Abstract
Background:
Breast cancer is one of the most common cancers and a major cause of death in women worldwide. Chemotherapy is mainly used to treat and control the progression of breast cancer. Leukopenia is the most common side effect of chemotherapy which may decrease immune function and further lead to serious fatal infections. The purpose of this study was to evaluate the effect of acupuncture on regulating hematopoietic function in chemotherapy-induced leukopenia among patients with breast cancer.
Methods:
PubMed, Embase, Cochrane Library, CINAHL Plus, Web of Science, and Chinese articles in the Airiti Library and China National Knowledge Infrastructure (CNKI) databases were searched to August 2021 for papers to include in a systematic review and meta-analysis. A random-effects model was applied. The effect size was calculated by Hedges’ g. Heterogeneity was determined using Cochran’s Q test. Moderator analyses were performed to examine potential sources of heterogeneity. A trial sequential analysis (TSA) was conducted to determine whether the current sample size was sufficient.
Results:
Ten randomized controlled trials involving 650 participants were eligible for inclusion. Analysis by the random-effects model showed a significant effect by acupuncture of ameliorating leukopenia during chemotherapy. Levels of white blood cells (WBCs) were increased (Hedges’ g = 0.70, P < .001, I2 = 34%), neutrophil counts (Hedges’ g = 0.80, P < .001, I2 = 0%) were significantly enhanced. Moreover, regardless of the manner through which acupuncture was applied, overall values of WBCs increased.
Conclusions:
The current meta-analysis supports acupuncture possibly ameliorating chemotherapy-induced leukopenia, as WBC and neutrophil values significantly increased after acupuncture in patients undergoing chemotherapy. Additionally, regardless of the type of acupuncture, values of WBCs increased. These findings are actionable and support both the clinical use of acupuncture to relieve chemotherapy-induced leukopenia and further research regarding the use of acupuncture in patients experiencing immunosuppression when undergoing chemotherapy.
Trial Registration: PROSPERO-CRD42020215759.
Keywords: leukopenia, chemotherapy, acupuncture, breast cancer, meta-analysis
Background
Breast cancer is one of the most common cancers and a major cause of death in women worldwide. Chemotherapy is the most effective therapeutic approach for treating cancer. It is administered as adjuvant or neoadjuvant therapy for early-stage breast cancer and is also administered for palliative purposes for metastatic breast cancer. 1 Many patients are treated with moderate to high toxicity chemotherapy, including doxorubicin/epirubicin, paclitaxel/docetaxel, and cyclophosphamide with or without 5-fluorouracil. Although it is an effective way to manage cancer cells, it is also harmful to the hematopoietic system.1,2
Previous clinical trials demonstrated that taxane and cyclophosphamide, 2 chemotherapeutic drugs, are the main regimens for breast cancer. They can cause bone marrow suppression (leukopenia, thrombocytopenia, and anemia)1,3 and severe immunosuppression, which may lead to serious and fatal infections, including respiratory infections and sepsis. 4
Once leukopenia occurs, treatment schedules should be interrupted and the treatment course may even be discontinued. In the clinic, granulocyte colony-stimulating factor (G-CSF) is the most common administered to reduce the risks of leukopenic complications associated with chemotherapy. 5 However, it can result in many problems, such as aching in the bones and muscles, dyspnea, chest pain, nausea, hypoxemia, anaphylaxis, syncope, and flushing.6,7 And also, the G-CSF injection is conditional only for patients with a white blood cell (WBC) count of <1000/µL or an absolute neutrophil count (ANC) of <500/µL. 8 Therefore, using a subcutaneous injection of G-CSF to prevent or treat chemotherapy-induced leukopenia cannot benefit all oncologic patients.
With regard to G-CSF, there are some other therapeutic approaches toward improving chemotherapy-induced leukopenia, such as mistletoe extracts, 9 Chinese herbal medicine decoctions (Sijunzi Tang), 10 chamomile, 11 Wenshen Shengbai decoction with moxibustion 12 and also acupuncture. Furthermore, acupuncture is the most commonly used as a complementary therapy in patients with leukopenia during chemotherapy. 13
Acupuncture is a traditional Chinese medicine (TCM) based approach for treating a wide variety of symptoms by triggering specific acupoints on the skin with needles, which has been observed to alleviate adverse effects of surgery or chemotherapy for cancer patients, including postoperative arm lymphedema, 14 chemotherapy-induced nausea and vomiting,15,16 and chemotherapy-induced peripheral neuropathy17,18 and leukopenia.19,20 It is strongly suggested to provide a comprehensive review of the effect of acupuncture in relieving chemotherapy-induced leukopenia in patients with breast cancer. Nevertheless, as of today, there was only one systematic review focused on the breast cancer population for acupoint stimulation-managed treatment- related side-effects in breast cancer, 21 although there are published academic studies evaluating the association between acupoint injection/acupuncture and chemotherapy related complications in patients with lung cancer regardless of the tumor type.13,19,22 There has been no systematic review with a meta-analysis study that has evaluated the association between the effects of acupuncture and chemotherapy-induced hematopoietic suppression in patients with breast cancer so far. Therefore, the purpose of this systematic review with a meta-analysis was to evaluate the effects of acupuncture on chemotherapy-induced hematopoietic suppression in patients with breast cancer, including changes in blood counts, and further, to use a trial sequential analysis (TSA) to determine whether the sample size is sufficient in the current study.
Methods
This review and meta-analysis were performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement guidelines. This review was registered with PROSPERO (International Prospective Register of Systematic Reviews: CRD42020215759).
