Abstract
Background
Arrhythmogenic left ventricular cardiomyopathy (ALVC) is a rare form of arrhythmogenic cardiomyopathy characterized by fibrofatty replacement of left ventricular myocardium, malignant arrhythmia, and sudden cardiac death. The definition incorporates several genetic causes, including pathogenic variation in the Filamin C gene (FLNC). Although awareness of ALVC has improved, identification remains challenging and diagnostic criteria continue to evolve.
Case summary
A 50-year-old athletic male was admitted following an out-of-hospital cardiac arrest due to ventricular tachycardia (VT) whilst playing football. Coronary angiography revealed unobstructed epicardial vessels and the diagnosis of ALVC was suggested by cardiovascular magnetic resonance imaging, which demonstrated a mildly dilated and moderately impaired left ventricle with epicardial late gadolinium enhancement in the basal to mid-lateral walls and subendocardial septum. Initial testing with a cardiomyopathy and arrhythmia gene panel was negative but extended testing uncovered a likely pathogenic variant in FLNC. Subsequently, the patient experienced a recurrence of sustained VT necessitating implantable cardioverter-defibrillator (ICD) therapies, ultimately undergoing a combined epicardial and endocardial VT ablation 4 years after presentation. Six months post-ablation, he was asymptomatic and his arrhythmia rendered quiescent.
Discussion
Arrhythmogenic cardiomyopathy should be considered in the evaluation of an initially unexplained cardiac arrest. This case characterizes the clinical features of a patient with FLNC cardiomyopathy and emphasizes the utility of genetic testing using modern gene panels in patients with comparable phenotypes. We also demonstrate that optimal medical therapy with antiarrhythmic drugs, exercise restriction, ICD insertion, and catheter ablation can be useful in the management of ALVC with positive outcomes
Keywords: Inherited cardiomyopathies, Filamin C, Arrhythmogenic left ventricular cardiomyopathy, Case report
For the podcast associated with this article, please visit https://academic.oup.com/ehjcr/pages/podcast
Learning points
Arrhythmogenic left ventricular cardiomyopathy is a distinct form of arrhythmogenic cardiomyopathy (ACM) characterized by left ventricular (LV) structural, electrical, and genetic abnormalities, including systolic dysfunction and arrhythmia, non-ischaemic LV late gadolinium enhancement, left precordial T-wave inversion, low limb lead QRS voltages, and pathogenic variants in ACM-related genes.
Phenotype-guided genetic testing can enhance diagnostic certainty, facilitate predictive testing of family members, and occasionally inform risk stratification, such as lowering the threshold for primary prevention defibrillator implantation in patients with Filamin C.
Catheter ablation is a reasonable approach in ACM patients with ventricular arrhythmia who have not responded to optimal antiarrhythmic drug therapy.
Introduction
Arrhythmogenic cardiomyopathy (ACM) is a rare heritable disorder characterized by ventricular arrhythmia and fibrofatty replacement of ventricular myocardium.1,2 Classically considered a disease of the right ventricle (RV), biventricular and left ventricular ACM phenotypes are increasingly recognized. The term ‘arrhythmogenic left ventricular cardiomyopathy’ (ALVC) has since been adopted as a distinct clinical entity within the spectrum of ACM.
Given its rarity, clinical features and outcomes of patients with ALVC are scarce and identification can prove challenging. However, pathogenic variants affecting Filamin C (FLNC), an essential protein for myocyte integrity and cell signalling, have been increasingly implicated in patients presenting with characteristic hallmarks of ALVC, including LV predominant disease, malignant arrhythmia, and sudden cardiac death.3,4 In this case report, we discuss the challenges of diagnosis and management in a patient who presented with cardiac arrest and later diagnosed with ALVC.
