Abstract
Hemostatic parameters have been investigated as molecular determinants of tumor progression. To analyze the dynamics of microparticle-associated tissue factor activity (MPTF), tissue factor antigen (TF-Ag), and angiopоietin-2 (ANG-2) in cancer patients before, during, and after active treatment and to explore their potential as biomarkers for metastatic occurrence and death. Blood for the analysis of MPTF, TF-Ag, ANG-2, and conventional hemostatic tests was sampled in 111 patients with various cancers at 4 consecutive visits: before first chemotherapy cycle, after 3 courses, at the sixth course, and 3 months after chemotherapy cessation. Patients were followed up until metastatic progression/death or the end of the study. MPTF did not change during chemotherapy, but increased significantly after treatment cessation. Total TF-Ag and ANG-2 decreased throughout active treatment. Significant drop of their levels was observed 3 months post therapy cessation. Progressive disease was significantly associated with higher pre-chemotherapy TF-Ag and fibrinogen. Elevated baseline levels of fibrinogen were associated with increased risk of shortened progression free survival. Cessation of chemotherapy is associated with significant change of hemostatic parameters. Pre-chemotherapy levels of TF-Ag and fibrinogen may be informative of disease state and prognosis.
Keywords: hemostasis, angiopoietins, progression-free survival, neoplasms
Introduction
Malignancy is associated with hypercoagulability as evidenced by the observation that cancer patients have increased risk of developing thromboembolic events. 1 Extensive research has elucidated some of the biological mechanisms linking hemostatic system and neoplasia. 2 It is now recognized that oncogenic mechanisms intertwine with hemostatic processes, leading to shift of hemostatic equilibrium towards either increased thrombogenicity or bleeding tendency. Basic research has shown that factors of hemostatic system facilitate tumor inherent processes such as tumor initiation, growth, neo-angiogenesis, anoikis, epithelial-to-mesenchymal transition, invasion, and metastasis.3–6 It is also suggested that cancer-associated hemostatic imbalance is largely driven by the tumor itself through the release of procoagulant substances, prothrombotic conversion of endothelium, and suppression of natural anticoagulants.
Tissue factor (TF) is one of the major mediators of the crosstalk between malignancy and hemostatic system. In situ TF expression by tumor cells is a hallmark of cancer and is found to be defined by oncogenic events. Circulating isoforms of TF: total TF antigen (TF-Ag), alternatively spliced TF, and extracellular vesicles expressing TF activity (microparticle-associated TF activity, MPTF) have been studied for their implication in cancer-associated thrombosis. 7 Fewer studies have assessed their utility as markers for biologic activity in tumors.
Proangiogenic switch of cancers is coupled with coagulation processes. It is well known that positive regulators of angiogenesis such as VEGF enhance TF endothelial expression and inversely, TF downregulates the negative regulator of angiogenesis thrombospondin. 8 Angiopoietin-1,2/Tie-2 system is a crucial regulator of angiogenesis and controls VEGF production. Angiopoietin-1 suppresses TF expression in HUVECs by putative mechanism of PI3k/Akt signaling.9,10 Angiopietin-2 derepression has been implicated in the loss of vascular quiescence and thrombus formation in endotoxemic mice. 11 However, data on TF and Angiopoietin-2 relationship in cancer is still scarce.
We hypothesized that markers of coagulation (MPTF, TF-Ag) and angiogenesis (ANG-2) in cancer are in a straight relationship and can be informative of disease state and tumor activity. We expected MPTF, TF-Ag, ANG-2 to be higher in patients with metastatic spread and to decrease throughout chemotherapy mirroring suppression of the disease. We also expected to observe different profiles of coagulation activity according to the achieved response—hypercoagulability maintained in patients with progression as opposed to decreased levels of those markers in patients with responsive disease.
Material and Methods
Study Design and Setting
The study was conducted as prospective, observational, diagnostic, and longitudinal single-center study at the Clinic of Medical Oncology, University Hospital “Sveti Georgi,” Plovdiv, Bulgaria and was approved by the Ethics Committee of Medical University of Plovdiv, Bulgaria. From all cancer patients admitted to the clinic for initiation of systemic treatment between April 2013 and April 2015 a total of 128 cancer patients were recruited and included in the study. Patients were followed up from the date of first chemotherapy cycle until the occurrence of event of interest or end of study. If patients did not attend for their regular clinic visit, an attempt to contact them was made to register patient status. If no contact was achieved and patients were lost to follow-up, the last date of registered clinic visit was considered end of follow-up.
Participants
Patients with lung, ovary, colorectal, and breast cancer expected to receive chemotherapy were assessed for study eligibility, if following inclusion criteria were met: age ≥ 18 years, histologically verified cancer, newly diagnosed or indicated for a new line of chemotherapy. Patients were excluded from the study, if they had one or more exclusion criteria: history of arterial or venous thrombosis in the last 3 months prior to study inclusion, history of significant cardio-vascular disease: heart failure NYHA class III/IV, arterial bypass angioplasty, stenting, valve prosthesis; ECOG/Performance status ≥ 4; oral contraceptive use; concomitant malignancy, acute viral or bacterial infection during the last 2 weeks prior to study entry; anticoagulant treatment with vitamin K antagonists and DOACs during last 3 months prior to study inclusion, vitamin K treatment 1 month before study entry.
Study consisted of 4 planned visits defined as follows: timepoint 1 (TP1)—baseline at study inclusion and prior to chemotherapy initiation; timepoint 2 (TP2)—after third chemotherapy cycle (prior to infusion of fourth chemotherapy cycle); timepoint 3 (TP3)—at sixth chemotherapy cycle; timepoint 4 (TP4)—at the third-month follow-up post chemotherapy cessation. During the first visit data on patient demographic, disease stage, histology type, planned chemotherapy regimen, date of cancer diagnosis, surgical volume (if applicable), date of progression/ metastasis (if applicable) was obtained from patient records. Blood was drawn for the analysis of microparticle-associated tissue factor activity (MPTF), total tissue factor antigen (TF-Ag), angiopoietin-2 (ANG-2), CBC, creatinine, screening coagulation tests: thrombin time, activated partial thromboplastin time, prothrombin time (%), INR, fibrinogen, tumor markers—CEA, CA19-9, CA15-3, CA125. During each subsequent visit (TP 2-4) blood was drawn for the analysis of MPTF, TF-Ag, and ANG-2, screening coagulation tests, and CBC. Assessment of disease activity and response to treatment was performed within routine clinical care which adheres to the National Standards of Bulgarian Oncology Society and Response Evaluation Criteria in Solid Tumors (RECIST). 12
Endpoints
Metastatic occurrence and/or death (irrespective of cause) were chosen as primary clinical endpoints for the evaluation of progression free survival (PFS). PFS was defined as time between chemotherapy initiation and either metastatic progression or death (excluding local/locoregional relapse).
All patient relevant data was captured on individual hard copy records. Patients were enrolled in the study after written informed consent was obtained.
