Abstract
Background
The emergence of the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 and the reduced effectiveness over time of the BNT162b2 vaccine (Pfizer–BioNTech) led to a resurgence of coronavirus disease 2019 (Covid-19) cases in populations that had been vaccinated early. On July 30, 2021, the Israeli Ministry of Health approved the use of a third dose of BNT162b2 (booster) to cope with this resurgence. Evidence regarding the effectiveness of the booster in lowering mortality due to Covid-19 is still needed.
Methods
We obtained data for all members of Clalit Health Services who were 50 years of age or older at the start of the study and had received two doses of BNT162b2 at least 5 months earlier. The mortality due to Covid-19 among participants who received the booster during the study period (booster group) was compared with that among participants who did not receive the booster (nonbooster group). A Cox proportional-hazards regression model with time-dependent covariates was used to estimate the association of booster status with death due to Covid-19, with adjustment for sociodemographic factors and coexisting conditions.
Results
A total of 843,208 participants met the eligibility criteria, of whom 758,118 (90%) received the booster during the 54-day study period. Death due to Covid-19 occurred in 65 participants in the booster group (0.16 per 100,000 persons per day) and in 137 participants in the nonbooster group (2.98 per 100,000 persons per day). The adjusted hazard ratio for death due to Covid-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% confidence interval, 0.07 to 0.14; P<0.001).
Conclusions
Participants who received a booster at least 5 months after a second dose of BNT162b2 had 90% lower mortality due to Covid-19 than participants who did not receive a booster.
The deployment of the BNT162b2 messenger RNA vaccine (Pfizer–BioNTech) and other vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) resulted in a significant decrease in mortality due to coronavirus disease 2019 (Covid-19).1 Israel deployed the vaccines rapidly, and accordingly, the incidence of Covid-19 dropped from almost 1000 cases per 1 million persons per day in January 2021 to 1 to 2 cases per 1 million persons per day in June 2021.2 However, the emergence of the B.1.617.2 (delta) variant3 and the reduced efficacy of BNT162b2 over time4 led to a resurgence of Covid-19 cases in Israel5 and in other populations that had been vaccinated early, such as the U.S. health care workforce.6 By August 2021, Israel had the highest incidence of Covid-19 worldwide.2 On July 30, 2021, the Israeli Ministry of Health approved the use of a third dose of BNT162b2 (booster). The booster was initially indicated for use in persons 60 years of age or older who had received a second dose at least 5 months earlier. Two weeks later, the age of eligibility was lowered to 50 years. This local regulatory approval was made despite the lack of robust evidence of efficacy and the absence of regulatory approval by the Food and Drug Administration and by the European Medicines Agency.
A global debate regarding the approval of Covid-19 vaccine boosters is ongoing.7 Data regarding the effectiveness of the BNT162b2 booster in lowering mortality due to Covid-19 are still unavailable in all age groups. Therefore, our objective was to assess for any decrease in mortality associated with the use of the BNT162b2 booster.
Methods
Study Design
The study period started on August 6, 2021, which was 7 days after the approval of the booster for use in persons 60 years of age or older in Israel. The study period ended on September 29, 2021, which was the last date for which data regarding confirmed deaths due to Covid-19 were available on the day the data were extracted (October 3, 2021). The study timeline is depicted in Figure S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
The Clalit Health Services (CHS) Community Helsinki Committee and the CHS Data Utilization Committee approved the study. The study was exempt from the requirement to obtain informed consent.
