Abstract
Increased levels of fetal hemoglobin (HbF) lessen the severity of symptoms and increase the life span of patients with sickle cell disease (SCD). More effective strategies to increase HbF are needed because the current standard of care, hydroxyurea, is not effective in a significant proportion of patients. Treatment of the millions of patients projected worldwide would best be accomplished with an orally administered drug therapy that increased HbF. LSD1 is a component of corepressor complexes that repress γ-globin gene expression and are a therapeutic target for HbF reactivation. We have shown that subcutaneous administration of RN-1, a pharmacological LSD1 inhibitor, increased γ-globin expression in SCD mice and baboons, which are widely acknowledged as the best animal model in which to test the activity of HbF-inducing drugs. The objective of this investigation was to test the effect of oral administration of a new LSD1 inhibitor, ORY-3001. Oral administration of ORY-3001 to SCD mice (n = 3 groups) increased γ-globin expression, Fetal Hemoglobin (HbF)-containing (F) cells, and F reticulocytes (retics). In normal baboons (n = 7 experiments) treated with ORY-3001, increased F retics, γ-globin chain synthesis, and γ-globin mRNA were observed. Experiments in anemic baboons (n = 2) showed that ORY-3001 increased F retics (PA8695, predose = 24%, postdose = 66.8%; PA8698: predose = 13%, postdose = 93.6%), γ-globin chain synthesis (PA8695: predose = 0.07 γ/γ+β, postdose = 0.20 γ/γ+β; PA8698: predose = 0.02 γ/γ+β, postdose = 0.44 γ/γ+β), and γ-globin mRNA (PA8695: predose = 0.06 γ/γ+β, postdose = 0.18 γ/γ+β; PA8698: predose = 0.03 γ/γ+β, postdose = 0.33 γ/γ+β). We conclude that oral administration of ORY-3001 increases F retics, γ-globin chain synthesis, and γ-globin mRNA in baboons and SCD mice, supporting further efforts toward the development of this drug for SCD therapy.
The β-hemoglobinopathies, consisting of sickle cell disease (SCD) and the β-thalassemias, are the most common genetic diseases worldwide. These syndromes cause wide-spread suffering and significant reduction in lifespan in patients. The β-thalassemia syndromes comprise a spectrum of disorders characterized by deficiencies in the production of the adult β-globin chain [1]. Increased expression of the fetal γ-globin chains and fetal hemoglobin [(HbF(α2γ2)] can augment reduced β-globin chain deficiencies and is therefore considered a desirable therapeutic target that can ameliorate the symptoms of this disease [2,3]. SCD is caused by a point mutation in the β-globin gene that triggers polymerization of deoxygenated sickle hemoglobin (HbS). SCD patients suffer from chronic hemolytic anemia, painful crises, multisystem organ damage, and reduced life expectancy [4], with millions of patients projected worldwide in future years [5]. Hematopoietic stem cell transplantation can be curative, but the majority of patients do not have a suitable donor. Although advanced gene-editing technologies also offer the possibility of a cure, the likelihood that these strategies can be mobilized to treat the large numbers of patients residing in third-world countries is remote [6]. A pharmacological treatment to increase HbF as a therapy for SCD has been a long sought goal because increased levels of HbF inhibit the polymerization of HbS and are associated with reduced symptoms and an increased lifespan of SCD patients [7]. Hydroxyurea, the first drug approved for the treatment of SCD, does not increase HbF to effective levels in a large percentage of patients and we have therefore pursued the development of an orally available, easily administered drug regimen to increase HbF for therapy of both SCD and β-thalassemia.