Biological Material, Sampling, and Storage
Blood sampling for all laboratory parameters was performed in the morning before infusion of any chemotherapy by sterile atraumatic venipuncture. For determination of MPTF activity, TF-Ag levels and screening clotting tests blood was sampled in vacuum tubes containing 0.106 molar solution sodium citrate (Monovette Sarstedt Sodium Citrate, 2.9 mL) and mixing ratio of 1:10 was strictly observed; for ANG-2 determination blood was collected in serum tubes with sillicate beads (S-Monovette Sarstedt, 2.6 mL) and for CBC analysis—in EDTA K3 tubes with concentration 1.6 mg EDTA/mL blood (Monovette Potassium EDTA, 2.7 mL). After sample processing plasma/serum aliquots were stored at −80 °C for MPTF and TF-Ag or at −20 °C for ANG-2 for up to 6 months until serial measurements.
Microparticle-Associated Tissue Factor Activity
MPTF procoagulant activity was determined by immune-chromogenic assay (Zymuphen-MPTF, HYPHEN Biomed, France). Plasma supernatant was removed after centrifugation for 15 min at 1500 g at room temperature and then centrifuged again for 2 min at 13 000 g. MPTF activity measurement proceeded as per manufacturer's specifications described elsewhere. 13 In brief: enhancer solution and patient sample were added to wells of polyvinylchloride plate coated with mouse antihuman tissue factor monoclonal antibody that does not interfere with TF activity. After incubation for 18 h (to allow TF expressing microparticles to bind to the plate) f.VIIa and f.X were added. Finally, yellow-color chromogenic substrate for f.Xa was added. Absorption at 405 nm that is directly proportional to MPTF concentration in sample was determined spectrophotometrically. MPTF activity was measured in pg/mL. The major characteristics of analytic reliability of ELISA for MPTF procoagulant activity were: intraassay imprecision—CV 4% to 6%; interassay imprecision—CV 9% to 10%.
Tissue Factor Antigen Levels
Plasma concentration of tissue factor antigen levels was determined with quantitative ELISA (ZYMUTEST Total Tissue Factor, HYPHEN Biomed France). Plasma supernatant was removed after centrifugation for 20 min at 2500 g. Measurement of TF-Ag levels was performed according to manufacturer's instructions and TF-Ag concentration was expressed in pg/mL. Characteristics of analytic reliability of ELISA for TFAg were: intraassay imprecision—CV 5% to 7%; interassay imprecision—CV 6% to 9%.
Angiopoietin-2
Serum Angiopoietin-2 levels were measured with quantitative ELISA (Angiopoietin-2 Human ELISA kit, Abcam, UK). Serum was removed from venous blood after centrifugation at 1500 g for 10 min. ANG-2 levels were expressed in ng/mL. Characteristics of analytic reliability of ELISA for ANG-2 were: intraassay imprecision—CV 4.2% to 6.9%; interassay imprecision—CV 7.4% to 10.4%.
Prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen, complete blood count, creatinine, CEA, CA15-3, CA125, CA19-9 were immediately analyzed. PT, aPTT, and TT were measured on a Sysmex CS 2000 analyzer (Siemens Diagnostica), fibrinogen was measured according to Clauss. 14 CBC was measured on ADVIA 2120i hematology analyzer (Siemens Diagnostica), tumor markers—on automatic Immulite 2000 system (Siemens Diagnostica). Analytical coefficients of variation for PT were 4% to 7%, aPTT—4% to 6%, fbg—3.05% to 4.54%.
Statistical Methods
In order to account both for the unbalanced number of subjects at each subsequent visit and repeated measures over time we chose linear mixed effect analysis to assess the effect of disease and therapy-related factors on MPTF, TF-Ag, and ANG-2 levels. The analysis was performed using R packages lme4 and lmerModTest.15,16 We followed the procedure outlined by Zuur et al: first, with all variables of interest in the model we chose random error structure using REML. Next, we searched for the optimal fixed components in a top-down approach by gradually reducing variables using ML and AIC. 17 The final model is presented using REML estimation. We entered visit, diagnosis, disease stage, age, gender, and interaction term of targeted therapy with visit as fixed effects. As random effects we included both intercept (subjects) and slope (visit). After selecting the final model a likelihood ratio test was performed on the final model with the effect in question against the null model without the effect in question. To compare whether dynamics of MPTF, TF-Ag, and ANG-2 parallels changes of conventional blood parameters with respect to chemotherapy, dynamics of hemoglobin (HGB), white blood cells (WBC) and platelets (PLT) was analyzed according to the aforementioned procedure. A separate model was fit to evaluate differences in studied parameters with respect to response to therapy where response was added as fixed effect for intercept and an interaction term between visit and response as fixed effect on slope with random effects for both intercept (subject) and slope (visit).
To analyze relationships between MPTF, TF-Ag, and ANG-2 Pearson correlation was performed by each visit. For the purpose of the analysis MPTF and TF-Ag were square root transformed and ANG-2 was log-transformed.
Cox regression analysis was performed to quantitate the effect of tested hemostatic biomarkers on progression free survival. Occurrence of distant metastases and /or death were defined as primary event of progression free survival. ROC analysis was applied to determine discriminatory threshold values of studied parameters for the occurrence of a PFS event. Then based on cutoff values significant variables were dichotomized and the Kaplan–Meier method was applied to build survival curves and compare survival distributions. Linear mixed effect analysis, correlation, Kaplan–Meier survival analysis, and Cox regression were performed in R programing environment v.4.0.4 and packages lme4, lmerModTest, survival, survminer, ggplot, tidyverse, lattice, sjPlot were used.18–23 ROC analysis was performed on MedCalc 12.3 (mariakerke, Belgium).
Results
Participants
A total of 128 patients enrolled in the study and completed first visit. Of them, 17 patients were excluded from longitudinal and survival analysis because twelve patients did not undergo chemotherapy and 5 patients did not return for treatment continuation after their first chemotherapy cycle and could not be followed up beyond their discharge date. Final number of patients completing follow-up was 111: breast (n = 37), lung (n = 25), ovary (n = 17), colorectal (n = 32). Of them 79 patients completed visit TP2, 56 completed visit TP3, and 37—Visit TP4, accounting for totally of 277 samples. Median follow-up was 220 days. Distribution of patients by tumor site and visit is presented in Table 1. 70 patients received adjuvant chemotherapy and 41—first or next line of therapy. Overall, 39 patients (35.1%) experienced PFS event, 46 patients (41.4%) had progressive disease as defined by RECIST. Patients’ demographic and disease-related characteristics are presented in Table 2 and Supplemental Table S1. Median age was 59.31 years (21-81), females represented 64% (n = 71).
Table 1.
Distribution of Patients by Tumor Type and Visit.
| Visit | Tumor type | Total | |||
|---|---|---|---|---|---|
| Breast (n) | Lung (n) | Ovary (n) | Colorectal (n) | ||
| Visit 1 | 37 | 25 | 17 | 32 | 111 |
| Visit 2 | 28 | 15 | 13 | 23 | 79 |
| Visit 3 | 19 | 12 | 9 | 16 | 56 |
| Visit 4 | 14 | 7 | 6 | 10 | 37 |
Abbreviation: n, number of patients.
Table 2.