Study Population
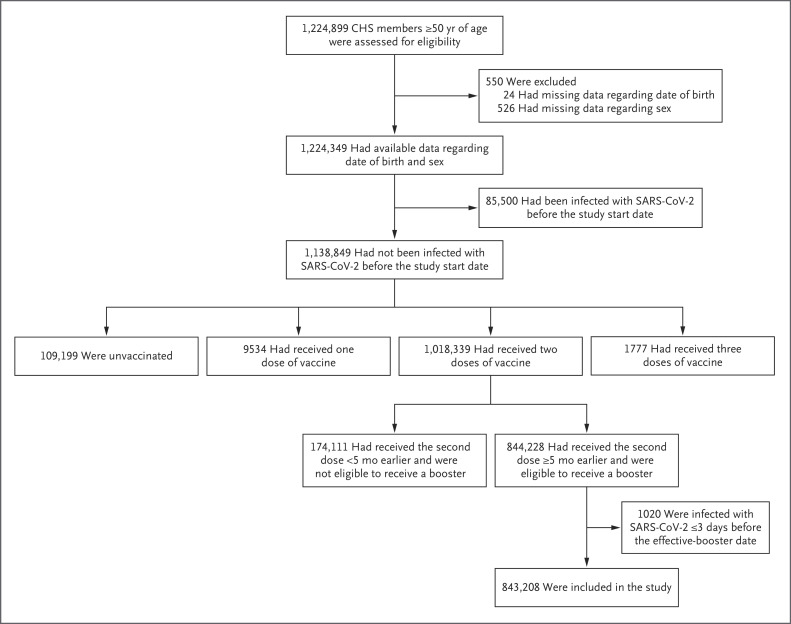
The study included all CHS members who were 50 years of age or older on the study start date and had received two doses of BNT162b2 at least 5 months earlier. CHS covers approximately 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care. Participants with missing data regarding date of birth or sex were excluded from the study. In addition, participants were excluded if they had been infected with SARS-CoV-2 or had received a booster before August 6, 2021; early administration of the booster was indicated in immunocompromised persons. Finally, participants who received the booster and had a confirmed case of Covid-19 within 3 days before the effective-booster date (defined as 7 days after the booster was administered) were excluded.
The study population was divided into two groups: those who had received a booster during the study period (booster group) and those who had not received a booster (nonbooster group). Participants were included in the booster group on the effective-booster date to allow time for antibodies to build effectively.4,8 Up to 7 days after receiving the booster, participants were still included in the nonbooster group. A description of the transition of participants from the nonbooster group to the booster group is provided in Figure S2.
Data Sources and Organization
We analyzed patient-level data that were extracted from CHS electronic medical records. A specific database was created for this study that integrated patient-level data from two primary sources: the CHS operational database and the CHS Covid-19 database. The CHS operational database includes sociodemographic data and comprehensive clinical information, such as coexisting chronic conditions, community-care visits, hospitalizations, medications, and results of laboratory tests and imaging studies. The CHS Covid-19 database includes information that is collected centrally by the Israeli Ministry of Health and transferred daily to CHS, such as vaccination dates, reverse-transcriptase–quantitative polymerase-chain-reaction (RT-qPCR) test dates and results, and hospitalizations and deaths related to Covid-19.
The CHS databases were used in the primary studies that evaluated the effectiveness1 and safety9 of the BNT162b2 vaccine in a real-world setting. In addition, the Israeli Ministry of Health Covid-19 database was used as the basis of the initial study that evaluated the effectiveness of the BNT162b2 booster among persons 60 years of age or older.10 A description of the CHS data repositories that were used in this study is provided in the Supplementary Appendix.
For each participant in the study, the following sociodemographic data were extracted: age, sex, population sector (general Jewish population, Arab population, or ultra-Orthodox Jewish population), and score for socioeconomic status (scores range from 1 [lowest] to 10 [highest]; details are provided in the Supplementary Appendix). The following clinical data were extracted: vaccination dates (first, second, and booster doses), RT-qPCR test dates and results, death due to Covid-19, and any clinical risk factors for death due to Covid-19 that have been identified in the general population,11 such as diabetes mellitus, chronic obstructive pulmonary disease, asthma, chronic kidney failure, hypertension, ischemic heart disease, chronic heart failure, obesity, lung cancer, or a history of cerebrovascular accident, transient ischemic attack, or smoking.
Study Outcomes
The primary outcome was death due to Covid-19. In the primary analysis of the effectiveness of the booster with respect to this outcome, we compared the mortality due to Covid-19 in the booster group with that in the nonbooster group.
Because the initial approval of the booster by the Food and Drug Administration was for use in persons 65 years of age or older, we performed a subgroup analysis according to age group. We performed an additional subgroup analysis according to sex.
In a secondary analysis of the effectiveness of the booster in preventing SARS-CoV-2 infection, we compared the frequency of positive RT-qPCR tests in the booster group with that in the nonbooster group.
