Abstract
The MLL-ELL chimeric gene is the product of the (11;19)(q23p13.1) translocation associated with de novo and therapy-related acute myeloid leukemias (AML). ELL is an RNA polymerase II elongation factor that interacts with the recently identified EAF1 (ELL associated factor 1) protein. EAF1 contains a limited region of homology with the transcriptional activation domains of three other genes fused to MLL in leukemias, AF4, LAF4, and AF5q31. Using an in vitro transformation assay of retrovirally transduced myeloid progenitors, we conducted a structure-function analysis of MLL-ELL. Whereas the elongation domain of ELL was dispensable, the EAF1 interaction domain of ELL was critical to the immortalizing properties of MLL-ELL in vitro. To confirm these results in vivo, we transplanted mice with bone marrow transduced with MLL fused to the minimal EAF1 interaction domain of ELL. These mice all developed AML, with a longer latency than mice transplanted with the wild-type MLL-ELL fusion. Based on these results, we generated a heterologous MLL-EAF1 fusion gene and analyzed its transforming potential. Strikingly, we found that MLL-EAF1 immortalized myeloid progenitors in the same manner as that of MLL-ELL. Furthermore, transplantation of bone marrow transduced with MLL-EAF1 induced AML with a shorter latency than mice transplanted with the MLL-ELL fusion. Taken together, these results indicate that the leukemic activity of MLL-ELL requires the EAF1 interaction domain of ELL, suggesting that the recruitment by MLL of a transactivation domain similar to that in EAF1 or the AF4/LAF4/AF5q31 family may be a critical common feature of multiple 11q23 translocations. In addition, these studies support a critical role for MLL partner genes and their protein-protein interactions in 11q23 leukemogenesis.
11q23 translocations occur frequently in hematologic malignancies. The MLL gene spans the 11q23 chromosomal translocation breakpoint and contains significant homology with the Drosophila trithorax gene (9, 26, 29, 32). More than 30 different recurring cytogenetic aberrations that affect the MLL gene have been described (5). The critical feature of these chromosomal rearrangements is the generation of a chimeric transcript consisting of 5′ MLL and 3′ sequences of the gene on the partner chromosome. At present, more than 20 MLL partner genes at 11q23 partner chromosomal breakpoints have been cloned (3, 5). The functions of most MLL partner genes are not yet known. Although no consistent homologies or motifs among the partner gene sequences have been identified that might explain how their fusion to MLL results in leukemia, certain groups of partner genes have similar features. These include ENL and AF9, which are serine- and proline-rich and share extensive amino acid homology (15, 29). AF4, LAF4, and AF5q31 are also rich in serines and prolines and exhibit homology with ENL and AF9 (8, 14, 25). AF4, ENL, and AF9 contain transcriptional activation domains with similar properties in reporter gene assays (17, 20).
The (11;19)(q23;p13.1) translocation is a recurring chromosomal aberration in de novo and therapy-related acute myeloid leukemias (AML). This translocation juxtaposes the 5′ sequences of the MLL gene to the 3′ sequences of the ELL gene and results in the formation of an in-frame MLL-ELL fusion protein (27). Subsequent studies revealed that ELL functions as an RNA polymerase II (Pol II) transcriptional elongation factor (21). Several functional domains within ELL have been identified. The Pol II elongation activity maps to the amino-terminal 373 amino acids of ELL (22). A domain that inhibits the initiation of transcription localizes to the first 60 amino acids. A lysine-rich domain with a potential bipartite nuclear localization signal spans from amino acids 447 to 465, and a region of homology with occludin maps to amino acids 521 to 616. The chimeric MLL-ELL protein that results from the (11;19)(q23;p13.1) translocation contains the amino-terminal region of MLL, including its AT hooks, methyltransferase domain, and repression domain, fused to amino acids 46 to 621 of ELL, including its elongation domain, lysine-rich region, and occludin homology domain.
Characterization of ELL in murine development revealed that it is expressed diffusely in early embryonic development. In late embryonic development and in mature mice, ELL is expressed in multiple tissues with the highest levels in the liver and gut epithelium (28). ELL is a nuclear protein that exhibits a speckled pattern by indirect immunofluorescence and confocal microscopy. ELL2 was identified by sequence homology to ELL and has been shown to exhibit similar activities to ELL in transcriptional elongation assays (23). However, ELL2 has not been observed in association with chromosome translocations in leukemia or in other malignancies. In addition to ELL and ELL2, several different factors with elongation activity have been identified, including TFIIS, P-TEFb, TFIIF, Elongin, and FACT. TFIIS and P-TEFb each prevent specific types of transcriptional arrest. TFIIS is involved in the maintenance of transcriptional fidelity, and P-TEFb protects against the inhibition of elongation by DSIF (10, 30). FACT facilitates elongation through its interactions with chromatin (16). In contrast, ELL, ELL2, TFIIF, and Elongin function as general elongation factors and serve to prevent transient pausing of Pol II along the DNA template (1, 18) Using ELL as the bait in a yeast two-hybrid screen, we recently identified a novel protein that we named EAF1 for ELL associated factor 1 (23a). We found that endogenous ELL and EAF1 coimmunoprecipitated as a complex in multiple cell types. In addition, ELL and EAF1 colocalized in a distinct speckled pattern within nuclei. Interestingly, EAF1 contains a limited region of homology with the AF4, LAF4, and AF5q31 proteins that fuse to MLL in 11q23 chromosome translocations. This domain is rich in serine, aspartic acid, and glutamic acid residues and has been shown to activate transcription. Thus, we examined EAF1 for its transactivation potential and identified a transcriptional activation domain that maps to this region of homology.
Expression of the MLL-AF9, MLL-ENL, and MLL-CBP fusion genes in the mouse models results in the development of AML (4, 11, 12). In addition, retroviral gene transfer of MLL-ENL into murine hematopoietic progenitor cells resulted in an increased capacity for self-renewal and proliferation in vitro. Serial passage of these cells demonstrated the potential of MLL-ENL to immortalize myelomonocytic progenitors. Using this assay, an analysis of deletion mutants revealed that the DNA binding domains of MLL, namely, the AT hooks and the DNA methyltransferase homology domains, were essential to the transforming properties of MLL-ENL. This analysis also showed that the C-terminal 84 amino acids of ENL defined the essential contribution of ENL to the chimeric protein (24). This region of ENL was found to exhibit transcriptional activation potential in reporter gene assays. Recently, we have shown that retroviral infection of MLL-ELL into murine hematopoietic progenitor cells, followed by transplantation into lethally irradiated littermates, results in the development of AML (13). Similarly, infection of murine hematopoietic progenitor cells in vitro with MLL-ELL results in their immortalization.
