Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a rare life-threatening clinical condition that can develop in patients younger than 21 years of age with a history of infection/exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The cardiovascular system is a main target of the inflammatory process that frequently causes myocardial dysfunction, myopericarditis, coronary artery dilation, hypotension, and shock. Multisystem inflammatory syndrome in children-associated myocarditis is usually characterized by fever, tachycardia, non-specific electrocardiogram abnormalities, and left ventricular dysfunction, but serious tachyarrhythmias may also occur. We report two cases of patients with MIS-C-associated myocarditis who developed severe bradycardia.
Case summary
Two female adolescents with recent history of coronavirus disease 2019 (COVID-19) were initially hospitalized for long-lasting high-grade fever and severe gastrointestinal symptoms. Both patients were diagnosed with MIS-C-associated myocarditis for elevation of markers of myocardial injury (mean highly-sensitive cardiac troponin 2663 pg/mL, mean N-terminal-pro-brain natriuretic peptide 5097 pg/mL) and left ventricular dysfunction, which was subsequently confirmed by cardiac magnetic resonance. Both patients developed a severe sinus bradycardia (lowest heart rate 36 and 42, respectively), which appeared refractory to the treatment with intravenous Methylprednisolone and Immunoglobulins, despite a clinical and biochemical improvement. The use of Anakinra (a recombinant interleukin-1 receptor antagonist), was associated with a rapid improvement of cardiac rhythm and excellent clinical outcome at 6 months of follow-up.
Discussion
In patients with MIS-C-associated myocarditis, a continuous cardiac monitoring is mandatory to promptly identify potential conduction abnormalities. Adolescents may present bradycardia as a rhythm complication. We experienced a rapid recovery after treatment with Anakinra, to be considered as add-on therapy in cases refractory to standard anti-inflammatory treatment.
Keywords: Bradycardia, Myocarditis, COVID-19, SARS-CoV-2, MIS-C, Children, Case series
Learning points
Sinus bradycardia may be an early and likely immune-mediated complication of multisystem inflammatory syndrome in children-associated myocarditis.
A continuous cardiac monitoring is mandatory to identify conduction abnormalities in those patients.
Early administration of Anakinra may reduce the progression of myocardial damage and the risk of cardiac conduction disorders.
Introduction
Multisystem inflammatory syndrome in children (MIS-C) is a rare but potentially fatal clinical condition associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or exposure.1
Regardless of the various definitions, the main clinical manifestations include long-lasting fever with increased inflammatory markers associated with at least two of the following: rash, bilateral non-purulent conjunctivitis, mucocutaneous inflammation in the oral cavity or extremities, coagulopathy, signs or symptoms of organ damage in cardiovascular, respiratory, gastrointestinal, renal, and neurological systems.2
The diagnosis of MIS-C also requires the exclusion of other plausible alternative diagnoses (infectious, rheumatologic, and immunologic diseases) and a temporal correlation with infection/exposure to SARS-CoV-2.3,4
Multisystem inflammatory syndrome in children mainly occurs about 2–6 weeks after SARS-COV-2 exposure, and a significant proportion of patients requires intensive care, with an estimated mortality rate that ranges between 1.7% and 5%.1,5
Up to 80% of children with MIS-C show cardiovascular involvement,6 although the pathophysiological mechanisms and risk factors for severe courses are still unclear.2 In addition, pathological data and advanced imaging (cardiac magnetic resonance imaging—MRI) findings are scarce in children.7
According to a recent systematic review, 75.3% of patients with MIS-C developed myocarditis,8 with mild-to-severe increase of myocardial injury markers (highly-sensitive cardiac troponin—hs-cTn, brain natriuretic peptide—BNP, N-terminal-pro-BNP, and muscle brain creatine kinase—CK-MB).
Children with MIS-C-associated myocarditis usually present with fever, chest pain, sinus tachycardia, non-specific T-wave abnormalities, left ventricular ejection fraction impairment, and cardiogenic shock.9,10 Similarly to other viral and non-viral myocarditis, a minority of patients may develop electrical conduction disorders like life-threatening arrhythmias, variable degrees of atrioventricular block, ventricular fibrillation/flutter, or ventricular tachycardia.11,12
We describe two cases of myocarditis in MIS-C complicated by unusual and severe bradycardia.