Extensive efforts to elucidate the mechanism responsible for repression of γ-globin expression during adult erythropoiesis have identified three trans-acting factors, the orphan nuclear receptors TR2/TR4, BCL11A, and LRF (ZBTB7A), which are capable of binding to sequences within the γ-globin gene promoter and/or other regulatory regions within the β-globin gene complex to repress γ-globin expression. These factors recruit multiprotein corepressor complexes containing enzymes such as DNMT1, LSD1, and HDACs that catalyze repressive epigenetic modifications of DNA and histones to silence γ-globin gene expression [8–10]. Pharmacological inhibition of these enzymes can increase HbF expression in vivo. Our experiments showing that DNMT inhibitors were potent activators of γ-globin expression pioneered the use of pharmacological inhibitors of enzymes that catalyze repressive epigenetic modifications to increase HbF [11]. A recently completed clinical trial demonstrated that a noncytotoxic, orally administered combinatorial regimen consisting of the DNMT inhibitor decitabine and the cytidine deaminase inhibitor tetrahydrouridine increased HbF in patients with SCD [12]. We have shown that subcutaneous administration of the LSD1 inhibitor RN-1 increased HbF in SCD mice, albeit to low levels [13,14]. However, the regulation of human globin gene expression is not faithfully reproduced in transgenic mouse models. Because of the conservation of structure and developmental regulation of the β-like globin gene locus among all simian primates, nonhuman primate animal models such as the baboon are widely regarded as the best in vivo models in which to test the activity of HbF-inducing agents [7]. In baboons rendered anemic by repeated phlebotomy, RN-1 administered subcutaneously increased HbF to high levels, whereas prolonged RN-1 treatment (>265 days) of normal baboons increased Fetal Hemoglobin (HbF) containing (F) cells and HbF in the absence of significant hematological toxicity [15,16]. Drugs inhibiting DNMT1 and LSD1 are currently among the most promising agents for in vivo HbF induction therapies in SCD patients. The objective of this investigation was to test the effect of oral administration of a new LSD1 inhibitor, ORY-3001, which has increased activity and specificity compared with RN-1 (Supplementary Table 1, online only, available at www.exphem.org), on HbF expression in SCD transgenic mice and baboons.
Methods
SCD mice
SCD mice purchased from The Jackson Laboratory (B6;129-Hbatm1(HBA)Tow Hbbtm2(HBG1,HBB*)Tow/J) and bred at the University of Illinois–Chicago (UIC) were housed under standard conditions according to institutional guidelines. All experiments were approved by the UIC Animal Care Committee. Blood samples were collected by retro-orbital bleeding of isoflurane anesthetized mice in vacutainer EDTA tubes (BD Biosciences, San Jose, CA). Complete blood count was analyzed with complete blood count with differential (CBC-Diff).
Baboons
Female baboons (Papio anubis; 10–15 kg body weight) were maintained at UIC according to institutional guidelines. Animals were housed under standard conditions. All experiments were approved by the UIC Animal Care Committee. Peripheral blood for hematological analyses was collected from the femoral vein of anesthetized animals in EDTA-containing tubes (approximately 10 mL per sample). CBC-Diff was performed by the Biologic Resources Laboratory (BRL) staff.
For detailed descriptions of drug preparation and administration, RNA purification, and analysis of globin mRNA, F cells, F reticulocytes (F retics), and globin chain synthesis, see the supplementary data (online only, available at www.exphem.org).
Results and discussion
Our primary goal in these studies was to determine whether ORY-3001 increased HbF when administered orally in vivo to SCD mice and baboons because effective treatment of millions of SCD patients projected worldwide demands an easily administered oral agent. The real potential of a drug for SCD therapy requires an evaluation of its HbF-inducing activity in vivo in animal models. Although studies in cell line models such as HuDEP-2 and primary BM cultures can provide valuable data that encourage further in vivo experiments, the ability of LSD1 inhibitors to increase γ-globin expression in cultured primary erythroid cell cultures was previously demonstrated [13,17], so we chose to pursue in vivo studies of the effects of oral administration of ORY-3001, a more advanced drug.
The effect of ORY-3001 on HbF was initially tested in SCD-transgenic mice. Three groups of mice (three mice per group) were treated with varying doses of ORY-3001 (0.25, 0.5, 1.0, and mg/kg/d) for 4 days. An identical volume of vehicle (H2O) was administered to a fourth group. On the fifth day, levels of F retics and F cells were measured by flow cytometry and levels of γ-globin mRNA were determined by real-time polymerase chain reaction. CBC analysis was also performed on each mouse. Results showed that oral administration of ORY-3001 resulted in dose-dependent increases in F retics, F cells, and γ-globin mRNA (Table 1). Reticulocytes were decreased at the two higher doses of drug (40%, 0.5 mg dose, p < 0.01; 52%, 1.0 mg dose, p < 0.02), whereas platelets were decreased 71% in the group treated with 1.0 mg/kg ORY-3001. No significant effects on ANC were observed.
