Abstract
Amyotrophic lateral sclerosis is a devastating neurodegenerative disease characterized by progressive loss of upper and lower motor neurons. Diagnosis, management and therapeutic trials are hampered by a lack of informative biomarkers. Troponins are components of skeletal and cardiac muscles. Acute elevation of cardiac isoforms of troponin I and T in serum indicates myocardial injury. Case reports suggested that serum levels of cardiac troponin T, but not cardiac troponin I are chronically elevated in myotrophic lateral sclerosis and other neuromuscular disorders. Using standard clinical laboratory methodologies, we studied serum troponin levels in a multicentric cross-sectional cohort of 75 amyotrophic lateral sclerosis patients and 30 Alzheimer’s disease controls and 29 healthy controls (DESCRIBE-ALS cohort) and in a real-world cohort of 179 consecutive patients from our amyotrophic lateral sclerosis clinic at the University Hospital Bonn. We found that serum cardiac troponin T is elevated in >60% of amyotrophic lateral sclerosis patients, while cardiac troponin I is always normal. Serum cardiac troponin T levels increase over time and correlate with disease severity as measured with the revised Amyotrophic Lateral Sclerosis Functional Rating Scale score. There was no correlation with the phosphorylated neurofilament heavy chain levels in the cerebrospinal fluid. We propose that cardiac troponin T elevations in amyotrophic lateral sclerosis are of non-cardiac origin and may serve as a proxy of lower motor neuron or skeletal muscle involvement. They potentially help to stratify patients according to lower motoneuron involvement. Further research will determine the biological origin of the cardiac troponin T elevation and its validity as a diagnostic and/or prognostic marker. Our finding also serves as a reminder to interpret cardiac troponin T elevations in patients with neuromuscular diseases with caution.
Keywords: motor neuron disease, biomarker, troponin, amyotrophic lateral sclerosis
Castro-Gomez et al. report elevated serum Troponin T in ALS patients and suggest this can be used as a biomarker of neuromuscular involvement, complementing the neuroaxonal damage marker neurofilament. This finding has immediate implications for the laboratory work-up of ALS and for the design of clinical trials.
Graphical Abstract
Graphical Abstract.
Introduction
Amyotrophic lateral sclerosis (ALS), the most common form of adult-onset motoneuron diseases, remains a clinical diagnosis and is defined as the combination of progressive upper and lower motor neuron symptoms.1 There is an urgent need for biomarkers that inform diagnosis, prognosis and the design of interventional trials.2
Troponins (Tn) are essential structural and functional components of skeletal and cardiac muscle. Expression of cardiac troponin I (cTnI) and T (cTnT) is highly tissue specific and their elevation in serum is a sensitive and specific indicator of acute myocardial injury.3 The interpretation of troponin serum levels is complicated by a range of chronic conditions associated with persistently elevated cardiac troponins. Serum troponin levels in ALS are rarely reported. A limited number of case reports and a case–control study, however, suggest that cTnT is elevated in ALS even in the absence of evidence for cardiac damage.4–6 This prompted us to speculate that serum cTnT levels might have an unexpected, informative value for establishing the diagnosis and prognosis of ALS, especially if cTnI is measured simultaneously to control for potentially undetected myocardial injury.
Here, we combined an observational cross-sectional biomarker study and real-world evidence from our ALS and memory clinics to test the hypothesis that serum cTnT elevation is a hallmark of ALS and reflects disease severity.
Materials and methods
Subject samples
We analysed clinical information and serum samples from two resources: (i) the DZNE Clinical Registry Study of Neurodegenerative Diseases (DESCRIBE) cohort is a multicentric observational study maintained by the German Center for Neurodegenerative Diseases. It recruits patients with neurodegenerative conditions, including ALS, and age- and sex-matched healthy controls. All DESCRIBE participants provided written informed consent (University of Bonn Ethics Board statement 113/19)7 and (ii) The Department of Neurodegenerative Diseases and Gerontopsychiatry at Bonn University Hospital runs a large memory and ALS unit where cTnT and cTnI serum levels are part of the routine work-up. For the longitudinal data, the interval was 90 ± 30 days, as patients are typically seen every 3 months. For this study, we included 117 consecutive patients from the ALS clinic who were seen between January 2019 and June 2019. As the laboratory measurements were part of the routine clinical work-up and retrospectively analysed, no formal consent was needed per statement of our institutional ethics review board (Ethics Board decision letter 324/20). All ALS patients had progressive upper and lower motor neuron signs, but the El Escorial criteria were not formally recorded. The Alzheimer’s disease patients fulfilled the clinical and CSF biomarker-based criteria according to National Institute on Aging and Alzheimer's Association (NIA-AA)Research Framework.8 They ranged clinically from mild cognitive impairment to moderate dementia. All Huntington’s disease patients had one confirmed abnormally expanded CAG repeat in the huntingtin gene locus (>39 CAG repeats) and were motor symptomatic as assessed by an Unified Huntington’s Disease Rating Score motor score >5.