Timeline
| Time | Events |
|---|---|
| Patient 1 | |
| Days 0 | Onset of fever, asthenia, abdominal pain, and diarrhoea. |
| Day 5 | Hospital admission. Finding of significant increase in acute phase proteins and myocardial injury. Ecocardiography revealed a global reduction in left ventricular ejection fraction (EF 36%) and mild ventricular dysfunction with tricuspid insufficiency. Diagnosis of multisystem inflammatory syndrome in children (MIS-C). Starting treatment with Methylprednisolone, IVIG, Enoxaparine, and Enalapril. |
| Day 8 | Onset of bradycardia. 24-h Holter monitoring showed mean heart rate (HR) 51 with sinus rhythm. |
| Day 12 | Starting treatment with Anakinra. |
| Day 14 | Improvement of HR and systolic function. |
| Day 17 | Cardiac magnetic resonance imaging (MRI) showed sub-acute phase myocarditis, with sub-epicardial involvement of left ventricle. EF 63%. |
| Day 28 | 24-h Holter monitoring confirmed the normalization of HR. |
| Patient 2 | |
| Day 0 | Onset of asthenia, laterocervical lymphadenomegaly, fever, and diarrhoea. |
| Day 3 | Hospital admission. Laboratory tests showed significant increase in acute phase proteins and myocardial injury markers. Diagnosis of MIS-C. |
| Day 4 | Starting treatment with Methylprednisolone, IVIG, Enoxaparine, and Enalapril. |
| Day 6 | Ecocardiography revealed thin flap of pericardial effusion (4 mm) with normal cardiac morphology and contractility (EF 62%). |
| Day 8 | Onset of bradycardia. 24-h Holter monitoring showed mean HR 55 with sinus rhythm. |
| Day 13 | Starting treatment with anakinra. |
| Day 15 | Improvement of HR and systolic function. |
| Day 27 | Cardiac MRI showed outcomes of myocarditis with sub-epicardial involvement of the mid-ventricular area. EF 59%. |
Case presentation
Patient 1
A previously healthy non-obese Caucasian 12-year-old girl was admitted to our department for a 5-day fever unresponsive to antibiotic therapy, asthenia, severe abdominal pain, and acute diarrhoea characterized by about 10 loose stools in 24 h.
At hospital admission, the girl was in fair clinical conditions, with the following clinical parameters; heart rate (HR) 90 b.p.m., respiratory rate (RR) 20 b.p.m., peripheral oxygen saturation (SpO2) 99%, and blood pressure (BP) 110/70 mmHg.
Physical examination showed mild dehydration with capillary refill time of about 3 s, moderate–severe abdominal pain in the epigastrium (Numerical Rate Scale 8/10), regular cardio-respiratory activity, and no signs of respiratory distress.
Significant increase in acute phase proteins (ferritin, C-reactive protein, procalcitonin, fibrinogen, and D-dimer) and markers of myocardial injury (hs-cTn, BNP, NT-pro-BNP, and CK-MB) with neutrophilia, lymphopenia, and thrombocytopenia were detected (Table 1).
Table 1.
General characteristics, clinical features, and laboratory findings of two paediatric patients with MIS-C
| General characteristics | Patient 1 | Patient 2 |
|---|---|---|
| Gender/age (years) | Female/12 | Female/12 |
| SARS-CoV-2 testing | ||
| SARS-CoV-2 IgG | Positive | Positive |
| Real-time PCR on nasopharyngeal swab | Negative | Positive |
| Clinical features | ||
| Duration of fever (days) | 5 | 3 |
| Cough | No | No |
| Interstitial pneumonia | No | No |
| Diarrhoea | Yes | Yes |
| Abdominal pain | Yes | Yes |
| Mucosal/skin lesions | Yes | No |
| Serositis | Yes | Yes |
| Neurocognitive symptoms | No | No |
| Cervical lymphadenopathy >1.5 cm diameter | No | Yes |
| Laboratory findings | ||
| C-reactive protein, mg/L (maximum) | 278 | 71.5 |
| Ferritin, ng/mL (maximum) | 858 | 912 |
| D-dimer, mg/L (maximum) | 4.29 | 2.88 |
| White blood cells (highest levels) | 8470 | 10230 |
| Neutrophils (highest levels) | 7290 | 6820 |
| Lymphocytes (lowest levels) | 590 | 1180 |
| Thrombocytes (lowest levels) | 111 000 | 202 000 |
| Haemoglobin, g/dL (lowest levels) | 9.4 | 12 |
| Cardiac involvement | ||
| hs-cTn, pg/mL (highest levels) | 1494 | 3833 |
| CK-MB, ng/mL (highest levels) | 4.7 | 0.6 |
| NT-proBNP, pg/mL (highest levels) | 8184 | 2011 |
| BNP, ng/mL (highest levels) | 470 | 230 |
| 24-h Holter monitoring | ||
| Lowest HR (b.p.m.) | 36 | 42 |
| Mean HR (b.p.m.) | 50 | 55 |
| Highest HR (b.p.m.) | 104 | 94 |
| Prolonged QTc | Yes | No |
| Nonspecific T-wave abnormalities | No | Yes |
| Tricuspidal insufficiency | Yes | No |
| Ejection fraction (lowest rate %) | 36 | 59 |
| Mitral regurgitation | No | No |
| Coronary dilation | No | No |
| Pericardial effusion | Yes | Yes |
CK-MB, muscle brain creatine kinase; HR, heart rate; Hs-cTn, highly-sensitive cardiac troponin; NT-pro-BNP, N-terminal-pro-brain natriuretic peptide.