Timeline
| Timeline | Clinical events |
|---|---|
| Premorbid state | Recent symptoms of exertional pre-syncope; playing 5-a-side football and cycling 60–100 miles regularly |
| Presentation | Out-of-hospital ventricular tachycardia (VT) arrest whilst playing football |
| Coronary angiography: unobstructed epicardial vessels | |
| Cardiac magnetic resonance imaging: mildly dilated left ventricle (LV) (Left ventricular end diastolic volume 214 mL) with moderately impaired LV systolic function (LVEF 41%) and no regional wall motion abnormalities. Non-dilated right ventricle (RV) with preserved systolic function. Low-grade late gadolinium enhancement within the septum and basal lateral subepicardium | |
| Implantation of secondary prevention defibrillator | |
| Started Bisoprolol 5 mg o.d. | |
| Blood sample sent for Lamin A (LMNA) gene testing and family referred for clinical screening | |
| 26 months | LMNA gene studies negative |
| Fluorodeoxyglucose (FDG) positron emission cardiac computed tomography: negative for sarcoid and active inflammation | |
| Implantable cardioverter-defibrillator (ICD) interrogation: brief runs of non-sustained ventricular tachycardia (NSVT) and one appropriate shock for sustained run of fast VT | |
| Switched to Nadolol 80 mg b.i.d. | |
| 31 months | ICD interrogation: no recorded ventricular arrhythmia |
| Genetic testing performed using a panel of 77 cardiomyopathy and arrhythmia-related genes | |
| 41 months | Genetic testing unable to identify a cause |
| Echo: mildly dilated LV [left ventricular end-diastolic diameter (LVEDD) 59 mm] with mild-moderately impaired LV systolic function (LVEF 45%). Markedly hypokinetic inferolateral and anterolateral walls. Mildly dilated RV with moderately impaired systolic function | |
| Nadolol not tolerated due to fatigue, changed to Sotalol 40 mg b.i.d. | |
| Started Ramipril 1.25 mg o.d. for left ventricular systolic dysfunction | |
| 42 months | Two episodes of NSVT and one of fast VT (250 b.p.m.) treated by antitachycardia pacing |
| Increased Sotalol to 40/80 mg b.i.d. | |
| Genetic testing to include Filamin C (FLNC) | |
| 52 months | Patient tests positive for likely pathogenic FLNC variant |
| One episode of fast VT (cycle length 220 ms) within the ventricular fibrillation defibrillator zone whilst climbing a ski slope, shock delivered | |
| Increased Sotalol to 80 mg b.i.d. | |
| Referred for VT ablation | |
| 57 months | Combined epicardial and endocardial VT ablation |
| 58 months | Echo: normal LV size and wall thickness (LVEDD 49 mm) with mildly impaired LV systolic function (LVEF 50–54%). Normal RV size with impaired radial function |
| 63 months | No recurrence of arrhythmia at 6-month follow-up post-ablation |
Case presentation
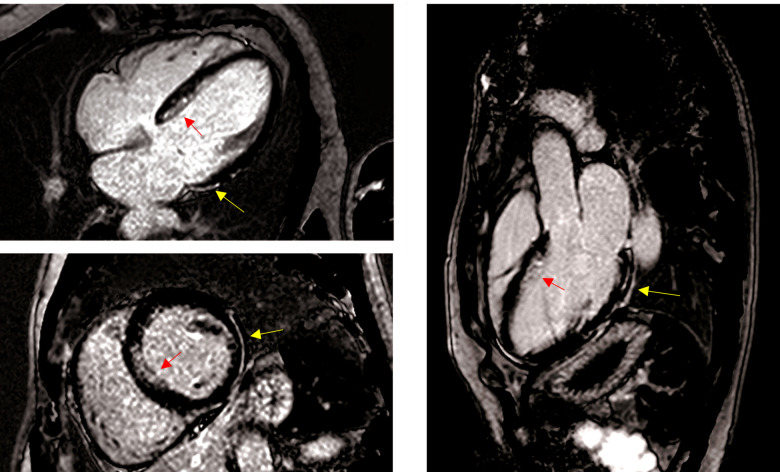
A 50-year-old Caucasian male collapsed unexpectedly whilst playing football. He was brought to hospital following successful resuscitation for an out-of-hospital ventricular tachycardia (VT) arrest. He had no significant past medical or family history of cardiac disease and was a keen recreational athlete, playing 5-a-side football regularly and cycling 60–100 miles per week. In the months leading up to his cardiac arrest, he recalled three brief episodes of exertional pre-syncope whilst playing sports. On admission, coronary angiography showed unobstructed epicardial vessels and echocardiography revealed mild LV impairment with normal heart valves. An FDG PET–computed tomography scan was negative for active myocardial inflammation. However, cardiovascular magnetic resonance imaging (CMRI) demonstrated a mildly dilated and moderately impaired LV [left ventricular ejection fraction (LVEF) 41%] with epicardial late gadolinium enhancement of the lateral wall and subendocardial enhancement of the septum. The RV was non-dilated with no regional wall motion abnormalities (Figure 1) (Videos 1–3).