Laboratory markers
High-sensitivity cardiac cTnT and cTnI measurements were performed in a fully accredited commercial laboratory (Labor Volkmann, Karlsruhe) or at the central laboratory of the University Hospital Bonn. All other parameters were routinely determined through the University Hospital Bonn central laboratory. The phosphorylated neurofilament heavy chain (pNfH) in CSF was measured at the University Medical Center of Ulm, Germany using standardized ELISA, as described previously.9 Disease severity was assessed with the revised ALS-Functional Rating Scale (ALS-FRSr).10
Statistical analysis
Statistical analysis was performed using IBM SPSS 21 and GraphPad Prism 9. χ2 and Fisher’s exact tests were used for categorical variables as appropriate. Significance values are shown in the tables uncorrected for multiple comparisons. Groups were compared for cTnT, cTnI levels and age using non-parametric Kruskal–Wallis test with pairwise comparisons adjusted for multiple comparisons controlling false discovery rate. Correlation analyses were performed using non-parametric Spearman correlations. Receiver operating characteristics (ROC) curves were calculated using non-parametric settings.
Data availability
All details regarding the presented datasets are available from the corresponding author upon request.
Results
To ascertain the prevalence of troponin level elevations in ALS, based on a power analysis informed by the Mach et al.11 study, we obtained serum samples from three diagnosis groups in the DESCRIBE cohort: ALS (n = 75), Alzheimer’s disease (n = 30) and matched controls (n = 29) (Table 1).
Table 1.
DESCRIBE cohort
| Control | AD | ALS | P-value | |
|---|---|---|---|---|
| Number | 29 | 30 | 75 | |
| Sex, female:malea | 16:15 | 14:16 | 34:41 | 0.8396 |
| Age at test, yearsb | 66.7 (±9.7) | 72,07 (±8.7) | 59.5 (±11.9) | <0.0001*** |
| cTnT (ng/l)b | 5.7 (±4.4) | 12.05 (±16.3) | 35.99 (±62.4) | <0.0001*** |
| cTnT > cutoffa | 3 () | 6 (16.7%) | 44 (62,7%) | <0.0001*** |
| cTnI (ng/l)b | 4.7 (±2.9) | 9.1 (±18.9) | 4.7 (±3.1) | 0.1155 |
P < 0.05;
P < 0.001.
χ2 test of independence.
Kruskal–Wallis test.
AD, Alzheimer’s disease;
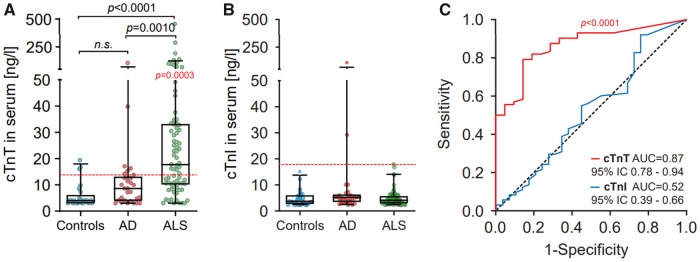
The mean (SD) cTnT level in the ALS samples was 35.99 pg/ml (±62.4), significantly higher than in controls (5.7 pg/ml ± 4.4 SD) or Alzheimer’s disease (12.05 pg/ml ± 16.3) (Fig. 1A). The mean cTnT level of the ALS cohort (35.99 pg/ml) was more than double the upper reference limit (14.0 ng/ml). In total, 44 of the 75 ALS samples (62.7%) were above the upper reference limit. cTnI levels did not differ between the three conditions (Fig. 1B) and were always within normal limits, except for two Alzheimer’s disease cases where, however, cTnT levels were also elevated (15.2/29.2 and 89.2/105.7, respectively).
Figure 1.
