Abstract
Background:
Hyponatremia (serum Na+ < 135 mmol/L) is the most common electrolyte abnormality detected in clinical practice and an important cause of mortality and morbidity in hospital settings. Hyponatremia in patients with pneumonia is usually mild but is associated with increased risk of intensive care unit (ICU) admission, prolonged hospital stays, and increased mortality rates. The purpose of this study is to understand the impact of varying degrees of hyponatremia and various other inflammatory markers on the severity and outcome of coronavirus disease-19 (COVID-19).
Objective:
The main objective of this study is to evaluate the prevalence of hyponatremia in COVID-19 patients and to assess the correlation between hyponatremia and severity and outcome of COVID-19. The other objective is to evaluate the correlation between various inflammatory markers and outcome (ICU vs non-ICU admission, discharged vs deceased) in patients with COVID-19 pneumonia.
Methods:
A total of 113 participants who have been diagnosed with COVID-19 infection by reverse transcriptase-polymerase chain reaction test were included in the study. Epidemiological, demographic, clinical, investigative work-up, and outcome data were extracted from electronic health records using a standard data collection form. Based on serum sodium levels, patients were divided into two groups: normonatremic (serum Na+ ≥ 135 mEq/L) and hyponatremic (serum Na+ < 135 mEq/L). Various clinical, laboratory, and outcome parameters were compared between the two groups.
Results:
Hyponatremia was present in 50 out of 113 (44%) patients in our study, and it was generally mild. There were more male patients in hyponatremia group (P = .006), and hyponatremic patients were older than normonatremic patients (P = .001). Forty (35%) of the 113 patients were transferred to the ICU, and 17 (15%) needed mechanical ventilation during their hospitalization. Interleukin-6 (IL-6) levels were higher in the hyponatremic group (P = .022). Intensive care unit admissions and oxygen requirement were significantly higher in hyponatremic patients (P = .001 and .016, respectively). Ferritin, lactate dehydrogenase (LDH), IL-6, total leucocyte count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels were significantly elevated in those patients requiring ICU admission and those who died due to COVID-19.
Conclusions:
Our study revealed that demography, clinical features, radiographic findings, complications like renal insufficiency, and inflammatory markers like IL-6 play a considerable role in hyponatremic COVID-19 patients. Hyponatremia patients required significantly higher rates of ICU admissions and oxygen support. Our results suggest that monitoring inflammatory markers such as ESR, CRP, total white blood cell (WBC) count, ferritin, LDH, and IL-6 may serve as an early warning system for progression to severe COVID-19.
Keywords: hyponatremia, COVID-19, SARS-CoV-2, coronavirus, IL-6
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was declared as a pandemic by the World Health Organization (WHO) on March 11, 2020. Since its outbreak in December 2019 in Wuhan city, Hubei province, China, about 141 057 106 cases were reported, and over 3 million deaths were reported globally since the start of the pandemic as of April 20, 2021. 1 The clinical spectrum of the disease is highly variable, ranging from asymptomatic to respiratory failure and other potentially fatal complications.
Although most patients have mild disease and recover spontaneously without the need for hospitalization, few patients may rapidly and unpredictably progress to a critical disease requiring intensive care treatment. 2 In this context, clinical tools that help in accurately predicting the severity and mortality among hospitalized patients are invaluable in efficient utilization of hospital resources and improvement in patient outcomes. Various clinical and laboratory prognostic factors have been reported in COVID-19.
