Abstract
Myelodysplastic syndrome (MDS) evolves due to genomic instability, dysregulated signaling pathways, and overproduction of inflammatory markers. Reactive oxygen species contribute to the inflammatory response, which causes gene damage, cellular remodeling, and fibrosis. MDS can be a debilitating condition, and management options in patients with MDS aim to improve cytopenias, delay disease progression, and enhance quality of life. High serum ferritin levels, a source of iron for reactive oxygen species production, correlate with a higher risk of progression to acute myeloid leukemia, and iron overload is compounded by blood transfusions given to improve anemia. 6-shogaol is a natural phenolic compound formed when ginger is exposed to heat and/or acidic conditions, and it has been shown to possess anti-tumor activity against leukemia cell lines and antioxidant effects. This narrative review assessed the potential benefits of this phytochemical in lower-risk MDS patients through examining the current evidence on the pharmacological and therapeutic properties of ginger and 6-shogaol.
Keywords: (max 6) Zingiber officinale, 6-Shogaol, 6-gingerol, hematopoiesis, hepcidin, anemia
Introduction
Myelodysplastic syndrome (MDS) is a heterogeneous group of clonal stem cell disorders characterized by dysplastic and ineffective hematopoiesis and peripheral cytopenias. 1 Anemia occurs in greater than 85% of MDS patients, while more than 50% are neutropenic, and at least one third have moderate or severe thrombocytopenia. 2 The revised International Prognostic Scoring System (IPSS-R) categorizes the prognostic risk of the primary untreated adult MDS patients into 5 levels (Very low, Low, Intermediate, High, and Very High), with the majority of the patients (77%) belonging to the 3 lower-risk categories. 3 Survival is less than 6 months for those over 60 years of age who have the most advanced forms of the disease to more than 8 years in younger patients with no adverse risk factors. 4 The only form of curative therapy is allogeneic hematopoietic stem cell transplantation, but most patients are not eligible for this due to advanced age or co-existing medical conditions. 1 Thus, the treatment goals in lower-risk MDS are to improve cytopenias, prolong survival, and enhance health-related quality of life (QoL). Consequently, interventions focus on increasing blood counts, delaying progression to acute myeloid leukemia and maintaining functional capacities. 2
Compared to a reference population, patients newly diagnosed with lower-risk MDS experience a pronounced reduction in QoL, which is only partly explained by the presence of anemia. 2 The most debilitating MDS symptom reported was fatigue, but this was poorly correlated with hemoglobin level regardless of whether patients had received any blood transfusions. 2 Fatigue was reported to be associated with significant impairment of both QoL and the ability to work or participate in desired activities. 2 In a recent review by Stauder et al, 5 patients with lower-risk MDS reported moderate to severe impairments to pain/discomfort, mobility, anxiety/depression, and their usual activities. So, improving QoL is an essential management factor for lower-risk MDS patients.
In traditional Chinese medicine, “Qi” is a fundamental theory closely related to blood circulation and nutritional deficiency. 6 Dry ginger is considered a warm herb that can dispel cold and dampness and promote the flow of blood and Qi to remove blood stasis and ease weakness and fatigue. 7 Thus, ginger is a choice ingredient in many Chinese herbal remedies for blood circulation, anemia, and hemorheological conditions. For example, ginger is an ingredient in the Jianpi Buxue formula, which has been shown to reverse chemotherapy-induced myelosuppression through increasing hematopoietic stem cells in the bone marrow. 8 Ginger is also a common ingredient in the blood tonic formulas of Guizhi Tang, Neibu Dangguijianzhong Tang and Zao Tang, which promote the hematopoietic function through stimulation of the protein expressions of erythropoietin via the hypoxia response elements. 9 In Southern China, the “ginger vinegar soup” consisting of sweet vinegar, ginger, egg, and pig’s trotters is a traditional dietary practice recommended during the early postpartum period for women to recover from blood loss during delivery. 10 Chan et al 10 showed that the “ginger vinegar soup” was rich in iron, with its content comparable to other iron-rich foods. From these traditional usages, it appears that ginger may potentially benefit the hematopoietic function of lower-risk MDS patients to alleviate fatigue and improve QoL.
Shogaols are biologically active constituents of ginger. The most common constituent is 6-shogaol, a promising anti-cancer and anti-inflammatory agent that also possesses hepatoprotective effects. 11 A recent study by Golombick et al 12 found 6-shogaol supplementation to lower the elevated serum ferritin levels in early-stage, transfusion-independent patients with MDS. In light of the preceding information, it is plausible that ginger extract rich in 6-shogaol, a natural remedy, can potentially benefit lower-risk MDS patients. This article examines the existing literature to explore the therapeutic properties of 6-shogaol as a low-intensity supportive treatment in MDS.
Ginger and Shogaols
Ginger is the rhizome of Zingiber officinale Roscoe, a perennial herbaceous plant of the Zingiberaceae family. It has been used as a tonic root and flavoring agent for more than 5000 years by the Indians and Chinese, long before history was formally recorded. 13 Today, ginger is a culinary spice and a herbal remedy widely used around the world. Research to date has demonstrated that ginger possesses many biological activities such as antioxidant, anti-inflammatory, antimicrobial, antiemetic, and anti-cancer activities. 14 Furthermore, ginger is also proven beneficial in the prevention and management of cardiovascular disease, 15 obesity, 16 neurodegenerative conditions, 17 diabetes mellitus, 18 respiratory disorders, 19 and in nausea relief. 20
Ginger contains both volatile and non-volatile components. The non-volatile compounds include major biologically active constituents such as shogaols, gingerols, paradols, and zingerone, which are the primary ingredients that give ginger its unique pungent flavor. 21 Through drying, heat or storage, gingerols can dehydrate into shogaols. 22 Hence, the composition of fresh ginger varies with variety, agronomic practices, curing and drying methods and storage conditions. 23
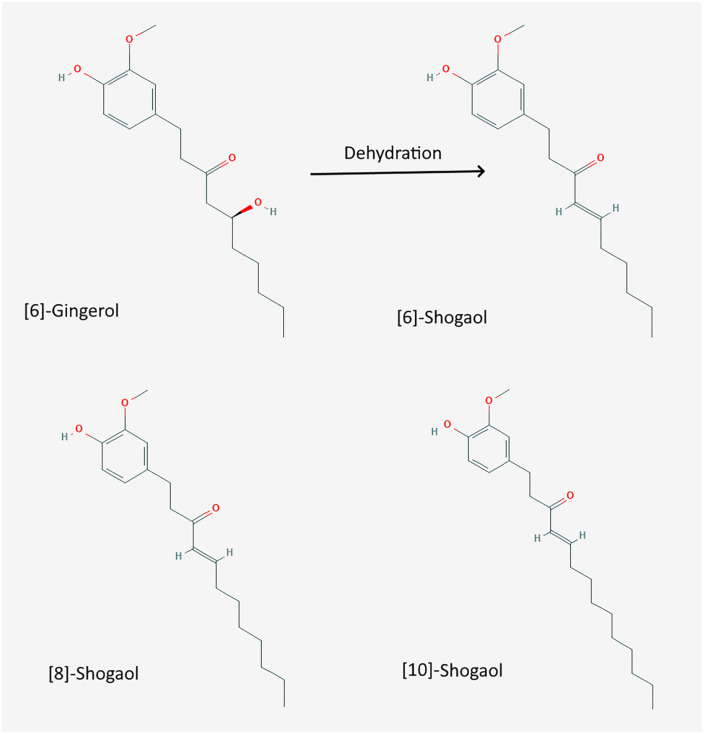
The chemical structures of different forms of shogaols are shown in Figure 1. They form due to the unstable beta-hydroxy ketone in gingerols when exposed to heat and/or acidic conditions. 24 Thus, both gingerols and shogaols have different bioactivities, molecular targets and metabolic pathways. 24 The processing of fresh ginger is essential to produce the most efficacious quantity of non-volatile compounds, especially shogaols. Ho and Su observed that the optimal temperature in the preparation of 6-shogaol from ginger was 125◦C for 40 to 60 minutes. 25 There was an increase in shogaol content by 9.34-fold at this temperature. Li et al 26 also compared the chemical characteristics and antioxidant activities of fresh, dried, stir-fried and carbonized ginger. The highest antioxidant activity, highest phenolic, and highest level of shogaols were found in dried ginger. When dried ginger was then carbonized, the amount of 6-shogaol was the most abundant, being around 114-fold higher than in fresh ginger. Moreover, 6-shogaol exhibited the most potent antioxidant and anti-inflammatory properties in vitro compared with 6, 8, and 10-gingerol. 27
Figure 1.
