Abstract
Background
Non-bacterial thrombotic endocarditis is characterized by the presence of sterile vegetations on a cardiac valve. We present a case of multi-territory stroke caused by embolism of a non-bacterial thrombotic aortic valve endocarditis, leading to the diagnosis of a prostate adenocarcinoma with bone metastases.
Case summary
A 66-year-old patient was diagnosed with pulmonary embolism, first attributed to an asymptomatic coronavirus disease 2019 infection. Edoxaban was started, which was discontinued by the patient. Four weeks later, he presented with subacute vertigo and balance disorders. Magnetic resonance imaging showed a multi-territory stroke. A transoesophageal echocardiogram demonstrated a small vegetation on the aortic valve with moderate aortic insufficiency. Blood cultures remained negative. Malignancy screening showed a markedly elevated prostate-specific antigen. Prostate adenocarcinoma was confirmed on biopsy. A positron emission tomography revealed metastatic disease. A diagnosis of non-bacterial thrombotic endocarditis and paraneoplastic pulmonary embolism secondary to prostate cancer was made. Edoxaban was restarted and the patient was referred for treatment of the prostate adenocarcinoma. Follow-up after 5 months showed no evidence of aortic valve vegetations.
Discussion
Coronavirus disease 2019 in ambulatory patients may be insufficient as a predisposing factor for venous thrombo-embolism and these patients, especially the elderly, should undergo a screening for malignancy. Non-bacterial thrombotic endocarditis is a rare cause of multi-territory stroke. When related to cancer, the prostate can be the primary tumour.
Keywords: Stroke, Non-bacterial thrombotic endocarditis, Prostate cancer, Pulmonary embolism, COVID-19, Case report
Learning points
COVID-19 in ambulatory patient may be insufficient as a predisposing factor for venous thrombo-embolism.
Non-bacterial thrombotic endocarditis is a rare cause of multi-territory stroke. When it is related to cancer, the prostate can be the primary tumour.
Introduction
Ischaemic stroke represents a large medical burden causing death and disability worldwide.1 A cardioembolic source is found in 20–30% of cases.2 Possible causes include, among others, atrial dysrhythmias, intracardiac shunts, and valvulopathy.2 We present a case of a patient with multi-territory stroke due to non-bacterial thrombotic endocarditis, preceded by a pulmonary embolism and asymptomatic coronavirus disease 2019 (COVID-19), leading to a new diagnosis of metastatic prostate adenocarcinoma.
Timeline
| Relative point in time | Case course |
|---|---|
| Day −30 | 66-year-old male admitted with chest pain, diagnosis of subsegmental pulmonary embolism. Edoxaban is started after 5 days of enoxaparin. Coronavirus disease 2019 polymerase chain reaction is compatible with previous infection. |
| Day −23 | At home discontinuation of edoxaban. |
| Day 1 | Admitted with subacute balance disorders. Magnetic resonance imaging confirms multi-territory stroke. Re-initiation of edoxaban. |
| Day 3 | A transthoracic echocardiogram shows no significant abnormalities. |
| Day 14 | Ambulatory transoesophageal echocardiogram shows a small vegetation on the aortic valve with moderate aortic regurgitation. |
| Day 16 | Blood cultures remain negative. Prostate specific antigen is significantly elevated (317 ng/mL). |
| Day 17 | Prostate biopsies are taken. Histology confirms prostate carcinoma. |
| Day 19 | Positron emission tomography shows multiple fluoro-deoxy-glucose avid osteoblastic bone lesions. |
| Day 31 | Androgen deprivation therapy is initiated. |
| Day 120 | Our patient is in good general condition. His gait has improved. Prostate specific antigen is 0.84 ng/mL. |
| Day 150 | A control transthoracic echocardiogram shows no visible vegetations. |
Case presentation
A 66-year-old Caucasian male with no previous medical history was hospitalized for chest and interscapular pain. Blood pressure and oxygen saturation on admission were normal. Electrocardiography showed normal sinus rhythm. D-dimers were > 10 000 ng/mL (normal < 500 ng/mL) and additional computed tomography (CT) angiography showed small, non-occlusive subsegmental pulmonary embolisms in the right lower lobe. A routine polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on a nasopharyngeal swab on admission was positive with cycle threshold values >36, compatible with a previous infection. There were no signs of coronavirus disease 2019 (COVID-19) pneumonia on CT imaging. Besides chest pain, the patient was asymptomatic, oxygen saturations stayed >96% at room air. A duplex ultrasound revealed extensive superficial thrombosis in the veins of the left lower leg, with limited deep venous thrombosis in the lateral gastrocnemius. After 5 days of therapeutic enoxaparine, treatment with edoxaban 60 mg once daily was initiated.