Literature Sources and Search Strategy
Studies were identified by a comprehensive literature search of the following electronic databases up to August 2021: PubMed, Embase, Cochrane Library, CINAHL Plus, Web of Science, and Chinese articles in the Airiti Library and China National Knowledge Infrastructure (CNKI). The search was restricted to human populations, and only in the English or Chinese language, and there was no time restriction on publications. We also reviewed references, government publications, and recommendations from the included studies.
The strategy combined or separated Medical Subject Heading (MeSH) terms and all fields of white blood cell, bone marrow suppression, breast cancer and acupuncture. The detailed search strategy for PubMed is shown in Supplemental Table 1.
Inclusion Criteria
All RCTs in English or Chinese were included without a time restriction. Participants of any race or gender and who were at least 18 years old with a diagnosis of breast cancer at any stage and under chemotherapy were included. Interventions of studies which evaluated any type of invasive (with/without electrical stimulation) or non-invasive acupuncture (moxibustion) were included. A study had to include a hematopoietic outcome indicator such as WBCs.
Exclusion Criteria
Observational, cohort, and case-control studies, case reports, qualitative studies, non-RCTs, review articles, and laboratory (animal) studies were excluded. Also, studies with participants with a disease of the hematopoietic system or immune deficiency, or with participants duplicated from other included studies were excluded.
Data Extraction and Quality Assessment
Two investigators (Y.W.S. and J.Y.S.) independently identified titles and abstracts of articles obtained using the above described search strategy and extracted the general characteristics of eligible studies. Discrepancies were resolved by discussion with a third investigator (H.T.T.).
Studies for further review were selected on the basis of the following inclusion criteria: (1) humans who had been diagnosed with breast cancer at any stage and were undergoing chemotherapy; (2) with an RCT study design; (3) studies that used acupoint stimulation as the intervention, such as, acupuncture, moxibustion, which are invasive or non-invasive interventions; and (4) studies that investigated regulation of hematopoietic indicator, for example WBCs.
Evaluation of the methodological quality of the included studies was based on the Cochrane risk of bias tool (RoB). 23 The following elements in trials were assessed: random sequence generation, allocation concealment, blinding of participants and staff, blinding of outcome assessments, incomplete outcome data, selective reporting and other sources of bias. The tool ranks evidence from research studies as having “high,” “low,” or “unclear” levels of bias. The rank of high risk of bias is interpreted as bias that may affect the research results dramatically; for low risk of bias, the bias does not affect the research results dramatically; for unclear of bias, there is insufficient information to assess whether an important risk of bias exists. 24 This assessment tool also is appropriate for the evaluation of the methodological quality of RCTs.23,24 Two investigators (Y.W.S and J. Y. S.) independently extracted data from the studies (Table 1), and any disagreements were resolved by discussion or consultation with a third independent reviewer (H.T.T.).
Table 1.
Characteristics of the Included Studies.
| No | Authors | Country | No. of subjects in each arm (experimental: control) | Chemotherapy drug | Acupuncture group | Control group intervention | Assessment of outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| Acupoints | Intervention | Intervention dose (mins) | |||||||
| 1 | Beith et al 29 | Australia | 14:16 | AC FEC |
PC6/IL4/ST36 | Electro-acupuncture | 80 | Sham Electro-acupuncture | Nausea and vomiting/WBC/Neutrophil |
| 2 | Hu and Zhao 31 | China | 30:30 | TAC | ST36/SP6/SP10/BL23 | Acupuncture | 150 | Western medicine routine | WBC/Neutrophil |
| 3 | Ji et al 38 | China | 48:44 | Anthracycline-taxane | ST36/SP6/CV8/ST35/ST41 | Moxibustion | 2400 | Without moxibustion | WBC/ANC |
| 4 | Li 36 | China | 36:36 | Anthracycline-taxane | ST36/SP6/CV8/CV4/CV6 | Moxibustion | 2835 | Western medicine routine | WBC/QoL/Neutrophil |
| 5 | Lu et al 34 | China | 30:30 | n/a | BL20/BL24/CV12/CV4 | Moxibustion | 420 | Routine care | WBC/BFI |
| 6 | Liu 35 | China | 50:50 | TEC | CV12/CV4/ST36/CV6/SP10/BL20/BL21/BL17 | Moxibustion | 320 | Western medicine routine | WBC/vomiting |
| 7 | Meng and Lü 30 | China | 27:27 | CAF | ST36/ST25/BL21/BL17/CV6/SP6/SP4/HT7 | Acupoint catgut-embedding + moxibustion | 10080 | Western medicine routine | WBC/ nausea/vomiting |
| 8 | Shi 37 | China | 30:28 | n/a | GV20/CV12/CV4/CV6/CV3/GV4/GV3/GV2 | Electro-acupuncture | 210 | Sham Electro-acupuncture | WBC/Neutrophil nausea/vomiting |
| 9 | Xie et al 33 | China | 23:23 | FEC | ST36/BL23 | Acupoint catgut-embedding | NA | Western medicine and Chinese decoction | WBC/ Neutrophil |
| 10 | Zhu et al 32 | China | 40:38 | CAT, CET | ST36/SP10/BL26/SP6/BL23/BL18 | Acupuncture | 150 | Western medicine | WBC |
Abbreviations: BFI, brief fatigue inventory; n/a, not mentioned; CAF, cyclophosphamide/Adriamycin/5-fluorouracil; FEC, 5-Fluorouracil/Epirubicin /cyclophosphamide; AC, Adriamycin/Cyclophosphamide; TAC, Taxotere/Adriamycin/Cyclophosphamide; TAC, Taxotere/Adriamycin/cyclophosphamide; TEC, Taxotere/epirubicin/cyclophosphamide; CAT, cyclophosphamide/Adriamycin/Taxotere; CET, cyclophosphamide/epirubicin/Taxotere.