Two dimensions structure of 6-gingerol, 6-, 8-, and 10-shogaol.
Source: PubChem.
Pharmacokinetics and Toxicity of 6-Shogaol
The pharmacokinetics and toxicity of 6-shogaol have been studied in animal models and humans. Studies in rats show 6-shogaol is well absorbed from the gastrointestinal tract (GIT), with most (64%) excretion occurring via the fecal route through biliary excretion. 28 Urinary excretion is on the order of 20%. 6-shogaol was rapidly and extensively metabolized when given to healthy human subjects; its glucuronic acid conjugates were detected in plasma from 15 minutes to 72 hours post-administration. 28 In a review by Kou et al 29 looking at both animal and human studies, the authors noted that 6-shogaol was extensively metabolized after oral administration and some metabolites were reabsorbed from the GIT. Phase 1 and phase 2 enzymes take part in the first-pass metabolism with the conjugation of glucuronide as the dominant product. 30 6-shogaol also had inhibitory effects on phase 1 and phase 2 enzymes which could be important in drug-drug interactions. A study by Yu et al 31 on the pharmacokinetics of the active ingredients of ginger in humans showed that 6-shogaol and its metabolites had short half-lives between 1 and 3 hours, and there was no accumulation of these compounds after repeated dosing.
Concerning toxicity, ginger appears to be a safe product. In a systematic review of 109 randomized controlled trials, Anh et al 32 found that most adverse effects were GIT related, namely heartburn, nausea, diarrhea, abdominal pain, bloating, gas, and epigastric distress. Moreover, doses ranging from 10 to 100 mg/kg of 6-shogaol were administered for 14 days to albino mice. No mortality was observed at any dose, and only mild clinical changes were observed at doses of 100 mg/kg. 33 The recommended doses of ginger range from 200 mg to 2.5 g per day of the dried extract, containing between 1% to 4% shogaols. 34 Side effects include mainly GIT symptoms, and there may be potentiation of anticoagulant effects. It is noteworthy that a dose of ginger between 0.5 g and 1.0 g, 2 to 3 times daily over periods ranging from 3 months to 2.5 years, did not cause any adverse side effects. 35 Larger intakes are noted in various populations over the world. In animal models, doses of 2.5 g/kg were tolerated with no mortality, but at doses of 3 to 3.5 g/kg, mortality rates were 10% to 30%. 36 Nota bene, for a 70 kg person, 2.5 g/kg equates to 7 g shogaols per dose based on a 4% content of shogaols.
Therapeutic Properties of 6-Shogaol
Anti-inflammatory Effect
MDS is the result of genomic instability, dysregulated signaling pathways and overproduction of inflammatory markers. 37 This process is a feature of MDS due to genetic defects within pluripotent stem cell populations, with chronic inflammation causing deoxyribonucleic acid damage, cellular remodeling and fibrosis. 38 The pro-inflammatory signaling within the malignant clone and the bone marrow microenvironment, which activate aberrant innate immune responses, are key pathogenic drivers of MDS. 39 Symptom development in MDS has been associated with inflammation due to an abnormal elevation of cytokine expression. 40 Certain inflammatory markers are associated with specific symptoms, for example, ferritin with pruritis and interleukin-8 with elevated blasts. Fatigue is the most debilitating symptom reported to be induced by cytokines. 38 Moreover, autoimmune and inflammatory conditions, such as inflammatory arthritis, are common comorbidities in up to 25% of MDS patients. 41
Ginger is well-known for its anti-inflammatory effect. The systematic review by Anh et al included 8 clinical trials that reported the anti-inflammatory effect of ginger supplementation. 32 These studies, primarily conducted among arthritis patients, commonly reported pain relief as a promising benefit. Other observed effects include reducing pro-inflammatory cytokines, promoting forkhead box P3 gene expression, and decreasing ferritin and malondialdehyde levels. 32
The anti-inflammatory property of 6-shogaol was studied in many in vivo and in vitro studies. 6-shogaol was shown to attenuate inflammatory process in vivo with murine macrophages, microglia cells, epidermal keratinocytes, and oral squamous cell carcinoma through multiple signaling pathways, including nuclear factor-kappa B, activator protein 1, mitogen-activated protein kinase cascades, and peroxisome proliferator-activated receptor gamma.42 -45 6-shogaol also demonstrated its anti-inflammatory effect to lower lung inflammation in a murine asthma model, 46 protect against acute lung injury in mice, 47 downregulate inflammatory markers in hamster buccal pouch carcinogenesis, 44 inhibit monosodium urate crystal-induced inflammation in mice, 48 and reduce neuroinflammation in rodent models of dementia. 49 A recent review of 30 in vivo and in vitro studies further confirmed 6-shogaol to be a promising anti-inflammatory substance, but conclusive clinical data remained lacking. 50 Nevertheless, it is reasonable to assume that 6-shogaol can potentially reduce chronic inflammation associated with MDS and alleviate inflammation-related comorbidities.
Antioxidant Effect
Chronic inflammation is due to oxidative stress partly related to the free radical production of reactive oxygen species (ROS) generated by iron, an essential but potentially toxic element. 51 The literature shows that ROS, as a result of iron overload, are involved in the pathogenesis of MDS. 52 The high ROS levels within the hemopoietic niche can impact clonal evolution. Oxidative stress affects the self-renewal, proliferation and differentiation of hematopoietic stem cells and impairs cell growth, leading to defective hemopoietic mechanisms in MDS. 52 A study by Saigo et al among transfusion-independent MDS patients showed that 85% of them (34 out of 40) had higher than normal ROS levels in serum as measured using derivatives of reactive oxygen metabolites. 53 The study also detected a positive correlation between ROS levels and serum ferritin levels and a negative correlation between ROS levels and hemoglobin levels. 53
Gonçalves et al 54 observed that bone marrow cell types from MDS patients had increased intracellular peroxide levels and decreased glutathione content, compared with control cells. Furthermore, oxidative stress levels were risk group-dependent, with the lower-risk patients having the highest ROS levels characterized by high bone marrow apoptosis. As such, oxidative stress parameters, such as malondialdehyde, were suggested to be predictive biomarkers for treatment response in lower-risk MDS patients. 55 Hoeks et al 56 followed the progress of 256 lower-risk MDS patients over 3 years and found a progressive increase of malondialdehyde levels in the patients over time. Oxidative stress due to iron toxicity, especially among transfusion-dependent patients, damaged organs and led to inferior outcomes.