Demographic and Disease-related Patient Characteristics (n = 111).
| Patient characteristic | Descriptive value |
|---|---|
| Age (years) | |
| Median | 60.00 |
| Range | 21-81 |
| Gender, n (%) | |
| Male | 40 (36) |
| Female | 71 (64) |
| Localization, n (%) | |
| Breast | 37 (33.3) |
| Lung | 25 (22.5) |
| Ovary | 17 (15.3) |
| Colorectal | 32 (28.8) |
| Disease stage, n (%) | |
| I | 15 (13.5) |
| II | 36 (32.4) |
| III | 35 (31.5) |
| IV | 25 (22.5) |
| Chemotherapy line, n (%) | |
| Adjuvant | 70 (63.1) |
| First | 36 (32.4) |
| Second | 2 (1.8) |
| Third | 3 (2.7) |
| Targeted therapy | |
| Yes | 17 (84.7) |
| No | 94 (84.7) |
| BMI ≥ 35, n (%) | 4 (3.6) |
| Hgb < 100 g/l, n (%) | 7 (6.3) |
| WBC ≥ 11 G/l, n (%) | 18 (16.2) |
| PLT ≥ 350 G/l, n (%) | 36 (32.4) |
| Response, n (%) | |
| Disease progression | 46 (41.4) |
| Without progression | 65 (58.5) |
| Follow-up (days) | |
| Median | 220 |
| Range | 9-688 |
Abbreviations: PLT, platelets; WBC, white blood cells; BMI, body mass index.
Longitudinal Analysis of MPTF, TF-Ag, and ANG-2
MPTF Activity Levels Do Not Change Throughout Active Treatment and Increase After Chemotherapy Cessation
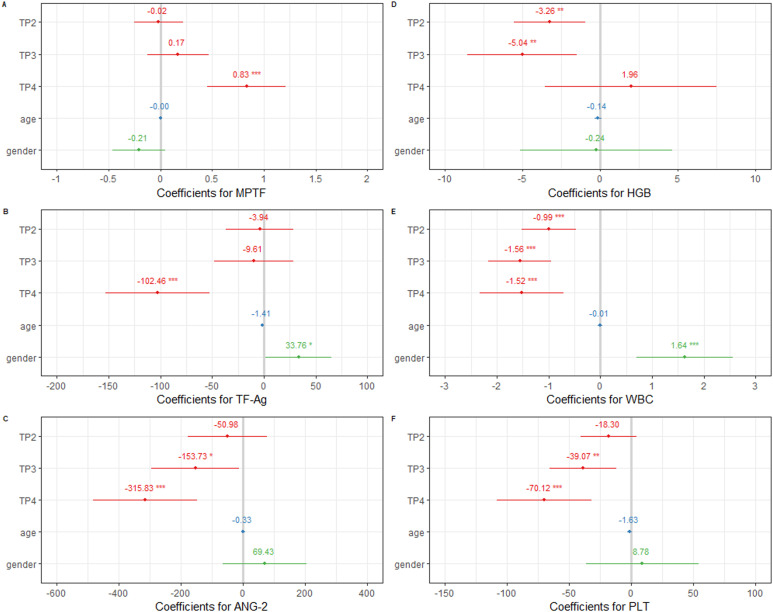
The estimated initial MPTF activity was 1.065 pg/mL (± 0.338) and the estimated subject-to-subject variation in initial MPTF corresponded to a standard deviation of 0.182 pg/mL (Supplemental Tables S2 and S3). During chemotherapy administration MPTF activity fluctuated nonsignificantly compared to pre-chemotherapy levels (BTP2 = −0.018, P = .87, BTP3 = 0.171, P = .25). A statistically significant increase of 0.834 pg/mL (± 0.194) was observed at the third-month follow-up post chemotherapy cessation (P < .001), Figure 1A. Estimated subject-to-subject individual dynamics varied within standard deviation of 0.261 pg/mL. Subsequent models indicated that tumor site and stage were not predictive of initial MPTF activity levels and administration of targeted therapy was not associated with changes of MPTF levels over time. The final model that described longitudinal change of MPTF included age, gender, and visit as fixed effects, random effect for intercept, and slope (time) by subject. Loglikelihood ratio test indicated that the model was significant compared to a null model without visit (AIC 737. 66 vs 724.3, LogLik −361.53 vs −352.17, χdf=3 = 19.323, P = .0002).
Figure 1.
Forrest plots of model coefficients for studied parameters, REML estimation. Significance codes for P-value are “***” < .001 < “**” < .01 < “*” < .05. TP2—timepoint 2, TP3—timepoint 3, TP4—timepoint 4. Reference category is timepoint 1 (TP1) for visit, female for gender.
TF-Ag Levels Decrease After Chemotherapy Cessation
As indicated by AIC comparison the best model representing longitudinal dynamics of TF-Ag levels included factors visit, age, and gender (Supplemental Tables S2 and S3). TF-Ag decreased nonsignificantly after 3 (at TP2) and after 5 (at TP3) chemotherapy cycles when compared to its pre-chemotherapy levels (TP1). On average TF-Ag levels decreased with 3.94 pg/mL after 3 cycles of chemotherapy and with ∼ 9.61 pg/mL after 5 cycles. Significant decrease with > 50% of pre-chemotherapy values was observed 3 months after chemotherapy cessation (102.46 pg/mL ± 25.67 pg/mL), Figure 1B. Gender-related changes were observed. Male patients showed significantly higher intercept levels than female patients (33.76 pg/mL ± 16.25). Stage and tumor site were not predictive of TF intercept levels. Administration of targeted therapy was not associated with different dynamics of TF. Loglikelihood ratio test indicated that the model was significant compared to a null model without visit (AIC 2537.3 vs 2547.4, LogLik −1258.7 vs −1266.7, χdf=3 = 16.03, P = .001).
ANG-2 Levels Decrease During Active Treatment and After Chemotherapy Cessation
Angiopoietin-2 exhibited clear dynamics of decrease during antineoplastic treatment and at follow-up. The best model describing longitudinal change of angiopoietin levels with respect to treatment included fixed effects of age, gender, tumor site and visit on intercept, fixed effects of visit and targeted therapy on the slope and random effects of individual dynamics over time and intercept (Supplemental Tables S2 and S3). Removal of either tumor site or targeted therapy from the model did not change AIC in subsequent models and therefore they were both kept in the final model. On average, initial angiopoietin-2 levels were 551.78 pg/mL (± 196.5). Individual subject-to-subject variation in initial values corresponded to standard deviation of 303.82 pg/mL. Angiopoietin-2 decreased throughout chemotherapy administration and during follow-up, Figure 1C. Compared to pre-chemotherapy levels decrease was significant at the sixth course (TP2) and at 3 months post chemotherapy cessation (TP4) (BTP3 = −152.15, P = .03, BTP4 = −307.67, P = .0003, ML estimation). Administration of targeted therapy did not exhibit significant effect on ANG-2 level dynamics. No significant effect on intercept was found for age, gender, stage, and primary tumor site as well. Loglikelihood ratio test was significant in favor of the model with visit versus without (AIC 2996.9 vs 2996.4, LogLik −1488.4 vs −1481.2, χ7 = 14.498, P = .042).
TF-Ag and ANG-2 Parallel WBC and PLT Dynamics During Longitudinal Follow-up
Longitudinal analysis of blood parameters revealed steady and significant decrease of WBC and PLT counts over time compared to pre-chemotherapy levels, Figure 1E and F. Dynamics of Hgb levels showed significant drop during active treatment, whereas nonsignificant increase of Hgb was observed 3 months post chemotherapy cessation in comparison to baseline levels, Figure 1D.