Statistical Analysis
A chi-square test was used to compare categorical variables according to study group. Given that the independent variable (booster status) varied over time, univariate and multivariate survival analyses were performed with time-dependent covariates, in accordance with the study design.12 A Kaplan–Meier analysis with a log-rank test was used for the univariate analysis. Comparison of the survival curves and Schoenfeld’s global test were used to test the proportional-hazards assumption for each dependent variable. Variables that met the testing criteria served as inputs for multivariate regression analysis.
A Cox proportional-hazards regression model with time-dependent covariates was used to estimate the association of booster status with death due to Covid-19. The regression model was used to estimate the hazard ratio for death due to Covid-19 in the booster group, as compared with the nonbooster group, with the use of sociodemographic and baseline clinical characteristics as independent variables.
The assumption of a 7-day lag time between the administration of the booster and the effective-booster date, during which participants were included in the nonbooster group, was further tested to verify that this grouping did not create any bias. Validation of the lag time used to ensure booster effectiveness was performed through estimation of the hazard ratio for death due to Covid-19 in participants up to 7 days after the administration of the booster, as compared with the nonbooster group. Use of an alternative 14-day lag time was also tested with the same method.
R statistical software, version 3.5.0 (R Foundation for Statistical Computing), was used for the univariate and multivariate survival analyses with time-dependent covariates. SPSS software, version 26 (IBM), was used for all other statistical analyses. A P value of less than 0.05 was considered to indicate significance in all analyses.
Results
Patient Population
A total of 843,208 participants met the eligibility criteria (Figure 1). The characteristics of the study population are shown in Table 1. The mean age was 68.5 years; 60% of the participants were 65 years of age or older. The most common coexisting conditions were hypertension (46%), obesity (33%), and diabetes (29%). For most sociodemographic and clinical characteristics, the difference between the booster group and the nonbooster group was significant.
Figure 1. Assessment for Eligibility.
CHS denotes Clalit Health Services, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
Table 1. Characteristics of the Participants at Baseline.*.
| Characteristic | All Participants (N=843,208) | Booster (N=758,118) | No Booster (N=85,090) | P Value |
|---|---|---|---|---|
| Age — yr | 68.5±10.6 | 68.9±10.5 | 64.8±10.9 | <0.001 |
| Age group — no. (%) | ||||
| ≥65 yr | 506,016 (60) | 470,808 (62) | 35,208 (41) | <0.001 |
| 50–64 yr | 337,192 (40) | 287,310 (38) | 49,882 (59) | <0.001 |
| Female sex — no. (%) | 448,272 (53) | 400,300 (53) | 47,972 (56) | <0.001 |
| Population sector — no. (%) | ||||
| General Jewish population | 732,493 (87) | 674,266 (89) | 58,227 (68) | <0.001 |
| Arab population | 86,162 (10) | 62,042 (8) | 24,120 (28) | <0.001 |
| Ultra-Orthodox Jewish population | 24,297 (3) | 21,633 (3) | 2,664 (3) | <0.001 |
| Unknown | 256 (<1) | — | — | — |
| Score for socioeconomic status — median (SD)† | 5.9 (2.2) | 6 (2.2) | 4.8 (2.2) | <0.001 |
| Clinical risk factors — no. (%) | ||||
| Diabetes | 244,746 (29) | 220,959 (29) | 23,787 (28) | <0.001 |
| Chronic obstructive pulmonary disease | 41,449 (5) | 37,291 (5) | 4,158 (5) | 0.68 |
| Asthma | 51,360 (6) | 46,198 (6) | 5,162 (6) | 0.75 |
| Chronic kidney failure | 51,636 (6) | 47,187 (6) | 4,449 (5) | <0.001 |
| Hypertension | 391,654 (46) | 358,517 (47) | 33,137 (39) | <0.001 |
| Ischemic heart disease | 142,742 (17) | 131,058 (17) | 11,684 (14) | <0.001 |
| Chronic heart failure | 37,297 (4) | 33,524 (4) | 3,773 (4) | 0.87 |
| Obesity | 278,097 (33) | 249,152 (33) | 28,945 (34) | <0.001 |
| Lung cancer | 5,661 (1) | 5,132 (1) | 529 (1) | 0.06 |
| History of cerebrovascular accident | 60,343 (7) | 54,328 (7) | 6,015 (7) | 0.30 |
| History of transient ischemic attack | 29,145 (3) | 26,586 (4) | 2,559 (3) | <0.001 |
| History of smoking | 348,654 (41) | 314,226 (41) | 34,428 (40) | <0.001 |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding.