The antioxidant effect of 6-shogaol is due to the alpha, beta-unsaturated ketone moiety, which confers increased potency and efficacy to this compound compared to its precursor, 6-gingerol. 27 6-shogaol possesses a strong metal binding capacity to reduce lipid peroxidation, scavenge free radicals, and inhibit the action of xanthine oxidase, a generator of oxygen free radicals. 29 An in vitro study with a neuron-like rat pheochromocytoma cell line by Peng et al 57 showed that 6-shogaol upregulated a series of phase II antioxidant molecules, such as glutathione, heme oxygenase 1 and thioredoxin 1. 6-shogaol exhibited remarkable cytoprotection against oxidative stress-induced cell damage. The likely mechanism of this cytoprotection is mediated by activating the transcription factor called nuclear factor-erythroid 2-related factor 2 (Nrf2). 58 Consistently, other studies also demonstrated that 6-shogaol was able to enhance cellular antioxidant activities through increasing glutathione levels and antioxidant response element promoter activities via the Nrf2 signaling pathway, in vivo and in vitro.59,60
Phenolic compounds have been shown to counter oxidative stress due to free radicals caused by iron overload. 61 Dried ginger extract was shown to possess free radical scavenging for iron nanoparticles. 62 The water-soluble extracts of red and white gingers were shown to protect against Fe (2+)-induced lipid peroxidation in the rat brain in vitro. 63 Moreover, another hydroalcoholic ginger extract was shown in rats to protect against ferrous sulfate-induced hepatic and renal toxicity in vivo by reducing lipid peroxidation and chelating iron. 64 Currently, there is a lack of direct study on how 6-shogaol may protect against iron-induced oxidative stress in bone marrow cells or MDS. This topic requires further research.
Hematopoietic Effect
The hematopoietic effect of ginger has been studied in a zebrafish embryo model. 65 This model looks at tissues in the zebrafish embryo that are equivalent to the mammalian site of hematopoiesis. The bone morphogenetic protein (bmp), a tissue growth factor-beta superfamily member, is involved in a signaling pathway that plays a critical role in hematopoiesis. 65 This pathway regulates the development of human hematopoietic stem cells and early embryonic hematopoiesis during early vertebrate development. The zebrafish embryo model has been used to study the mechanism of hemopoiesis and erythropoiesis in vivo. In an inducible hemolytic anemia model, ginger extract and 10-gingerol were shown to promote hemopoietic recovery via the bmp signaling pathway. 65 Further investigation with the zebrafish model showed that 10-gingerol could rescue the hemogenic endothelium. 10-gingerol was showed to upregulate nitric oxide production crucial for arteriogenesis and hematopoiesis in hematopoietic stem/progenitor cells with genetic defects. 66
Conversely, in an in vitro experiment, Alamri et al 67 showed that 6-gingerol could stimulate premature death of red blood cells at 100 µM concentration. Cell apoptosis was characterized by loss of membrane asymmetry, elevated cytosolic calcium, cell shrinkage, and casein kinase 1α activation. 67 Since both gingerols are extensively metabolized after ingestion, such findings are more relevant for intravenous applications of ginger derivatives as anti-cancer agents rather than administration through the oral route.
Ferri-Lagneau et al 65 also demonstrated that 6-shogaol is less effective in promoting hemopoiesis than 10-gingerol. Such findings prompted the suggestion that 6-shogaol had no effect on improving anemia and that fresh ginger (rich in gingerols) was more effective than dried ginger (rich in shogaols). 24 However, a cross-sectional study among 4628 residents in China found no significant correlation between daily consumption of fresh ginger as a spice and the prevalence of anemia. 68 In contrast, intervention studies among anemic patients using dried ginger powder did produce promising results.
Table 1 summarizes the available human intervention studies with ginger extract on disorders due to ineffective erythropoiesis. Kulkarni et al 69 studied the effect of ginger supplementation in anemic patients requiring iron supplementation. The patients (n = 30) who took 250 mg of ginger extract (1.5 g of pure ginger powder) and iron supplements daily had higher percentages rise in hematological and iron-related parameters (iron and ferritin) than the control group (n = 32) on iron supplements alone after 30 days. The results showed that dried ginger powder assisted in iron absorption in patients with iron-deficiency anemia. 69 Similar findings were reported by Kumar et al 70 in patients with tuberculosis-associated anemia. Patients (n = 36) who took dried ginger powder supplement (250 mg/day for 30 days) along with an anti-tubercular drug had higher percentages increase in hemoglobin, and decrease in ferritin and C-reactive protein (CRP), compared to the control group (n = 36) on an anti-tubercular drug alone. Hence, the authors concluded that ginger supplementation helped regulate iron metabolism by decreasing systemic inflammation in tuberculosis. 70
Table 1.
Human Intervention Studies With Ginger Extract on Disorders Due to Ineffective Erythropoiesis.
| Study | Study design | Participants | Interventions | Efficacy findings |
|---|---|---|---|---|
| Raza et al 71 | Prospective single-arm clinical trial | Patients with low or intermediate-1 risk MDS (N = 9). | Gingerol (350 mg twice daily increasing to 1.4 g/day) plus
curcumin (starting with 2 g in 4 divided doses/day,
increasing to 8 g/day). Duration: >6 months. |
Available data from 6 patients only. Four out of 6 patients shown overall hematologic improvement, with 3 showing trilineage improvement in Hb, platelets, and ANC. |
| Kulkarni et al 69 | Open-label control trial | Patients with moderate to severe iron deficiency anemia
(N = 62). Ginger + iron (n = 30). Iron only (n = 32). |
250 mg of ginger extract (equivalent to 1.5 g of pure ginger
powder) twice daily. Iron supplement (dosage not specified). Duration: 30 days. |
Ginger + iron group achieved a higher percentage increase in
hematological and iron parameters compared to the iron only
group. Hb: 8.23% versus 2.3%, Iron: 19.63% versus 5.54%, TIBC: 7.23% versus 4.47%, Ferritin: 45.11% versus 34.11%. |
| Kumar et al 70 | Open-label control trial | Newly diagnosed pulmonary tuberculosis patients having
significant anemia (N = 68). Ginger + ATT (n = 36). ATT only (n = 32). |
250 mg of ginger extract (equivalent to 1.5 g of pure ginger
powder) twice daily in capsule form. Duration: 30 days. |
Ginger + ATT group achieved a higher percentage improvement in CRP (17.52% lower), ferritin (5.08% lower) and Hb (1.39% higher) parameters compared to ATT only group. |
| Golombick et al 12 | Case series | Patients with low or intermediate-1 risk MDS (N = 6). | One gel capsule of 20 mg ginger extract standardized for 20%
6-shogaol once daily. Duration: 6 + 6 months. |
The study detected a decrease in ferritin levels in 3 of the 6 patients whose ferritin levels were elevated at baseline. The decrease in ferritin in the 3 patients was accompanied by an increase in serum hepcidin level in repeat studies in the next 6 months. |
Abbreviations: ANC, absolute neutrophil count; TIBC, transferrin and iron-binding capacity; ATT, anti-tuberculosis treatment; CRP, C-reactive protein; Hb, hemoglobin; MDS, myelodysplastic syndrome.