To determine the domains of ELL that are essential for leukemogenesis when fused to MLL, we have undertaken a detailed structure-function analysis. Using the hematopoietic progenitor cell immortalization assay, we demonstrate that the elongation domain of ELL is dispensable but that the EAF1 interaction domain is required for the immortalization of hematopoietic progenitor cells. Since the essential contribution of ELL to the MLL-ELL fusion appears to be its EAF1 interaction domain, we analyzed a heterologous MLL-EAF1 fusion protein in the myeloid progenitor cell assay and by transplantation studies. MLL-EAF1 demonstrates the capacity to immortalize hematopoietic progenitor cells in vitro, and transplantation of transduced progenitor cells leads to the development of AML that resembles that induced by MLL-ELL. Taken together, our data suggest that recruitment of interacting factors by MLL partner proteins may be essential to the transformation of hematopoietic cells into leukemic cells.
MATERIALS AND METHODS
Retroviral constructs and methylcellulose colony-forming assays.
To facilitate the assembly of a series of chimeric fusions containing 5′ MLL, a SalI site was engineered at the 3′ end of exon 7 of MLL by site-directed mutagenesis within a BamHI fragment that extended from MLL nucleotides 3750 to 4609. Nucleotide 4209 of MLL was mutated from a G to a C, but this did not result in an amino acid change. To exclude the formation of other mutations, this construct was sequenced in its entirety, and the BamHI-SalI fragment was ligated to MLL nucleotides 1 to 3750. For the structure-function studies of ELL and for the MLL-EAF1 construct, individual domains were amplified using Pfu polymerase with oligonucleotide primers containing a SalI site at the 5′ end and a BglII site at the 3′ end. The ELL and EAF1 fragments were ligated to 5′ MLL and cloned in either the MSCV retroviral expression vector for in vitro studies or upstream of the internal ribosomal entry site (IRES) of the MIE (MSCV-IRES-enhanced green fluorescent protein [EGFP]) vector for in vivo expression (11, 12). Production of retroviral supernatants in Bosc23 cells was performed as described elsewhere (11). Viral titers were determined by infection of 3T3 cells with Bosc23 retroviral supernatants. Infection of lineage-depleted (Lin−) bone marrow (BM) cells obtained from BA.1 mice 5 days after 5-fluorouracil treatment and culture of the transduced progenitors in methylcellulose culture were performed as previously described (13).
Reconstitution assay and characterization of leukemias.
Reconstitution of lethally irradiated BA.1 (C57BL/Ka-Ly5.2, Thy1.1) mice with transduced progenitors was performed as described earlier (12), with the following modifications. Each mouse was inoculated by tail vein injection with 30 × 103 Lin− BM cells from congenic BS/BA (C57BL/Ka-Ly5.1, Thy1.1) mice retrovirally transduced with the MIE vector, MLL-ELL, MLL-ELL508-621, or MLL-EAF1, together with 105 normal BA.1 BM cells to ensure radioprotection. Precise determination of the transduction efficiency is not possible at this step because there is no antibiotic selection with this vector and the level of expression of EGFP is too low for accurate measurement by fluorescence-activated cell sorting in the cells transduced with the MLL fusion constructs. However, titering experiments with these constructs in the MSCVneo vector resulted in equivalent transduction efficiencies. The degree of engraftment was assessed by flow cytometric analysis of peripheral blood (PB) stained with anti-Ly5.1 antibodies (Pharmingen). PB counts were measured using a Cell-Dyn 3500R (Abbott). Immunophenotypic characterization of the leukemic cells was done by costaining the circulating leukocytes with phycoerythrin-conjugated anti-CD11b/Mac-1 antibodies and allophycocyanin-conjugated antibodies against Gr-1 or cKit (Pharmingen). Stained cells were examined with a FACSCalibur instrument (Becton Dickinson) following the exclusion of dead cells by high propidium iodide staining and forward light scatter. Tissues were fixed in formalin, sectioned, and stained with hematoxylin and eosin for histological analysis. Blood smears and cytospin preparations of the BM cells were stained with Wright-Giemsa.
DNA and RT-PCR analysis.
Cells from primary colonies grown in the presence of G418 were harvested, and total RNA was isolated using RNA STAT-60 (Tel-Test) as recommended by the manufacturer. A total of 1 μg of RNA was reversed transcribed into cDNA with enhanced avian myeloblastosis virus reverse transcriptase (RT; Sigma). PCR reactions were performed with Taq polymerase using a forward primer from MLL exon 7 and a reverse primer from ELL and EAF1. To exclude a false-positive signal from DNA contamination of the RNA, PCR reactions were also performed on 1-μg aliquots of RNA that had not been incubated with RT. As a control for the integrity of the RNA, PCR reactions were also performed using primers from the actin gene. DNA was prepared from tumor-infiltrated spleens and digested with BamHI. Southern blot analysis for clonality using a 2.2-kb MLL HindIII fragment was performed as previously described (11).
Production of a polyclonal antibody to MLL.
To produce a histidine-tagged MLL protein in bacteria, amino acids 1 to 371 of MLL were cloned into the pET-19b expression vector (Novagen) and transformed in the Escherichia coli strain BL21(DE3). The histidine-tagged MLL protein was purified on a nickel column and eluted in 1 M imidazole–500 mM NaCl–20 mM Tris-HCl (pH 7.9). The protein was dialyzed against Tris-buffered saline (TBS; pH 7.5) and injected into rabbits using standard methods. Approximately 1 mg of histidine-tagged MLL protein was electrophoresed on a preparative gel, transferred to nitrocellulose, blocked in TBST containing 10% normal goat serum, and incubated with 2 ml of MLL antiserum overnight at 4°C. The strips were washed in 1% Tween and then phosphate-buffered saline (PBS). Bound antibody was eluted in 0.2 M glycine (pH 2.8), neutralized with 1 M Tris (pH 10.5), and dialyzed against PBS.
Western blot analysis.
Nuclear extracts were prepared from Bosc23 cells, electrophoresed in sodium dodecyl sulfate (SDS)-7.5% polyacrylamide gels, and blotted onto nitrocellulose membranes (Bio-Rad) using a transfer buffer with 25 mM Tris, 192 mM glycine, 0.1% SDS, and 20% methanol (pH 8.3). The membranes were blocked in 5% nonfat dry milk in TBS with 0.05% Tween 20 and incubated with the indicated primary antibody (anti-ELL at 1:500, anti-MLL at 1:100, and anti-EAF1 at 1:10). The membranes were washed, incubated with biotin-conjugated goat anti-rabbit antibody (Santa Cruz), and then washed and incubated with horseradish peroxidase-conjugated streptavidin (Jackson). After five washes, the protein bands were detected with an enhanced chemiluminescence protocol (Amersham).
Immunoprecipitation.
