Abstract
Background
Regional anaesthesia (spinal or epidural anaesthesia) for caesarean section is the preferred option when balancing risks and benefits to the mother and her fetus. Spinal anaesthesia for caesarean section is thought to be advantageous due to simplicity of technique, rapid administration and onset of anaesthesia, reduced risk of systemic toxicity and increased density of spinal anaesthetic block.
Objectives
To assess the relative efficacy and side‐effects of spinal versus epidural anaesthesia in women having caesarean section.
Search methods
The Cochrane Pregnancy and Childbirth Group Trials Register (February 2003) and the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 1, 2003).
Selection criteria
Types of studies considered for review include all published randomised controlled trials involving a comparison of spinal with epidural anaesthesia for caesarean section.
Data collection and analysis
Two reviewers independently assessed trials for inclusion. Review Manager software was used for calculation of the treatment effect represented by relative risk (RR) and weighted mean difference (WMD) using a random effects model with 95% confidence intervals (CI).
Main results
Ten trials (751 women) met our inclusion criteria. No difference was found between spinal and epidural techniques with regards to failure rate (RR 0.98, 95% CI 0.23 to 4.24; four studies), need for additional intraoperative analgesia (RR 0.88, 95% CI 0.59 to 1.32; five studies), need for conversion to general anaesthesia intraoperatively, maternal satisfaction, need for postoperative pain relief and neonatal intervention. Women receiving spinal anaesthesia for caesarean section showed reduced time from start of the anaesthetic to start of the operation (WMD 7.91 minutes less (95% CI ‐11.59 to ‐4.23; four studies), but increased need for treatment of hypotension RR 1.23 (95% CI 1.00 to 1.51; six studies).
Authors' conclusions
Both spinal and epidural techniques are shown to provide effective anaesthesia for caesarean section. Both techniques are associated with moderate degrees of maternal satisfaction. Spinal anaesthesia has a shorter onset time, but treatment for hypotension is more likely if spinal anaesthesia is used. No conclusions can be drawn about intraoperative side‐effects and postoperative complications because they were of low incidence and/or not reported.
Plain language summary
Spinal versus epidural anaesthesia for caesarean section
Effective regional anaesthesia for caesarean section can be achieved by both spinal or epidural techniques.
Compared to epidural, spinal anaesthesia allows surgery to begin earlier, but increases the need to treat hypotension. There was no difference shown with respect to failure rate, need for additional intraoperative analgesia, conversion to general anaesthesia intraoperatively, maternal satisfaction, and neonatal intervention. Differences in side‐effects such as post dural puncture headache, nausea and vomiting, and postoperative complications needing anaesthetic intervention were inconclusive due to the small numbers reported. No studies reported breastfeeding ability and time to ambulation post surgery.
Background
Anaesthesia‐related maternal mortality is decreased when general anaesthesia is avoided (Hawkins 1997; Hibbard 1996). Maternal mortality related to anaesthesia fell from 12.8 to 1.7 per one million live births in the UK and 4.3 to 1.9 per one million live births in the US between the late 1970s and the late 1980s. This is believed to be partly due to the increasing use of regional anaesthesia for caesarean delivery (Ezri 2001). Therefore regional anaesthesia (spinal or epidural anaesthesia) for elective caesarean section is often the preferred option of caregivers when balancing risks and benefits to the mother and her fetus. However some women prefer a general to a regional anaesthetic (e.g. the mother may request to be asleep during the operation). General anaesthesia may also be required for elective caesarean sections if regional anaesthesia is contraindicated.
With regional anaesthesia, the mother and partner are able to share in the experience of the delivery, which may enhance parental‐baby bonding (Reisner 1987). The incidence and indications for elective caesarean section in different countries vary, depending on resources available and attitudes towards 'natural' birth. The incidence of caesarean section can be as high as 55% in South America (Behaque 2002), or as low as 15.5% in England (Chamberlain 1999). Epidural anaesthesia was the regional anaesthetic of choice for caesarean section in North America in 1992, but this had changed to spinal anaesthesia by 1997 (Hawkins 1999). Spinal anaesthesia has been the preferred technique in the UK for over a decade (Scott 1995). In a recent survey of hospitals (total 37,000 births a year) in the south‐west Thames region of the UK, the rate of regional anaesthesia for elective caesarean section was 94.9%, with spinal anaesthesia being used in 86.6% of these cases; for emergency caesarean section, the regional anaesthesia rate was 86.7% with spinal anaesthesia being used in 44.1% of cases (Jenkins 2003).