The host receptor for SARS-CoV-2 cell entry is an angiotensin-converting enzyme 2 (ACE2). SARS-CoV-2 binds to ACE2 through the receptor binding gene region of its spike protein. ACE2 is a membrane protein widely distributed in different tissues like epithelial cells of the lung, heart, small intestine, kidney, and vascular endothelium.3,4 ACE2 is the main anti-regulatory factor of the renin-angiotensin system, which is an essential factor in controlling blood pressure and electrolyte balance. The counteract of ACE2 is reduced by binding SARS-CoV-2, which results in hyponatremia and other electrolyte abnormalities. 5
Hyponatremia (serum Na+ < 135 mmol/L) is the most common electrolyte abnormality detected in clinical practice and an important cause of mortality and morbidity in hospital settings. The prevalence of hyponatremia in in-patient settings is about 20% to 30%, while it is up to 42% for intensive care patients.6,7 Hyponatremia in hospital settings may occur due to infectious agents; endocrine, cardiovascular, renal, and liver pathologies; as well as nutritional and metabolic causes. 8
In 1962, Stormont and Waterhouse, 9 for the first time, described the association between pneumonia and hyponatremia. Most common infectious diseases associated with hyponatremia include bacterial infections like community-acquired pneumonia (CAP; mainly Legionella), spontaneous bacterial peritonitis, tuberculosis, murine typhus, sepsis, meningitis, skin infections, and soft tissue infections. Influenza virus and other respiratory viruses, human immunodeficiency virus, human herpes virus-6, hantavirus, severe fever with thrombocytopenia syndrome virus, Ebola virus, fungal infections like cryptococcosis, and protozoan infections like malaria are other infectious agents commonly associated with hyponatremia. 8 Recent studies have shown association of hyponatremia with COVID-19. 10
Previous studies have reported that 8% to 28% of the pneumonia patients had hyponatremia at admission.11,12 It is more common with some infectious agents like Legionella pneumophila, in which hyponatremia was reported in 44% to 46% of the patients while it is 8% to 14% of patients with other causes of CAP.13,14
Hyponatremia in patients with CAP is usually mild but is associated with increased risk of intensive care unit (ICU) admission, prolonged hospital stays, and increased mortality rates. However, there is very limited data on the prevalence and impact of hyponatremia in COVID-19. The purpose of this study is for understanding the impact of varying degrees of hyponatremia and other inflammatory markers (interleukin-6 [IL-6], ferritin, lactate dehydrogenase [LDH]) on the severity and outcome of COVID-19. Although a few cases were reported, to the best of our knowledge, this is one of the few studies on the prevalence and impact of hyponatremia in COVID-19 patients in India.
Methods
The main objective of this study is to evaluate the prevalence of hyponatremia in COVID-19 patients and to assess the correlation between hyponatremia and severity and outcome of COVID-19. The other objective is to evaluate the correlation between various inflammatory markers and outcome (ICU vs non-ICU admission, discharged vs deceased) in patients diagnosed with COVID-19 pneumonia.
Both male and female patients aged above 18 years who have been diagnosed with COVID-19 infection by reverse transcriptase-polymerase chain reaction (RT-PCR) test between June 2020 and September 2020 (4 months) at Apollo Hospitals, Chennai, were included in the study. The test was performed using the CoviPath COVID-19 RT-PCR kit from Thermo Fisher Scientific that is intended for the qualitative detection of ORF1ab and N genes of the SARS-CoV-2 genome by real time RT-PCR. As per the stated manufacturer’s performance, the sensitivity and specificity are 100%. Both male and female patients less than 18 years of age and those patients who were admitted elsewhere and received intravenous fluids prior to presentation to our hospital were excluded from the study.
The Institutional Ethics Committee approved the study protocol. Informed consent was waived due to the nature of the retrospective study. All patients with a diagnosis of COVID-19 were evaluated as per the proforma. Epidemiological, demographic, clinical, investigative work-up, and outcome data were extracted from electronic health records using a standard data collection form. Extracted data include demographic data of the patients with COVID-19, the time between symptoms onset and hospital admission, comorbidities, and presenting symptoms. Vital parameters like temperature, systolic and diastolic blood pressure, respiratory rate, oxygen saturation, and oxygen flow rate recorded at the time of admission were collected. Laboratory investigations including complete blood count, serum creatinine, serum sodium, alanine transaminase (ALT), aspartate transaminase (AST), IL-6, ferritin, and LDH levels performed during the first 24 h were extracted. Computed tomography (CT) scan of the chest performed during the hospitalization, focusing on the extension of COVID-19 lesions, was also extracted. Serum sodium was measured by using ion selective electrode-indirect method. All data were entered into the computerized database for further statistical analyses. Hyponatremia is defined by serum sodium levels below 135 mEq/L. Based on serum sodium levels, patients were divided into two groups: normonatremic (serum Na+ ≥ 135 mEq/L) and hyponatremic (serum Na+ < 135 mEq/L).
All continuous variables were tested for normality using Shapiro-Wilk’s test. Normally distributed continuous variables were represented by mean ± SD. Non-normally distributed continuous variables were expressed as median (interquartile range [IQR]). Categorical variables were mentioned as percentage. Comparison of normally distributed continuous variables were done by independent t test. Non-normally distributed continuous variables were compared by Mann-Whitney U test. Comparison of categorical variables was done by Chi-square test. Univariate analysis was carried out to find the best predictors of the outcome. Only those univariately significant predictors were considered for multivariate analysis. Data entry was done by using Microsoft Excel 2007. Data validation and analysis was carried out by IBM SPSS statistics for Windows, Armonk, NY; Version 25.0. All P values <.05 were considered as statistically significant.
