Abstract
Objective.
To investigate the mechanism(s) responsible for increased γ-globin expression in vivo in decitabine-treated baboons and in vitro in cultured erythroid progenitor cells (EPC) from adult baboon bone marrow (BM).
Materials and Methods.
Fetal liver, adult BM erythroid cells pre- and post-decitabine, and cultured EPCs were analyzed for distribution of RNA polymerase II, histone acetylation, and histone H3 (lys4) trimethyl throughout the γ-globin gene complex by chromatin immunoprecipitation. DNA methylation of the γ-globin promoter was determined by bisulfite sequencing. Expression of the baboon Iγ- and Vγ-globin chains was determined by high performance liquid chromatography (HPLC). Expression of BCL11A, a recently identified repressor of γ-globin expression, was analyzed by Western blot.
Results.
Increased γ-globin expression in decitabine-treated baboons and cultured EPC correlated with increased levels of RNA polymerase II, histone acetylation, and histone H3 (lys4) trimethyl associated with the γ-globin gene consistent with a transcriptional activation mechanism. Cultured EPC expressed the Iγ- and Vγ-globin chains in a pattern characteristic of fetal development. The level of DNA methylation of the γ-globin gene promoter in EPC cultures was similar to BM erythroid cells from normal adult baboons. Different BCL11A isoforms were observed in BM erythroid cells and cultured EPC.
Conclusion.
The mechanism responsible for increased γ-globin expression in cultured EPC was unexpectedly not associated with increased DNA hypomethylation of the γ-globin gene promoter compared to normal BM erythroid cells, in contrast to BM erythroid cells of decitabine-treated baboons. Rather, increased fetal hemoglobin in EPC cultures was associated with a fetal Iγ/Vγ chain ratio and a difference in the size of the BCL11A protein compared to normal BM erythroid cells.
The human β-globin gene complex is contained within 70 kb DNA on chromosome 11. Expression of the β-like globin genes located within the complex is developmentally regulated [1,2]. The ϵ-globin gene is expressed during the first 8 weeks of gestation, the two γ-globin genes are expressed at high levels during the fetal period and at very low levels in adults, while the adult β-globin gene is expressed at high levels in adults. The switch from ϵ- to γ-to β-globin expression during ontogeny is the primary level of developmental regulation of genes within this locus. A secondary level of developmental regulation is superimposed upon the duplicated γ-globin genes. The two γ-globin genes are distinguished by an amino acid substitution at aa136. The 5′ gene, denoted Gγ, contains glycine at this position, while the 3′ gene, denoted Aγ, contains alanine. The Gγ- and Aγ-globin genes are expressed in a ratio of 7:3 during fetal development and 2:3 in adults. Because increased fetal hemoglobin (HbF) levels are associated with decreased symptoms associated with sickle cell disease and increased lifespan of patients [3,4], a complete understanding of the mechanism responsible for the regulation of globin gene expression during development would be important for the design of future pharmacologic and molecular therapies to increase HbF for sickle cell disease and also β-thalassemia.
Previous results firmly established that HbF synthesis was reactivated in burst-forming unit erythroid (BFU-E) colonies derived from adult peripheral blood progenitors [5–8]. Higher γ/γ + β ratios observed in BFU-E derived from fetal and neonatal blood compared to adult showed that the level of HbF synthesis in cultured BFU-E remained influenced by developmental controls [8–11]. It was also observed that the γ/γ + β ratio decreased as erythroid maturation progressed, showing that the level of HbF was also modulated by events occurring during erythroid maturation in addition to developmental controls [12]. While HbF was increased in adult-derived BFU-E, the Gγ/Aγ ratio corresponded to that of normal adult peripheral blood [13]. Analysis of individual bursts from neonatal and adult blood showed a positive correlation between γ/γ + β and Gγ expression, although expression of Gγ was lower in adult BFU-E compared to neonatal BFU-E, regardless of the level of γ/γ β expression. These results demonstrated that the Gγ/A+γ ratio was influenced by the developmental stage of the BFU-E more strongly than the γ/γ + β ratio [14]. The results suggested, however, that a strict quantitative relationship between the γ/γ + β and Gγ/Aγ ratios did not necessarily exist and that a considerable amount of flexibility in both HbF and Gγ expression could be observed between individual BFU-E [14]. In contrast to changes in the γ/γ β ratio observed during erythroid maturation, no changes+in the Gγ/Aγ ratio were observed, suggesting that the level of total γ-globin expression and Gγ/Aγ ratio were regulated by different mechanisms [14]. The mechanism responsible for increased HbF synthesis in adult-derived BFU-E was shown to involve transcriptional activation of the γ-globin genes associated with changes in chromatin structure [15]. The level of HbF expression in adult-derived BFU-E is heavily influenced by specific culture conditions that include factors present in fetal serum [16,17], and various growth factors, including stem cell factor and transforming growth factor-β [18–20].
The baboon is an important animal model to study regulation of expression of genes within the β-globin gene complex because the structure of the β-globin locus and the developmental pattern of expression of genes within the locus is almost identical to man [21,22]. The two baboon γ-globin genes are distinguished at aa75 by the presence of isoleucine in the 5′ Iγ-globin gene and valine in the 3′ Vγ-globin gene [23]. The developmental change in Iγ- and Vγ-globin expression is very similar to human Gγ/Aγ as the ratio of Iγ/Vγ expression shifts from 3:2 during fetal development to 1:2 in adults [23,24]. Although reactivation of HbF synthesis to high levels was observed in BFU-E derived from adult baboon bone marrow (BM) [25,26], the relative levels of Iγ and Vγ expression were not studied.
DNA methylation is an important factor in the regulation of γ-globin expression. CpG dinucleotides within the 5′ γ-globin gene are unmethylated in fetal liver when the genes are expressed but methylated in adult bone marrow when the genes are silenced in both man and baboon [27–29]. Treatment with inhibitors of DNA methyltransferase reduced DNA methylation of the γ-globin promoter and increased HbF in baboons [30,31] and in patients with β-thalassemia [32] and sickle cell disease [33,34]. In transgenic mice harboring the human with β-globin locus in the context of a yeast artificial chromosome model using a construct that allowed specific manipulation of DNA methylation of the Aγ-globin promoter, DNA methylation repressed Aγ-globin expression in adult erythroid cells by altering histone acetylation levels to prevent the binding of transcription factors to the γ-globin promoter [35]. Recently, however, it was suggested that reducing DNA methylation of the γ-globin promoter by RNAi targeting DNMT1 was not sufficient to induce γ-globin expression and that decitabine increased γ-globin expression through a posttranscriptional mechanism independent of DNA methylation [36].
A genome-wide association mapping strategy recently identified the zinc finger protein BCL11A as a quantitative trait locus involved in F-cell production [37] and RNAi and chromatin immunoprecipitation (ChIP) experiments demonstrated that BCL11A acted as a repressor of γ-globin expression that bound to multiple sites within the β-globin gene locus [38].
To gain insight into the molecular mechanism of globin gene regulation, we have compared the level of association of RNA polymerase II, histone H3 acetylation, and histone H3 (lys4) trimethyl throughout the β-globin gene complex and the level of γ-globin promoter DNA methylation in: 1) erythroblasts expressing high levels of HbF generated from CD34+ adult baboon BM erythroid progenitors in a liquid culture system that can produce large numbers of cells (0.5 – 1 × 109 total nucleated cells; >90% erythroid); 2) baboon fetal liver; and 3) primary bone marrow (BM) erythroblasts from phlebotomized adult baboons expressing low levels of HbF and baboons treated with the DNA methyltransferase inhibitor decitabine expressing very high levels of HbF. In addition, the ratio of expression of the 5′Iγ- and 3′ Vγ-globin chains was determined by high performance liquid chromatography (HPLC) and expression of the BCL11A protein in cultured erythroid progenitor cells (EPC) and BM were compared by Western blot analysis.
Our results demonstrate that increased γ-globin expression in cultured EPC is not correlated with DNA hypomethylation. Increased γ-globin expression in EPC cultures is characterized by a fetal Iγ/Vγ chain ratio and expression of a 125-kDa BCL11A isoform, rather than the 220-kDa isoform observed in BM erythroid cells.
Materials and methods
Baboon treatments
Cord blood and liver samples were obtained from fetal baboons, P. anubis, produced by timed matings at the University of Illinois Biologic Resources Laboratory. Fetuses were euthanatized by injection with euthobarb prior to the removal of tissue. BM samples from the hips of 3- to 4-year-old baboons were obtained by aspiration. Acute phlebotomy was performed by daily removal of 16% to 18% of the packed cell volume to a hematocrit of 20 and hematocrit was maintained at 20 for the reminder of the experiment by periodic bleeding. Ten days after beginning phlebotomy, BM aspiration was performed prior to 10 days of treatment with decitabine. Animals were injected subcutaneously with decitabine (0.52 mg/kg/d) for 10 days. Postdecitabine BM sample was obtained following the last day of treatment. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago.
