Abstract
Objective.
Augmentation of the number of cord blood (CB) hematopoietic stem cells (HSC) present in a unit is required before it can be considered as an alternative graft for hematopoietic reconstitution for adult patients. In order to further optimize strategies to augment HSC numbers, we examined whether expansion of HSC mediated by epigenetic mechanisms remains permissive to external environmental cues.
Materials and Methods.
The chromatin-modifying agents 5-aza-2′-deoxycytidine (5azaD) and trichostatin A (TSA) were used to ameliorate epigenetic alteration of CB cells during ex vivo culture by adding various cytokines. After culture, CD34+CD90+ cell numbers, their division history, in vitro clonogenic potential, and in vivo hematopoietic reconstitution potential and frequency were determined.
Results.
5azaD/TSA-treated, CD34+CD90+ cells were greatly influenced in terms of their degree of expansion, clonogenic potential, cell-division rate, and transplantability by the combination of cytokines used in culture. Furthermore, our current results verify that the sequential addition of 5azaD followed by TSA is crucial for expansion of HSC. We demonstrate that following 5azaD/TSA treatment, the rate of CD34+CD90+ cell division is also dependent on the cytokine cocktail and that this is associated with functional changes, including alteration of in vitro clonogenic potential and in vivo reconstitution potential.
Conclusions.
Our studies indicate there are interactions between intrinsic factors influenced by epigenetic mechanisms and external environmental signals in the regulation of HSC expansion. Epigenetic influences on HSC can be accentuated by environmental factors. Regulation of the rate of divisions may be a critical determinant for the maintenance of HSC functional potency during ex vivo expansion.
Silencing of gene transcription has been shown to be accompanied by DNA methylation of a gene’s promoter and by histone deacetylation in regions controlling specific genes [1–3]. Hypomethylating agents and histone deacetylase (HDAC) inhibitors have been shown to be capable of reversing epigenetically mediated loss of gene function [4,5]. Our previous results indicate that epigenetic mechanisms likely play a critical role in the loss of the in vivo repopulation potential of hematopoietic stem cells (HSC) during ex vivo culture, and that this can be circumvented by the addition of chromatin-modifying agents [6]. In order to determine the role of external humoral influences during chromatin-modifying agent-induced expansion of HSC, we further investigated the role of various cytokine combinations that may influence HSC expansion. Currently, there is no consensus about the optimal combination of cytokines to be used for HSC expansion. Most investigators have concluded that a cytokine cocktail containing stem cell factor (SCF), Flt3 ligand (FL), and thrombopoietin (TPO) is effective, or at least serves as a core cytokine combination to which additional cytokines may be added [7,8]. To date, expansion of HSC retaining in vivo marrow repopulating potential has not been successful, likely due to the irreversible loss of homing/engraftment-related activities that may be related to the transit of HSC through specific phases of the cell cycle [9–11].
It has been suggested that epigenetic mechanisms may serve to allow an organism to adapt to its environment by altering its gene expression pattern [12]. Our current studies indicate that exogenous cytokines added to the culture influence the epigenetic regulations that alter the degree of expansion of stem/progenitor cells and that, with time, epigenetic modifications are reversible. Because epigenetic changes are reversible, they can also be modulated by environmental factors, resulting in the altered fate or phenotype of a cell population [12].
In our previous studies, we have demonstrated that cord blood (CB) cells treated with 5-aza-2′-deoxycytidine (5azaD)/trichostatin A (TSA) with cytokines including TPO, SCF, and FL results in significant expansion of CD34+ CD90+ cells, including transplantable HSC [13,14]. In our current studies, we intend to examine whether changes in environmental conditions can influence epigenetic regulation of HSC fate choices as determined by the degree of expansion of transplantable HSC and their relationship to the rate of cell divisions. Our current studies provide evidence that epigenetic events governing the expansion of transplantable HSC are capable of modulation by the presence of exogenous cytokines added to the culture.
Materials and methods
Isolation and ex vivo cultures of CB CD34+ cells
Human CB collections were obtained from the New York Blood Center (New York, NY, USA) according to guidelines established by the Institutional Review Board. Low-density cells were enriched using the CD34 progenitor isolation kit (Miltenyi Biotech, Inc., Auburn, CA, USA) as described previously [13,14]. Purity of CD34+ cells ranged between 90% and 99%.
CD34+ cells were cultured in medium containing fetal bovine serum (HyClone Laboratories, Logan, UT, USA) supplemented with 100 ng/mL SCF, 100 ng/mL FL, 100 ng/mLTPO, and 50 ng/mL interleukin (IL)-3 (CellGenix USA, Antioch, IL, USA). In some experiments, 50 ng/mL granulocyte-macrophage colony-stimulating factor, or 50 ng/mL IL-6, or 5 U/mL erythropoietin was added. Cells were exposed to 5azaD (Pharmachemie B.V., Haarlem, Holland) and TSA (Sigma, St Louis, MO, USA) at the concentrations of 1 μM and 5 ng/mL, respectively, as described previously [13].
Flow cytometric analysis
Briefly, cells were stained with antihuman CD34 monoclonal antibody conjugated to fluorescein isothiocyanate and antihuman CD90 monoclonal antibody conjugated to phycoerythrin. Lineage markers included antigens associated with terminally differentiated hematopoietic cells (CD2, CD14, CD15, CD19, and glycophorin A). In some experiments, cells were stained with antihuman CD90 fluorescein isothiocyanate and antihuman CD11a, CD26, CD31, CD44, CD49d, CD62L, or CXCR-4 conjugated to phycoerythrin and antihuman CD34 conjugated to allophycocyanin. All monoclonal antibody were purchased from Becton Dickinson Pharmingen (San Diego, CA, USA). Cell-cycle status was determined by propidium iodide staining (Sigma).
Western blotting analysis
Primary CB CD34+ cells or CB cells expanded in various conditions were harvested and total cellular extracts or nuclear extracts were prepared by using the Mammalian Cell Extraction Kit and Nuclear/Cytosol Fractionation Kit (BioVision, Mountain View, CA, USA), respectively, according to manufacturer’s instructions. Samples were separated by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The blot was probed with antiacetylated histone H4 antibody, antihistone H4 antibody (Upstate Biotechnology, Lake Placid, NY, USA), anti-P21 antibody (Santa Cruz Technologies, Santa Cruz, CA, USA), or anti – β-actin antibody (Sigma) and developed using an enhanced chemiluminescence system and horseradish peroxidase–conjugated secondary antibody (Pierce Biotechnology, Rockford, IL, USA) as described previously [13].
In vitro progenitor cell assays
Colony-forming cell assays were performed in semisolid media as described previously [14]. Duplicate cultures were set up in Methocult (Stem Cell Technologies, Vancouver, BC, Canada), to which SCF, FL, IL-3, IL-6, granulocyte-macrophage colony-stimulating factor, and erythropoietin were added.
To quantitate the number of cobblestone area-forming cells (CAFC), primary CD34+ cells and ex vivo cultured CB cells were plated in limiting dilution onto an irradiated murine stromal fibroblast line (M2–10B4). CAFC frequency was computed by means of minimization of chi regression as described previously [14].
Carboxyfluorescein diacetate succinimidyl ester labeling to assess cell division
Primary CB CD34+ cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR, USA) on day 0 as described previously [13]. After 5 and 9 days of culture, cells were labeled with anti–CD34-allophycocyanin and anti–CD90-phycoerythrin and analyzed for a progressive decline of fluorescence intensity of CFSE, using flow cytometry [13].
In vivo marrow repopulating potential of ex vivo expanded CB cells
Immunodeficient nonobese diabetic/ltsz-scid/scid (NOD/SCID) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). NOD/SCID assay was performed as described previously [13]. Bone marrow (BM) cells from each mouse were analyzed after 8 weeks by flow cytometry to detect human hematopoietic cell engraftment.
Matrigel assay
In vitro cell migration was studied using a modified Matrigel-based assay as described previously [15]. Briefly, the lower chambers were filled with medium supplemented with 0.1% bovine serum albumin with 100 ng/mL stromal cell–derived factor-1 (R & D Systems, Inc. Minneapolis, MN, USA), and 8-μm filters coated with 20 μg Matrigel were placed between the compartments. Cells were placed in the upper compartments, and incubated for 5 hours at 37°C in 5% CO2 and allowed to migrate through the filters. The percentage of cell migration was calculated and clonogenic assays were also performed on the cells transmigrated and the nonmigrated cells.