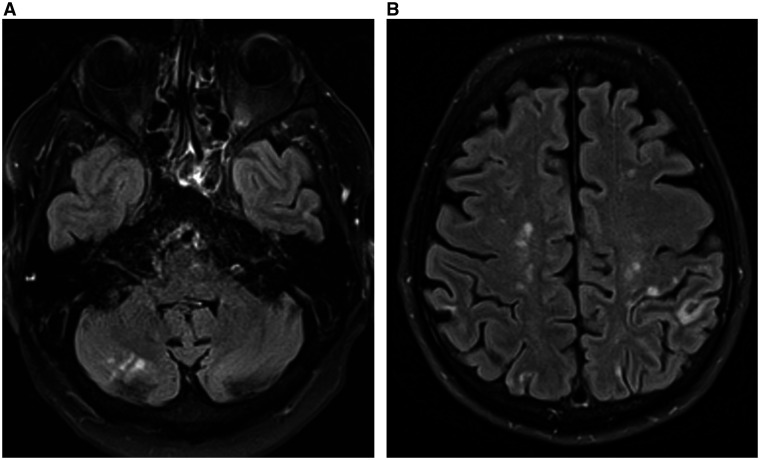
Four weeks later our patient presented in the emergency department because of subacute vertigo, gait difficulties, and diplopia since 2 weeks. He admitted to have discontinued edoxaban as soon as one week after hospital discharge. Clinical findings included dysmetria on finger-to-nose testing in the right arm and a difficult tandem gait. Magnetic resonance imaging showed numerous ischaemic brain lesions in the cerebral (sub)cortical regions, corpus callosum and in the right cerebellar hemisphere, the latter matching with the right-sided ataxia (Figure 1).
Figure 1.
Axial T2 fluid-attenuated inversion recovery magnetic resonance imaging images showing multiple hyperintense lesions in the cerebellum and the (sub)cortical regions, suggestive of recent ischaemic stroke of cardio-embolic source.
Because of the multifocal pattern, an embolic source was suspected. Our patient reported to have an unintentional weight loss of around 4 kg (8.8 pounds), but there was no history of palpitations or fever. On physical examination, he appeared overweight (body mass index 28 kg/m2). No signs of peripheral embolism were found, there was no fever or cardiac murmur. The most important differential diagnoses were patent foramen ovale with paradoxical embolism, atrial fibrillation, and endocarditis. Rhythm monitoring showed sinus rhythm without episodes of supraventricular tachycardia. Atrial size, valvular function, and ventricular size and function appeared within the normal range on a transthoracic echocardiogram. Blood biochemistry showed a high alkaline phosphatase of 463 U/L (normal 45–117 U/L) and a mildly elevated C-reactive protein of 24 mg/L (normal < 3.0 mg/L). Sedimentation rate, complete blood count, electrolyte panel, creatinine, other liver tests, and protein electrophoresis were normal. Anticoagulation therapy with edoxaban 60 mg once daily was re-initiated.
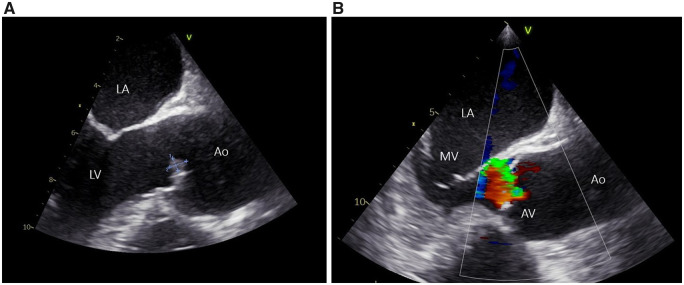
A transoesophageal echocardiogram showed a small vegetation of 6.7 mm × 5.2 mm on the aortic side of the right coronary cusp (Figure 2A, Video 1). There was a moderate eccentric aortic valve regurgitation with a jet in the direction of the anterior leaflet of the mitral valve (Figure 2B, Video 2). There was no patent foramen ovale or atrial septal defect. Multiple sets of blood cultures were taken but remained negative. Testing for causes of culture-negative infectious endocarditis endemic in our region (among others Coxiella burnetii and Bartonella spp.) were negative. Antiphospholipid antibodies testing turned out negative.
