DOI] [PubMed] [Google Scholar]
- 22. Bramante S, Koski A, Liikanen I, Vassilev L, Oksanen M, Siurala M, et al. Oncolytic Virotherapy for Treatment of Breast Cancer, Including Triple-Negative Breast Cancer. Oncoimmunology (2016) 5(2):e1078057. doi: 10.1080/2162402X.2015.1078057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gürlevik E, Woller N, Strüver N, Schache P, Kloos A, Manns MP, et al. Selectivity of Oncolytic Viral Replication Prevents Antiviral Immune Response and Toxicity, But Does Not Improve Antitumoral Immunity. Mol Ther (2010) 18(11):1972–82. doi: 10.1038/mt.2010.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bauerschmitz GJ, Ranki T, Kangasniemi L, Ribacka C, Eriksson M, Porten M, et al. Tissue-Specific Promoters Active in CD44+CD24-/Low Breast Cancer Cells. Cancer Res (2008) 68(14):5533–9. doi: 10.1158/0008-5472.CAN-07-5288 [DOI] [PubMed] [Google Scholar]
- 25. González M, van de Ven R, Haan H, Sluijs J, Dong W, Beusechem V, et al. Oncolytic Adenovirus ORCA-010 Increases the Type-1 T Cell Stimulatory Capacity of Melanoma-Conditioned Dendritic Cells. Clin Exp Immunol (2020) 201:145–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yano S, Tazawa H, Hashimoto Y, Shirakawa Y, Kuroda S, Nishizaki M, et al. A Genetically Engineered Oncolytic Adenovirus Decoys and Lethally Traps Quiescent Cancer Stem-Like Cells in s/G2/M Phases. Clin Cancer Res (2013) 19(23):6495–505. doi: 10.1158/1078-0432.CCR-13-0742 [DOI] [PubMed] [Google Scholar]
- 27. Chen CY, Wang PY, Hutzen B, Sprague L, Swain HM, Love JK, et al. Cooperation of Oncolytic Herpes Virotherapy and PD-1 Blockade in Murine Rhabdomyosarcoma Models. Sci Rep (2017) 7(1):2396. doi: 10.1038/s41598-017-02503-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghouse SM, Nguyen H-M, Bommareddy PK, Guz-Montgomery K, Saha D. Oncolytic Herpes Simplex Virus Encoding IL12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front Oncol (2020) 10:384. doi: 10.3389/fonc.2020.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang W, Hu X, Liang J, Zhu Y, Zeng B, Feng L, et al. Ohsv2 can Target Murine Colon Carcinoma by Altering the Immune Status of the Tumor Microenvironment and Inducing Antitumor Immunity. Mol Ther Oncolytics (2020) 16:158–71. doi: 10.1016/j.omto.2019.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benencia F, Courreges M, Fraser N, Coukos G. Herpes Virus Oncolytic Therapy Reverses Tumor Immune Dysfunction and Facilitates Tumor Antigen Presentation. Cancer Biol Ther (2008) 7:1194–205. doi: 10.4161/cbt.7.8.6216 [DOI] [PubMed] [Google Scholar]
- 31. Li H, Dutuor A, Tao L, Fu X, Zhang X. Virotherapy With a Type 2 Herpes Simplex Virus–Derived Oncolytic Virus Induces Potent Antitumor Immunity Against Neuroblastoma. Clin Cancer Res (2007) 13(1):316–22. doi: 10.1158/1078-0432.CCR-06-1625 [DOI] [PubMed] [Google Scholar]