Statistical Analysis
Two-sided P values were calculated, with P < .05 set as the level of statistical significance. First, pre- to post-test changes in scores were derived for the intervention group and control group from each included study. 23 Then, the effect size for the difference between the intervention and control groups was calculated for each study. Hedges’ g was used as the effect size index using the formula: g = d(1−(3/(4(n1 + n2)−9))).23,25 Results are expressed as the effect size (Hedges’ g) with the 95% confidence interval (CI). A Hedges’ g value of 0.2 to 0.4 represents a small effect size, 0.5 to 0.7 a moderate effect size, and ≥0.8 a large effect size. 25 A random-effects model was chosen because all included study characteristics differed: stage of cancer, different cancers, type of acupuncture (manual acupuncture or electro-acupuncture), theoretical principles of acupuncture points selected (fixed or unfixed points), total treatment dose (min), and the quality of the study.
Heterogeneity was determined using Cochran’s Q test, with statistical significance at P < 0.1, and the I2 statistic was used to determine the proportion of total variance in the pooled effect size that was caused by heterogeneity among studies. An I2 value of 0% indicates no heterogeneity, 25% low heterogeneity, 50% moderate heterogeneity, and ≥75% high heterogeneity. 26
Moderator analyses were performed to examine potential sources of heterogeneity. Once heterogeneity was detected, subgroup analyses and meta-regression analyses were conducted to determine the possible influences of certain effects. 23 Evaluation of publication bias using funnel plots was performed if the number of studies more than 10. 27 Comprehensive Meta-analysis (CMA) vers. 3.0 was used for all statistical analyses.
TSA is a methodology in which the evidence required is quantified, providing a value for the required information size. We applied a TSA to determine whether the sample size was sufficient to detect the specific effect, which can only be calculated using three or more trials. TSA was performed at the level of an overall 5% risk of a type I error and with 80% power. In the TSA, if the cumulative Z curve entered the futility area or crossed the trial sequential monitoring boundary, the anticipated intervention effect might reach a sufficient level of evidence, and further trials would not need to be included in the current meta-analysis. 28 We calculated the required information size based on the mean difference and variance in change of blood cell count levels. The TSA was conducted using TSA vers. 0.9 beta software.
Results
Study Selection
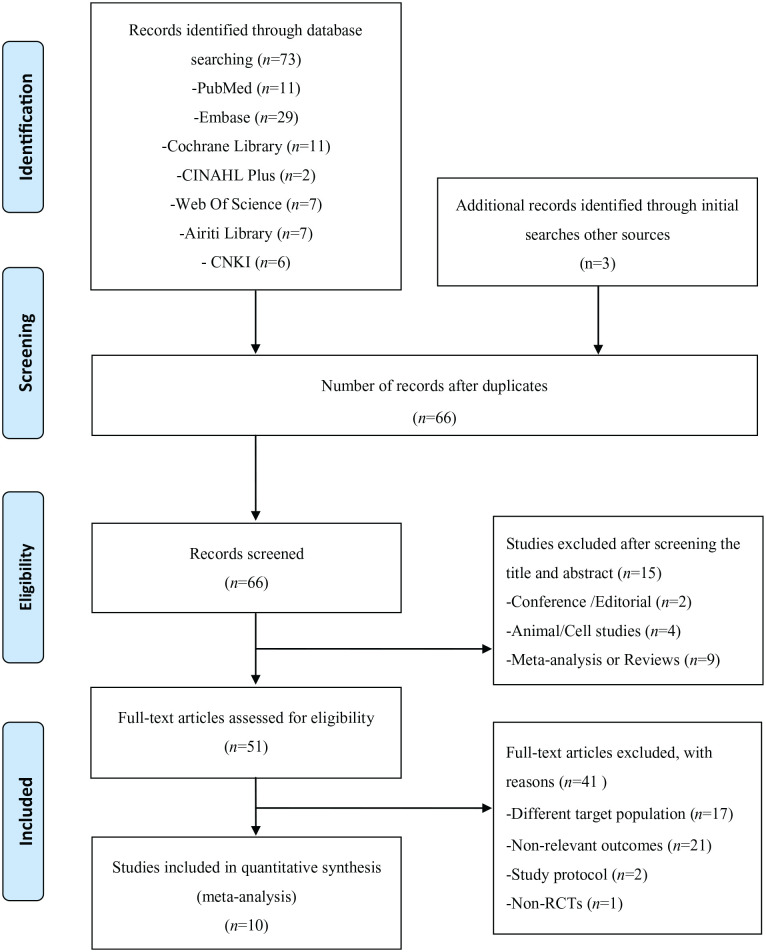
In total, 73 studies were identified from an initial search, and additional records identified initial searches other sources (n = 3), 10 duplicate articles were excluded. Records were screened for 66 articles, and a further 15 articles were excluded due to the following reasons: conference or editorial (n = 2), animal/cell studies (n = 4), meta-analyses or reviews (n = 9). In total, there were 51 studies remaining for full manuscript review, of which 41 studies were excluded due to the following reasons: different target population (n = 17), study protocols (n = 2), non-relevant outcomes (n = 21), non-RCT study design (n = 1). Finally, ten studies29-38 were included for further qualitative and quantitative analyses. The PRISMA flow diagram of the review process is presented in Figure 1.
