Abstract
Non-convulsive seizures (NCSs) are highly treatable, but appropriate management is usually delayed because of inaccurate diagnoses as a result of variable clinical presentations, including an altered mental state. It is difficult to detect NCSs in patients with dementia. We report a case of NCS superimposed on cognitive decline caused by Alzheimer’s dementia. The patient’s history was carefully recorded. An electroencephalogram was recorded with sphenoidal electrodes, which showed epileptiform discharges in the right mesial temporal lobe and focal, sharply contoured, slow wave activity in the left fronto-temporal area, suggesting an epileptic origin contributing to the patient’s cognitive decline. After treatment with antiepileptic drugs, the patient’s cognitive functioning gradually improved. An accurate diagnosis of NCS relies on performing a detailed inventory of a patient’s history, thorough physical and neurological examinations, and electroencephalogram recordings. In patients with cognitive decline, testing for NCS should always be included in the differential diagnosis of cognitive impairment, even in the case of dementia. Early administration of antiepileptic drug therapy is the mainstay treatment for reversing the condition and for preventing prolonged insults from neurological sequelae.
Keywords: Non-convulsive seizure, dementia, cognitive decline, case report, epilepsy, electroencephalography
Introduction
Dementia is a clinical syndrome, usually of a chronic or progressive nature, in which deterioration in cognitive functioning occurs beyond that expected as a result of normal aging. This mental health condition can affect memory, thinking, orientation, comprehension, calculation, learning capacity, language, and judgement. 1 In contrast, epilepsy is characterized by recurrent seizures as a result of excessive electrical discharges in a group of brain cells. It is important to note that several factors contribute to cognitive impairment in patients with epilepsy, including trait-dependent factors, such as the type of epilepsy and the presence of structural brain damage, as well as state-dependent factors, such as interictal epileptiform discharges recorded by electroencephalography (EEG).
It has recently been reported that patients with dementia are at higher risk of generalized or focal seizures than patients without dementia. 2 Patients who experience non-convulsive seizures (NCSs) more often than other types of seizures frequently present with cognitive decline, and the diagnosis of NCS is usually delayed because of its variable clinical presentations. 3 Such seizures can co-exist with dementia and may either precede or follow its onset,4,5 which is suggestive of a probable bidirectional relationship between seizure activity and dementia. 6 Furthermore, a recent study showed that epileptiform abnormalities on EEG are common in Alzheimer’s disease (AD), 7 but they are not all equivalent. In this study, we report the case of a patient with NCSs superimposed on dementia. In patients with cognitive decline, testing for NCSs should always be included in the differential diagnosis of cognitive impairment, even in the case of dementia.
Case report
The patient provided verbal informed consent for this case report to be published, and the reporting of this study conforms to the CARE guidelines. 8 Institutional review board approval was not required for a descriptive study of a single case. A 62-year-old man with underlying hypertension was admitted to the Neurology Department of National Cheng Kung University Hospital to investigate memory impairment and topographic disorientation that had persisted for approximately 1 year. A bedside neurological examination yielded unremarkable results, with the exception of elevated cortical functioning. Both the Mini-Mental State Examination (MMSE) and the Cognitive Abilities Screening Instrument (CASI) showed abnormalities in cognitive functioning. The total MMSE score was 25/30, and that of the CASI was 80/100 (remote memory = 10/10; recent memory = 11/12; attention = 6/8; mental manipulation = 5/10; orientation = 18/18; abstract thinking = 7/12; language = 10/10; drawing = 8/10; and verbal fluency = 5/10). Finally, the initial awake EEG results were essentially normal, and magnetic resonance imaging of the brain showed changes to the non-specific white matter in the bilateral periventricular regions.