- 32. Bommareddy PK, Zloza A, Rabkin SD, Kaufman HL. Oncolytic Virus Immunotherapy Induces Immunogenic Cell Death and Overcomes STING Deficiency in Melanoma. OncoImmunology (2019) 8(7):e1591875. doi: 10.1080/2162402X.2019.1591875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sobol PT, Boudreau JE, Stephenson K, Wan Y, Lichty BD, Mossman KL. Adaptive Antiviral Immunity is a Determinant of the Therapeutic Success of Oncolytic Virotherapy. Mol Ther (2011) 19(2):335–44. doi: 10.1038/mt.2010.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Warner SG, Haddad D, Au J, Carson JS, O'Leary MP, Lewis C, et al. Oncolytic Herpes Simplex Virus Kills Stem-Like Tumor-Initiating Colon Cancer Cells. Mol Ther Oncolytics (2016) 3:16013. doi: 10.1038/mto.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wakimoto H, Kesari S, Farrell CJ, Curry WT, Jr., Zaupa C, Aghi M, et al. Human Glioblastoma-Derived Cancer Stem Cells: Establishment of Invasive Glioma Models and Treatment With Oncolytic Herpes Simplex Virus Vectors. Cancer Res (2009) 69(8):3472–81. doi: 10.1158/0008-5472.CAN-08-3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, et al. Phase I/II Study of Oncolytic HSVGM-CSF in Combination With Radiotherapy and Cisplatin in Untreated Stage III/IV Squamous Cell Cancer of the Head and Neck. Clin Cancer Res (2010) 16(15):4005–15. doi: 10.1158/1078-0432.CCR-10-0196 [DOI] [PubMed] [Google Scholar]
- 37. Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, et al. A Phase I Study of Oncovexgm-CSF, a Second-Generation Oncolytic Herpes Simplex Virus Expressing Granulocyte Macrophage Colony-Stimulating Factor. Clin Cancer Res (2006) 12(22):6737–47. doi: 10.1158/1078-0432.CCR-06-0759 [DOI] [PubMed] [Google Scholar]
- 38. Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II Clinical Trial of a Granulocyte-Macrophage Colony-Stimulating Factor-Encoding, Second-Generation Oncolytic Herpesvirus in Patients With Unresectable Metastatic Melanoma. J Clin Oncol (2009) 27(34):5763–71. doi: 10.1200/JCO.2009.24.3675 [DOI] [PubMed] [Google Scholar]
- 39. Xia M, Luo D, Dong J, Zheng M, Meng G, Wu J, et al. Graphene Oxide Arms Oncolytic Measles Virus for Improved Effectiveness of Cancer Therapy. J Exp Clin Cancer Res (2019) 38(1):408. doi: 10.1186/s13046-019-1410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bach P, Abel T, Hoffmann C, Gal Z, Braun G, Voelker I, et al. Specific Elimination of CD133+ Tumor Cells With Targeted Oncolytic Measles Virus. Cancer Res (2013) 73(2):865–74. doi: 10.1158/0008-5472.CAN-12-2221 [DOI] [PubMed] [Google Scholar]
- 41. Li H, Peng K-W, Dingli D, Kratzke R, Russell SJ. Oncolytic Measles Viruses Encoding Interferon β and the Thyroidal Sodium Iodide Symporter Gene for Mesothelioma Virotherapy. Cancer Gene Ther (2010) 17(8):550–8. doi: 10.1038/cgt.2010.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T Cells as Carriers for Systemic Measles Virotherapy in the Presence of Antiviral Antibodies. Gene Ther (2007) 14(4):324–33. doi: 10.1038/sj.gt.3302880 [DOI] [PubMed] [Google Scholar]
- 43. Lal G, Rajala MS. Combination of Oncolytic Measles Virus Armed With Bnip3, a Pro-Apoptotic Gene and Paclitaxel Induces Breast Cancer Cell Death. Front Oncol (2019) 8:676. doi: 10.3389/fonc.2018.00676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdullah SA, Al-Shammari AM, Lateef SA. Attenuated Measles Vaccine Strain Have Potent Oncolytic Activity Against Iraqi Patient Derived Breast Cancer Cell Line. Saudi J Biol Sci (2020) 27(3):865–72. doi: 10.1016/j.sjbs.2019.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heinzerling L, Künzi V, Oberholzer P, Kündig T, Naim H, Dummer R. Oncolytic Measles Virus in Cutaneous T-Cell Lymphomas Mounts Antitumor Immune Responses In Vivo and Targets Interferon-Resistant Tumor Cells. Blood (2005) 106:2287–94. doi: 10.1182/blood-2004-11-4558 [DOI] [PubMed] [Google Scholar]
