Abstract
Aims
Heart failure (HF) is an ongoing epidemic and a serious clinical and public health issue. Currently, little is known about prospective associations between insomnia symptoms and HF incidence. We investigated the longitudinal associations between time-varying insomnia symptoms (difficulty initiating sleep, difficulty maintaining sleep, early-morning awakening, non-restorative sleep) and incident HF.
Methods and results
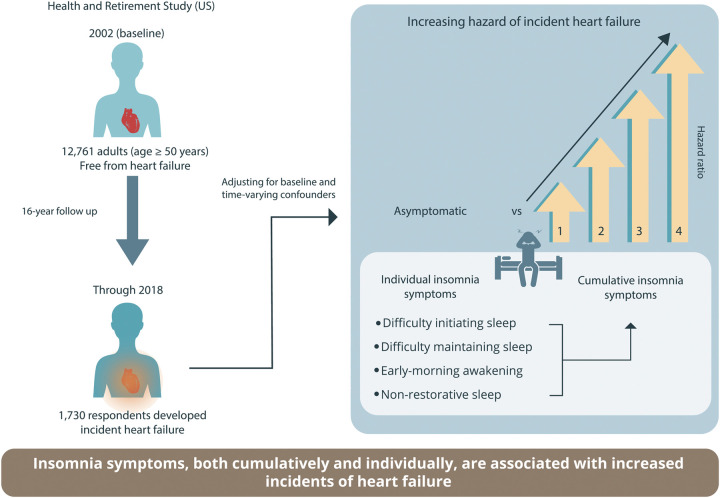
Data were obtained from the Health and Retirement Study in the US for a population-representative sample of 12,761 middle-aged and older adults (age ≥ 50 years; mean [SD] age, 66.7 [9.4] years; 57.7% females) who were free from HF at baseline in 2002. Respondents were followed for 16 years for incident HF. We employed marginal structural discrete-time survival analyses to adjust for potential time-varying biological, psycho-cognitive, and behavioral factors and to account for bias due to differential loss to follow-up. At baseline, 38.4% of the respondents reported experiencing at least one insomnia symptom. During the 16-year follow-up, 1,730 respondents developed incident HF. Respondents experiencing one (hazard ratio [HR]=1.22; 95% CI: 1.08–1.38), two (HR=1.45; 95% CI: 1.21–1.72), three (HR=1.66; 95% CI: 1.37–2.02), or four (HR=1.80; 95% CI: 1.25–2.59) insomnia symptoms had a higher hazard of incident HF than asymptomatic respondents. Respondents that had trouble initiating sleep (HR=1.17; 95%CI: 1.01–1.36), maintaining sleep (HR=1.14; 95% CI: 1.01–1.28), early-morning awakening (HR=1.20; 95% CI: 1.02–1.43), or non-restorative sleep (HR=1.25; 95% CI: 1.06–1.46) had a higher hazard of incident HF than asymptomatic respondents.
Conclusion
Insomnia symptoms, both cumulatively and individually, are associated with incident HF. Public health awareness and screening for insomnia symptoms in at-risk populations should be encouraged to reduce HF incidence.
Keywords: Heart failure, Insomnia symptoms, Sleep disturbance, Risk factor, Marginal structural models
Graphical Abstract
 Listen to the audio abstract of this contribution
Listen to the audio abstract of this contribution
See page 4177 for the editorial comment for this article ‘Insomnia, a new modifiable risk factor for heart failure?’, by M. Berger, G. Solelhac, F. Roche, and R. Heinzer, https://doi.org/10.1093/eurheartj/ehab570.
Introduction
Heart failure (HF) is a serious clinical and public health issue.1–3 In the USA, about 6.2 million adults are living with HF.3 Among middle-aged and older adults, HF is a leading cause of emergency department visits,4 hospital admissions, readmissions3,5 and mortality,1 and its economic costs are approximately $32 billion annually.6 Despite advances in HF treatment and an increased understanding of its risk factors, prognosis and patients’ quality of life remain poor.1 Notably, about 70% of all cardiovascular morbidities and mortalities are attributed to modifiable risk factors.7 Healthy lifestyle habits, such as maintaining a normal body weight, quitting or not smoking, engaging in regular exercise, moderate or no alcohol intake, and maintaining a healthy diet, reduce the lifetime risk of HF,8 and maintaining healthy habits positively influences or prevents the occurrence of other major risk factors for HF, including coronary heart disease,9–11 hypertension,11 obesity, and diabetes.11,12 Thus, identifying and targeting these modifiable factors could reduce HF incidence and HF-related morbidity and improve the overall quality of life.13,14
Insomnia symptoms, such as difficulty initiating sleep, difficulty maintaining sleep, early morning awakening, and non-restorative sleep, are prevalent among middle-aged and older adults,15–17 and up to 50% of middle-aged and up to 75% of older adults report experiencing at least one insomnia symptom annually.16,17 Individuals with insomnia symptoms have a 41–55% higher risk of myocardial infarction, stroke, coronary heart disease, and cerebrovascular disease, and they are more likely to experience cardiovascular-related mortality.18–20 Therefore, an association between insomnia symptoms and HF is plausible owing to their common underlying mechanisms and pathophysiology.21 However, these links remain unclear, despite the extensive review of HF risk factors.1,22–24 Specifically, little is known about the effects of insomnia symptoms on future risk of HF among individuals without HF at baseline.
We used longitudinal data from a large population-based cohort in the USA to investigate the direct associations between insomnia symptoms and incident HF while strictly controlling for confounding factors and selection bias. We hypothesized that respondents with more insomnia symptoms would have a higher incidence of HF, and experiencing each insomnia symptom individually (i.e. difficulty initiating or maintaining sleep, early morning awakenings, or non-restorative sleep) would be associated with a higher incidence of HF, compared to that in asymptomatic respondents.
