Abstract
Background
Cardiac magnetic resonance (CMR) has a unique role in evaluating pericardial disease, permitting non-invasive tissue analysis, and haemodynamic assessment.
Case summary
In Case 1 of recurrent pericarditis, CMR confirmed reactivation of inflammation with late gadolinium enhancement and native T1/T2 mapping techniques, prompting therapeutic changes. In constrictive pericarditis, CMR is the only modality capable of differentiating a subacute potentially reversible form (Case 2), from a chronic, burnt out irreversible phase characterized by constrictive physiology (Case 3).
Discussion
Cardiac magnetic resonance is an effective tool to tailor individual therapy, particularly in cases of recurrent and constrictive pericarditis. Late gadolinium enhancement provides diagnostic and prognostic information, and multiparametric mapping has emerged as a promising tool with incremental diagnostic value.
Keywords: Cardiac magnetic resonance, Pericardial disease, Pericarditis, Constrictive pericarditis, Case series
Learning points
Cardiac magnetic resonance with late gadolinium enhancement and native T1/T2 mapping has the unique ability to provide a differential diagnosis in the whole spectrum of pericardial diseases;
Cardiac magnetic resonance provides incremental diagnostic and prognostic information and the possibility of individualizing therapy;
Cardiac magnetic resonance is particularly useful in cases of suspected recurrent pericarditis and constrictive pericarditis.
Introduction
Inflammatory diseases of the pericardium constitute a pathological spectrum ranging from acute pericarditis to chronic constrictive pericarditis (CP).1
The interaction between inflammation and haemodynamics is optimally characterized with multimodality non-invasive imaging [echocardiography, cardiac computed tomography (CCT), and cardiac magnetic resonance (CMR)].1
Cardiac magnetic resonance represents the most versatile modality due to high spatial resolution and image contrast devoid of ionizing radiation. Cardiac magnetic resonance allows tissue characterization, haemodynamic assessment, and provides prognostic information.1–3
We describe three clinical cases that illustrate the indispensable role of CMR in the evaluation of the broad spectrum of pericardial diseases.
Timeline
| Time | Progress |
|---|---|
| Case 1 | |
| Day 1 | Acute chest pain |
| Day 2 | Admission to the emergency department |
| Diffuse concave-upward ST-segment elevation in electrocardiography | |
| Blood analysis with elevated inflammatory and cardiac biomarkers | |
| Transthoracic echocardiography showed moderate pericardial effusion | |
| Thoracic radiography with mild pleural effusion | |
| Days 2–7 | Admission to the cardiology department |
| Started colchicine 0.5 mg/day and ibuprofen | |
| Regression of chest pain and discharge with colchicine and ibuprofen | |
| Day 9 | Fever and recurrence of chest pain |
| Day 10–30 | Hospital readmission |
| Thoracic radiography with moderate pleural effusion (increased) | |
| Transthoracic echocardiography showed mild pericardial effusion | |
| Investigation of auto-immune, auto-inflammatory, and bacterial infectious causes: negative | |
| Started prednisone 0.5 mg/kg/day | |
| Discharge with prednisone and colchicine | |
| Day 37 | Tapering 5 mg/day of prednisone each week |
| Month 3 | Stop prednisone |
| Month 3 | Recurrence of chest pain |
| Admission to the emergency department | |
| Transthoracic echocardiography without pericardial pleural effusion | |
| No alterations on electrocardiography | |
| Blood analysis with elevated inflammatory markers (Polymerase chain reaction 14.3 mg/dL, 12 700 leukocytes) and negative cardiac biomarkers | |
| Month 4 | Cardiac magnetic resonance (CMR) study showed myopericarditis: |
| Late gadolinium enhancement (LGE)+ | |
| T1 and T2 mapping + | |
| Restarted prednisolone | |
| Case 2 | |
| 10 years prior to the pericardial disease diagnosis | Prostate cancer, treated with surgery and hormonotherapy |