Raza et al 71 showed promising results in combining curcumin and gingerol to improve lower-risk MDS patients’ hematologic parameters. Nine patients with low or intermediate-1 risk MDS took curcumin (2 g/day increasing to 8 g/day) and gingerol (350 mg/day-1.4 g/day) supplements in incremental doses. Six patients were evaluated for their response after supplementing for a minimum of 6 months. Four out of six showed overall hematologic improvement, with 3 of the 4 showing trilineage improvement. 71
Golombick et al 12 carried out a small investigative study on 6 patients with low to intermediate-1 risk MDS. Their study investigated the effect of 6-shogaol (20 mg/day of a ginger extract standardized for 20% 6-shogaol) on serum ferritin levels, changes in liver function tests, blood hepcidin levels and blood cell levels in transfusion-independent patients. Results indicated a reduction in serum ferritin level in 3 patients, improved liver function tests in 1 patient, and increased serum hepcidin level in 2 patients. 12 This small investigative study has raised the question of whether 6-shogaol could be beneficial in patients with lower-risk MDS.
Hepatoprotective Effect
The liver is the primary iron store of the body. It regulates the absorption of iron through hepcidin production and synthesizes iron metabolism proteins such as transferrin. 72 MDS patients often have lower hepcidin/ferritin ratios than healthy people, a sign of blunted response in hepcidin production in the liver. 73 Patients with MDS who have not received blood transfusions have been shown to have inappropriately low hepcidin levels.74,75 This self-sustaining response was driven by ineffective erythropoiesis and caused increased intestinal iron absorption. Adding to this is the effect of growth differentiation factor 15 secreted by mature erythroblasts that suppressed hepcidin production by the liver. 76 Camaschella et al 72 also noted a vicious cycle in MDS patients due to the inhibition of hepcidin by erythroferrone released by erythropoietin stimulated erythroblasts. This high serum level of erythroferrone led to high iron levels, which interfered with erythroblast maturation aggravating the anemia of MDS. 74
The Canadian Consensus Guidelines state that iron overload is defined as a serum ferritin level greater than 1000 micrograms/liter. 77 Level II evidence obtained from at least one well-designed randomized controlled study shows that iron overload may worsen overall and leukemia-free survival in MDS patients. The effect of iron overload is more apparent in low risk or intermediate-1 risk MDS groups, as these patients usually live long enough to experience iron toxicity. 77
Iron overload begins to develop even before a patient becomes transfusion-dependent because of the suppressed hepcidin production, leading to excessive intestinal iron uptake. Regular transfusion to ameliorate anemia further exacerbates iron overload. 74 Excessive iron store in the liver is toxic and can lead to cirrhosis, liver failure, and hepatocellular carcinoma. 78
The hepatoprotective effects of ginger were seen in ameliorating age-related changes, 79 reducing liver injury due to unhealthy diet and alcohol,80,81 and improving chemical-induced hepatotoxicity and liver fibrosis in many in vivo studies.82 -88 These studies commonly reported improved aspartate aminotransferase and alanine aminotransferase enzyme levels and preserved hepatocyte structure after ginger administration.82,84,88 More specifically, 6-shogaol has been shown to provide liver protection against aflatoxin B1 induced hepatotoxicity 89 and diclofenac induced liver damage in rats. 90 The hepatoprotective effect of 6-shogaol was even more pronounced when it was delivered in nanoparticles. Zhang et al 11 demonstrated in vivo that 6-shogaol loaded micelles had a 3.2-fold increase in oral bioavailability, improved tissue distribution, and better hepatoprotective effect against carbon tetrachloride-induced hepatic injury than free 6-shogaol. Notwithstanding, Hikino et al 91 found gingerols to exert a stronger antihepatotoxic effect in vitro than the corresponding shogaols.
Anti-cancer Effect
MDS is considered a type of age-related myeloid malignancy. MDS patients also present a higher incidence of malignant tumors than the general population. 92 Prior treatment for other cancers with radiation therapy or chemotherapy also increases the risk of secondary MDS.92,93 Inability to suppress inflammation and the continuous exposure to inflammatory mediators also increase the rate of transformation and disease progression of MDS to acute myeloid leukemia. 37
Ginger and its bioactive constituents have been studied extensively for their anti-cancer effects. Research has shown that ginger protects against aberrant gene expressions and mutations within the transforming cell population. It also reduces inflammation and abnormal gene expressions by the cells within the surrounding lesion. 94 Specifically, 6-shogaol has shown anti-cancer activity in prostate, pancreatic and breast cancer via cell arrest and apoptosis. 95 Liu et al 96 showed that 6-shogaol induces apoptosis in leukemia cells in vitro and in vivo, but not normal cells. The selective toxicity of shogaols for tumor cells appears to be differential activation of the glycosylated metabolites by higher levels of β-glucuronidase in the tumor cells. 94 Zhu et al 97 examined 12 synthesized metabolites of 6-shogaol and found that specific metabolites were comparable with 6-shogaol in growth inhibitory effects and discriminatory effects against cancer cells but not causing toxicity toward normal cells. Hence, 6-shogaol could be a potential anti-tumor agent against hematologic malignancies even via oral administration.
Discussion
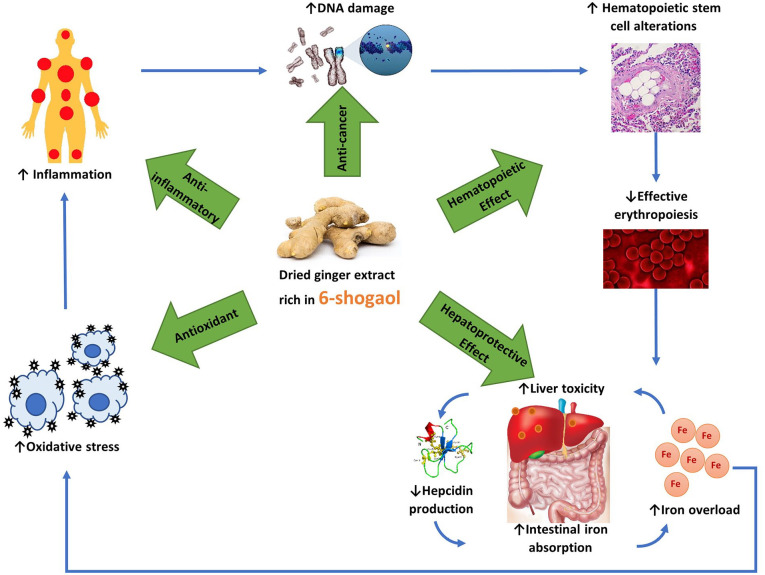
This study has identified a lack of direct evidence evaluating the use of 6-shogaol in patients with lower-risk MDS, apart from a small investigative study carried out by Golombick et al. 12 This literature review found evidence that ginger extract, with 6-shogaol as an active constituent, is a safe, natural, phytochemical compound with anti-inflammatory, antioxidant, hematopoietic, hepatoprotective and anti-tumorigenic properties. Therefore, this is the first in-depth review of the therapeutical properties of ginger and 6-shogaol and their potential therapeutic applications in lower-risk MDS indicating an existing knowledge gap in the literature. Currently, there is evidence showing a link between MDS development and chronic inflammation secondary to oxidative stress caused by ROS generated by iron overload. Figure 2 is a schematic depiction of how dried ginger extract rich in 6-shogaol can potentially benefit lower-risk MDS patients.
Figure 2.
