Abstract
Background
Wound and bone infections are frequently associated with open fractures of the extremities and may add significantly to the resulting morbidity. The administration of antibiotics is routinely practised in developed countries as an adjunct to a comprehensive management protocol that also includes irrigation, surgical debridement and stabilisation when indicated, and is thought to reduce the frequency of infections.
Objectives
To review the evidence for the effectiveness of antibiotics in the initial treatment of open fractures of the limbs.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (26 July 2009), Clinical Trials (The Cochrane Library 2009, Issue 3), MEDLINE (1950 to July 2009), EMBASE (1980 to 2009 Week 30), LILACS (1992 to July 2009), International Pharmaceutical Abstracts (1970 to July 2009), and reference lists of articles. We handsearched proceedings of the American Academy of Orthopaedic Surgeons (1980 to 2001), the Orthopaedic Trauma Association (1990 to 2001) and the Société Internationale de Chirurgie Orthopedique et Traumatologique (1980 to 2001). We also contacted published researchers in the field.
Selection criteria
Randomised or quasi‐randomised controlled trials involving: participants ‐ people of any age with open fractures of the limbs; intervention ‐ antibiotic administered before or at the time of primary treatment of the open fracture compared with placebo or no antibiotic; outcome measures ‐ early wound infection, chronic drainage, acute or chronic osteomyelitis, delayed unions or non‐unions, amputations and deaths.
Data collection and analysis
Two review authors independently screened papers for inclusion, assessed trial quality using an eight item scale, and extracted data. Additional information was sought from three trialists. Pooled data are presented graphically.
Main results
Data from 1106 participants in eight studies were analysed. The use of antibiotics had a protective effect against early infection compared with no antibiotics or placebo (risk ratio 0.43 (95% confidence interval (CI) 0.29 to 0.65); absolute risk reduction 0.07 (95% CI 0.03 to 0.10). There were insufficient data in the included studies to evaluate other outcomes.
Authors' conclusions
Antibiotics reduce the incidence of early infections in open fractures of the limbs. Further placebo controlled randomised trials are unlikely to be justified in middle and high income countries, except for open fractures of the fingers. Further research is necessary to the determine the avoidable burden of morbidity in countries where antibiotics are not used routinely in the management of open fractures.
Plain language summary
Antibiotics for preventing infection in open limb fractures
Wound and bone infections are common complications following open fractures of the limbs. For more than 20 years in developed countries, the use of antibiotics has been a part of a standard management protocol that also includes washing the wound (irrigation), cleaning up the wound and fracture (surgical debridement), and stabilisation of the fracture, as required. This review, which included data from 1106 participants in eight trials, found that antibiotics are effective in decreasing the incidence of wound infections, as compared with no antibiotics or placebo. No studies reporting bone infection or long‐term ill health (morbidity) were identified.
Background
The use of antibiotics in the initial treatment of open fractures of the extremities is almost universal in all high‐income countries, where benefits are assumed to outweigh potential risks. In the USA, a 100% compliance level is one of many required criteria for hospital re‐credentialing by the Joint Committee for Accreditation of Healthcare Organisations (JCAHO). In many lower‐income countries antibiotics may not be used routinely for various reasons: costs, lack of knowledge, low levels of suspicion of infection or poor case‐recognition from first‐line health care workers, availability, accessibility, and use of traditional or alternative health care. In some of these countries, the use of antibiotics for open fractures is delayed until the patient is seen in the secondary or even the tertiary care centres. This delay often exceeds the accepted "golden period" for treatment of six to eight hours used in high income countries. Since an open fracture is by definition contaminated, the use of antibiotics is therapeutic, not prophylactic, and aims at preventing subsequent infectious problems such as cellulitis, myositis, acute or chronic osteomyelitis (bone infection), infected non‐unions, recurrent abscesses, chronic drainage with fistula formation, and their associated impairment and disability.
A related systematic review (Gillespie 2001) concluded that antibiotic prophylaxis should be offered to those undergoing surgical treatment of closed hip and other long bone fractures. Our purpose is to review the evidence for use of antibiotics in the treatment of open fractures of the extremities.
Objectives
To review the evidence for the effectiveness of antibiotics in the initial treatment of open fractures of the limbs.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised (e.g. date of birth, alternation) controlled trials.
Types of participants
People of any age with open fractures of the limbs.
Types of interventions
Antibiotic administered before or at the time of primary treatment of the open fracture compared with placebo or no antibiotic. Trials comparing different antibiotics, different antibiotic dosages, route of administration or differences in timing or duration of administration were excluded.
Types of outcome measures
Primary outcome
early wound infection (as defined in individual study reports)
Secondary outcomes
chronic wound discharge
acute or chronic osteomyelitis
delayed union or non‐union
amputation
death
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (26 July 2009), the Cochrane Central Register of Controlled Trials/Clinical Trials (The Cochrane Library 2009, Issue 3), MEDLINE (1950 to July Week 3 2009), EMBASE (1980 to 2009 Week 30), Latin American and Caribbean Literature on the Health Sciences (LILACS) (1982 to July 2009), and International Pharmaceutical Abstracts (1970 to July 2009).
The search strategy for MEDLINE combined a subject‐specific section with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE (sensitivity‐maximizing version) (Lefebvre 2008), and was modified for use in other databases (seeAppendix 1). The MEDLINE search strategy for the previous version is shown in Appendix 2.
We searched the WHO International Clinical Trials Registry Platform (July 2009) for ongoing trials.
Searching other resources
Proceedings of meetings of the American Academy of Orthopaedic Surgeons (1980 to 2001), the Orthopaedic Trauma Association (1990 to 2001) and the Société Internationale de Chirurgie Orthopedique et Traumatologique (1980 to 2001) were handsearched. We also searched reference lists of articles and contacted published researchers in the field.