Methods
Data source and study population
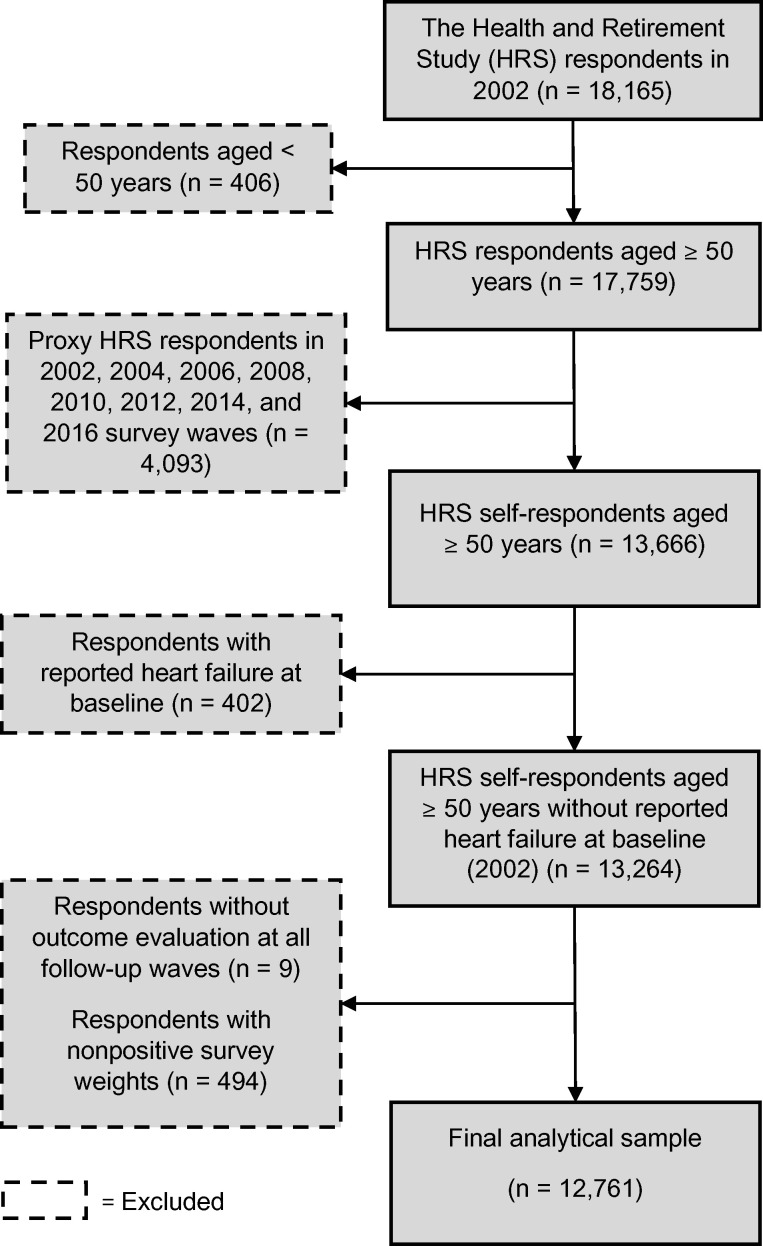
We obtained data from the 2002 through the 2018 waves of the Health and Retirement Study (HRS), which is an ongoing longitudinal, biennial, nationally representative survey on health, employment, income, and family structure of individuals aged ≥50 years and their spouses, regardless of age in the USA.25 The ongoing survey is conducted by the Institute for Social Research, and its protocol was approved by the Institutional Review Board at the University of Michigan.25 Before each wave’s new interviews or re-interviews, verbal informed consent was obtained from all participants.26 Details of the survey methodology and documentation are presented elsewhere.25,27,28 The 2002 survey wave was selected as the baseline for the current study because it was the first wave to include insomnia symptoms questions. Only self-respondents without HF at baseline were included. Proxy respondents (e.g. participants’ family members or other next-of-kin) were excluded from all waves, since they usually do not answer the cognitive and depressive symptoms assessment questions for the participants. Figure 1 depicts the inclusion and exclusion flowchart for the study participants. Respondents were censored at a fatal or first non-fatal incident HF, loss to follow-up or dropout, non-HF death, or the end of the 2018 survey wave, whichever came first. This study followed the reporting guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology.
Figure 1.
Inclusion and exclusion flowchart for the study participants. HRS, Health and Retirement Study.
Heart failure
Heart failure was defined as a fatal or first non-fatal HF incident during follow-up. The HF status was ascertained through respondents’ self-report of a physician’s diagnosis at each follow-up wave or from proxy informants’ reports of a physician’s diagnosis during post-mortem exit interviews. For details regarding the survey question and HF case ascertainment, see Supplementary material online, Methods S1.
Insomnia symptoms
Questions about insomnia symptoms assessed difficulties in initiating and maintaining sleep, early morning awakening, and non-restorative sleep. Respondents were asked how often they have trouble with ‘falling asleep’, ‘waking up during the night’, and ‘waking up too early and not being able to fall asleep again’, and how often they feel ‘really rested’ when they wake up in the morning. The response options for each question include ‘most of the time’, ‘sometimes’, and ‘rarely or never’. We defined individuals as experiencing insomnia symptoms when they answered, ‘most of the time’ in the first three questions and answered, ‘rarely or never’ in the last question, as described by prior studies.29–33 Each insomnia symptom status was then represented with a binary variable, ‘yes vs. no’, in the analyses. To examine the quantity of insomnia symptoms, we categorized respondents based on the number of reported symptoms. This was performed for each wave by summing across the four symptoms and categorizing the respondents into those experiencing no symptoms or one, two, three, or all four symptoms. Measures of insomnia symptoms were time-varying; however, in survey waves 2008 and 2012, three of the four insomnia symptoms' questions were not asked; hence, we carried forward the respondents' last observation for those symptoms. To address the issue of temporal sequence of events and make the measures of time-varying insomnia symptoms and confounders precede incident HF at each wave,34 we used insomnia symptoms and covariate measurements of waves 2002 through 2016 and subsequent evaluations for incident HF in waves 2004 through 2018.