Table 1.
Effect of OG-S1335 on F retics, F cells, and γ-globin mRNA in SCD mice
| Treatment | Dose (mg/kg/d) | F retics (%) | F cells (%) | γ-globin mRNA (γ/γ+β) |
|---|---|---|---|---|
| Vehicle | 0 | 3.57 ± 0.57 | 3.53 ± 0.55 | 0.003 ± 0.000 |
| ORY-3001 | 0.25 | 8.77 ± 1.20 | 6.40 ± 1.45 | 0.010 ± 0.001 |
| ORY-3001 | 0.5 | 10.17 ± 2.23 | 7.78 ± 1.85 | 0.0173 ± 0.005 |
| ORY-3001 | 1.0 | 11.23 ± 2.91 | 9.30 ± 0.46 | 0.029 ± 0.007 |
To test the effect of ORY-3001 in baboons, dose escalation was initially performed by administering the drug by gavage to a single animal in cycles of 3 days, followed by 1–2 weeks of observation to measure effects on blood parameters. Increased levels of F retics were initially observed at a dose of 100 μg/kg/d and, subsequently, four additional baboons were treated with ORY-3001 (by oral gavage) at a dose of either 100 or 200 μg/kg/d (seven separate experiments in total). The data presented are in the mean ± standard deviation (SD) format, with “postdose” indicating the time point when the highest variation from baseline levels was reported. Increased F retics (predose = 8.4 ± 8.9%, postdose = 43.0 ± 27.9%; p < 0.01), γ-globin chain synthesis (predose = 0.03 ± 0.03 γ/γ+β, postdose = 0.28 ± 0.18 γ/γ+β; p < 0.01), and γ-globin mRNA (predose = 0.02 ± 0.02 γ/γ+β, postdose = 0.20 ± 0.11 γ/γ+β; Figure 1A) were observed with maximal levels achieved 7 days after administration of the first dose (day 8). A decrease in the absolute number of reticulocytes (predose = 72.8 ± 31.8 × 104/μL, postdose = 35.4 ± 17.8 × 104/μL; p < 0.05; nadir on days 6–8), neutrophils (predose = 4603 ± 911/μL, postdose = 2332 ± 1429/μL; p < 0.01; nadir on days 13–17), and platelets (predose = 406 ± 86 × 103/μL, postdose = 168 ± 98 × 103/μL; p < 0.01; nadir on day 8) was also observed, together with increased monocytes (predose = 200 ± 68/μL, postdose = 541 ± 213/μL; p < 0.01; peak on days 10–15; Figure 1B).
Figure 1.