No language restriction was applied.
Data collection and analysis
Selection of studies
Two reviewers independently screened each record for eligibility by initially examining titles, abstracts and key words. Reports identified by either reviewer were retrieved. We searched the reference lists of relevant trials and reviews in which the largest number of eligible trials had been published. We contacted the authors of eligible trials and reviews to ask about unpublished trials. Reports of potentially eligible trials were obtained and two reviewers independently assessed each one for eligibility.
Data extraction and management
Two reviewers independently extracted data from included reports using a standard pro forma. Data were extracted on the type of participants, the type of antibiotic used, the number randomised to intervention or control groups, the quality of allocation concealment, and the outcome measures stated in the protocol. We wrote to the authors of reports if relevant information was missing. Trials in which we could not confirm that random or quasi‐random allocation had been used to allocate participants were excluded.
Assessment of risk of bias in included studies
Methodological quality for each study was independently assessed by two reviewers using a schedule derived from the former generic evaluation tool developed by the Cochrane Bone, Joint and Muscle Trauma Group (seeTable 1), from which a risk of bias assessment was derived. Disagreements were resolved by consensus. The scores for individual items were designed to give readers a general impression of trial quality and were not used in any quantitative manner.
1. Quality assessment tool.
| Items | Scores |
| A. Was the assigned treatment adequately concealed prior to allocation? | 1 = states random, but no description, or quasi‐randomisation 2 = small but real chance of disclosure of assignment 3 = method did not allow disclosure of assignment |
| B. Were the outcomes of patients who withdrew described and included in the analysis (intention‐to‐treat)? | 1 = not mentioned 2 = states numbers and reasons for withdrawal, but analysis unmodified 3 = primary analysis based on all cases as randomised |
| C. Assessment of outcome. Were assessors of outcome blinded to treatment status? | 1 = not done or not mentioned 2 = moderate chance of unblinding of assessors 3 = action taken to blind assessors, or outcomes such that bias is unlikely |
| D. Were treatment and control groups comparable at entry? | 1 = large potential for confounding or not discussed 2 = confounding small; mentioned but not adjusted for 3 = unconfounded; good comparability of groups or confounding adjusted for |
| E. Was a placebo treatment assigned as part of the randomisation? | 1 = no 3 = yes |
| F. Were exclusion criteria clearly defined? | 1 = not defined 2 = poorly defined 3 = well defined |
| G. Was the method of assessment of wound infection stated? | 1 = not stated 2 = clinical decision, or definite criteria without a microbiological diagnosis 3 = definite criteria including a microbiological diagnosis |
| H. Was the method and duration of surveillance stated? | 1 = not stated, or not active 2 = active, but less than three months 3 = active, and at least one year |
Data synthesis
For each intervention, we estimated the pooled risk ratio in a fixed‐effect model. We calculated 95% confidence intervals for each outcome. Selection bias was assessed using Egger's weighted regression method and Begg's rank correlation test and funnel plot. Heterogeneity was assessed using visual inspection of overlap in the forest plot and consideration of the Chi² test and I² statistic. We planned three subgroup analyses exploring whether the effect differed between placebo‐controlled and no‐placebo studies, whether the effect of antimicrobials differed depending on the location of the fracture (specifically comparing use in phalangeal fractures in the hand with use in fractures of major limb bones), and whether the timing of antibiotic administration was a critical factor.
Results
Description of studies
The updated search strategy identified 13 trials in which the use of any antibiotics for open fractures of the limbs was compared with placebo or no antibiotics. Five studies were excluded (seeCharacteristics of excluded studies) either because there was no randomisation (two studies), patients were randomised after an initial dose of antibiotics (one), or it was impossible to disaggregate data on open fractures and soft tissue injuries only (two). The remaining eight studies were included and are described in detail in Characteristics of included studies.
There were three randomised double‐blinded trials (Bergman 1982; Braun 1987; Stevenson 2003), three quasi‐randomised trials (Rojczyk 1983; Dickey 1989; Suprock 1990), and two trials where the randomisation methods were unclear (Patzakis 1974; Sloan 1987).
Three studies were limited to open fractures of fingers (Sloan 1987; Stevenson 2003; Suprock 1990), while four excluded hand and finger fractures (Bergman 1982; Braun 1987; Dickey 1989; Rojczyk 1983). Patzakis 1974 included all fractures of the extremities. Four studies included only patients in the ill‐defined 'adult age group' (Bergman 1982; Braun 1987; Dickey 1989; Sloan 1987). Two studies included patients of all ages (Patzakis 1974; Rojczyk 1983) and one study did not mention age (Suprock 1990). Participants in Stevenson 2003 were aged over 16 years.
Four studies used a placebo (Bergman 1982; Braun 1987; Sloan 1987; Stevenson 2003). Antibiotic regimens differed but all employed penicillin derivatives or first generation cephalosporins, active against gram‐positive organisms: Penicillin/streptomycin and cephalothin (Patzakis 1974); penicillin and dicloxacillin (Bergman 1982); cloxacillin (Braun 1987); flucloxacillin (Stevenson 2003); cefazolin (Dickey 1989; Rojczyk 1983); first generation cephalosporin, dicloxacillin or erythromycin (Suprock 1990); or cephradine (Sloan 1987). There were also differences in the duration and route of administration of treatment.
Risk of bias in included studies
The risk of bias in the included studies varied (seeFigure 1 and Figure 2). Only Stevenson 2003 provided information on sequence generation for randomisation. Concealment of allocation and blinding were judged adequate in three studies (Bergman 1982; Braun 1987; Stevenson 2003). Hence, these three trials were considered to be at a lower risk of bias than the other five trials. The trialists' reported definitions of outcome varied, as did length of follow‐up periods. Wound infection required microbiological confirmation in five studies (Bergman 1982; Braun 1987; Patzakis 1974; Rojczyk 1983; Sloan 1987).