To map the EAF1 interaction domain within ELL more precisely, different regions of ELL were cloned in the pFLAG-CMV2 expression vector and transiently transfected in the human 293 cell line by the calcium phosphate method using 20 μg of plasmid DNA. Cell pellets were resuspended in 1 ml of TEN (40 mM Tris, 1 mM EDTA, 150 mM NaCl) buffer, centrifuged for 5 min at 1,200 × g at 4°C, lysed with 500 μl of NETN (100 mM NaCl; 20 mM Tris, pH 8.0; 1 mM EDTA; 0.2% NP-40) containing a cocktail of protease inhibitors (Sigma), incubated on ice for 10 min, and centrifuged at 2,500 × g for 30 min at 4°C. To precipitate the complexes, supernatants were precleared with 30 μl of A/G-agarose beads (Santa Cruz) for 30 min and then incubated for 1 h with the indicated antibody. We then added 30 μl of a 50% slurry of protein A/G-agarose beads, followed by incubation overnight at 4°C, five washes at 4°C with lysis buffer, boiling in Laemmli sample buffer, fractionatation by SDS-polyacrylamide gel electrophoresis (PAGE), and transfer to nitrocellulose membranes (Bio-Rad). To immunoprecipitate endogenous EAF1, the cell extracts were incubated with the anti-FLAG monoclonal antibody (Sigma) at 1:500, and the Western blots were incubated with the EAF1 monoclonal antibody at 1:10.
RESULTS
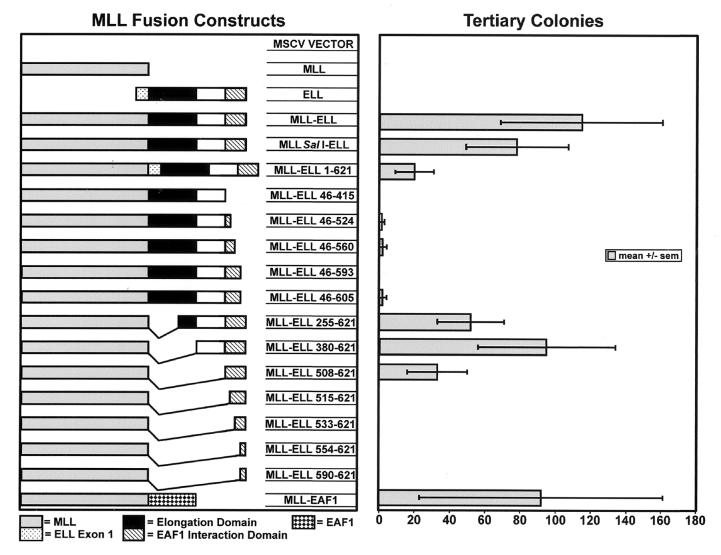
To determine the domains within ELL that are essential for leukemogenesis, we prepared a series of constructs to assess for the potential to immortalize primary murine hematopoietic progenitor cells. Previously, we showed that the MLL-ELL fusion has the potential to immortalize hematopoietic progenitor cells in vitro and to generate AML in mice transplanted with transduced progenitor cells in vivo (13). The MLL-ELL cDNA in these studies corresponded to the fusions isolated from patients with the (11;19)(q23;p13.1) translocation and contained exons 1 through 7 of MLL fused to exons 2 through the stop codon of ELL. To facilitate the construction of a series of mutants, we generated a unique SalI site at nucleotide 4209 (see Materials and Methods). Because this eliminates the last two MLL amino acids of exon 7, we verified that this mutation did not affect the transforming capacity of MLL-ELL. We compared MLL-ELL to the MLLΔ SalI-ELL construct containing the same ELL sequences from exon 2 to its stop codon and found similar numbers of colonies on tertiary plating in methylcellulose cultures for both constructs (Fig. 1).
FIG. 1.
Determination of the sequences of ELL essential to the immortalization of hematopoietic progenitor cells by MLL-ELL in a myeloid clonogenic assay. The bars in the right column represent the number of tertiary colonies generated by the respective mutants depicted in the left column.
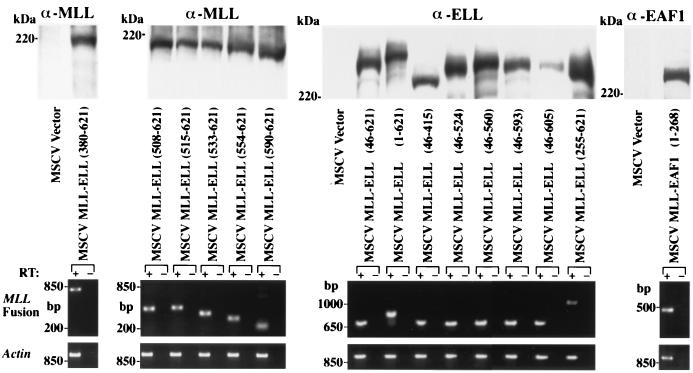
To assess the contribution of known functional domains and to determine whether uncharacterized regions of ELL might be essential to the development of AML, a series of amino- and carboxy-terminal deletion mutants were cloned into the MLLΔ SalI construct. Expression of each of these constructs within cell nuclei was confirmed by Western blotting (Fig. 2). For constructs that included the central region of ELL, we used an affinity-purified polyclonal antiserum to ELL that recognizes amino acids 250 to 400 of ELL. To detect expression of the constructs lacking this region of ELL, we generated a polyclonal antiserum to MLL. In addition, we used RT-PCR of primary hematopoietic progenitors to confirm expression in the cells transduced by the individual retroviral constructs (Fig. 2).
FIG. 2.
Western blot and RT-PCR analysis of the expression of the various constructs. The upper panels show expression of the various MLL fusion protein constructs in transiently transfected BOSC cells using antibodies to MLL, ELL, or EAF1. The lower panels show RT-PCR expression analysis in the transduced myeloid clonogenic cells harvested from primary methylcellulose cultures. Amplification from reverse transcribed cDNA is indicated by a plus symbol (+). To exclude amplification of integrated retroviral genomic DNA, a no-RT control is indicated by a minus symbol (−).
To examine the potential requirement of the elongation domain of ELL, we assayed a construct that completely deleted this domain from the MLL-ELL fusion protein (MLL-ELL380-621). Although a minor decrease in colony formation compared to full-length ELL was observed, inclusion of the elongation domain was not found to be required for immortalization (Fig. 1). Similarly, retention of the basic, lysine-rich domain of ELL was also dispensable. The minimal contribution of ELL that retained the capacity to immortalize hematopoietic progenitor cells spanned from amino acids 508 to 621. The MLL-ELL515-621 construct included the occludin homology domain at the carboxy terminus of ELL but failed to immortalize progenitor cells. In addition, constructs with truncations of the carboxy terminus of ELL all failed to produce tertiary colonies in this assay, including the smallest deletion of only 16 carboxy-terminal amino acids of ELL (MLL-ELL46-605) (Fig. 1). This was not due to a lack of protein stability, since these constructs exhibited equivalent levels of expression as seen by Western blotting (Fig. 2). As the genomic breaks within ELL in patients with (11;19)(q23;p13.1) translocations occur 3′ of the first exon of ELL, exon 1 is not retained in the MLL-ELL fusion gene. To assess the effect of inclusion of exon 1 of ELL within the MLL-ELL fusion gene, we examined a construct that included the entire open reading frame of ELL. Inclusion of exon 1 did not abrogate immortalization and did not affect the morphology of the colonies, although it somewhat reduced the number of tertiary colonies formed (Fig. 1).