Results
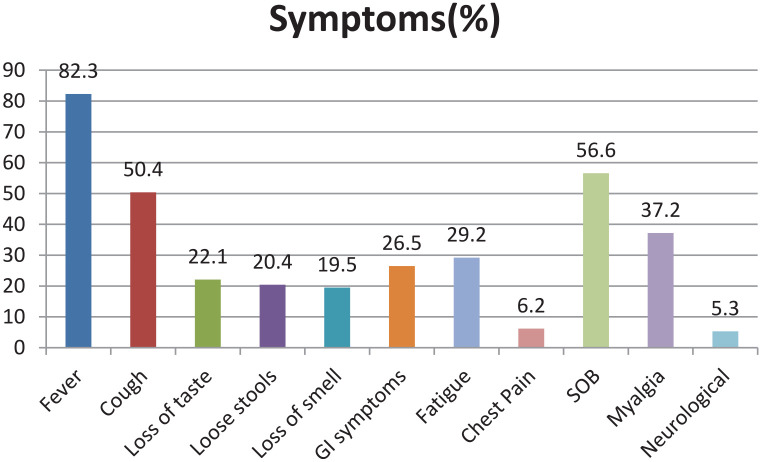
A total of 113 hospitalized patients with COVID-19, confirmed by RT-PCR test, were included in the study. In the COVID-19 cohort, 75 (66.4%) of the patients were males and 38 (33.6%) were females, with a mean age of 57.39 ± 12.8 years. The most frequent presenting symptoms were fever (82.3%), shortness of breath (56.6%), cough (50.4%), and myalgia (37.2%), and the gastrointestinal symptoms were seen in 26.5% of the patients and 5.3% of patients presented with neurological symptoms (Figure 1).
Figure 1.
A bar diagram showing most frequent symptoms in our cohort.
Note. GI = gastrointestinal; SOB = shortness of breath.
In all, 86 (76.1%) of the patients had at least one comorbidity, and the most frequent comorbidities were diabetes mellitus (54%), hypertension (43.4%), and coronary arterial diseases (15.9%). Forty (35.4%) of the patients were transferred to the ICU, and 17 (15%) needed mechanical ventilation during their hospitalization. Furthermore, 17 (15%) of the 113 patients in our study died.
Hyponatremia was present in 50 out of 113 (44.2%) patients in our study. According to serum sodium levels at the time of admission, patients were divided into two groups: hyponatremic (natremia < 135 mEq/L, n = 50, 44.2% of the cohort) and normonatremic (natremia ≥ 135 mEq/L, n = 63, 55.8% of the cohort) patients. Hyponatremia was generally mild (32 [28.3%]), while 13 (11.5%) patients had moderate hyponatremia. Only four (3.5%) patients had serum sodium levels less than 120 mEq/L.
Hyponatremic patients were much older than normonatremic patients (62.0 ± 12.4 vs 53.6 ± 11.9 years old; P = .001). There were significantly more male patients in the hyponatremic group (P = .006). Males had more severe hyponatremia compared with females (P = .027).
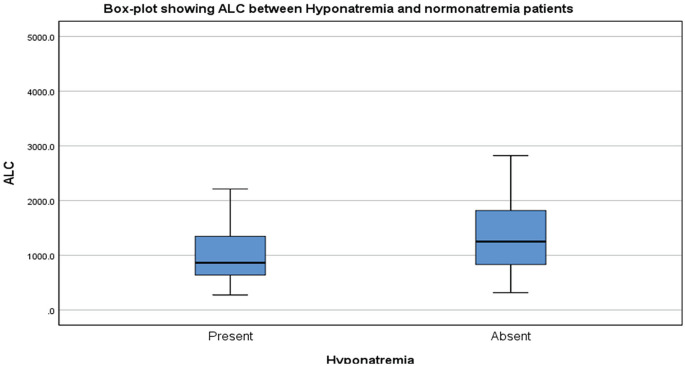
Hyponatremic patients had significantly higher respiratory rate and lowered oxygen saturation levels (P = .0001 and P = .006, respectively). In addition, patients with gastrointestinal symptoms had more severe hyponatremia (P = .028). Patients in the hyponatremia group exhibited lower median lymphocyte counts, and all the patients with severe hyponatremia had lymphopenia (Figure 2). The mean total white blood cell (WBC) count was higher in hyponatremia group (10 222 ± 5770) than normonatremia group (9511.7 ± 5464), but there was no statistical significance.