Cell purification and culture
For ChIP analysis, low-density mononuclear cells were cells were enriched from bone marrow aspirates as described previously [29]. Cells were prepared from fetal livers by passing finely minced tissue through a wire screen. Erythroid cells were purified from low-density mononuclear cells from BM aspirates (10 – 20 mL) and from suspensions of fetal liver cells by immunomagnetic column separation using baboon anti-red blood cell (bRBC) mouse monoclonal antibody (anti-bRBC; #551299, BD Pharmingen, San Jose, CA, USA) in combination with rat anti-mouse IgG1 magnetic microbeads (#130-047-101, Miltenyi Biotec, Bergisch Gladbach, Germany). Purity of cell suspensions were evaluated by microscopic examination of Wright’s stained cytospin preparation.
For EPC cultures, low-density mononuclear cells were enriched from BM aspirates (30 – 40 mL) obtained from normal, untreated baboons by two cycles of Percoll gradient sedimentation. Cells were incubated with the mouse monoclonal 12.8 antibody (a gift of Dr. R. Andrews), reactive with baboon CD34+ cells, followed by incubation with rat anti-mouse IgM bound to magnetic microbeads (Miltenyi) and magnetic column separation performed using LS columns (Miltenyi) to obtain a cell population enriched in CD34+ BM cells. CD34+-enriched populations were cultured in Iscove’s media containing 30% fetal bovine serum, 2 U/mL erythropoietin, 200 ng/mL stem cell factor, and 1 μM dexamethasone. Additional media was added on day 4 of culture and every 2 days thereafter to maintain cell numbers between 0.25 and 1 × 106/mL and maintain high concentrations of the growth factors.
Flow cytometric analysis
Flow cytometric analysis of the distribution of CD36 and bRBC on the surface of cells grown in liquid culture was performed as described previously [39]. Cells harvested on varying days of culture were incubated with 30% rabbit serum for 10 minutes on ice to block Fc receptors. Cells were incubated with fluorescein isothiocyanate–conjugated mouse anti-human CD36 antibody (Immunotech, Fullerton, CA, USA) and phycoerythrin-conjugated anti-bRBC antibody (Pharmingen) for 30 minutes on ice. Cells were washed twice in phosphate-buffered saline followed by addition of propidium iodide. The proportion of CD36+ bRBC+, CD36+ bRBC−, and CD36−bRBC+ cells was determined by flow cytometry at the University of Illinois at Chicago Flow Cytometry Facility. Appropriate isotype controls were always included to assess the specificity of binding of specific antibodies.
HPLC analysis of globin chains
For analysis of globin chain expression in cultured baboon erythroid progenitor cells, cells (5 – 10 × 106) were harvested and washed three times in PBS. Lysates were prepared by addition of H2O to the packed cell pellet followed by three cycles of freezing and thawing in a dry ice-methanol bath. Analysis of globin chains was performed on a TSP Spectra HPLC system using a LiChristopher 100 RP-8 column and a gradient of acetonitrile-methanol-NaCl [40].
ChIP assays
Immunoprecipitation of formaldehyde-fixed, sonically sheared chromatin was performed as described previously [29,41]. Antibodies used were anti-pol II (sc-899; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-acetylated histone H3 (06–599; Upstate Biotechnology, Lake Placid, NY, USA), anti-acetylated histone H4 (06–866; Upstate), and anti-histone H3 lysine 4 trimethyl (07–745; Upstate Biotechnology). No chromatin, no antibody, and rabbit IgG control samples were included for each sample. Salmon sperm DNA-Protein A agarose beads (Upstate Biotechnology) were used as the secondary reagent. Eluted samples were heated to 68°C for 4 hours to reverse cross-links. Samples were digested with Proteinase K overnight at 45°C followed by extraction with 50:50:1 phenol:ChCl3:isoamyl alcohol to purify DNA. Samples were analyzed by real-time polymerase chain reaction with SYBR Green Reagent mix (Applied Biosystems, Foster City, CA, USA) using a 7500 Real-Time PCR System (Applied Biosystems). A series of 18 primer sets spanning the baboon β-globin gene complex were used. The sequence of each primer set and their relative location within the β-globin gene complex is shown in Figure 3. For real-time analysis, a standard curve for each primer set was prepared from dilutions (1:3, 1:12, 1:48, 1:192, 1:768) of input DNA samples removed prior to immunoprecipitation. DNA samples were analyzed in triplicate. In all cases, the amount of each specific sequence amplified by a specific set of primers was determined by comparison to the standard curve derived from serial dilutions of formaldehyde-fixed input DNA (removed prior to immunoprecipitation) from the same sample. The amount of DNA in each immunoprecipitated sample was extrapolated from the standard curves and expressed as the fraction of 20% input DNA immunoprecipitated following subtraction of background DNA in the rabbit IgG sample. Within any single sample precipitated with a specific antibody, data between different primer sets is therefore quantitative. To compare data between different samples that may differ in the absolute amount of DNA immunoprecipitated due to differences in efficiency of immunoprecipitation, extent of cross-linking, and unknown factors such as erythrocyte contamination, relative values are presented that were calculated by setting the primer set with highest amount immunoprecipitated to 100 and calculating values for other primer sets relative to this within a single sample [29,42].
Figure 3.
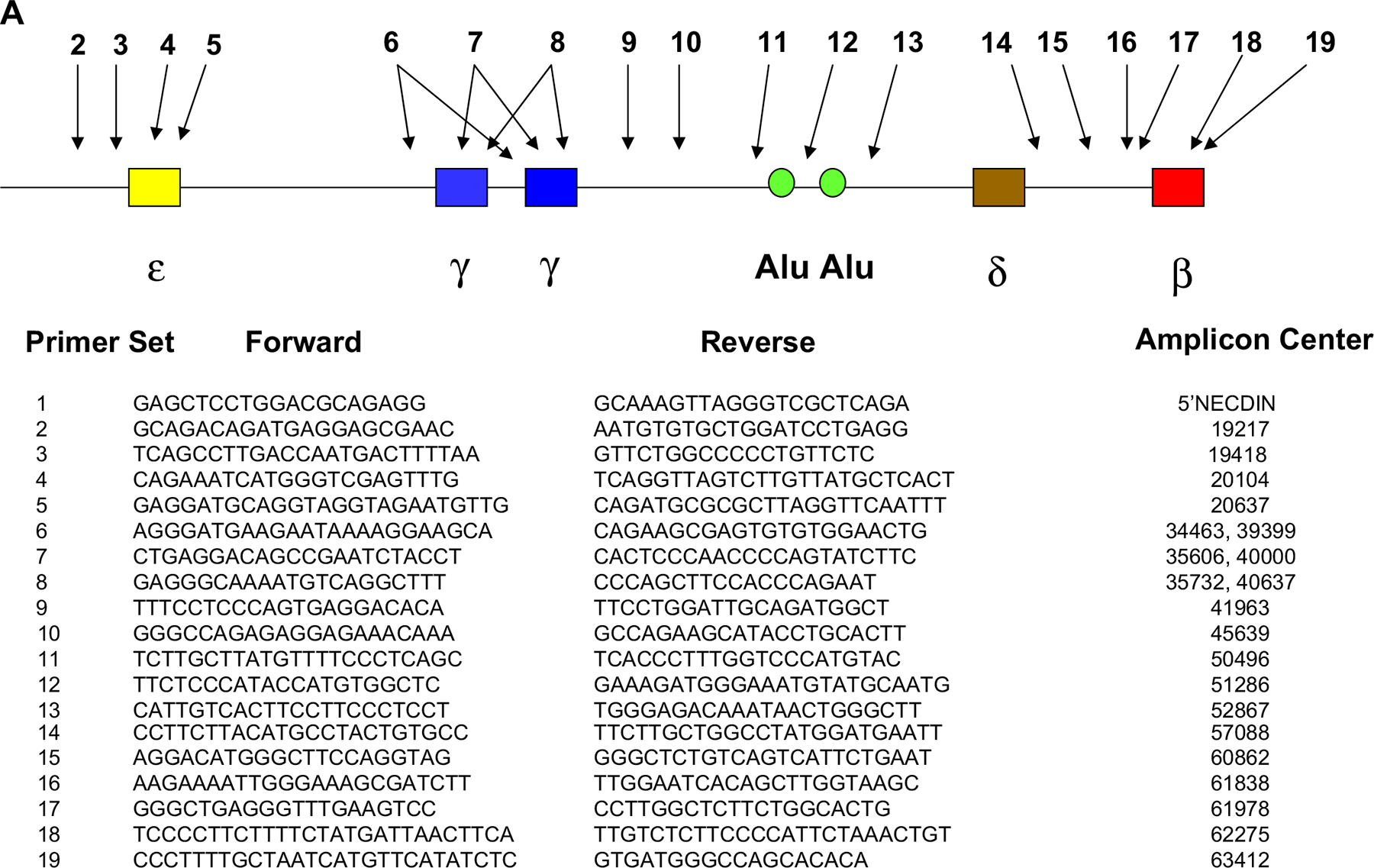
(A) Location of primer sets used in real-time polymerase chain reaction analysis of chromatin immunoprecipitation (ChIP) assays.