Statistical analysis
Results are expressed as mean ± standard error when appropriate. Statistical differences were evaluated using the Student’s t-test with significance at p ≤ 0.05.
Results
Optimal culture conditions for ex vivo expansion of CB cells
CB CD34+ cells were cultured in media supplemented with SCF, FL, TPO, and IL-3 for the initial 48 hours. Cells were exposed to 5azaD (at 16 hours) followed by TSA at 48 hours when the cytokine cocktail was changed to various combinations of cytokines during the final 7 days of the 9-day culture period. Because we have previously demonstrated that only the CD34+CD90+ cell population gives rise to in vivo repopulating cells when transplanted in NOD/SCID mice, not the relatively committed CD34+ CD90− population, we have used CD34+CD90+ cell number within CD34+ cells for determination of expansion in this article [14]. The combination consisting of SCF, FL, and TPO (SFT) promoted the greatest expansion of CD34+CD90+ cells following 5azaD/TSA treatment in comparison to other cytokine combinations tested (Fig. 1A, Table 1). Addition of cytokines like IL-3, IL-6, granulocyte-macrophage CSF, and erythropoietin blunted the degree of CD34+CD90+ cell expansion, indicating that the fate of CB cells treated with 5azaD/TSA can be influenced by the addition of exogenous cytokine stimuli to the culture (Fig. 1A). On day 5 of culture, SFT in combination with 5azaD/TSA resulted in a 5.07 ± 0.62-fold expansion of CD34+CD90+ cells, however, when IL-3 and IL-6 were added with SFT (SFT36) with 5azaD/TSA treatment, 3.53 ± 0.09-fold expansion was observed (p = 0.069) (Fig. 1B). This difference became more pronounced by day 9, when the SFT and SFT36 cytokine combinations used after 5azaD/TSA treatment resulted in 11.7 ± 1.18-fold and 4.78 ± 0.7-fold expansions of CD34+CD90+ cells, respectively (p = 0.005) (Fig. 1B). Although expansion of primitive CD34+CD90+ cells in 5azaD/TSA-treated culture is significantly greater than the control cultures (cytokines alone), the 5azaD/TSA-treated cultures contained much fewer total nucleated cells (Table 1). Whether the lower total nucleated cells number in 5azaD/TSA-treated cultures is due to differential killing of more mature hematopoietic cells or a delay in differentiation or proliferation would require further investigation. When the culture period was extended to 14 days, there was no difference in the expansion of CD34+CD90+ cells, regardless of the cytokine cocktail used, with or without 5azaD/TSA treatment (Fig. 1B). Irrespective of the type of cytokine cocktail added to the culture, treatment of the culture with 5azaD/TSA resulted in greater expansion of CD34+CD90+ cells in comparison to control cultures at day 9 (Fig. 1A). These data clearly indicate that the cytokine-induced CD34+ CD90+ cell expansion is variable with the culture conditions and this is likely mediated by epigenetic mechanisms because it can be circumvented by use of inhibitors of methylation and acetylation.
Figure 1.
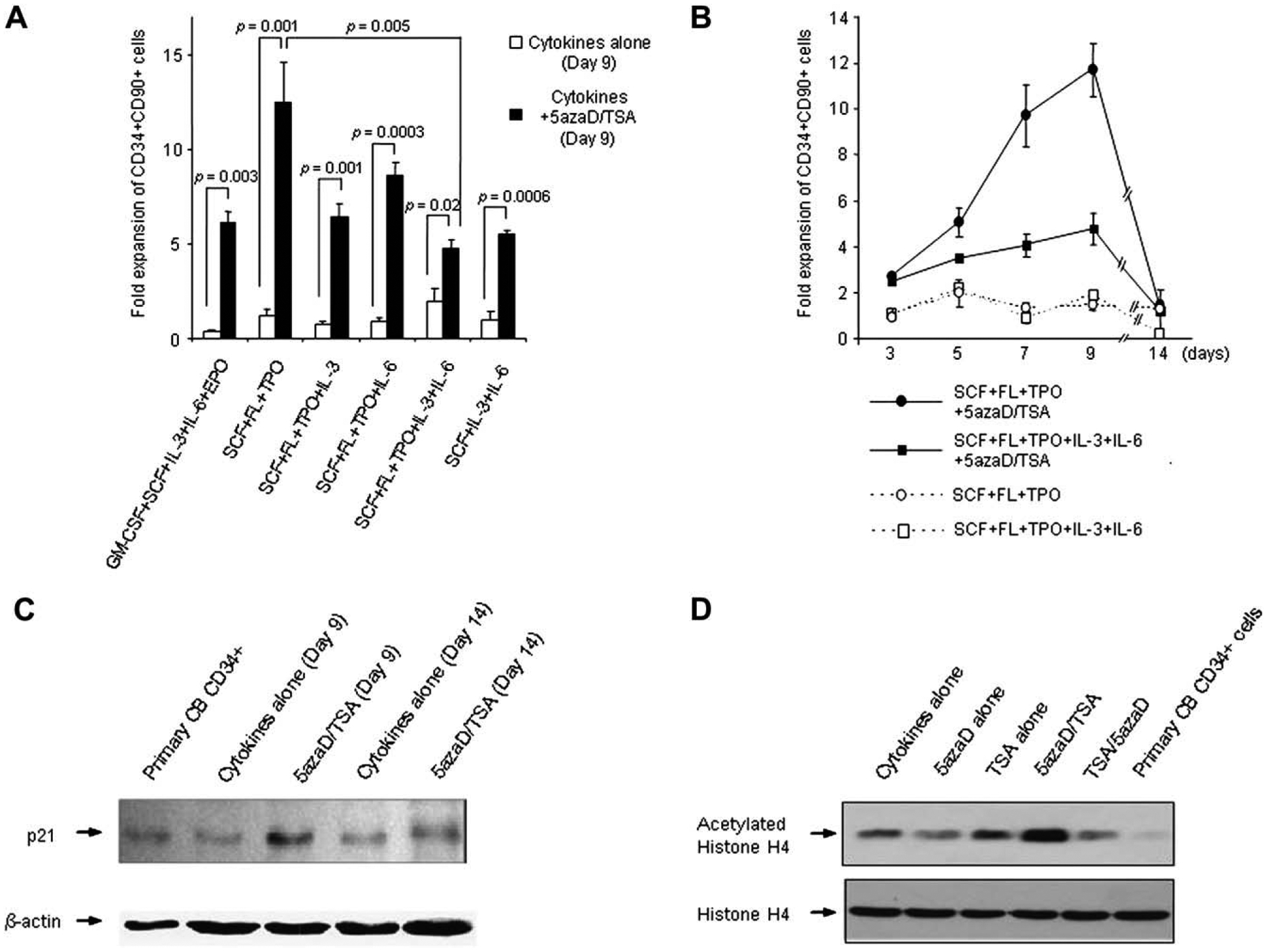
Effects of different cytokine combinations on the expansion of cord blood (CB) CD34+CD90+ cells. (A) Effect of 5-aza-2′-deoxycytidine (5azaD)/trichostatin A (TSA) on the number of CB CD34+CD90+ cells following 9 days of culture in the presence of various cytokine combinations. Cytokine combinations tested during the final 7 days of the 9 day culture were as follows: granulocyte-macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), interleukin (IL)-3, IL-6, erythropoietin (EPO); SCF, FL, TPO (SFT); SCF, FL, TPO, IL-3; SCF, FL, TPO, IL-6; SCF, FL, TPO, IL-3, IL-6 (SFT36); SCF, IL-3, IL-6. CD34+ cells (5 × 104/well) were cultured in the presence of SCF, FL, TPO, and IL-3 for the first 48 hours and then the cocktail of cytokines was changed to the designated combinations. The graph represents mean ± standard error of three independent experiments. (B) Effect of 5azaD/TSA on the number of CD34+CD90+ cells following ex vivo culture in various cytokine combinations. The fold expansion was determined at various time points by dividing the output number of CB CD34+CD90+ cells by the input number of CD34+CD90+ cells. (C) Protein levels of P21 were examined on CB cells at day 0, and on cultured CB cells with cytokines (SFT) alone or cytokines (SFT) and 5azaD/TSA at day 9 and day 14 by Western blot analysis. Equal loading of protein was verified with anti – β-actin antibody on the same membrane. (D) Status of histone H4 acetylation of CD34+ cells was examined by Western blot analysis to test the effects of the alteration of the sequence of addition of 5azaD and TSA. Parallel cultures were set up with CB CD34+ cells with cytokines (SFT) alone and SFT with the addition of chromatin-modifying agents; 5azaD alone, TSA alone, TSA followed by 5azaD and 5azaD followed by TSA for 72 hours. Cells were harvested and equal amounts of proteins were electrophoresed on 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. Acetylated-histone H4 level was checked by using anti-acetylated H4 antibody. Histone H4 level was used as a loading control.