A schematic depiction of how dried ginger extract rich in 6-shogaol can potentially benefit lower-risk MDS patients. Chronic inflammation leads to genetic aberrations in these patients, altering the hematopoietic stem cells’ ability to produce erythrocytes. Ineffective erythropoiesis causes iron overload and accumulation of excess iron in the liver, which lowers the liver’s ability to produce hepcidin. Intestinal absorption of iron increases as a result and further exacerbates iron overload. Excess iron is toxic, causing oxidative stress, which worsens inflammation. Dried ginger extract rich in 6-shogaol provides the much-needed antioxidants to scavenge the free radicals and reduce inflammation. It has anti-cancer properties to reduce the spread of malignancies. Ginger also promotes hematopoiesis and protects against liver damage and preserves hepcidin production to reduce iron overload.
Iron overload measured by elevated serum ferritin is commonly observed in lower-risk MDS patients. Serum ferritin is an acute-phase reactant, and its levels are elevated when inflammation is present, especially during hepatocellular damage. Thus, the anti-inflammatory action of 6-shogaol would be expected to reduce the level of serum ferritin. Moreover, its hepatoprotective effect could reverse the blunted response on hepcidin due to iron overload, resulting in an elevation of hepcidin levels. These effects were seen in Golombick et al 12 and warrant further research.
Both traditional use and available clinical evidence suggest that dried ginger extract can potentially improve anemic conditions. Moreover, Raza et al 71 found promising results in improving hematologic parameters of MDS patients with curcumin and gingerol. However, the study by Golombick et al 12 did not report improvement in blood cell counts, possibly due to its small sample size. Preclinical evidence of ginger extract and 10-gingerol shows positive effects on hematopoietic recovery in a zebrafish model but not 6-shogaol. Therefore, future research should investigate the hematopoietic effect of 6-shogaol in both preclinical and human studies with a larger cohort. Furthermore, MDS affects patients’ QoL. There is a lack of information on whether ginger or 6-shogaol has any benefits on fatigue and any QoL dimensions of MDS patients, and further studies are needed to clarify this aspect.
6-shogaol is stable in the GIT, rapidly and extensively metabolized, but could potentially involve in drug-drug interactions. In general, ginger extract has been reported in multiple case studies to interact with blood-thinning medication, notably warfarin, increasing bleeding risk. One case reported that consuming 48 mg of a ginger supplement caused a sharp rise in the clotting time of a 76-year-old patient. 98 Another 2 cases with ginger-anticoagulation interaction did not specify the dosage of ginger and its mode of ingestion.99,100 Moreover, a prospective longitudinal study did not find ginger to have a significant risk of increasing clotting time but was associated with an increased risk of self-reported bleeding. 101 However, an open-label, three-way crossover randomized study by Jiang et al showed that taking ginger extract at 0.4 g did not affect the pharmacokinetics or pharmacodynamics of either S-warfarin or R-warfarin in humans, nor did it affect coagulation status. 102 Hence, there may exist a dose-dependent relationship between the interaction of ginger and anticoagulant agents. This topic requires further investigation. Given that MDS predominates in the elderly population over 70 years old, many of them also have numerous comorbid conditions that may require blood-thinning agents. Any clinical application or study with 6-shogaol among MDS patients should consider the potential interaction risk with warfarin.
Conclusion
This the first in-depth review of the therapeutical properties of ginger and 6-shogaol and their potential therapeutic applications in lower-risk MDS. It fills an existing knowledge gap and thus provides the basis for further research in this area. Based on the evidence presented, the phenolic compound 6-shogaol in ginger appears to have various pharmacological properties, suggesting it may be a beneficial supportive treatment in lower-risk MDS patients. It appears to be safe, well-tolerated, addresses the pathophysiological process driving the cause of MDS, and affects the major risk factor that causes progression to acute myeloid leukemia. Given the potential therapeutic values of 6-shogaol, there is an immediate need for more human clinical trials with larger sample sizes to evaluate the therapeutic effects of 6-shogaol in lower-risk MDS patients.
Acknowledgments
In the spirit of reconciliation, the authors acknowledge the Traditional Custodians of country throughout Australia and their connections to land, sea and community. We pay our respect to their Elders past and present and extend that respect to all Aboriginal and Torres Strait Islander peoples today.
Footnotes
Author Contributions: The first two authors contributed equally to this work. All authors reviewed and approved the final version of the manuscript for publication.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Soo Liang Ooi  https://orcid.org/0000-0002-0534-3673
https://orcid.org/0000-0002-0534-3673
Vladimir Badmaev  https://orcid.org/0000-0003-3737-2847
https://orcid.org/0000-0003-3737-2847
References
- 1. Gangat N, Patnaik MM, Tefferi A. Myelodysplastic syndromes: contemporary review and how we treat. Am J Hematol. 2016;91:76-89. doi: 10.1002/ajh.24253 [DOI] [PubMed] [Google Scholar]
- 2. Steensma DP, Heptinstall KV, Johnson VM, et al. Common troublesome symptoms and their impact on quality of life in patients with myelodysplastic syndromes (MDS): results of a large internet-based survey. Leuk Res. 2008;32:691-698. doi: 10.1016/j.leukres.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 3. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454-2465. doi: 10.1182/blood-2012-03-420489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Germing U, Kobbe G, Haas R, Gattermann N. Myelodysplastic syndromes: diagnosis, prognosis, and treatment. Dtsch Arztebl Int. 2013;110:783-790. doi: 10.3238/arztebl.2013.0783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stauder R, Yu G, Koinig KA, et al. Health-related quality of life in lower-risk MDS patients compared with age- and sex-matched reference populations: a European LeukemiaNet study. Leukemia. 2018;32:1380-1392. doi: 10.1038/s41375-018-0089-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yao W, Yang H, Ding G. Mechanisms of qi-blood circulation and qi deficiency syndrome in view of blood and interstitial fluid circulation. J Tradit Chin Med. 2013;33:538-544. doi: 10.1016/s0254-6272(13)60162-4 [DOI] [PubMed] [Google Scholar]