- 46. Hu L, Sun S, Wang T, Li Y, Jiang K, Lin G, et al. Oncolytic Newcastle Disease Virus Triggers Cell Death of Lung Cancer Spheroids and Is Enhanced by Pharmacological Inhibition of Autophagy. Am J Cancer Res (2015) 5(12):3612–23. [PMC free article] [PubMed] [Google Scholar]
- 47. Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized Oncolytic Virotherapy Overcomes Systemic Tumor Resistance to Immune Checkpoint Blockade Immunotherapy. Sci Transl Med (2014) 6(226):226ra32. doi: 10.1126/scitranslmed.3008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ye T, Jiang K, Wei L, Barr MP, Xu Q, Zhang G, et al. Oncolytic Newcastle Disease Virus Induces Autophagy-Dependent Immunogenic Cell Death in Lung Cancer Cells. Am J Cancer Res (2018) 8(8):1514. [PMC free article] [PubMed] [Google Scholar]
- 49. García-Romero N, Palacيn-Aliana I, Esteban-Rubio S, Madurga R, Rius-Rocabert S, Carriَn-Navarro J, et al. Newcastle Disease Virus (NDV) Oncolytic Activity in Human Glioma Tumors Is Dependent on CDKN2A-Type I IFN Gene Cluster Codeletion. Cells (2020) 9(6):1405. doi: 10.3390/cells9061405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang G, Kang X, Chen KS, Jehng T, Jones L, Chen J, et al. An Engineered Oncolytic Virus Expressing PD-L1 Inhibitors Activates Tumor Neoantigen-Specific T Cell Responses. Nat Commun (2020) 11(1):1–14. doi: 10.1038/s41467-020-15229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakao S, Arai Y, Tasaki M, Yamashita M, Murakami R, Kawase T, et al. Intratumoral Expression of IL-7 and IL-12 Using an Oncolytic Virus Increases Systemic Sensitivity to Immune Checkpoint Blockade. Sci Trans Med (2020) 12(526):eaax7992. doi: 10.1126/scitranslmed.aax7992 [DOI] [PubMed] [Google Scholar]
- 52. Parato KA, Breitbach CJ, Le Boeuf F, Wang J, Storbeck C, Ilkow C, et al. The Oncolytic Poxvirus JX-594 Selectively Replicates in and Destroys Cancer Cells Driven by Genetic Pathways Commonly Activated in Cancers. Mol Ther (2012) 20(4):749–58. doi: 10.1038/mt.2011.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu Y, Li K, Zhu W-b, Zhang H, Huang W-t, Liu X-c, et al. Suppression of CCDC6 Sensitizes Tumor to Oncolytic Virus M1. Neoplasia (2021) 23(1):158–68. doi: 10.1016/j.neo.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feist M, Zhu Z, Dai E, Ma C, Liu Z, Giehl E, et al. Oncolytic Virus Promotes Tumor-Reactive Infiltrating Lymphocytes for Adoptive Cell Therapy. Cancer Gene Ther (2020) p:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ochiai H, Moore SA, Archer GE, Okamura T, Chewning TA, Marks JR, et al. Treatment of Intracerebral Neoplasia and Neoplastic Meningitis With Regional Delivery of Oncolytic Recombinant Poliovirus. Clin Cancer Res (2004) 10(14):4831–8. doi: 10.1158/1078-0432.CCR-03-0694 [DOI] [PubMed] [Google Scholar]