Table 1.
Effects of different cytokine combinations on the number of TNCs, CD34+CD90+ cells and percentage of CD34+CD90+ cells
| Culture condition | No. of TNCs/well (×104) | % of CD34+CD90+ | No. of CD34+CD90+/well (×104) | |
|---|---|---|---|---|
| CB CD34+ (day 0) | 5.00 ± 0.00 | 29.2 ± 2.38 | 1.46 ± 0.12 | |
| GM-CSF + SCF + IL-3 + IL-6 + EPO | Cytokines alone | 734 ± 284 | 0.10 ± 0.03* | 0.56 ± 0.10 |
| Cytokines +5azaD/TSA | 91.3 ± 12.0 | 10.3 ± 1.95* | 8.93 ± 0.80 | |
| SCF + FL + TPO | Cytokines alone | 192 ± 79.1 | 1.40±0.32† | 2.23 ± 0.45 |
| Cytokines +5azaD/TSA | 53.5 ± 6.80 | 35.4 ± 5.83†,** | 18.3 ± 2.0 | |
| SCF + FL + TPO +IL-3 | Cytokines alone | 350 ± 138 | 0.55 ± 0.18‡ | 1.40 ± 0.15 |
| Cytokines +5azaD/TSA | 61.6 ± 8.20 | 15.9 ± 2.97‡ | 9.37 ± 0.98 | |
| SCF + FL + TPO +IL-6 | Cytokines alone | 296 ± 108 | 0.70 ± 0.21§ | 1.67 ± 0.27 |
| Cytokines +5azaD/TSA | 55.6 ± 6.80 | 23.1 ± 1.50§ | 12.6 ±0.89 | |
| SCF + FL + TPO + IL-3 + IL-6 | Cytokines alone | 411 ± 142 | 0.79 ± 0.23¶ | 2.90 ± 0.95 |
| Cytokines +5azaD/TSA | 119 ± 25.2 | 6.23 ± 1.00¶,** | 6.93 ± 0.61 | |
| SCF+IL-3 + IL-6 | Cytokines alone | 355 ± 135 | 0.35 ± 0.15‖ | 1.38 ± 0.62 |
| Cytokines +5azaD/TSA | 87.8 ± 17.0 | 9.77 ± 1.64‖ | 8.03 ± 0.27 | |
The effect of 5-aza-2′-deoxycytidine (5azaD)/trichostatin A (TSA) on the number of total nucleated cells (TNCs), CD34+CD90+ cells and percentage of CD34+CD90+ cells following 9 days of culture in the presence of various cytokine combinations were determined. CD34+ cells (5 × 104/well) were cultured in the presence of stem cell factor (SCF), Flt3 ligand (FL), thrombopoietin (TPO), and interleukin (IL)-3 for the first 48 hours and then the cocktail of cytokines was changed to the designated combinations. The cytokine combinations tested during the final 7 days of the 9 days culture were as follows: granulocyte-macrophage colony-stimulating factor (GM-CSF), SCF, IL-3, IL-6, erythropoietin (EPO); SCF, FL, TPO (SFT); SCF, FL, TPO, IL-3; SCF, FL, TPO, IL-6; SCF, FL, TPO, IL-3, IL-6 (SFT36); SCF, IL-3, IL-6. Each value presents the mean ± standard error of three independent experiments.
p = 0.006;
p = 0.004;
p = 0.007;
p = 0.0001;
P = 0.007;
p = 0.005;
p = 0.008.
Expression of P21 protein level correlates with the degree of expansion of CD34+CD90+ cells
Our current studies demonstrated that, despite treatment with 5azaD/TSA, expansion of CD34+CD90+ cells declines after 9 days of culture (Fig. 1B). Based on our previous studies, we examined the protein levels of the cyclin-dependent kinase inhibitor P21 in CB cells treated with 5azaD/TSA at day 9 and day 14 of ex vivo culture in comparison to cells expanded in SFT alone. We observed a dramatic decline in the number of CD34+CD90+ cells in both control and 5azaD/TSA-treated cultures at day 14 (Fig. 1B). At day 9 of cultures, while 5azaD/TSA-treated cells displayed greater levels of P21 protein in comparison to control cultures, at day 14 P21 levels decreased significantly in both cultures, corresponding with a decline in CD34+CD90+ cells (Fig. 1B, C). These findings indicate that a correlation may exist between maintenance of P21 protein levels and expansion of CD34+CD90+ cells mediated by epigenetic mechanisms.
Sequential addition of 5azaD followed by TSA is essential for expansion of CD34+CD90+ cells
It is known that DNA methylation and histone deacetylation appear to act in a synergistic manner in silencing genes in mammalian cells [16]. Thus, we studied the importance of the order of addition of chromatin-modifying agents to ex vivo expansion cultures using CB CD34+ cells. When we altered the sequence of addition of the chromatin-modifying agents by adding TSA first instead of 5azaD, the optimal degree of expansion of CD34+CD90+ cells was not achieved (Table 2), suggesting that sequential addition of 5azaD followed by TSA is crucial to promote the maximal expansion of CD34+CD90+ cells. Furthermore, acetylation of histone H4 is significantly reduced in cells in which the order of addition of 5azaD/TSA were reversed (Fig. 1D). Interestingly, ex vivo culture of CD34+ cells in cytokines alone (SFT) also induced increased histone H4 acetylation in comparison to unamnipulated fresh CB CD34+ cells. The significance of this remains unclear and needs to be explored in the future. Although the hypomethylating drug 5azaD alone was not capable of increasing acetylation of histone H4. When TSA was added, followed by 5azaD, it resulted in significant enhancement of histone H4 acetylation. Our data indicate that methylation needs to be targeted prior to targeting HDAC to achieve reacetylation of histones, which is required for gene reactivation. This is consistent with layers of histone deacetylation followed by DNA methylation of gene promoter sites needed for gene silencing [16].
Table 2.
Effects of 5-aza-2′-deoxycytidine/trichostatin A on the numbers of CD34+CD90+ cells
| Culture condition | Total nucleated cells (×104) | (%) CD34+CD90+ cells | Fold expansion of CD34+CD90+ cellsa | (%) S/G2/M |
|---|---|---|---|---|
| SFT + IL-3/SFT +5azaD/TSA (16 h 5azaD/48 h TSA) | 42.7 ± 9.7 | 31.4 ± 4.1 | 10.9 ± 0.8*,† | 12.5 ± 3.8‡ |
| SFT/SFT +5azaD/TSA (16 h 5azaD/48 h TSA) | 7.0 ± 1.3 | 42.9 ± 3.9 | 2.0 ± 0.3* | 6.6 ± 2.9‡ |
| SFT + IL-3/SFT +TSA/5azaD (16 h TSA/48 h 5azaD) | 8.3 ± 0.5 | 4.6 ± 0.6 | 0.3 ± 0.1† | ND |
The 5 × 104 CD34+ cells/well were cultured using the protocol described below.
SFT/SFT indicates that SCF + FL + TPO were added to culture for the first 48 hours that lacked interleukin-3 (IL-3), and stem cell factor (SCF)+Flt3 ligand (FL)+thrombopoietin (TPO) were added to culture for the final 7 days of the 9-day culture period.
SFT + IL-3/SFT indicates culturing in SCF + FL + TPO + IL-3 for the first 48 hours, followed by SCF + FL + TPO for the terminal 7 days; 16 hours 5-aza-2′-deoxycytidine (5azaD)/48 hours TSA indicates that 5azaD was added to the culture at 16 hours and trichostatin A (TSA) was added at 48 hours; 16-hour TSA/48-hour 5azaD indicates that TSA was added to culture at 16 hours and 5azaD was added at 48 hours.
Each value represents the mean of three independent experiments ± standard error.
Fold expansion was calculated as the total numbers of CD34+CD90+ cells at the end of culture period (output cells) over the number of primary CD34+CD90+ cell numbers (input number of cells started at the beginning of the culture).
ND = not determined.
p < 0.01;
p < 0.01;
p < 0.05.