Figure 2.
Box-plot showing the comparison of lymphocyte counts (cells/mm3) between hyponatremic and normonatremic patients.
Note. ALC = absolute lymphocyte count.
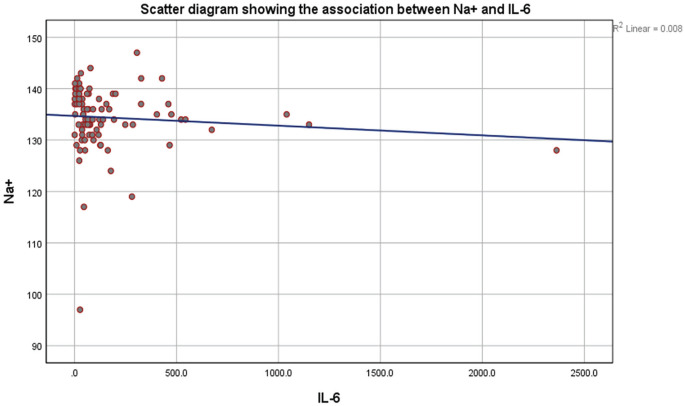
Patients in the hyponatremia group had significantly lower serum uric acid, higher creatinine levels, and significantly higher C-reactive protein (CRP) levels than normonatremic patients (Table 1). Interleukin-6 was significantly higher in the hyponatremia group (P = .022; Figure 3). We compared pulmonary lesions between hyponatremic and normonatremic groups, and hyponatremia was significantly higher in patients with consolidation on CT scan of the chest (P = .012).
Table 1.
Correlation of Clinical and Laboratory Parameters Between Hyponatremic and Normonatremic COVID-19 Patients.
| Blood parameter | Hyponatremia group |
Normonatremia group |
|||
|---|---|---|---|---|---|
| Mean | 95% confidence interval | Mean | 95% confidence interval | P value | |
| CRP (mg/L) (N = 81; 39 vs 42) | 114.2 | 95.5-132.8 | 70.9 | 50.1-91.6 | .0001 |
| Serum creatinine (mg/dL) | 1.1 | 0.4-1.8 | 0.8 | 0.5-1.1 | .016 |
| Lymphocyte count (cells/mm3) | 989.3 | 449.4-1529.2 | 1434.2 | 591.1-2277.3 | .002 |
| Serum uric acid levels (mg/dL) | 3.9 | 2.2-5.6 | 4.7 | 3-6.4 | .033 |
| Respiratory rate (per minute) | 27.6 | 21-34.2 | 22.7 | 17-28.4 | .0001 |
| SpO2 levels (%) | 89 | 83.5-94.5 | 91.5 | 87.3-95.7 | .006 |
Note. CRP = C-reactive protein.
Figure 3.
Scatter diagram showing the association between Na+ (mEq/L) and IL-6 (pg/mL).
Note. IL-6 = interleukin-6.
Ferritin, LDH, erythrocyte sedimentation rate (ESR), CRP, total WBC count, and IL-6 were significantly higher in patients requiring ICU admission (Table 2). ICU admissions were significantly higher in hyponatremic than normonatremic patients (52% vs 22.2%; P = .001). Depending on oxygen requirement, patients were divided into three groups (none, <6 L/min, and >6 L/min). A significant number of patients in the hyponatremia group required more than 6 L of oxygen support (P = .016). Most of the patients requiring >6 L oxygen had moderate hyponatremia, more commonly.
Table 2.
Inflammatory Markers in ICU Versus Non-ICU Admissions.