- 56. Garant K, Shmulevitz M, Pan L, Daigle R, Ahn D, Gujar S, et al. Oncolytic Reovirus Induces Intracellular Redistribution of Ras to Promote Apoptosis and Progeny Virus Release. Oncogene (2016) 35(6):771–82. doi: 10.1038/onc.2015.136 [DOI] [PubMed] [Google Scholar]
- 57. Ma J, Ramachandran M, Jin C, Quijano-Rubio C, Martikainen M, Yu D, et al. Characterization of Virus-Mediated Immunogenic Cancer Cell Death and the Consequences for Oncolytic Virus-Based Immunotherapy of Cancer. Cell Death Dis (2020) 11(1):1–15. doi: 10.1038/s41419-020-2236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Streby KA, Geller JI, Currier MA, Warren PS, Racadio JM, Towbin AJ, et al. Intratumoral Injection of HSV1716, an Oncolytic Herpes Virus, Is Safe and Shows Evidence of Immune Response and Viral Replication in Young Cancer Patients. Clin Cancer Res (2017) 23(14):3566–74. doi: 10.1158/1078-0432.CCR-16-2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, et al. Recurrent Glioblastoma Treated With Recombinant Poliovirus. N Engl J Med (2018) 379: (2):150–61. doi: 10.1056/NEJMoa1716435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garcia-Carbonero R, Salazar R, Duran I, Osman-Garcia I, Paz-Ares L, Bozada JM, et al. Phase 1 Study of Intravenous Administration of the Chimeric Adenovirus Enadenotucirev in Patients Undergoing Primary Tumor Resection. J Immunother Cancer (2017) 5(1):71. doi: 10.1186/s40425-017-0277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Husseini F, Delord JP, Fournel-Federico C, Guitton J, Erbs P, Homerin M, et al. Vectorized Gene Therapy of Liver Tumors: Proof-of-Concept of TG4023 (MVA-FCU1) in Combination With Flucytosine. Ann Oncol (2017) 28(1):169–74. doi: 10.1093/annonc/mdw440 [DOI] [PubMed] [Google Scholar]
- 62. Dispenzieri A, Tong C, LaPlant B, Lacy MQ, Laumann K, Dingli D, et al. Phase I Trial of Systemic Administration of Edmonston Strain of Measles Virus Genetically Engineered to Express the Sodium Iodide Symporter in Patients With Recurrent or Refractory Multiple Myeloma. Leukemia (2017) 31(12):2791–8. doi: 10.1038/leu.2017.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cohn DE, Sill MW, Walker JL, O'Malley D, Nagel CI, Rutledge TL, et al. Randomized Phase IIB Evaluation of Weekly Paclitaxel Versus Weekly Paclitaxel With Oncolytic Reovirus (Reolysin®) in Recurrent Ovarian, Tubal, or Peritoneal Cancer: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol Oncol (2017) 146(3):477–83. doi: 10.1016/j.ygyno.2017.07.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mahalingam D, Fountzilas C, Moseley J, Noronha N, Tran H, Chakrabarty R, et al. A Phase II Study of REOLYSIN(®) (Pelareorep) in Combination With Carboplatin and Paclitaxel for Patients With Advanced Malignant Melanoma. Cancer Chemother Pharmacol (2017) 79(4):697–703. doi: 10.1007/s00280-017-3260-6 [DOI] [PubMed] [Google Scholar]
- 65. Packiam VT, Lamm DL, Barocas DA, Trainer A, Fand B, Davis RL, et al. An Open Label, Single-Arm, Phase II Multicenter Study of the Safety and Efficacy of CG0070 Oncolytic Vector Regimen in Patients With BCG-Unresponsive non-Muscle-Invasive Bladder Cancer: Interim Results. Urol Oncol (2018) 36(10):440–7. doi: 10.1016/j.urolonc.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 66. Geletneky K, Hajda J, Angelova AL, Leuchs B, Capper D, Bartsch AJ, et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/Iia Glioblastoma Trial. Mol Ther (2017) 25(12):2620–34. doi: 10.1016/j.ymthe.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol (2015) 33(25):2780–8. doi: 10.1200/JCO.2014.58.3377 [DOI] [PubMed] [Google Scholar]