Covariate measures
Potential confounders of the associations between insomnia symptoms and HF were referred from the extant literature.3,7,23,35–37 A set of sociodemographic covariates were incorporated as time-invariant ‘baseline’ characteristics, including respondents' self-reported sex, race and ethnicity, educational attainment, marital status, and poverty level. The time-varying characteristics included a series of socioeconomic, biological, functional, psychological, cognitive, psychophysiological, and behavioural factors. Socioeconomic factors included whether the respondent was working for pay. Biological factors included age in years, and body mass index (BMI), calculated as weight in kilograms divided by height in metres squared. Other biological factors included self-report of doctor's diagnosis of hypertension, diabetes, cancer (excluding skin cancers), chronic lung disease, and arthritis; each was dichotomized and used as a binary variable.
Functional limitation variables were limitations in any of the five activities of daily living (dressing, bathing, eating, getting in/out of bed, and walking across a room) and five instrumental activities of daily living (managing money, shopping for groceries, taking medications, preparing meals, and using phone). Psychological and cognitive measures were the modified Center for Epidemiologic Studies Depression scale (CES-D) score and cognitive functioning score; both were evaluated using a continuous scale. These measures, which have been proven valid and reliable for assessing depression and cognition,38–42 were retained in our analyses because they have demonstrated significant correlations with insomnia symptoms or HF.36,43–45 The modified CES-D scale in the HRS is the summation of eight items; however, we removed one item (which evaluates ‘restless sleep’) from the score to minimize operational confounding46,47 of the CES-D scale with outcomes. The cognitive functioning score was assessed through a range of tests adopted from the modified Telephone Interview for Cognitive Status.38,39 A higher CES-D score indicates higher depressive symptomatology, and a higher cognitive score represents better cognitive performance for respondents. Detailed descriptions of these two measures are provided elsewhere.27,38,40,48 Moreover, we incorporated one psychophysiological element—pain—which was assessed if the respondent was frequently troubled with pain. Finally, time-varying health behaviour variables included smoking status, drinking alcohol, and engaging in vigorous physical activity.
Statistical analysis
We first analysed the time-invariant and time-varying general characteristics of the respondents at baseline. The associations between respondent characteristics at baseline and incident HF status during the 16-year follow-up were then calculated. Longitudinal associations between the number and type of insomnia symptoms and incident HF were analysed using a marginal structural model (MSM) approach.49–51 Marginal structural models developed by Robins et al. are a class of statistical models used for causal inferencing in epidemiology.49–52 Since measures of insomnia symptoms and covariates in our study were time-dependent, insomnia symptoms might influence their subsequent values and the covariates' values during the follow-up, and time-varying covariates might simultaneously become confounders and intermediate factors in the association analysis. Furthermore, attrition in longitudinal studies could induce selection bias if there are systematic differences between subjects that remain in the study until it concludes and those who are lost to follow-up.50 In these circumstances, standard regression methods fail to adjust for time-varying confounding and selection bias appropriately.50,51 Thus, we adopted an MSM approach to minimize or avoid the influence of confounding factors and selection bias (MSM notation in Supplementary material online, Methods S2).
Two inverse probability weights were calculated to perform an MSM analysis: the inverse probability of treatment weight (IPTW) to account for time-varying confounding and the inverse probability of censoring weight (IPCW) to account for differential loss to follow-up due to study withdrawal, loss to follow-up, or non-HF deaths. Final MSM weights were derived from multiplying IPTWs by IPCWs and incorporated both time-dependent confounding and censoring adjustments. Final weights were truncated at the 1st and 99th percentiles to have a mean weight of 1.00 [standard deviation (SD) = 0.30; minimum = 0.67; maximum = 2.55]. More detailed information on calculations of IPTWs and IPCWs is provided in Supplementary material online, Methods S3.
The final MSM weights were then applied to a discrete-time hazard model with a complementary log-log link using the pooled data (77 107 person-waves) to generate the hazard ratios (HRs) and 95% confidence intervals (CIs) for HF. The model predicts the average causal effects of the number of insomnia symptoms on incident HF. In this instance, since the measures are recorded in discrete times, employing a complementary log-log link will generate HRs that resemble those obtained from a continuous-time Cox proportional hazards model.34,53 The proportionality assumption was tested by an interaction term between the cumulative number of insomnia symptoms variable and the time indicator, and there were no violations of the proportionality assumption. A similar marginal structural discrete-time hazard model was fit to investigate the associations between each independent insomnia symptom and incident HF. Both models were only adjusted for the time-invariant, baseline variables because the effects of time-varying variables were incorporated into the final MSM weights.54 Usually, analysing pooled data may bring up a concern related to dependence within the clusters (i.e. respondents); however, it was not an issue in our models since the respondents were censored at the initial incident HF.53 Several sensitivity analyses were conducted to test the validity of the weights and robustness of the results (Supplementary material online, Methods S4). The analyses were conducted using SAS 9.4 statistical software (SAS Institute Inc., Cary, NC, USA). All P-values were two-tailed, and the significance threshold was set at ≤0.05.
Results
The final analytical sample included 12 761 respondents aged ≥50 years [mean (SD) age, 66.7 (9.4) years] without HF at baseline. Table 1 shows respondent characteristics at baseline. About 38.4% of the respondents reported having at least one insomnia symptom, and 11.5%, 25.0%, 11.5%, and 13.2% of respondents reported having difficulty initiating sleep, difficulty maintaining sleep, early morning awakening, and non-restorative sleep, respectively.
Table 1.