- 7. Han Y, Li Y, Wang Y, Gao J, Xia L, Hong Y. Comparison of fresh, dried and stir-frying gingers in decoction with blood stasis syndrome in rats based on a GC-TOF/MS metabolomics approach. J Pharm Biomed Anal. 2016;129:339-349. doi: 10.1016/j.jpba.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 8. Huang Q, Feng L, Li H, et al. Jian-Pi-Bu-Xue-Formula alleviates cyclophosphamide-induced myelosuppression via up-regulating NRF2/HO1/NQO1 signaling. Front Pharmacol. 2020;11:1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lam CTW, Chan PH, Lee PSC, et al. Chemical and biological assessment of Jujube ( Ziziphus jujuba )-containing herbal decoctions: induction of erythropoietin expression in cultures. J Chromatogr B, Anal Technol Biomed life Sci. 2016;1026:254-262. doi: 10.1016/j.jchromb.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 10. Chan SM, Nelson EA, Leung SS, Cheung PC, Li CY. Special postpartum dietary practices of Hong Kong Chinese women. Eur J Clin Nutr. 2000;54:797-802. doi: 10.1038/sj.ejcn.1601095 [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Wang Q, Sun C, et al. Enhanced oral bioavailability, anti-tumor activity and hepatoprotective effect of 6-shogaol loaded in a type of novel micelles of polyethylene glycol and linoleic acid conjugate. Pharmaceutics. 2019;11:107. doi: 10.3390/pharmaceutics11030107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golombick T, Diamond TH, Manoharan A, Ramakrishna R, Badmaev V. Effect of the ginger derivative, 6-shogaol, on ferritin levels in patients with low to intermediate-1-risk myelodysplastic Syndrome-A small, investigative study. Clin Med Insights Blood Disord. 2017;10:1179545X17738755. doi: 10.1177/1179545X17738755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bode AM, Dong Z. The amazing and mighty ginger. In: Benzie IFF, Wachtel-Galor S. eds. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. CRC Press/Taylor & Francis; 2011;131-156. https://www.ncbi.nlm.nih.gov/books/NBK92775/ [Google Scholar]
- 14. Mao Q-Q, Xu XY, Cao S-Y, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. 2019;8:185. doi: 10.3390/foods8060185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fakhri S, Patra JK, Das SK, Das G, Majnooni MB, Farzaei MH. Ginger and heart health: from mechanisms to therapeutics. Curr Mol Pharmacol. 2020. Advance online publication. doi: 10.2174/1874467213666201209105005 [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Ke W, Bao R, Hu X, Chen F. Beneficial effects of ginger Zingiber officinale Roscoe on obesity and metabolic syndrome: a review. Ann N Y Acad Sci. 2017;1398:83-98. doi: 10.1111/nyas.13375 [DOI] [PubMed] [Google Scholar]
- 17. Mohd Sahardi NFN, Makpol S. Ginger (Zingiber officinale Roscoe) in the prevention of ageing and degenerative diseases: review of current evidence. Evid Based Complement Alternat Med. 2019;2019:5054395. doi: 10.1155/2019/5054395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang F-Y, Deng T, Meng L-X, Ma XL. Dietary ginger as a traditional therapy for blood sugar control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine. 2019;98:e15054-e15054. doi: 10.1097/MD.0000000000015054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Townsend EA, Siviski ME, Zhang Y, Xu C, Hoonjan B, Emala CW. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. Am J Respir Cell Mol Biol. 2013;48:157-163. doi: 10.1165/rcmb.2012-0231OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nikkhah Bodagh M, Maleki I, Hekmatdoost A. Ginger in gastrointestinal disorders: a systematic review of clinical trials. Food Sci Nutr. 2019;7:96-108. doi: 10.1002/fsn3.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiyama R. Nutritional implications of ginger: chemistry, biological activities and signaling pathways. J Nutr Biochem. 2020;86:108486. doi: 10.1016/j.jnutbio.2020.108486 [DOI] [PubMed] [Google Scholar]
- 22. Ghasemzadeh A, Jaafar HZE, Baghdadi A, Tayebi-Meigooni A. Formation of 6-, 8- and 10-shogaol in ginger through application of different drying methods: altered antioxidant and antimicrobial activity. Molecules. 2018;23:1646. doi: 10.3390/molecules23071646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agrahari P, Panda P, Verma NK, Khan WU, Darbari S. A brief study on zingiber officinale: a review. J Drug Discov Ther. 2015;3:20-27. https://www.jddt.in/index.php/jddt/article/view/238 [Google Scholar]
- 24. Sang S, Snook HD, Tareq FS, Fasina Y. Precision research on ginger: the type of ginger matters. J Agric Food Chem. 2020;68:8517-8523. doi: 10.1021/acs.jafc.0c03888 [DOI] [PubMed] [Google Scholar]
- 25. Ho S-C, Su M-S. Optimized heat treatment enhances the anti-inflammatory capacity of ginger. Int J Food Prop. 2016;19:1884-1898. doi: 10.1080/10942912.2015.1084633 [DOI] [Google Scholar]
- 26. Li Y, Hong Y, Han Y, Wang Y, Xia L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J Chromatogr B. 2016;1011:223-232. doi: 10.1016/j.jchromb.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 27. Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515-520. doi: 10.1016/j.jep.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 28. Asami A, Shimada T, Mizuhara Y, et al. Pharmacokinetics of [6]-shogaol, a pungent ingredient of Zingiber officinale Roscoe (part I). J Nat Med. 2010;64:281-287. doi: 10.1007/s11418-010-0404-y [DOI] [PubMed] [Google Scholar]
- 29. Kou X, Wang X, Ji R, et al. Occurrence, biological activity and metabolism of 6-shogaol. Food Funct. 2018;9:1310-1327. doi: 10.1039/c7fo01354j [DOI] [PubMed] [Google Scholar]
- 30. Ionescu C, Caira MR, eds. Pathways of biotransformation: phase II reactions. In: Ionescu C, Caira MR, eds. Drug Metabolism: Current Concepts. Springer Netherlands; 2005;129-170. [Google Scholar]
- 31. Yu Y, Zick S, Li X, Zou P, Wright B, Sun D. Examination of the pharmacokinetics of active ingredients of ginger in humans. AAPS J. 2011;13:417-426. doi: 10.1208/s12248-011-9286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anh NH, Kim SJ, Long NP, et al. Ginger on human health: a comprehensive systematic review of 109 randomized controlled trials. Nutrients. 2020;12:157. doi: 10.3390/nu12010157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hassan SMA, Hassan AH. Assessment of toxicological effect of shogaol in albino mice. Pak Vet J. 2018;38:377-383. doi: 10.29261/pakvetj/2018.095 [DOI] [Google Scholar]
- 34. Cicero AFG, Colletti A. Handbook of Nutraceuticals for Clinical Use. Springer; 2018. [Google Scholar]
- 35. Kumar S, Saxena K, Uday I, Singh N, Saxena R, Singh UN. Anti-inflammatory action of ginger: a critical review in anemia of inflammation and its future aspects. Int J Herb Med. 2013;1:16-20. [Google Scholar]
- 36. Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12:684-701. doi: 10.1016/j.phymed.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 37. Banerjee T, Calvi LM, Becker MW, Liesveld JL. Flaming and fanning: the spectrum of inflammatory influences in myelodysplastic syndromes. Blood Rev. 2019;36:57-69. doi: 10.1016/j.blre.2019.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geyer HL, Dueck AC, Scherber RM, Mesa RA. Impact of inflammation on myeloproliferative neoplasm symptom development. Mediators Inflamm. 2015;2015:1-9. doi: 10.1155/2015/284706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sallman DA, List A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood. 2019;133:1039-1048. doi: 10.1182/blood-2018-10-844654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi X, Zheng Y, Xu L, Cao C, Dong B, Chen X. The inflammatory cytokine profile of myelodysplastic syndromes: a meta-analysis. Medicine. 2019;98:e15844-e15844. doi: 10.1097/MD.0000000000015844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolach O, Stone R. Autoimmunity and inflammation in myelodysplastic syndromes. Acta Haematol. 2016;136:108-117. doi: 10.1159/000446062 [DOI] [PubMed] [Google Scholar]