Cardiac troponin levels in serum from ALS patients. (A) cTnT levels in serum of control, Alzheimer’s disease and ALS patients (German Center for Neurodegenerative Diseases Cohort). The dashed horizontal red line is the conventional cut-off value (14 ng/l) for myocardial injury. Reported P (in black) is from Kruskal–Wallis tests on cTnT serum concentrations in comparisons between controls, Alzheimer’s disease and ALS patients. Reported P (in red) is from Wilcoxon Signed Rank Test on cTnT serum concentrations in comparison to the theoretical median of cut-off value of 14 ng/l for myocardial injury. Box plots represent the median and whiskers the 5–95th percentile. (B) cTnI levels in serum of control, Alzheimer’s disease and ALS patients. The dashed horizontal red line is the conventional cut-off value of 17 ng/l for myocardial injury. Box plots represent the median and whiskers the 5–95th percentile. (C) ROC curves illustrate cTnT (Redline) association with ALS diagnosis in comparison to cTnI levels in serum (blue line).
To determine the diagnostic accuracy of the two troponin tests for discriminating ALS from non-disease controls, we generated ROC curves. The cTnT ROC curve had an area-under-the-curve (AUC) of 0.87. The cTnI ROC AUC was 0.52, and thus carried no information concerning the ALS diagnosis (Fig. 1C).
To better understand cTnT levels in the context of ALS, we interrogated real-world records from 117 consecutive patients that frequented our ALS unit from January 2019. In addition, 5 patients with benign fasciculation syndrome (BFS) were included as disease-mimics, and 38 Alzheimer’s disease and 19 Huntington’s disease patients as disease-controls (Table 2). Because of a lag in implementing the test, cTnI levels were only available for a subset of the patients.
Table 2.
Real-world cohort—University Hospital Bonn
|
ALS
|
|||||||
|---|---|---|---|---|---|---|---|
| Spinal-onset | Bulbar-onset | PLS | BFS | AD | HD | P-value | |
| Number | 85 | 24 | 8 | 5 | 38 | 19 | |
| Sex, female:malea | 37:48 | 15:9 | 8:0 | 0:5 | 24:14 | 11:8 | 0.0025* |
| Age, yearsb | 64.4 (±11.6) | 64.2 (±8.5) | 59.9 (±8.2) | 41.5 (±9.0) | 73.8 (±8.4) | 52.4 (±10.6) | <0.0001*** |
| Duration, years | 3,1 (±2.8) | 2.5 (±1.8) | 7.4 (±7.9) | 3.5 (±4.2) | NA | NA | 0.3199 |
| BMI (kg/m2)b | 24.2 (±4.5) | 22.2 (±2.7) | 24.4 (±6.1) | 29.3 (±7.1) | ND | 27.9 (±6.7) | 0.1714 |
| ALSFRS-Rb | 31.4 (±8.4) | 29.4 (±10.5) | 11.3 (±1.5) | 48 | ND | ND | <0.0001*** |
| cTnT (ng/l)b | 31.8 (±25.4) | 22.5 (±22.0) | 4.6 (±2.8) | 4.4 (±1.8) | 9.2 (±5.1) | 5.3 (±3.2) | <0.0001*** |
| cTnT > cut-offa | 64 (75,3%) | 11 (45,8%) | 0 (0%) | 0 (0%) | 7 (18.4%) | 0 (0%) | <0.0001*** |
| cTnI (ng/l)b | 6.6 (±4.3) | 5.5 (±2.2) | 5.4 (±2.7) | 5 (±1.3) | ND | 6.1 (±5.1) | 0.7577 |
| pNfH (pg/ml)b | 3058 (±2583) | 2330 (±1162) | 2541 (±1613) | 240.8 (±280.7) | ND | 355.4 (±295.5) | 0.0003*** |
χ2 test of independence.
Kruskal–Wallis test.
P < 0.05;
P < 0.001.
AD, Alzheimer’s disease; BFS, benign fasciculation syndromeHD, Huntington's disease.
The mean cTnT level in the ALS cohort was 29.79 ng/ml (±24.89 SD), double the upper reference limit and similar to the DESCRIBE cohort (35.99 ng/ml). The mean cTnI level was 6.41 ng/ml (±3.95 SD), again similar to the DESCRIBE cohort (4.74 ng/ml).
The routine data collected in our clinic include time and site of symptom-onset (bulbar versus spine) and degree of upper motor neuron involvement, allowing us to classify three subgroups including the pure upper motor neuron variant primary laterals sclerosis (PLS) (Fig. 2A). The highest absolute (131 ng/ml) and median (23.0 ng/ml) cTnT levels were detected in the spinal-onset group. The bulbar-onset group (which usually also had limb involvement at the time of assessment), had a maximum cTnT level of 77.9 ng/ml and a median of 13.6 ng/ml. Alzheimer’s disease patients presented with a cTnT mean value of 9.2 (±5.1 SD). All PLS, BFS and Huntington’s disease values were well below the upper limit of the reference range (<14 ng/ml).