- 68. Chesney J, Awasthi S, Curti B, Hutchins L, Linette G, Triozzi P, et al. Phase Iiib Safety Results From an Expanded-Access Protocol of Talimogene Laherparepvec for Patients With Unresected, Stage IIIB-IVM1c Melanoma. Melanoma Res (2018) 28(1):44–51. doi: 10.1097/CMR.0000000000000399 [DOI] [PubMed] [Google Scholar]
- 69. Boasso A. Type I Interferon at the Interface of Antiviral Immunity and Immune Regulation: The Curious Case of HIV-1. Scientifica (2013) 2013:580968. doi: 10.1155/2013/580968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Apelbaum A, Yarden G, Warszawski S, Harari D, Schreiber G. Type I Interferons Induce Apoptosis by Balancing Cflip and Caspase-8 Independent of Death Ligands. Mol Cell Biol (2013) 33(4):800–14. doi: 10.1128/MCB.01430-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting Tumor-Specific Defects in the Interferon Pathway With a Previously Unknown Oncolytic Virus. Nat Med (2000) 6(7):821–5. doi: 10.1038/77558 [DOI] [PubMed] [Google Scholar]
- 72. Everts B, van der Poel HG. Replication-Selective Oncolytic Viruses in the Treatment of Cancer. Cancer Gene Ther (2005) 12(2):141–61. doi: 10.1038/sj.cgt.7700771 [DOI] [PubMed] [Google Scholar]
- 73. Evgin L, Vنhن-Koskela M, Rintoul J, Falls T, Le Boeuf F, Barrett JW, et al. Potent Oncolytic Activity of Raccoonpox Virus in the Absence of Natural Pathogenicity. Mol Ther (2010) 18(5):896–902. doi: 10.1038/mt.2010.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cho D-Y, Lin S-Z, Yang W-K, Lee H-C, Hsu D-M, Lin H-L, et al. Targeting Cancer Stem Cells for Treatment of Glioblastoma Multiforme. Cell Transplant (2013) 22(4):731–9. doi: 10.3727/096368912X655136 [DOI] [PubMed] [Google Scholar]
- 75. Balachandran S, Porosnicu M, Barber GN. Oncolytic Activity of Vesicular Stomatitis Virus is Effective Against Tumors Exhibiting Aberrant P53, Ras, or Myc Function and Involves the Induction of Apoptosis. J Virol (2001) 75(7):3474–9. doi: 10.1128/JVI.75.7.3474-3479.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Christian SL, Zu D, Licursi M, Komatsu Y, Pongnopparat T, Codner DA, et al. Suppression of IFN-Induced Transcription Underlies IFN Defects Generated by Activated Ras/MEK in Human Cancer Cells. PloS One (2012) 7(9):e44267. doi: 10.1371/journal.pone.0044267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Martin T, Watkins G, Jiang WG. The Coxsackie-Adenovirus Receptor has Elevated Expression in Human Breast Cancer. Clin Exp Med (2005) 5(3):122–8. doi: 10.1007/s10238-005-0076-1 [DOI] [PubMed] [Google Scholar]
- 78. Sanjuán X, Fernández Pl, Miquel R, Muñoz J, Castronovo V, Ménard S. Overexpression of the 67-Kd Laminin Receptor Correlates With Tumour Progression in Human Colorectal Carcinoma. J Pathol (1996) 179(4):376–80. doi: [DOI] [PubMed] [Google Scholar]