Figure 1.
PRISMA flow diagram of the review process.
Characteristics of the Included Studies
All 10 of the included studies were conducted with a randomized control design, with a total of 650 participants (intervention, n = 328; control, n = 322). The populations among the included studies were all breast cancer with a wide variety of chemotherapeutic regimens. Papers were published between 2012 and 2019. Most were carried out in China30-38; only one took place in Australia. 29 The interventions used different types of acupuncture, including 2 trials with manual acupuncture, 2 trials with electroacupuncture, 2 with acupoint catgut embedding, and 4 moxibustion. Characteristics of the included studies are presented in Table 1.
Methodological Quality Assessment
The methodological quality of the selected studies is reported in Table 2. All included studies reported random sequence generation. Three studies31-33 reported neither the procedure for allocation concealment nor blinding of participants and personnel in the research, so they presented a high risk of bias. For the item of blinding of the outcome assessment, four29,33,36,38 were classified as having low risk, and one had high risk. In terms of how the studies addressed incomplete outcome data, 8 studies29-35,38 were classified as having low risk with reported rates of follow-up and withdrawal, and the remaining 2 had high risk. Seven studies29,30,32,34,36-38 had a low risk of selective reporting. And also, 2 studies37,38 had low risk of bias with respect to other sources of bias.
Table 2.
Risk of Methodological Bias of the Included Studies.
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data addressed | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Beith et al 29 | L | L | L | L | L | L | H |
| Hu and Zhao 31 | L | H | H | U | L | H | H |
| Ji et al 38 | L | L | L | L | L | L | L |
| Li 36 | L | L | L | L | H | L | H |
| Lu et al 34 | L | L | H | U | L | L | H |
| Liu 35 | L | L | H | U | L | H | H |
| Meng and Lü 30 | L | L | H | U | L | L | H |
| Shi 37 | L | L | H | H | H | L | L |
| Xie et al 33 | L | H | H | L | L | H | H |
| Zhu et al 32 | L | H | H | U | L | L | H |
Abbreviations: L, low risk; H, high risk; U, unclear risk of bias.
The Effect of Acupuncture on Relieving Hematopoietic-Suppression Outcomes
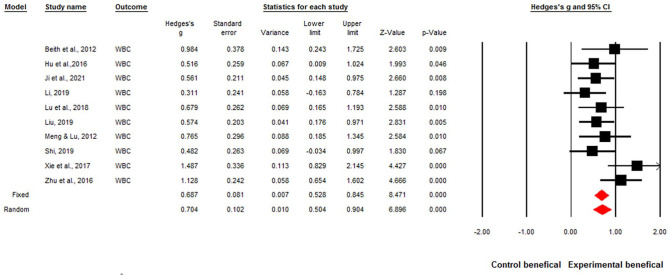
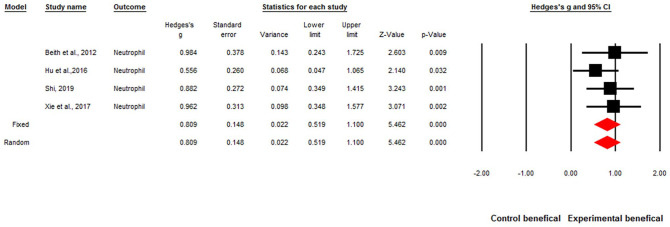
The overall effect sizes of the ten studies were significant for acupuncture improving WBCs and neutrophils when patients were undergoing chemotherapy. The overall effect size was Hedges’ g of 0.68, P < .001, and I2 = 34% for increased WBCs by acupuncture (Figure 2). The overall effect size for increased neutrophils was Hedges’ g of 0.80, P < .001, I2 = 0% by acupuncture (Figure 3).
Figure 2.
Forest plot of the overall effect sizes for studies measuring white blood cell (WBC) counts and acupuncture.
Q = 13.83, P = .12, I2 = 34%. Fixed-effects Hedges’ g = 0.68, P < .001. Random-effects Hedges’ g = 0.70, p < .001.
Figure 3.
Forest plot of the overall effect sizes for studies measuring Neutrophil counts and acupuncture.
Q = 1.47, P = .68, I2 = 0%. Fixed-effects Hedges’ g = 0.80, P < .001. Random-effects Hedges’ g = .80, P < .001.
Subgroup Analyses
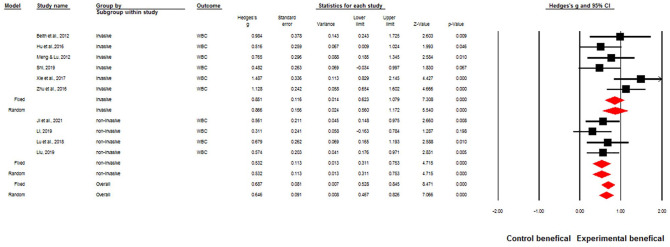
In this study, subgroup analyses were performed on different types of acupuncture. Mixed effects showed Q = 2.99, P = .08. This indicated that regardless of the various types of acupuncture, overall WBCs were increased. Data are shown in Figure 4.
Figure 4.
Mixed-effects model for different types of acupuncture.
Mixed-effect: Q = 2.99, P = .08.
Publication Bias
Publication bias did not exist for the WBC models. Egger’s test showed that the t value was 1.78, P = .11. Egger’s regression test results are shown in Figure 5.
Figure 5.