Figure 2.
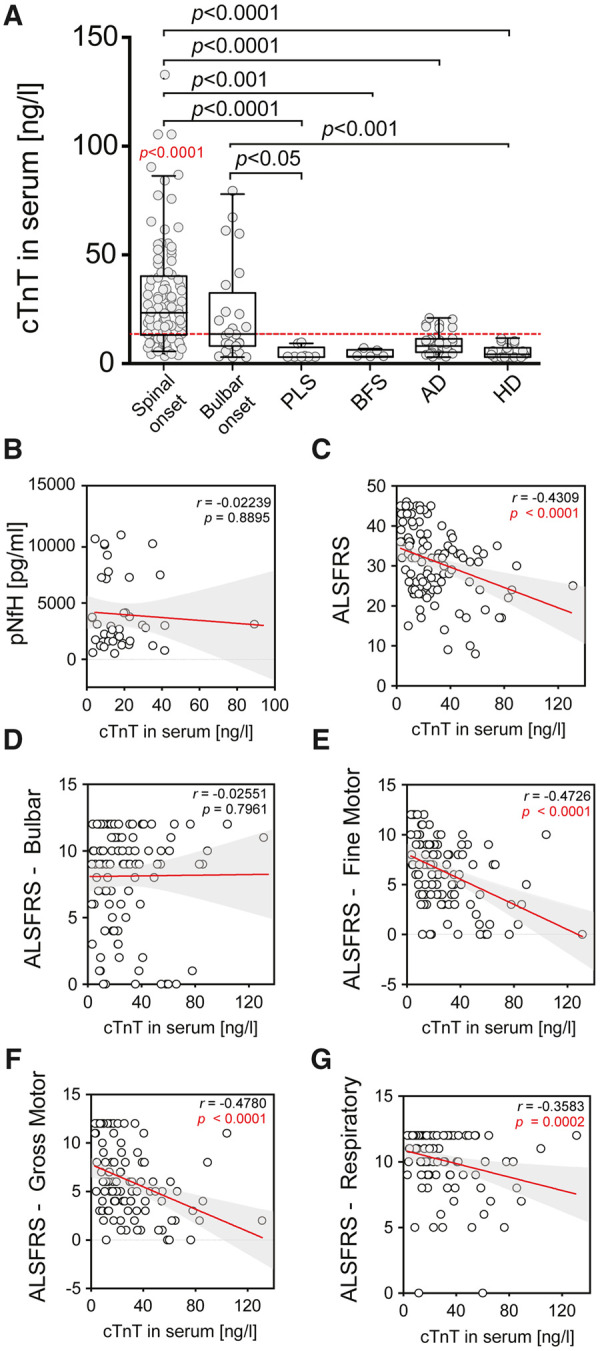
Real-life cohort and correlation of cTnT in serum with ALS-FRS and sub-scores. (A) cTnT levels in serum of patients with ALS with limb-onset, bulbar-onset, PLS, BFS, Alzheimer’s disease and Huntington’s disease. Reported P (in black) is from Kruskal–Wallis tests on cTnT serum concentrations in comparisons between subgroups by Dunn's multiple comparisons test. Reported P in red is from Wilcoxon Signed Rank Test on cTnT serum concentrations in comparison to the theoretical median of cut-off value of 14 ng/l for myocardial injury. The dashed horizontal red line is the established cut-off value of 14 ng/l for myocardial injury. Box plots represent the median and whiskers the 5–95th percentile. (B–G) Correlation analyses were performed using non-parametric Spearman correlations (r). Curves were drawn by a linear regression model with an interaction term for cTnT in serum by (B) ALSFRS Global Score, (C) ALS-FRS Bulbar Score, (D) ALS-FRS Gross Motor Score, (E) ALS-FRS Gross Motor Score, (F) ALS-FRS Respiratory Score and (F) pNfH in CSF. Shaded areas represent 95% CIs..