Transthoracic echocardiography showed a global reduction in left ventricular ejection fraction [ejection fraction (EF) 36%] with normal cavity size and parietal thickness. Mild right ventricular dysfunction and tricuspid insufficiency with normal estimated pulmonary systolic pressure were also detected. A minimal pericardial detachment was present.
Electrocardiography (ECG) showed normal sinus rhythm. QTc interval, calculated according to Bazett’s formula, was prolonged (547 ms).13
Ultrasonographic examination of the abdomen showed mesenteric thickening and a fluid collection in subumbilical region with inhomogeneous content (max diameter 35 mm). Urgent surgical conditions were ruled out.
No evidence of infiltrative parenchymal lesions was observed at Chest X-ray.
An extensive work up was performed to exclude other aetiologies potentially responsible for the clinical status, including blood cultures, specific test for EBV-DNA, CMV-DNA, Mycoplasma pneumoniae and Chlamydia pneumoniae, and serology for coeliac disease. All of these were found to be normal or negative. Stool were negative for enteric pathogens (including SARS-CoV-2).
The patient had history of exposure to coronavirus disease 2019 (COVID-19) cases approximately 1 month before, so a nasopharyngeal swab for SARS-CoV-2 RNA and specific serology were performed. Positive IgG antibodies against SARS-CoV-2 in the absence of RNA on nasopharyngeal swab were detected. Both tests were positive in the mother, who took care of the daughter during hospitalization.
The presence of persistent fever, elevation in laboratory markers of inflammation, signs and symptoms of organ dysfunction, lack of alternative diagnoses, and infection/exposure to SARS-CoV-2,5 defined the diagnosis of MIS-C complicated by myocarditis.
Immediately after diagnosis, Methylprednisolone as bolus of 20 mg/kg body weight was started followed by progressive oral tapering, intravenous immunoglobulins (IVIG; 2 g/kg), and Enoxaparine (80 UI/kg/day). Enalapril (0.2 mg/kg/day) was also introduced to reduce cardiac workload and treat the left ventricular dysfunction and remodelling.
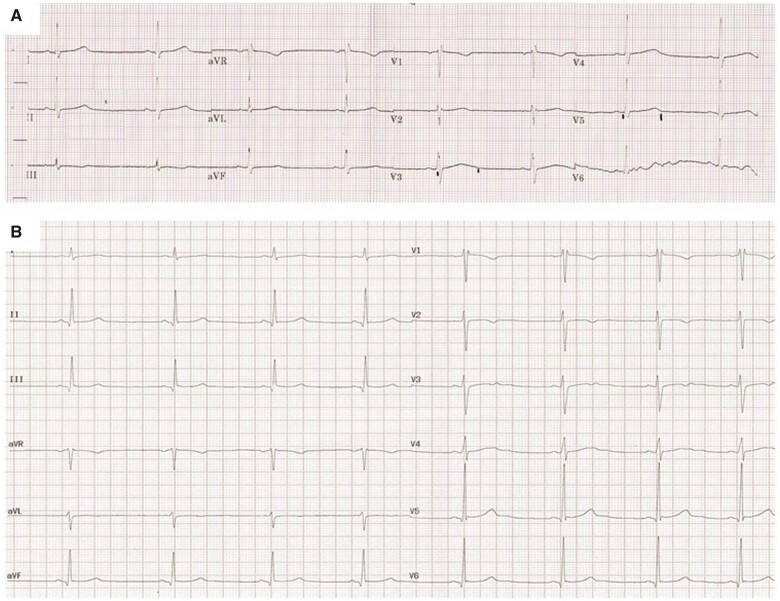
During the following days, general clinical conditions and laboratory tests progressively improved. QTc normalized 5 days after the beginning of treatment, however, a further ECG and the continuous cardiac monitoring revealed the presence of asymptomatic bradycardia, with HR below 45 b.p.m. (Figure 1A).