(B) ChIP analysis showing distribution throughout the β-globin gene complex. Panel A: RNA polymerase. Panel B: Acetyl histone H3. Panel C: Histone H3 (lys 4) trimethyl.
Bisulfite sequence analysis
Bisulfite sequence analysis was performed to determine the level of DNA methylation of the γ-globin promoter region in purified erythroid cells generated in baboon EPC cultures. Erythroid cells were purified from liquid cultures using immunomagnetic column selection with a mouse monoclonal antibody reactive with baboon red blood cells (anti-bRBC) and rat anti-mouse IgG1 magnetic microbeads as described here. DNA was isolated from these purified, nucleated erythroid cells and bisulfite modification performed as described previously [29,43]. Following two rounds of amplification using semi-nested primers that amplify a 105-bp segment of baboon γ-globin promoter region, PCR products were cloned in the pCR4 vector. Inserts of at least 10 randomly isolated clones were sequenced using an ABI Prism 300 genetic analyzer at the University of Illinois DNA Sequence Facility. Five CpG sites (−54, −51, +5, +16, +48) within the baboon γ-globin promoter were analyzed for cytosine methylation.
Western blot analysis
To analyze expression of BCL11A, whole-cell extracts were prepared from purified BM erythroid cells and day 11 cultured EPC. Cells were extracted in lysis buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1.5 mM MgCl2, 10 mM sodium pyrophosphate, 1 mM ethylenediamine tetraacetic acid, 100 mM sodium fluoride, 100 μM sodium orthovanadate, 1% Triton X-100, 10% glycerol, 20 μg/mL aprotinin, 0.5 mM phenylmethylsulfonyl fluoride, and 20 mM N-ethylmaleimide [44,45]. Extracts containing equal amounts of protein were separated by electrophoresis in sodium dodecyl sulfate polyacrylamide gels. Western blot analysis was performed using three different monoclonal antibodies (Abcam, Cambridge, MA, USA), 14B5, 18B12DE6, and 15E3AC11, recognizing determinants in the core region, C terminus, and N terminus, respectively.
Results
HbF expression in cultured baboon BM erythroid progenitors
Purified baboon BM CD34+ cells were grown in liquid culture using conditions designed to mimic conditions associated with stress erythropoiesis and allow preferential expansion and differentiation of erythroid progenitor cells [46]. Cultures initiated from 0.5 to 1 × 106 CD34+ cells generated >5 × 108 total cells by day 14 and >90% were reactive with a mouse monoclonal anti-bRBC by flow cytometric analysis. Flow cytometric analysis of cultured cells on days 4, 8, 11, and 14 were consistent with progressive differentiation through CD36+bRBC−, CD36+bRBC+, and CD36−bRBC+ stages (Fig. 1A) and Wright’s stained cytospin preparations also showed progressive erythoid differentiation of cultured cells (Fig. 1B). Mean enucleation in three cultures on day 14 was 37.2% ± 14.6%. A high level of γ-globin gene expression was observed in lysates of these cultures (Fig. 2; Table 1). HPLC analysis of lysates prepared from cells of cultures initiated from CD34+ BM cells of three different animals ranged from 33.3 to 60.5 γ/γ ± β.
Figure 1.
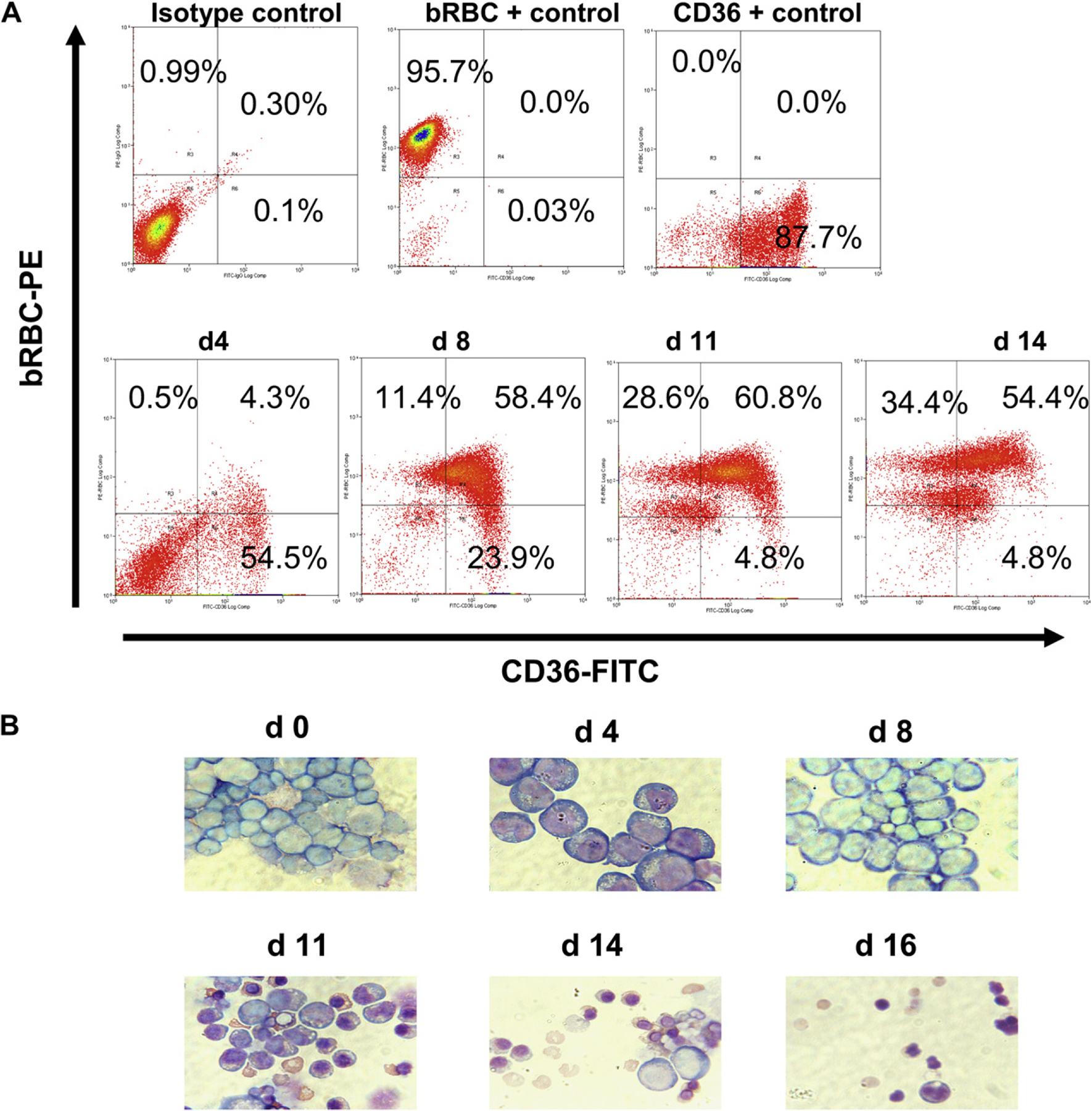
Erythroid progenitor cell cultures derived from baboon CD34+ bone marrow (BM) cells. (A) Kinetics of changes in expression of CD36 and baboon red blood cell (bRBC) on varying days of culture (bottom panel). The top panels shows reactivity of isotype controls and anti-bRBC and anti-CD36 with BM mononuclear cells (+ controls). (B) Wright’s stained cytospin preparations show changes in morphology on varying days of culture.
Figure 2.