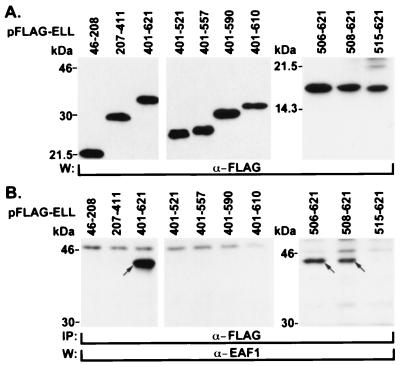
Previously, we had determined that the EAF1 interaction domain mapped to the carboxy terminus of ELL (23a). To map this domain more precisely, we prepared a series of ELL deletion mutants and cloned them in the pFLAG-CMV2 expression vector. These were transiently transfected in 293 cells, and the cell lysates were immunoprecipitated using an anti-FLAG antibody and immunoblotted with the EAF1 monoclonal antibody. As we established previously, we could immunoprecipitate endogenous EAF1 with the carboxy-terminal one-third of ELL. Using a series of deletion mutants, we found that the smallest region of ELL that retained binding to EAF1 included amino acids 508 to 621 of ELL. However, a construct that deleted 7 further amino acids (ELL515-621) failed to bind to EAF1 (Fig. 3). In addition, we also found that truncation from the carboxy terminus of ELL of as few as 11 amino acids (ELL401-610) also prevented EAF1 binding (Fig. 3). Thus, the capacity to bind to EAF1 localized to amino acids 508 to 621 of ELL and corresponded precisely with the region of ELL required for the immortalizing activity of MLL-ELL.
FIG. 3.
Mapping of the EAF1 interaction domain within ELL. (A) The human 293 cell line was transfected with FLAG-tagged constructs containing different regions of ELL. Western blot analysis of cell lysates confirmed the expression of each of the constructs. (B) Immunoprecipitation of cell lysates was performed with the FLAG antibody, followed by stringent washes of the immunoprecipitated complexes. The minimal domain that coprecipitated with endogenous EAF1 mapped to amino acids 508 to 621 within ELL. Endogenous EAF1 migrates at approximately 43 kDa.
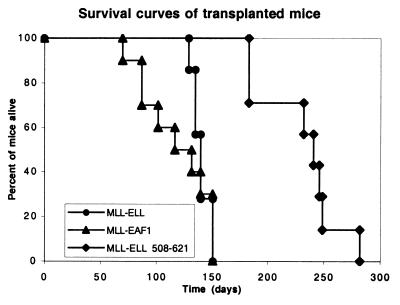
To determine whether the transforming activity of the minimal MLL-ELL508-621 mutant relied on the fusion of an EAF1 interacting domain to MLL, we tested the effect of an artificial construct obtained by fusing MLL exons 1 through 7 to the open reading frame of EAF1. Expression of this construct was confirmed by Western blotting extracts of transfected 293 cells using the EAF1 monoclonal antibody and by performing RT-PCR analysis of the transduced colony-forming cells. We found that MLL-EAF1 immortalized primary hematopoietic progenitor cells in vitro and generated a similar number of secondary and tertiary colonies as full-length MLL-ELL (Fig. 1). We then compared the ability of MLL-ELL, MLL-ELL508-621, and MLL-EAF1 to induce leukemias in mice transplanted with retrovirally transduced hematopoietic progenitors. To this end, we used the MIE (MSCV-IRES-EGFP) vector that expresses EGFP and facilitates detection of the transduced cells in the reconstituted mice. We transplanted lethally irradiated Ly5.2 animals with a relatively high dose of lineage-depleted congenic Ly5.1 BM cells transduced with MLL-EAF1 (10 mice), MLL-ELL (7 mice), MLL-ELL508-621 (7 mice), or the empty MIE vector (8 mice). The mice were first bled 10 weeks after transplantation to perform cell counts and analyze engraftment, as well as the contribution of the donor cells to the myeloid compartment by flow cytometry. As previously described (12) the MLL-ELL mice displayed a higher level of engraftment compared to the MIE controls, but no increase in the percentage of myeloid cells was observed at this time point. On the other hand, 5 of the 10 MLL-EAF1 mice displayed a considerable increase (ranging from 17 to 92%) in the fraction of myeloid cells of donor origin compared to the MIE or MLL-ELL mice (average, 12% ± 3% and 12% ± 1%, respectively). The MLL-EAF1 mouse with the highest percentage of myeloid cells of donor origin also displayed a more than fivefold increase in leukocyte count and was found dead 2 days later with an enlarged spleen. By 15 weeks posttransplantation, 4 of the 10 MLL-EAF1 mice had died of myeloid leukemia. At that time point, an increase in myeloid cells of donor origin was observed in two of the seven MLL-ELL mice, but it was not before 18 weeks posttransplantation that these mice first began to die of acute myeloid leukemia. All of the MLL-EAF1 mice died between 70 and 151 days, all of the MLL-ELL mice died between 129 and 151 days, and all of the MLL-ELL508-621 mice died between 183 and 282 days (Fig. 4). The mice in each of the three cohorts exhibited splenomegaly, leukocytosis, anemia, and thrombocytopenia compared to the MIE vector controls (Table 1). The MLL-ELL508-621 mice exhibited a lesser degree of leukocytosis but a greater degree of anemia and thrombocytopenia compared to the MLL-ELL and MLL-EAF1 mice.
FIG. 4.
Survival curves of the mice transplanted with BM progenitors transduced by the MLL-ELL, MLL-ELL508–621, and MLL-EAF1 encoding vectors.
TABLE 1.
Characteristics of leukemias in recipients of MLL-ELL, MLL-EAF1, and MLL-ELL508-621 transduced BM cells
| Cohort | Mean values ± SDa
|
||||
|---|---|---|---|---|---|
| Latency (days) | Spleen wt (g) | WBC (103/μl) | RBC (106/μl) | PLT (103/μl) | |
| MLL-ELL | 140 ± 8.3 | 0.39 ± 0.17 | 29.2 ± 33.0 | 10.2 ± 4.1 | 669 ± 144 |
| MLL-EAF1 | 119 ± 30.6 | 0.44 ± 0.24 | 45.9 ± 44.4 | 7.7 ± 2.0 | 464 ± 450 |
| MLL-ELL508–621 | 231 ± 36.2 | 0.27 ± 0.22 | 12.6 ± 20.2 | 3.0 ± 2.8 | 86 ± 58 |
| MIE vector | NA | 0.08 ± 0.02 | 4.7 ± 1.0 | 10.2 ± 0.3 | 1,334 ± 337 |
Means with standard deviations are shown for each cohort of mice. WBC, white blood cells; RBC, red blood cells; PLT, platelets. NA, not applicable.