1.
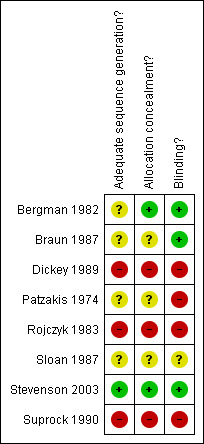
Risk of bias summary: review authors' judgements.
2.
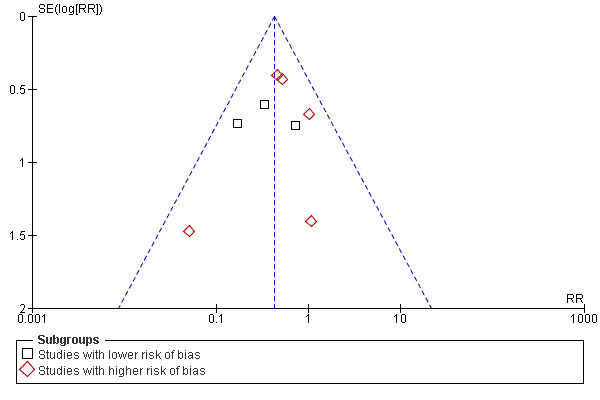
Funnel plot of comparison: 1 Any antibiotic treatment versus control, outcome: 1.1 Early wound infection.
Effects of interventions
Although there was no uniform definition of the primary outcome measure, we considered that those measures used in each study were clinically sufficiently consistent to permit pooling. The data did not permit the analysis of secondary outcomes (osteomyelitis, chronic drainage, infected non‐union, amputation for infection, or infection‐related death).The antibiotic regimens varied significantly in terms of agents used and duration, but we found each regimen likely to be effective at the time of the study against the gram‐positive organisms usually associated with early infection in open fractures.
The data from 1106 participants in the eight studies were pooled (Analysis 1.1), subgrouped by lower and higher risk of bias (seeFigure 1). In the overall analysis, antibiotics significantly reduced the incidence of wound infection (risk ratio (RR) 0.43, 95% confidence interval (CI) 0.29 to 0.65). The absolute risk of wound infection was 0.11 (53/461) in the controls (no antibiotic) and 0.05 (33/645) in those receiving any antibiotics, giving an absolute risk reduction of 0.07 (95% CI 0.03 to 0.10) (Analysis 1.2). In the subgroup analysis, the lower risk of bias group had an absolute risk of wound infection of 0.13 (22/169) for the controls and 0.04 (9/201) for those receiving antibiotics (risk difference ‐0.09 (95% CI ‐0.03 to ‐0.15), while the no‐placebo group had an absolute risk of 0.11 (31/292) for the controls and 0.05 (24/444) for those receiving antibiotics (risk difference ‐0.05 (95%CI ‐0.01 to ‐0.10). The difference between these subgroups was not statistically significant.
1.1. Analysis.
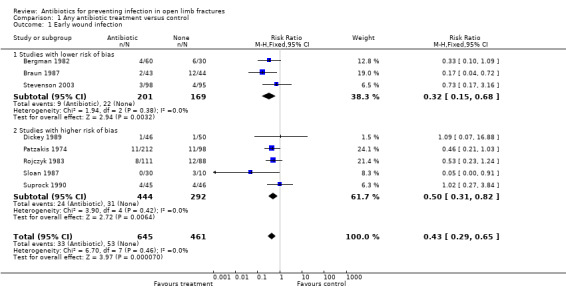
Comparison 1 Any antibiotic treatment versus control, Outcome 1 Early wound infection.
1.2. Analysis.
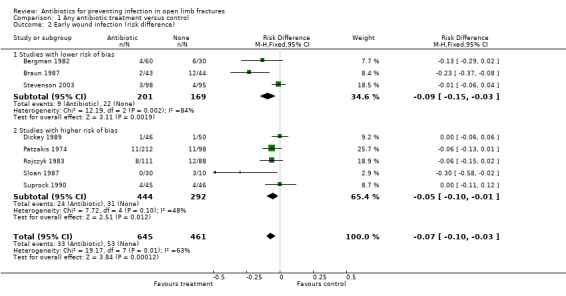
Comparison 1 Any antibiotic treatment versus control, Outcome 2 Early wound infection (risk difference).
Subgroup analysis (Analysis 1.3) explored the importance of fracture location and found that in open finger fractures, there was no evidence of significant benefit from antibiotics (3 trials, 367 participants; RR 0.56, 95% CI 0.26 to 1.23). In trials which did not include open finger fractures, antibiotics significantly reduced early infection (4 trials, 472 participants, RR 0.37, 95% CI 0.21 to 0.68). However, the difference between the two groups was not statistically significant.
1.3. Analysis.
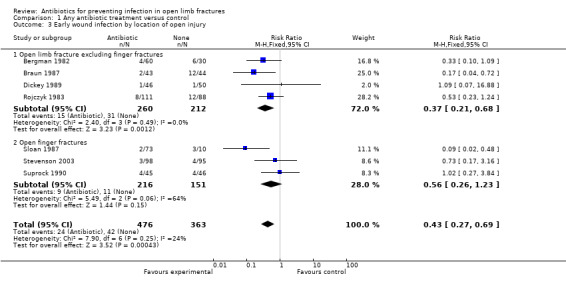
Comparison 1 Any antibiotic treatment versus control, Outcome 3 Early wound infection by location of open injury.
Subgroup analysis based on the timing of antibiotic administration was not possible as the necessary data were not available.