- 79. Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, et al. Overexpression of the CD155 Gene in Human Colorectal Carcinoma. Gut (2001) 49(2):236–40. doi: 10.1136/gut.49.2.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anderson BD, Nakamura T, Russell SJ, Peng K-W. High CD46 Receptor Density Determines Preferential Killing of Tumor Cells by Oncolytic Measles Virus. Cancer Res (2004) 64(14):4919–26. doi: 10.1158/0008-5472.CAN-04-0884 [DOI] [PubMed] [Google Scholar]
- 81. Kim J-S, Lee S-H, Cho Y-S, Choi J-J, Kim YH, Lee J-H. Enhancement of the Adenoviral Sensitivity of Human Ovarian Cancer Cells by Transient Expression of Coxsackievirus and Adenovirus Receptor (CAR). Gynecol Oncol (2002) 85(2):260–5. doi: 10.1006/gyno.2002.6607 [DOI] [PubMed] [Google Scholar]
- 82. Tseng J-C, Levin B, Hirano T, Yee H, Pampeno C, Meruelo D. In Vivo Antitumor Activity of Sindbis Viral Vectors. J Natl Cancer Inst (2002) 94(23):1790–802. doi: 10.1093/jnci/94.23.1790 [DOI] [PubMed] [Google Scholar]
- 83. Ohka S, Matsuda N, Tohyama K, Oda T, Morikawa M, Kuge S, et al. Receptor (CD155)-Dependent Endocytosis of Poliovirus and Retrograde Axonal Transport of the Endosome. J Virol (2004) 78(13):7186–98. doi: 10.1128/JVI.78.13.7186-7198.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dörig RE, Marcil A, Chopra A, Richardson CD, et al. The Human CD46 Molecule is a Receptor for Measles Virus (Edmonston Strain). Cell (1993) 75(2):295–305. doi: 10.1016/0092-8674(93)80071-L [DOI] [PubMed] [Google Scholar]
- 85. Hughes J, Wang P, Alusi G, Shi H, Chu Y, Wang J, et al. Lister Strain Vaccinia Virus With Thymidine Kinase Gene Deletion is a Tractable Platform for Development of a New Generation of Oncolytic Virus. Gene Ther (2015) 22(6):476–84. doi: 10.1038/gt.2015.13 [DOI] [PubMed] [Google Scholar]
- 86. Bommareddy PK, Shettigar M, Kaufman HL. Integrating Oncolytic Viruses in Combination Cancer Immunotherapy. Nat Rev Immunol (2018) 18(8):498. doi: 10.1038/s41577-018-0014-6 [DOI] [PubMed] [Google Scholar]
- 87. Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going Viral With Cancer Immunotherapy. Nat Rev Cancer (2014) 14(8):559–67. doi: 10.1038/nrc3770 [DOI] [PubMed] [Google Scholar]
- 88. Versteeg GA, García-Sastre A. Viral Tricks to Grid-Lock the Type I Interferon System. Curr Opin Microbiol (2010) 13(4):508–16. doi: 10.1016/j.mib.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang S, Huang W, Zhou X, Zhao Q, Wang Q, Jia B. Seroprevalence of Neutralizing Antibodies to Human Adenoviruses Type-5 and Type-26 and Chimpanzee Adenovirus Type-68 in Healthy Chinese Adults. J Med Virol (2013) 85(6):1077–84. doi: 10.1002/jmv.23546 [DOI] [PubMed] [Google Scholar]
- 90. Nwanegbo E, Vardas E, Gao W, Whittle H, Sun H, Rowe D, et al. Prevalence of Neutralizing Antibodies to Adenoviral Serotypes 5 and 35 in the Adult Populations of the Gambia, South Africa, and the United States. Clin Diagn Lab Immunol (2004) 11(2):351–7. doi: 10.1128/CDLI.11.2.351-357.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Harada JN, Berk AJ. P53-Independent and-Dependent Requirements for E1B-55K in Adenovirus Type 5 Replication. J Virol (1999) 73(7):5333–44. doi: 10.1128/JVI.73.7.5333-5344.