Conventional Hemostatic Tests
Longitudinal analysis of conventional hemostatic parameters revealed significant effect of chemotherapy administration on INR, thrombin time, and MPV (Supplemental Table S8). During active treatment significant change was observed only for MPV. Compared to pre-chemotherapy values MPV decreased with 0.28 fl after 3 courses of chemotherapy and with 0.3 fl—after 6. Cessation of chemotherapy was associated with further decrease in MPV (BTP4 = −0.47, P = .03), increase in thrombin time (BTP4 = 0.71, P = .02), and increase in INR (BTP4 = 0.185, P = .046). Lung cancer patients had significantly increased fibrinogen levels and INR (Bfbg = 1.84, P < .001, BINR = 0.244, P = .01), as well as aPTT, but with borderline significance (BAPTT = 2.24, P = .05); PT% values were significantly lower (BPT = −14.27, P = .003). MPV was significantly higher in colorectal cancer patients (BMPV = 0.50, P = .043). Advanced disease stage was associated with decrease in MPV (Bstage = −0.19, P = .043) and borderline decrease in INR (BINR = 0.059, P = .057). Male patients had significantly longer aPTT and lower PT% values than female patients (BAPTT = 1.80, P = .049).
Differences in Longitudinal Dynamics According to Response
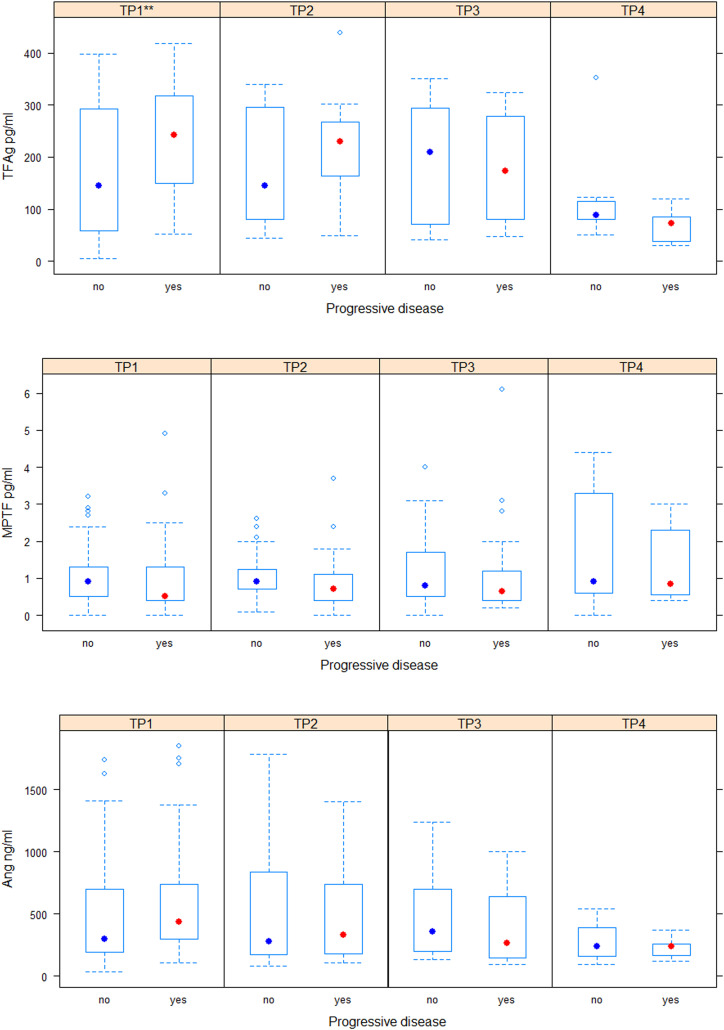
No significant difference was observed in baseline values of MPTF (B = 0.02 ± 0.04, P = .67) and ANG-2 (B = 118.39 ± 93.18, P = .2) between patients who progressed and those who did not. In patients with progressive disease TF-Ag levels were significantly higher compared to patients with stable disease, partial remission, and complete remission (B = 64.57 ± 23.28, P = .006). No significant difference was found in dynamics of MPTF (BTP2 = 0.015 ± 0.07, P = .84; BTP3 = −0.002 ± 0.101, P = .9; BTP4 = 0.13 ± 0.13), TF-Ag (BTP2 = −25.52 ± 34.92, P = .46; BTP3 = −64.54 ± 40.15, P = .11; BTP4 = −97.16 ± 52.17, P = .06) and ANG-2 (BTP2 = −106.41 ± 118.90, P = .37; BTP3 = −180.6 ± 133.023, P = .17; BTP4 = −145.231 ± 158.8, P = .3) with respect to disease response (Figure 2). Significantly higher baseline values of fibrinogen (B = 0.82 ± 0.25, P = .001) and significantly decreased PT% values (B = −10.32 ± 4.56) at TP2 were observed in in patients with progressive disease. No difference was found in other conventional parameters with respect to baseline values or dynamics.
Figure 2.
Levels of investigated parameters in patients with and without progressive disease at each visit /timepoint/. Significance codes for P-value are “***” < .001 < “**” < .01 < “*” < .05. TP1—timepoint 1, TP2—timepoint 2, TP3—timepoint 3, TP4—timepoint 4.
Correlation Between Parameters
No significant correlations were observed between investigated parameters at baseline and at the last visit. At the second visit TF-Ag and ANG-2 exhibited moderate straight significant correlation, which was also maintained at the next visit (ρTP2 0.41, P = .007; ρTP3 0.52, P = .005). Significant correlations were found for MPTF at the third visit: moderate inverse with both TF-Ag (ρTP3 −0.50, P = .001) and Ang-2 (ρTP3 −0.42, P = .009). Overall MPTF and TF-Ag showed a trend of inverse relationship, whereas TF-Ag and ANG-2 maintained trend of positive relationship over time (Supplemental Table S7).