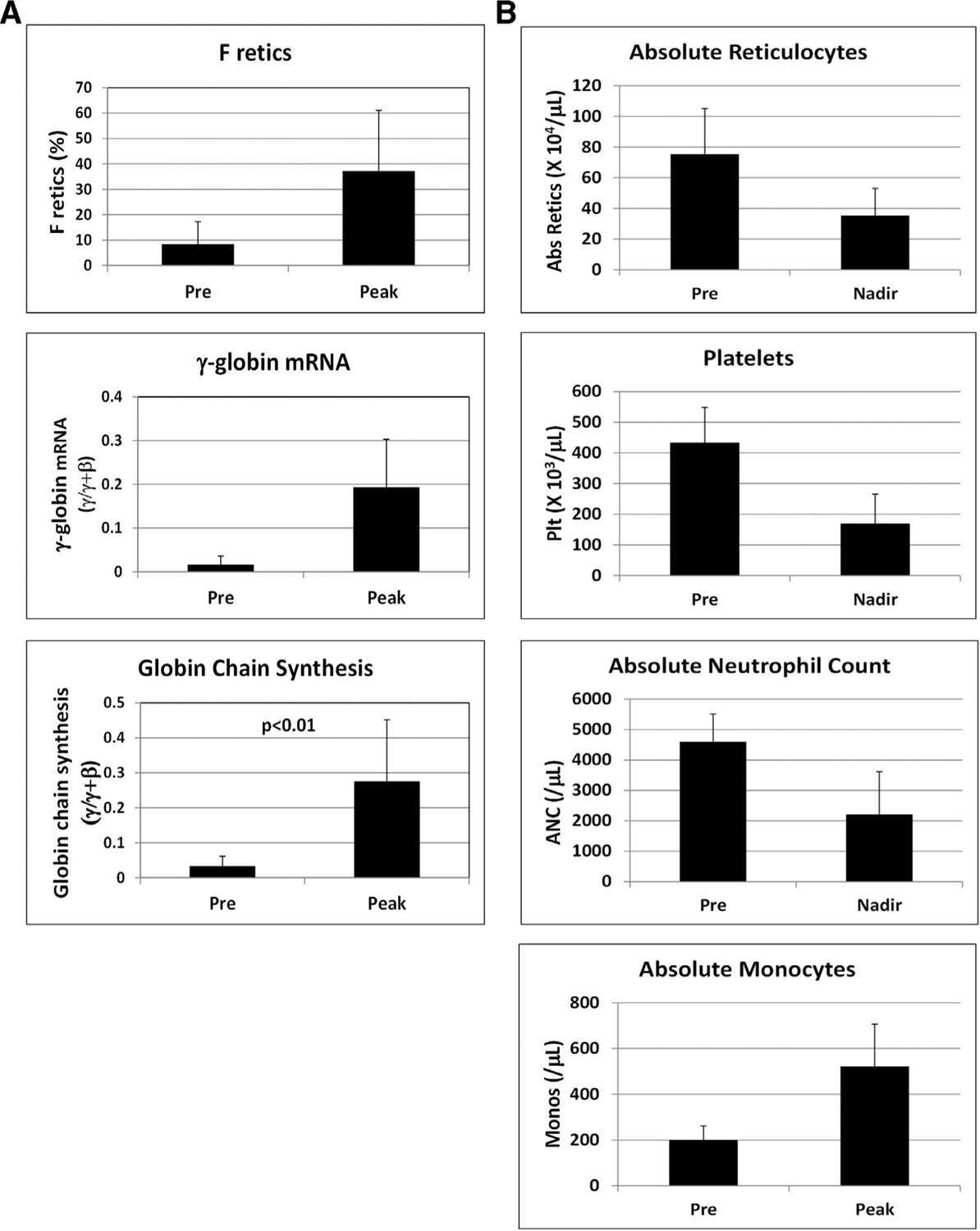
(A) Effect of oral administration of ORY-3001 on F retics, γ-globin mRNA, and globin chain synthesis in the peripheral blood. Results from the study of four baboons treated for three days with 100 μg/kg/d and three baboons treated with 200 μg/kg/d ORY-3001 were combined. Total Hb levels were 12.1 ± 1.0 g/dL predose and 12.0 ± 0.9 g/dL postdose for animals treated with 100 μg/kg/d and 11.6 ± 0.4 g/dL predose and 11.8 ± 0.5 g/dL postdose for animals treated with same 200 μg/kg/d. (B) Effect of oral administration of ORY-3001 in these baboons on absolute reticulocytes, platelets, absolute neutrophil count, and absolute monocyte count as determined by CBC analysis.
Additional experiments were performed in two phlebotomized anemic baboons to test the effect of the drug in a model with increased relevance to SCD. ORY-3001 (100 μg/kg/d for 3 days) induced high levels of F retics (PA8695, predose = 24%, postdose = 66.8%; PA8698: predose = 13%, postdose = 93.6%), γ-globin chain synthesis (PA8695: predose = 0.07 γ/γ+β, postdose = 0.20 γ/γ+β; PA8698: predose = 0.02 γ/γ+β, postdose = 0.44 γ/γ+β), and γ-globin mRNA (PA8695: predose = 0.04 γ/γ+β, postdose = 0.18 γ/γ+β; PA8698: predose = 0.03 γ/γ+β, postdose = 0.33 γ/γ+β) in each animal (Figure 2). Future experiments are planned to address the effect of ORY-3001 on global gene expression and genome-wide histone modifications in bone marrow cells that are outside the scope of this current study. We conclude that oral administration of the LSD1 inhibitor ORY-3001 significantly increased F retics, γ-globin chain synthesis, and γ-globin mRNA in SCD mice and in baboons, supporting further efforts toward the further development of this drug for SCD therapy.