Figure 2.
(A) Transoesophageal echocardiogram (mid-oesophageal aortic valve LAX view) showing a vegetation of 6.7 mm × 5.2 mm on the aortic side of the right coronary cusp. (B) Demonstration of the eccentric aortic valve regurgitation with a jet in the direction of the anterior leaflet of the mitral valve. (Ao, ascending aorta; AV, aortic valve; LA, left atrium; LV, left ventricle; MV, mitral valve).
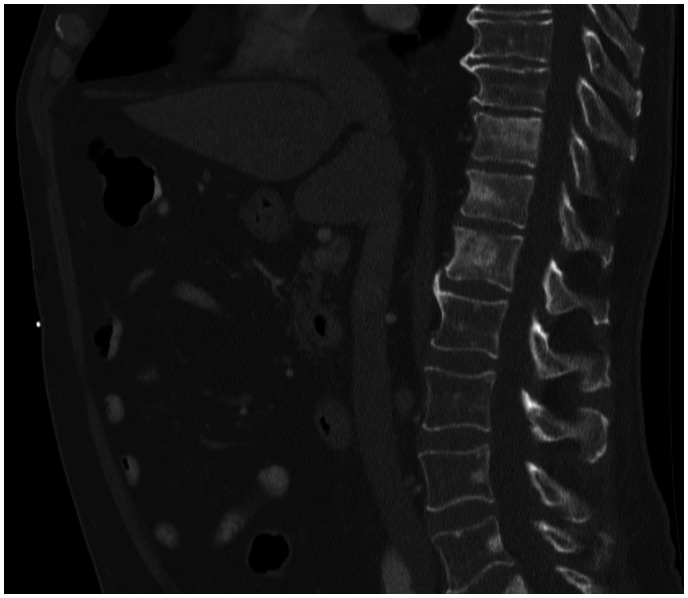
A screening for malignancy was performed. Computed tomography imaging of the chest had showed no suspicious lesions. Faecal occult blood test was negative. Serum prostate-specific antigen (PSA) appeared significantly elevated (317 ng/mL) (normal < 4.50 ng/mL). An induration on the right side of the prostate was noted during digital rectal examination. Histological examination of prostate biopsies confirmed a prostate adenocarcinoma. Staging with a fluoro-deoxy-glucose (FDG) positron emission tomography-CT scan revealed extensive FDG avid osteoblastic bone metastases and small retroperitoneal, inguinal, and axillary lymphadenopathies (Figures 3 and 4). There was no significant increased uptake of FDG in the aortic valve vegetation. A diagnosis of advanced metastatic prostate carcinoma with secondary non-bacterial thrombotic aortic valve endocarditis was made. The previous pulmonary embolism was considered to be paraneoplastic.
Figure 3.
Computed tomography scan demonstrating diffuse osteoblastic bone lesions in the vertebral column.
Figure 4.