| Day 1 | Progressive worsening dyspnoea, peripheral oedema, and increased abdominal perimeter |
| Month 4 | Cardiology outpatient clinic first observation |
| Transthoracic echocardiography with pericardial thickening and respiratory septal shift | |
| Month 4 | CMR study: |
| LGE + | |
| T1 mapping + | |
| Pericardial thickness of 8 mm | |
| Constrictive physiology | |
| Computed tomography study: no pericardial calcification and severe pleural effusion | |
| Blood analysis: mild increased in C-reactive protein (4.3 mg/dL) with normal white blood cell count, haemoglobin 13.6 g/dL, hyponatraemia (129 mmol/L), and normal serum immunoelectrophoresis | |
| Started heart failure treatment, including diuretics, and aetiology investigation | |
| Month 5 | Submitted to thoracentesis—pleural effusion analysis compatible with neoplastic origin |
| Case 3 | |
| Day 1 | First hospitalization for heart failure and atrial flutter |
| Transthoracic echocardiography with preserved left ventricle ejection fraction, diastolic dysfunction, and biatrial enlargement | |
| Until Month 24 | New York Heart Association (NYHA) classification I, under treatment with diuretics |
| Month 25 | Worsening dyspnoea (NYHA II–III), peripheral oedema, and ascites |
| Cardiology outpatient clinic observation and started aetiology investigation | |
| Blood analysis without increased inflammatory markers (C-reactive protein 0.3 mg/dL) nor cardiac biomarkers | |
| Month 26 | Transthoracic echocardiography and cardiac catheterization with signs of pericardial constriction |
| Month 29 | Bronchoscopy: bronchoalveolar lavage fluid analysis excluded infectious pulmonary disease |
| Month 30 | CMR study: |
| Pericardial thickness of 8 mm | |
| Constrictive physiology | |
| Pericardial adherence | |
| Month 36 | Partial pericardiectomy |
| Month 37 | 2nd CMR study: No constrictive physiology |
| Regression of symptoms |
Case reports
Case 1
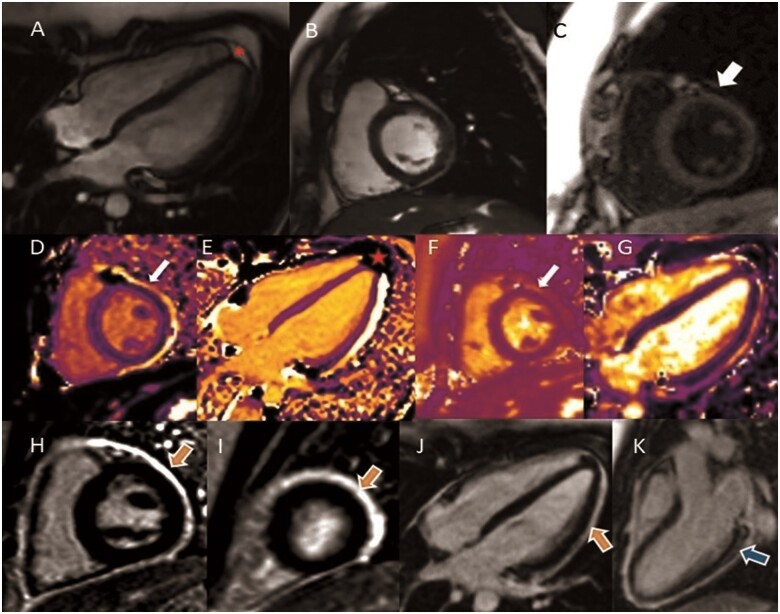
A 23-year-old female, with no relevant past medical history except Polycystic Ovary Syndrome, previously hospitalized with polyserositis (pericardial and pleural effusions) and treated with colchicine and prednisolone had recurrent chest pain after tapering and discontinuing corticosteroid therapy. While the physical examination, transthoracic echocardiogram and electrocardiography showed no abnormalities, a CMR study (Figure 1) confirmed recurrent pericarditis and concomitant myocardial involvement (myopericarditis) with preserved left ventricle ejection fraction (69%). Cardiac magnetic resonance showed increased pericardial thickness (maximum 2.5 mm) with marked pericardial late gadolinium enhancement (LGE). As it may be hard to differentiate between pericardial LGE and fat in this sequence, native T1 mapping distinguishes pericardial inflammation (high signal—bright image) from fat (low signal—dark image)—Figure 1D,E. Myocardial involvement was demonstrated by the increased T2 signal [52 ms—normal value 47.69 (4.28) ms, Figure 1G] and LGE with a subepicardial pattern in the basal segment of left ventricular inferolateral wall. Given these results which were compatible with acute inflammation, the patient was commenced on immunosuppressive therapy (prednisolone 15 mg/day). A comprehensive aetiological study was carried out, which excluded bacterial infections, namely tuberculosis and zoonotic pathogens. A viral aetiology was suspected, although viral serology and molecular assays did not show any acute viral infection. While spontaneous ovarian hyperstimulation can be associated with polyserositis, thyroid function and hormonal investigations were in the normal range. The initial autoimmunity panels were negative and the patient had no other concomitant symptoms (such as arthralgias). The patient is being followed up in the Rheumatology department and autoimmunity panels remain unchanged and progressive weaning from corticosteroid therapy was initiated without recurrence of symptoms.