Respondents’ characteristics at baseline (n = 12 761)
| Respondent characteristics | Frequency (weighted %)a |
|---|---|
| Time-invariant characteristics | |
| Female sex | 7592 (57.7) |
| Race and ethnicity | |
| Non-Hispanic White | 9986 (83.8) |
| Non-Hispanic Black | 1628 (8.3) |
| Hispanic | 900 (5.7) |
| Non-Hispanic other | 242 (2.2) |
| Level of education | |
| Less than high school or GED | 3329 (22.9) |
| High-school graduate | 4210 (32.6) |
| Some college | 2674 (22.0) |
| College and above | 2546 (22.5) |
| Marital status | |
| Married or living as married | 8281 (63.7) |
| Otherb | 4469 (36.3) |
| Whether in poverty | |
| Household income above poverty threshold | 11 757 (92.6) |
| Household income below poverty threshold | 1004 (7.4) |
| Time-varying characteristics | |
| Number of insomnia symptoms | |
| No symptoms | 7862 (61.6) |
| One | 3025 (23.4) |
| Two | 1094 (8.8) |
| Three | 565 (4.4) |
| Four | 213 (1.8) |
| Type of insomnia symptomc | |
| Difficulty initiating sleep | 1484 (11.5) |
| Difficulty maintaining sleep | 3154 (25.0) |
| Early morning awakening | 1491 (11.5) |
| Non-restorative sleep | 1632 (13.2) |
| Working for pay | |
| Yes | 4455 (40.2) |
| Age (years), mean (SD) (range: 50–109) | 66.7 (9.4) |
| BMI (kg/m2) | |
| Normal or underweight (<25) | 4377 (34.8) |
| Overweight (25–29.9) | 4917 (39.2) |
| Obese (≥30) | 3264 (26.0) |
| Hypertension | 6722 (50.3) |
| Diabetes | 2131 (15.4) |
| Cancer (excluding skin cancers) | 1751 (13.2) |
| Chronic lung disease | 1216 (9.3) |
| Arthritis | 7746 (58.1) |
| ADL limitations | 1751 (12.9) |
| IADL limitations | 1340 (10.0) |
| CES-D scale score, median (IQR) (range: 0–7)d | 0.0 (0.0–1.25) |
| Cognitive functioning score, mean (SD) (range: 0–27)e | 16.1 (4.4) |
| Often troubled with pain | 3649 (28.8) |
| Smoking status | |
| Current | 1791 (14.7) |
| Former | 5728 (45.1) |
| Never | 5149 (40.2) |
| Alcohol consumption | 6121 (51.1) |
| Vigorous physical activityf | 5474 (44.2) |
The data were obtained from the Health and Retirement Study (age of respondents ≥50 years, USA, 2002).
ADL, activities of daily living; BMI, body mass index (calculated as weight in kilograms divided by height in metres squared); CES-D, Center for Epidemiologic Studies Depression scale; GED, general educational development; IADL, instrumental activities of daily living; IQR, interquartile range; SD, standard deviation.
Weighted N = 48.2 million adults (age ≥50 years) at the US national level, estimated through adjustments for HRS complex survey design features.
Other marital statuses include separated, divorced, widowed, and never married.
Only affirmative responses are reported to maintain table brevity.
Higher scores indicate worse depression symptoms.
Higher scores indicate better cognitive performance.
At least 1–3 times per month.
Table 2 illustrates the baseline characteristics of the respondents who had or did not have an incident HF during follow-up. During the 16-year follow-up, 1730 respondents (12.7% at the US national level) had incident HF.
Table 2.
Heart failure incidents during follow-up and their associations with respondents’ characteristics at baseline in 2002
| Respondent characteristics | Frequency (weighted %) |
|
|---|---|---|
| Heart failure during follow-up (n = 1730) | No heart failure during follow-up (n = 11 031) | |
| Time-invariant characteristics | ||
| Female sex | 966 (55.7) | 6626 (58.0) |
| Race and ethnicity | ||
| Non-Hispanic White | 1393 (85.5) | 8593 (83.6) |
| Non-Hispanic Black | 212 (8.4) | 1416 (8.3) |
| Hispanic | 91 (4.3) | 809 (5.9) |
| Non-Hispanic other | 32 (1.8) | 210 (2.2) |
| Level of education | ||
| Less than high school or GED | 580 (31.3) | 2749 (21.7) |
| High-school graduate | 568 (32.2) | 3642 (32.6) |
| Some college | 338 (21.1) | 2336 (22.1) |
| College and above | 244 (15.4) | 2302 (23.6) |
| Marital status | ||
| Married or living as married | 985 (54.1) | 7296 (65.1) |
| Othera | 743 (45.9) | 3726 (34.9) |
| Whether in poverty | ||
| Household income above the poverty threshold | 1553 (90.3) | 10 204 (93.0) |
| Household income below the poverty threshold | 177 (9.7) | 827 (7.0) |
| Time-varying characteristics | ||
| Number of insomnia symptoms | ||
| No symptoms | 935 (54.8) | 6927 (62.6) |
| One | 460 (25.3) | 2565 (23.2) |
| Two | 185 (11.0) | 909 (8.5) |
| Three | 111 (6.4) | 454 (4.1) |
| Four | 39 (2.5) | 174 (1.6) |
| Type of insomnia symptomb | ||
| Difficulty initiating sleep | 270 (16.0) | 1214 (10.8) |
| Difficulty maintaining sleep | 525 (30.1) | 2629 (24.2) |
| Early morning awakening | 258 (14.5) | 1233 (11.1) |
| Non-restorative sleep | 266 (15.5) | 1366 (12.9) |
| Working for pay | 387 (24.4) | 4068 (42.6) |
| Age (years), mean (SD) | 71.0 (9.7) | 66.1 (9.1) |
| BMI (kg/m2) | ||
| Normal or underweight (<25) | 540 (31.9) | 3837 (35.2) |
| Overweight (25–29.9) | 635 (37.4) | 4282 (39.5) |
| Obese (≥30) | 530 (30.7) | 2734 (25.3) |
| Hypertension | 1187 (68.4) | 5535 (47.7) |
| Diabetes | 495 (26.8) | 1636 (13.7) |
| Cancer (excluding skin cancers) | 295 (16.8) | 1456 (12.7) |
| Chronic lung disease | 283 (16.4) | 933 (8.2) |
| Arthritis | 1215 (69.6) | 6531 (56.4) |
| ADL limitations | 397 (22.8) | 1354 (11.4) |
| IADL limitations | 330 (18.6) | 1010 (8.8) |
| CES-D scale score, median (IQR)c | 0.37 (0.0–1.84) | 0.0 (0.0–1.11) |
| Cognitive functioning score, mean (SD)d | 14.9 (4.4) | 16.3 (4.3) |
| Often troubled with pain | 668 (38.6) | 2981 (27.4) |
| Smoking status | ||
| Current | 234 (14.5) | 1557 (14.7) |
| Former | 876 (50.5) | 4852 (44.4) |
| Never | 605 (35.0) | 4544 (40.9) |
| Alcohol consumption | 646 (39.0) | 5475 (52.9) |
| Vigorous physical activitye | 601 (35.6) | 4873 (45.4) |
The data were obtained from the Health and Retirement Study (n = 12 761, USA, 2002–2018).