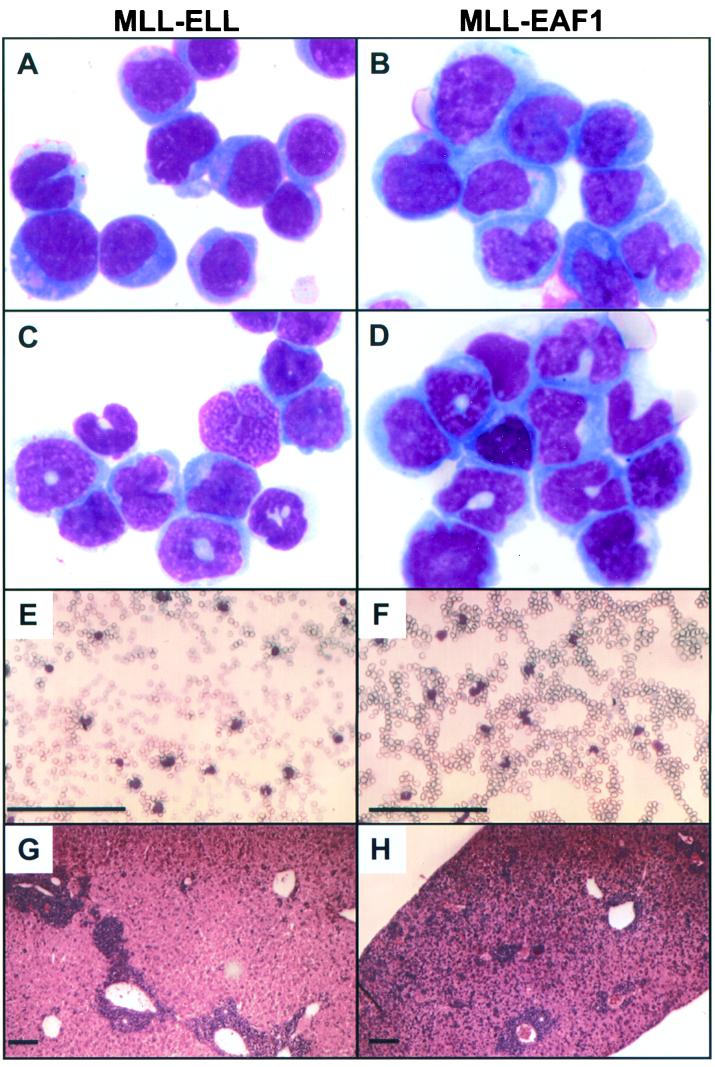
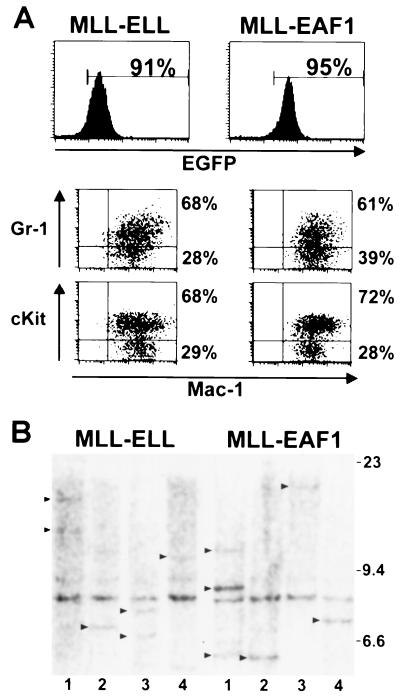
To compare the diseases induced by MLL-ELL and MLL-EAF1, we analyzed the morphology and the flow cytometry profile of blood and BM from terminally ill mice. In each of the cohorts, all of the mice eventually developed AML. The mice exhibited AML subtypes that are similar morphologically to either acute myelomonocytic leukemia (FAB-M4) or acute monocytic leukemia (FAB-M5) (Fig. 5A to D). This is quite similar to the morphologies observed in human AML with the (11;19)(q23;p13.1) translocation. In the MLL-ELL cohort, there were two mice with acute myelomonocytic leukemia, four mice with acute monoblastic leukemia (poorly differentiated), and one mouse with acute monocytic leukemia (differentiated). In the MLL-EAF1 cohort, there were three mice with acute myelomonocytic leukemia and three with acute monocytic leukemia (differentiated). We studied the expression of Mac-1, Gr-1, and cKit to further characterize the degree of maturation between the MLL-ELL and MLL-EAF1 mice but found no consistent differences (Fig. 6A). Namely, the leukemic cells were all Mac-1 positive with a subpopulation (varying from 44 to 86%) that coexpressed the primitive marker cKit and a gradient of cells expressing the granulocytic marker Gr-1. In the majority of both MLL-ELL and MLL-EAF1 mice we found that leukemic cells infiltrated the spleen, liver, thymus, lymph nodes, and kidneys. However, the microscopic aspects of the liver seemed to differ consistently between the two groups of mice. Whereas the leukemic infiltrate was essentially restricted to the liver parenchyma immediately adjacent to the blood vessels in the MLL-ELL mice, it was much more diffusely spread out in the liver sinuses of the MLL-EAF1 mice (compare Fig. 5G and H). Altogether, this study demonstrates that both MLL-EAF1 and MLL-ELL have potent tumorigenic activity toward myeloid precursors. To assess the clonal composition of the leukemias, we digested spleen DNA from MLL-ELL and MLL-EAF1 mice with BamHI and probed with an MLL cDNA probe. As a single BamHI site is present in the MLL-ELL and MLL-EAF1 proviruses, this permitted an assessment of the number of different proviral integration sites found in the leukemia-infiltrated spleens. We observed one to three integration sites in both groups of mice, suggesting that the leukemias were mono- or pauciclonal (Fig. 6B). We did not observe a difference in the number of integration sites between the MLL-ELL and MLL-EAF1 mice.
FIG. 5.
Morphology of the neoplastic cells in MLL-ELL (left column) and MLL-EAF1 leukemic mice (right column). Wright-Giemsa staining of BM cytospin preparation (A, B, C, and D). (A) MLL-ELL mouse with acute monoblastic leukemia (poorly differentiated). (B) MLL-EAF1 mouse with acute monocytic leukemia (differentiated). (C and D) MLL-ELL and MLL-EAF1 mice, respectively, with acute myelomonocytic leukemia. (E and F) PB smears showing circulating blast cells. (G and H) Histological analysis of the liver showing infiltration by leukemia cells. Bar, 300 μm.
FIG. 6.