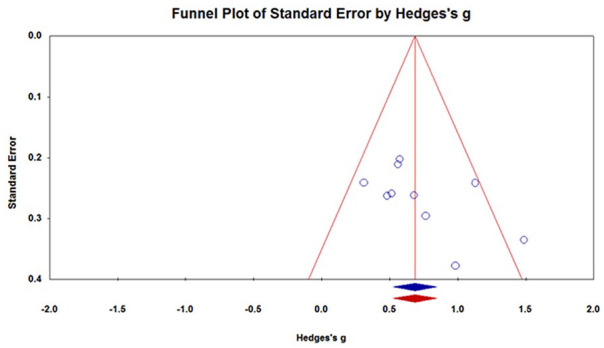
Funnel plot for assessing publication bias.
Egger’s test showed that the t value was 1.78, P = .11.
Trial sequential analysis (TSA)
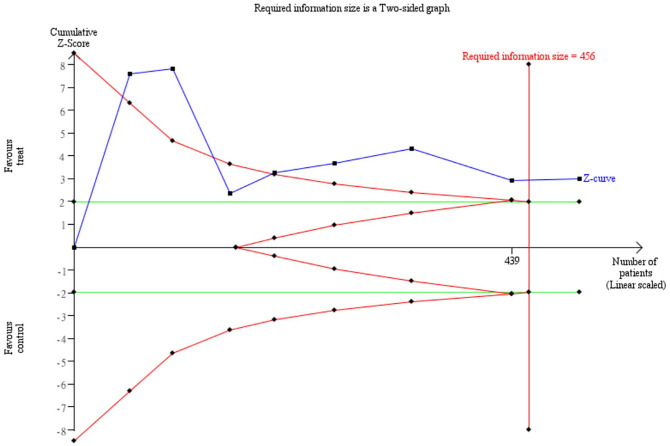
The TSA was used to determine whether the sample size was sufficient to detect a specific effect, which could only be calculated by 3 or more trials. We examined the effects of WBCs and acupuncture in 8 trials30-33,35-38 to determine the sufficiency of the sample size. With an α value of 5% (two-sided) and β of 20%, a cumulative z-curve (blue) was constructed using a random-effects model. The required information size of WBC levels was calculated to be 456, the z-curve crossed both the conventional and trial sequential monitoring boundaries, and the required information size was achieved; thus, there was sufficient evidence to reach a conclusion with no need for additional trials (Figure 6). To our knowledge the TSA of acupuncture on chemotherapy-induced leukopenia was applied for the first time, strengthening the conclusions achieved.
Figure 6.
Trial sequential analysis of the effects of white blood cell (WBC) counts and acupuncture.
Discussion
This meta-analysis of ten RCTs revealed a significant effect of acupuncture on the numbers of WBCs, which could ameliorate chemotherapy-induced hematopoietic suppression-related leukopenia in patients with breast cancer who were undergoing chemotherapy. In addition, regardless of the various types of acupuncture, overall WBCs increased.
Leukopenia often occurs in breast cancer patients who are treated with anthracyclines combined with taxane regimens, which is the primary regimen for breast cancer, thus the degree of leukopenia during chemotherapy is important because it is correlated with a reduction in resistance to infections, particularly in breast cancer.4,39 As the current meta-analysis revealed, the value of WBCs could be improved by acupuncture during chemotherapy in breast cancer patients. This result is consistent with a pilot RCT by Pais et al, 40 who investigated the effects of acupuncture on the immune system. All participants were randomized into acupuncture or control groups, and participants in the acupuncture group received 6 sessions of acupuncture point stimulation, twice a week, beginning a week prior to a cycle of chemotherapy and ending at the second cycle of chemotherapy. As their results indicated, the effects on WBCs, the ANC, and NK cells showed statistically significant increases overall compared to the non-acupuncture group (P = .036, P = .046, and P < .0001, respectively). 40 Additionally, our result was similar to a pilot RCT conducted by Lu et al, 20 in which 21 gynecologic malignancy patients undergoing chemotherapy were randomly assigned to an acupuncture or sham acupuncture group (sham needle sites were inserted away from standard TCM acupuncture points). The outcome measures on WBC values were significantly higher (P = .04) than the sham group after 6 interventions. 20
Neutrophils are a type of white blood cell that is very important to defense and against infection. In breast cancer chemotherapy regimens, a drop in neutrophil blood levels typically occurs when the body uses immune cells faster than it produces them, or when the bone marrow is suppressed after chemotherapy treatment. 5 As our results indicated, the levels of neutrophil were increased by acupuncture significantly during chemotherapy. This finding was consistent with a systematic literature review that evaluated 67 relevant papers, claiming that total leukocytes were increased 43% by acupuncture with a population of leukopenic patients after chemotherapy. 41 In this review articles, the most measured outcome representative were used total leukocytes, followed by lymphocytes and neutrophils; in addition, 2 relevant papers showed neutrophils were enhanced after the intervention of acupuncture or electroacupuncture.42,43 Another RCT conducted by Beith et al 29 also supported our results, in which 32 breast cancer patients were randomly assigned to true electroacupuncture or sham electroacupuncture while they receiving chemotherapy. There was a statistically significant increase in neutrophil counts compare to the sham electroacupuncture group at week 6 (median 3.55 × 109/L vs. 1.90 × 109/L, P = .01).