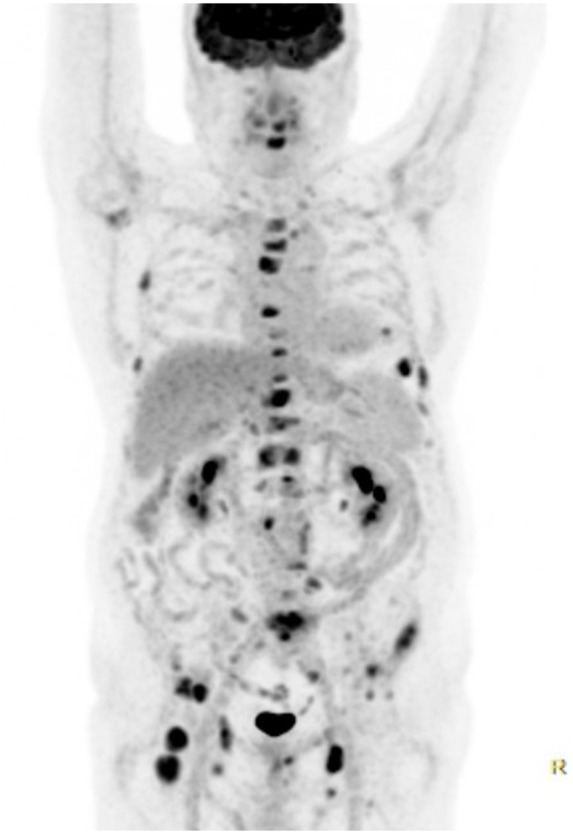
18-Fluoro-deoxy-glucose positron emission tomography demonstrating widespread fluoro-deoxy-glucose avid lesions in the proximal skeleton and small lymphadenopathies. There is no visible uptake of fluoro-deoxy-glucose in the aortic valve.
The prostate adenocarcinoma was treated with androgen deprivation therapy. After dental sanitation denosumab and calcium–vitamin D were started to prevent pathological fractures. Because of persistent balance disorders and writing difficulties, our patient was included in a neurological rehabilitation program. There was a good tumour response with a decrease of the PSA after 4 months to 0.84 ng/mL. His gait improved with gait revalidation training. A follow-up transthoracic echocardiogram 5 months after admission for his balance disorders showed no visible vegetations of the aortic valve.
Discussion
The pulmonary embolism was first thought to be secondary to COVID-19, as it is shown that patients with COVID-19 have an increased thrombo-embolic risk. However, this relation is mostly described in patients with severe disease, requiring supplemental oxygen or intensive care.3 For ambulatory COVID-19 patients, as in our case, there are only a few reports of venous thromboembolism, and a causal relationship is not clear.3–5 Our patient was, besides chest pain, asymptomatic and did not develop other COVID-19 related symptoms during hospitalization. Hence, COVID-19 could insufficiently explain the observed pulmonary embolism. All patients who are considered to have venous thromboembolism without identifiable risk factors, such as the patient presented in this case, should undergo a clinically guided screening for occult cancer, especially older patients, since their higher prevalence of occult cancer. Screening includes at least clinical evaluation, laboratory analysis, chest imaging, faecal occult blood test, and gender-specific testing, including prostate-specific antigen testing in men.6,7
Non-bacterial thrombotic endocarditis (NBTE), also known as marantic endocarditis, is a rare form of culture-negative endocarditis characterized by a sterile vegetation on a cardiac valve. The pathophysiology of NBTE most likely involves damage of the cardiac valve endothelium with subsequent deposition of sterile fibrin and platelets.8 It can be caused by hypercoagulability, autoimmunity, or cancer, the latter most frequently pancreatic and lung cancer.8,9 To our current knowledge, there have only been three cases reported of non-bacterial thrombotic endocarditis secondary to prostate cancer.10–12 Non-bacterial thrombotic endocarditis is clinically characterized by systemic embolism, most commonly to the brain, while fever is mostly absent. Non-bacterial thrombotic endocarditis should therefore be considered as a rare cause of multi-territory ischaemic stroke. In patients with stroke with a suspected cardioembolic origin without other causes, a transoesophageal echocardiogram can be indicated, even when a transthoracic echocardiogram is within normal range.13,14 Vegetations in NBTE are small (typically 3–10 mm), heterogeneous in shape, and can appear anywhere on the valve surface.8,15 Valve destruction and dysfunction are usually mild or absent.8,15 Treatment of the primary cancer is paramount in patients with NBTE secondary to cancer. Traditionally, anticoagulation with low molecular weight heparines (LMWHs) is used to prevent further thrombo-embolism in patients with NBTE, which is mostly based on expert opinions by extrapolation of data from paraneoplastic venous thromboembolism.8,9 However, recent evidence showed non-inferiority of edoxaban compared with LMWHs in patients with paraneoplastic thromboembolism, making it a valid alternative for our patient.16
Conclusion
Coronavirus disease 2019 may be considered insufficient as a predisposing factor for venous thromboembolism in ambulatory patients. In patients with unprovoked pulmonary embolism, a screening for malignancy should be performed according to current guidelines. Non-bacterial thrombotic endocarditis should be considered as a rare cause of multi-territory stroke. The diagnosis is made by demonstrating a vegetation on a cardiac valve and an associated condition, such as cancer, after excluding infectious endocarditis. Prostate cancer can be the primary tumour causing non-bacterial thrombotic endocarditis.