Discussion
Summary of main results
Despite the risk of bias and relative lack of power of most studies, meta‐analysis supports the effectiveness of antibiotics active against gram‐positive organisms in reducing the incidence of early infection when administered before or at the time of primary treatment of an open limb fracture, at least in the short term. All authors agree that antibiotic therapy is an adjunct to, not a replacement for, a comprehensive open fracture management protocol that includes early lavage, surgical debridement, fracture stabilisation when appropriate, and bone grafting and wound coverage if necessary. Subgroup analysis indicates that antibiotic prophylaxis administered to people with open finger fractures may not reduce the incidence of early infection, but the difference between subgroups is not significant. We did not include trials comparing one antimicrobial agent against another, or comparing single dose or short course administration with longer periods of prophylaxis.
Overall completeness and applicability of evidence
Although the number of included studies is small, and the review has a number of limitations in overall completeness and applicability, it establishes with some confidence the effectiveness of antibiotic prophylaxis directed at gram‐positive organisms in reducing early infection after open fracture in the extremities.
We feel confident that our search strategy was thorough and comprehensive, although limited to Europe and the Americas. We did not apply any language restriction, but we were unable to search Chinese or Japanese databases. We cannot rule out the possibility of publication bias.
The limitations in completeness and applicability arise from the limited scope of the included studies as much as from their quality. They include:
1. Data from the included studies does not permit the analysis of a number of longer‐term outcomes, such as chronic osteomyelitis, infected non‐union, amputation for infection, or infection‐related death.
2. Although raising the hypothesis that antibiotics may possibly not be effective in the case of relatively low energy injuries (phalangeal fractures in the finger), the available data did not allow exploration of whether effectiveness differs with the severity or grade of the open wound.
3. Available data did not allow exploration of the optimal duration of prophylaxis in respect of either effectiveness or adverse effects.
4. This review does not address some of the important issues faced in the management of major trauma in the first decade of the 21st century, in which the treatment of infection caused by antimicrobial resistant organisms, both gram‐positive and gram‐negative, plays an important part. The included studies did not address the question of whether antibiotic prophylaxis contributes to the development of antimicrobial resistance, as a number of studies in the last decade have suggested. Nor did they address the question of whether prophylaxis should take into account the increasing incidence of multiple antibiotic resistance amongst gram‐positive organisms, particularly Staphylococcus aureus. Nor did they provide useful data on the incidence of hypersensitivity reactions to the antibiotic agents used.
5.The extent to which results from studies carried out in developed countries, where antibiotics are part of a comprehensive open fracture management protocol, are applicable to developing countries remains debatable. We found no studies from developing countries comparing antibiotics as the main, or even the only initial treatment of open fractures of the limbs, to placebo or no treatment. Nevertheless, it seems reasonable to assume that, at least to some extent, the findings of this review would apply to low‐resource settings. If good data on open fracture prevalence and incidence were available for a given population, it could be possible to estimate the morbidity burden that might be avoidable. An economic analysis taking the local context into account could then evaluate the costs and benefits.
Although it seems somewhat unlikely that further placebo‐controlled trials of antibiotic prophylaxis for open fracture management could be justified, future studies could certainly address longer‐term outcomes and duration of prophylaxis (probably single dose versus a longer but restricted regimen). Dellinger 1991 listed recommendations for the conduct and reporting of clinical trials on the management of open fractures which should improve both study quality and applicability of data.
Quality of the evidence
Only three placebo‐controlled included studies (Bergman 1982; Braun 1987; Stevenson 2003 ) were judged to have both adequate allocation concealment and blinding. The subgroup analysis which shows a larger effect in these studies with lower risk of bias than in those with higher risk of bias is reassuring. Another potential weakness comes from the relatively short periods of follow‐up in most studies, although it is impossible to estimate in which direction this would bias the results. On the other hand, very few patients were lost to follow‐up, and all data analyses appear to have been intention‐to‐treat analyses.
The precision of the results of this systematic review may be decreased by the relatively small numbers of included studies, and participants. This is unlikely to change in the future. It is doubtful whether further trials using placebo or no antibiotics will be justifiable, as the use of antibiotics has been part of the standard care of open fractures of the extremities since the mid 1970s, despite some controversy about its use in open fractures of the fingers.
Agreements and disagreements with other studies or reviews
The Eastern Association for the Surgery of Trauma (EAST 2000) has published a literature review leading to "practice management guidelines for prophylactic antibiotic use in open fractures" which is in agreement with the findings of this review. More recently, Hauser 2006, although noting that "current antibiotic management of open fractures is based on a small number of studies that generally are more than 30 years old and do not reflect current management priorities in trauma and critical care", concluded, as this review does, that a short course of an agent effective against gram‐positive organisms such as a first‐generation cephalosporin "begun as soon as possible after injury, significantly lowers the risk of infection when used in combination with prompt, modern orthopedic fracture wound management".
A recent systematic review and economic analysis (Cranny 2008) has addressed the issue of whether the choice of agent for routine antibiotic prophylaxis for surgery should take account of multiple antibiotic resistance in Staphylococcus aureus (MRSA) by a switch from non‐glycopeptide to glycopeptide prophylaxis. It concluded that "There is insufficient evidence to determine whether there is a threshold prevalence of MRSA at which switching from non‐glycopeptide to glycopeptide antibiotic prophylaxis might be clinically effective and cost‐effective. Future research needs to address the complexities of decision‐making relating to the prevention of MRSA and infection control in general. Research including evidence synthesis and decision modelling comparing a full range of interventions for infection control, which extends to other infections, not just MRSA, is needed."
The problem of adverse effects of antibiotic prophylaxis, in particular hypersensitivity reactions, the development of antibiotic associated diarrhoea, and the development of antibiotic resistance to which this review was unable to contribute any evidence has recently been reviewed in a contemporary Practice Guideline on antibiotic prophylaxis in surgery (SIGN 2008).