IL-3 is essential during the first 48 hours of culture
Because 5azaD is a cell-cycle–specific drug, the efficacy of this substance is dependent up on a proportion of cells in active DNA synthesis phase (S/G2/M) of the cell cycle [17]. In our current studies, after 48 hours of culture CD34+ cells exposed to 5azaD/TSA either in the presence or absence of IL-3 were determined to be 12.5% ± 3.8% and 6.6% ± 2.9%, respectively, in the S/G2/M phases of the cell cycle (p < 0.05; mean ± standard error) (Table 2). We also questioned whether these mitogenic effects of IL-3 might blunt the effects of 5azaD/TSA on HSC expansion. As shown in Table 2, addition of IL-3 during the first 48 hours of culture was absolutely necessary for the 5azaD/TSA treatment to be effective in expanding CD34+CD90+ cells, generating about 11-fold more CD34+CD90+ cells than the starting cell number. However, the absence of IL-3 in the same culture resulted in a significant decrease of CD34+CD90+ cells (5.5-fold decrease) (Table 2). These findings suggest that use of IL-3 during the first 48 hours of culture increases the number of actively cycling cells, promoting the incorporation of 5azaD into the cells.
In vitro functional potential of 5azaD/TSA-treated expanded CB cells is influenced by cytokine cocktails used in culture
In order to ensure that the CD34+CD90+ cells expanded using 5azaD/TSA with cytokines possess functional potency comparable to unmanipulated primary CB CD34+CD90+ cells, we evaluated both short-term (colony-forming cells) and long-term (CAFC) clonogenic potential in the presence of cytokine combination SFT in comparison to the cytokine combination that supported the least degree of expansion, SFT36. The plating efficiency for colony-forming cells as well as the frequency of CAFC declined significantly when IL-3 and IL-6 (SFT36) were added to the optimal cytokine combination (SFT) in spite of 5azaD/TSA treatment (Table 3). In the presence of SFT, 5azaD/TSA treatment resulted in near-complete restoration of the primitive colony-forming unit (CFU)-Mix colony number to that of primary CB cells. Cultures without exposure to 5azaD/TSA (SFT alone) resulted in loss of almost 94% of long-term in vitro clonogeneic cells (CAFC), while cultures exposure to 5azaD/TSA and SFT cytokines retained 81.7% of CAFC from unmanipulated primary CD34+ cells. Despite containing only 50% CD34+ cells (day 9), the 5azaD/TSA-treated expanded cultures possessed a CAFC frequency, which is 81.7% of the frequency of CAFC assayed from the primary CD34+ cells (day 0), which are 90% to 99% CD34+. Moreover, we have shown that cytokines SFT in combination with 5azaD/TSA results in a 10- to 12-fold expansion of CD34+CD90+ cells, which possess a CAFC frequency comparable to primary CB cells. These data confirm that CD34+CD90+ cells expanded using 5azaD/TSA retained their in vitro functional potential as evidenced by their retention of comparable CAFC and CFU-Mix generation potential, potentially resulting in net expansion of both CFU-Mix and CAFC numbers.
Table 3.
Effects of 5-aza-2′-deoxycytidine/trichostatin A and cytokines combinations on cloning efficiency after ex vivo culture
| Culture condition | CFCs per 500 cellsa | ||||||
|---|---|---|---|---|---|---|---|
| CFU-GM | BFU-E | CFU-Mix | Total | Plating efficiencyb | CAFCs per 104 cellsa | ||
| CB CD34+ (Day 0) | 35.5 ± 1.8 | 43.0 ± 2.7 | 8.5 ± 0.8 | 87.0 ± 5.0 | 17.4 ± 1.0 | 70.2 ± 20.0 | |
| SCF + FL + TPO (Day 9) | Cytokines alone | 26.0 ± 1.3 | 8.0 ± 0.3 | 0.67 ± 0.3 | 34.3 ± 1.3 | 6.9 ± 0.3 | 4.2 ± 1.2 |
| Cytokines +5azaD/TSA | 37.7 ± 1.2 | 29.5 ± 4.3 | 7.0 ± 1.5* | 74.2 ± 6.9 | 14.8 ± 1.4 | 57.2 ± 10.3† | |
| SCF + FL + TPO +IL-3 +IL-6 (Day 9) | Cytokines alone | 18.3 ± 1.4 | 3.3 ± 1.7 | 0.0 ± 0.0 | 22.3 ± 2.0 | 4.3 ± 0.3 | 2.2 ± 1.2 |
| Cytokines +5azaD/TSA | 31.0 ± 3.1 | 19.8 ± 1.2 | 3.5 ± 0.3* | 54.3 ± 3.8 | 10.9 ± 0.2 | 24.8 ± 8.0† | |
5azaD = 5-aza-2′-deoxycytidine; BFU-E = burst-forming unit erythroid; CAFC = cobblestone area-forming cells; CB = cord blood; CFC = colony-forming cell; CFU-GM = colony-forming unit granulocyte-macrophage; FL = Flt3 ligand; IL = interleukin; SCF = stem cell factor; TPO = thrombopoietin; TSA = trichostatin A.
Each value presents the mean ± standard error of three independent experiments.
Plating efficiency is defined as (total number of hematopoietic colonies/ total cells plated) × 100.
p < 0.05;
p < 0.05.
Effects of cytokine combination on the cell division history of CD34+CD90+ cells
We have previously shown that the CD34+CD90+ cells expanded in 5azaD/TSA undergoing four or fewer cell divisions, including a significant fraction of CD34+CD90+ cells undergoing five or more divisions, retain higher clonogenic potential as well as in vivo hematopoietic reconstitution potential [13]. In our current studies, the rate of CD34+CD90+ cells cultured in SFT or SFT36 with or without 5azaD/TSA treatment were tracked using CFSE and their absolute numbers at day 5 and day 9 were compared. It was apparent that the cytokine combination SFT with 5azaD/TSA treatment had a significantly higher proportion of CD34+CD90+ cells (41%) that had undergone four or fewer cell divisions, in contrast to cultures containing only SFT (10%), SFT36 (6%) alone, or cultures treated with 5azaD/TSA with SFT36 (27%) at day 9 (Fig. 2A).
Figure 2.
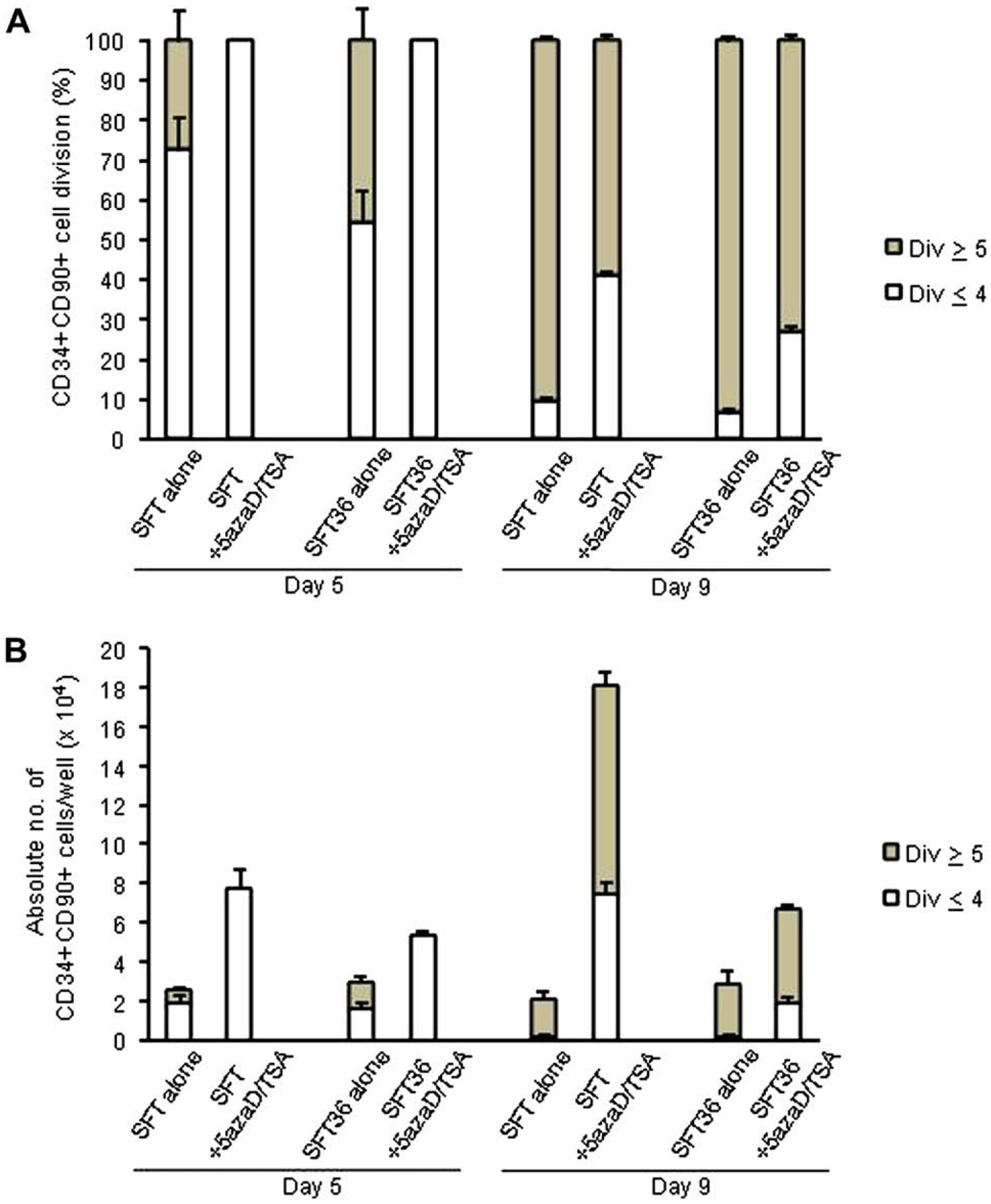
Effects of 5-aza-2′-deoxycytidine (5azaD)/trichostatin A (TSA) treatment and cytokine combinations on cell division history of CD34+CD90+ cells after 5 and 9 days of culture. (A) Effects of 5azaD/TSA treatment and cytokine combinations on the cell division history of CD34+CD90+ cells. (B) Absolute number of CD34+CD90+ cells generated from CD34+CD90+ cells undergoing five or fewer divisions or five or more divisions present in an individual well were determined at day 5 and at day 9. Primary CB CD34+ cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) on day 0. After 5 and 9 days of culture, cells were labeled with anti–CD34-allophycocyanin and anti–CD90-phycoerythrin and CD34+CD90+ gated cells were analyzed for a progressive decline of CFSE fluorescence intensity. Data represents the mean ± standard error of three independent experiments.