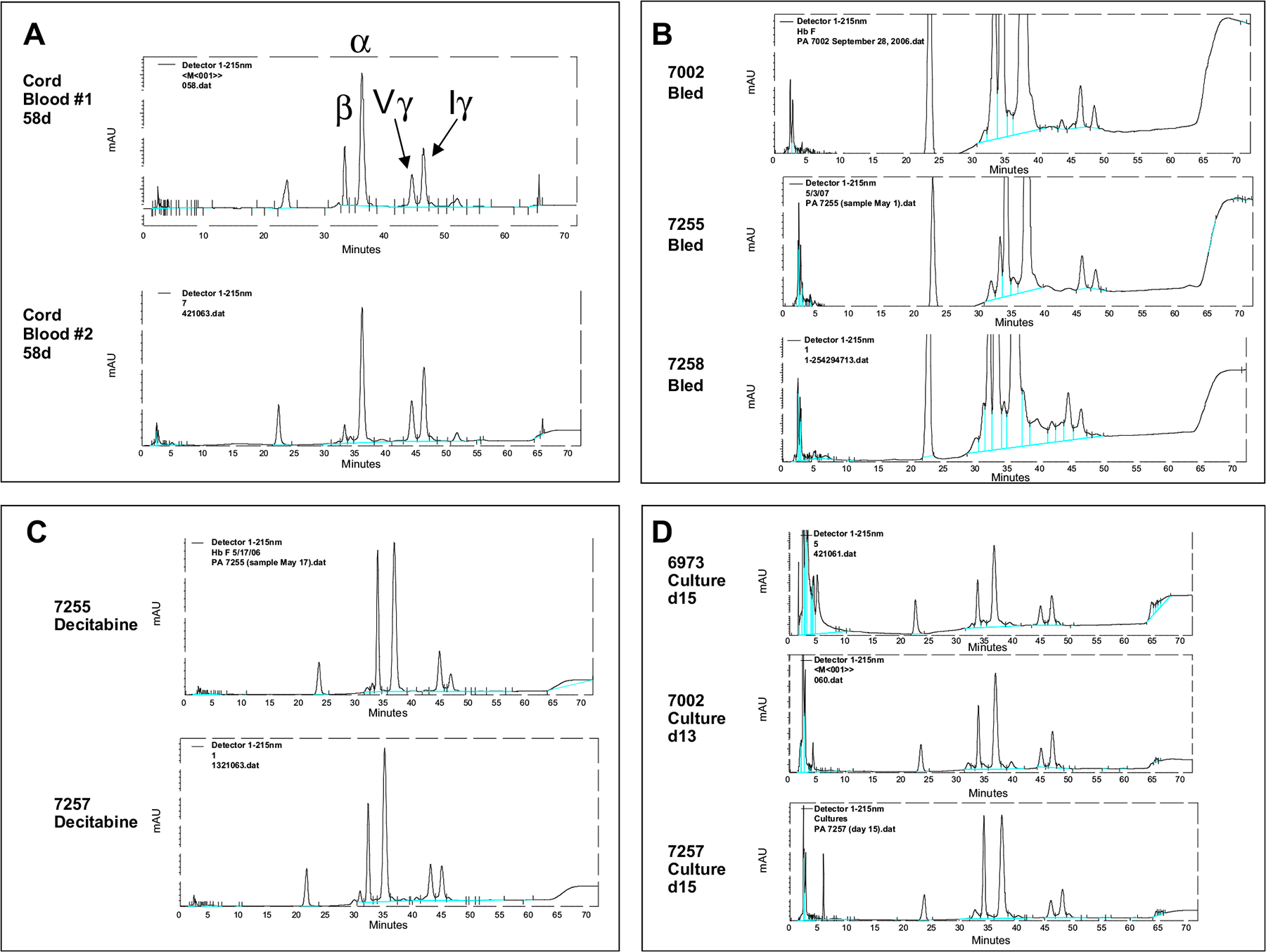
HPLC analysis of globin chain expression in (A) fetal cord blood, (B) peripheral blood of phlebotomized baboons, (C) peripheral blood of decitabine-treated baboons, and (D) lysates of cultured baboon bone marrow erythroid progenitor cells.
Table 1.
Expression of individual γ-globin chains
| Sample | γ/γ + β | α/γ + β | Iγ/Vγ | |
|---|---|---|---|---|
| Fetal cord blood | 58d #1 | 72.6 | 1.09 | 1.85 |
| 58d #2 | 90.1 | 1.04 | 1.85 | |
| Mean | 81.4 | 1.07 | 1.85 | |
| Baboon (bled) | 7254 | 4.1 | 1.09 | 0.64 |
| 7255 | 9.6 | 1.05 | 0.56 | |
| 7258 | 7.4 | 0.99 | 0.76 | |
| Mean 6 SD | 7.03 ± 2.77 | 1.04 ± 0.05 | 0.65 ± 0.10 | |
| Baboon (decitabine) | 7001 | 55.2 | 0.98 | 0.40 |
| 7002 | 55.3 | 1.24 | 1.12 | |
| 7254 | 50.5 | 1.05 | 0.90 | |
| 7255 | 41.9 | 1.05 | 0.45 | |
| 7257 | 55.1 | 1.06 | 0.95 | |
| Mean ± SD | 51.6 ± 5.79 | 1.08 ± 0.09 | 0.75 ± 0.32 | |
| CD34+ BM cultures | 6973 | 60.5 | 1.16 | 1.55 |
| 7002 | 54.2 | 1.08 | 2.10 | |
| 7255 | 33.3 | 1.04 | 1.52 | |
| 7256 | 49.5 | 1.07 | 1.71 | |
| 7257 | 42.9 | 1.01 | 1.79 | |
| 7258 | 45.2 | 1.05 | 1.46 | |
| Mean ± SD | 47.6 ± 9.44 | 1.07 ± 0.07 | 1.69 ± 0.23 |
Expression of individual γ-globin chains in hemolysates from cord blood, peripheral blood of bled and decitabine-treated baboons, and adult baboon CD34+ bone marrow erythroid progenitor cell cultures.
BM = bone marrow; SD = standard deviation.
Iγ- and Vγ -globin genes in adult BM -derived EPC cultures are expressed in a ratio characteristic of fetal development
The baboon Iγ-globin and Vγ-globin chains differ by the presence of either an isoleucine or valine at amino acid 75. The individual chains are expressed at characteristic ratios that differ in fetuses and adults in a manner similar to the developmental difference in Gγ and Aγ-globin chain expression in man. The Iγ/Vγ chain ratio, a measurement of the pattern of expression of the individual Iγ-globin and Vγ-globin chains, was 1.69 ± 0.24 (n = 4) in EPC cultures (Table 1; Fig. 2D). This ratio was not significantly different compared to cord blood samples of two early gestational age 58-day fetuses (1.85; Table 1; Fig. 2A), but differed significantly from the chain ratio observed upon reactivation of low levels of HbF expression in vivo in adult baboons induced by phlebotomy (0.65 ± 0.10; n = 3; Table 1; Fig. 2B) or high levels of HbF induced by treatment with the DNA demethylating drug decitabine (0.75 ± 0.32; n = 5; Table 1; Fig. 2C). In three of five baboons treated with decitabine, an increase in the Iγ/Vγ ratio was observed, but the fetal ratio was not attained. Reversion to the fetal pattern of expression was observed in all cultures at levels of HbF that ranged between 33% and 60%, but not in baboons treated with decitabine where HbF levels ranging from 42% to 55% were attained. Reversion to the fetal pattern of γ-globin chain synthesis in cultured erythroid progenitors was therefore not dependent on the level of HbF reactivation.