Scores for socioeconomic status range from 1 (lowest) to 10 (highest).
Primary Outcome
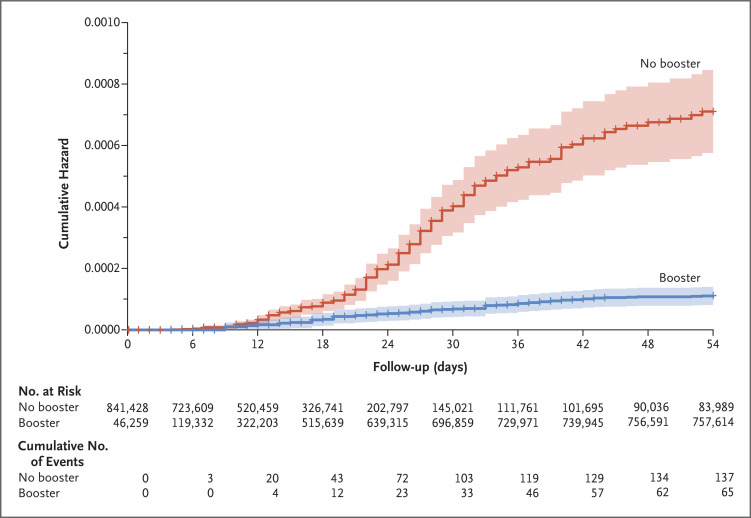
During the study period, death due to Covid-19 occurred in 65 participants in the booster group (0.16 per 100,000 persons per day) and in 137 participants in the nonbooster group (2.98 per 100,000 persons per day). The adjusted hazard ratio for death due to Covid-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% confidence interval [CI], 0.07 to 0.14; P<0.001). Cumulative hazard-ratio curves are shown in Figure 2. At the end of the study period, 758,118 participants (90%) had received the booster.
Figure 2. Cumulative Hazard Ratio for Death Due to Covid-19.
The shaded area indicates the 95% confidence interval. Covid-19 denotes coronavirus disease 2019.
The results of the Cox proportional-hazards regression model with time-dependent covariates are shown in Table 2. The model included only variables that met the criteria for the proportional-hazards assumption on the basis of the results of Schoenfeld’s global test (Table S1). Therefore, population sector, asthma, and hypertension were not incorporated into the model. In the Cox regression model, age, male sex, chronic kidney failure, lung cancer, and history of cerebrovascular accident were confounding variables that had a significant association with death due to Covid-19. Socioeconomic status, diabetes, chronic obstructive pulmonary disease, ischemic heart disease, chronic heart failure, obesity, history of transient ischemic attack, and history of smoking did not have a significant association with death due to Covid-19.
Table 2. Association of Confounding Variables with Death Due to Covid-19.*.
| Variable | Hazard Ratio for Death Due to Covid-19 (95% CI) | P Value |
|---|---|---|
| Booster received | 0.10 (0.07–0.14) | <0.001 |
| Age | 1.10 (1.09–1.12) | <0.001 |
| Male sex | 2.49 (1.82–3.41) | <0.001 |
| Socioeconomic status | 0.98 (0.92–1.04) | 0.45 |
| Diabetes | 1.29 (0.96–1.72) | 0.09 |
| Chronic obstructive pulmonary disease | 1.31 (0.86–1.99) | 0.22 |
| Chronic kidney failure | 2.27 (1.63–3.15) | <0.001 |
| Ischemic heart disease | 0.96 (0.69–1.32) | 0.79 |
| Chronic heart failure | 1.41 (0.95–2.09) | 0.09 |
| Obesity | 1.17 (0.87–1.58) | 0.30 |
| Lung cancer | 3.20 (1.49–6.87) | 0.003 |
| History of cerebrovascular accident | 1.54 (1.08–2.17) | 0.02 |
| History of transient ischemic attack | 0.87 (0.50–1.51) | 0.63 |
| History of smoking | 1.10 (0.82–1.49) | 0.52 |
Age was a continuous variable, and socioeconomic status was an ordinal variable; all other variables were dichotomous (present vs. absent). Covid-19 denotes coronavirus disease 2019.