(A) Immunophenotype of the leukemic BM of MLL-ELL (left) and MLL-EAF1 (right) mice. The histograms show the expression of the EGFP marker, and the region used to gate the EGFP-positive population is indicated, along with the percentage of cells it comprises. The dot plots represent the Mac-1 versus Gr-1 or cKit staining of the EGFP-expressing cells. Percentage values correspond to the content of the adjacent quadrants. (B) Southern blot analysis of spleen DNA obtained from leukemic mice, digested with BamHI, and probed with an MLL cDNA fragment. As indicated by the arrows, one to three integration sites could be detected, indicating that the leukemias were mono- or pauciclonal in the MLL-ELL and the MLL-EAF1 mice. A 9-kb band is also detected, corresponding to the endogenous murine Mll gene.
DISCUSSION
We have previously demonstrated the leukemic properties of the MLL-ELL fusion protein in a transduction-transplantation model (13). To investigate the mechanism of this leukemogenic activity, we have exploited a myeloid colony-forming assay and focused on the discrete functional domains that have been identified within ELL. Since ELL has been shown to function as a Pol II elongation factor, one hypothesis had been that the fusion of the elongation domain of ELL to the amino terminus of MLL may lead to increased levels of expression of target genes regulated by MLL (21). The elongation activity of ELL was mapped to amino acids 1 to 373 with deletions of amino acids 50 to 100, 100 to 150, and 150 to 200 abolishing elongation as measured by in vitro transcription assays (22). Using a series of deletion mutants, we found that amino acids 1 to 507 of ELL are dispensable to the capacity of MLL-ELL to immortalize hematopoietic progenitor cells. In addition to the elongation domain, this region includes the basic, lysine-rich domain of ELL spanning amino acids 447 to 465, which may represent a bipartite nuclear localization signal. However, proper nuclear localization of constructs lacking this signal was confirmed by Western blotting of nuclear extracts. The nuclear localization signal of MLL has been mapped to its amino terminus and is retained in the formation of MLL fusion proteins (31). In addition, MLL fusion proteins exhibit the subnuclear localization pattern of MLL rather than that of its various partner proteins (2, 19). A region of homology to the occludin gene spans from amino acids 521 to 616 of ELL, but the functional significance of this homology is not known. A construct that retained the occludin homology domain (MLL-ELL515-621) but lacked the potential to bind to EAF1 was insufficient to maintain the capacity for immortalization. Since the occludin homology domain is contained within the minimal region sufficient for immortalization (MLL-ELL508-621), a potential contribution to the transforming capacity cannot be excluded. Recently, DiMartino et al. used a similar hematopoietic progenitor cell immortalization assay to examine the contribution of ELL to the MLL-ELL fusion protein and found that amino acids 461 to 621 of ELL were required and that the elongation activity of ELL was not essential for immortalization (6). In this report, we extend these findings by determining precisely the critical contribution of ELL to transformation both in vitro and in vivo, establishing that this domain within ELL mediates binding to the EAF1 protein and demonstrating that a heterologous MLL-EAF1 fusion protein recapitulates the immortalization and leukemogenic capacities of the MLL-ELL fusion protein.
Exon 1 of ELL spans from amino acids 1 to 45 and is excluded from the MLL-ELL fusion gene because the genomic breaks in patients with (11;19)(q23;p13.1) translocations occur 3′ of the first exon of ELL. A domain that inhibits promoter-specific initiation localizes to the first 60 amino acids of ELL and successive deletions of groups of 10 residues between amino acids 10 and 60 of ELL all disrupt this activity (22). Thus, the MLL-ELL fusion protein would lack this domain and its inhibitory effect on initiation, which requires an intact exon 1. A hypothesis formulated based on the lack of inclusion of exon 1 in the MLL-ELL fusion was that its exclusion from the fusion might be required for transformation by MLL-ELL (22). Although our data indicate that inclusion of exon 1 of ELL in the MLL-ELL fusion does not abrogate immortalization by MLL-ELL, it somewhat inhibits total tertiary colony formation. Thus, we cannot exclude that the lack of ELL exon 1 in the wild-type MLL-ELL fusion may contribute to its potency as an oncogene.
Precise mapping of the contribution of specific domains of ELL to the capacity to immortalize hematopoietic progenitor cells revealed that amino acids 508 to 621 of ELL were necessary and sufficient for transformation. To delineate the domain of ELL that binds to EAF1, we transfected a series of deletion mutants of ELL and examined their ability to bind to the endogenous EAF1 protein. Strikingly, the minimal domain of ELL essential for immortalization coincided exactly with the capacity of ELL to bind to the EAF1 protein. To confirm that the minimal essential domain identified in our in vitro assays was also critical to leukemogenesis in vivo, we transplanted BM cells transduced with MLL fused to ELL508-621. These mice also developed acute myeloid leukemia, with a longer latency compared to the wild-type MLL-ELL. These data suggest that recruitment of an interacting protein rather than a specific functional domain may be the critical contribution of ELL to the leukemogenic effect of MLL-ELL. However, we cannot exclude that another as-yet-unknown function maps to amino acids 508 to 621 of ELL or that this domain might mediate interaction with additional proteins other than EAF1. To address whether EAF1 recruitment was indeed the critical function contained in this region of ELL, we examined the transforming properties of a heterologous MLL-EAF1 fusion. Although EAF1 has not been identified as a partner gene in 11q23 translocations, the chimeric MLL-EAF1 fusion recapitulates the phenotype observed with MLL-ELL. MLL-EAF1 demonstrated the capacity to immortalize primary hematopoietic cells in vitro. Moreover, mice transplanted with cells transduced with MLL-EAF1 developed AML morphologically similar to those induced by MLL-ELL. The MLL-ELL and MLL-EAF1 mice all developed either acute myelomonocytic or acute monocytic leukemias, which are observed in human acute leukemias with the (11;19)(q23;p13.1) translocation. The more rapid progression of myeloproliferation and leukemogenesis induced by MLL-EAF1 compared to MLL-ELL in our murine model may indicate that MLL-EAF1 has stronger tumorigenic activity in vivo. In addition, the MLL-ELL508-621 mice develop acute leukemia after a longer interval than the wild-type MLL-ELL and the MLL-EAF1 mice. This may reflect a possible diminished capacity to bind EAF1 compared to full-length ELL. Alternatively, other domains within ELL between amino acids 46 and 508 may increase its potency as an oncogene. However, the difference in kinetics between the three cohorts of mice could also result from differences in retroviral titers obtained with the different MLL fusion genes.
The generation of a model of AML using retroviral gene transfer of MLL-EAF1 is the first example of a leukemia model involving an interacting protein of an MLL partner gene rather than the partner gene itself. However, another example involving the fusion of MLL to a gene not involved in chromosome translocations has recently been reported. In these experiments, an Mll-lacZ fusion gene was generated by gene targeting in embryonic stem cells and compared to mice generated with an Mll-AF9 fusion. In contrast to Mll-AF9 mice, which all developed acute leukemia, only 35% of the mice generated with the Mll-lacZ fusion gene were found to develop leukemia, and this manifested after a much longer latency compared to mice expressing an Mll-AF9 fusion gene (7). The Mll-AF9 mice developed acute leukemia beginning at 4 months, which is comparable to that of the MLL-ELL and MLL-EAF1 mice (5 and 4 months, respectively). In contrast, the 35% of the Mll-lacZ mice that eventually developed leukemia did not exhibit the leukemia phenotype until much later, i.e., between the ages of 8 and 20 months.