A spinal anaesthetic involves inserting a fine needle in the lower back and passing it beyond the epidural space through the dura in order to enter the subarachnoid (spinal or intrathecal) space within which is contained the spinal nerves and cerebrospinal fluid. Local anaesthetic drugs such as bupivacaine are typically injected through the spinal needle into the subarachnoid space. Following injection, the spinal needle is removed. An epidural anaesthetic typically involves inserting a larger diameter needle than a spinal needle in order to allow epidural catheter placement. The epidural needle passes through the same tissues as a spinal needle but stops short of penetrating the dura. The tip of the epidural needle is thus positioned in the epidural space which lies just before the dura and subarachnoid space. An epidural catheter is often passed through the epidural needle which is then removed. The epidural catheter can then be used for injecting local anaesthetic medications to allow caesarean section to take place comfortably for the mother and for the administration of pain‐relieving medications postoperatively (Reisner 1999).
Although the medications used in both spinal and epidural techniques are similar, approximately ten times the volume of anaesthetic is required for an epidural technique to achieve a similar level of anaesthesia for caesarean section compared to spinal anaesthesia. As well as local anaesthetics, other analgesic medications such as fentanyl are sometimes added to enhance the duration and quality of both spinal and epidural anaesthesia (Cousins 1998). The spinal or epidural medications act on nerves supplying the uterus, abdominal wall and lower chest, thus allowing caesarean section to be performed without discomfort while the mother is awake (Bridenbaugh 1998).
Potential adverse effects common to both spinal and epidural anaesthetic techniques include: failure to provide adequate anaesthesia, maternal hypotension, post dural puncture headache (PDPH) (Weeks 1999), itching and transient backache over the injection site. Rare serious complications include meningitis, compression of the spinal cord from a blood clot or abscess and damage to nerve roots causing paraesthesia or weakness. Spinal needles are designed to minimise the incidence of PDPH (Weeks 1999), which is approximately 1%. Epidural needles are not designed to enter the subarachnoid space and if they do so accidentally, which occurs in approximately 1% of women, they are associated with an 80% chance of developing a PDPH (Brown 1999). This complication can sometimes be disabling (Weir 2000). If the headache fails to resolve spontaneously or with symptomatic treatment, an epidural blood patch is permanently effective in 60% to 70% (Weeks 1999).
Provision of postoperative analgesia can be achieved by the addition of analgesic medications such as pethidine via the epidural catheter left in situ, or by the addition of morphine to the spinal anaesthetic (MacKay 1996; Reisner 1999; Rout 2000). Good post‐caesarean analgesia can enhance ambulation, breastfeeding and early maternal‐infant bonding (Sinatra 1999).
The available data indicate a trend toward spinal anaesthesia for elective caesarean section (Hawkins 1999; Riley 1995; Stamer 1999). This is thought to be due to the perceived advantages of simplicity of technique, rapid administration and onset of anaesthesia, reduced risk of systemic toxicity and density of spinal anaesthetic block. The primary outcome for this review is to compare spinal versus epidural anaesthesia to allow completion of elective caesarean section.
Objectives
To assess the relative effects of spinal versus epidural anaesthesia in women having caesarean section.
Methods
Criteria for considering studies for this review
Types of studies
All published randomised controlled trials that compare spinal with epidural anaesthesia for caesarean section.
Types of participants
Women having spinal or epidural anaesthesia for caesarean section.
Types of interventions
Spinal or epidural anaesthesia techniques used to provide anaesthesia for caesarean section. Combined spinal‐epidural techniques are excluded.
Types of outcome measures
The main outcome of interest will be the provision of adequate anaesthesia during surgery. This will be determined by the number of women:
failing to achieve adequate anaesthesia to begin surgery;
who required another anaesthetic technique (e.g. general anaesthesia) during the course of surgery;
who received additional interventions for pain relief during surgery; e.g. intravenous opioids, gaseous analgesia such as nitrous oxide/oxygen, or local anaesthetic infiltration by the surgeon;
who were unsatisfied with their anaesthetic.
Secondary outcomes will include the number of women receiving:
treatment for hypotension after commencement of the anaesthetic;
any other intervention, e.g. for nausea and vomiting during surgery;
treatment for post dural puncture headache postoperatively;
unplanned interventions for pain relief postoperatively;
anaesthetic intervention postoperatively for any reason, e.g. nerve damage, delayed respiratory depression.
Other outcomes:
the number of women able to breastfeed satisfactorily;
time to ambulation post surgery.
For the neonate, the outcomes to be examined include:
any requirement for neonatal intervention, e.g. admission to neonatal unit, intubation etc.
The difference in time taken for surgery to commence between epidural and spinal patients was determined where possible (when means and standard deviations were provided).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group trials register (February 2003).
The Cochrane Pregnancy and Childbirth Group's trials register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
monthly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness search of a further 37 journals.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Search strategies for identification of studies' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are given a code (or codes) depending on the topic. The codes are linked to review topics. The Trials Search Co‐ordinator searches the register for each review using these codes rather than keywords.