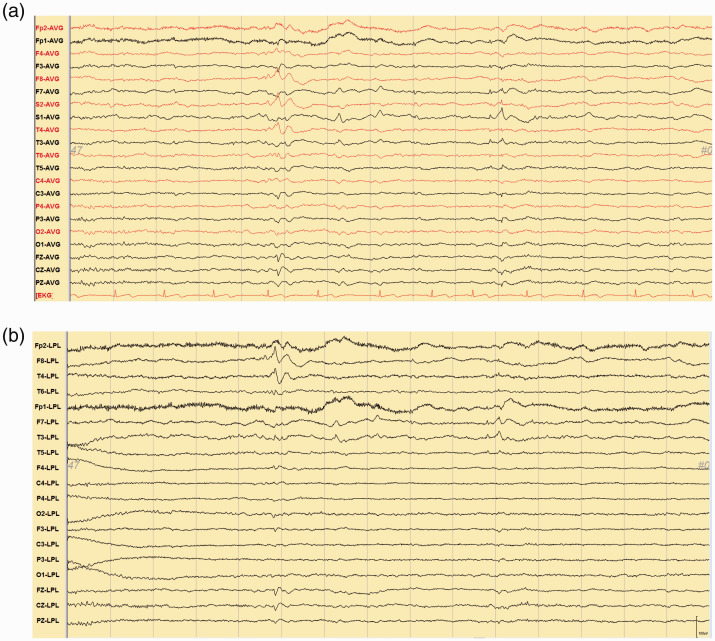
Following the initial examination, the patient was diagnosed with probable AD with or without vascular dementia, and drugs were prescribed (10 mg of Aricept and 20 mg of nicergoline per day). During a follow-up examination at our dementia outpatient clinic, one episode of confusion and vacant staring followed by post-event amnesia were reported by the patient’s daughter. In addition, two episodes of sudden “dullness” during conversation lasting for 10 minutes accompanied with a nonsensical smile and delayed and incoherent responses had been observed in previous months. Further investigations were then scheduled. EEG with sphenoidal electrodes showed paroxysmal spikes and sharpish waves over the right mesial temporal lobe and sharply contoured theta waves over the left temporal lobe (See Figure 1a and b). A diagnosis of dementia with epileptiform discharges was then recorded, with the manifestation of NCSs. Interictal single-photon emission computed tomography revealed mild hypoperfusion in the bilateral temporoparietal areas.
Figure 1.
(a) Electroencephalogram with sphenoidal electrodes in a common average referential montage showed paroxysmal spikes and sharply contoured wave activity over the right mesial temporal region and focal, sharply contoured theta waves over the left temporal area. (b) The Laplacian montage showed high amplitude localized spikes at F8 and T4 and low to medium amplitude localized sharp waves at F7 and T3.
AVG: average; EKG: electrocardiography; LPL: Laplacian.
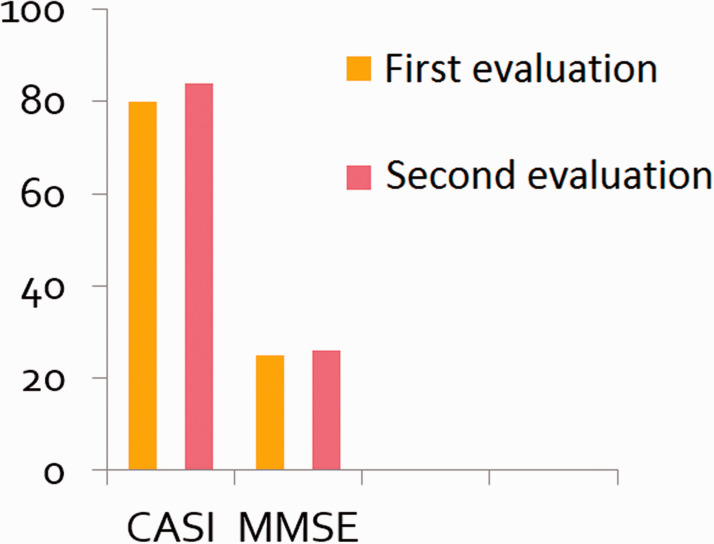
On the basis of the clinical presentation of this patient and the epileptiform discharges recorded on EEG, an epileptic origin of the NCSs was suspected. An initial dose of 300 mg per day of oxcarbazepine (Trileptal, 300 mg/tablet), to be taken orally, was prescribed, followed by a maintenance dose of 600 mg per day. The frequency of the patient’s seizure events decreased substantially, but he developed hyponatremia, with blood sodium levels of 128 mEq2/L, 1 month later. The use of oxcarbazepine was maintained, but at a reduced dose of 300 mg per day, and we added 2 mg of perampanel at bedtime, which was later increased to 4 mg following titration because of the epileptiform discharges and his poor sleep maintenance. Afterwards, he exhibited significant memory improvements, and no additional similar events were reported 1 month later. Follow-up EEG was performed 3 months after the addition of perampanel, which revealed only a few small, sharply contoured waves in the left temporal area. Six months later, follow-up MMSE and CASI assessments produced total scores of 26/30 and 84/100, respectively. The CASI score was as follows: remote memory = 10/10; recent memory = 10/12; attention = 7/8; mental manipulation = 6/10; orientation = 16/18; abstract thinking = 8/12; language = 10/10; drawing = 10/10; and verbal fluency = 7/10. These scores show a mild improvement in cognitive performance (see Figure 2). At the time of this follow-up cognitive evaluation, no further seizure events similar to those that prompted this treatment course had been observed.