The development of leukemia in the Mll-lacZ mice suggests a potential lack of specificity in the contribution of MLL partner genes, and perhaps that fusion of the amino terminus of MLL to any stable sequence is sufficient for leukemogenesis. However, our data showed that numerous constructs expressing fusions of MLL to ELL constructs lacking the capacity to bind EAF1 were insufficient to immortalize hematopoietic progenitors in vitro. Expression of these constructs was confirmed at the RNA and protein levels, and protein expression levels were indistinguishable between transforming and nontransforming constructs. Similarly, previous functional studies of ENL identified that its transactivation domain was necessary and sufficient for immortalization, and constructs lacking this domain failed to immortalize despite detectable protein expression. An alternative hypothesis is that MLL partner genes contribute specific functions and that lacZ contributes a domain present in naturally occuring MLL partner genes. Dobson et al. note that lacZ functions as a tetramer and that two MLL partner genes, AF10 and AF17, contain leucine zipper dimerization domains, suggesting that dimerization of the MLL fusion protein may be a critical feature of these leukemias (7). The close homology between certain MLL partner genes also supports the hypothesis that a specific functional contribution from these partner genes is essential.
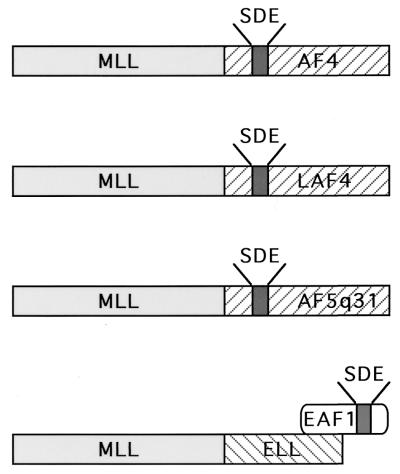
Taken together, our data indicate that the elongation domain of ELL is dispensable, whereas the EAF1 interaction domain of ELL is necessary and sufficient for the leukemogenic effect of the MLL-ELL fusion protein. Strikingly, a heterologous MLL-EAF1 fusion protein recapitulates the phenotype of MLL-ELL in vitro and in vivo. These data suggest that MLL may fuse directly with a member of a common family of transactivators including AF4, LAF4, and AF5q31 or, alternatively, with a partner protein that has a direct physical interaction with a transactivator in this class, e.g., EAF1 (Fig. 7 and 8). Thus, the MLL-ELL fusion may serve to recruit a transactivation domain of the AF4/LAF4/AF5q31/EAF1 family to MLL and suggests that a common transcriptional pathway may be disrupted in several different types of 11q23 translocations. Moreover, our data suggest that the protein-protein interactions of MLL partner genes may have important functional contributions to leukemias that result from 11q23 chromosome translocations.
FIG. 7.
Model of 11q23 leukemogenesis involving AF4 family members and ELL. A subset of 11q23 translocations involves a fusion of MLL to the AF4, LAF4 and AF5q31 genes that contain transcriptional activation domain rich in serine (S), aspartic acid (D), and glutamic acid (E) residues. In the MLL-ELL fusion that results from the t(11;19)(q23;p13.1), ELL retains its interaction domain with EAF1, a transcriptional activator with homology to the serine (S)-, aspartic acid (D)-, and glutamic acid (E)-rich activation domains of AF4, LAF4, and AF5q31.
FIG. 8.
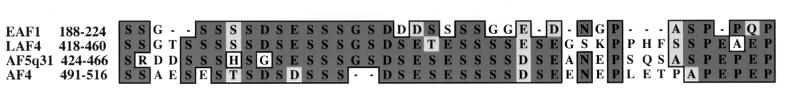
CLUSTALW alignment of EAF1 with LAF4, AF5q31, and AF4. Amino acid identity is indicated by dark gray boxes, and amino acid similarity is indicated by light gray boxes.
ACKNOWLEDGMENTS
We thank John Anastasi for assistance in analyzing the morphologies of the bone marrows from the leukemic mice.
The first two authors contributed equally to this work.
This work was supported by grants to M.J.T. from the National Institutes of Health (CA78431), the Burroughs-Wellcome Fund, the American Society of Hematology, and the family of Robert A. Chapski.
REFERENCES
- 1.Aso T, Lane W S, Conaway J W, Conaway R C. Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II. Science. 1995;269:1439–1443. doi: 10.1126/science.7660129. [DOI] [PubMed] [Google Scholar]
- 2.Butler L H, Slany R, Cui X, Cleary M L, Mason D Y. The HRX proto-oncogene product is widely expressed in human tissues and localizes to nuclear structures. Blood. 1997;89:3361–3370. [PubMed] [Google Scholar]
- 3.Bernard O A, Berger R. Molecular basis of 11q23 rearrangements in hematopoietic malignant proliferations. Genes Chromosomes Cancer. 1995;13:75–85. doi: 10.1002/gcc.2870130202. [DOI] [PubMed] [Google Scholar]
- 4.Corral J, Lavenir I, Impey H, Warren A J, Forster A, Larson T A, Bell S, McKenzie A N, King G, Rabbitts T H. An Mll-Af9 fusion gene made by homologous recombination causes acute leukemia in chimeric mice: a method to create fusion oncogenes. Cell. 1996;85:853–861. doi: 10.1016/s0092-8674(00)81269-6. [DOI] [PubMed] [Google Scholar]
- 5.DiMartino J F, Cleary M L. MLL rearrangements in haematological malignancies: lessons from clinical and biological studies. Br J Haematol. 1999;106:614–626. doi: 10.1046/j.1365-2141.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 6.DiMartino J F, Miller T, Ayton P M, Landewe T, Hess J L, Cleary M L, Shilatifard A. A carboxy-terminal domain of ELL is required and sufficient for immortalization of myeloid progenitors by MLL-ELL. Blood. 2000;96:3887–3893. [PubMed] [Google Scholar]
- 7.Dobson C L, Warren A J, Pannell R, Forster A, Rabbitts T H. Tumorigenesis in mice with a fusion of the leukaemia oncogene Mll and the bacterial lacZ gene. EMBO J. 2000;19:843–851. doi: 10.1093/emboj/19.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domer P H, Fakharzadeh S S, Chen C S, Jockel J, Johansen L, Silverman G A, Kersey J H, Korsmeyer S J. Acute mixed-lineage leukemia t(4;11)(q21;q23) generates an MLL-AF4 fusion product. Proc Natl Acad Sci USA. 1993;90:7884–7888. doi: 10.1073/pnas.90.16.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Croce C M, Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;71:701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- 10.Jeon C, Agarwal K. Fidelity of RNA polymerase II transcription controlled by elongation factor TFIIS. Proc Natl Acad Sci USA. 1996;93:13677–13682. doi: 10.1073/pnas.93.24.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavau C, Szilvassy S J, Slany R, Cleary M L. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 1997;16:4226–4237. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavau C, Du C, Thirman M, Zeleznik-Le N. Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukemia. EMBO J. 2000;19:4655–4664. doi: 10.1093/emboj/19.17.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavau C, Luo R T, Du C, Thirman M J. Retrovirus-mediated gene transfer of MLL-ELL transforms primary myeloid progenitors and causes acute myeloid leukemias in mice. Proc Natl Acad Sci USA. 2000;97:10984–10989. doi: 10.1073/pnas.190167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma C, Staudt L M. LAF-4 encodes a lymphoid nuclear protein with transactivation potential that is homologous to AF-4, the gene fused to MLL in t(4;11) leukemias. Blood. 1996;87:734–745. [PubMed] [Google Scholar]