ADL, activities of daily living; BMI, body mass index (calculated as weight in kilograms divided by height in metres squared); CES-D, Center for Epidemiologic Studies Depression scale; GED, general educational development; IADL, instrumental activities of daily living; IQR, interquartile range; SD, standard deviation.
Other marital statuses include separated, divorced, widowed, and never married.
Only affirmative responses are reported to maintain table brevity.
Higher scores indicate worse depression symptoms.
Higher scores indicate better cognitive performance.
At least 1–3 times per month.
Table 3 shows the results of the discrete-time survival MSMs. Respondents experiencing one (HR: 1.22; 95% CI: 1.08–1.38; P = 0.0012), two (HR: 1.45; 95% CI: 1.21–1.72; P < 0.001), three (HR: 1.66;95% CI: 1.37–2.02; P < 0.001), or four (HR: 1.80; 95% CI: 1.25–2.59; P = 0.0011) insomnia symptoms had a higher hazard of incident HF than those not experiencing any insomnia symptoms. For each symptom individually, experiencing difficulty initiating sleep (HR: 1.17; 95% CI: 1.01–1.36; P = 0.028), maintaining sleep (HR: 1.14; 95% CI: 1.01–1.28; P = 0.028), early morning awakening (HR: 1.20; 95% CI: 1.02–1.43; P = 0.03), or non-restorative sleep (HR: 1.25; 95% CI: 1.06–1.46; P = 0.006) was associated with a higher hazard of incident HF than not experiencing the symptom. Time-varying crude rates for HF are provided in Results—Supplementary material online, Table S1, and the HRs obtained from traditional (standard) discrete-time survival analysis are provided in Results—Supplementary material online, Table S5 for comparison.
Table 3.
Marginal structural discrete-time survival analyses modelling longitudinal associations between the cumulative number and type of insomnia symptoms and incident heart failure
| Respondent characteristics | Adjusted HR (95% CI)a | P-value |
|---|---|---|
| Number of insomnia symptoms (ref: no symptoms) | ||
| One | 1.22 (1.08–1.38) | 0.0012 |
| Two | 1.45 (1.21–1.72) | <0.001 |
| Three | 1.66 (1.37–2.02) | <0.001 |
| Four | 1.80 (1.25–2.59) | 0.0011 |
| Individual insomnia symptoms | ||
| Difficulty initiating sleep (ref: no) | 1.17 (1.01–1.36) | 0.028 |
| Difficulty maintaining sleep (ref: no) | 1.14 (1.01–1.28) | 0.028 |
| Early morning awakening (ref: no) | 1.20 (1.02–1.43) | 0.03 |
| Non-restorative sleep (ref: no) | 1.25 (1.06–1.46) | 0.006 |
The data were obtained from the Health and Retirement Study (n = 12 761, age of respondents ≥50 years, 2002–2018, USA).
CI, confidence interval; HR, hazard ratio; MSM, marginal structural model; ref, reference.
The stabilized MSM weights were applied. The models incorporated a general specification for the main effect of time and were adjusted for all time-invariant ‘baseline’ respondent characteristics (sex, race and ethnicity, level of education, marital status, and family poverty threshold).
Sensitivity analyses
Results from the sensitivity analyses did not show qualitatively different estimates compared with those from our selected MSMs, and the statistical conclusions for the estimated HRs for HF did not notably differ (see Supplementary material online, Tables S2 to S4). Thus, the results of sensitivity analyses support the robustness and reliability of our selected MSMs in investigating the study objectives and confirm the validity of the products of discrete-time hazard models.
Discussion
We investigated prospective associations between experiencing insomnia symptoms and the risk of incident HF in a population-representative sample of middle-aged and older adults (age ≥50 years) in the USA. Our robust design revealed direct associations between time-varying insomnia symptoms and incident HF over 16 years of follow-up. Our results showed that respondents who experienced more insomnia symptoms had a higher-graded HF incidence. Experiencing each symptom independently was associated with a higher hazard of developing HF during the follow-up. The present findings indicate that insomnia symptoms are significantly associated with future incident HF in middle-aged and older adults and are potential targets for preventing and reducing HF incidence (Graphical abstract).
To our best knowledge, only a few studies have examined longitudinal associations between insomnia symptoms and HF incidence,55–57 and none have incorporated a comprehensive set of insomnia symptoms, accounted for the recurrent and time-dependent nature of the symptoms, or adjusted for the evolving effects of time-varying confounding factors. The course of insomnia symptoms is usually characterized by a waxing and waning pattern,15 and they sometimes co-occur with other health and medical conditions.16,45,58 Thus, any effort to investigate longitudinal associations between insomnia symptoms and incident HF should consider the time-dependent nature of the symptoms and confounding factors and adjust for selection bias. Using a marginal structural modelling analysis, we found that all primary insomnia symptoms were associated with subsequent HF in middle-aged and older adults. Those previous longitudinal studies that used only few insomnia symptoms and covariate assessments at baseline also showed some positive associations. For example, Laugsand et al.57 screened for difficulty initiating sleep, difficulty maintaining sleep, and non-restorative sleep at baseline and found that the hazards of incident HF during follow-up were only significantly higher among those who reported all three symptoms compared to asymptomatic respondents. Similarly, Ingelsson et al.55 showed that men with difficulty initiating sleep at baseline had an increased risk of incident HF. Moreover, Newman et al.56 found that baseline daytime sleepiness was significantly associated with age-adjusted incident HF in both men and women. Taken together, these findings suggest that insomnia symptoms potentially contribute to HF development.