Figure 1.
Patient 1 (A) and 2 (B) 12-lead electrocardiograms performed when bradycardia was first detected.
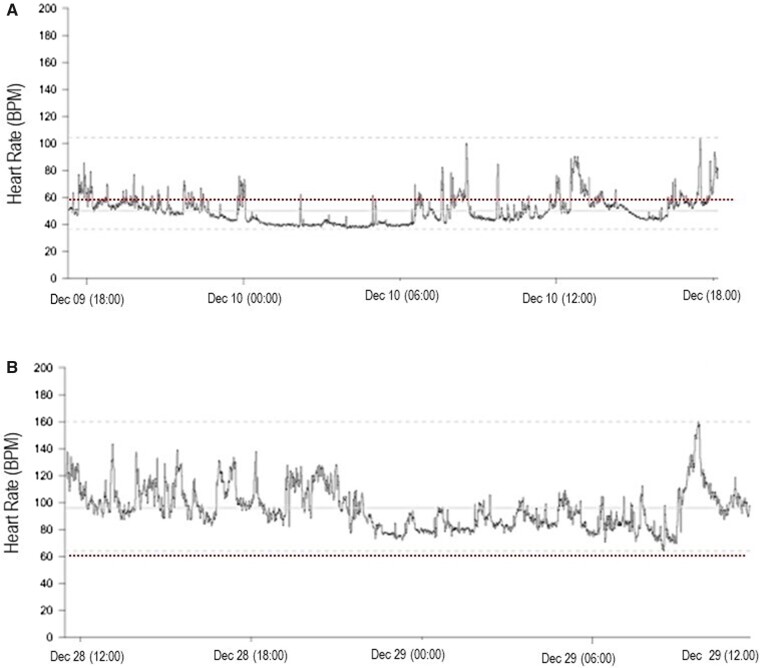
For this reason, a 24-h Holter monitoring was obtained, which showed a sinus rhythm with bradycardia throughout the day (minimum HR 36 b.p.m.; mean HR 50 b.p.m.; maximum HR 104 b.p.m.) (Figure 2A). Pauses lasting longer than 2 s were not detected. Atrioventricular and intraventricular conduction was regular and QTc interval was confirmed within normal limits.
Figure 2.
24-h Holter monitoring of Patient 1 obtained during bradycardia (A) and after recovery (B).
Thyroid function was normal.
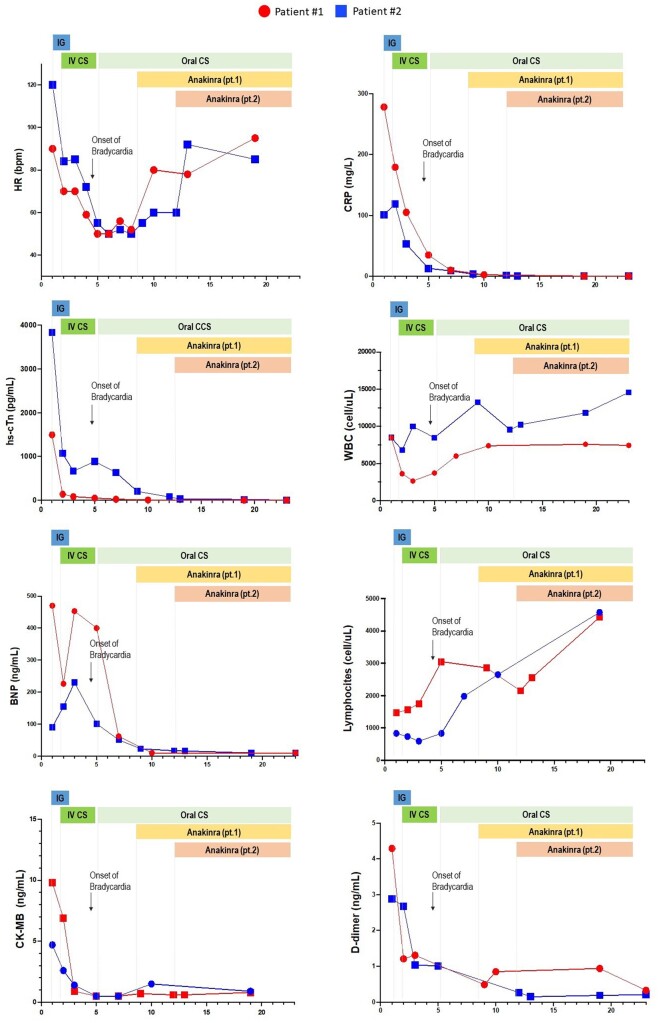
The persistence of severe bradycardia for more than 72 h despite the treatment with steroids and IVIG was considered as a clinical parameter of potential treatment failure. In that scenario, the use of Anakinra, a recombinant interleukin (IL)-1 receptor antagonist, was considered as second-line therapy. The administration of Anakinra (dose of 4 mg/kg/day), was related with a subsequent rapid improvement of HR, and a further reduction of the markers of inflammation, until complete normalization (Figure 3).