We further quantitated the fraction of CD34+CD90+ cells undergoing four or fewer cell divisions or five or more divisions in various culture conditions with or without 5azaD/TSA treatment. CB cells treated with 5azaD/TSA in SFT cytokines had 32-fold more CD34+CD90+ cells, which had undergone four or fewer divisions than cells cultured in SFT, lacking 5azaD/TSA treatment following 9 days of culture (7.46 ± 1.3 × 104 cells vs 0.23 ± 0.06 × 104 cells) (Fig. 2B). CB cells treated with 5azaD/TSA in the presence of SFT resulted in 3.8-fold more CD34+CD90+ cells undergoing four or fewer cell divisions when compared to cells treated with 5azaD/TSA with SFT36 (7.46 ± 1.3 × 104 cells vs 1.95 ± 0.06 × 104 cells) (Fig. 2B). 5azaD/TSA-treated expanded CB CD34+CD90+ cells contained a large fraction of cells that underwent less than four divisions, which possessed greater in vivo reconstitution potential in comparison to control cultures (Fig 2B). However, there were relatively more CD34+CD90+ cells that had divided five or more times than the CD34+CD90+ cells undergoing four or fewer divisions (Fig. 2B). Our data suggest that SFT permits slower division of CD34+CD90+ cells in comparison to culture expanded in SFT36. The addition of 5azaD/TSA results in a further slowing of cell division rate in CD34+CD90+ cells as evidenced by a greater fraction of CD34+CD90+ cells undergoing four or fewer divisions (Fig. 2A). Our data indicate that the cytokines used in the culture can alter the rate of cycling of primitive CD34+CD90+ cells, which is further accentuated by the addition of chromatin-modifying agents. Alternatively, it is conceivable that 5azaD/TSA results in expansion of a subfraction of more primitive transplantable HSC within CD34+CD90+ cells, which divides relatively slowly due to higher P21 protein content in a cell-intrinsic manner.
In vivo marrow repopulating potential of 5azaD/TSA-treated expanded CB cells is influenced by the cytokine cocktail used in the culture
Frequency of SCID mouse repopulating cells (SRC) was quantitated by limiting dilution analysis using NOD/SCID mouse xenogeneic transplantation assay. The SRC frequency was determined in CD34+CD90+ cells after culturing CD34+ cells for 5 and 9 days with SFT with or without 5azaD/TSA treatment. Eight weeks after transplantation the frequency of SRC in primary CB CD34+CD90+ cells was 1 in 26,251, 1 in 54,568 in the culture containing cytokines SFT (day 5), and 1 in 123,315 in the culture containing cytokines SFT (day 9). Frequency of SRC was 1 in 27,906 in the 5azaD/TSA-treated cells cultured for 5 days and 1 in 3,147 in the 5azaD/TSA-treated cells cultured for 9 days (Fig. 3A). These results indicate that 5azaD/TSA is capable of expanding SRC during ex vivo culture and that there is a net loss of SRC in the absence of 5azaD/TSA both at day 5 and day 9 of culture.
Figure 3.
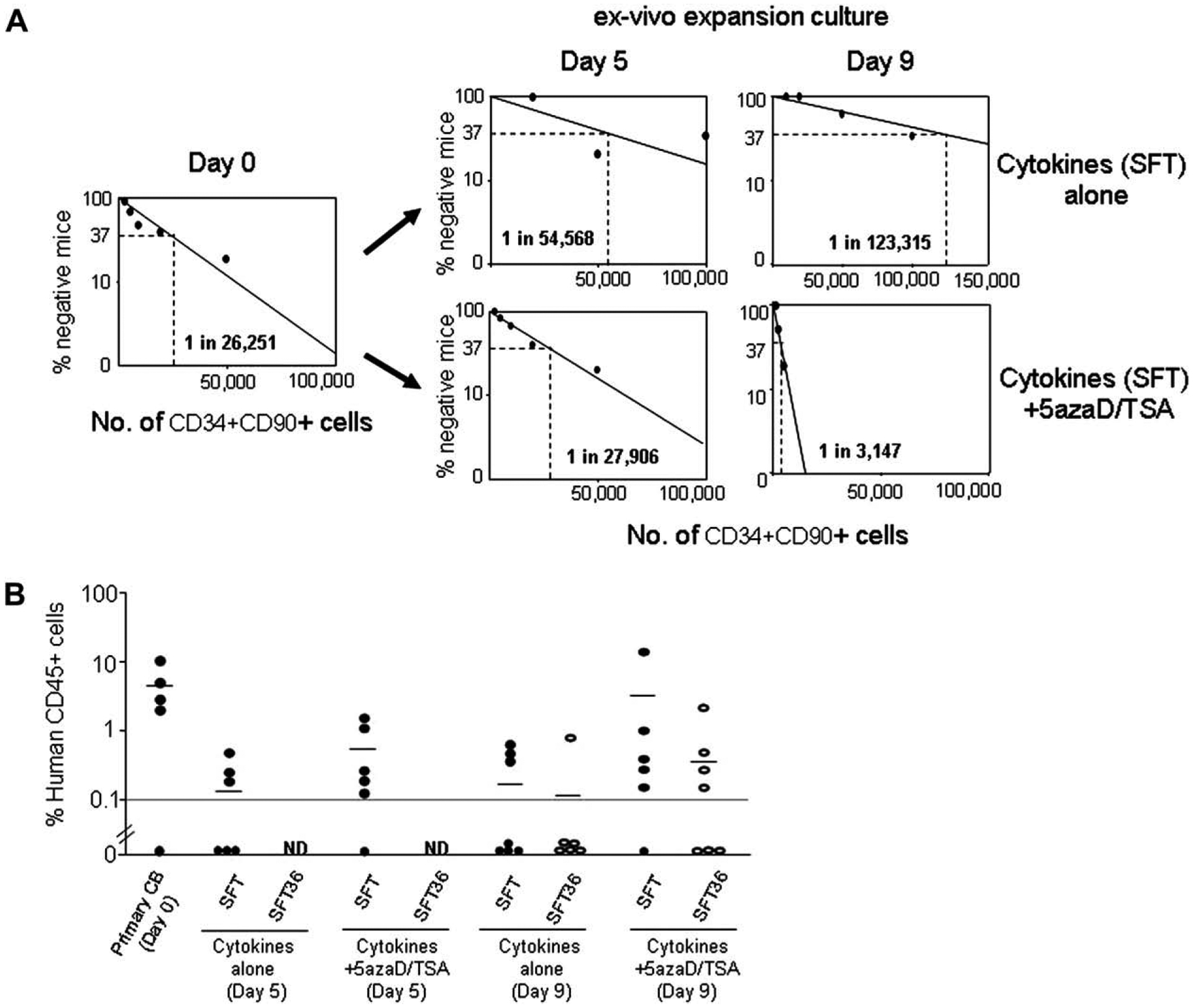
Marrow repopulating potential of the CD34+CD90+ cell population following ex vivo culture. (A) Frequency of SCID mouse repopulating cells (SRC) present in the primary CD34+CD90+ cells prior to (day 0) and following culture in the presence or absence of 5-aza-2′-deoxycytidine (5azaD)/trichostatin A (TSA) treatment (day 5 and day 9) were determined by limiting dilution analysis following transplantation of cord blood (CB) cells in a NOD/SCID mouse assay. Increasing numbers (1,000; 2,000; 5,000; 10,000; 20,000; 50,000; 100,000) of primary CB CD34+CD90+ cells or the progeny of the same number of input CD34+CD90+ cells after 5 and 9 days of ex vivo culture in the presence of SFT were transplanted into NOD/SCID mice. NOD/SCID mice were transplanted with primary CB CD34+ cell fraction or the cellular products of culture lacking 5azaD/TSA treatment (cytokines alone, SFT) or 5azaD/TSA-treated cultures containing cytokines (SFT) initiated with identical numbers of CD34+CD90+ cells. The frequency of SRC in primary CB CD34+CD90+ cells was 1 in 26,251 (95% confidence interval: 1/10,627 – 1/64,850), 1 in 54,568 (95% confidence interval: 1/16,909 − 1/176,101) in the culture containing cytokines alone (day 5) and 1 in 123,315 (95% confidence interval: 1/46,617 – 1/326,200) in the culture containing cytokines alone (day 9). The frequency of SRC was 1 in 27,906 (95% confidence interval: 1/10,280 – 1/75,748) in the 5azaD/TSA-treated cells cultured for 5 days and 1 in 3,147 (95% confidence interval: 1/1,602 – 1/6,189) in the 5azaD/TSA-treated cells cultured for 9 days. Data was analyzed by applying Poisson statistics according to the single-hit model. (B) NOD/SCID engraftment observed with the transplantation of expanded cells containing 5 × 104 of CD34+CD90+ cells per mouse following 5 and 9 days of culture in the presence or absence of 5azaD/TSA with SCF + FL + TPO (SFT) (●) or SCF + FL + TPO + IL- 3 + IL-6 (SFT36) (○).