Figure 1.
Late gadolinium enhancement cardiovascular magnetic resonance images showing horizontal long axis (four-chamber) (A) mid-ventricular short axis (B) and left ventricular outflow tract (three-chamber) (C) views. The yellow arrows indicate regions of epicardial lateral fibrosis and the red arrows point to septal subendocardial enhancement.
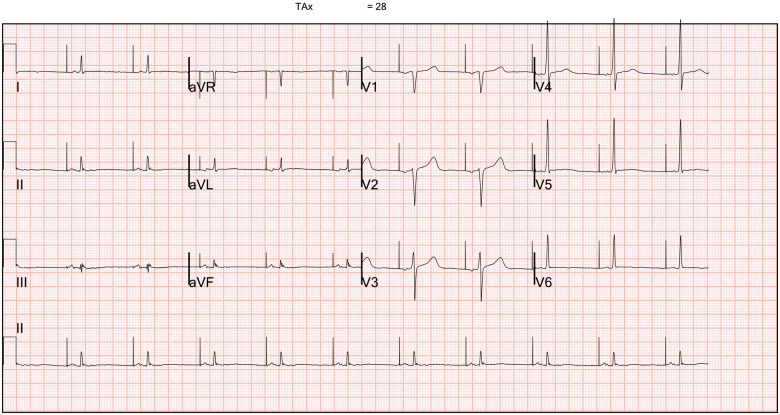
A diagnosis of ACM was suspected, and he received an implantable cardiac defibrillator (ICD) for secondary prevention. Post-implantation, his 12-lead electrocardiogram (ECG) showed an atrial paced rhythm with normal ventricular conduction (Figure 2). The limb lead complexes were of low voltage and demonstrated fractionated QRS complexes. There were flattened inferolateral T waves and his signal-averaged ECG was negative for late potentials. He was discharged on Bisoprolol 5 mg daily, lifestyle advice to limit his exercise intensity, and referred for genetic testing along with a recommendation for clinical screening of his family members. He was asymptomatic for a significant period, but at 26 months follow-up device interrogation demonstrated three runs of non-sustained ventricular tachycardia (NSVT) and he received one shock for sustained fast VT. He was then trialled on Nadolol therapy. Genetic testing for pathogenic Lamin A variants and subsequent testing against a panel of 77 cardiomyopathy and arrhythmia-related genes were negative.
Figure 2.
Twelve-lead electrocardiogram post-implantable cardioverter-defibrillator implantation demonstrating an atrial paced rhythm with inferolateral T-wave flattening and low voltage QRS complexes in the limb leads.
At 41 months, an echocardiogram demonstrated a mildly dilated LV with mild-moderate systolic impairment (LVEF 45%). Interrogation of his ICD revealed two episodes of NSVT and one fast sustained VT treated by antitachycardia pacing. He was switched to Sotalol for its Class III antiarrhythmic properties and started Ramipril 1.25 mg once daily. Given his electrical and structural phenotype, genetic testing was extended to include FLNC. Subsequently, he tested positive for a likely pathogenic FLNC frameshift variant [c.8107del; p.(Asp2703ThrfsTer69)], thus confirming the diagnosis of ALVC. Predictive testing also uncovered the variant in his three children, all of whom had variable clinical expression of the disease.
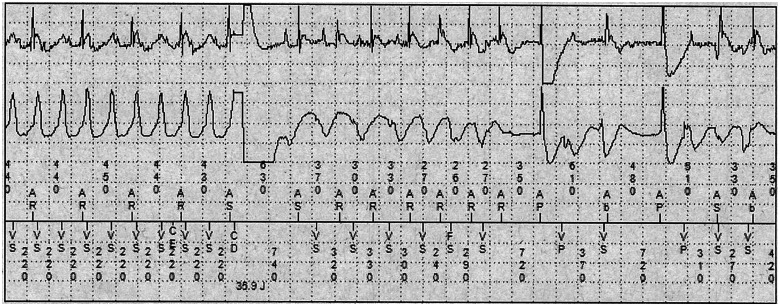
At 52 months he was climbing a ski slope and experienced an episode of fast VT requiring an ICD shock (Figure 3). Following urgent electrophysiology outpatient review, he was referred for VT ablation utilizing a combined endocardial and epicardial approach. During the procedure, two types of VT arising from the LV were readily induced and radiofrequency ablation was targeted to abnormal local potentials and the region surrounding epicardial and endocardial scar. At 6 months post-ablation, he was asymptomatic and arrhythmia free on Sotalol 80 mg twice daily, and echocardiography showed some improvement in LV systolic function (LVEF 50–54%).
