Abstract
Background
Mitral valve (MV) repair or replacement surgery is indicated for a variety of conditions. Although uncommon, damage to the left circumflex (LCx) coronary artery, which courses in close proximity to the MV annulus, is a devastating complication.
Case summary
This report describes the case of a 63-year-old woman following re-operative MV replacement. Shortly after being transferred to the surgical intensive care unit after MV replacement, her EKG was notable for persistent inferolateral ST-segment elevations and reciprocal ST-segment depressions. Emergency transthoracic echocardiogram revealed a reduced left ventricular ejection fraction of 35–40% and mid to distal lateral wall motion hypokinesis. She was emergently taken to the cardiac catheterization laboratory where coronary angiography demonstrated complete occlusion of her mid LCx artery. She underwent urgent percutaneous coronary intervention of the lesion and was started on dual antiplatelet treatment, anticoagulation for comorbid atrial fibrillation, as well as guideline directed medical therapy with improvement in her EKG changes and cardiac function.
Conclusion
Prompt diagnosis and recognition of LCx injury is crucial. Management involves immediate percutaneous recanalization or surgical coronary bypass grafting.
Keywords: Left circumflex artery, Mitral valve replacement, Percutaneous coronary intervention, Coronary artery bypass grafting, Case report
Learning points
Left circumflex (LCx) artery flow may be compromised due to persistent mechanical kinking of the vessel from an abutting suture during and after surgical mitral valve (MV) surgery.
Patients with pre-existing MV replacements undergoing reoperation are particularly susceptible to these complications.
Early diagnostic and management approaches can mitigate long-term adverse sequalae, such as left ventricular remodelling, heart failure, and arrhythmias in patients with LCx artery injury.
Introduction
Injury to the left circumflex (LCx) artery occurs rarely during mitral valve (MV) surgery.1–3 It was first described by Danielson et al. in 1967 and has been subsequently confirmed in a variety of case reports. There are multiple mechanisms by which damage to the LCx artery may occur. These include direct injury to the artery with an encircling suture, kinking of the vessel during valve or ring implantation given the anatomic proximity of the annulus of the valve to the circumflex artery, and compression from an over-sized prosthesis.2 Other mechanisms include embolism of air or particulate matter and coronary vasospasm.3,4
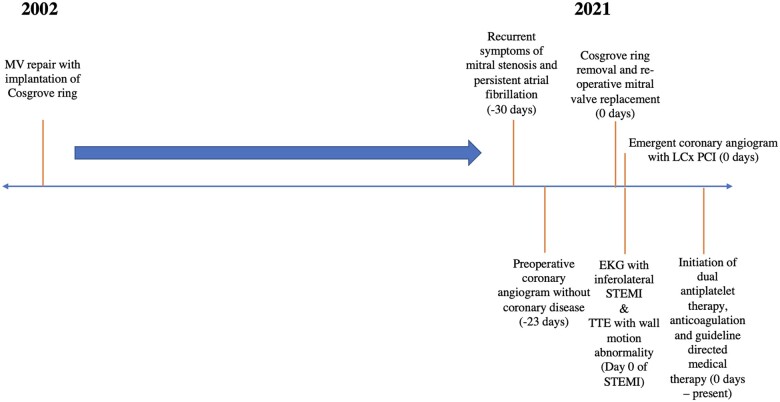
Timeline
Case presentation
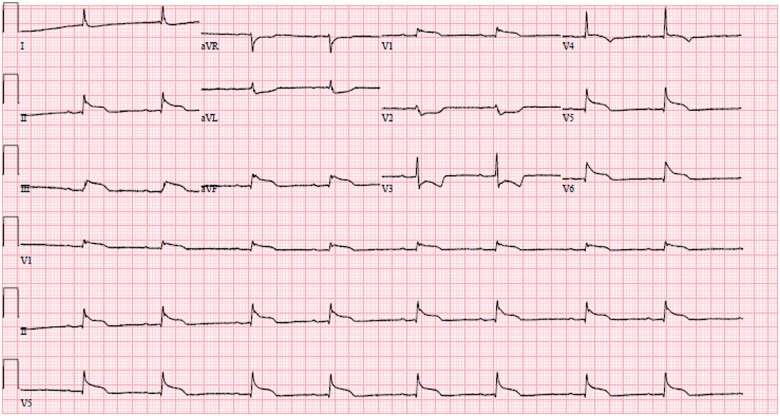
A 63-year-old woman was transferred to the surgical intensive care unit (ICU) for routine monitoring and postoperative care following re-operative MV replacement (MVR, 25 mm On-X mechanical valve), left atrial maze, and appendage closure via a right minithoracotomy. She was afebrile and normotensive with a heart rate of 58 beats per minute and room air oxygen saturation of 97%. Her jugular venous pressure was 12 cm of H2O. Breath sounds were grossly clear anteriorly. A mechanical S1 was audible without an appreciable murmur. Electrocardiogram (ECG) revealed sinus bradycardia with inferolateral ST-segment elevations (Figure 1) and reciprocal ST-segment depressions in leads avL, V2, and V3.