Authors' conclusions
Implications for practice.
The use of a prophylactic antibiotic regimen effective against gram‐positive organisms (for example a narrow spectrum beta‐lactam agent such as dicloxacillin or flucloxacillin, or a first generation cephalosporin), begun as soon as possible after injury, significantly lowers the risk of early infection after an open fracture when used in combination with good wound management.
Although no relevant data could be provided by this review, health professionals and people at risk should ensure their awareness of the adverse effects of antibiotic prophylaxis.
Implications for research.
1. Further placebo controlled trials to evaluate the effectiveness of antibiotics for open fractures of the limbs proximal to the phalanges are unwarranted. There may be a case for further evaluation of the effectiveness of antibiotic prophylaxis in open finger fractures.
2. For those populations for which antibiotics are not universally used in the management of open fractures, reliable epidemiological data on the incidence and prevalence of open fractures, prevalence of antibiotic utilisation, and incidence and prevalence of infectious complications would allow quantifying the avoidable burden associated with this problem.
3. Both in these low‐resource settings, where management of open fractures is inconsistent, and in settings where antibiotic administration is routine, there may be justification for further studies comparing the benefits of single versus multiple doses of antibiotics, and which antibiotic by which route.
4. Economic analysis would then allow the most cost‐effective intervention regimen to be determined, and to value the costs and benefits associated with burden reduction.
What's new
| Date | Event | Description |
|---|---|---|
| 28 July 2009 | New search has been performed | Search updated to July 2009 and one new trial (Stevenson 2003) included. Comparison with other reviews updated. Consequential changes to text entered. No overall change to conclusions but additional subgroup analysis conducted. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 25 September 2008 | Amended | Converted to new review format. |
| 29 October 2003 | New search has been performed | First review version published |
Acknowledgements
The authors would like to acknowledge the following for their help in the preparation of the original review, and this update: Ms Leeann Morton, Mrs Lesley Gillespie and Ms Frances Bunn. We would also like to thank the following for their useful comments during the editorial review process: Professor John Stothard, Professor Marc Swiontkowski, Professor Rajan Madhok, Associate Professor Peter Herbison and Dr Janet Wale.
Appendices
Appendix 1. Search strategies
The Cochrane Library (Wiley InterScience interface)
#1 MeSH descriptor Antibiotic Prophylaxis, this term only #2 MeSH descriptor Anti‐Bacterial Agents explode all trees #3 (antibiotic* or antimicrob*):ti,ab,kw in Clinical Trials #4 (#1 OR #2 OR #3) #5 MeSH descriptor Infection, this term only #6 MeSH descriptor Wound Infection explode all trees #7 MeSH descriptor Sepsis explode all trees #8 (infect*):ti,ab,kw in Clinical Trials #9 (#5 OR #6 OR #7 OR #8) #10 MeSH descriptor Fractures, Bone explode all trees #11 MeSH descriptor Fracture Fixation explode all trees #12 (fractur*):ti,ab,kw in Clinical Trials #13 (#10 OR #11 OR #12) #14 (#4 AND #9 AND #13) (99 records identified)
MEDLINE (Ovid interface)
1 Antibiotic Prophylaxis/ 2 exp Antibiotics/ 3 (antibiotic$ or antimicrob$).tw. 4 or/1‐3 5 Infection/ 6 exp Wound Infection/ 7 Sepsis/ 8 infect$.tw. 9 or/5‐8 10 exp Fractures, Bone/ 11 exp Fracture Fixation/ 12 fractur$.tw. 13 or/10‐12 14 and/4,9,13 15 Randomized Controlled Trial.pt. 16 Controlled Clinical Trial.pt. 17 randomized.ab. 18 placebo.ab. 19 drug therapy.fs. 20 randomly.ab. 21 trial.ab. 22 groups.ab. 23 or/15‐22 24 exp Animals/ not Humans/ 25 23 not 24 26 and/14,25 (515 records identified)
EMBASE (Ovid interface)
1 exp Antibiotic Agent/ 2 (antibiotic$ or antimicrob$).tw. 3 or/1‐2 4 exp Infection/ 5 Infection Prevention/ 6 Infection Complication/ 7 or/4‐6 8 exp Fracture/ 9 exp Fracture Treatment/ 10 fractur$.tw. 11 or/8‐10 12 and/3,7,11 13 exp Randomized Controlled Trial/ 14 exp Double Blind Procedure/ 15 exp Single Blind Procedure/ 16 exp Crossover Procedure/ 17 Controlled Study/ 18 or/13‐17 19 ((clinical or controlled or comparative or placebo or prospective$ or randomi#ed) adj3 (trial or study)).tw. 20 (random$ adj7 (allocat$ or allot$ or assign$ or basis$ or divid$ or order$)).tw. 21 ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw. 22 (cross?over$ or (cross adj1 over$)).tw. 23 ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw. 24 or/19‐23 25 or/18,24 26 limit 25 to human 27 and/12,26 (524 records identified)
LILACS (Clinical Trials in LILACS)
((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE‐BLIND METHOD OR Mh SINGLE‐BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) [Palavras] and (antibiotic$ OR antimicrob$) [Palavras] and (fractur$) [Palavras] (1 record identified)
International Pharmaceutical Abstracts (Ovid interface)
1. (antibiotic$ or antimicrob$ or infect$ or septic or sepsis).tw. 2. (fractur$ and open).tw. 3. and/1‐2 (10 records identified)
Appendix 2. Search strategy for MEDLINE (OVID WEB) in previous versions
1. Antibiotic Prophylaxis/ 2. exp Antibiotics/ 3. (antibiotic$ or antimicrob$).tw. 4. or/1‐3 5. Infection/ 6. exp Wound Infection/ 7. Sepsis/ 8. infect$.tw. 9. or/5‐8 10. and/4,9 11. Fractures, Open/ 12. exp Fractures/ 13. (open or compound).tw. 14. and/12‐13 15. (infect$ adj3 (bone$ or fracture$)).tw 16. or/11,14,15 17. and/10,16
Data and analyses
Comparison 1. Any antibiotic treatment versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early wound infection | 8 | 1106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.29, 0.65] |
| 1.1 Studies with lower risk of bias | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.15, 0.68] |
| 1.2 Studies with higher risk of bias | 5 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.82] |
| 2 Early wound infection (risk difference) | 8 | 1106 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.10, ‐0.03] |
| 2.1 Studies with lower risk of bias | 3 | 370 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.15, ‐0.03] |
| 2.2 Studies with higher risk of bias | 5 | 736 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.10, ‐0.01] |
| 3 Early wound infection by location of open injury | 7 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.27, 0.69] |
| 3.1 Open limb fracture excluding finger fractures | 4 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.68] |
| 3.2 Open finger fractures | 3 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.26, 1.23] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bergman 1982.