Figure 3.
Time course and response to therapies of heart rate, markers of myocardial damage, and C-reactive protein in two paediatric patients with multisystem inflammatory syndrome in children.
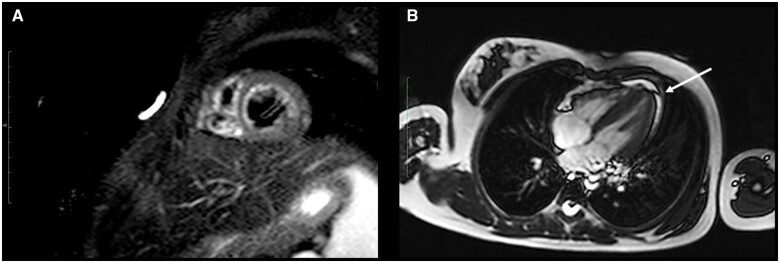
After 12 days, following the stabilization of general clinical conditions and vital parameters, cardiac MRI was also performed. Sub-acute phase myocarditis, with sub-epicardial involvement in lateral and postero-lateral walls of left ventricle was detected, in the absence of areas of myocardial fibrosis/necrosis. Left ventricle showed normal size and parietal thickness with preserved global systolic function at rest (EF 63%). No alteration in right chambers and valves was found. A minimal pericardial effusion was observed (Figure 4A and B).
Figure 4.
Cardiac magnetic resonance imaging scans of Patient 1. (A) Short inversion time inversion-recovery sequences showed myocardial signal hyperintensity of the left ventricle, suggesting interstitial oedema. Absence of late gadolinium enhancement suggestive of focal myocardial necrosis/fibrosis. (B) Thin flap of pericardial effusion along the inferior wall of the left ventricle (arrow).
Before discharge, a new 24-h Holter ECG confirmed the normalization of HR (minimum HR 60 b.p.m.; mean HR 96 b.p.m.; maximum HR 160 b.p.m.) (Figure 2B).
Cine MRI sequences of Patient 1 were captured after 2 weeks from symptoms onset (Videos 1–2).
At a 9-month follow-up, the girl showed a good clinical outcome with optimal cardiac function and normal HR values for age.
Patient 2
A previously healthy non-obese Caucasian 12-year-old girl with SARS-CoV-2 infection was admitted to our department for suspected MIS-C: the patient had asthenia, pharyngodynia, 3-day antibiotic-resistant fever, diarrhoea, and laterocervical lymphadenomegaly that presented approximately 4 weeks after the diagnosis of COVID-19.
On admission, she was in good general conditions, with HR 120 b.p.m., SatO2 98%, RR 22 b.p.m., BP 110/65 mmHg.
Physical examination showed moderate abdominal pain in the epigastrium (NRS 5/10), mild dehydration with capillary refill time of about 3 s, regular cardio-respiratory activity without signs of respiratory distress.
Laboratory tests showed significant increase in acute phase proteins and myocardial injury markers (Table 1).
A thin flap of pericardial effusion (4 mm) in the absence of alterations in cardiac morphology and contractility (EF 62%) was observed at transthoracic echocardiography.
Electrocardiography showed normal sinus rhythm with non-specific atypia in the ventricular recovery phase in Patient 2. QTc interval, calculated according to Bazett’s formula, was borderline (453 ms).13
Abdominal ultrasonography showed a fluid collection in the pelvic cavity with inhomogeneous content (50 × 40 × 30 mm) associated with mesenteric thickening. Chest X-ray was found to be normal. The patient showed a positive serology and nasopharyngeal real-time PCR for SARS-CoV-2 (cycle threshold 39).
Other main aetiologies potentially responsible for the clinical status were ruled out.
Based on clinical and biochemical parameters, a diagnosis of MIS-C complicated by myocarditis was made and initial treatment with Methylprendisolone, IVIG, Enoxaparine, and Enalapril was promptly started.