| Blood parameter | ICU admission |
Non-ICU admission |
|||
|---|---|---|---|---|---|
| Median | Range | Median | Range | P value | |
| Ferritin (ng/mL) (N = 88; 36 vs 52) | 687 | 405-1061.2 | 288.2 | 142.8-618.6 | .001 |
| LDH (U/L) (N = 100; 37 vs 63) | 499 | 407-590 | 358.5 | 305-463 | .0001 |
| IL-6 (pg/mL) (N = 88; 34 vs 54) | 124 | 62.8-306.2 | 41.5 | 78.8-99.2 | .0001 |
| Total white blood cells count (cells/mm3) (N = 113; 40 vs 73) | 6800 | 4160-11 700 | 5435 | 4800-7265 | .001 |
| ESR (mm/h) (N = 81; 37 vs 44) | 33 | 14.4-51 | 10.5 | 7-26 | .0001 |
| CRP (mg/L) (N = 81; 37 vs 44) | 79.3 | 12.5-122 | 12.3 | 5-59.9 | .0001 |
Note. ICU = intensive care unit; LDH = lactate dehydrogenase; IL-6 = interleukin-6; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
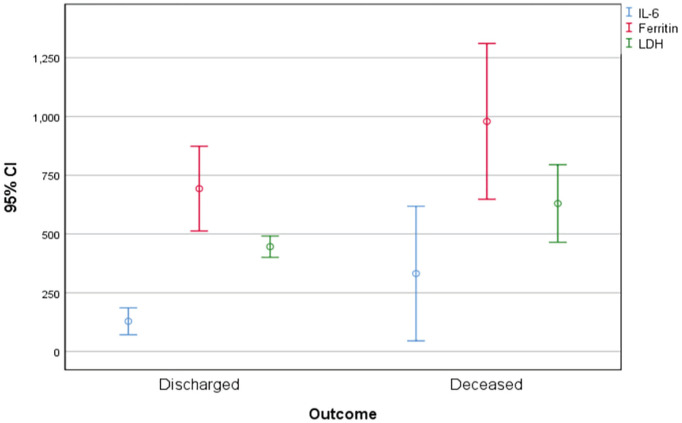
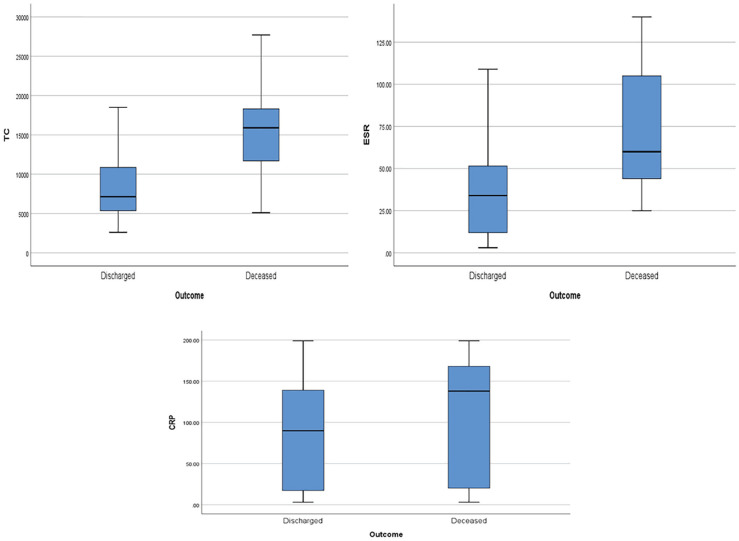
Ferritin, LDH, IL-6, total WBC count, ESR, and CRP levels were significantly elevated in those patients who died due to COVID-19 (Figures 4 and 5; Table 3). In multivariate regression analysis, high CRP was found to be an independent predictor for ICU admission (P = .029), lymphopenia count was found to be associated with hyponatremia (P = .023), and elevated ESR was associated with poor outcome (P = .002).
Figure 4.
Error bar showing the comparison between IL-6 (pg/mL), ferritin (ng/mL), and LDH (U/L) in hyponatremic and normonatremic patients.
Note. IL-6 = interleukin-6; LDH = lactate dehydrogenase; CI = confidence interval.
Figure 5.
Box-plots showing total white blood cells count (cells/mm3), ESR (mm/h), and CRP (mg/L) versus outcome, respectively.
Note. ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; TC = total leucocyte count.
Table 3.