Figure 1.
Steady-state free precession (SSFP) sequence in long-axis (A) and short-axis (B). (C) Black blood T1 weighted (double inversion recovery) showing maximum pericardium thickness of 2.5 mm (arrow). Native T1 mapping [980 ms—normal value 990.66 (17.5) ms] in short-axis (D) and long-axis (E), contributing to differentiate between fat (red star) from fluid/inflammation (bright/arrow). (F) Native T2 mapping sequence showing pericardial inflammation (arrow). (G) Increased myocardium native T2 mapping [52 ms—normal value 47.69 (4.28) ms], suggestive of concomitant myocardial oedema. (H–K) Late gadolinium enhancement (LGE) sequence in short-axis (median and apical slices) and long-axis views showing hyperenhancement in the pericardium (orange arrows) and in myocardium (subepicardial pattern in the basal infero-lateral wall—blue arrow).
Case 2
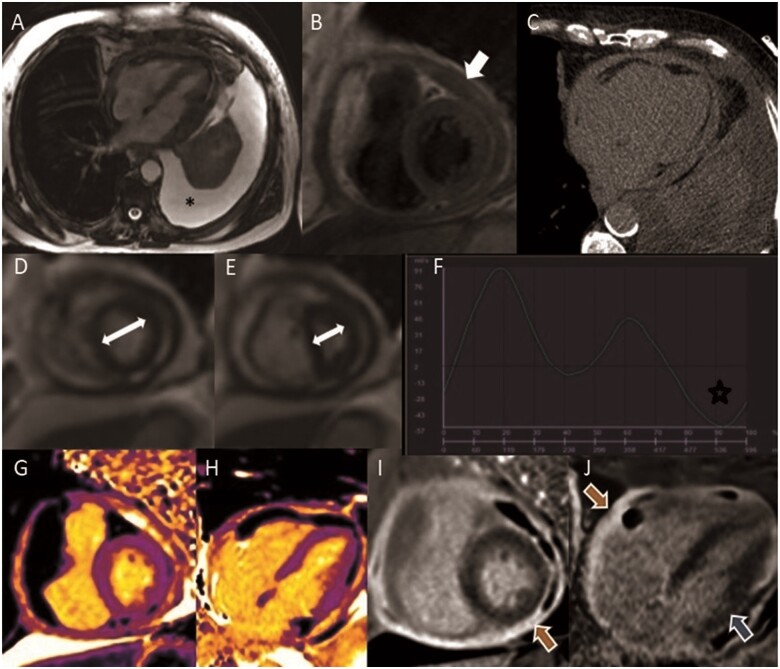
A 75-year-old-man with a diagnosis of heart failure with preserved ejection fraction, with medical history of multiple cardiovascular risk factors (type 2 diabetes mellitus, arterial hypertension, smoking, and dyslipidaemia), chronic obstructive pulmonary disease, and prostate cancer (treated with local surgery and hormone therapy), was referred for a CMR study due to worsening dyspnoea, peripheral oedema, pleural effusion, ascites, and evidence of pericardial thickening on echocardiography. Cardiac magnetic resonance confirmed a marked increase in pericardial thickness (8 mm) (Figure 2), signs of constrictive physiology (respirophasic septal shift in real-time cine imaging—Video 1, dilatation of inferior vena cava with late reverse diastolic flow in with phase-contrast study) with ongoing inflammation (severe pericardial LGE and elevated native T1 signal in the pericardium). The study was complemented with a CT scan that excluded pericardial calcification. The analysis of the pleural fluid was compatible with neoplastic aetiology. The patient died for a non-cardiac cause before the conclusion of all investigations.