Figure 2.
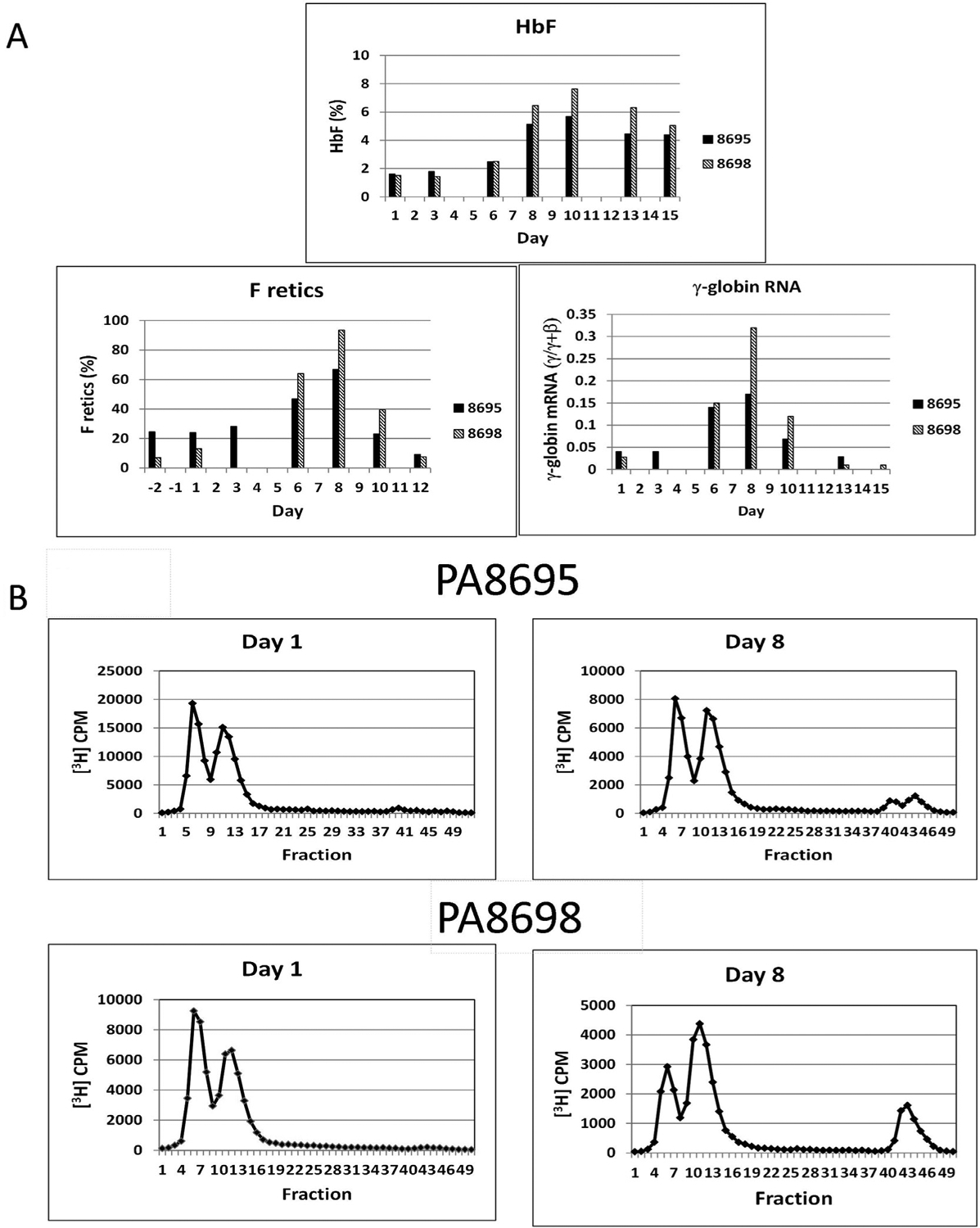
(A) Effect of oral administration of ORY-3001 on HbF, F retics, and γ-globin mRNA in two anemic baboons, PA8695 and PA8698. The drug was administered for three consecutive days on days 1–3 at a dose of 100 μg/kg/d. Total hemoglobin levels were: PA8698: 6.1 g/dL predose, 6.3 g/dL postdose; PA8695: 7.0 g/dL predose, 6.7 g/dL postdose. (B) HPLC analysis of globin chain synthesis in 8695 and 8698 predose and 8 days after administration of the first dose of drug. Absolute HbF levels were: PA 8698: 0.9 g/dL predose; 4.8 g/dL postdose; PA8695 1.1 g/dL predose, 3.8 g/dL postdose. Levels of α-globin chain synthesis were: PA8698: 1.01 α/γ+β predose, 1.23 α/γ+β postdose; PA8695: 1.09 α/γ+β predose, 1.09 α/γ+β postdose.