Next, we compared the in vivo marrow repopulating potential of equal numbers (5 × 104 cells per mouse) of CD34+CD90+ cells transplanted in mice following 5 and 9 days of culture in the presence or absence of 5azaD/TSA, either with SFT or SFT36 cytokines. CD34+ cells expanded with 5azaD/TSA and SFT for 5 days resulted in human multilineage hematopoietic engraftment (data not shown) in five of six mice (chimerism 0.14% to 1.35%), while mice receiving equal numbers of cells that were expanded for 5 days with SFT alone displayed hematopoietic engraftment in only three of six mice (chimerism 0.2% to 0.45%). On the other hand, mice receiving an equal number of CD34+CD90+ cells expanded with 5azaD/TSA for 9 days in the presence of SFT showed the greatest frequency of engraftment (five of six mice vs four of seven mice) and greater degree of hematopoietic cell chimerism (0.15% to 13.9% vs 0.12% to 1.73%) when compared to mice receiving cells expanded in SFT36 with 5azaD/TSA (Fig. 3B). Although this did not reach a statistical significance likely because of the small sample size. In the absence of 5azaD/TSA treatment, the cytokine combination SFT still showed a greater number of engrafted mice and higher degree of human hematopoietic cell chimerism (SFT: three of seven mice, chimerism 0.4% to 0.56% vs SFT36: one of six mice engrafted having 0.83% chimerism) when compared with the cytokines SFT36 (Fig. 3B). These data indicate that the in vivo repopulation potential of CB cells is diminished based on the cytokine cocktail used in the culture. However, use of chromatin-modifying agents in the culture is not only capable of retaining the repopulation potential, but results in the net expansion of transplantable HSC.
Migration of primary and ex vivo expanded CB cells
In order to test the possible cause of poor engraftability of CB cell-expanded cultures lacking 5azaD/TSA treatment (SFT alone), the migration ability and expression of adhesion molecules of primary CB cells as well as CB cells expanded with or without 5azaD/TSA treatment was examined. We monitored the transmigration of the CB cells across a reconstituted basement membrane (Matrigel) in a transwell assay as a surrogate. Cells expanded with 5azaD/TSA showed a significantly greater migrating ability than did the cells expanded without 5azaD/TSA treatment in SFT alone (2.25% ± 0.44% vs 0.95% ± 0.22%) (Fig. 4A; p < 0.05). Both migrating and nonmigrating cells possessed comparable CFU-plating efficiency and multilineage differentiation potential (Fig. 4B).
Figure 4.
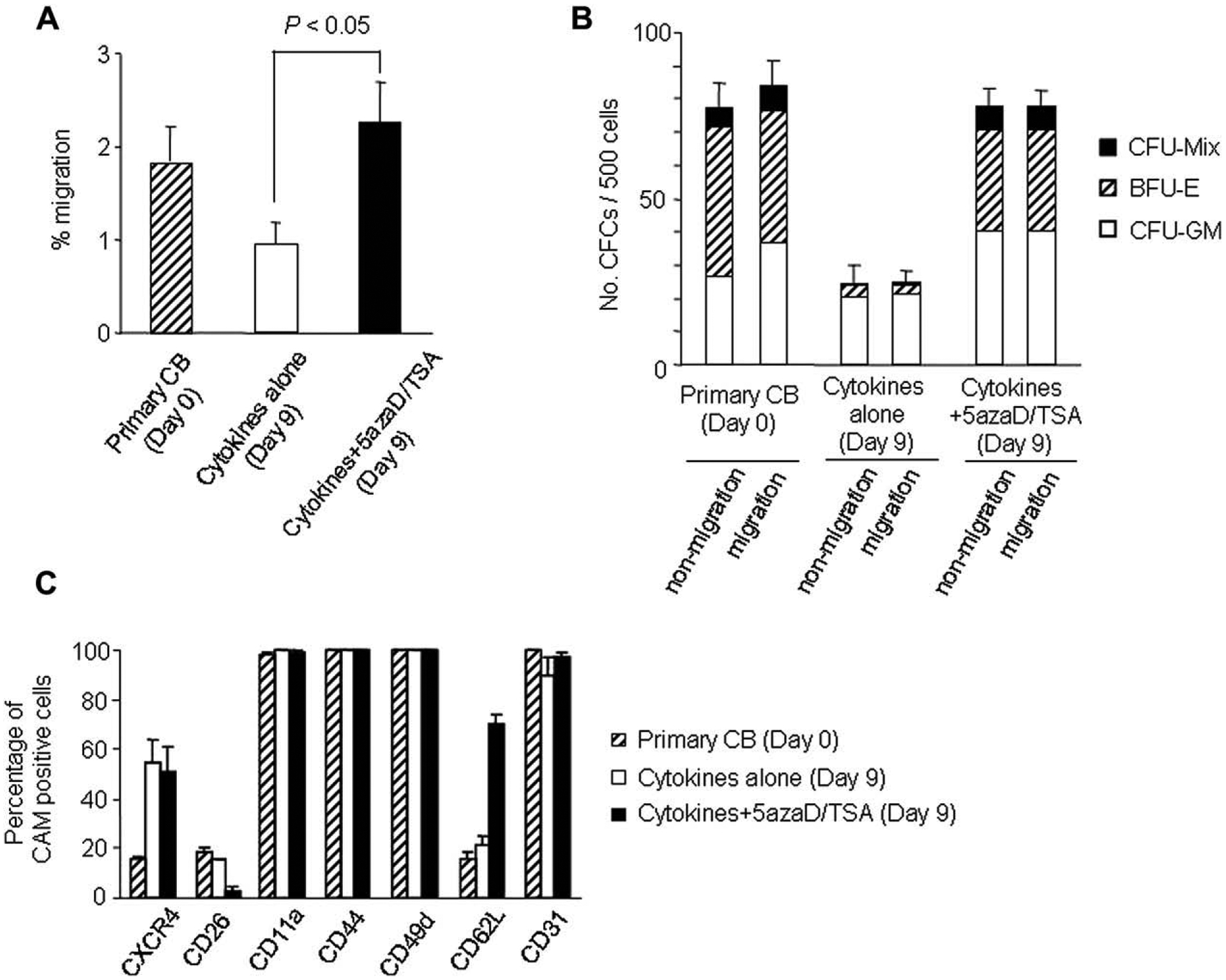
Migration and levels of cell adhesion molecules expressed in primary cord blood (CB) CD34+ cells cultured with or without 5-aza-2′-deoxycytidine (5azaD)/trichostatin A (TSA). (A) Migration of cells through a preformed basement membrane (Matrigel). (B) Plating efficiency of the cells that migrated through Matrigel compared with that of nonmigrating cells. Equal numbers of cells (500 cells) from the upper (nonmigrating) and lower (migrating) compartments of the Boyden chamber were plated and colonies were scored after 14 days of incubation. (C) Primary CD34+CD90+ cells and CD34+CD90+ cells cultured with or without 5azaD/TSA were analyzed for cell adhesion molecules and CXCR4 expression profiles. Cytokine cocktail used for these experiments included interleukin-3 (IL-3), stem cell factor (SCF), Flt3 ligand (FL), and thrombopoietin (TPO) for the initial 48 hours, while for the remaining 7 days of culture the cytokine cocktail changed to SCF, FL, and TPO. After 9 days of ex vivo culture, CD34+CD90+ cells were examined for cell adhesion molecules and CXCR4 expression using three-color fluorescence analysis. Each value represents mean ± standard error of three independent experiments.