The exact pathophysiologic pathways through which insomnia symptoms are linked to incident HF are largely unknown.21,35 Insomnia symptoms induce a state of hyperarousal during sleep and wakefulness and are often accompanied by increased heart rate and decreased heart rate variability, increased blood pressure, and increased cortisol secretion, and could subsequently lead to the development of adverse health conditions, including insulin resistance, diabetes, hypertension, coronary heart disease, and mental health disorders, such as anxiety and depression.21,35,36 The direct and indirect adverse health outcomes of insomnia could then mediate or moderate the pathways between insomnia symptoms and HF. For example, accumulating evidence indicates that patients with hypertension, myocardial infarction, diabetes, coronary artery disease, or other impaired biomarkers are significantly more likely to develop HF.59,60 Further, experiencing insomnia symptoms results in increased pro-inflammatory biomarkers,35 which have been implicated in HF development.1 Another potential mechanism that indirectly links insomnia symptoms to incident HF is the presence of maladaptive or inadequate health behaviours among individuals with insomnia, which are well-established risk factors for HF.59 Experiencing insomnia symptoms could trigger these behaviours, which could put individuals on the path of developing cardiovascular diseases.15 Therefore, dysregulation of the hypothalamic-pituitary-adrenal axis, abnormal modulation of the autonomic nervous system, increased sympathetic nervous system activity, and increased systemic inflammation could provide mechanistic bases linking insomnia symptoms and HF.21,35,61–63 Most of these mechanisms are not mutually exclusive and may synergize in concert or result in a vicious cycle of events, thereby affecting heart health.35
This study has few limitations. Insomnia symptoms were self-reported and subjective measures of sleep. Although the use of devices, such as actigraphy and polysomnography, can provide accurate and additional data on sleep,44 sleep measures derived from these devices are less sensitive or specific than self-reports in identifying insomnia symptoms64 and are not feasible in large-scale epidemiological studies. Thus, although subjective perceptions of sleep might be prone to bias, they are one of the only means of capturing insomnia symptoms. Further, the survey assesses HF and most other health conditions through self-report of a doctor’s diagnosis. Nevertheless, these self-report measures are valid in accurately diagnosing health conditions, specifically cardiovascular diseases, and demonstrated to be reliable across diverse populations in prior scientific studies.1,65–68 Moreover, this study only used the information provided in the HRS dataset. Assessments of sleep duration and other secondary insomnia complaints, such as excessive daytime sleepiness, irritability, and fatigability, were absent in the dataset, and considering these factors would have otherwise enriched our analyses.21,64,69 Furthermore, we did not know the prevalence of obstructive sleep apnoea (OSA) among study participants. Obstructive sleep apnoea could be an essential contributing factor to insomnia symptoms16 and might have a significant, usually bidirectional, correlation with HF.70 Although we could not directly account for OSA, other respondent characteristics, such as age and BMI, were used as proxy measures of OSA. Indeed, current evidence indicates a strong, positive correlation between increasing age or BMI and the probability of OSA.16,71
Conclusion
Insomnia symptoms, both individually and cumulatively, were associated with incident HF among middle-aged and older adults. Thus, these symptoms are potentially modifiable factors that—if properly managed—could reduce the risk of HF and HF-related morbidity. As insomnia symptoms can be assessed and identified early and are relatively manageable,16,35 healthcare providers and clinicians should screen for insomnia symptoms to prevent adverse health outcomes such as HF and other cardiovascular diseases. Further, since sleep quality is an integral part of preventive medicine,58 good quality sleep can be made part of wellness information, in addition to diet and regular exercise.58 Patients and individuals at risk of HF should be provided sufficient information about sleep quality and insomnia symptoms and guidance regarding insomnia prevention and treatment. Currently, several non-pharmacological behavioural treatment modalities such as Cognitive Behavioral Therapy for Insomnia are available for insomnia symptoms management. Cognitive Behavioral Therapy for Insomnia is proven to be effective and have longer lasting effects than medications.16,35 Thus, effective treatment options exist that should be recommended for individuals complaining of insomnia symptoms. Future studies could investigate the mechanisms linking insomnia symptoms and incident HF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The Health and Retirement Study is sponsored by grant (U01AG009740) from the US National Institute on Aging (NIA). However, the current study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The NIA had no role in the design and conduct of the present study nor in data collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest: none declared.
Data availability
The data underlying this article were obtained from the Health and Retirement Study (HRS), publicly available to access and download at https://hrs.isr.umich.edu/data-products.