Figure 2.
Follow-up Mini-Mental State Examination (MMSE) and Cognitive Abilities Screening Instrument (CASI) assessments showed improvements in cognitive performance scores after antiepileptic drug therapy.
Discussion
NCSs typically involve an absence of major motor symptoms (such as involuntary tonic-clonic movements of the limbs) and have a diverse spectrum of clinical presentations, including an altered mental state, changes in behavior, subtle motor signs, speech disturbances, autonomic dysfunction, and changes in the sensorium. 3 Among these symptoms, NCS-related behavioral changes typically occur in the form of a paroxysmal event accompanied by an altered sensorium, 5 as observed in our case study. This type of seizure event has been found to resemble transient global amnesia, and therefore, subtle interictal autobiographical memory loss may be observed.9,10 In addition, a patient presenting with insidious onset of cognitive decline may actually be suffering from temporal lobe epilepsy, which thus mimics dementia.11,12 In this specific situation, EEG is the key to confirming the diagnosis. Therefore, in the case under consideration in the present study, NCS was diagnosed through a detailed recording of the patient’s medical history, physical and neurological examinations, and the use of EEG with sphenoidal electrodes.
Patients with mild to moderate AD have been reported to have an 8% to 18% incidence rate of seizures. 13 AD not only leads to an increased risk of developing epilepsy, 14 but a recent study revealed that patients with AD also show significantly greater subclinical epileptiform activity. 15 Furthermore, it has also been reported that up to 42% of patients with AD monitored using overnight video-EEG showed evidence of epileptiform activity. 16 In our opinion, epileptiform activity recorded by EEG is suggestive of neuronal hyperexcitability, which is consistent with the findings of the present case.
Cognitive impairment is an important issue in patients with epilepsy because multiple factors contribute to their cognitive performance.1,17 Although several models predict cognitive worsening in patients with epilepsy, 15 we have previously reported that patients with cryptogenic epilepsy exhibiting declines in cognitive performance showed significant improvements in performance after adequate antiepileptic drug (AED) treatment. This finding suggests that AED treatments may be helpful in improving cognitive impairment in patients with cryptogenic epilepsy. 18 Despite the higher risk of developing epilepsy associated with AD and other forms of dementia,19–20 the excellent response of seizure activity to AEDs supports this conclusion. 21
The clinical course of neurodegenerative diseases such as dementia generally progresses slowly, requiring regular follow-up evaluations at neurology outpatient departments, including laboratory workups, cognitive assessments, and imaging studies. Patients with dementia complicated with epileptic seizures require increased attention and medical care because unexpected injuries may occur during motor seizures or NCSs, 22 which may impair awareness and arousal. Furthermore, the existence of epileptic seizures increases the long-term burden on caregivers.
In conclusion, our case study showed that NCSs can occur in patients with dementia. The early identification of NCS relies on performing a detailed inventory of a patient’s history, thorough physical and neurological examinations, and EEG recordings. Furthermore, the timely administration of AED therapy may improve the prognosis by minimizing epileptiform discharges and their associated cognitive impairment. Finally, teaching caregivers how to detect subtle abnormalities in a patient is as important as relying solely on the professional observations of a well-trained medical staff.
This case report was presented at the Annual Meeting of the Taiwan Neurological Society in 2019.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported in part by grants from the Ministry of Science and Technology, Taiwan (109-2314-B-006-034-MY3).