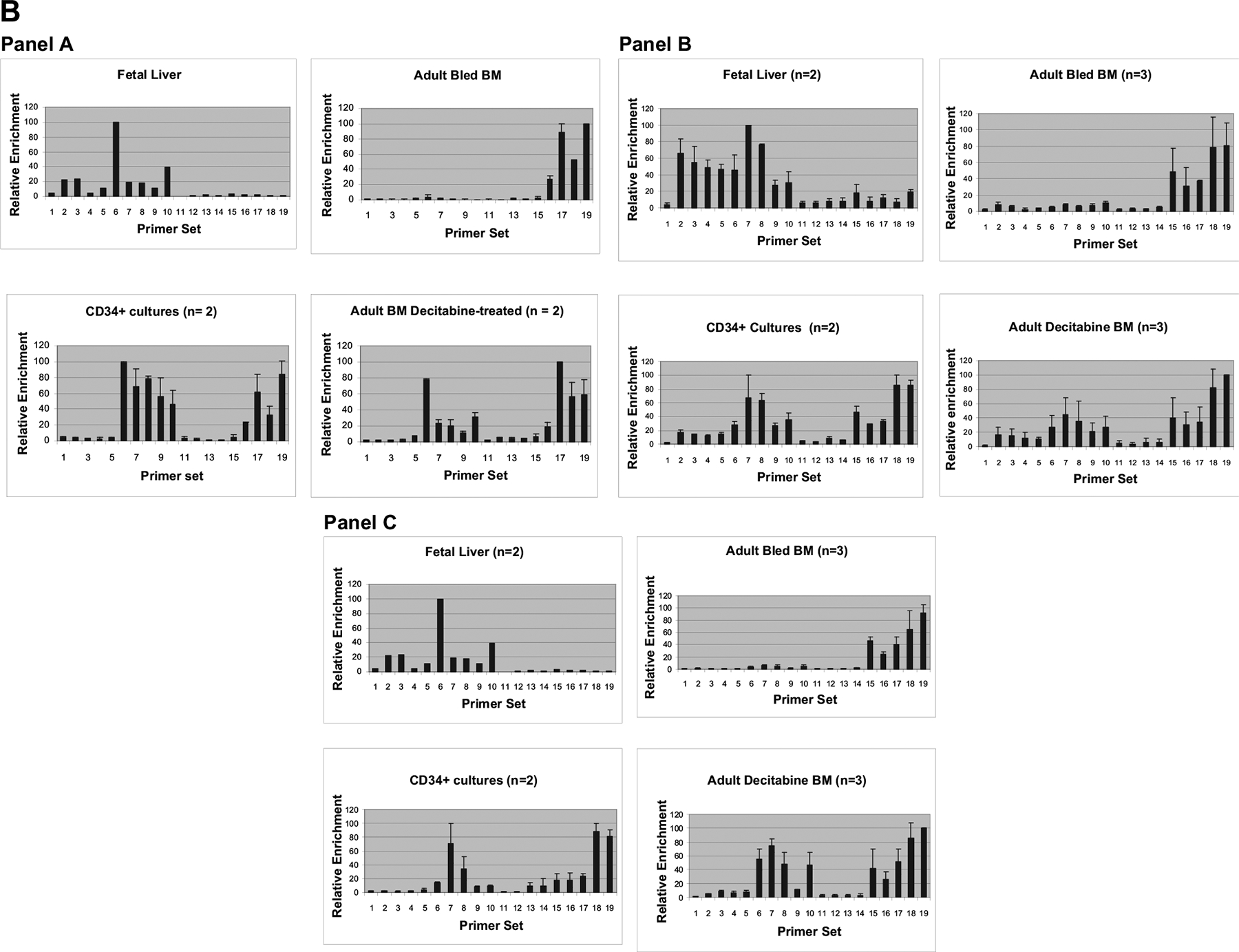
RNA polymerase II
To better understand the molecular events responsible for reactivation of HbF expression, the pattern of distribution of RNA polymerase II throughout the baboon β-globin locus in purified erythroblasts from baboon fetal liver, adult baboon BM pre- and postdecitabine, and from EPC cultures was determined by ChIP. ChIP analysis was performed on day 11 of EPC culture when approximately 90% of cells were bRBC+. The sequence of the 18 primer sets used in these experiments and their relative location within the β-globin gene locus is depicted in Figure 3A. The pattern of distribution of RNA polymerase II throughout the β-globin gene locus in purified erythroblasts from baboon fetal liver (n = 1), adult baboon BM predecitabine (bled; n = 2), baboon BM postdecitabine treatment (n = 2), and baboon BM EPC cultures (n = 2) correlated with the pattern of globin gene expression (Fig. 3B, panel A). In fetal liver erythroblasts, RNA polymerase II was associated primarily with the γ-globin promoter and lesser amounts were detected near the promoter of the pseudo – β-globin gene, the ϵ-globin promoter, and ϵ- and γ-globin intervening sequence 2 (IVS2) regions. In the adult BM erythroblasts purified from phlebotomized baboons (HbF = 5 – 7%), RNA polymerase II was associated primarily with the β-globin gene promoter and IVS2 regions. In baboon BM, EPC cultures and BM erythroblasts purified following decitabine treatment, RNA polymerase II was associated with both the γ-globin and β-globin gene promoters and IVS regions and also with the Aγ-globin enhancer and pseudo β-globin gene promoter.
Acetyl histone H3
Levels of acetylated histone H3 (ac-H3) throughout the β-globin gene locus were determined in purified fetal liver erythroblasts (n = 2) and adult BM erythroblasts predecitabine (bled; n = 3) and postdecitabine (n = 3) and cultured erythroid progenitors (n = 2) (Fig. 3B, panel B). Ac-H3 was associated primarily with the γ-globin gene in fetal liver erythroblasts and with the β-globin gene in BM from phlebotomized baboons. Following decitabine treatment, ac-H3 was distributed at approximately equal levels between the γ-globin and β-globin genes. Decitabine treatment was also associated with a smaller increase in the level of acetylation of the ϵ-globin gene and sequences within the Aγ-enhancer and pseudo β-globin gene, while acetylation of chromatin surrounding the duplicated Alu sequences in the γ – β intergenic region remained low. The pattern of distribution of ac-H3 in EPC cultures (day 11; n = 2) was similar to that of BM cells of decitabine-treated animals. A domain of chromatin characterized by a relatively low level of acetylation of histone H3 within the γ- to β-globin intergenic region appears to separate the active γ-globin and β-globin genes in postdecitabine erythroblasts and erythroid progenitor day 11 cultures.
Histone H3 lysine 4 trimethyl
Distribution of histone H3 (lys4) trimethyl throughout the β-globin gene locus was also analyzed in fetal liver erythroblasts (n = 2), and adult BM erythroblasts pre- and postdecitabine treatment (n = 2) and cultured erythroid progenitors (day 11; n = 2) (Fig. 3B, panel C). Distribution of the histone H3 (lys4) trimethyl modification was highly correlated with differences in expression levels of the γ- and β-globin genes. In fetal liver erythroblasts (n = 2), histone H3 (lys4) trimethyl was highly enriched near γ-globin gene and was not detectable near the β-globin gene. In adult BM erythroblasts from phlebotomized baboons (n = 2), histone H3 (lys4) trimethyl was highly enriched near the γ-globin gene, while very low levels were observed associated with the β-globin gene. In BM postdecitabine (n = 2), and also in adult baboon BM EPC cultures where HbF was reactivated, histone H3 (lys4) trimethyl was distributed at nearly equal levels between the γ- and β-globin genes, reflecting the similar levels of expression of these genes. The level of histone H3 (lys4) trimethyl associated with the ϵ-globin promoter and IVS regions was low and similar to that associated with the brain-specific necdin gene and the γ β intergenic region.
DNA methylation of the γ-globin gene promoter in cultured baboon erythroid progenitors
In previous studies, we have observed a correlation between the level of DNA methylation of five CpG residues within the γ-globin gene promoter region and the level of γ-globin gene expression. In previously published studies [31], the level of DNA methylation (calculated as percentage of total CpG methylated) of these five CpG residues in purified BM erythroblasts obtained from phlebotomized adult baboons (n = 4; HbF = 6.45% ± 1.75%) was 70.8% ± 10.0%. Decitabine treatment was associated with increased HbF and decreased DNA methylation of the γ-globin promoter (33.4% ± 10.9%). DNA methylation of these CpG sites was absent in purified fetal liver erythroblasts harvested at 50 to 55 days gestation [9]. Bisulfite sequence analysis of DNA isolated from purified erythroid cells present in baboon EPC cultures on days 7, 11, and 14 was performed to determine to what extent HbF reactivation in cultured baboon erythroid progenitors was also associated with a decreased γ-globin gene methylation. Erythroid cells were purified from cultures by immunomagnetic chromatography using the bRBC antibody. Cells analyzed were >90% erythroid as evaluated by either examination of Wright staining or flow cytometry. In EPC cultures initiated from CD34+ BM cells of three different baboons, overall level of DNA methylation of five CpG residues (as a percentage of total CpG residues) within the γ-globin promoter in purified bRBC+ cells on days 7, 11, and 14 of culture was 75.4% ± 3.15%, 83.4% ± 5.85%, and 82.4% ± 11.25%, respectively (Table 2; Fig. 4A). This level of DNA methylation was not significantly different from that of purified normal BM erythroid cells (O90% erythroid; 77.7% ± 14.5%) or BM erythroid cells harvested from phlebotomized baboons (76.2% ± 3.16%; Table 2). Analysis of the extent of methylation of the individual −54, +51, +5, +16, and +48 CpG sites in BM erythroid cells from three representative samples of normal and phlebotomized baboons, and purified erythroid cells from days 7, 11, and 14 EPC cultures, showed only minor differences between sites that were not associated with differences in γ-globin expression (Fig. 4B). Examination of the average number of methyl CpG per sequenced clone also showed only minor differences between samples that were also not associated with differences in γ-globin expression (Fig. 4C). The average number of methyl CpG within the 105-bp γ-globin promoter fragment in day-7 cultures was 2.94, nearly identical to the number in BM erythroid cell from normal (2.93) and bled baboons (2.84), and only slightly less that levels in day-11 (3.23) and day-14 cultures (3.20). In contrast, the level of γ-globin promoter methylation in BM erythroid cells from decitabine-treated baboons (33.4% ± 10.9%) and the number of methyl CpG residues per clone in decitabine-treated BM erythroid cells (1.16) was significantly less than in cultured EPC and BM erythroid cells from bled and normal baboons. Despite this difference in the level of γ-globin methylation, no significant difference in γ-globin expression between cultured erythroid progenitors (47.6% ± 9.4%) and decitabine-treated baboons (51.6% ± 5.8%) was observed.