| Methods | RCT Double‐blinded Placebo Cultures for diagnosis | |
| Participants | Sweden 90 adults with open extremity fractures, excluding hand and finger fractures, recruited over 30 months 60 cases treated with dicloxacillin or benzyl penicillin 30 controls treated with placebo | |
| Interventions | 1. Intervention: 2 g dicloxacillin: first dose given pre‐operatively with infusions repeated every six hours for two days. 2. Intervention: 3 million units benzyl penicillin: first dose given pre‐operatively with infusions repeated every six hours for two days. 3. Control: 100 ml saline: in the same dosing regimen as for the intervention groups. | |
| Outcomes | Early wound infection: a wound was considered infected when signs of inflammation were present, i.e. supra‐fascial drainage and a positive bacterial culture. Deep infection was defined as a subfascial process going down to the bone or osteosynthesis material. Superficial thrombophlebitis was defined as a palpable fibrotic vessel or visible inflammation along the course of the vessel. | |
| Notes | F/U until wound healed or drainage | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "The patients were randomly allocated to treatment" |
| Allocation concealment? | Low risk | "The drugs were packed in coded boxes according to random number, each box containing divided doses for a treatment period for one patient" |
| Blinding? All outcomes | Low risk | Double blind. "The patients were treated with either dicloxacillin, benzyl penicillin or saline (placebo)". |
Braun 1987.
| Methods | RCT Double‐blinded Placebo Cultures for diagnosis | |
| Participants | Switzerland 100 consecutive adults with open extremity fractures 13 excluded (hand fractures, skull fractures, colon injury, previous use of antibiotics) 87 participants: 43 cases treated with cloxacillin 44 controls treated with placebo | |
| Interventions | 1. Intervention: During the first four days, four 1 g doses of cloxacillin were administered intravenously. Thereafter, four 1.5 g doses of cloxacillin were given orally for six days. 2. Control: Indistinguishable placebo given in the same dosing regimen as for the intervention group. | |
| Outcomes | Early wound infection: (up to 6 weeks). Wound swabs were taken at weekly intervals from the base of the wound and the surrounding skin. | |
| Notes | F/U up to 10 months Cloxacillin group: ‐ 2 urticaria ‐ 3 Gastro‐intestinal symptoms ‐ 1 phlebitis Placebo group: ‐ 7 phlebitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear; no description given |
| Allocation concealment? | Unclear risk | Placebo controlled |
| Blinding? All outcomes | Low risk | Double blind |
Dickey 1989.
| Methods | CCT quasi‐randomised (alternate days) No blinding No placebo No cultures | |
| Participants | USA
96 adults with open extremity fractures, excluding hand and finger fractures, recruited over 20 months:
46 cases with treatment
50 controls without treatment
Results reported on 32 cases treated with cefazolin, 35 without treatment Hand injuries excluded. |
|
| Interventions | 1. Intervention: One day of intravenous cefazolin, three 1 g doses given eight hourly. 2. Control: No antibiotics | |
| Outcomes | Early wound infection: any wound complication including prolonged drainage, erythema, or any physical findings such as cellulitis, localised fluctuance or drainage. | |
| Notes | F/U until bony union 30% loss to F/U low‐velocity gunshot wounds only case‐definition? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate. Alternate days |
| Allocation concealment? | High risk | Inadequate. Alternate days. |
| Blinding? All outcomes | High risk | Not blinded |
Patzakis 1974.
| Methods | RCT No blinding No placebo Cultures for diagnosis | |
| Participants | USA 310 consecutive patients with open extremity fractures, including hand and finger fractures, recruited over 12 months 212 cases treated with penicillin and streptomycin, or cephalotin 98 controls without treatment | |
| Interventions | 1. Intervention: Adults received 10 million units benzyl penicillin per day in a continuous infusion and 0.5 g streptomycin intramuscularly every 12 hours. Children received 100,000 units of penicillin per kilogram of body weight per day in a continuous infusion and 7.5 mg of streptomycin per kilogram of body weight every 12 hours intramuscularly. 2. Intervention: Both adults and children received cephalothin, 100 mg per kilogram of body weight per day in divided dosage intravenously every six hours. 3. Control: No antibiotics. | |
| Outcomes | Early wound infection: clinical signs and symptoms of wound infection present such as fever, erythema, tenderness, and wound drainage with either a positive gram stain or a positive culture. Either of the latter two had to be present before the wound was classified as infected. | |
| Notes | Length of F/U not specified. If gunshot wounds excluded (none got an infection) infections in cases: 11 in 176 Infections in controls: 11 in 79 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Consecutive patients "randomly assigned to one of three study groups: Group I, no antibiotics; Group II, penicillin and streptomycin; and Group III, cephalothin (Keflin)". |
| Allocation concealment? | Unclear risk | Consecutive patients "randomly assigned to one of three study groups: Group I, no antibiotics; Group II, penicillin and streptomycin; and Group III, cephalothin (Keflin)". |
| Blinding? All outcomes | High risk | No placebo |
Rojczyk 1983.