Our subgroup analysis was conducted with different types of acupuncture and the rise in WBC levels, which showed that regardless of the mode of application of acupuncture, overall leukocytes increased (mixed-effects model: Q = 2.99, P = .08). In our study, the type of acupuncture included invasive, such as, acupuncture, electroacupuncture, catgut; and non-invasive such as moxibustion. Traditional Chinese acupuncture methods include a variety modalities. The principle of acupuncture (invasive/non-invasive) is through acupoint stimulation-increased cytokines and neurochemicals released.8,21 In addition, acupoint stimulation enhances the antitumor efficacy on breast cancer by regulating the microvascular system and immunosuppression. 36 The clinical effects of acupoint stimulation may be attributable to enhancing immunity, relieving bone marrow suppression, and producing an antioxidant effect. 21 Moxibustion could attenuate inflammatory impairment and have an anti-inflammatory effect. 36 Moreover, there is an effect of acupuncture-moxibustion on preventing bone marrow suppression and recovering the leukocyte count after chemotherapy. 38 These effects have been proposed as potential mechanisms by which acupoint stimulation appears to improve leukopenia.36,38,44 Another RCT also confirmed the previous statement. Shih et al 8 found that there was a significant interactive effect between acupoint stimulation and time to increasing blood levels of stem cell factor (SCF). The SCF is a mediating cytokine, which can stimulate growth and differentiation of prognostic cells, viz., the common myeloid progenitor and common lymphoid progenitor, and then produce mature blood cell components of granulocytes, lymphocytes, megakaryocytes, and erythrocytes. 8 This possibly implies acupoints stimulate efficacy of activity of cytokines-SCF and following the stimulation of blood cells produce, for example, neutrophils and WBCs.
The subgroup analyses were performed on different types of acupuncture, resulting in significant effects: regardless of the various modes of acupuncture, WBCs were increased overall. Our result was similar to a previous meta-analysis conducted by Chen et al 13 which pooled 31 studies and showed that various modes of acupuncture (acupoint needle insertion, acupoint injection, plaster application, and moxibustion) could ameliorate chemotherapy-induced bone marrow suppression (preventing reductions in Hb, platelets, and WBCs) through immunomodulation of cancer patients undergoing chemotherapy. A comprehensive systematic review by Chao et al 21 also scrutinized 26 studies to confirm that acupoint stimulation by any modality could manage adverse events related to treating breast cancer patients, which also showed that chemotherapy-induced leukopenia could be relieved through any type of stimulation of acupoints.
Limitations and Recommendations
Previous studies presented cytokines indicators for hematopoiesis, such as SCF, GM-CSF, and G-CSF.8,20 Some scholarly research indicated that possible mechanism for acupuncture relieving chemotherapy-induced leukopenia include that acupuncture might enhance SCF and G-CSF, which would have the potential to relieve chemotherapy-induced myelosuppression.3,8 However, these hematopoietic cytokines mentioned above could not be demonstrated in this study to further clarify the relationship between regulation of hematopoietic cytokines and acupuncture due to the limited number of studies included. Additionally, the included studies mostly were all carried out in China, with only one taking place in Australia. There were high percentages of included studies with high risk for blinding of participants, and unclear descriptions of the blinding of outcome assessments, which were considered risks of performance bias and detection bias. However, in treatment trials, the blinding of participants is hard to implement.
Given the unclear methodological quality of the included studies, our results should be replicated in additional high quality research. More-rigorous trials should pay attention to quality issues noted in the quality assessment, in particular blinding of outcome assessments.
Conclusions
This is the first systematic review and meta-analysis to evaluate the effect of acupuncture on chemotherapy-induced hematopoietic suppression in patients with breast cancer. To our knowledge, the TSA of acupuncture on chemotherapy-induced leukopenia was applied for the first time to strengthen the conclusions achieved. The current meta-analysis with updated evidences supports acupuncture possibly ameliorating chemotherapy-induced leukopenia, since WBC and neutrophil values significantly increased after acupuncture in breast cancer patients undergoing chemotherapy. Additionally, regardless of the type of acupuncture used, values of WBCs increased. These novel findings are actionable and support both the clinical use of acupuncture to relieve chemotherapy-induced leukopenia and further research regarding the use of acupuncture in patients experiencing immunosuppression when undergoing chemotherapy.
Supplemental Material
Supplemental material, sj-docx-1-ict-10.1177_15347354211063884 for Effectiveness of Acupuncture in Relieving Chemotherapy-induced Leukopenia in Patients With Breast Cancer: A Systematic Review With A Meta-Analysis and Trial Sequential Analysis by Ya Wen Shih, Jui Yuan Su, Yu Shan Kung, Yu Huei Lin, Duong Thi To Anh, Edi Sampurno Ridwan and Hsiu Ting Tsai in Integrative Cancer Therapies
Footnotes
Author Note: Yu Shan Kung is now affiliated to Department of Nursing, Taipei Veterans General Hospital, Taipei 11217, Taiwan and Department of Obstetrics and Gynecology, Taipei Veterans General Hospital, Taipei 11217, Taiwan.
Duong Thi To Anh is now affiliated to Personnel Department, Thai Nguyen Medical College, Thai Nguyen, Viet Nam.
Edi Sampurno Ridwan is now affiliated to Department of Nursing, Faculty of Health Sciences, Universitas Alma Ata, Yogyakarta, Indonesia.
Hsiu Ting Tsai is now affiliated toSchool of Nursing, College of Nursing, National Yang Ming Chiao Tung University, Taipei 11221, Taiwa.