Figure 2.
Cardiac magnetic resonance study of Case 2 constrictive pericarditis. (A) Steady-state free precession sequence, noticing the large left pleural effusion (*). (B) Black blood T1 weighted Turbo Spin Echo sequences showing circumferential thickening of the pericardium (arrow). (C) Computed tomography scan excluded calcification of the pericardium. (D, E) Real-time cine imaging functional assessment of ventricular inter-dependence (D) normal septal position during expiration and (E) marked leftward septal shift seen immediately after deep inspiration (white arrows demonstrate the relative change in cavity size due to the septal shift). (F) Phase contrast study showing reverse diastolic flow in inferior vena cava (star). (G and H) Native T1 mapping with high native T1 values in the pericardium. (I and J) Late gadolinium enhancement sequence in short-axis view and four-chamber view with severe hyperenhancement in the pericardium (orange arrow) and in the myocardium (subendocardial pattern in the basal inferolateral wall—blue arrow).
Case 3
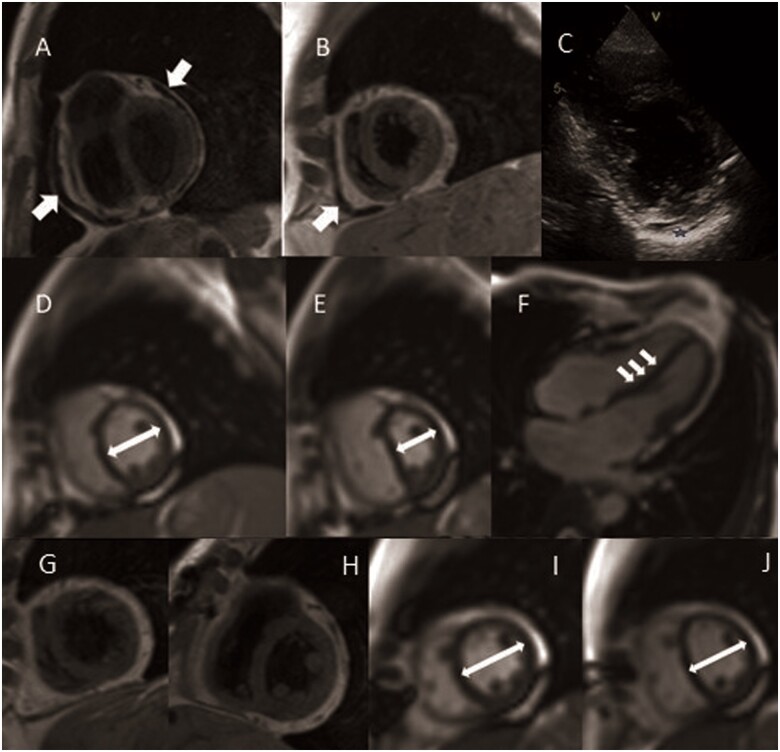
A 59-year-old-female with a previous history of acute decompensated heart failure in the context of atrial tachycardia, 2 years ago, was admitted due to progressive exertional dyspnoea [New York Heart Association (NYHA) Class III]. At physical examination, the patient had elevated jugular venous pressure and peripheral oedema and a transthoracic echocardiography and subsequent cardiac catheterization was diagnostic of constrictive pericarditis (CP). A CMR study (Figure 3) confirmed a chronic form of CP without myocardial involvement: thickened pericardium (8 mm-anterior; 6 mm-lateral), biatrial enlargement (left atrium> right atrium), constrictive physiology (‘septal bounce’, respirophasic septal shift—Video 2) and pericardial adherence (lack of slippage between the parietal and visceral pericardium in tissue-tagging sequences—Video 3). After partial pericardiectomy, the patient’s functional class improved to NYHA Class I and CMR demonstrated reduction in pericardial thickness and, by real time cine imaging, significant improvement in constrictive physiology (Figure 3J–M). The aetiology remains idiopathic, since autoimmune and infectious causes (including analysis of bronchoalveolar lavage fluid acquired by bronchoscopy) were excluded. The histopathological analysis of the pericardium reported characteristics of a chronic, non-inflammatory, constrictive phase (dense fibrosis and calcification).