Association of Hemostatic Parameters With PFS
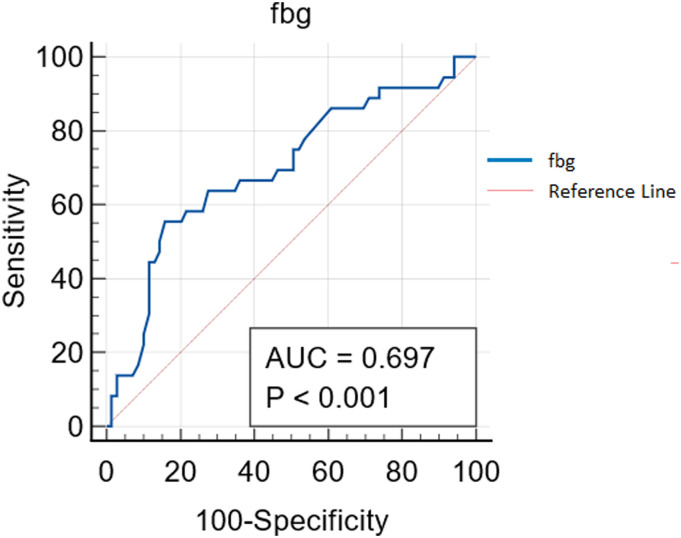
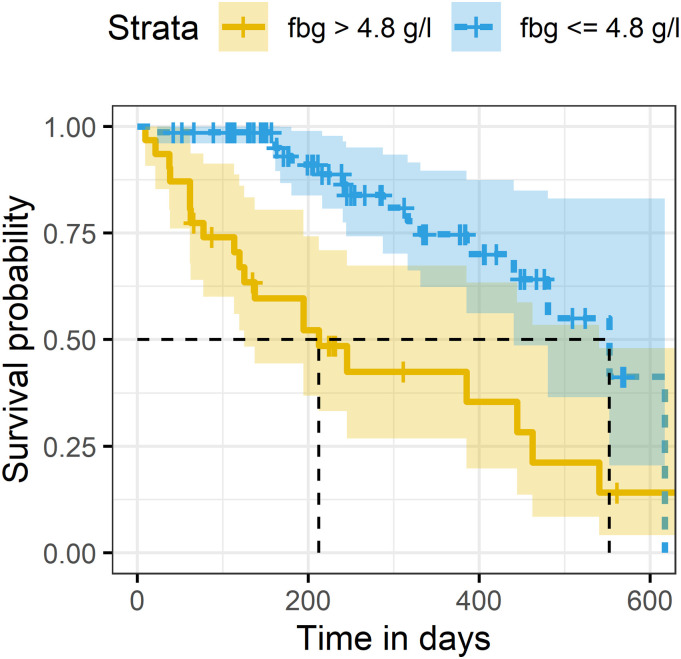
Cox regression analysis revealed significant association for elevated levels of fibrinogen with increased risk for metastatic progression and/or death (HR 1.53, 95% CI 1.18-1.97, P = .001). No statistically significant associations were found between PFS and other investigated parameters (Table 3). ROC analysis determined the cutoff values of fibrinogen for occurrence of PFS event. Fibrinogen levels > 4.08 g/l correctly identified 69.7% of cases with PFS event (AUC 95% CI 0.60-0.78, P = .005, Figure 3). Further, Kaplan–Meier survival analysis showed significantly shorter PFS time for patients with fibrinogen > 4.08 g/l (median 212 days, 95% CI 125-540) compared to patients with fibrinogen ≤ 4.08 g/l (median 552 days, 95% CI 440—not reached, χ2[1] = 15.7, P < .005), Figure 4.
Table 3.
Univariate Cox Regression of Investigated Parameters.
| HR | 95% CI | P-value | |
|---|---|---|---|
| MPTF | 1.182 | 0.82-1.7 | .4 |
| TFAg | 1.002 | 0.99-1.005 | .3 |
| Ang-2 | 1 | 0.99-1.001 | .6 |
| aPTT | 1.011 | 0.96-1.064 | .7 |
| PT% | 0.98 | 0.97-1.002 | .09 |
| INR | 1.079 | 0.65-1.77 | .8 |
| TT | 0.90 | 0.64-1.25 | .5 |
| *Fbg | 1.53 | 1.18-1.97 | .001 |
| MPV | 0.86 | 0.57-1.31 | .5 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 3.
ROC curve for fibrinogen cutoff value of 4.08 g/l for binary classification of PFS event.
Figure 4.
Kaplan–Meier survival curves of patients with fibrinogen levels below and above the cutoff value.
Discussion
In this study we aimed to determine the longitudinal dynamics of hemostatic (MPTF, TF-Ag) and angiogenic (ANG-2) markers during active treatment and after chemotherapy-free interval in patients with breast, lung, colorectal, and ovary cancer. We also assessed longitudinal dynamics with respect to disease progression and analyzed potential associations with metastatic occurrence and adverse outcome.
Contrary to our initial assumptions, during active treatment we did not observe significant changes of MPTF procoagulant activity and found significant increase in MPTF activity after chemotherapy cessation. Other studies have reported no significant change in MPTF activity over the course of cytolytic therapy and disconnectedness was observed between MPTF activity and thrombin generation.24–26 Serial measurements of microparticle-associated tissue factor procoagulant activity in thirteen patients with metastatic testicular cancer did not find change at days 1, 2, and 4 of 2 consecutive chemotherapy cycles. 24 Study on breast cancer patients assessing impact of chemotherapy on coagulation has shown that MPTF activity remained unchanged 24 h and 8 days post infusion of chemotherapy, while TAT levels were significantly elevated at the same timepoints compared to baseline. 25 In the RASTEN trial no change of TF-extra vesicle associated procoagulant activity was observed during active treatment while all parameters of thrombin generation were significantly higher at the third chemotherapy cycle compared to their pretreatment levels. 26 This study also assessed TF-extra vesicle activity 2 months post therapy cessation, but found no difference in procoagulant activity levels. Based on this data we suggest that unchanged MPTF activity during chemotherapy is not necessarily indicative of suppressed coagulation. It may rather be the result of consumed coagulation potential during ongoing thrombin generation and the observed increase in MPTF activity several months post treatment may be reflective of its reinstatement after removal of the perturbing stimulus. One limitation of our study is that no dynamic assessment of thrombin generation parameters was employed and thus no direct comparisons can be made.
In our study total TF and ANG-2 showed a trend of decrease throughout course of chemotherapy and dropped significantly during the 3-month treatment free interval. Importantly, TF-Ag and ANG-2 exhibited similar dynamics of decrease as leukocytes and platelets. One aspect to consider is the enhanced cancer-associated myeloproliferation of solid tumors driven by tumor-derived cytokines. 27 With disease control exerted by chemotherapy over time this effect is expected to be abrogated. This is in line with our observation on dynamics of decrease for platelets and leukocytes over the span of several months. Furthermore, platelets have been established as the major source of TF in circulation and blood-borne TF was also found to originate from monocytes and neutrophils.28,29 Given these findings we find it plausible to consider that TF dynamics reflects disease- and treatment-related changes of WBC and PLT longitudinally through their contribution to TF concentration. Since ANG-2 is exclusively secreted by endothelial cells, its dynamics can be attributed to related effects on endothelium. Thus, as another aspect we suppose that the drop of total TF and ANG-2 levels after cessation of chemotherapy is attributed at least in part to suspension of vascular and cellular damage induced by cytostatics. Vascular toxicity is a well-known effect of cancer therapies. Chemotherapy induces endothelial apoptosis, decreased production of nitric oxide, secretion of inflammatory cytokines, and conversion to prothrombotic phenotype. 30 Release of TF-positive apoptotic vesicles by endothelial and tumor cells is considered a potential mechanism for cytotoxic thrombogenicity. Preclinical studies have demonstrated increased TF activity in cultured endothelial cells after treatment with cisplatin, gemcitabine, and anthracyclines.31,32 Doxorubicin, 5FU, and vincristine have been shown to induce the release of apoptotic vesicles with TF-dependent procoagulant effect by tumor cells.33,34 Clinical studies have notwithstanding detected seemingly constant TF-Ag levels throughout chemotherapy. Stable levels of TF-Ag throughout chemotherapy have been reported in pancreatic cancer patients who did not develop VTE. 35 TF-Ag levels measured at baseline and 4 to 6 months after treatment onset showed no significant difference in patients with various cancers. 36 Dynamics of total TF blood levels during active treatment have been assessed in metastatic prostate cancer patients. No difference in serum levels was observed between baseline and after 2 to 5 weeks of treatment, but TF levels dropped significantly 6 to 8 weeks after treatment initiation. 37 Likely, kinetics of TF-Ag depends on the timing of measurement to detect subtle changes in plasma levels and is in temporal relationship with chemotherapy administration. With respect to angiopoietin-2 effect of chemotherapy has also been investigated. Immunohistochemical evaluation of Ang-2 expression in cervical cancer patients showed lower scores in patients who underwent neoadjuvant chemotherapy compared to surgical patients. 38 Serum ANG-2 levels decreased significantly after 4 cycles of bortezomib-based therapy in relapsed/refractory multiple myeloma patients. 39 During the course of neoadjuvant chemotherapy in breast cancer patients significant increase in ANG-2 levels was observed, but levels decreased several weeks thereafter, prior to surgery. 40 That was accompanied by the same dynamics of endothelial progenitor cells and other angiogenic factors—VEGF, erythropoietin, and endoglin. Serum concentrations of ANG-2, ANG-1, and Tie-2 did not differ before chemotherapy and 28 days postinfusion in nonsmall cell lung cancer patients. 41 Altogether these findings support the notion that removal of the cytotoxic stimulus leads to decrease of hemostatic and angiogenic factors.