- 42. Han Q, Yuan Q, Meng X, Huo J, Bao Y, Xie G. 6-Shogaol attenuates LPS-induced inflammation in BV2 microglia cells by activating PPAR-γ. Oncotarget. 2017;8:42001-42006. doi: 10.18632/oncotarget.16719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen F, Tang Y, Sun Y, Veeraraghavan VP, Mohan SK, Cui C. 6-shogaol, a active constiuents of ginger prevents UVB radiation mediated inflammation and oxidative stress through modulating NrF2 signaling in human epidermal keratinocytes (HaCaT cells). J Photochem Photobiol B Biol. 2019;197:111518. doi: 10.1016/j.jphotobiol.2019.111518 [DOI] [PubMed] [Google Scholar]
- 44. Annamalai G, Suresh K. [6]-Shogaol attenuates inflammation, cell proliferation via modulate NF-κB and AP-1 oncogenic signaling in 7,12-dimethylbenz[a]anthracene induced oral carcinogenesis. Biomed Pharmacother. 2018;98:484-490. doi: 10.1016/j.biopha.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 45. Pan MH, Hsieh MC, Hsu PC, et al. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol Nutr Food Res. 2008;52:1467-1477. doi: 10.1002/mnfr.200700515 [DOI] [PubMed] [Google Scholar]
- 46. Yocum GT, Hwang JJ, Mikami M, Danielsson J, Kuforiji AS, Emala CW. Ginger and its bioactive component 6-shogaol mitigate lung inflammation in a murine asthma model. Am J Physiol Cell Mol Physiol. 2020;318:L296-L303. doi: 10.1152/ajplung.00249.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang JC, Zhou LH, Zhao HJ, Cai SX. Examination of the protective effect of 6-shogaol against LPS-induced acute lung injury in mice via NF-κB attenuation. Arch Biol Sci. 2016;68:633-639. doi: 10.2298/ABS151012055W [DOI] [Google Scholar]
- 48. Sabina EP, Rasool M, Mathew L, EzilRani P, Indu H. 6-Shogaol inhibits monosodium urate crystal-induced inflammation: An in vivo and in vitro study. Food Chem Toxicol. 2010;48:229-235. doi: 10.1016/j.fct.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 49. Moon M, Kim HG, Choi JG, et al. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem Biophys Res Commun. 2014;449:8-13. doi: 10.1016/j.bbrc.2014.04.121 [DOI] [PubMed] [Google Scholar]
- 50. Bischoff-Kont I, Fürst R. Benefits of ginger and its constituent 6-shogaol in inhibiting inflammatory processes. Pharmaceuticals. 2021;14:571. doi: 10.3390/ph14060571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9-17. doi: 10.1038/nchembio.1416 [DOI] [PubMed] [Google Scholar]
- 52. Pilo F, Angelucci E. A storm in the niche: iron, oxidative stress and haemopoiesis. Blood Rev. 2018;32:29-35. doi: 10.1016/j.blre.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 53. Saigo K, Takenokuchi M, Hiramatsu Y, et al. Oxidative stress levels in myelodysplastic syndrome patients: their relationship to serum ferritin and haemoglobin values. J Int Med Res. 2011;39:1941-1945. doi: 10.1177/147323001103900539 [DOI] [PubMed] [Google Scholar]
- 54. Gonçalves AC, Cortesão E, Oliveiros B, et al. Oxidative stress and mitochondrial dysfunction play a role in myelodysplastic syndrome development, diagnosis, and prognosis: a pilot study. Free Radic Res. 2015;49:1081-1094. doi: 10.3109/10715762.2015.1035268 [DOI] [PubMed] [Google Scholar]
- 55. Gonçalves AC, Alves R, Baldeiras I, et al. Oxidative stress parameters Can predict the response to erythropoiesis-stimulating agents in myelodysplastic syndrome patients. Front Cell Dev Biol. 2021;9:701328. doi: 10.3389/fcell.2021.701328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoeks M, Bagguley T, Marrewijk CV, Smith A, Bowen D, Culligan D. Toxic iron species in lower-risk myelodysplastic syndrome patients : course of disease and effects on outcome. Leukemia. 2021;35:1745-1750. doi: 10.1038/s41375-020-01022-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peng S, Yao J, Liu Y, Duan D, Zhang X, Fang J. Activation of Nrf2 target enzymes conferring protection against oxidative stress in PC12 cells by ginger principal constituent 6-shogaol. Food Funct. 2015;6:2813-2823. doi: 10.1039/c5fo00214a [DOI] [PubMed] [Google Scholar]
- 58. Yang L, Yang F, Teng L, Katayama I. 6-Shogaol protects human melanocytes against oxidative stress through activation of the Nrf2-antioxidant response element signaling pathway. Int J Mol Sci. 2020;21:3537. doi: 10.3390/ijms21103537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim JK, Jang HD. 6-Shogaol attenuates H2O2 -induced Oxidative stress via up- regulation of Nrf2-mediated γ -glutamylcysteine synthetase and Heme Oxygenase expression in HepG2 cells. Food Sci Biotechnol. 2016;25:319-327. doi: 10.1007/s10068-016-0045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bak MJ, Ok S, Jun M, Jeong WS. 6-Shogaol-Rich extract from ginger Up-regulates the antioxidant defense systems in cells and mice. Molecules. 2012;17:8037-8055. doi: 10.3390/molecules17078037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Imam MU, Zhang S, Ma J, Wang H, Wang F. Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients. 2017;9:671. doi: 10.3390/nu9070671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mohamed NFR, Jeevitha M, Rajeshkumar S, Preetha S. Free radical scavenging activity of iron nanoparticles synthesized using dried ginger. Int J Pharm Res. 2020;12:3252-3256. doi: 10.31838/ijpr/2020.12.04.442 [DOI] [Google Scholar]
- 63. Oboh G, Akinyemi AJ, Ademiluyi AO. Antioxidant and inhibitory effect of red ginger (Zingiber officinale var. Rubra) and white ginger (Zingiber officinale Roscoe) on Fe2+ induced lipid peroxidation in rat brain in vitro. Exp Toxicol Pathol. 2012;64:31-36. doi: 10.1016/j.etp.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 64. Gholampour F, Behzadi Ghiasabadi F, Owji SM, Vatanparast J. The protective effect of hydroalcoholic extract of ginger (Zingiber officinale Rosc.) against iron-induced functional and histological damages in rat liver and kidney. Avicenna J Phytomedicine. 2017;7:542-553. [PMC free article] [PubMed] [Google Scholar]
- 65. Ferri-Lagneau KF, Moshal KS, Grimes M, et al. Ginger stimulates hematopoiesis via Bmp pathway in zebrafish. PLoS One. 2012;7:e39327. doi: 10.1371/journal.pone.0039327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferri-Lagneau KF, Haider J, Sang S, Leung T. Rescue of hematopoietic stem/progenitor cells formation in plcg1 zebrafish mutant. Sci Rep. 2019;9:244. doi: 10.1038/s41598-018-36338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Alamri HS, Alsughayyir J, Akiel M, et al. Stimulation of calcium influx and CK1α by NF-κB antagonist [6]-gingerol reprograms red blood cell longevity. J Food Biochem. 2021;45:e13545. doi: 10.1111/jfbc.13545 [DOI] [PubMed] [Google Scholar]
- 68. Wang Y, Yu H, Zhang X, et al. Evaluation of daily ginger consumption for the prevention of chronic diseases in adults: a cross-sectional study. Nutrition. 2017;36:79-84. doi: 10.1016/j.nut.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 69. Kulkarni R, Deshpande A, Saxena K, Varma M, Sinha ARS. Ginger supplementary therapy for iron absorption in iron deficiency anemia. Indian J Tradit Knowl. 2012;11:78-80. [Google Scholar]