Figure 3.
Cardiac magnetic resonance study of Case 3—(A–I) Chronic pericardial constriction, previous to pericardiectomy. Black blood T1 weighted Turbo Spin Echo showing thickening of the pericardium (A, B). (C) Echocardiography, in parasternal short axis view, showing bright and thickened pericardium (blue star). (D–F) Functional assessment of ventricular inter-dependence (short axis in midcavity and long-axis view): (D) normal septal position during expiration, (E) marked leftward septal shift after deep inspiratory effort (white arrows demonstrate the relative change in cavity size due to the septal shift); (F) evidence of septal bounce in long-axis four-chamber views. (G–J) Cardiac magnetic resonance study after pericardiectomy. (G and H) Black blood T1 weighted turbo spin echo confirmed successful partial pericardiectomy. Real-time free breathing: end-expiratory (I) and end-inspiratory (J) septal position, revealing almost completely resolution of constriction signs.
Discussion
Although transthoracic echocardiography is the first-line imaging modality in pericardial diseases4 its inability to characterize tissue is a limitation.1
In Case 1, CMR confirmed reactivation of pericarditis with techniques of LGE and mapping. LGE is highly sensitive for identifying inflammation and neovascularization of the pericardium.1,5 In patients with recurrent pericarditis, quantification of LGE provides incremental diagnostic value to conventional clinical criteria, predicting recurrence at 6 months.6 Furthermore, treatment tailored by CMR findings results in quicker remission and less steroid therapy.7
Inflammation may also be depicted by multiparametric mapping. High values of native T1 and T2 mapping (bright image) suggest pericardial oedema/inflammation. The comparison of mapping signal through consecutive CMR during patient’s follow-up allows the evaluation of treatment response and relapse, without the use of gadolinium-containing contrasts.4,5 Native T1 has also the potential of detailed tissue characterization, allowing the distinction between pericardial enhancement and pericardial fat (both positive in LGE sequences).3
The diagnosis of CP is often challenging, and CMR may constitute the modality of choice, since transthoracic echocardiography cannot reliably visualize the pericardium unless there is marked thickness (>5 mm).1 Cardiac magnetic resonance allows the measurement of the thickness of the entire pericardium and the evaluation of the extent of pericardial involvement.1,5 Although pericardial thickness >5–6 mm is highly specific (∼100%) for constriction, up to 18% of patients with histologically proven CP have pericardial thickness <2 mm.8 Thickening is usually most pronounced over the right heart (right ventricle and anterior atrioventricular groove), as seen in Case 3.8
Cardiac magnetic resonance also contributes to the physiological assessment by documenting ventricular interdependence, using real time free-breathing sequences in short axis views where a septal bounce can be detected on deep inspiration.5 Cardiac magnetic resonance better differentiates small effusions from pericardial thickening and has higher temporal resolution than CT, enabling detection of rapid haemodynamic processes that characterize constrictive physiology.1 In addition, phase-contrast acquisition in caval veins demonstrates constrictive physiology with diminished or absent forward, or even reversed systolic flow, and increased early diastolic forward flow and late backward flow.8
Tissue tagging facilitates the identification of patients with CP by defining visceral–parietal adherence patterns as the failure for orthogonally aligned tagged stripes to ‘slide’ past each other.9
The versatility of CMR permits staging the pericardial disease. The demonstration of oedema and/or inflammation on T2 images and LGE sequences points towards an acute inflammatory process. The absence of high signal in T2 images suggests a healing process, without active inflammation. If pericardial enhancement remains in LGE acquisition, without increases T2 signal, a subacute process should be taken into consideration. In the late stage, when fibrotic component predominates, neither T2 or LGE show increase signal (Table 1).1
Table 1.