We found inverse correlation between MPTF activity and total TF-Ag levels. A positive straight correlation between TF-Ag levels and TF mediated procoagulant activity of MPs has been reported in 11 pancreatic cancer patients with and without VTE. 35 Another study found no association between TF-Ag levels and MPTF activity in 252 patients with various tumors and showed that most of TF procoagulant activity was present in MP free plasma.35,36 Evaluation of plasma TF-Ag and MPTF procoagulant activity in patients with ovarian carcinoma found inverse, albeit nonsignificant correlation between the 2 variables. 42 We suppose that the observed dissociation between TF-Ag levels and MPTF activity in our study can be attributed to the measurement of different fractions of TF. MPTF activity assay captures only membrane bound TF, whereas ZYMUTEST captures total antigenic form (full length and truncated). Important to note is that lower specificity and sensitivity of TF-dependent procoagulant activity have been reported for the commercial MPTF assay used in our study compared to “in-house” TF activity assay.43,44 Inability to capture all TF+MPs and higher f.VIIa related background noise was outlined as potential reasons and they may contribute partly to the observed dissociation. Even though that this does not fully explain the inverse relationship between activity and total antigen in the context of their dynamic monitoring, this finding must be interpreted with caution until further evidence on the concomitant changes of TF-Ag and MPTF activity is accumulated.
Significant positive correlation was observed between TF-Ag levels and ANG-2 that also reflects their simultaneous dynamics during longitudinal follow-up. Several lines of evidence suggest that ANG-2 controls VEGF expression and function and that the two angiogenic factors work in concert to promote tumor growth and angiogenesis.45,46 Given the established synergistic relationship between TF and VEGF,6,47 we expected that TF and ANG-2 will exhibit the same pattern of dynamic changes. Our data support the observation that angiogenesis and coagulation are tightly coupled processes. No data on the simultaneous dynamics of TF-Ag and ANG-2 were found in the literature.
We did not find significant differences in MPTF, TF-Ag, and ANG-2 with respect to disease stage and primary site. In contrast, elevated numbers of TF+-MPs in patients with metastatic disease have been reported by other studies.48,49 Significantly higher TF-Ag levels have been measured in patients with advanced stage (III-IV) versus localized disease (I-II), but MP-associated TF activity was not different regarding stage or presence of metastases. 36 Same study found no difference in TF across cancer types. Elevated levels of ANG-2 have been reported in stage III CRC patients compared to stage II. 50 Also, elevated levels ANG-2 were reported across disease stages in lung cancer patients in a meta-analysis. 51 In this study significant association with disease stage was observed only for MPV. Advancing stage was associated with decrease in MPV. In a retrospective study of DLBCL patients no difference was found in the proportional distribution of patients according to stage between MPV below or above the cutoff of 6.11 fl. However, lower MPV was significantly associated with adverse survival outcome. 52 In the Vienna CAT Study significantly lower MPV was found in patients with metastasis compared to those without and was associated with increased risk of VTE. 53
With respect to disease course and outcome longitudinal analysis of hemostatic parameters revealed that only TF-Ag and fibrinogen levels were significantly elevated at baseline in patients who subsequently progressed. Our findings are consistent with results from another longitudinal study, which showed that fibrinogen levels in patients who achieved complete remission remain lower over time than in patients without. 54 In our study throughout the course of time fibrinogen levels remained higher in patients with progressive disease, whereas TF-Ag levels varied, albeit those differences did not reach statistical significance. This might also imply that chemotherapy contributes to leveling out differences between groups by exerting procoagulant effect and disease control at the same time.
In keeping with the association between fibrinogen and progressive disease there was also significant association of heightened risk for shorter progression free survival with increasing levels of fibrinogen. While elevated preoperative fibrinogen has been consistently associated with mortality in patients with various cancers, several studies have also shown that pre-chemotherapy fibrinogen is linked to increased risk of shortened survival in patients with multiple myeloma, lung cancer, and melanoma.55–60 Altogether this supports observations from animal models that fibrinogen promotes metastasis in cancer and is in accordance with the concept that heightened thrombotic risk mirrors propensity for disease evolution. 61
Our study has several limitations one of which is that no direct assessment with thrombin generation parameters could be made with respect to the measured MPTF activity. Next, studied population is heterogenous, comprising of primary sites with varying thrombotic risk and longitudinal cohort is relatively small, thus limiting the opportunity to perform separate analyses by tumor site. However, conduct of longitudinal studies faces several challenges among which are serial sampling procedures and keeping patient in follow-up especially after treatment cessation. We consider simultaneous measurement of angiogenic and hemostatic markers as well as extended follow-up beyond treatment cessation strengths of our study.
Conclusion
Our study presents data on longitudinal dynamics of TF-Ag, MPTF, and ANG-2 that suggests possible effect of chemotherapy on the levels of hemostatic and angiogenic biomarkers that significantly change after cessation of chemotherapy. Pre-chemotherapy levels of fibrinogen and tissue factor antigen are higher in patients who subsequently progress, implying that hypercoagulability is indicative of aggressive disease. There is evidence that elevated pre-chemotherapy fibrinogen levels are associated with increased risk for metastatic progression and death, but this association needs to be further investigated for the other hemostatic parameters.
Supplemental Material
Supplemental material, sj-docx-1-cath-10.1177_10760296211056637 for Longitudinal Dynamics of Coagulation and Angiogenesis Markers in Cancer Patients During and After Chemotherapy by Elina A. Beleva, Tanya I. Deneva, Snezhana S. Stoencheva and Zhanet G. Grudeva-Popova in Clinical and Applied Thrombosis/Hemostasis
Supplemental material, sj-tiff-2-cath-10.1177_10760296211056637 for Longitudinal Dynamics of Coagulation and Angiogenesis Markers in Cancer Patients During and After Chemotherapy by Elina A. Beleva, Tanya I. Deneva, Snezhana S. Stoencheva and Zhanet G. Grudeva-Popova in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Author Contributions: E.A. Beleva was responsible for study concept and design, grant acquisition, patient inclusion and management, data acquisition, statistical analysis, data interpretation, drafting the manuscript, and critical revision for intellectual content; T.I. Deneva was responsible for laboratory analysis and critical revision for intellectual content; S.S. Stoencheva was responsible for laboratory analysis and critical revision for intellectual content; Z.G. Grudeva-Popova was responsible for critical revision for intellectual content and supervision of the study.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received financial support for the research, authorship, and/or publication of this article: This study was funded by grant support from Medical University of Plovdiv, Bulgaria: project SDP-01/2013 and project BG051PO001-3.3.06-0011.