Supplementary Material
References
- 1.Groenewegen A, Rutten FH, Mosterd A, Hoes AW.. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN.. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 4.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD.. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail 2018;11:e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM.. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013;309:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, Brauer M, Kutty VR, Gupta R, Wielgosz A, AlHabib KF, Dans A, Lopez-Jaramillo P, Avezum A, Lanas F, Oguz A, Kruger IM, Diaz R, Yusoff K, Mony P, Chifamba J, Yeates K, Kelishadi R, Yusufali A, Khatib R, Rahman O, Zatonska K, Iqbal R, Wei L, Bo H, Rosengren A, Kaur M, Mohan V, Lear SA, Teo KK, Leong D, O'Donnell M, McKee M, Dagenais G.. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020;395:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Djoussé L, Driver JA, Gaziano JM.. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA 2009;302:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiuve SE, McCullough ML, Sacks FM, Rimm EB.. Clinical perspective. Circulation 2006;114:160–167. [DOI] [PubMed] [Google Scholar]
- 10.Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB.. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol 2015;65:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L.. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 12.Del Gobbo LC, Kalantarian S, Imamura F, Lemaitre R, Siscovick DS, Psaty BM, Mozaffarian D.. Contribution of major lifestyle risk factors for incident heart failure in older adults: the Cardiovascular Health Study. JACC Heart Fail 2015;3:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd-Jones DM, Wilkins JT.. Hypertension, obesity, diabetes, and heart failure-free survival: the cardiovascular disease lifetime risk pooling project. JACC Heart Fail 2016;4:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, Rosamond WD, Heiss G.. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol 2012;60:1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Mendoza J. Insomnia and cardiometabolic disease risk. In: Grandner MA, ed. Sleep and Health. 1st ed. London/San Diego/Cambridge/Oxford: Elsevier; 2019. p391–407. [Google Scholar]
- 16.Li J, Gooneratne NS.. Sleep and health in older adults. In: Grandner MA, ed. Sleep and Health. 1st ed. London/San Diego/Cambridge/Oxford: Elsevier; 2019. p31–43. [Google Scholar]
- 17.Nguyen V, George T, Brewster GS.. Insomnia in older adults. Curr Geriatr Rep 2019;8:271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Q, Zhang P, Li G, Dai H, Shi J.. The association between insomnia symptoms and risk of cardio-cerebral vascular events: a meta-analysis of prospective cohort studies. Eur J Prev Cardiol 2017;24:1071–1082. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Zhang X-W, Hou W-S, Tang Z-Y.. Insomnia and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol 2014;176:1044–1047. [DOI] [PubMed] [Google Scholar]
- 20.Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF.. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol 2014;21:57–64. [DOI] [PubMed] [Google Scholar]
- 21.Javaheri S, Redline S.. Insomnia and risk of cardiovascular disease. Chest 2017;152:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas MC. Type 2 diabetes and heart failure: challenges and solutions. Curr Cardiol Rev 2016;12:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chahal H, Bluemke DA, Wu CO, McClelland R, Liu K, Shea SJ, Burke G, Balfour P, Herrington D, Shi PB, Post W, Olson J, Watson KE, Folsom AR, Lima JAC.. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart 2015;101:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubicki DM, Xu M, Akwo EA, Dixon D, Muñoz D, Blot WJ, Wang TJ, Lipworth L, Gupta DK.. Race and sex differences in modifiable risk factors and incident heart failure. JACC Heart Fail 2020;8:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR.. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol 2014;43:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.University of Michigan Institute for Social Research. Health and Retirement Study: Institutional Review Board Information. 2018. https://hrs.isr.umich.edu/sites/default/files/biblio/HRS_IRB_Information%28web%29_08_2018.pdf (12 July 2020).
- 27.Bugliari D, Campbell N, Chan C, Hayden O, Hayes J, Hurd M, Main R, Mallett J, McCullough C, Meijer E.. RAND HRS Longitudinal File 2016 (V1) Documentation. St Monica, CA: RAND Cent Study Aging; 2019. [Google Scholar]
- 28.Fisher GG, Ryan LH.. Overview of the Health and Retirement Study and introduction to the special issue. Work Aging Retire 2018;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canham SL, Kaufmann CN, Mauro PM, Mojtabai R, Spira AP.. Binge drinking and insomnia in middle-aged and older adults: the Health and Retirement Study. Int J Geriatr Psychiatry 2015;30:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L, Agnew J, Mojtabai R, Surkan PJ, Spira AP.. Insomnia as a predictor of job exit among middle-aged and older adults: results from the Health and Retirement Study. J Epidemiol Community Health 2017;71:750–757. [DOI] [PubMed] [Google Scholar]
- 31.Kim ES, Hershner SD, Strecher VJ.. Purpose in life and incidence of sleep disturbances. J Behav Med 2015;38:590–597. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer M, Ma J, Gao X.. Association between insomnia symptoms and mortality: a prospective study of US men. Circulation 2014;129:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min Y, Nadpara PA, Slattum PW.. The association between sleep problems, sleep medication use, and falls in community-dwelling older adults: results from the Health and Retirement Study 2010. J Aging Res 2016;2016:3685789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer JD, Willett JB, Willett JB.. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. 1st ed. New York: Oxford University Press; 2003. [Google Scholar]
- 35.Choi O, Irwin MR.. Insomnia in aging. In: Avidan AY, Alessi C, eds. Geriatric Sleep Medicine. 1st ed. Florida: CRC Press; 2008. p.89–112. [Google Scholar]