Inflammatory Markers in Deceased Versus Discharged Patients.
| Blood parameter | Deceased |
Discharged |
|||
|---|---|---|---|---|---|
| Median | Range | Median | Range | P value | |
| Ferritin (ng/mL) (N = 88; 17 vs 71) | 924.6 | 683.5-1292.4 | 377 | 154.7-706.5 | .002 |
| LDH (U/L) (N = 100; 17 vs 83) | 562 | 407-776 | 392 | 318.5-488.5 | .005 |
| IL-6 (pg/mL) (N = 88; 17 vs 71) | 140 | 78-326.9 | 23 | 1-127 | .009 |
| Total white blood cells count (cells/mm3) (N = 113; 17 vs 96) | 10 950 | 6700-15 900 | 5340 | 4040-7135 | .0001 |
| ESR (mm/h) (N = 81; 17 vs 64) | 41 | 26.6-60 | 12 | 8-34 | .001 |
| CRP (mg/L) (N = 81; 17 vs 64) | 18.3 | 12.1-138 | 16.3 | 5.6-89.8 | .028 |
Note. LDH = lactate dehydrogenase; IL-6 = interleukin-6; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
Hyponatremia was more prevalent in patients who died than in survivors (10 out of 17, 58.8% vs 40 out of 96, 41.7%, respectively), but there was no statistically significant difference. Although our data confirmed an elevated prevalence of hyponatremia in COVID-19 patients during their hospitalization and increased need for ICU admission and higher oxygen requirement in the hyponatremic group, we were unable to specifically relate hyponatremia state to patient outcome.
Discussion
Hyponatremia reported in patients with lower respiratory tract infections may be due to different underlying pathophysiological mechanisms. Recent studies have reported hyponatremia in association with COVID-19. The causes of hyponatremia in COVID-19 are multifactorial. It may be due to increased gastrointestinal loss (diarrhea, vomiting), decreased oral intake, or syndrome of inappropriate antidiuretic hormone secretion (SIADH). It can be hypervolemic, euvolemic, or hypovolemic hyponatremia. 5 Aggarwal et al 10 reported hyponatremia in 50% of the hospitalized COVID-19 patients in the United States, which is in concordance with our study (44.2%). This is similar to the incidence of hyponatremia seen in association with Legionella where it is much higher compared with other causes of CAP.13,14
It is important to establish the cause of hyponatremia early in the course of the disease to guide initial management. We analyzed the relevant clinical and laboratory parameters to evaluate the possible etiopathogenetic mechanism underlying hyponatremia in our COVID-19 patients. Various studies done earlier have shown that gastrointestinal loss and renal insufficiency are the most notable causes of sodium disorders.15,16
The consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup, after analyzing various studies, stated that COVID-19-associated acute kidney injury likely affects >20% of hospitalized patients and >50% of patients in the ICU. 17 Similar to these findings, in our study, patients with hyponatremia had higher levels of serum creatinine levels, and patients with gastrointestinal symptoms had more severe hyponatremia. Moreover, hyponatremic patients were much older, and 76.1% of our population had at least one comorbidity; this might have resulted in reduced renal reserve function and reduced the kidney’s ability to maintain electrolyte balance.
The total body water is more than the total body sodium ion that occurs in hypervolemic hyponatremia, which is seen in association with congestive cardiac failure, cirrhosis, and nephrotic syndrome. In a study by Hu et al, 5 there was a significant association between hyponatremia and hypertension and coronary artery disease. In our study, even though albumin levels were lower in the hyponatremic group, they were within the normal range in both groups, and there was no association between hyponatremia and hypertension or any other cardiac disease.
Bartter and Schwartz first described SIADH as a common complication of a wide range of clinical disorders. Viral pneumonia is one of the common causes of SIADH that can be broadly due to infections, tumors, pulmonary or central nervous system disorders, medications, or other causes such as inflammation and the post-operative state. A few case reports of COVID-19-associated SIADH have been documented. 18
SIADH in the setting of COVID-19 may be due to physical or psychological distress induced by disease, and it may be secondary to cytokine storm/IL-6-mediated non-osmotic release of antidiuretic hormone (ADH) secretion. 19 Berni et al 20 reported an inverse relation between sodium and IL-6 levels, and similarly, hyponatremia patients in our study had significantly higher levels of IL-6. The presence of hypouricemia with an elevated fractional excretion of urate can aid in the diagnosis. 21 In our patient series, the hyponatremic group had significantly lower uric acid levels than the normonatremic group.
The presence of tachypnea was independently associated with hyponatremia. Tachypnea increases insensible body fluid loss. In addition, difficulty in solid food and fluid intake increases due to tachypnea, which may be further worsened by the reduction in oral intake secondary to the anorexia described in few patients with COVID-19. This may explain the increased incidence of hyponatremia in COVID-19 patients. 22 In patients with COVID-19, both hyponatremia and acute kidney injury may be secondary to febrile illness and poor oral liquid intake. The intravascular volume loss in these patients further contributes to hyponatremia via two mechanisms—an increase in ADH secretion and elevated levels of angiotensin II which promotes thirst and the action of aquaporin-2 channel-mediated water retention.