Figure 3.
Intracardiac implantable cardioverter-defibrillator electrograms (top channel: atrial near-field; middle channel: ventricular far-field; bottom channel: markers) of an appropriate shock (Charge Delivered (CD) marker) for fast monomorphic ventricular tachycardia (Ventricular sensed (VS) event marker; cycle length 220 ms) occurring within the ventricular fibrillation zone. Recurrence of ventricular tachycardia shortly after shock delivery with intermittent Atrioventricular (AV) sequential pacing, the arrhythmia later spontaneously terminating without the requirement for additional therapy.
Discussion
Arrhythmogenic left ventricular cardiomyopathy is an under-characterized phenotype of ACM and few studies have evaluated clinical markers of the disease.5–7 The prevalence is also likely underestimated as findings of LV systolic impairment and myocardial fibrosis may overlap clinically with DCM and other ACM phenotypes. This difficulty in characterization was addressed by a statement from the Heart Rhythm Society,1 who proposed broadening the definition of ACM to an ‘arrhythmogenic heart muscle disorder not explained by ischaemic, hypertensive, or valvular heart disease’. The recently introduced ‘Padua criteria’ propose an update from the 2010 Task Force criteria for diagnosing ACM in order to improve differentiation between ACM phenotypes and identification of left-sided genetic causes.8,9 It utilizes a combination of structural, histological, electrocardiographic, and genetic parameters along with the patient’s presentation and family history. For a definitive diagnosis of ALVC, the patient must have a confirmed genetic mutation in one of the genes implicated in disease pathogenesis (Table 1).
Table 1.
Minimum set of genes implicated in the development of arrhythmogenic cardiomyopathy
| Gene | Protein type | Mutation type |
|---|---|---|
| BLC2-Associated Athanogene 3 (BAG3) | Chaperone | Truncating and missense |
| Desmin | Intermediate filament | Truncating and missense |
| Desmocollin-2 (DSC2) | Desmosomal | Truncating and missense |
| Desmoglein-2 (DSG2) | Desmosomal | Truncating and missense |
| Desmoplakin (DSP) | Desmosomal | Truncating and missense |
| Filamin C (FLNC) | Actin crosslink | Truncating and missense |
| Junction Plakoglobin (JUP) | Desmosomal | Missense |
| LIM Domain Binding 3 (LBD3) | Z-band | Missense |
| Lamin A/C (LMNA) | Nuclear envelope | Truncating and missense |
| NK2 Homeobox 5 (NKX2-5) | Homeobox | Truncating and missense |
| Plakophilin-2 (PKP2) | Desmosomal | Truncating |
| Phospholamban (PLN) | Calcium handing | Missense, nonsense, and deletion |
| RNA-Binding Motif Protein 20 (RBM20) | Splice factor | Missense |
| Sodium Voltage-Gated Channel Alpha Subunit 5 (SCN5A) | Sodium channel | Mostly missense |
| Transmembrane Protein 43 (TMEM43) | Nuclear envelope | Missense |
Adapted from HRS guidelines.1
The presenting feature in this case was an initially unexplained cardiac arrest secondary to ventricular arrhythmia. In conjunction with the scarring pattern on CMRI, a diagnosis of ALVC was suspected but could not be confirmed without genetic evidence. Although cardiovascular magnetic resonance has a fundamental role in delineating phenotype, additional investigative modalities such as molecular genetic studies, family screening, and rarely endomyocardial biopsy, may improve diagnostic certainty. Other suggestive features of ALVC include the presence of inferolateral T-wave inversion or apparent DCM with an arrhythmic burden incongruous with myocardial function.7
The elusive genetic basis of this case supports evolving evidence that the genetic architecture underlying ACM are more complex than previously thought. Consequently, the use of existing and potentially ‘outdated’ gene panels may omit newly identified genes that are implicated in disease pathogenesis, thereby precluding a formal diagnosis. Therefore, it is prudent for clinicians to have a low threshold for broadened genetic testing in patients with a suggestive phenotype, as seen here where the decision to extend gene testing yielded the diagnosis of FLNC-induced ALVC. Notably, our patient’s variant had been reported in two other patients with comparable phenotypes.10,11