Validation of the 7-Day Lag Time to Ensure Booster Effectiveness
The hazard ratio for death due to Covid-19 in participants up to 7 days after the administration of the booster, as compared with the nonbooster group, was 0.95 (95% CI, 0.86 to 1.05; P=0.32). However, the hazard ratio for death due to Covid-19 in participants up to 14 days after the administration of the booster, as compared with the nonbooster group, was 0.67 (95% CI, 0.60 to 0.74; P<0.001). Therefore, our assumption of a 7-day lag time between the administration of the booster and booster effectiveness was confirmed. Details of the results of these analyses are provided in Tables S2 and S3.
Subgroup Analysis
Among participants 65 years of age or older, death from Covid-19 occurred in 60 of 470,808 participants in the booster group and in 123 of 35,208 participants in the nonbooster group (adjusted hazard ratio, 0.09; 95% CI, 0.07 to 0.13; P<0.001) (Table S4). Among participants younger than 65 years of age, death from Covid-19 occurred in 5 of 287,310 participants in the booster group and in 14 of 49,882 participants in the nonbooster group (adjusted hazard ratio, 0.13; 95% CI, 0.04 to 0.40; P<0.001) (Table S5).
Among female participants, death from Covid-19 occurred in 13 of 400,300 participants in the booster group and in 54 of 47,972 participants in the nonbooster group (adjusted hazard ratio, 0.06; 95% CI, 0.03 to 0.11; P<0.001) (Table S6). Among male participants, death from Covid-19 occurred in 52 of 357,818 participants in the booster group and in 83 of 37,118 participants in the nonbooster group (adjusted hazard ratio, 0.12; 95% CI, 0.08 to 0.18; P<0.001) (Table S7).
Secondary Outcome
During the study period, confirmed SARS-CoV-2 infection was observed in 2888 participants in the booster group and in 11,108 participants in the nonbooster group. The adjusted hazard ratio for SARS-CoV-2 infection in the booster group, as compared with the nonbooster group, was 0.17 (95% CI, 0.16 to 0.18; P<0.001) (Table S8).
Discussion
Our study showed that among participants who were 50 years of age or older and had received a second dose of the BNT162b2 vaccine at least 5 months earlier, those who received a booster had 90% lower mortality due to Covid-19 than those who did not receive a booster.
Israeli authorities approved the administration of a booster on July 30, 2021. In Israel, the decision to receive the booster is based entirely on personal preference. Delays in getting a booster may be related to logistic issues, including the ability to make an appointment at a convenient time and at a clinic close to home or at work. Delays in, or avoidance of, getting a booster may also be related to personal safety concerns. However, we found that by the end of our study period, most (90%) of the eligible persons 50 years of age or older had received the booster.
The waning vaccine effect that was observed in Israel and in other populations that had been vaccinated early5,6,13,14 may occur in upcoming months in many other populations, in concordance with the timing of the first two doses of BNT162b2 in the mass vaccination campaign. Nevertheless, regulatory approval or recommendation of the booster, especially for participants younger than 65 years of age, is still under debate in many countries. The evidence generated in this study, which shows significant lifesaving potential from providing the booster, may help to resolve this issue.
The 90% lower mortality due to Covid-19 with the use of the booster is somewhat less substantial than the effect observed in a preliminary study based on data from the Israeli Ministry of Health,15 which showed approximately 93% lower mortality due to Covid-19 with the booster. The difference could reflect the different study designs; the use of a 12-day lag time to ensure booster effectiveness in the Ministry of Health study, as compared with a 7-day lag time in our study; the 32-day follow-up period in the Ministry of Health study, which was considerably shorter than the 54-day follow-up period in our study; and our use of the CHS operational database to adjust for coexisting conditions.