We correlated the cTnT serum levels with total ALS-FRSr score and each of the subdomains, and—where available—with the pNfH levels in the CSF from the time of cTnT serum measurements (Fig. 2B–G). cTnT showed a significant negative correlation with the total ALS-FRSr score (Fig. 2C). There was no correlation between cTnT and bulbar function, while the correlation with the fine and gross motor domain and with the respiratory domain was highly significant. There was no correlation between serum cTnT and CSF-pNfH (Fig. 2B) or between cTnT and ΔALS-FRSr/month (points decline per month, Supplementary Fig. 1A–C).
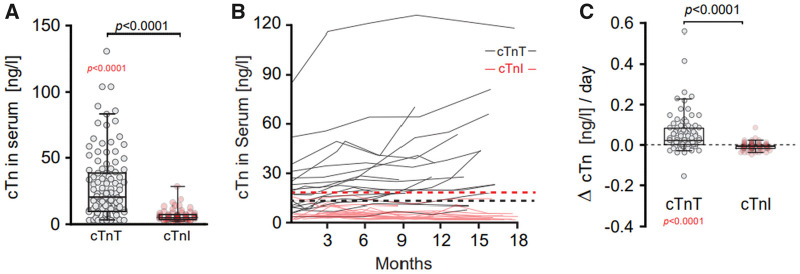
In the real-world cohort, cTnT but not cTnI serum levels were elevated ALS patients, fully confirming the findings from the DESCRIBE cohort (Fig. 3A). In a small subset of ALS patients from the real-world cohort (n = 14), longitudinal data allowed us to examine the dynamics of cTnT and cTnI (Fig. 3B). As the disease progressed over time the cTnT levels tended to increase, whereas the cTnI levels stayed stable. We then normalized the data by plotting the change of cTnT between the longest available interval (minimum 30 days) as ΔcTn/day. The scatter of ΔTnT/day was significantly larger than ΔTnI/day (Fig. 3C), which did not show a significant change over time.
Figure 3.
Correlation of cTnT Serum with ALSFRSr and sub-scores. (A) cTnT in comparison to cTnI levels in serum of ALS patients (MND Clinic). Reported P (in black) is from Mann–Whitney test on cTnT serum concentrations in comparisons to cTnI of ALS patients. Reported P in red is from Wilcoxon Signed rank test on cTnT serum concentrations in comparison to the theoretical median of cut-off value of 14 ng/l for myocardial injury. Box plots represent the median and whiskers the 5–95th percentile. (B) Spaghetti plot of cTn values versus time (months). Each black line represents longitudinal cTnT values of an individual patient. Each red line represents longitudinal cTnT values of an individual patient over time. Dash lines represent the theoretical median of cut-off value of 14 ng/l (cTnT in black) and 17 ng/l (cTnI in red) for myocardial injury. (C) Change in time of cTnI and cTnT levels in serum of ALS patients when two measurements were available with a min. interval >30 days. Reported P (in black) is from Mann–Whitney test on ΔcTnT serum concentrations in comparison to cTnT. Reported P in red is from Wilcoxon Signed Rank Test on ΔcTnT serum concentrations in comparison to the theoretical median of no change. The dashed horizontal red line represents Δ = 0. cTnT, cardiac troponin T; cTnI, cardiac troponin I. ΔcTn/day = (cTn first − cTn last)/days. Box plots represent the median and whiskers the 5–95th percentile.
Discussion
This is, to the best of our knowledge, the first systematic report of a discordance between cTnT and cTnI levels in patients with ALS. We believe it has important and immediate implications for the laboratory work-up of patients with confirmed or suspected ALS.
The prior literature reveals a total of 11 cases with a similar cTnT versus cTnI discordance in four independent publications.4–6,12 As case reports, none of these studies allow any conclusions on prevalence. A cohort study by Mach and colleagues found elevated cTnT in 68% of their population (n = 40) versus 5% in the case–control population.13 This is in remarkably close agreement with our observations, but as Mach et al. did not measure cTnI, they had to speculate on the cardiac health of their subjects.
Our study offers the first opportunity to view the chronic cTnT elevation in ALS as a source of information rather than a confounding factor. The observation that cTnT levels correlate well with gross and fine motor deficits in the ALS-FRSr, but not with bulbar symptoms points to peripheral skeletal muscle as potential driver. Indeed, two independent research groups have put forward compelling arguments that degenerating or regenerating skeletal muscle is the likely source of the cTnT elevation in neuromuscular disorders, including ALS.4,5 Of note, Bodor et al.13 report diaphragm muscle fibres expressing cardiac cTnT, while cardiac TnI is not detectable in any of the human skeletal muscles tested.13
The lack of correlation between serum cTnT and pNfH in CSF is intriguing, as we may have two complementary biomarkers at hand: pNfH as a marker of neuroaxonal damage and cTnT elevation as a proxy of neuromuscular, i.e. lower motoneuron affection. This is supported by the observation that patients with the upper motoneuron variant PLS had low normal serum cTnT. Future studies should include lower motoneuron dominant variants such as progressive muscular atrophy and spinal and bulbar muscular atrophy to help support or dismiss our interpretation.