Whilst rare, ACM-associated FLNC presents with a distinctive phenotype of abnormalities including LV dilation and myocardial fibrosis, systolic dysfunction, inferolateral negative T waves, low QRS voltages, and ventricular arrhythmias,1,3,10 all of which were present in this case. Truncating variants in FLNC have been shown to exhibit a dominant inheritance pattern and high penetrance amongst genotype-positive individuals (>97% in carriers older than 40 years of age).10 As such, extending genetic testing to family members is essential to allow for early intervention. Moreover, genetic testing can occasionally enhance risk stratification and management. In lower risk FLNC–ACM patients without a history of sustained arrhythmia, international consensus currently supports ICD implantation in those exhibiting moderate LV systolic impairment (LVEF < 45%).1
Overall, the aim of medical therapy in ACM is to treat arrhythmia, optimize ventricular function, and manage symptoms.1 Exercise is also known to accelerate disease progression and competitive sport is associated with an increased risk of ventricular arrhythmia and SCD.12 Several observational studies also suggest a dose-dependent relationship between participation in endurance exercise and risk of developing ACM in genotype-positive family members.1,13 Therefore, a comprehensive therapeutic approach includes restriction from endurance and high intensity sports, medical therapy (including antiarrhythmic agents and prognostic medications in patients with LV systolic impairment) and ICD implantation, where indicated. Ventricular tachycardia ablation may also be utilized to good effect, as seen here where our patient experienced multiple ICD-treated arrhythmias despite optimized medical therapy.
Lead author biography

Navneet Kandhari graduated from Newcastle University with an MBBS and MRes degree in 2019. He obtained a PgDip in Medical Education in 2021. He now works as a junior doctor in the UK. His current interests include cardiology, academic research, and medical education.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We would like to thank the patient for their valuable input to the study.
Funding
C.M. was the recipient of a British Heart Foundation clinical research training fellowship (FS/18/28/33549). The funders had no role in the study design, data collection, analysis and interpretation, the preparation of the manuscript, or in the decision to submit the article for publication.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
References
- 1.Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC. et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019;16:e301–e372. [DOI] [PubMed] [Google Scholar]
- 2.Miles C, Finocchiaro G, Papadakis M, Gray B, Westaby J, Ensam B. et al. Sudden death and left ventricular involvement in arrhythmogenic cardiomyopathy. Circulation 2019;139:1786–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall CL, Akhtar MM, Sabater-Molina M, Futema M, Asimaki A, Protonotarios A. et al. Filamin C variants are associated with a distinctive clinical and immunohistochemical arrhythmogenic cardiomyopathy phenotype. Int J Cardiol 2020;307:101–108. [DOI] [PubMed] [Google Scholar]
- 4.Verdonschot JAJ, Vanhoutte EK, Claes GRF, Helderman-van den Enden ATJM, Hoeijmakers JGJ, Hellebrekers DMEI. et al. A mutation update for the FLNC gene in myopathies and cardiomyopathies. Hum Mutat 2020;41:1091–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norman M, Simpson M, Mogensen J, Shaw A, Hughes S, Syrris P. et al. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 2005;112:636–642. [DOI] [PubMed] [Google Scholar]
- 6.Saguner AM, Buchmann B, Wyler D, Manka R, Gotschy A, Medeiros-Domingo A. et al. Arrhythmogenic left ventricular cardiomyopathy: suspected by cardiac magnetic resonance imaging, confirmed by identification of a novel plakophilin-2 variant. Circulation 2015;132:e38–e40. [DOI] [PubMed] [Google Scholar]
- 7.Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D. et al. Left-dominant arrhythmogenic cardiomyopathy. An under-recognized clinical entity. J Am Coll Cardiol 2008;52:2175–2187. [DOI] [PubMed] [Google Scholar]
- 8.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari MD. et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–114. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz-Genga MF, Cuenca S, Dal Ferro M, Zorio E, Salgado-Aranda R, Climent V. et al. Truncating FLNC mutations are associated with high-risk dilated and arrhythmogenic cardiomyopathies. J Am Coll Cardiol 2016;68:2440–2451. [DOI] [PubMed] [Google Scholar]
- 11.Dal Ferro M, Stolfo D, Altinier A, Gigli M, Perrieri M, Ramani F. et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart 2017;103:1704–1710. [DOI] [PubMed] [Google Scholar]
- 12.Ruwald A-C, Marcus F, Estes NAM, Link M, McNitt S, Polonsky B. et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2015;36:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H. et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.