Figure 1.
Electrocardiogram with inferolateral ST elevation and reciprocal ST depression noted.
Her medical history was notable for primary mitral regurgitation due to a congenital cleft of the A2 scallop for which she underwent MV repair in 2002 with implantation of a Cosgrove-Edwards ring. She subsequently developed post-repair mitral stenosis and AF, leading to multiple cardioversion procedures and catheter ablation in 2014. Re-operative MVR was undertaken in 2021. As part of the surgery, the anterior leaflet of the MV was removed as was the previously placed mitral ring. A 23 mm Onyx valve was sutured into place without obvious complications.
The differential diagnosis for the inferolateral injury current included intra-procedural LCx coronary artery compromise, air or particulate matter embolism, or coronary artery vasospasm.
The repolarization changes persisted on repeat ECG approximately 1 h later. Emergency bedside transthoracic echocardiogram revealed a reduced left ventricular ejection fraction of 35–40% and mid to distal lateral wall motion hypokinesis, significantly more marked than the mild lateral wall hypokinesis noted on intraoperative transoesophageal echocardiography on separation from cardiopulmonary bypass. A rapid heart team discussion was held between the cardiothoracic surgery and interventional cardiology teams. Together, they felt coronary angiography and percutaneous coronary intervention (PCI) would provide the swiftest restoration of myocardial blood flow and would prevent repeat sternotomy, as a result of which the cardiac catheterization laboratory was activated for emergency angiography.
Coronary angiography demonstrated complete occlusion of her mid LCx artery (Video 1). This finding was new compared with her preoperative catheterization a month prior (Video 2). There was concern for intraoperative injury to the LCx artery. Percutaneous reperfusion was undertaken. The initial attempt to cross the lesion was unsuccessful with a work-horse wire (Sion Blue) (Video 3); a subsequent antegrade wire escalation strategy with a Pilot 50 wire supported by a Turnpike LP micro-catheter was successful in navigating the lesion. Balloon angioplasty of the region of interest was performed with a variety of semicompliant, non-compliant, and scoring balloons with recoil and without re-establishment of distal flow (Supplementary material online, Video S1). Intravascular coronary ultrasound (IVUS) was performed and demonstrated significant narrowing of the mid LCx artery (Supplementary material online, Video S2). Potential causes for the lack of antegrade flow included large thrombus burden (not appreciated on IVUS), no-reflow phenomenon (unlikely as good distal flow from micro-catheter injection was seen), severe coronary vasospasm, or persistent mechanical kinking of the vessel related to MVR. Intracoronary nitroglycerine, nitroprusside, and epinephrine were trialed to ensure there was no component of coronary spasm. Coronary stenting was felt indicated to provide sufficient radial strength in the LCx to maintain vessel patency. A 2.5 × 38 mm Synergy® drug-eluting stent was deployed and post-dilated with both 2.5 mm and 3.0 mm non-compliant balloons with restoration of TIMI III flow of both the LCx and the branching large obtuse marginal vessels (Supplementary material online, Videos S3 and S4).