Table 2.
Results of bisulfite sequence analysis of DNA methylation of five CpG sites located in 5′ region of the γ-globin gene
| Sample | Clones analyzed | CpG sites analyzed | DNA methylation (% CpG methylated) |
|---|---|---|---|
| Normal BM (n = 3) | 33 | 128 | 77.6 ± 14.5 |
| Bled BM (n = 4) | 60 | 240 | 76.2 ± 3.16 |
| Decitabine BM (n = 4) | 40 | 166 | 33.5 ± 9.4 |
| Culture day 7 (n = 3) | 36 | 141 | 75.4 ± 3.15 |
| Culture day 11 (n = 3) | 34 | 131 | 83.4 ± 5.85 |
| Culture day 14 (n = 3) | 32 | 125 | 82.4 ± 11.25 |
Results were obtained by DNA sequence analysis of polymerase chain reaction amplicons containing the γ-globin gene 5′ region following bisulfite modification cloned in the pCR4 vector.
BM = bone marrow.
Figure 4.
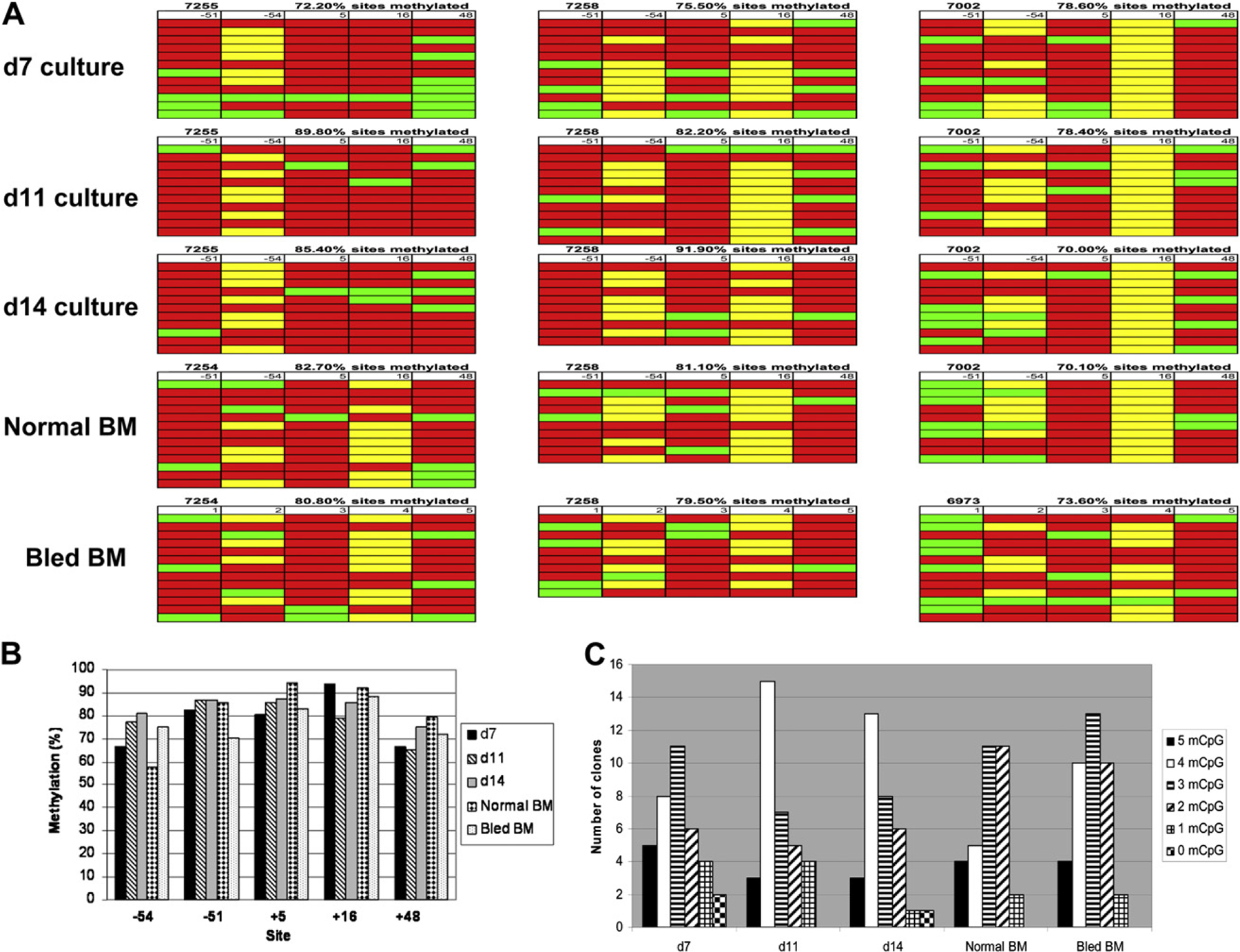
(A) results of bisulfite sequence analysis of purified erythroid cells of days 7, 11, and 14 cultured erythroid progenitor cells (EPC) and 3 representative samples of purified erythroid cells of bone marrow (BM) aspirates of normal and bled baboons. Each row represents the sequence analysis of a single pCR4-derived clone containing the polymerase chain reaction (PCR) amplicon of the γ-globin promoter region following bisulfite modification. Columns show the level of DNA methylation at the respective −54, −51, +5, +16, and +48 CpG sites within the 5′ region of the γ-globin gene. Methylated CpG (red); unmethylated CpG (green); baboon-specific polymorphic sites resulting in the absence of the CpG dinucleotide (yellow). (B) Analysis of the proportion of DNA methylation at each respective CpG site (−54, −51, +5, +16, +48) in purified erythroid cells of day 7, 11, 14 EPC, and normal and bled bone marrow (BM). (C) Analysis of the number of methyl CpG present in sequenced clones of amplified, bisulfite-treated DNA purified from purified erythroid cells from day 7, day 11, day 14 EPC, and normal and bled BM.
Different BCL11A isoforms are expressed in cultured EPC and BM erythroid cells
We then investigated whether a difference in BCL11A expression between cultured EPC and BM erythroid cells might be associated with the difference in HbF expression. Expression of BCL11A in cultured EPC and purified erythroid BM cells was compared by Western blot. Extracts prepared from two independent BM aspirates and two EPC cultures (day 11) showed a distinct difference in molecular weight of the BCL11A protein (Fig. 5). In cultured EPC cells, the molecular weight of BCL11A protein detected with three different monoclonal antibodies recognizing different determinants in the N-terminal, core, and C-terminal regions of the protein was 125 kDa. In contrast, the molecular weight of BCL11A observed in extracts of purified erythroid BM cells was 220 kDa. Therefore, the difference in the level of γ-globin expression in cultured EPC compared to BM cells was associated with a distinct difference in the size of the BCL11A protein.
Figure 5.
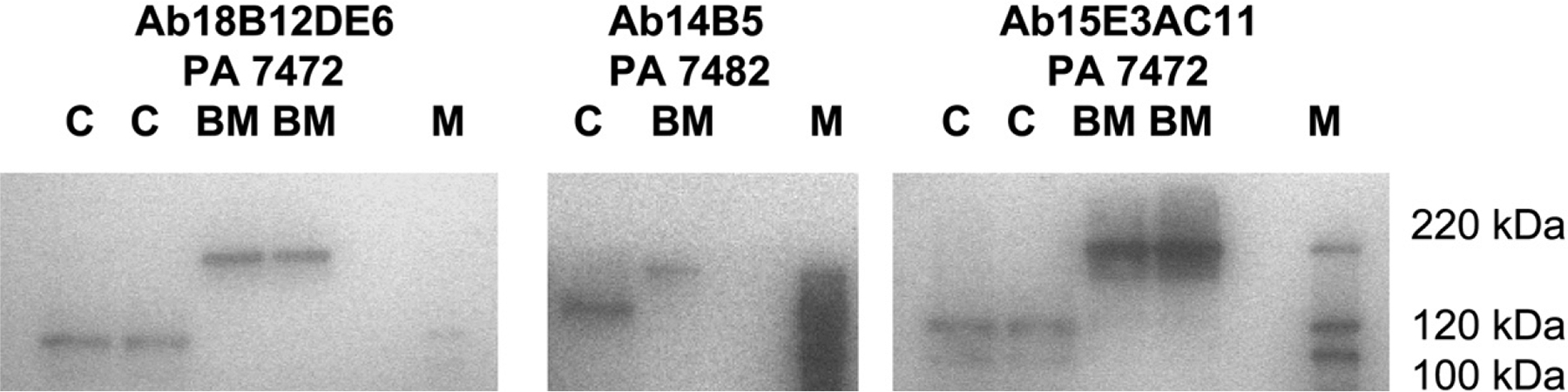
Western blot analysis showing differences in the size of the BCL11A protein in purified erythroid cells from baboon bone marrow (BM) (7472, 7482) and cultured erythroid progenitor cells (EPC) (C, day 11). Blots were developed with three different anti-BCL11A monoclonal antibodies.