Contribution of cardiac magnetic resonance in the classification of constrictive pericarditis in acute, subacute, or chronic
| Acute | Subacute | Chronic | |
|---|---|---|---|
| T2 | + | − | − |
| LGE | + | + | − |
LGE, late gadolinium enhancement.
A case series with CMR and histopathologic correlation in CP showed pericardial LGE correlates with greater fibroblastic proliferation, chronic inflammation, and neovascularization, whereas patients without pericardial LGE had more pericardial fibrosis and calcification, and lesser degrees of pericardial thickening.2 Reversible CP was associated with pericardial and systemic inflammation, with reduction in pericardial LGE with anti-inflammatory therapy, concomitant with resolution of CP physiology and symptoms.10
Finally, CMR can contribute to pre-operative planning, as seen in Case 3.4
Conclusion
The unique capability of CMR to combine the evaluation of anatomy, haemodynamic and inflammatory status, provides superior diagnostic information. These inflammatory syndromes are not mutually exclusive, but rather a continuum in the phenotypes in a wide spectrum of pericardial diseases, sometimes overlapping or transitioning from one phase to the other. Cardiac magnetic resonance is an effective tool to tailor individual therapy, particularly in cases of recurrent and constrictive pericarditis, given that the presence of ongoing inflammation has been found to be the best predictor of reversibility.
Lead author biography

Tânia Branco Mano is cardiology Internship in Santa Marta Hospital of Centro Hospitalar Universitário de Lisboa Central.
Supplementary material
Supplementary material is available at European Heart Journal—Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of these case reports including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
References
- 1. Chetrit M, Xu B, Kwon D, Ramchand J, Rodriguez RE, Tan CD. et al. Imaging-guided therapies for pericardial diseases. JACC Cardiovasc Imaging 2020;13:1422–1437. [DOI] [PubMed] [Google Scholar]
- 2. Zurick A, Bolen M, Kwon D, Tan CD, Popovic ZB, Rajeswaran J. et al. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy - a case series with histopathological correlation. J Am Coll 2011;4:1180–1191. [DOI] [PubMed] [Google Scholar]
- 3. Vidalakis E, Kolentinis M, Gawor M, Vasquez M, Nagel E.. CMR in pericardial diseases - an update. Curr Cardiovasc Imaging Rep 2020;13:14. [Google Scholar]
- 4. Cosyns B, Plein S, Nihoyanopoulos P, Smiseth O, Achenbach S, Andrade MJ, et al. ; European Society of Cardiology Working Group (ESC WG) on Myocardial and Pericardial diseases. European Association of Cardiovascular Imaging (EACVI) position paper: multimodality imaging in pericardial disease. Eur Heart J Cardiovasc Imaging 2015;16:12–31. [DOI] [PubMed] [Google Scholar]
- 5. Hassan O, Kwon D.. Update on MRI techniques for evaluation of pericardial disease. Curr Cardiol Rep 2020;22:147. [DOI] [PubMed] [Google Scholar]
- 6. Kumar A, Sato K, Verma B, Ala CK, Betancor J, Yzeiraj E. et al. Quantitative assessment of pericardial delayed hyperenhancement help identify patients with ongoing recurrences of pericarditis. Open Heart 2018;5:e000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alraies M, AlJaroudi W, Yarmohammadi H, Yingchoncharoen T, Schuster A, Senapati A. et al. Usefulness of cardiac magnetic resonance guided management in patients with recurrent pericarditis. Am J Cardiol 2015;115:542–e547. [DOI] [PubMed] [Google Scholar]
- 8. Grizzard J. Magnetic resonance imaging of pericardial disease and intracardiac thrombus. Heart Fail Clin 2009;5: 401–419. [DOI] [PubMed] [Google Scholar]
- 9. Power J, Thompson D, Rayarao G, Doyle M, Biederman RWW.. Cardiac magnetic resonance radiofrequency tissue tagging for diagnosis of constrictive pericarditis: a proof of concept study. J Thorac Cardiovasc Surg 2016;151:1348–1355. [DOI] [PubMed] [Google Scholar]
- 10. Feng D, Glockner J, Kim K, Martinez M, Syed IS, Araoz P. et al. Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy - a pilot study. Circulation 2011;124:1830–1837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.