Expression of several homing molecules, including the chemokine receptor CXCR4 and the adhesion molecule CD62L (L-selectin), have been implicated as an indicator of BM homing ability [18,19]. Expression of CXCR4 on CD34+CD90+ cells was not altered after ex vivo culture, irrespective of 5azaD/TSA treatment (Fig. 4C). Another molecule implicated in HSC homing is CD26. It has been shown that endogenous CD26 expression on donor cells can decrease homing and engraftment in BM [20]. These results show that after ex vivo culture in the presence of 5azaD/TSA in SFT (3.2% ± 1.8%), expression of CD26 is significantly lower than that of primary CD34+CD90+ cells (18.5% ± 1.6%) or cells expanded in SFT alone (15.7% ± 0.3%) (Fig. 4C). In addition, expression of CD62L in 5azaD/TSA-treated expanded CD34+CD90+ cells (70.7% ± 3.6%) was significantly higher than primary CB cells (16.0% ± 2.4%) or cells expanded in SFT alone (21.0% ± 4.2%). Our results indicate that 5azaD/TSA-treated expanded CD34+CD90+ cells migration ability as well as expression of adhesion molecules implicated as critical for BM homing are not diminished after ex vivo culture, rather it was higher than primary CB cells. These data also indicate that the higher migration ability and higher level of expression of CD62L in conjunction with low level of expression of CD26 in 5azaD/TSA-treated expanded CD34+CD90+ cells may, in part, serve as favorable factors for better engraftment capacity of these cells.
Discussion
Ex vivo expansion of CB grafts could theoretically augment the number of HSC and likely shorten the time to hematopoietic engraftment after transplantation in humans. However, ex vivo expansion of HSC has been met with limited success [21]. Widespread use of expanded CB grafts is limited by a lack of understanding of the factors regulating symmetric self-renewing HSC divisions, as well as the culture conditions capable of maintaining HSC in a noncommitted state [22].
Here we show that HSCs expanded in the presence of chromatin-modifying agents remain permissive to environmental cues, as shown by the effects of various cytokine cocktails added to the cultures. The culture conditions described in previous studies present a possible mechanism by which chromatin-modifying agents can alter the methylation and acetylation status of gene regulatory sites to activate critical genes (HoxB4, Bmi1, etc.), which might be pivotal in the expansion of transplantable HSC [13]. Our current findings suggest possible mechanistic interactions between intrinsic factors (HoxB4, Bmi-1, etc.) induced by chromatin-modifying agents and extrinsic factors (cytokines) that influence HSC fate decisions during ex vivo culture. Furthermore, our data suggest that the sequence of administration of a hypomethylating drug (5azaD) followed by the addition of an HDAC inhibitor (TSA) is crucial in promoting expansion of transplantable HSC, which is associated with the alteration of histone H4 acetylation. Methylation and histone deacetylation appear to act as layers that must be sequentially removed in order to reverse epigenetic gene silencing. Our results support the concept that methylation needs to be targeted prior to targeting acetylation with an HDAC inhibitor because reversing this sequence results in abrogation of HSC expansion, which is consistent with the findings of other investigators [16].
Lack of P21 has been shown to accelerate progression of HSC from quiescence to the active phases of the cell cycle. This may transiently result in expansion of HSC, but ultimately leads to the eventual exhaustion of the HSC pool [23,24]. Relatively slow cycling is an important attribute of stem/progenitor cells, which contain high P21 levels for maintenance of quiescence or relatively slow cycling [23,25,26]. Chromatin-modifying agent–treated expanded CB cells contained higher P21 protein levels than CD34+CD90+ cells expanded without 5azaD/TSA (SFT alone), and divide slowly and possess greater in vivo hematopoietic reconstitution potential. Alternatively, it is conceivable that 5azaD/TSA causes the expansion of the more primitive subpopulation of CD34+CD90+ cells, which divides relatively slowly because of an intrinsically higher P21 protein level. However, with extended culture periods, the differences in P21 levels diminished. This is likely a result of the diminishing effects of 5azaD/TSA over time and corresponds with an abrogation of HSC expansion. Cells cultured with 5azaD/TSA in the presence of SFT resulted in the greatest expansion of CD34+CD90+ cells retaining their in vitro and in vivo functional potential in comparison to cells cultured with 5azaD/TSA in the presence of SFT36, indicating that modification of HSC fate by 5azaD/TSA is not fully independent of external humoral influences. In order to determine the cause of the diminished functional potential dependent on the cytokine combination used, we studied the rate of division of CD34+CD90+ cells in the culture. 5azaD/TSA-treated CD34+CD90+ cells cultured in the presence of SFTunderwent the slowest rate of division and are capable of maintaining their in vivo repopulation potential in contrast to CD34+CD90+ cells expanded in SFTalone or in SFT36 with or without 5azaD/TSA. This data is also consistent with our earlier observation demonstrating a relatively slower rate of division of 5azaD/TSA-treated expanded CD34+ cells using a BrdU pulse chase assay [13]. It has been shown previously that the number of cell divisions plays a significant role in retaining the in vivo reconstitution potential of HSC [27–29]. Furthermore, it has been reported previously that the marrow-repopulating potential of CB cells generated using such ex vivo culture systems resides within the population originated during the first few divisions or cells that remained quiescent [27–29]. We have previously reported that a large fraction of the CD34+CD90+ cells in the 5azaD/TSA-treated culture that underwent one to four cell divisions possessed higher functional potential than the cells that divided further [13]. In our current studies, we show that cytokine combinations containing cytokines like IL-3 and IL-6 (SFT36) resulted in more divisions, reduced expansion of progenitors, and diminished retention of in vivo marrow repopulating potential, despite treatment with chromatin-modifying agents. The effects of IL-3 on HSC function appear to be influenced by the target cell and the presence of other cytokines or serum [30]. We have shown that absence of IL-3 during the initial 48 hours of the culture results in abrogation of HSC expansion. The pivotal effect of IL-3 is likely a result of its ability to promote HSC cycling, which is crucial for the incorporation of 5azaD into the cell [5,17]. In the absence of chromatin-modifying agents, loss of SRC was more prominent when the culture period was extended beyond 5 days. Loss of SRC varied depending on culture conditions, and this can be ameliorated by use of chromatin-modifying agents. Interestingly, the degree of expansion of SRC was also most evident between 5 and 9 days of culture and was almost completely lost at later time points (day 14).