Discussion
Our studies show clearly that high levels of RNA polymerase II, acetyl histone H3, and histone H3 (lys4) trimethyl are associated with the γ-globin gene when it is expressed at high levels in early gestational age fetal liver, in BM erythroid cells following decitabine treatment, and in baboon erythroid progenitor cell cultures and not when it is expressed at low levels in phlebotomized adults. Therefore, increases in γ-globin expression in baboons following decitabine treatment and in EPC cultures derived from CD34+ BM cells involved increased transcription activation of the γ-globin gene.
In EPC cultures, the two baboon γ-globin chains, Iγ and Vγ, are expressed in a ratio characteristic of fetal development. The fetal pattern of expression is not dependent on the level of HbF synthesized. The γ-globin chain ratio is altered in baboons treated with decitabine that reactivate HbF to even higher levels than culture, but the ratio characteristic of fetal development was not attained. Although previous studies of human adult-derived BFU-E showed that the ratio of Gγ/Aγ produced was similar to that of adult peripheral blood, it was suggested that a strict quantitative relationship between γ/γ + β and Gγ/Aγ synthesis may not exist and that a considerable amount of flexibility in each program could reflect differences in the regulatory mechanisms [14]. Whether the fetal pattern of expression in baboon EPC cultures represents a species-specific difference in regulatory mechanisms or a difference in response to culture conditions remains to be determined.
Because DNA hypomethylation of the γ-globin gene promoter is clearly correlated with increased expression of HbF in fetal liver [29] and decitabine-treated BM [31], we expected that reactivation of HbF synthesis in cultured erythroid progenitors would also be associated with extensive γ-globin promoter DNA hypomethylation. Surprisingly, we observed that the level of DNA methylation of CpG residues within the γ-globin promoter in baboon EPC cultures was not significantly different from the levels in purified BM erythroid cells from bled and normal, unbled baboons expressing low levels of HbF. Therefore, the difference in γ-globin gene expression and levels of RNA polymerase II, ac-H3, and histone H3 lys 4 trimethyl associated with the γ-globin genes between purified erythroblasts from baboon EPC cultures and bled BM samples was not dependent on additional DNA hypomethylation of the γ-globin promoter beyond that observed in normal BM erythroblasts. Recent experiments have shown that reactivation of genes silenced by DNA methylation may not necessarily require extensive changes in DNA methylation. Increased γ-globin expression in human BFU-E treated with the histone deactylase inhibitor butyrate was associated with increased histone acetylation and partial loss of DNA methylation of the γ-globin gene [47]. Loss of MBD2, a component of the NURD co-repressor complex, in MBD2 knockout mice containing the human βYAC transgene resulted in a modest reduction of DNA methylation of the γ-globin gene while γ-globin expression was induced to a level similar to that following 5-azacytdtine treatment [48].
In contrast to the lack of difference in γ-globin promoter DNA methylation, a clear difference in the size of the BCL11A protein expressed in cultured EPC and BM cells was observed. In cultured EPC, the molecular weight of BCL11A was 125 kDa, compared to the 220-kDa form observed in BM cells. Alternative splicing is known to produce different transcripts encoding various BCl11A isoforms. The longest known transcript, designated XL, encodes a protein of 835 amino acids corresponding to a predicted size of 91.8 kDa. The size of the BCL11A XL transcript expressed by in vitro transcription/translation was 125 kDa [49]. Posttranslational modification of BCL11A by SUMOylation, a modification important in transcriptional regulation, was shown to result in isoforms of up to 220 kDa [50]. Our observation of a 220-kDa isoform in BM erythroid cells and a 125-kDa isoform suggest that BCL11A may be modified by SUMOylation in BM cells, but not in cultured EPC. SUMOylation has an important role regulation of gene expression, particularly in gene repression [51], and its potential role in BCL11A-mediated γ-globin gene repression will require further studies.
In conclusion, our results demonstrate that transcriptional activation of the γ-globin gene in baboons following decitabine treatment and in cultured erythroid progenitors is likely increased through different mechanisms. Repression of the γ-globin gene may be achieved by multiple mechanisms involving both epigenetic modifications (DNA methylation) and transacting factors such as BCL11A. These multiple mechanisms of repression might be important in regulating differences in levels of expression occurring during fetal development and following erythropoietic stress [52,53]. Detailed knowledge of these mechanisms may identify new therapeutic targets to increase HbF levels in patients with sickle cell disease and β-thalassemia.
Acknowledgments
This was work was supported by VA Merit Review, National Institutes of Health (Bethesda, MD, USA) 5U54HL090513-02, and State of Illinois Sickle Cell Center (Chicago, IL, USA) Research funds.
Footnotes
Conflict of Interest
No authors of this article have any commercial, proprietary, or financial interest in the products or companies described in this article.
References
- 1.Stamatoyannopoulos G Molecular and cellular basis of hemoglobin switching. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, eds. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge:Cambridge University Press; 2001. p. 131–145. [Google Scholar]
- 2.Bank A Regulation of human fetal hemoglobin: new players, new complexities. Blood. 2006;107:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. [DOI] [PubMed] [Google Scholar]
- 4.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994; 330:1639–1644. [DOI] [PubMed] [Google Scholar]
- 5.Papayannopoulou T, Brice M, Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci U S A. 1976;73:2033–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papayannopoulou T, Nakamoto B, Buckley J, Kurachi S, Nute PE, Stamatoyannopoulos G. Erythroid progenitors circulating in the blood of adult individuals produce fetal hemoglobin in culture. Science. 1978;199:1349–1350. [DOI] [PubMed] [Google Scholar]
- 7.Papayannopoulou T, Brice M, Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977;74: 2923–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidoguchi K, Ogawa M, Karam JD. Hemoglobin biosynthesis in individual erythropoietic bursts in culture. Studies of adult peripheral blood. J Clin Invest. 1979;63:804–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidoguchi K, Ogawa M, Karam JD, Wilson JM, Fitch MS. Synthesis of fetal and adult hemoglobin in culture by human umbilical cord blood erythropoietic precursors. Hemoglobin. 1978;2:503–512. [DOI] [PubMed] [Google Scholar]
- 10.Stamatoyannopoulos G, Rosenblum BB, Th Papayannopoulou, Brice M, Nakamoto B, Shepard TH. HbF and HbA production in erythroid cultures from human fetuses and neonates. Blood. 1979;54:440–450. [PubMed] [Google Scholar]
- 11.Comi P, Giglioni B, Ottolenghi S, et al. Globin chain synthesis in single erythroid bursts from cord blood: Studies on γ-β and Gγ-Aγ switches. Proc Natl Acad Sci U S A. 1980;77:362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papayannopoulou T, Kalmantis T, Stamatoyannopoulos G. cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979;76:6420–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terasawa T, Ogawa M, Porter PN, Karam JD. Gγ and Aγ globin chain biosynthesis by adult and umbilical cord blood erythropoietic bursts and reticulocytes. Blood. 1980;56:93–97. [PubMed] [Google Scholar]
- 14.Papayannopoulou T, Kurachi S, Brice M, Nakamoto B, Stamatoyannopoulos G. Asynchronous synthesis of HbF and HbA during erythroblast maturation. II. Studies of Gγ, Aγ, and β chain synthesis in individual erythroid clones from neonatal and adult BFU-E cultures. Blood. 1981;57:531–536. [PubMed] [Google Scholar]