As previously described, despite a progressive improvement in general clinical conditions and laboratory tests, asymptomatic bradycardia was detected during cardiac monitoring (HR below 45 b.p.m.) (Figure 1B), this finding was confirmed by a 24-h Holter monitoring (minimum HR 42 b.p.m.; mean HR 55 b.p.m.; maximum HR 94 b.p.m.). Pauses lasting longer than 2 s were not observed. Atrioventricular and intraventricular conduction was regular and QTc interval was within normal limits. Thyroid function was normal.
In consideration of the successful response to the IL-1 receptor antagonist observed in patient 1, Anakinra (4 mg/kg/day) was promptly started, with a subsequent rapid and persistent improvement of HR, and progressive normalization of inflammatory markers (Figure 3).
A cardiac MRI performed 15 days after showed outcomes of myocarditis with sub-epicardial involvement in lateral and postero-lateral walls of the mid-ventricular area, in the absence of myocardial fibrosis or necrosis. Left ventricle showed normal size and parietal thickness with preserved global systolic function at rest (EF 59%).
At a 9-month follow-up, the patient maintained optimal cardiac function and normal HR values for age.
Discussion
Multisystem inflammatory syndrome in children is a severe and potentially fatal clinical condition temporarily associated with SARS-CoV-2 infection in children. Its incidence significantly varies among countries, with few cases reported in Southeast Asia and higher incidence in European, Northern and Southern American countries. A recent cohort study in USA estimated an incidence rate of 3/10 000 SARS-CoV-2 infected patients younger than 21 years of age.14
Risk factors for developing MIS-C are currently unknown; however, the presence of underlying comorbidities (excluding obesity) does not apparently expose children to higher risk or worst outcome.
Although usually affect otherwise healthy children and adolescents, MIS-C may be lethal in 1.7% of patients and 71% of affected subjects require intensive care support.5
Together with gastrointestinal tract (abdominal pain, diarrhoea, and serositis), the cardiovascular system is the main target organ of the immune-mediated inflammatory process accounting for more than 80% of MIS-C patients.
Multisystem inflammatory syndrome in children has overlapping features with Kawasaki Disease (KD); however, there are several epidemiological and clinical differences. Multisystem inflammatory syndrome in children predominantly affects children older than 5 years without a clear sex bias, results in a more common and severe gastrointestinal involvement, and a more frequent cardiovascular impairment resulting in higher rates of myocardial dysfunction and shock. A similar rate of coronary artery dilations and aneurysms (4–20%) has been reported, although persistent aneurysms seem to be less frequent in MIS-C patients if compared to Kawasaki.15
As reported in adult COVID-19 patients, who more frequently present previous cardiovascular diseases, cardiac involvement is related with a severe prognosis and increased mortality rate.16 The pathogenic mechanisms supporting the cardiovascular injury in children and adolescents with MIS-C, as well as factors affecting the clinical presentation and outcomes are far from being clear.
Therefore, it is critical to ensure an extensive cardiological, biochemical, and imaging work-up for all patients with MIS-C.
The main cardiovascular disorders diagnosed in this population are myocarditis, pericarditis, mitral regurgitation, arrhythmias, coronary artery aneurysms, hypotension, and shock.1
Myocardial injury results in a broad spectrum of clinical manifestations, ranging from mild non-specific symptoms to chest pain, lethargy, tachycardia, or even cardiogenic shock.17 Heart rate often seems to be disproportionately elevated compared with the overall appearance of the child and sinus tachycardia represents the most frequent presenting sign of myocarditis. Available evidence indicates that low-specific ECG changes such as repolarization abnormalities and alterations in the electrical conduction system can be frequently found in myocarditis, but life-threatening arrhythmias and conduction abnormalities, including variable degrees of atrioventricular block, ventricular fibrillation/flutter, or ventricular tachycardia can occur.11,18 Echocardiography shows ventricular systolic dysfunction, pericardial effusion, impaired diastolic release, and impaired biventricular strain in most cases.9
Only in rare cases cardiac magnetic resonance (MRI) has been performed in COVID-19-related myocarditis. An atypical pattern with evidence of myocardial signal hyperintensity in T2-short inversion time inversion-recovery sequences, suggesting interstitial oedema, with no evidence of late gadolinium enhancement suggestive of replacement fibrosis or focal necrosis has been identified.7
Limited histological data from myocardial biopsies is available in adults. The presence of SARS-CoV-2 in myocardiocytes was not detected in all patients, suggesting that myocardial damage is not necessarily virus-induced but most likely immune-mediated.19
According to Lake-Louis criteria, the diagnosis of myocarditis can be made in a patient presenting with suggestive symptoms and signs, specific laboratory alterations, and coherent cardiac MRI findings.17
In both our patients, despite the absence of prominent clinical signs or symptoms, the diagnosis of myocarditis was based on the elevation of markers of myocardial injury in association to echocardiographic findings. Bradycardia was a late and unexpected sign detected during clinical visits and continuous cardiac monitoring. Sinus bradycardia alone is an extremely rare manifestation of cardiac involvement in patients with MIS-C. Its occurrence is reported in a very small number of case series and is quite poorly described.20 The exact pathogenetic mechanism underlying the development of conduction abnormalities is currently unknown.12 We could infer that the damage of electrical conduction system cells could be secondary to a viral propagation within the myocardial cells,8 although being MIS-C an immune-mediated response to SARS-CoV-2, bradycardia might be more likely sustained by an inflammatory mechanism, even after complete clearance of viral infection.