ORCID iD: Elina A. Beleva https://orcid.org/0000-0002-2426-6589
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Falanga A. Thrombophilia in cancer. Semin Thromb Hemost. 2005;31(01):104-110. doi: 10.1055/s-2005-863812 [DOI] [PubMed] [Google Scholar]
- 2.Langer F, Bokemeyer C. Crosstalk between cancer and haemostasis: implications for cancer biology and cancer-associated thrombosis with focus on tissue factor. Hämostaseologie. 2012;32(02):95-104. doi: 10.5482/ha-1160 [DOI] [PubMed] [Google Scholar]
- 3.Garnier D, Magnus N, Lee TH, et al. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287(52):43565-43572. doi: 10.1074/jbc.M112.401760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakasaki T, Wada H, Shigemori C, et al. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol. 2002;69(4):247-254. doi: 10.1002/ajh.10061 [DOI] [PubMed] [Google Scholar]
- 5.Milsom C, Anderson GM, Weitz JI, Rak J. Elevated tissue factor procoagulant activity in CD133-positive cancer cells. J Thromb Haemost. 2007;5(12):2550-2552. doi: 10.1111/j.1538-7836.2007.02766.x [DOI] [PubMed] [Google Scholar]
- 6.Poon RT-P, Lau CP-Y, Ho JW-Y, Yu W-C, Fan S-T, Wong J. Tissue Factor Expression Correlates with Tumor Angiogenesis and Invasiveness in Human Hepatocellular Carcinoma. Published online 2003:8. [PubMed]
- 7.Davila M, Robles-Carrillo L, Unruh D, et al. Microparticle association and heterogeneity of tumor-derived tissue factor in plasma: is it important for coagulation activation? J Thromb Haemost. 2014;12(2):186-196. doi: 10.1111/jth.12475 [DOI] [PubMed] [Google Scholar]
- 8.Ruf W, Yokota N, Schaffner F. Tissue factor in cancer progression and angiogenesis. Thromb Res. 2010;125(Supplement 2):S36-S38. doi: 10.1016/S0049-3848(10)70010-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinhardt C, Bergentall M, Greiner TU, et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature. 2012;483(7391):627-631. doi: 10.1038/nature10893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim I, Oh J-L, Ryu YS, et al. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. FASEB J. 2002;16(1):1-24. doi: 10.1096/fj.01-0556fje [DOI] [PubMed] [Google Scholar]
- 11.Higgins SJ, De Ceunynck K, Kellum JA, et al. Tie2 protects the vasculature against thrombus formation in systemic inflammation. J Clin Invest. 2018;128(4):1471-1484. doi: 10.1172/JCI97488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13.Amiral J, Seghatchian J. The diagnostic usefulness of capture assays for measuring global/specific extracellular micro-particles in plasma. Transfus Apher Sci. 2015;53(2):127-136. doi: 10.1016/j.transci.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 14.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematol. 1957;17(4):237-246. doi: 10.1159/000205234 [DOI] [PubMed] [Google Scholar]
- 15.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 16.Kuznetsova A, Brockhoff PB, Christensen RHB. Lmertest package: tests in linear mixed effects models. J Stat Softw. 2017;82(13):1-26. doi: 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- 17.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. Springer-Verlag; 2009. doi: 10.1007/978-0-387-87458-6 [DOI] [Google Scholar]
- 18.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. https://www.R-project.org/ [Google Scholar]
- 19.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Springer; 2000. [Google Scholar]
- 20.Therneau TM. Survival: Survival Analysis. 2021. https://github.com/therneau/survival
- 21.Kassambara A, Kosinski M, Biecek P. Survminer: Drawing Survival Curves Using Ggplot2. 2021. https://rpkgs.datanovia.com/survminer/index.html
- 22.Sarkar D. Lattice: Multivariate Data Visualization with R. Springer; 2008. http://lmdvr.r-forge.r-project.org [Google Scholar]
- 23.Lüdecke D. SjPlot: Data Visualization for Statistics in Social Science. 2021. https://strengejacke.github.io/sjPlot/
- 24.van den Hengel LG, van Tol AQMJS, Bertina RM, Versteeg HH, Osanto S. Microparticle-associated tissue factor activity in plasma is unaffected by cytolytic chemotherapy treatment in metastatic testicular cancer patients. Thromb Res. 2013;131(2):187-189. doi: 10.1016/j.thromres.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee SD, Swystun LL, Mackman N, et al. Impact of chemotherapy on thrombin generation and on the protein C pathway in breast cancer patients. Pathophysiol Haemost Thromb. 2009;37(2–4):88-97. doi: 10.1159/000324166 [DOI] [PubMed] [Google Scholar]
- 26.Gezelius E, Flou Kristensen A, Bendahl PO, et al. Coagulation biomarkers and prediction of venous thromboembolism and survival in small cell lung cancer: a sub-study of RASTEN – A randomized trial with low molecular weight heparin. Karamanos NK, ed. PLOS ONE. 2018;13(11):e0207387. doi: 10.1371/journal.pone.0207387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcox RA. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clin Proc. 2010;85(7):656-663. doi: 10.4065/mcp.2010.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maugeri N, Manfredi AA. Tissue factor expressed by neutrophils: another piece in the vascular inflammation puzzle. Semin Thromb Hemost. 2015;41(7):728-736. doi: 10.1055/s-0035-1564043 [DOI] [PubMed] [Google Scholar]
- 29.Lösche W. Platelets and tissue factor. Platelets. 2005;16(6):313-319. doi: 10.1080/09537100500140190 [DOI] [PubMed] [Google Scholar]
- 30.Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol. 2020;17(8):503-522. doi: 10.1038/s41569-020-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma L, Francia G, Viloria-Petit A, et al. In vitro procoagulant activity induced in endothelial cells by chemotherapy and antiangiogenic drug combinations: modulation by lower-dose chemotherapy. Cancer Res. 2005;65(12):5365-5373. doi: 10.1158/0008-5472.CAN-04-3156 [DOI] [PubMed] [Google Scholar]
- 32.Swystun LL, Shin LYY, Beaudin S, Liaw PC. Chemotherapeutic agents doxorubicin and epirubicin induce a procoagulant phenotype on endothelial cells and blood monocytes. J Thromb Haemost JTH. 2009;7(4):619-626. doi: 10.1111/j.1538-7836.2009.03300.x [DOI] [PubMed] [Google Scholar]
- 33.Muhsin-Sharafaldine M-R, Kennedy BR, Saunderson SC, et al. Mechanistic insight into the procoagulant activity of tumor-derived apoptotic vesicles. Biochim Biophys Acta BBA – Gen Subj. 2017;1861(2):286-295. doi: 10.1016/j.bbagen.2016.11.020 [DOI] [PubMed] [Google Scholar]
- 34.Pluchart C, Poitevin G, Colinart-Thomas M, et al. Vincristine induces procoagulant activity of the human lymphoblastic leukemia cell line jurkat through the release of extracellular vesicles. J Thromb Thrombolysis. 2019;48(2):195-202. doi: 10.1007/s11239-019-01894-x [DOI] [PubMed] [Google Scholar]