- 15.Groudine M, Peretz M, Nakamoto B, Papayannopoulou T, Stamatoyannopoulos G. The modulation of HbF synthesis in adult erythroid progenitor (burst-forming-unit) cultures reflects changes in γ-globin gene transcription and chromatin structure. Proc Natl Acad Sci U S A. 1986;83:6887–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamatoyannopoulos G, Nakamoto B, Kurachi S, Th Papayannopoulou. Direct evidence for interaction between human erythroid progenitor cells and a hemoglobin switching activity present in fetal sheep serum. Proc Natl Acad Sci U S A. 1983;80:5650–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constantoulakis P, Nakamoto B, Papayannopoulou T, Stamatayannopoulos G. Fetal calf serum contains activities that induce fetal hemoglobin in adult erythroid cell cultures. Blood. 1990;75:1862–1869. [PubMed] [Google Scholar]
- 18.Wojda U, Leigh KR, Njoroge JM, et al. Fetal hemoglobin modulation during human erythropoiesis: stem cell factor has “late” effects related to the expression pattern of CD117. Blood. 2002;101:492–497. [DOI] [PubMed] [Google Scholar]
- 19.Bhanu NV, Trice TA, Lee YT, et al. A sustained and pancellular reversal of gamma-globin gene silencing in adult human erythroid cells. Blood. 2005;105:387–393. [DOI] [PubMed] [Google Scholar]
- 20.Bohmer RM, Campbell TA, Bianchi DW. Selectively increased growth of fetal hemoglobin-expressing adult erythroid progenitors after brief treatment of early progenitors with transforming growth factor beta. Blood. 2000;95:2967–2974. [PubMed] [Google Scholar]
- 21.Barrie PA, Jeffreys AJ, Scott AF. Evolution of the beta-globin gene cluster in man and primates. J Mol Biol. 1981;149:319–336. [DOI] [PubMed] [Google Scholar]
- 22.DeSimone J, Mueller AL. Fetal hemoglobin synthesis in baboons (Papio cynocephalus). J Lab Clin Med. 1978;91:862–871. [PubMed] [Google Scholar]
- 23.Schroeder WA, DeSimone J, Shelton JB, et al. Changes in the gamma chain heterogeneity of hemoglobin F of the baboon (Papio cynocephalus) postnatally and after partial switching to hemoglobin F production by various stimuli. J Biol Chem. 1983;258:3121–3125. [PubMed] [Google Scholar]
- 24.DeSimone J, Schroeder WA, Shelton JB, et al. Speciation in the baboon and its relation to gamma-chain heterogeneity and to the response to induction of HbF by 5-azacytidine. Blood. 1984;63:1088–1095. [PubMed] [Google Scholar]
- 25.DeSimone J, Heller P, Adams JG. Hemopoietic stress and fetal hemoglobin synthesis: comparative studies in vivo and in vitro. Blood. 1979;54:1176–1181. [PubMed] [Google Scholar]
- 26.Torrealba de Ron A, Papayannopoulou T, Stamatoyannopoulos G. Studies of HbF in adult nonanemic baboons: HbF expression in erythroid colonies decreases as the level of maturation of erythroid progenitors advances. Exp Hematol. 1985;13:919–925. [PubMed] [Google Scholar]
- 27.van der Ploeg LH, Flavell RA. DNA methylation in the human gamma delta beta-globin gene locus in erythroid and nonerythroid tissues. Cell. 1980;19:947–958. [DOI] [PubMed] [Google Scholar]
- 28.Mavilio F, Giampaolo A, Care A, et al. Molecular mechanisms of human hemoglobin switching: selective undermethylation and expression of globin genes in embryonic, fetal, and adult erythroblasts. Proc Natl Acad Sci U S A. 1983;80:6907–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavelle D, Vaitkus K, Hankewych M, Singh M, DeSimone J. Developmental changes in DNA methylation and covalent histone modifications of chromatin associated with the ϵ-, γ-, and β-globin gene promoters in Papio anubis. Blood Cell Mol Dis. 2006;36: 269–278. [DOI] [PubMed] [Google Scholar]
- 30.DeSimone J, Heller P, Hall L, Zwiers D. 5-azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982;67:4428–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavelle D, Vaitkus K, Hankewych M, Singh M, DeSimone J. The effect of 5-aza-2′-deoxycytidine (Decitabine) on covalent histone modifications of chromatin associated with the ϵ-, γ-, and β-globin genes in baboon (P. anubis). Exp Hematol. 2006;34:339–347. [DOI] [PubMed] [Google Scholar]
- 32.Ley TJ, DeSimone J, Anagnou NP, et al. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta + thalassemia. N Engl J Med. 1982;307:1469–1475. [DOI] [PubMed] [Google Scholar]
- 33.Ley TJ, DeSimone J, Noguchi CT, et al. 5-azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patents with sickle cell anemia. Blood. 1983;62:370–380. [PubMed] [Google Scholar]
- 34.Saunthararajah Y, Hillery CA, Lavelle D, et al. Effects of 5-aza-2’-deoxycytidine on fetal hemoglobin levels, red cell adhesion, and hematopoietic differentiation in patients with sickle cell disease. Blood. 2003;102:3865–3870. [DOI] [PubMed] [Google Scholar]
- 35.Goren A, Simchen G, Fibach E, et al. Fine tuning of globin gene expression by DNA methylation. PloS One. 2006;1:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mabaera R, Greene MR, Richardson CA, Conine SJ, Kozul CD, Lowrey CH. Neither DNA hypomethylation nor changes in the kinetics of erythroid differentiation explain 5-azacytidine’s ability to induce fetal hemoglobin. Blood. 2008;111:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thein SL, Menzel S. Discovering the genetics underlying foetal hemoglobin production in adults. Br J Haematol. 2008;145:455–467. [DOI] [PubMed] [Google Scholar]
- 38.Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–1842. [DOI] [PubMed] [Google Scholar]
- 39.Singh M, Lavelle D, Vaitkus K, Mahmud N, Hankewych M, DeSimone J. The γ-globin gene promoter progressively demethylates as the hematopoietic stem progenitor cells differentiate along the erythroid lineage in baboon fetal liver and adult bone marrow. Exp Hematol. 2007;35: 48–55. [DOI] [PubMed] [Google Scholar]
- 40.Leone L, Monteleone M. Reversed-phase high-performance liquid chromatography of human hemoglobin chains. J Chromatogr. 1985; 321:407–419. [DOI] [PubMed] [Google Scholar]
- 41.Im H, Grass JA, Johnson KD, Boyer ME, Wu J, Bresnick EH. Measurement of protein-DNA interactions in vivo by chromatin immunoprecipitation. Methods Mol Biol. 2004;284:129–146. [DOI] [PubMed] [Google Scholar]
- 42.Bulger M, Sawado T, Schubeler M, Groudine M. ChIPs of the β-globin locus: unraveling gene regulation within an active domain. Curr Opin Genet Dev. 2002;12:170–177. [DOI] [PubMed] [Google Scholar]
- 43.Raizis AM, Schmitt F, Jost J-P. A bisulfite method of 5-metylcytosine mapping that minimizes template degradation. Anal Biochem. 1995; 226:161–166. [DOI] [PubMed] [Google Scholar]
- 44.Hilgarth RS, Sarge KD. Analysis of protein sumoylation. Curr Protoc Protein Sci. 2006;14:14.8.1–14.8.7. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y-H, Lavelle D, DeSimone J, Uddin S, Platanias LC, Hankewych M. Growth inhibition of a human myeloma cell line by all-trans retinoic acid is not mediated through downregulation of interleukin-6 receptors but through upregulation of p21WAF1. Blood. 1999;94:251–259. [PubMed] [Google Scholar]
- 46.von Lindern M, Zauner W, Mellitzer G, et al. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94:550–559. [PubMed] [Google Scholar]
- 47.Fathallah H, Weinberg R,Galperin Y, SuttonM, Atweh GF. Roleof epigenetic modifications in normal globin gene regulation and butyrate-mediated induction of fetal hemoglobin. Blood. 2007;110:3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rupon JW, Wang SZ, Gaensler K, Lloyd J, Ginder GD. Methyl binding domain protein 2 mediates g-globin gene silencing in adult human βYAC transgenic mice. Proc Natl Acad Sci U S A. 2006;103:6617–6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H, Ippolito GC, Wall JK, et al. Functional studies of BCL11A: characterization of the conserved BCL11A-XL splice variant and its interaction with BCL6 in nuclear paraspeckles of germinal center B cells. Mol Cancer. 2006;5:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuwata T, Nakamura T. BCL11A is a SUMOylated protein and recruits SUMO-conjugation enzymes in its nuclear body. Gene Cells. 2008;13:931–940. [DOI] [PubMed] [Google Scholar]
- 51.Gill G Something about SUMO inhibits transcription. Curr Opin Genet Dev. 2005;15:536–541. [DOI] [PubMed] [Google Scholar]
- 52.DeSimone J, Biel SI, Heller P. Stimulation of fetal hemoglobin synthesis in baboons by hemolysis and hypoxia. Proc Natl Acad Sci U S A. 1978;75:2937–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeSimone J, Biel M, Heller P. Maintenance of fetal hemoglobin (HbF) elevations in the baboon by prolonged erythropoietic stress. Blood. 1982;60:519–523. [PubMed] [Google Scholar]