| Methods | CCT quasi‐randomised (alternate days) No blinding No placebo Cultures for diagnosis | |
| Participants | Germany 199 participants of all ages with open extremity fractures, excluding hand and finger fractures, recruited over 30 months: 111 cases treated with cefazolin 88 controls without treatment | |
| Interventions | 1. Intervention: Cefazolin 1 g intravenously every six hours for five days. 2. Control: no antibiotics | |
| Outcomes | Early wound infection | |
| Notes | Hand and feet open fractures excluded. 80% F/U at 1 year | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate. Alternate days |
| Allocation concealment? | High risk | Inadequate. Alternate days |
| Blinding? All outcomes | High risk | Not blinded |
Sloan 1987.
| Methods | RCT No blinding Placebo controlled Cultures for diagnosis | |
| Participants | UK
40 adults with open finger fractures 85 planned, but stopped after 40 because of 30% infection rate in placebo group Study continued with remaining three antibiotics groups ‐ time? 30 cases treated with one of three regimens of cephradine 10 controls treated with placebo |
|
| Interventions | 1. Intervention: Cephradine 500 mg orally every six hours for five days 2. Intervention: Cephradine 1 g intravenously followed by 500 mg orally every six hours for five days 3. Intervention: Cephradine 1 g intravenously followed by one dose of 1 g orally 4. Control: Placebo | |
| Outcomes | Early wound infection: Swabs were taken if there was any evidence of infection (erythema, pus or exudate). | |
| Notes | Only fingers F/U 5 days 8 lost to F/U | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Insufficient information |
| Allocation concealment? | Unclear risk | Insufficient information |
| Blinding? All outcomes | Unclear risk | Placebo controlled but insufficient information on early stop. |
Stevenson 2003.
| Methods | RCT Placebo controlled Double blind Cultures for diagnosis (infection was defined on clinical grounds) | |
| Participants | UK
200 randomised. 7 did not meet criteria on review, or were lost. 193 analysed.
98 treated with flucloxacillin
93 controls treated with placebo Inclusion criteria: recent fracture of a distal phalanx in a finger with an overlying wound, including fractures associated with subungual hematoma bleeding externally or trephined as part of the treatment process. Exclusion criteria: less than 16 years of age, wound was more than 12 hours old, history of diabetes or symptomatic peripheral vascular disease, taking oral steroids, fracture caused by a bite, already coincidentally taking an antibiotic or had a known allergy to penicillin. |
|
| Interventions | 1. Flucloxacillin 250 mg capsule 2. Lactose placebo | |
| Outcomes | Infection, defined using clinical parameters of erythema, pain, swelling, wound discharge, presence of pus or cellulitis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Two hundred identical bottles of study medication were prepared in the hospital pharmacy department. The bottles were randomised into blocks of ten using a random number table and sequentially labelled. Each group of ten bottles consisted of five of placebo and five of flucloxacillin." |
| Allocation concealment? | Low risk | "Sealed code identifying each bottle was kept in the pharmacy department and not opened until completion" |
| Blinding? All outcomes | Low risk | "Each patient entered in the study was given the next sequential bottle containing the study medication and instructed to take two capsules four times daily for five days." |
Suprock 1990.
| Methods | CCT quasi‐randomised (alternate days) No blinding No placebo No cultures | |
| Participants | USA 91 patients with open finger fractures recruited over 25 months 45 cases treated with cephalosporin, dicloxacillin or erythromycin 46 controls without treatment | |
| Interventions | 1. Intervention: either a first generation cephalosporin, dicloxacillin or erythromycin for three days ‐ dose and route of administration were not reported. 2. Control: no antibiotics. | |
| Outcomes | Early wound infection: clinical signs of infection ‐ full details not reported. | |
| Notes | Only fingers F/U up to one year No antibiotic dosage reported. Cultures done only if clinical suspicion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Inadequate. Alternate days |
| Allocation concealment? | High risk | Inadequate. Alternate days |
| Blinding? All outcomes | High risk | Not blinded |
F/U: follow‐up
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almanza 1999 | CCT. Non randomised comparison. |
| Altergott 2008 | RCT. Antibiotic therapy versus no antibiotic for fingertip injuries in children. Only 39% of 146 randomised had fracture. No separate data presented. |
| Cutler 1944 | CCT. Non randomised comparison. Fractures are not separated from soft tissue injuries only. |
| Miller 1986 | RCT. All participants received an initial dose of antibiotics, then were randomised to more or no more antibiotics. |
| Peacock 1988 | RCT. Fractures are not separated from soft tissue injuries only. |
Contributions of authors
All reviewers contributed to the protocol development and the editing of the review. One reviewer (RG) executed the search strategy. All reviewers independently assessed trial quality and extracted data. WJG contributed to the drafting of this update. Richard Gosselin is the guarantor of the review.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bergman 1982 {published data only}