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Goodrum FD, Ornelles DA. P53 Status Does Not Determine Outcome of E1B 55-Kilodalton Mutant Adenovirus Lytic Infection. J Virol (1998) 72(12):9479–90. doi: 10.1128/JVI.72.12.9479-9490.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ries S, Korn W. ONYX-015: Mechanisms of Action and Clinical Potential of a Replication-Selective Adenovirus. Br J Cancer (2002) 86(1):5–11. doi: 10.1038/sj.bjc.6600006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Goodrum FD, Ornelles DA. The Early Region 1B 55-Kilodalton Oncoprotein of Adenovirus Relieves Growth Restrictions Imposed on Viral Replication by the Cell Cycle. J Virol (1997) 71(1):548–61. doi: 10.1128/jvi.71.1.548-561.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Schietinger A, Philip M, Liu RB, Schreiber K, Schreiber H. Bystander Killing of Cancer Requires the Cooperation of CD4+ and CD8+ T Cells During the Effector Phase. J Exp Med (2010) 207(11):2469–77. doi: 10.1084/jem.20092450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA, et al. Targeted Inflammation During Oncolytic Virus Therapy Severely Compromises Tumor Blood Flow. Mol Ther (2007) 15(9):1686–93. doi: 10.1038/sj.mt.6300215 [DOI] [PubMed] [Google Scholar]
- 97. Breitbach CJ, Arulanandam R, De Silva N, Thorne SH, Patt R, Daneshmand M, et al. Oncolytic Vaccinia Virus Disrupts Tumor-Associated Vasculature in Humans. Cancer Res (2013) 73(4):1265–75. doi: 10.1158/0008-5472.CAN-12-2687 [DOI] [PubMed] [Google Scholar]
- 98. Sarkar D, Su Zz, Park ES, Vozhilla N, Dent P, Curiel DT, et al. A Cancer Terminator Virus Eradicates Both Primary and Distant Human Melanomas. Cancer Gene Ther (2008) 15(5):293–302. doi: 10.1038/cgt.2008.14 [DOI] [PubMed] [Google Scholar]
- 99. Qian W, Liu J, Tong Y, Yan S, Yang C, Yang M, et al. Enhanced Antitumor Activity by a Selective Conditionally Replicating Adenovirus Combining With MDA-7/Interleukin-24 for B-Lymphoblastic Leukemia via Induction of Apoptosis. Leukemia (2008) 22(2):361–9. doi: 10.1038/sj.leu.2405034 [DOI] [PubMed] [Google Scholar]
- 100. Azab BM, Dash R, Das SK, Bhutia SK, Sarkar S, Shen XN, et al. Enhanced Prostate Cancer Gene Transfer and Therapy Using a Novel Serotype Chimera Cancer Terminator Virus (Ad.5/3-CTV). J Cell Physiol (2014) 229(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bhoopathi P, Lee N, Pradhan AK, Shen XN, Das SK, Sarkar D, et al. Mda-7/IL-24 Induces Cell Death in Neuroblastoma Through a Novel Mechanism Involving AIF and ATM. Cancer Res (2016) 76(12):3572–82. doi: 10.1158/0008-5472.CAN-15-2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bhoopathi P, Lee N, Pradhan AK, Shen XN, Das SK, Sarkar D, et al. Theranostic Tripartite Cancer Terminator Virus for Cancer Therapy and Imaging. Cancers (Basel) (2021) 13: (4):857. doi: 10.3390/cancers13040857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Greco A, Di Benedetto A, Howard CM, Kelly S, Nande R, Dementieva Y, et al. Eradication of Therapy-Resistant Human Prostate Tumors Using an Ultrasound-Guided Site-Specific Cancer Terminator Virus Delivery Approach. Mol Therapy: J Am Soc Gene Ther (2010) 18(2):295–306. doi: 10.1038/mt.2009.252 [DOI] [PMC free article] [PubMed] [Google Scholar]