It has been reported previously that ex vivo expansion of CD34+ cells induces extensive changes in expression of adhesion molecules implicated in BM homing [31]. However, our data show that CB cells expanded with 5azaD/TSA possess comparable migration potential to primary CB cells. 5azaD/TSA-treated expanded cells did not show diminished expression of the chemokine receptor CXCR4, which is considered to be an important candidate molecule for HSC homing. Expression of CXCR4 in 5azaD/TSA-treated expanded cells was, in fact, higher than that observed in primary CB cells. Similar expression of CXCR4 was also observed in CD34+CD90+ cells expanded in conditions lacking 5azaD/TSA treatment, although these cells displayed significantly less migration ability and poor expression of CD62L, an important molecule for BM homing. The increased expression of CXCR4 in ex vivo expanded cells suggests that expression of CXCR4 is unlikely to be responsible for the lower engraftment potential of cells expanded without 5azaD/TSA. Recently, it has been observed that unlike primary CD34+ cells, cytokine-expanded CD34+ cells display dampened stromal cell–derived factor-1/CXCR4 signaling due to constitutive activation of α4 integrin (CD49d) [32]. The exact cause of the loss of in vivo repopulation potential of cells expanded in the absence of 5azaD/TSA treatment or in the presence of cytokines favoring an increased rate of divisions requires further investigation. On the other hand, the lower expression of CD26 or increased levels of CD62L in 5azaD/TSA expanded CB cells would likely favor homing to BM following transplantation.
Taken together, our data suggest that epigenetically mediated HSC expansion is not independent of external humoral influences and that the rate of cell division may be an important determinant in maintenance of SRC potency. In addition, this study provides further insight about possible cross-talk between extrinsic and intrinsic factors regulating HSC behavior mediated by epigenetic mechanisms.
Acknowledgments
We gratefully acknowledge Dr. Ronald Hoffman for his helpful comments and facilitating initial part of the work involved in this article. We would also like to thank Rifat Rahman for her technical assistance. We are highly indebted to Dr. Ludy Dobrila and Dr. Pablo Rubinstein of the National Cord Blood Program at New York Blood Center, New York, NY, for providing umbilical cord blood units for research. Drs. John Quigley, Dolores Mahmud, and Anwar Khan are acknowledged for critical reading of the manuscript. This work was supported in part by grants from the State of Illinois (Illinois Regenerative Medicine Institute; Springfield, IL, USA) and the Leukemia & Lymphoma Society (White Plains, NY, USA) (Translational Research Program) to N.M.
Footnotes
Financial disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Blau HM. Differentiation requires continuous active control. Annu Dev Biochem. 1992;61:1213–1230. [DOI] [PubMed] [Google Scholar]
- 2.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. [DOI] [PubMed] [Google Scholar]
- 3.Marks PA, Richon VM, Rifkind RA. Histone deacetylases inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. [DOI] [PubMed] [Google Scholar]
- 4.Kass SU, Pruss D, Wolffe AP. How dose methylation mediated repress transcription? Trends Genet. 1997;13:444–449. [DOI] [PubMed] [Google Scholar]
- 5.Issa JP. Decitabine. Curr Opin Oncol. 2003;15:446–451. [DOI] [PubMed] [Google Scholar]
- 6.Mahmud N, Milhem M, Araki H, Hoffman R. Alteration of hematopoietic stem cell fates by chromatin-modifying agents. In: Ho AD, Hoffman R, Zanjani ED, eds. Frontiers in Stem Cell Transplantation. Weinheim, Germany: Wiley-VCH; 2006. p. 27–42. [Google Scholar]
- 7.Ueda T, Tsuji K, Yoshino H, et al. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest. 2000;105: 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piacibello W, Sanavio F, Severino A, et al. Engraftment in nonobese diabetic severe combined immunodeficient mice of human CD34+ cord blood cells after ex vivo expansion: evidence for the amplification and self-renewal of repopulating stem cells. Blood. 1999;93: 3736–3749. [PubMed] [Google Scholar]
- 9.Habibian HK, Peters SO, Hsieh CC, et al. The fluctuating phenotype of the lymphohematopoietic stem cell with cell cycle transit. J Exp Med. 1998;188:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takatoku M, Sellers S, Agricola BA, et al. Avoidance of stimulation improves engraftment of cultured and retrovirally transduced hematopoietic cells in primates. J Clin Invest. 2001;108:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glimm H, Oh IH, Eaves CJ. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0). Blood. 2000;96: 4185–4193. [PubMed] [Google Scholar]
- 12.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Gene. 2003;33:245–254. [DOI] [PubMed] [Google Scholar]
- 13.Araki H, Yoshinaga K, Boccuni P, Zhao Y, Hoffman R, Mahmud N. Chromatin modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood. 2007;109:3570–3578. [DOI] [PubMed] [Google Scholar]
- 14.Araki H, Mahmud N, Milhem M, et al. Expansion of human umbilical cord blood SCID-repopulating cells using chromatin modifying agents. Exp Hematol. 2006;34:140–149. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Bruno E, Chao J, et al. MPD research consortium. Constitutive mobilization of CD34+ cells into the peripheral blood in idiopathic myelofibrosis may be due to the action of a number of proteases. Blood. 2005;105:4508–4515. [DOI] [PubMed] [Google Scholar]
- 16.Cameron EE, Bachman KE, Myöhänen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. [DOI] [PubMed] [Google Scholar]
- 17.Momparler RL. Pharmacology of 5-Aza-2′-deoxycytidine (decitabine). Semin Hematol. 2005;42:9–16. [DOI] [PubMed] [Google Scholar]
- 18.Dercksen MW, Gerritsen WR, Rodenhuis S, et al. Expression of adhesion molecules on CD34+ cells: CD34 + L-selectin+ cells predict a rapid platelet recovery after peripheral blood stem cell transplantation. Blood. 1995;85:3313–3319. [PubMed] [Google Scholar]
- 19.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. [DOI] [PubMed] [Google Scholar]
- 20.Christopherson KW 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. [DOI] [PubMed] [Google Scholar]
- 21.Devine SM, Lazarus HM, Emerson SG. Clinical application of hematopoietic progenitor cell expansion: current status and future prospects. Bone Marrow Transplant. 2003;31:241–252. [DOI] [PubMed] [Google Scholar]
- 22.Goff JP, Shields DS, Greenberger JS. Influence of cytokines on the growth kinetics and immunophenotype of daughter cells resulting from the first division of single CD34 + Thy+lin- cells. Blood. 1998;92:4098–4107. [PubMed] [Google Scholar]
- 23.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. [DOI] [PubMed] [Google Scholar]
- 24.Stier S, Cheng T, Forkert R, et al. Ex vivo targeting of p21Cip1/Waf1 permits relative expansion of human hematopoietic stem cells. Blood. 2003;102:1260–1266. [DOI] [PubMed] [Google Scholar]
- 25.Mahmud N, Katayama N, Itoh R, Tanaka R, Ohishi K, Masuya M, et al. A possible change in doubling time of haemopoietic progenitor cells with stem cell development. Br J Haematol. 1996;94:242–249. [DOI] [PubMed] [Google Scholar]
- 26.Mahmud N, Devine SM,Weller KP, et al. Therelative quiescenceof hematopoietic stem cells in nonhuman primates. Blood. 2001;97:3061–3068. [DOI] [PubMed] [Google Scholar]
- 27.Ho AD. Kinetics and symmetry of divisions of hematopoietic stem cells. Exp Hematol. 2005;33:1–8. [DOI] [PubMed] [Google Scholar]
- 28.Srour EF. Proliferative history and hematopoietic function of ex vivo expanded human CD34+ cells. Blood. 2000;96:1609–1612. [PubMed] [Google Scholar]
- 29.Young JC, Lin K, Hansteen G, et al. CD34+ cells from mobilized peripheral blood retain fetal bone marrow repopulating capacity within the Thy-1+ subset following cell division ex vivo. Exp Hematol. 1999;27:994–1003. [DOI] [PubMed] [Google Scholar]
- 30.Zandstra PW, Conneally E, Petzer AL, Piret JM, Eaves CJ. Cytokine manipulation of primitive human hematopoietic cell self-renewal. Proc Natl Acad Sci U S A. 1997;94:4698–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prosper F, Stroncek D, McCarthy JB, Verfaillie CM. Mobilization and homing of peripheral blood progenitors is related to reversible down-regulation of α4β1 integrin expression and function. J Clin Invest. 1998;101:2456–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foguenne J, Di Stefano I, Giet O, Beguin Y, Gothot A. Ex vivo expansion of hematopoietic progenitor cells is associated with downregulation of alpha 4 integrin and CXCR4-mediated engraftment in NOD/SCID beta2-microglobulin-null mice. Haematologica. 2009;94: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]