Author Contributions: Conceptualization: Y.W.S. and H.T.T.; methodology (screened papers for eligibility, appraised the quality of the studies, extracted the data, and sought additional information): Y.W.S., J.Y.S., and H.T.T.; software validation: Y.W.S., Y.S.K., Y.H.L., D.T.T.A., and E.S.R.; formal analysis: Y.W.S.; writing original draft preparation: Y.W.S.; writing-review and editing: Y.W.S., Y.H.L., and H.T.T; supervision: H.T.T. All authors read and agreed to the published version of the manuscript.
Availability of Data and Materials: All data collected and analyzed during this study are available from the corresponding author upon reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grant no. MOST 109-2314-B-038-110 from the Ministry of Science and Technology, Taiwan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD: Hsiu Ting Tsai  https://orcid.org/0000-0003-1137-9274
https://orcid.org/0000-0003-1137-9274
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Moo TA, Sanford R, Dang C, Morrow M. Overview of breast cancer therapy. PET Clin. 2018;13:339-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Epstein RS, Aapro MS, Basu Roy UK, et al. Patient burden and real-world management of chemotherapy-induced myelosuppression: results from an online survey of patients with solid tumors. Adv Ther. 2020;37:3606-3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu H, Chen B, Hong S, Guo Y. Acupuncture therapy for the treatment of myelosuppression after chemotherapy: a literature review over the past 10 Years. J Acupunct Meridian Stud. 2015;8:122-126. [DOI] [PubMed] [Google Scholar]
- 4. Brand JS, Colzani E, Johansson ALV, et al. Infection-related hospitalizations in breast cancer patients: risk and impact on prognosis. Infect J. 2016;72:650-658. [DOI] [PubMed] [Google Scholar]
- 5. Papakonstantinou A, Hedayati E, Hellström M, et al. Neutropenic complications in the PANTHER phase III study of adjuvant tailored dose-dense chemotherapy in early breast cancer. Acta Oncol. 2020;59:75-81. [DOI] [PubMed] [Google Scholar]
- 6. Aras E, Bayraktar-Ekincioglu A, Kilickap S. Risk assessment of febrile neutropenia and evaluation of G-CSF use in patients with cancer: a real-life study. Support Care Cancer. 2020;28:691-699. [DOI] [PubMed] [Google Scholar]
- 7. Khoury H, Adkins D, Brown R, et al. Adverse side-effects associated with G-CSF in patients with chronic myeloid leukemia undergoing allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2000;25:1197-1201. [DOI] [PubMed] [Google Scholar]
- 8. Shih YW, Yang SF, Chien MH, Chang CW, Chang VHS, Tsai HT. Significant effect of acupressure in elevating blood stem cell factor during chemotherapy in patients with gynecologic cancer. J Nurs Res. 2018;26:411-419. [DOI] [PubMed] [Google Scholar]
- 9. Pelzer F, Tröger W. Complementary treatment with mistletoe extracts during chemotherapy: safety, neutropenia, fever, and quality of life assessed in a randomized study. J Altern Complement Med. 2018;24:954-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan SH, Feng S, Xu Y, et al. Effectiveness of herbal medicine for leukopenia/neutropenia induced by chemotherapy in adults with colorectal cancer: a systematic review and meta-analysis. Integr Cancer Ther. 2021;20:15347354211021654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daneshfard B, Shahriari M, Heiran A, Nimrouzi M, Yarmohammadi H. Effect of chamomile on chemotherapy-induced neutropenia in pediatric leukemia patients: a randomized triple-blind placebo-controlled clinical trial. Avicenna J Phytomed. 2020;10:58-69. [PMC free article] [PubMed] [Google Scholar]
- 12. Ji Y, Li S, Zhang X, et al. The prophylactic and therapeutic effects of moxibustion combined with traditional Chinese medicine decoction for treating chemotherapy-induced myelosuppression in early-stage breast cancer: study protocol for a randomized controlled trial. Trials. 2020;21:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen HY, Li SG, Cho WC, Zhang ZJ. The role of acupoint stimulation as an adjunct therapy for lung cancer: a systematic review and meta-analysis. BMC Complement Altern Med. 2013;13:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Javdan B, Cassileth B. Acupuncture research at Memorial Sloan Kettering Cancer Center. J Acupunct Meridian Stud. 2015;8:115-121. [DOI] [PubMed] [Google Scholar]
- 15. Cheng Peiyu WX. The effects of acupuncture at different intervention time points on nausea and vomiting caused by cisplatin chemotherapy in patients with lung cancer. Int J Clin Exp Med. 2020;13:7965-7971. [Google Scholar]
- 16. Rithirangsriroj K, Manchana T, Akkayagorn L. Efficacy of acupuncture in prevention of delayed chemotherapy induced nausea and vomiting in gynecologic cancer patients. Gynecol Oncol. 2015;136:82-86. [DOI] [PubMed] [Google Scholar]
- 17. Molasiotis A, Cheng E. A randomized assessor-blinded wait-list controlled trial to assess the effectiveness and cost-effectiveness of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2019;27:S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bao T, Seidman AD, Piulson L, et al. A phase IIA trial of acupuncture to reduce chemotherapy-induced peripheral neuropathy severity during neoadjuvant or adjuvant weekly paclitaxel chemotherapy in breast cancer patients. Eur J Cancer. 2018;101:12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu W, Hu D, Dean-Clower E, et al. Acupuncture for chemotherapy-induced leukopenia: exploratory meta-analysis of randomized controlled trials. J Soc Integr Oncol. 2007;5:1-10. [DOI] [PubMed] [Google Scholar]