ORCID iD: Chin-Wei Huang https://orcid.org/0000-0003-3335-2187
References
- 1.Aldenkamp AP. Effects of antiepileptic drugs on cognition. Epilepsia 2001; 42: 46–49. [DOI] [PubMed] [Google Scholar]
- 2.Habeych ME, Falcone T, Dagar A, et al. Dementia, subtype of seizures, and the risk of new onset seizures: a cohort study of a national US managed care database. J Alzheimers Dis 2021; 81: 973–980. [DOI] [PubMed] [Google Scholar]
- 3.Kinney MO Craig JJ andKaplan P.. Non-convulsive status epilepticus: mimics and chameleons. Pract Neurol 2018; 18: 291–305. [DOI] [PubMed] [Google Scholar]
- 4.Sarkis RA, Dickerson BC, Cole AJ, et al. Clinical and neurophysiologic characteristics of unprovoked seizures in patients diagnosed with dementia. J Neuropsychiatry Clin Neurosci 2016; 28: 56–61. [DOI] [PubMed] [Google Scholar]
- 5.Boylan LS. Peri-ictal behavioral and cognitive changes. Epilepsy Behav 2002; 3: 16–26. [DOI] [PubMed] [Google Scholar]
- 6.Hesdorffer D, Hauser W, Annegers J, et al. Dementia and adult-onset unprovoked seizures. Neurology 1996; 46: 727–730. [DOI] [PubMed] [Google Scholar]
- 7.Lam AD, Sarkis RA, Pellerin KR, et al. Association of epileptiform abnormalities and seizures in Alzheimer disease. Neurology 2020; 95: e2259–e2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus‐based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 9.Zeman AZ Boniface SJ andHodges JR.. Transient epileptic amnesia: a description of the clinical and neuropsychological features in 10 cases and a review of the literature. J Neurol Neurosurg Psychiatry 1998; 64: 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuilleumier P Despland PA andRegli F.. Failure to recall (but not to remember): pure transient amnesia during nonconvulsive status epilepticus. Neurology 1996; 46: 1036–1039. [DOI] [PubMed] [Google Scholar]
- 11.Li BY andChen SD.. Potential similarities in temporal lobe epilepsy and Alzheimer’s disease: from clinic to pathology. Am J Alzheimers Dis Other Demen 2015; 30: 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tombini M, Koch G, Placidi F, et al. Temporal lobe epileptic activity mimicking dementia: a case report. Eur J Neurol 2005; 12: 805–806. [DOI] [PubMed] [Google Scholar]
- 13.Amatniek JC, Hauser WA, DelCastillo‐Castaneda C, et al. Incidence and predictors of seizures in patients with Alzheimer's disease. Epilepsia 2006; 47: 867–872. [DOI] [PubMed] [Google Scholar]
- 14.Imfeld P, Bodmer M, Schuerch M, et al. Seizures in patients with Alzheimer’s disease or vascular dementia: a population‐based nested case–control analysis. Epilepsia 2013; 54: 700–707. [DOI] [PubMed] [Google Scholar]
- 15.Sen A Capelli V andHusain M.. Cognition and dementia in older patients with epilepsy. Brain 2018; 141: 1592–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vossel KA, Beagle AJ, Rabinovici GD, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 2013; 70: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mula M. Cognitive dysfunction in patients with epilepsy: focus on clinical variables. Future Neurol 2015; 10: 41–48. [Google Scholar]
- 18.Huang CW, Hsieh YJ, Tsai JJ, et al. Cognitive performance in cryptogenic epilepsy. Acta Neurol Scand 2005; 112: 228–233. [DOI] [PubMed] [Google Scholar]
- 19.Sherzai D, Losey T, Vega S, et al. Seizures and dementia in the elderly: Nationwide Inpatient Sample 1999–2008. Epilepsy Behav 2014; 36: 53–56. [DOI] [PubMed] [Google Scholar]
- 20.Helmstaedter C andWitt JA.. Epilepsy and cognition–a bidirectional relationship? Seizure 2017; 49: 83–89. [DOI] [PubMed] [Google Scholar]
- 21.Rao SC, Dove G, Cascino GD, et al. Recurrent seizures in patients with dementia: frequency, seizure types, and treatment outcome. Epilepsy Behav 2009; 14: 118–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin J andScharfman HE.. Shared cognitive and behavioral impairments in epilepsy and Alzheimer's disease and potential underlying mechanisms. Epilepsy Behav 2013; 26: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]