The patient was started on triple anti-thrombotic therapy with aspirin, clopidogrel, and warfarin. Her troponin T level peaked at 6706 nanograms/litre. She was continued on triple therapy for 2 weeks after which aspirin was discontinued.
Discussion
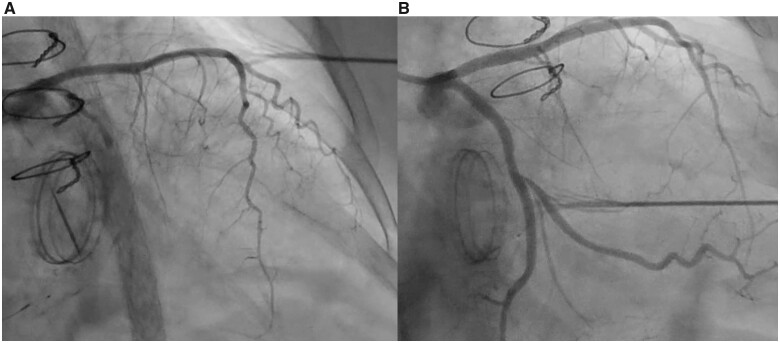
In our patient, the aetiology of LCx injury was felt to be due to kinking of the LCx (Figure 2A), given the rapid recoil after balloon inflation, the findings on IVUS, and the ultimate successful deployment of a stent. Full stent deployment would likely not have been possible in the case of complete suture-related occlusion. Patients with codominant or left dominant coronary circulations are particularly susceptible to LCx arterial damage due to the even closer proximity of the artery to the MV annulus. In a study by Virmani et al.,5 the average distance between LCx artery and the annulus was 4.1 mm in those with left dominance, 5.5 mm in those with codominance, and 8.4 mm in those with right dominance. Subsequent studies have confirmed these findings with some describing distances as close as 1 mm in patients with left or co-dominant anatomy.6 In addition, patients whom have had prior MV annuloplasty (as was the case in our patient) have a higher rate of complications at time of re-operative MVR due to excess commissure resection.
Figure 2.
(A) Coronary angiogram of the left circumflex artery abutting the mitral valve annulus with evidence of iatrogenic arterial kinking after contrast injection. (B) Coronary angiogram of the left circumflex artery abutting the mitral valve annulus with re-establishment of TIMI III flow after percutaneous coronary intervention.
Early diagnosis is crucial to mitigate the longer-term adverse sequalae of LCx occlusion. Intraoperative TEE is recommended during MV surgery and allows the surgical team to assess wall motion quickly and prior to chest closure.7 Recent innovations with three-dimensional TTE may allow operators to accurately measure horizontal and vertical distances of the LCx from the MV annulus, as well as detect any obstruction to flow within the artery with colour Doppler.7 Postoperative ECGs are a routine feature of postsurgical management. Lastly, the findings noted on preoperative cardiac computed tomography, if performed, can sometimes include recognition of the course of the LCx artery and coronary sinus relative to the MV annulus. These data may inform the technical approach to commissural resection and device implantation.
Management involves urgent re-establishment of coronary flow. When LCx compromise is detected intraoperatively and prior to chest closure, surgical correction is the preferred approach.2,7 However, when the complication is detected after chest closure and transfer to the ICU, both PCI, which was successfully performed in our patient’s case, and coronary artery bypass grafting (CABG) can be utilized to restore myocardial perfusion.8 PCI is preferred in cases of coronary arterial kinking given the faster nature of the procedure and avoidance of repeat sternotomy.9 However, when the vessel is obstructed by an encircling suture, CABG or repositioning of the prosthesis are reasonable alternatives.10
Repeat ECG after PCI demonstrated inferolateral Q waves. A TTE performed 2 days later showed a left ventricular ejection fraction of 40% with a persistent inferolateral wall motion abnormality. The patient was started on guideline directed medical therapy and ultimately discharged after achieving a therapeutic INR and confirming the absence of bleeding.