From these findings, we propose that SIADH and hypovolemic hyponatremia may be most likely the mechanisms of hyponatremia in COVID-19 patients. Hypovolemic hyponatremia should be distinguished from SIADH as these conditions require different management strategies, and urinary sodium, serum uric acid, and urine osmolality are needed to differentiate between these two.
Glatstein et al 23 reported a strong correlation between the consolidation of lung and hyponatremia, and a similar finding was reported by Natarajan et al 24 in a prospective observational study conducted in the southern part of India. A similar correlation between the consolidation of the lung on CT Chest and the presence of hyponatremia was observed in our study. Hyponatremia was mostly mild (64%), and elderly and males were more commonly affected. In our study, hyponatremia appears to be associated with poor outcome (ICU admission, higher levels of oxygen requirement) in COVID-19 patients. This is similar to previous studies regarding COVID-19.21,22
Khinda et al, 25 in a meta-analysis, noted strong associations between markers of inflammation (IL-6, ferritin) and tissue damage (LDH), with severe and fatal diseases. Another meta-analysis by Ji et al 26 noted elevated levels of IL-6 in COVID-19 patients who died than in those who survived. A similar observation was noted in our study; patients requiring ICU admission and patients with poor outcome had significantly elevated ferritin, LDH, and IL-6 levels.
Other inflammatory markers like ESR, CRP, and leukocytosis were significantly higher in those requiring ICU admission and in deceased patients. CRP was significantly higher in hyponatremic group. A meta-analysis of 56 studies involving 8719 COVID-19 patients showed higher levels of WBC, ESR, CRP, and IL-6, similar to the findings in our study. 26
Assessment of these inflammatory markers at admission helps in both understanding of the disease mechanisms and predicting severe illness, thus helping us in the identification of patients who are likely to benefit from early interventions. A few of these pathways may likely also be ideal targets for therapy, such as the IL-6 inhibitor tocilizumab to target inflammation. 26 Our results suggest that evaluation of sodium levels and inflammatory markers at the time of admission may serve as early warning signs for progression to severe COVID-19.
Limitations
Only hospitalized individuals were included in the study. Therefore, our data did not represent all COVID-19 patients. As the study was of a retrospective and observational design, we only examined sodium levels at the time of admission. We did not follow the results for the following days, so our findings are based on the initial lab results. Serum osmolality, urine electrolytes, and urine osmolality were not consistently available in our patient data.
Conclusions
Our study revealed that demography, clinical features, radiographic findings, complications like renal insufficiency, and inflammatory markers like IL-6 play a considerable role in hyponatremic COVID-19 patients. Hyponatremia patients required significantly higher rates of ICU admissions and oxygen support. Our results also suggest that monitoring inflammatory markers such as ESR, CRP, total WBC count, ferritin, LDH, and IL-6 may serve as an early warning system for progression to severe COVID-19. In conclusion, serum sodium imbalance is a common finding associated with severe illness and increased hospitalization in COVID-19 patients.
Acknowledgments
The authors acknowledge Dr Venkatachalam, Director of Medical Services; Dr Muralidharan M, Director of Medical Education; and all consultants in the Department of General Medicine, Apollo Hospitals, Chennai, and Mr Balasubramaniam, Senior Biostatistician, Apollo Hospitals, Chennai, Tamil Nadu, for their constant support.
Footnotes
Ethics Approval and Consent to Participate: The Institutional Ethical Committee Bio Medical Research, Apollo Hospitals, Chennai, approved the protocol (AMH-C-S-050/12-20, December 14, 2020). As all data were anonymously used and this study does not contain protected health information, the ethics committee approved a waiver of the requirement for informed consent.
Consent for Publication: The Institutional Ethics Committees approved the study protocol. Informed consent was waived off due to the retrospective nature of the study.
Availability of Data and Materials: The data supporting the findings of this article is not publically available. Questions about the data can be directed to the corresponding author.
Author Contributions: All authors contributed equally for the manuscript.
All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Phani Krishna Machiraju  https://orcid.org/0000-0002-9721-6996
https://orcid.org/0000-0002-9721-6996
Neetu Mariam Alex  https://orcid.org/0000-0002-8992-5784
https://orcid.org/0000-0002-8992-5784
References
- 1. https://covid19.who.int/table. Accessed on April 20, 2021.