- 70. Kumar S, Singh UN, Saxena K, Saxena R. Supplementation of ginger with anti- tuberculosis treatment (ATT): a better approach to treat anemic pulmonary tuberculosis patients. Int J Herb Med. 2013;1:17-20. [Google Scholar]
- 71. Raza A, Lagmay P, Mehdi M, et al. Multi-lineage response to a combination of gingerol and curcumin in low risk myelodysplastic syndromes (MDS). Cancer Res. 2008;68:5528. [Google Scholar]
- 72. Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272. doi: 10.3324/haematol.2019.232124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Santini V, Girelli D, Sanna A, et al. Hepcidin levels and their determinants in different types of myelodysplastic syndromes. PLoS One. 2011;6:e23109. doi: 10.1371/journal.pone.0023109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gattermann N. Iron overload in myelodysplastic syndromes (MDS). Int J Hematol. 2018;107:55-63. doi: 10.1007/s12185-017-2367-1 [DOI] [PubMed] [Google Scholar]
- 75. Lyle L, Hirose A. Iron overload in myelodysplastic syndromes: pathophysiology, consequences, diagnosis, and treatment. J Adv Pract Oncol. 2018;9:392-405. [PMC free article] [PubMed] [Google Scholar]
- 76. Cui R, Gale RP, Zhu G, et al. Serum iron metabolism and erythropoiesis in patients with myelodysplastic syndrome not receiving RBC transfusions. Leuk Res. 2014;38:545-550. doi: 10.1016/j.leukres.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Leitch HA, Buckstein R, Zhu N, et al. Iron overload in myelodysplastic syndromes: evidence based guidelines from the Canadian consortium on MDS. Leuk Res. 2018;74:21-41. doi: 10.1016/j.leukres.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 78. Bonkovsky HL, Lambrecht RW. Iron-induced liver injury. Clin Liver Dis. 2000;4:409-NaN29, vi. doi: 10.1016/s1089-3261(05)70116-1 [DOI] [PubMed] [Google Scholar]
- 79. Mahmoud Y, Hegazy H. Ginger and alpha lipoic acid ameliorate age-related ultrastructural changes in rat liver. Biotech Histochem. 2016;91:86-95. doi: 10.3109/10520295.2015.1076578 [DOI] [PubMed] [Google Scholar]
- 80. Zhuang X, Deng ZB, Mu J, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi: 10.3402/jev.v4.28713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leal DT, Fontes GG, Villa JKD, et al. Zingiber officinale formulation reduces hepatic injury and weight gain in rats fed an unhealthy diet. An Acad Bras Cienc. 2019;91:e20180975. doi: 10.1590/0001-3765201920180975 [DOI] [PubMed] [Google Scholar]
- 82. Elzwi S. Effect of Zingiber officinale (ginger) extract on acetaminophen-induced hepatotoxicity in mice. Pharm Methods. 2019;10:27-30. doi: 10.5530/phm.2019.1.5 [DOI] [Google Scholar]
- 83. Mukherjee S, Mukherjee N, Saini P, Roy P, Babu SP. Ginger extract ameliorates phosphamidon induced hepatotoxicity. Indian J Exp Biol. 2015;53:574-584. [PubMed] [Google Scholar]
- 84. Badawi MS. Histological study of the protective role of ginger on piroxicam-induced liver toxicity in mice. J Chin Med Assoc. 2019;82:11-18. doi: 10.1016/j.jcma.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 85. Sabina EP, Pragasam SJ, Kumar S, Rasool M. 6-gingerol, an active ingredient of ginger, protects acetaminophen-induced hepatotoxicity in mice. Zhong Xi Yi Jie He Xue Bao. 2011;9:1264-1269. doi: 10.3736/jcim20111116 [DOI] [PubMed] [Google Scholar]
- 86. Essawy AE, Abdel-Wahab WM, Sadek IA, Khamis OM. Dual protective effect of ginger and rosemary extracts against CCl(4)-induced hepatotoxicity in rats. Environ Sci Pollut Res Int. 2018;25:19510-19517. doi: 10.1007/s11356-018-2129-5 [DOI] [PubMed] [Google Scholar]
- 87. El-Sharaky AS, Newairy AA, Kamel MA, Eweda SM. Protective effect of ginger extract against bromobenzene-induced hepatotoxicity in male rats. Food Chem Toxicol. 2009;47:1584-1590. doi: 10.1016/j.fct.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 88. Motawi TK, Hamed MA, Shabana MH, Hashem RM, Aboul Naser AF. Zingiber officinale acts as a nutraceutical agent against liver fibrosis. Nutr Metab. 2011;8:40. doi: 10.1186/1743-7075-8-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vipin AV, Raksha RK, Kurrey NK, Anu Appaiah KA, Venkateswaran G. Protective effects of phenolics rich extract of ginger against aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed Pharmacother. 2017;91:415-424. doi: 10.1016/j.biopha.2017.04.107 [DOI] [PubMed] [Google Scholar]
- 90. Alqasoumi S, Yusufoglu H, Farraj A, Alam A. Effect of 6-shogaol and 6-gingerol on diclofenac sodium induced liver injury. Int J Pharmacol. 2011;7:868-873. doi: 10.3923/ijp.2011.868.873 [DOI] [Google Scholar]
- 91. Hikino H, Kiso Y, Kato N, et al. Antihepatotoxic actions of gingerols and diarylheptanoids. J Ethnopharmacol. 1985;14:31-39. doi: 10.1016/0378-8741(85)90025-X [DOI] [PubMed] [Google Scholar]
- 92. Sans-Sabrafen J, Buxó-Costa J, Woessner S, et al. Myelodysplastic syndromes and malignant solid tumors: analysis of 21 cases. Am J Hematol. 1992;41:1-4. doi: 10.1002/ajh.2830410102 [DOI] [PubMed] [Google Scholar]
- 93. Zeidan AM, Shallis RM, Wang R, Davidoff A, Ma X. Epidemiology of myelodysplastic syndromes: why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019;34:1-15. doi: 10.1016/j.blre.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 94. Lechner JF, Stoner GD. Gingers and their purified components as cancer chemopreventative agents. Molecules. 2019;24:2859. doi: 10.3390/molecules24162859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tuorkey MJ. Cancer therapy with phytochemicals: present and future perspectives. Biomed Environ Sci. 2015;28:808-819. doi: 10.3967/bes2015.112 [DOI] [PubMed] [Google Scholar]
- 96. Liu Q, Peng Y-B, Zhou P, et al. 6-Shogaol induces apoptosis in human leukemia cells through a process involving caspase-mediated cleavage of eIF2α. Mol Cancer. 2013;12:135. doi: 10.1186/1476-4598-12-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhu Y, Warin RF, Soroka DN, Chen H, Sang S. Metabolites of ginger component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells: chemical synthesis and biological evaluation. PLoS One. 2013;8:e54677. doi: 10.1371/journal.pone.0054677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rubin D, Patel V, Dietrich E. Effects of oral ginger supplementation on the INR. Case Rep Med. 2019;2019:8784029. doi: 10.1155/2019/8784029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lesho EP, Saullo L, Udvari-Nagy S. A 76-year-old woman with erratic anticoagulation. Cleve Clin J Med. 2004;71:651-656. doi: 10.3949/ccjm.71.8.651 [DOI] [PubMed] [Google Scholar]
- 100. Krüth P, Brosi E, Fux R, Mörike K, Gleiter CH. Ginger-associated overanticoagulation by phenprocoumon. Ann Pharmacother. 2004;38:257-260. doi: 10.1345/aph.1D225 [DOI] [PubMed] [Google Scholar]
- 101. Shalansky S, Lynd L, Richardson K, Ingaszewski A, Kerr C. Risk of Warfarin-related bleeding events and supratherapeutic international normalized ratios associated with complementary and alternative medicine: a longitudinal analysis. Pharmacotherapy. 2007;27:1237-1247. doi: 10.1592/phco.27.9.1237 [DOI] [PubMed] [Google Scholar]
- 102. Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi: 10.1111/j.1365-2125.2005.02322.x [DOI] [PMC free article] [PubMed] [Google Scholar]