- 15.Nakamura T, Alder H, Gu Y, Prasad R, Canaani O, Kamada N, Gale R P, Lange B, Crist W M, Nowell P C, Croce C M, Canaani E. Genes on chromosomes 4, 9, and 19 involved in 11q23 abnormalities in acute leukemia share sequence homology and/or common motifs. Proc Natl Acad Sci USA. 1993;90:4631–4635. doi: 10.1073/pnas.90.10.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orphanides G, Wu W H, Lane W S, Hampsey M, Reinberg D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature. 1999;400:284–288. doi: 10.1038/22350. [DOI] [PubMed] [Google Scholar]
- 17.Prasad R, Yano T, Sorio C, Nakamura T, Rallapalli R, Gu Y, Leshkowitz D, Croce C M, Canaani E. Domains with transcriptional regulatory activity within the ALL1 and AF4 proteins involved in acute leukemia. Proc Natl Acad Sci USA. 1995;92:12160–12164. doi: 10.1073/pnas.92.26.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reines D, Conaway J W, Conaway R C. Mechanism and regulation of transcriptional elongation by RNA polymerase II. Curr Opin Cell Biol. 1999;11:342–346. doi: 10.1016/S0955-0674(99)80047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogaia D, Grignani F, Carbone R, Riganelli D, LoCoco F, Nakamura T, Croce C M, Di Fiore P P, Pelicci P G. The localization of the HRX/ALL1 protein to specific nuclear subdomains is altered by fusion with its eps15 translocation partner. Cancer Res. 1997;57:799–802. [PubMed] [Google Scholar]
- 20.Rubnitz J E, Morrissey J, Savage P A, Cleary M L. ENL, the gene fused with HRX in t(11;19) leukemias, encodes a nuclear protein with transcriptional activation potential in lymphoid and myeloid cells. Blood. 1994;84:1747–1752. [PubMed] [Google Scholar]
- 21.Shilatifard A, Lane W S, Jackson K W, Conaway R C, Conaway J W. An RNA polymerase II elongation factor encoded by the human ELL gene. Science. 1996;271:1873–1876. doi: 10.1126/science.271.5257.1873. [DOI] [PubMed] [Google Scholar]
- 22.Shilatifard A, Haque D, Conaway R C, Conaway J W. Structure and function of RNA polymerase II elongation factor ELL. Identification of two overlapping ELL functional domains that govern its interaction with polymerase and the ternary elongation complex. J Biol Chem. 1997;272:22355–22363. doi: 10.1074/jbc.272.35.22355. [DOI] [PubMed] [Google Scholar]
- 23.Shilatifard A, Duan D R, Haque D, Florence C, Schubach W H, Conaway J W, Conaway R C. ELL2, a new member of an ELL family of RNA polymerase II elongation factors. Proc Natl Acad Sci USA. 1997;94:3639–3643. doi: 10.1073/pnas.94.8.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Simone F, Polak P E, Kaberlein J J, Luo R T, Levitan D A, Thirman M J. EAF1, a novel ELL-associated factor that is delocalized by expression of the MLL-ELL fusion protein. Blood. 2001;98:201–209. doi: 10.1182/blood.v98.1.201. [DOI] [PubMed] [Google Scholar]
- 24.Slany R K, Lavau C, Cleary M L. The oncogenic capacity of HRX-ENL requires the transcriptional transactivation activity of ENL and the DNA binding motifs of HRX. Mol Cell Biol. 1998;18:122–129. doi: 10.1128/mcb.18.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taki T, Kano H, Taniwaki M, Sako M, Yanagisawa M, Hayashi Y. AF5q31, a newly identified AF4-related gene, is fused to MLL in infant acute lymphoblastic leukemia with ins(5;11)(q31;q13q23) Proc Natl Acad Sci USA. 1999;96:14535–14540. doi: 10.1073/pnas.96.25.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thirman M J, Gill H J, Burnett R C, Mbangkollo D, McCabe N R, Kobayashi H, Ziemin-van der Poel S, Kaneko Y, Morgan R, Sandberg A A, Chaganti R S K, Larson R A, Le Beau M M, Diaz M O, Rowley J D. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med. 1993;329:909–914. doi: 10.1056/NEJM199309233291302. [DOI] [PubMed] [Google Scholar]
- 27.Thirman M J, Levitan D A, Kobayashi H, Simon M C, Rowley J D. Cloning of ELL, a gene that fuses to MLL in a t(11;19)(q23;p13.1) in acute myeloid leukemia. Proc Natl Acad Sci USA. 1994;91:12110–12114. doi: 10.1073/pnas.91.25.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thirman M J, Diskin E B, Bin S S, Ip H S, Miller J M, Simon M C. Developmental analysis and subcellular localization of the murine homologue of ELL. Proc Natl Acad Sci USA. 1997;94:1408–1413. doi: 10.1073/pnas.94.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tkachuk D C, Kohler S, Cleary M L. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71:691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 30.Wada T, Takagi T, Yamaguchi Y, Watanabe D, Handa H. Evidence that P-TEFb alleviates the negative effect of DSIF on RNA polymerase II-dependent transcription in vitro. EMBO J. 1998;17:7395–7403. doi: 10.1093/emboj/17.24.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano T, Nakamura T, Blechman J, Sorio C, Dang C V, Geiger B, Canaani E. Nuclear punctate distribution of ALL-1 is conferred by distinct elements at the N terminus of the protein. Proc Natl Acad Sci USA. 1997;94:7286–7291. doi: 10.1073/pnas.94.14.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziemin-van der Poel S, McCabe N R, Gill H J, III, Espinosa R, Patel Y, Harden A, Rubinelli P, Smith S D, LeBeau M M, Rowley J D, Diaz M O. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci USA. 1991;88:10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]