The finding of a sinus bradycardia as main and single rhythm disorder, in absence of conduction abnormalities (i.e. atrioventricular blocks), suggests extrinsic causes as a possible trigger of sinus node dysfunction, rather than a primary cardiac disease. Conduction disorders, more frequently than rhythm disturbances, have been reported in several autoimmune rheumatic diseases, and usually occur during clinical flares and regress in disease remission.
In patients with systemic lupus erythematosus (SLE), sinus node dysfunction is related to a small vessel vasculitis and infiltration by granulation tissue, and newborns to SLE mothers may develop rhythm and conduction disorders, up to complete heart block, due to the transplacental passage of anti-Ro/SSA and anti-La/SSB.21
It is plausible that a similar immune-mediated mechanism may determine conduction disturbances in children with MIS-C, who demonstrated a secondary autoreactive humoral response. In that scenario, Gruber et al.22 demonstrated IgG and IgA autoantibodies repertoire against endothelial, mucosal and immune antigens that can trigger potent inflammation and tissue damage. Particularly, the presence of anti-La/SSB and anti-Jo-1 in children with MIS-C, suggests that those patients may share similar pathophysiology with classic autoimmune disorders, including SLE or idiopathic inflammatory myopathies.
In addition, there is evidence that patients with SLE and positive anti-Ro/SSA antibodies have an increased likelihood of prolonged QTc, similarly to what we observed in the early inflammatory phase of Patient 1.
We also considered the opportunity that bradycardia may have an iatrogenic aetiology in patients receiving high doses of IVIG and steroids.
Bradycardia is known to be an extremely rare adverse event of IVIG infusion. As reported in a single case report of an adult patient, the reduction in HR occurred shortly after the start of the infusion and resolved spontaneously in less than 48 h.23 In children with Kawasaki, the combination of IGEV with intravenous steroids increases the risk of developing bradycardia compared with IGEV alone.24
In contrast with this evidence, in both our patients, the bradycardia occurred approximately 72 h after initiation of IVIG and persisted for 7 or more days. No other drugs potentially associated with bradycardia or vagal stimuli were administered. For this reason, we attributed the absence of spontaneous improvement in HR to the myocardial damage sustained by inflammation and interpreter this finding as a potential failure to first-line treatment.
Based on expert consensus and evidence coming from large case series, the use of IL-1 receptor antagonist has been proposed for patients refractory to first-line treatment.8,25
Furthermore, the biochemical-radiological evidence of myocarditis and the rapid normalization of HR after administration of Anakinra might further support this hypothesis.
The up-regulation of the IL-1b pathway, together with the presence of endothelial damage, plays a central role in the pathogenesis of postviral myocarditis.
Patient 1 came to our observation with a longer lasting fever and with higher inflammatory markers upon admission, and showed a more extensive myocardial damage and a more pronounced bradycardia. This may support the hypothesis that inflammation plays an important role in cardiac involvement in MIS-C and that an early diagnosis is crucial to avoid a sustained damage.
Of note, among all the patients who received a diagnosis of MIS-C at our department, both patients who had severe myocardial involvement showed bradycardia, although treatment was administered at the same timing and doses. This finding would suggest that bradycardia may represent an event more frequent than expected in children developing MIS-C-associated myocarditis. Since MIS-C is a relatively new condition and its long-term cardiac sequelae are still poorly understood, ongoing cardiologic follow-up is warranted.
At a 9-month follow-up, both patients showed a good clinical outcome with optimal cardiac function and normal HR values for age.
The clinical course of our patients with myocarditis suggests the need of a continuous cardiac monitoring as an essential diagnostic tool for a prompt detection of rhythm anomalies. In addition, since the use of Anakinra was associated with a concomitant improvement of bradycardia, clinical trials could be proposed to test the efficacy of Anakinra in patients with myocarditis and MIS-C, in order to reduce the progression of myocardial damage and the risk of cardiac conduction disorders.