- 35.Khorana AA, Francis CW, Menzies KE, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6(11):1983-1985. doi: 10.1111/j.1538-7836.2008.03156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernández C, Orbe J, Roncal C, et al. Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients. Thromb Haemost. 2013;110(09):598-608. doi: 10.1160/TH13-02-0122 [DOI] [PubMed] [Google Scholar]
- 37.Strijbos MH, Gratama JW, Schmitz PIM, et al. Circulating endothelial cells, circulating tumour cells, tissue factor, endothelin-1 and overall survival in prostate cancer patients treated with docetaxel. Eur J Cancer. 2010;46(11):2027-2035. doi: 10.1016/j.ejca.2010.03.030 [DOI] [PubMed] [Google Scholar]
- 38.Lv Q, Zhong W, Ye X, et al. Expression of angiopoietin and VEGF in cervical cancer and its clinical significance. Open Life Sci. 2018;13(1):527-532. doi: 10.1515/biol-2018-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anargyrou K, Terpos E, Vassilakopoulos TP, et al. Normalization of the serum angiopoietin-1 to angiopoietin-2 ratio reflects response in refractory/resistant multiple myeloma patients treated with bortezomib. Haematologica. 2008;93(3):451-454. doi: 10.3324/haematol.11852 [DOI] [PubMed] [Google Scholar]
- 40.Fürstenberger G, von Moos R, Lucas R, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94(4):524-531. doi: 10.1038/sj.bjc.6602952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naumnik W, Chyczewska E, Ossolinska M. Serum levels of angiopoietin-1, angiopoietin-2, and their receptor tie-2 in patients with nonsmall cell lung cancer during chemotherapy. Cancer Invest. 2009;27(7):741-746. doi: 10.1080/07357900802672704 [DOI] [PubMed] [Google Scholar]
- 42.Cohen JG, Prendergast E, Geddings JE, et al. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol. 2017;146(1):146-152. doi: 10.1016/j.ygyno.2017.04.021 [DOI] [PubMed] [Google Scholar]
- 43.Tatsumi K, Antoniak S, Monroe DM, Khorana AA, Mackman N. Evaluation of a new commercial assay to measure microparticle tissue factor activity in plasma: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1932-1934. doi: 10.1111/jth.12718 [DOI] [PubMed] [Google Scholar]
- 44.Hellum M, Øvstebø R, Trøseid A-MS, Berg JP, Brandtzaeg P, Henriksson CE. Microparticle-associated tissue factor activity measured with the zymuphen MP-TF kit and the calibrated automated thrombogram assay. Blood Coagul Fibrinolysis. 2012;23(6):520-526. doi: 10.1097/MBC.0b013e328354a256 [DOI] [PubMed] [Google Scholar]
- 45.Nicolini G, Forini F, Kusmic C, Iervasi G, Balzan S. Angiopoietin 2 signal complexity in cardiovascular disease and cancer. Life Sci. 2019;239:117080. doi: 10.1016/j.lfs.2019.117080 [DOI] [PubMed] [Google Scholar]
- 46.Hashizume H, Falcón BL, Kuroda T, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70(6):2213-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rickles FR, Shoji M, Abe K. The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int J Hematol. 2001;73(2):145-150. doi: 10.1007/BF02981930 [DOI] [PubMed] [Google Scholar]
- 48.Tseng J-C, Chang L-C, Jiang B-Y, et al. Elevated circulating levels of tissue factor-positive microvesicles are associated with distant metastasis in lung cancer. J Cancer Res Clin Oncol. 2014;140(1):61-67. doi: 10.1007/s00432-013-1544-8 [DOI] [PubMed] [Google Scholar]
- 49.Campello E, Spiezia L, Radu CM, et al. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb Res. 2011;127(5):473-477. doi: 10.1016/j.thromres.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 50.Engin H, Üstündağ Y, Tekin İÖ, Gökmen A, Ertop Ş, İlikhan SU. Plasma concentrations of angiopoietin-1, angiopoietin-2 and Tie-2 in colon cancer. Eur Cytokine Netw. 2012;23(2):68-71. doi: 10.1684/ecn.2012.0308 [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Zhang Y, Wang Z, Chen N, Zhou J, Liu L. The role of serum angiopoietin-2 levels in progression and prognosis of lung cancer: a meta-analysis. Medicine (Baltimore. 2017;96(37):e8063. doi: 10.1097/MD.0000000000008063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rupa-Matysek J, Gil L, Kroll-Balcerzak R, Barańska M, Komarnicki M. Mean platelet volume as a predictive marker for venous thromboembolism and mortality in patients treated for diffuse large B-cell lymphoma: mean platelet volume in diffuse large B-cell lymphoma. Hematol Oncol. 2017;35(4):456-464. doi: 10.1002/hon.2321 [DOI] [PubMed] [Google Scholar]
- 53.Riedl J, Kaider A, Reitter E-M, et al. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. Results from the Vienna cancer and thrombosis study (CATS). Thromb Haemost. 2014;111(4):670-678. doi: 10.1160/TH13-07-0603 [DOI] [PubMed] [Google Scholar]
- 54.Reitter E-M, Kaider A, Ay C, et al. Longitudinal analysis of hemostasis biomarkers in cancer patients during antitumor treatment. J Thromb Haemost. 2016;14(2):294-305. doi: 10.1111/jth.13218 [DOI] [PubMed] [Google Scholar]
- 55.Falanga A, Marchetti M. Hemostatic biomarkers in cancer progression. Thromb Res. 2018;164(Supplement 1):S54-S61. doi: 10.1016/j.thromres.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 56.Lin Y, Liu Z, Qiu Y, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44(10):1494-1503. doi: 10.1016/j.ejso.2018.07.052 [DOI] [PubMed] [Google Scholar]
- 57.Marchetti C, Romito A, Musella A, et al. Combined plasma fibrinogen and neutrophil lymphocyte ratio in ovarian cancer prognosis May play a role? Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2018;28(5):939-944. doi: 10.1097/IGC.0000000000001233 [DOI] [PubMed] [Google Scholar]
- 58.van Marion AMW, Auwerda JJA, Lisman T, et al. Prospective evaluation of coagulopathy in multiple myeloma patients before, during and after various chemotherapeutic regimens. Leuk Res. 2008;32(7):1078-1084. doi: 10.1016/j.leukres.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 59.Zhu L-R, Li J, Chen P, Jiang Q, Tang X-P. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients. Clin Transl Oncol. 2016;18(2):178-188. doi: 10.1007/s12094-015-1350-7 [DOI] [PubMed] [Google Scholar]
- 60.Tas F, Ciftci R, Kilic L, et al. Clinical and prognostic significance of coagulation assays in melanoma. Melanoma Res. 2012;22(5):368-375. doi: 10.1097/CMR.0b013e328357be7c [DOI] [PubMed] [Google Scholar]
- 61.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96(10):3302-3309. doi: 10.1182/blood.V96.10.3302 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cath-10.1177_10760296211056637 for Longitudinal Dynamics of Coagulation and Angiogenesis Markers in Cancer Patients During and After Chemotherapy by Elina A. Beleva, Tanya I. Deneva, Snezhana S. Stoencheva and Zhanet G. Grudeva-Popova in Clinical and Applied Thrombosis/Hemostasis
Supplemental material, sj-tiff-2-cath-10.1177_10760296211056637 for Longitudinal Dynamics of Coagulation and Angiogenesis Markers in Cancer Patients During and After Chemotherapy by Elina A. Beleva, Tanya I. Deneva, Snezhana S. Stoencheva and Zhanet G. Grudeva-Popova in Clinical and Applied Thrombosis/Hemostasis