Supplementary Material
Acknowledgements
This work was supported by a grant from the National Institutes of Health (U01 HL117658).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.exphem.2018.08.003.
References
- 1.Taher AT, Weatherall DJ, Cappellini MD. Thalassemia. Lancet. 8;391:155–67. [DOI] [PubMed] [Google Scholar]
- 2.Thein SL. Molecular basis of β thalassemia and potential therapeutic targets. Blood Cells Mol Dis. 2018;70:54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui S, Engel JD. Reactivation of fetal hemoglobin for treating β-thalassemia and sickle cell disease. Adv Exp Biol Med. 2017;1013:177–202. [DOI] [PubMed] [Google Scholar]
- 4.Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med. 2013;3:a011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10:e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer DE, Brendel C, Fitzhugh CD. Curative approached for sickle cell disease: A review of allogeneic and autologous strategies. Blood Cells Mol Dis. 2017;67:155–168. [DOI] [PubMed] [Google Scholar]
- 7.Schechter AN. Hemoglobin research and the origins of molecular biology. Blood. 2008;112:3927–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki M, Yamamoto M, Engel JD. Fetal globin gene repressors as drug targets for molecular therapies to treat the β-globinopathies. Mol Cell Biol. 2014;34:3560–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee WS, McColl B, Maksimovic J, Vadolas J. Epigenetic interplay at the β-globin locus. Biochim Biophys Acta. 2017;1860:393–400. [DOI] [PubMed] [Google Scholar]
- 10.Vinjamur DS, Bauer DE, Orkin SH. Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. Br J Haematol. 2018;180:630–643. [DOI] [PubMed] [Google Scholar]
- 11.DeSimone J, Heller PH, Hall L, Zwiers D. 5-azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci USA. 1982;79:4428–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molokie R, Lavelle D, Gowhari M, et al. Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: a randomized phase 1 study. PLoS Med. 2017;14:e1002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivers A, Vaitkus K, Ruiz MA, et al. RN-1, a potent and selective lysine-specific demethylase 1 inhibitor, increases γ-globin expression, F reticulocytes, and F cells in a sickle cell disease mouse model. Exp Hematol. 2015;43:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui S, Lim KC, Shi L, et al. The LSD1 inhibitor RN-1 induces fetal hemoglobin synthesis and reduces disease pathology in sickle cell mice. Blood. 2015;126:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivers A, Vaitkus K, Ibanez V, et al. The LSD1 inhibitor RN-1 recapitulates the fetal pattern of hemoglobin synthesis in baboons (P. anubis). Haematologica. 2016;101:688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibanez V, Vaitkus K, Rivers A, et al. Efficacy and safety of long-term RN-1 treatment to increase HbF in baboons. Blood. 2017;129:260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi L, Cui S, Engel JD, Tanabe O. Lysine-specific demethylase 1 is a therapeutic target for fetal hemoglobin induction. Nat Med. 2013;19:291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