- 36.Patel D, Steinberg J, Patel P.. Insomnia in the elderly: a review. J Clin Sleep Med 2018;14:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uijl A, Koudstaal S, Direk K, Denaxas S, Groenwold RH, Banerjee A, Hoes AW, Hemingway H, Asselbergs FW.. Risk factors for incident heart failure in age-and sex-specific strata: a population-based cohort using linked electronic health records. Eur J Heart Fail 2019;21:1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crimmins EM, Kim JK, Langa KM, Weir DR.. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci 2011;66B:i162–i171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langa KM, Kabeto M, Weir D.. Report on race and cognitive impairment using HRS. In: 2010 Alzheimer’s Disease Facts and Figures. Chicago: Alzheimer’s Association, 2009. p.46–61. [Google Scholar]
- 40.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 41.O’Halloran AM, Kenny RA, King-Kallimanis BL.. The latent factors of depression from the short forms of the CES-D are consistent, reliable and valid in community-living older adults. Eur Geriatr Med 2014;5:97–102. [Google Scholar]
- 42.Karim J, Weisz R, Bibi Z, Rehman SU.. Validation of the eight-item Center for Epidemiologic Studies Depression scale (CES-D) among older adults. Curr Psychol 2015;34:681–692. [Google Scholar]
- 43.Grandner MA. Social-ecological model of sleep health. In: Grandner MA, ed. Sleep and Health. 1st ed. London/San Diego/Cambridge/Oxford: Elsevier; 2019. p.45–53. [Google Scholar]
- 44.Lavoie CJ, Zeidler MR, Martin JL.. Sleep and aging. Sleep Sci Pract 2018;2:3. [Google Scholar]
- 45.Pigeon WR. Insomnia as a risk factor for disease. In: Sateia MJ, Buysse DJ, eds. Insomnia: Diagnosis and Treatment. New York: Informa Healthcare; 2010. p.31–41. [Google Scholar]
- 46.Sonnega A, Leggett A, Pepin R, Assari S.. Physical activity and insomnia symptoms over 10 years in a US national sample of late-middle-age and older adults: age matters. J Aging Phys Act 2020;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon GE, VonKorff M.. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry 1997;154:1417–1423. [DOI] [PubMed] [Google Scholar]
- 48.Eaton WW, Smith C, Ybarra M, Mutaner C, Tien A, Centre for epidemiologic studies depression scale: review and revision (CESD and CESD-R). In Maruish ME, ed. The Use of Psychological Testing for the Treatment Planning and Outcomes Assessment. Vol. 3. Mahwah, NJ: Lawrence Erlbaum Associates Inc.; 2004. p.363–377. [Google Scholar]
- 49.Hernán MA, Brumback BA, Robins JM.. Estimating the causal effect of zidovudine on CD4 count with a marginal structural model for repeated measures. Stat Med 2002;21:1689–1709. [DOI] [PubMed] [Google Scholar]
- 50.Robins JM. Association, causation, and marginal structural models. Synthese 1999;121: 151–179. [Google Scholar]
- 51.Robins JM. Marginal structural models versus structural nested models as tools for causal inference. In: Halloran ME, Berry D, eds. Statistical Models in Epidemiology, the Environment, and Clinical Trials. New York: Springer; 2000. p.95–133. [Google Scholar]
- 52.Cole SR, Hernán MA.. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allison PD. Survival Analysis Using SAS: A Practical Guide. 2nd ed. Cary, NC, USA: SAS Institute Inc.; 2010. [Google Scholar]
- 54.Faries D, Zhang X, Kadziola Z, Siebert U, Kuehne F, Obenchain RL, Haro JM.. Real World Health Care Data Analysis: Causal Methods and Implementation Using SAS. Cary, NC, USA: SAS Institute; 2020. [Google Scholar]
- 55.Ingelsson E, Lind L, Ärnlöv J, Sundström J.. Sleep disturbances independently predict heart failure in overweight middle-aged men. Eur J Heart Fail 2007;9:184–190. [DOI] [PubMed] [Google Scholar]
- 56.Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J.. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. J Am Geriatr Soc 2000;48:115–123. [DOI] [PubMed] [Google Scholar]
- 57.Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I.. Insomnia and the risk of incident heart failure: a population study. Eur Heart J 2014;35:1382–1393. [DOI] [PubMed] [Google Scholar]
- 58.Neubauer D, Pagel J, Zee P.. Comorbid conditions caused by sleeping disorders. Med Roundtable Gen Med Ed 2014;1:222–229. [Google Scholar]
- 59.Horwich TB, Fonarow GC.. Glucose, obesity, metabolic syndrome, and diabetes: relevance to incidence of heart failure. J Am Coll Cardiol 2010;55:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallner M, Eaton DM, von LD, Sourij H.. Revisiting the diabetes-heart failure connection. Curr Diab Rep 2018;18:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morin CM, Benca R.. Chronic insomnia. Lancet 2012;379:1129–1141. [DOI] [PubMed] [Google Scholar]
- 62.Pejovic S, Vgontzas AN.. Neurobiological disturbances in insomnia: clinical utility of objective measures of sleep. In: Sateia MJ, Buysse DJ, eds. Insomnia: Diagnosis and Treatment. 1st ed. New York: Informa Healthcare; 2010. p. 65–76. [Google Scholar]
- 63.Redline S, Foody J.. Sleep disturbances: time to join the top 10 potentially modifiable cardiovascular risk factors? Circulation 2011;124:2049–2051. [DOI] [PubMed] [Google Scholar]
- 64.Buysse DJ. Insomnia. JAMA 2013;309:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhingra R, Gaziano JM, Djoussé L.. Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail 2011;4:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glymour MM, Avendano M.. Can self-reported strokes be used to study stroke incidence and risk factors? Evidence from the Health and Retirement Study. Stroke 2009;40:873–879. [DOI] [PubMed] [Google Scholar]
- 67.Heliövaara M, Aromaa A, Klaukka T, Knekt P, Joukamaa M, Impivaara O.. Reliability and validity of interview data on chronic diseases. The mini-Finland health survey. J Clin Epidemiol 1993;46:181–191. [DOI] [PubMed] [Google Scholar]
- 68.Wallace RB, Herzog AR.. Overview of the health measures in the Health and Retirement Study. J Hum Resour 1995;30:S84–S107. [Google Scholar]
- 69.Seixas AA, Robbins R, Chung A, Popp C, Donley T, McFarlane SI, Moore J, Jean-Louis G.. Sleep health and diabetes: the role of sleep duration, subjective sleep, sleep disorders, and circadian rhythms on diabetes. In: Grandner MA, ed. Sleep and Health. 1st ed. London/San Diego/Cambridge/Oxford: Elsevier; 2019. p.213–225. [Google Scholar]
- 70.Kitcher A, Malhotra A, Sunwoo B.. Sleep apnea and cardiometabolic disease risk. In: Grandner MA, ed. Sleep and Health. 1st ed. London/San Diego/Cambridge/Oxford: Elsevier; 2019. p.409–417. [Google Scholar]
- 71.Grandner MA. Epidemiology of insufficient sleep and poor sleep quality. In: Grandner MA, ed. Sleep and Health. 1st ed. London/San Diego/Cambridge/Oxford: Elsevier; 2019. p.11–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were obtained from the Health and Retirement Study (HRS), publicly available to access and download at https://hrs.isr.umich.edu/data-products.