- 20. Lu W, Matulonis UA, Doherty-Gilman A, et al. Acupuncture for chemotherapy-induced neutropenia in patients with gynecologic malignancies: a pilot randomized, sham-controlled clinical trial. J Altern Complement Med. 2009;15:745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chao LF, Zhang AL, Liu HE, Cheng MH, Lam HB, Lo SK. The efficacy of acupoint stimulation for the management of therapy-related adverse events in patients with breast cancer: a systematic review. Breast Cancer Res Treat. 2009; 118:255-267. [DOI] [PubMed] [Google Scholar]
- 22. Hou L, Gu F, Gao G, Zhou C. Transcutaneous electrical acupoint stimulation (TEAS) ameliorates chemotherapy-induced bone marrow suppression in lung cancer patients. J Thorac Dis. 2017;9:809-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JPTG. Cochrane Handbook for Systematic Review of Interventions Version 5. 1. 0 [Updated March 2011]. The Cochrane Collabration; 2011. [Google Scholar]
- 24. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928-d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker DA. JMASM34: two group program for Cohen’s d, Hedges’ g, η2, Radj2, ω2, ɛ2, confidence intervals, and power. J Mod Appl Stat Methods. 2015;14:282-292. [Google Scholar]
- 26. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beith JM, Oh B, Chatfield MD, Davis E, Venkateswaran R. Electroacupuncture for nausea, vomiting, and myelosuppression in women receiving adjuvant chemotherapy for early breast cancer: a randomized controlled pilot trial. Med Acupunct. 2012;24:241-248. [Google Scholar]
- 30. Meng S, Lü JB. Twenty-seven cases of adverse reaction in postoperative chemotherapy of breast cancer treated with acupoint catgut-embedding therapy. World J Acupunct Moxibustion. 2012;22:64-67. [Google Scholar]
- 31. Hu GW, Wang JD, Zhao CY. Effect of acupuncture treatment on the first WBC reduction symptom after breast cancer chemotherapy. Beijing J Tradit Chin Med. 2016;35:777-778. [Google Scholar]
- 32. Zhu DL, Lu HY, Lu YY, Wu LY. Clinical observation of Qi-blood-supplementing needling for leukopenia after chemotherapy for breast cancer. Shanghai journal of acupuncture and moxibustion [shang hai zhen jiu za zhi]. 2016;35:964-966. [Google Scholar]
- 33. Xie F, Chen K, Li B, Zhu J. Clinical study of acupoint catgut-embedding therapy for prevention and treatment of bone marrow depression in breast cancer patients induced by FEC chemotherapy. J Guangzhou Univ Tradit Chin Med. 2017;34:530-534. [Google Scholar]
- 34. Lu L, Li WH, Guo XC, Fu WB. Thunder-fire moxibustion for Qi deficiency-induced fatigue in breast cancer patients under-going chemotherapy. Zhen CI yan jiu = Acupunct Res. 2018;43:110-113. [DOI] [PubMed] [Google Scholar]
- 35. Liu H-J. Observation of clinical effects of moxibustion on preventing and treatment of leukopenia, nausea, and vomiting after chemotherapy of breast cancer. Smart Healthcare. 2019;5:72-73. [Google Scholar]
- 36. Li S. Clinical Study of Moxibustion Combined With Tranditional Chinese Medicine to Improve Chemotherapeutic Bone Marrow Suppression and Quality of Life in Breast Cancer Patients [master]. Shanghai University of Traditional Chinese Medicine; 2019. [Google Scholar]
- 37. Shi X. Clinical Study on the Side-Effect of "TiaoRen TongDu" Acupuncture Method on Chemotherapeutic Drugs for Breast Cancer. chinese medicine, Nanjing University of Chinese Medicine; 2019. [Google Scholar]
- 38. Ji Y, Li S, Zhang X, et al. The efficacy of moxibustion for breast cancer patients with chemotherapy-induced myelosuppression during adjuvant chemotherapy: a randomized controlled study. Evid Based Complement Alternat Med. 2021;2021:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swain SM, Ewer MS, Viale G, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29:646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pais I, Correia N, Pimentel I, et al. Effects of acupuncture on leucopenia, neutropenia, NK, and B cells in cancer patients: a randomized pilot study. Evid Based Complement Alternat Med. 2014;2014:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mota SSr-LaMPGad. Acupuncture in Modulation of Immunity, Acupuncture in Modern Medicine (March 6th 2013). IntechOpen. [Google Scholar]
- 42. Han YF, Gong Z, Huang LQ, Xia X, Zhao WJ. [Clinical study on acupuncture for leukopenia induced by chemotherapy]. Zhongguo Zhen Jiu. 2010;30:802-805. [PubMed] [Google Scholar]
- 43. Ferreira Ade S, Lima JG, Ferreira TP, Lopes CM, Meyer R. Prophylactic effects of short-term acupuncture on Zusanli (ST36) in Wistar rats with lipopolysaccharide-induced acute lung injury. Zhong Xi Yi Jie He Xue Bao. 2009;7:969-975. [DOI] [PubMed] [Google Scholar]
- 44. NIH Consensus Conference. Acupuncture. JAMA. 1998;280: 1518-1524. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ict-10.1177_15347354211063884 for Effectiveness of Acupuncture in Relieving Chemotherapy-induced Leukopenia in Patients With Breast Cancer: A Systematic Review With A Meta-Analysis and Trial Sequential Analysis by Ya Wen Shih, Jui Yuan Su, Yu Shan Kung, Yu Huei Lin, Duong Thi To Anh, Edi Sampurno Ridwan and Hsiu Ting Tsai in Integrative Cancer Therapies