Lead author biography

Jakob Van Herck, M.D., is a resident internal medicine physician at the University of Leuven, Belgium, under supervision of Prof. Dr. Willy Peetermans. Dr. Van Herck has a special interest in systemic and cardiovascular disease.
Supplementary material
Supplementary material is available at European Heart Journal—Case Reports online.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report, including images and associated text, has been obtained from the patient in line with COPE guidance.
Funding: None declared.
Conflict of interest: None declared.
Supplementary Material
References
- 1.Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D. et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H, Nassif M, Khairy P, de Groot JR, Roos YBWEM, de Winter RJ. et al. Cardiac diagnostic work-up of ischaemic stroke. Eur Heart J 2018;39:1851–1860. [DOI] [PubMed] [Google Scholar]
- 3.Al-Ani F, Chehade S, Lazo-Langner A.. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res 2020;192:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laouan Brem F, Rasras H, Ouafi N, El Bazid Z.. Bilateral pulmonary embolism in patients recovered from asymptomatic COVID-19 infection. Cureus 2021;13:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overstad S, Tjonnfjord E, Garabet L, Fronas S, Bergan J, Aballi S. et al. Venous thromboembolism and coronavirus disease 2019 in an ambulatory care setting- A report of 4 cases. Thromb Res 2020;194:116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin-Romero S, Jara-Palomares L.. Screening for occult cancer: where are we in 2020? Thromb Res 2020;191:S12–S16. [DOI] [PubMed] [Google Scholar]
- 7.van Es N, Le Gal G, Otten H-M, Robin P, Piccioli A, Lecumberri R. et al. Screening for occult cancer in patients with unprovoked venous thromboembolism. Ann Intern Med 2017;167:410–417. [DOI] [PubMed] [Google Scholar]
- 8.Hurrell H, Roberts-Thomson R, Prendergast BD.. Non-infective endocarditis. Heart 2020;106:1023–1029. [DOI] [PubMed] [Google Scholar]
- 9.Patel MJ, Elzweig J.. Non-bacterial thrombotic endocarditis: a rare presentation and literature review. BMJ Case Rep 2020;13:e238585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan K, Wahid K, Qureshi S.. Nonbacterial thrombotic endocarditis as the initial presentation of prostate cancer - a case report. J Pak Med Assoc 2019;69:1737–1740. [DOI] [PubMed] [Google Scholar]
- 11.Doyoung K, Jarrar A, Nicholson C, Khan MZ, McLaughlin C.. Abstracts from the 2020 Annual Meeting of the Society of General Internal Medicine. J Gen Intern Med 2020;35:403–404.31705463 [Google Scholar]
- 12.Eftychiou C, Fanourgiakis P, Vryonis E, Golfinopoulou S, Samarkos M, Kranidis A. et al. Factors associated with non-bacterial thrombotic endocarditis: case report and literature review. J Heart Valve Dis 2005;14:859–862. [PubMed] [Google Scholar]
- 13.Marino B, Jaiswal A, Goldbarg S, Bernardini GL, Kerwin T.. Impact of transesophageal echocardiography on clinical management of patients over age 50 with cryptogenic stroke and normal transthoracic echocardiogram. J Hosp Med 2016;11:95–98. [DOI] [PubMed] [Google Scholar]
- 14.Katsanos AH, Bhole R, Frogoudaki A, Giannopoulos S, Goyal N, Vrettou A-R. et al. The value of transesophageal echocardiography for embolic strokes of undetermined source. Neurology 2016;87:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Frishman WH.. Nonbacterial thrombotic endocarditis: pathogenesis, diagnosis, and management. Cardiol Rev 2016;24:244–247. [DOI] [PubMed] [Google Scholar]
- 16.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. ; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018;378:615–624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.