- 2. Filipovic N, Saveljic I, Hamada K, Tsuda A. Abrupt deterioration of COVID-19 patients and spreading of SARS COV-2 virions in the lungs. Ann Biomed Eng. 2020;48(12):2705-2706. doi: 10.1007/s10439-020-02676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98(4):463-471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 5. Hu W, Lv X, Li C, et al. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Intern Emerg Med. 2021;16:853-862. doi: 10.1007/s11739-020-02515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rondon-Berrios H, Agaba EI, Tzamaloukas AH. Hyponatremia: pathophysiology, classification, manifestations and management. Int Urol Nephrol. 2014;46(11):2153-2165. [DOI] [PubMed] [Google Scholar]
- 7. Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119:S30-S35. [DOI] [PubMed] [Google Scholar]
- 8. Królicka AL, Kruczkowska A, Krajewska M, Kusztal MA. Hyponatremia in infectious diseases: a literature review. Int J Environ Res Public Health. 2020;17(15):5320. doi: 10.3390/ijerph17155320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stormont JM, Waterhouse C. Severe hyponatremia associated with pneumonia. Metabolism. 1962;11:1181-1186. [PubMed] [Google Scholar]
- 10. Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis. 2020;7(2):91-96. [DOI] [PubMed] [Google Scholar]
- 11. Nair V, Niederman MS, Masani N, Fishbane S. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007;27:184-190. doi: 10.1159/000100866. [DOI] [PubMed] [Google Scholar]
- 12. Zilberberg MD, Exuzides A, Spalding J, et al. Hyponatremia and hospital outcomes among patients with pneumonia: a retrospective cohort study. BMC Pulm Med. 2008;8:16. doi: 10.1186/1471-2466-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schuetz P, Haubitz S, Christ-Crain M, Albrich WC, Zimmerli W, Mueller B; ProHOSP Study Group. Hyponatremia and antidiuretic hormone in Legionnaires’ disease. BMC Infect Dis. 2013;13:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiumefreddo R, Zaborsky R, Haeuptle J, et al. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulm Med. 2009;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reynolds RM, Padfield PL, Seckl JR. Disorders of sodium balance. BMJ (Clin Res Ed). 2006;332(7543):702-705. doi: 10.1136/bmj.332.7543.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterns RH. Disorders of plasma sodium-causes, consequences, and correction. N Engl J Med. 2015;372(1):55-65. doi: 10.1056/NEJMra1404489. [DOI] [PubMed] [Google Scholar]
- 17. Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16:747-764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ravioli S, Niebuhr N, Ruchti C, Pluess E, Stoeckli T, Lindner G. The syndrome of inappropriate antidiuresis in COVID19 pneumonia: report of two cases. Clin Kidney J. 2020;13(3):461-462. doi: 10.1093/ckj/sfaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diaconu C. COVID-19 and hyponatremia. Arch Balk Med Union. 2020;55:373-374. doi: 10.31688/ABMU.2020.55.3.01. [DOI] [Google Scholar]
- 20. Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Invest. 2020;43(8):1137-1139. doi: 10.1007/s40618-020-01301-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem. 2020;57(3):262-265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, et al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. a HOPE-COVID-19 (Health Outcome Predictive Evaluation for COVID-19) registry analysis. Front Endocrinol (Lausanne). 2020;11:599255. doi: 10.3389/fendo.2020.599255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glatstein M, Rozen R, Scolnik D, et al. Radiologic predictors of hyponatremia in children hospitalized with community-acquired pneumonia. Pediatr Emerg Care. 2012;28(8):764-766. doi: 10.1097/PEC.0b013e3182624b98. [DOI] [PubMed] [Google Scholar]
- 24. Natarajan T, Sadique TNS, Shanmugham K. Incidence of hyponatremia and its utility as an indicator of morbidity in children hospitalized with community acquired pneumonia. Int J Contemp Pediatr. 2020;7:616. [Google Scholar]
- 25. Khinda J, Janjua NZ, Cheng S, van den Heuvel ER, Bhatti P, Darvishian M. Association between markers of immune response at hospital admission and COVID-19 disease severity and mortality: a meta-analysis and meta-regression. J Med Virol. 2021;93(2):1078-1098. doi: 10.1002/jmv.26411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ji P, Zhu J, Zhong Z, et al. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine (Baltimore). 2020;99(47):e23315. doi: 10.1097/MD.0000000000023315. [DOI] [PMC free article] [PubMed] [Google Scholar]