Lead author biography

Gian Paolo Ciccarelli is a pediatrician working at the Pediatric Infctious Diseaese Unit of the University of Naples Federico II (Italy), with a specific interest in paediatric cardiology and cardiac involvement of infectious diseases. He is currently part of the team at the Regional Reference Center for the management of COVID-19 in children.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patients’ parents in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J. et al. ; California MIS-C Response Team. COVID-19-associated multisystem inflammatory syndrome in children—United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020;69:1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K. et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020;20:e276–e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Scientific Brief. 2020. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (16 April 2021).
- 4. Centers for Disease Control and Prevention. Reporting Multisystem Inflammatory Syndrome in Children (MIS-C). 2020. https://www.cdc.gov/mis-c/hcp/index.html (16 April 2021).
- 5. Ahmed M, Advani S, Moreira A, Zoretic S, Martinez J, Chorath K. et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine 2020;26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF. et al. ; CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C. et al. Cardiac MRI in children with multisystem inflammatory syndrome associated with COVID-19. Radiology 2020;297:E283–E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McMurray JC, May JW, Cunningham MW, Jones OY.. Multisystem inflammatory syndrome in children (MIS-C), a post-viral myocarditis and systemic vasculitis—a critical review of its pathogenesis and treatment. Front Pediatr 2020;8:626182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S. et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020;142:429–436. [DOI] [PubMed] [Google Scholar]
- 10. Rogo T, Mathur K, Purswani M.. Systemic inflammation with cardiac involvement in pediatric patients with evidence of COVID-19 in a Community Hospital in the Bronx, New York. J Pediatric Infect Dis Soc 2020;9:502–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tunuguntla H, Jeewa A, Denfield SW.. Acute myocarditis and pericarditis in children. Pediatr Rev 2019;40:14–25. [DOI] [PubMed] [Google Scholar]
- 12. Choi NH, Fremed M, Starc T, Weller R, Cheung E, Ferris A. et al. MIS-C and cardiac conduction abnormalities. Pediatrics 2020;146:e2020009738. [DOI] [PubMed] [Google Scholar]
- 13. Rijnbeek PR, Witsenburg M, Schrama E, Hess J, Kors JA.. New normal limits for the paediatric electrocardiogram. Eur Heart J 2001;22:702–711. [DOI] [PubMed] [Google Scholar]
- 14. Payne AB, Gilani Z, Godfred-Cato S, Belay ED, Feldstein LR, Patel MM. et al. ; MIS-C Incidence Authorship Group. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open 2021;4:e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sancho-Shimizu V, Brodin P, Cobat A, Biggs CM, Toubiana J, Lucas CL. et al. SARS-CoV-2-related MIS-C: a key to the viral and genetic causes of Kawasaki disease? J Exp Med 2021;218:e20210446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A. et al. COVID-19 and cardiovascular disease. Circulation 2020;141:1648–1655. [DOI] [PubMed] [Google Scholar]
- 17. Dasgupta S, Iannucci G, Mao C, Clabby M, Oster ME.. Myocarditis in the pediatric population: a review. Congenit Heart Dis 2019;14:868–877. [DOI] [PubMed] [Google Scholar]
- 18. Kariyanna PT, Sutarjono B, Grewal E, Singh KP, Aurora L, Smith L. et al. A systematic review of COVID-19 and myocarditis. Am J Med Case Rep 2020;8:299–305.32747875 [Google Scholar]
- 19. Peretto G, Sala S, Caforio ALP.. Acute myocardial injury, MINOCA, or myocarditis? Improving characterization of coronavirus-associated myocardial involvement. Eur Heart J 2020;41:2124–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS. et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 2020;324:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plastiras SC, Moutsopoulos HM.. Arrhythmias and conduction disturbances in autoimmune rheumatic disorders. Arrhythm Electrophysiol Rev 2021;10:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F. et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C). Cell 2020;183:982–995.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raheja H, Kumar V, Hollander G, Shani J, Greenberg Y.. Intravenous immunoglobulin-induced profound bradycardia in a patient with idiopathic thrombocytopenic purpura. Am J Ther 2018;25:e572–e574. [DOI] [PubMed] [Google Scholar]
- 24. Akikusa JD, Feldman BM, Gross GJ, Silverman ED, Schneider R.. Sinus bradycardia after intravenous pulse methylprednisolone. Pediatrics 2007;119:e778-82–e782. [DOI] [PubMed] [Google Scholar]
- 25. Kaushik A, Gupta S, Sood M, Sharma S, Verma S.. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J 2020;39:e340–e346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.