Clearly, further research is necessary to determine the nature and origin of the elevated serum TnT levels in ALS patients. Also, our study reconfirms that in ALS, as in any neuromuscular disorder cTnT elevations need to be interpreted with caution. We propose that simultaneously measuring cTnI is an efficient heuristic to rule out undetected myocardial injury when the clinical setting does not allow for an immediate and full cardiac work-up.
Finally, we note that troponins are emerging as therapeutic targets in ALS. Ongoing trials are looking at troponin C activators as potential disease modifiers.14 We caution that troponin expression might be fundamentally altered in a large fraction of the ALS population and that this needs to be accounted for in trial design.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
In loving memory of Elliott Weydt (2006–2021).
Funding
The research was supported through unrestricted donations from our patients, especially Bruno Schmidt and the Initiative Alle-Lieben-Schmidt e. V. to Patrick Weydt.
Competing interests
The authors report no competing interests.
Glossary
- ALS =
amyotrophic lateral sclerosis
- ALS-FRSr =
revised ALS functional rating scale
- AUC =
area under the curve
- BFS =
benign fasciculation syndrome
- CAG =
cytosine-adenine-guanine (trinucleotide repeat sequence)
- DESCRIBE =
DZNE Clinical Registry Study of Neurodegenerative Diseases
- DZNE =
German Center for Neurodegenerative Diseases
- PLS =
primary lateral sclerosis
- pNfH =
phosphorylated neurofilament heavy chain
- ROC =
receiver operated characteristics
- Tn =
troponin
- TnI =
troponin I
- TnT =
troponin T
References
- 1.Ludolph A, Drory V, Hardiman O, et al. ; WFN Research Group On ALS/MND. A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Frontotemporal Degen. 2015;16(5-6):291–292. [DOI] [PubMed] [Google Scholar]
- 2.Verber NS, Shepheard SR, Sassani M, et al. Biomarkers in motor neuron disease: A state of the art review. Front Neurol. 2019;10:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park KC, Gaze DC, Collinson PO, et al. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc Res. 2017;113(14):1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rittoo D, Jones A, Lecky B, et al. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases. J Am Coll Cardiol. 2014;63(22):2411–2420. [DOI] [PubMed] [Google Scholar]
- 5.Schmid J, Liesinger L, Birner-Gruenberger R, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol. 2018;71(14):1540–1549. [DOI] [PubMed] [Google Scholar]
- 6.Casmiro M, Graziani A.. Serum troponin T in patients with amyotrophic lateral sclerosis. Acta Neurol Belg. 2019;119(2):285–288. [DOI] [PubMed] [Google Scholar]
- 7.Jessen F, Spottke A, Boecker H, et al. Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers Res Ther. 2018;10(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Bennett DA, Blennow K, et al. ; Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weydt P, Oeckl P, Huss A, et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis: Biomarkers in ALS. Ann Neurol. 2016;79(1):152–158. [DOI] [PubMed] [Google Scholar]
- 10.Castrillo-Viguera C, Grasso DL, Simpson E, et al. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11(1-2):178–180. [DOI] [PubMed] [Google Scholar]
- 11.Mach L, Konecny T, Helanova K, et al. Elevation of cardiac troponin T in patients with amyotrophic lateral sclerosis. Acta Neurol Belg. 2016;116(4):557–564. [DOI] [PubMed] [Google Scholar]
- 12.Hof D, Jung HH, Bloch KE.. Troponin T elevation in amyotrophic lateral sclerosis without cardiac damage. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(1):75–77. [DOI] [PubMed] [Google Scholar]
- 13.Bodor GS, Survant L, Vos EM, et al. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clin Chem. 1997;43(3):476–484. [PubMed] [Google Scholar]
- 14.Al-Chalabi A, Heunks LMA, Papp Z, et al. Potential of the cardiovascular drug levosimendan in the management of amyotrophic lateral sclerosis: An overview of a working hypothesis. J Cardiovasc Pharmacol. 2019;74(5):389–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All details regarding the presented datasets are available from the corresponding author upon request.