Conclusions
Injury to the LCx artery is a rare but potentially devastating complication of MV surgery and can occur by a variety of mechanisms, including direct injury from an encircling suture, kinking, or compression from an oversized prosthetic valve. Prompt diagnosis with a high index of suspicion is crucial to mitigate long-term adverse sequalae. Management involves immediate recanalization with PCI, CABG, and/or repositioning of the prosthesis.
Lead author biography

Dr Prakriti Gaba is a Cardiology Fellow at the Brigham and Women’s Hospital/Harvard Medical School in Boston, Massachusetts. She is passionate about pursuing a clinical career in Interventional and Structural Cardiology and her research involves understanding and improving clinical trials.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: D.L.B. discloses the following relationships: Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. The remaining authors have nothing to disclose relevant to the contents of this paper.
Funding: None declared.
Supplementary Material
References
- 1. Danielson GK, Cooper E, Tweeddale DN.. Circumflex coronary artery injury during mitral valve replacement. Ann Thorac Surg 1967;4:53–59. [Google Scholar]
- 2. Dillon J. A stitch too far: the circumflex artery in jeopardy during mitral valve repair. J Thorac Cardiovasc Surg 2017;154:1621–1623. [DOI] [PubMed] [Google Scholar]
- 3. Victor Arévalos MD, Luis Ortega-Paz MD, Daniel Pereda MD, Elena Sandoval MD, Salvatore Brugaletta MD.. Percutaneous treatment of a circumflex artery occlusion after minimally invasive barlow disease mitral valve repair. JACC Case Rep 2021;3:173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chacko M, Marrouche NF, Bhatt DL.. Asymptomatic acute inferior ST elevation myocardial infarction from thermal injury complicating radiofrequency ablation for atrioventricular re-entrant tachycardia. J Invasive Cardiol 2004;16:504–505. [PubMed] [Google Scholar]
- 5. Virmani R, Chun PK, Parker J, McAllister HA.. Suture obliteration of the circumflex coronary artery in three patients undergoing mitral valve operation. Role of left dominant or codominant coronary artery. J Thorac Cardiovasc Surg 1982;84:773–778. [PubMed] [Google Scholar]
- 6. Grande AM, Fiore A, Massetti M, Viganò M.. Iatrogenic circumflex coronary lesion in mitral valve surgery. Tex Heart Inst J 2008;35:179–183. [PMC free article] [PubMed] [Google Scholar]
- 7. Ender J, Selbach M, Borger MA, Krohmer E, Falk V, Kaisers UX. et al. Echocardiographic identification of iatrogenic injury of the circumflex artery during minimally invasive mitral valve repair. Ann Thorac Surg 2010;89:1866–1872. [DOI] [PubMed] [Google Scholar]
- 8. Husain A, Alsanei A, Tahir M, Dahdouh Z, AlHalees Z, AlMasood A.. Left circumflex artery injury postmitral valve surgery, single center experience. J Saudi Heart Assoc 2019;31:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bargagna M, Trumello C, Sala A, Blasio A, Castiglioni A, Alfieri O. et al. Left circumflex artery injury after mitral valve surgery: an algorithm management proposal. Ann Thorac Surg 2021;111:899–904. [DOI] [PubMed] [Google Scholar]
- 10. Dello SA, Leus SJ, Tan MES, Otterspoor LC, Botman C-J.. Percutaneous coronary intervention of an iatrogenic occlusion of the circumflex coronary artery after mitral valve replacement. Eur Heart J Acute Cardiovasc Care 2015;9:NP1–NP2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.