The primary limitation of our study is the relatively short study period (54 days). However, during this time, the incidence of Covid-19 in Israel was one of the highest in the world.2 Moreover, the social-distancing restrictions imposed on the public in Israel were limited. Therefore, exposure to SARS-CoV-2 was substantial, and accordingly, the number of deaths due to Covid-19 was sufficient to show a significant association between the use of the booster and lower mortality due to Covid-19.
Confounding sociodemographic and clinical characteristics may have led to bias in the analysis of effectiveness. We attempted to overcome such bias by adjusting for the variables known to affect mortality due to Covid-19. However, for some sources of bias, measurement or correction may not have been performed adequately.
Older participants (≥60 years of age) started to receive the booster earlier than younger participants (<60 years of age) and had higher mortality. This might have introduced bias in the estimation of survival, resulting in higher mortality in the booster group than would be expected in the overall study population. In addition, data from older participants were censored earlier in the survival analysis, as the participants were transitioned to the booster group. This may be a potential source of bias due to informative censoring. However, the inclusion of age as a covariate in the Cox regression model minimized such bias.
The main population sectors in Israel — the general Jewish population, Arab population, and ultra-Orthodox Jewish population — have different health-related behavioral patterns. Our analysis was adjusted for these subpopulations, but the adjustment did not significantly affect the study outcomes. This observation may be explained by the fact that all participants included in our study had chosen to receive the first two doses early in the vaccination campaign, and therefore, it is possible that they had similar health care–seeking behavior.
The incidence of Covid-19 and thus exposure to SARS-CoV-2 changed during the study period. However, we assume that after adjustment for all covariates, including socioeconomic status, these changes had a similar effect in the booster group and the nonbooster group.
Another major limitation of this study is the lack of data regarding serious adverse events. Future studies will be needed to assess the safety of the administration of the booster.
Finally, our findings are limited to the BNT162b2 vaccine. Other vaccines have shown different patterns of waning immunity over time.16 Ongoing research comparing the vaccines (e.g., a planned U.K. study17) may provide insight on this issue.
Despite these limitations, our study may provide meaningful answers to crucial questions regarding vaccination policy that remain partially unanswered by clinical trials.18 Although this study is observational in nature, we believe that the significant findings and the observed potential for saving many lives could assist decision makers in assessing the benefit of providing the booster to broad populations, especially persons 50 years of age or older.
Our study showed that participants who received a booster at least 5 months after a second dose of BNT162b2 had 90% lower mortality due to Covid-19 in the short term than participants who did not receive a booster. However, studies with longer-term follow-up periods to assess the effectiveness and safety of the booster are still warranted.
Supplementary Appendix
Disclosure Forms
This article was published on December 8, 2021, at NEJM.org.
Footnotes
The study did not receive any financial or in-kind support.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daily new confirmed COVID-19 cases per million people. Our World In Data. September 22, 2021. (https://ourworldindata.org/covid-cases?country=IND~USA~GBR~CAN~DEU~FRA~ISR~AFG~OWID_WRL#daily-confirmed-cases-per-million-people).
- 3.Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021;397:2461-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas SJ, Moreira ED Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 2021;385:1761-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. August 30, 2021. (https://www.medrxiv.org/content/10.1101/2021.08.24.21262423v1). preprint. [DOI] [PMC free article] [PubMed]
- 6.Keehner J, Horton LE, Binkin NJ, et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N Engl J Med 2021;385:1330-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021;398:1377-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021;385:1078-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS One 2021;16(3):e0247461-e0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med 2018;6:121-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021;385(24):e83-e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385(24):e84-e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar-On YM, Goldberg Y, Mandel M, et al. Protection across age groups of BNT162b2 vaccine booster against Covid-19. October 7, 2021. (https://www.medrxiv.org/content/10.1101/2021.10.07.21264626v1). preprint. [DOI] [PMC free article] [PubMed]
- 16.Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions — United States, March–August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1337-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahase E. Covid-19: Moderna and Novavax vaccines to be tested in mixing vaccines trial. BMJ 2021;373:n971-n971. [DOI] [PubMed] [Google Scholar]
- 18.Evans SJW, Jewell NP. Vaccine effectiveness studies in the field. N Engl J Med 2021;385:650-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.