- Bergman BR. Antibiotic prophylaxis in open and closed fractures: a controlled clinical trial. Acta Orthopaedica Scandinavica 1982;53(1):57‐62. [DOI] [PubMed] [Google Scholar]
Braun 1987 {published data only}
- Braun R, Enzler MA, Rittmann WW. A double‐blind clinical trial of prophylactic cloxacillin in open fractures. Journal of Orthopaedic Trauma 1987;1(1):12‐7. [DOI] [PubMed] [Google Scholar]
Dickey 1989 {published data only}
- Dickey RL, Barnes BC, Kearns RJ, Tullos HS. Efficacy of antibiotics in low‐velocity gunshot fractures. Journal of Orthopaedic Trauma 1989;3(1):6‐10. [DOI] [PubMed] [Google Scholar]
Patzakis 1974 {published data only}
- Patzakis MJ, Harvey JP, Ivler D. The role of antibiotics in the management of open fractures. Journal of Bone and Joint Surgery. American Volume 1974;56(3):532‐41. [PubMed] [Google Scholar]
- Patzakis MJ, Ivler D. Antibiotic and bacteriologic considerations in open fractures. Southern Medical Journal 1977;70 Suppl 1:46‐8. [DOI] [PubMed] [Google Scholar]
Rojczyk 1983 {published data only}
- Rojczyk M. Antibiotic doses in open fractures. Hefte zur Unfallheilkunde 1980;148:861‐2. [DOI] [PubMed] [Google Scholar]
- Rojczyk M. Treatment results in open fractures, aspects of antibiotic therapy [Behandlungsergebnisse bei offenen frakturen, aspekte der antibiotikatherapie]. Hefte zur Unfallheilkunde 1983;162:33‐8. [DOI] [PubMed] [Google Scholar]
- Rojczyk M, Malottke R. The effect of antibiotic prophylaxis in the treatment of open fractures [Untersuchungen uber den Einfluss einer Antibiotica‐prophylaxe bei der Behandlung offener Frakturen]. Hefte zur Unfallheilkunde 1979;138:355‐7. [PubMed] [Google Scholar]
Sloan 1987 {published data only}
- Sloan JP, Dove AF, Maheson M, Cope AN, Welsh KR. Antibiotics in open fractures of the distal phalanx?. Journal of Hand Surgery ‐ British Volume 1987;12(1):123‐4. [DOI] [PubMed] [Google Scholar]
Stevenson 2003 {published data only}
- Stevenson J, McNaughton G, Riley J. The use of prophylactic flucloxacillin in treatment of open fractures of the distal phalanx within an accident and emergency department: a double‐blind randomised placebo‐controlled trial. Journal of Hand Surgery ‐ British Volume 2003;28(5):388‐94. [DOI] [PubMed] [Google Scholar]
Suprock 1990 {published data only}
- Suprock MD, Hood JM, Lubahn JD. Role of antibiotics in open fractures of the finger. Journal of Hand Surgery ‐ American Volume 1990;15(5):761‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almanza 1999 {published data only}
- Almanza AJ, Reyes AG, Diaz RR. Antibiotic treatment in open fractures [Tratamiento antimicrobiano en las fracturas expuestas]. Revisa Mexicana Ortopedica Traumatologia 1999;13(5):470‐1. [Google Scholar]
Altergott 2008 {published data only}
- Altergott C, Garcia FJ, Nager AL. Pediatric fingertip injuries: do prophylactic antibiotics alter infection rates?. Pediatric Emergency Care 2008;24(3):148‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cutler 1944 {published data only}
- Cutler EC, Morton PC, Sandusky WR. Observations on the prophylactic use of penicillin in the wounds of aerial warfare. British Journal of Surgery 1944;32:207‐11. [Google Scholar]
Miller 1986 {published data only}
- Miller SD, Bray RC, Hughes GNF. Antibiotics in open fractures: a prospective randomised, double‐blind study of wound infection [abstract]. Journal of Bone and Joint Surgery ‐ British Volume 1986;68(5):850. [Google Scholar]
Peacock 1988 {published data only}
- Peacock KC, Hanna DP, Kirkpatrick K, Breidenbach WC, Lister GD, Firrell J. Efficacy of perioperative cefamandole with postoperative cephalexin in the primary outpatient treatment of open wounds of the hand. Journal of Hand Surgery ‐ American Volume 1988;13(6):960‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Cranny 2008
- Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al. A systematic review and economic model of switching from non‐glycopeptide to glycopeptide antibiotic prophylaxis for surgery. Health Technology Assessment 2008;12(1):i‐168. [DOI] [PubMed] [Google Scholar]
Dellinger 1991
- Dellinger EP. Antibiotic prophylaxis in trauma: penetrating abdominal injuries and open fractures. Reviews of Infectious Diseases 1991;13(Suppl 10):S847‐57. [DOI] [PubMed] [Google Scholar]
EAST 2000
- Luchette FA, Bone LB, Born CT, DeLong WG, Hoff WS, Mullins D, et al. EAST practice management guidelines work group: Practice management guidelines for prophylactic antibiotic use in open fractures. Eastern Association for the Surgery of Trauma www.east.org/tgp/openfrac.pdf (accessed 01 March 2002).
Gillespie 2001
- Gillespie WJ, Walenkamp G. Antibiotic prophylaxis for surgery for proximal femoral and other closed long bone fractures. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000244] [DOI] [PubMed] [Google Scholar]
Hauser 2006
- Hauser CJ, Adams CA Jr, Eachempati SR. Prophylactic antibiotic use in open fractures: An evidence‐based guideline. Surgical Infections 2006;7(4):379‐405. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies, Box 6.4.c. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
SIGN 2008
- Scottish Intercollegiate Guidelines Network (SIGN). Antibiotic prophylaxis in surgery. Edinburgh: SIGN, 2008. www.sign.ac.uk/pdf/sign104.pdf (accessed 26 July 2009). [ISBN 978 1 905813 34 6]
References to other published versions of this review
Gosselin 2004
- Gosselin RA, Roberts I, Gillespie WJ. Antibiotics for preventing infection in open limb fractures. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003764.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