In addition, we searched the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 1, 2003), using the following terms: (cesarean‐section.me or caesarean or cesarean or caesarian or cesarian) and (anesthesia‐obstetrical.me or anesth* or anaesth*) and (spinal or epidural). Additional relevant references referred to in the papers were also retrieved where appropriate to see if they met the criteria for inclusion in this review.
We performed a MEDLINE search (January 2003) using the following terms:
(caesarean or cesarean or caesarian or cesarian)
(epidural and spinal) and (1)
(anaesthesia or anesthesia)
(2 and 3)
limit (4) to randomised controlled trial
Data collection and analysis
Study identification
Types of studies to be considered for review included all published randomised controlled trials involving a comparison of spinal with epidural anaesthesia for caesarean section.
Quality assessment of included studies
Two reviewers independently assessed the quality of all relevant studies.
Trials under consideration were evaluated for appropriateness for inclusion and methodological quality without consideration of their results. There is no blinding of authorship.
Included trial data were processed as described in Clarke 2003. Included trials were assessed according to the following five main criteria:
adequate concealment of treatment allocation (e.g. opaque sealed numbered envelopes);
method of allocation to treatment (e.g. by computer randomisation, random number tables);
adequate documentation of how exclusions were handled after treatment allocation ‐ to facilitate intention to treat analysis;
adequate blinding of outcome assessment;
losses to follow up (trials with losses of data regarding certain outcomes (e.g. post dural puncture headache) greater than 20% had those particular outcomes excluded from the analysis).
Data extraction
We extracted data using a structured form that captured patient demographics (e.g. inclusion/exclusion criteria, number enrolled, number lost to follow up). The technique and drug details of the spinal and epidural groups were noted. Two reviewers independently extracted outcome data; differences were resolved by referring to the original study.
Data entry
Following agreement on which trials to include, one reviewer entered data into Review Manager (RevMan 2003) while the second reviewer checked it against the other's data extraction. Where clarification on any aspect of a study was needed, one reviewer contacted the author of the trial for further information.
Data analysis
We expressed dichotomous data as relative risks with 95% confidence intervals.
We expressed continuous data as weighted mean differences with 95% confidence intervals.
We used a random effects model.
We performed an intention to treat analysis (including all randomised patients) where possible.
We performed subgroup analyses for studies where different local anaesthetics were used (e.g. lignocaine instead of bupivacaine) or where other analgesic medications were added to the local anaesthetic (e.g. fentanyl or morphine used in the epidural and/or spinal groups).
We assessed possible sources of heterogeneity by sensitivity analyses. We performed sensitivity analyses omitting trials that did not report comparable groups to see if any differences could be detected between unconfounded and potentially confounded comparisons.
We also performed a sensitivity analysis including only the trials that specified a particular dose of fluid preload, and looking only at need for treatment for hypotension. The purpose of the analysis was to see if prophylactic fluid preload reduced the risk of hypotension. This analysis was not specified in our protocol.
We used the Review Manager software (RevMan 2003) for statistical analyses. In the event of differences in unspecified outcomes or subgroups being found, we analysed these post hoc, but identified them clearly as such to avoid drawing unjustified conclusions.
Results
Description of studies
See Tables 'Characteristics of included studies' and 'Characteristics of excluded studies' for details of individual studies.
Methods
Ten trials involving 751 women met our criteria for inclusion. All included studies reported obtaining informed consent from the participants and all had prior ethics committee approval. In two, studies verbal rather than written consent was obtained (Olofsson 1997; Vegfors 1992). While all studies stated that patients were randomly allocated to treatment groups, only three explicitly stated the methods used (Ledan 1993; Lertakyamanee 1999; Mahajan 1992). Operators and outcome assessors in the included studies were not blinded to the technique, but in two trials the assessor was reported as being blinded to group allocation for at least some outcome assessments (Lertakyamanee 1999; Olofsson 1997). (See Table 'Characteristics of included studies').
Participants
All but two of the included trials studied healthy women at term scheduled to have an elective caesarean section. Two trials included women who were not having elective caesarean sections. The first studied 31 women not in labour but who were undergoing caesarean delivery (including three women with ruptured membranes) (Saito 1998). The second trial studied women at term with "normal pregnancies scheduled to have elective or emergency caesarean sections during office hours" (Lertakyamanee 1999). (See Table 'Characteristics of included studies').
Interventions
All included studies compared spinal with epidural anaesthesia for caesarean delivery. The majority of the studies used 0.5% bupivacaine as the local anaesthetic for both spinal and epidural groups. One trial used lignocaine for both groups (Lertakyamanee 1999), two trials used different local anaesthetics for its spinal and epidural groups (Saito 1998; Vegfors 1992) and one trial used different concentrations of bupivacaine for both groups (Mahajan 1992). Only one trial studied fentanyl in addition to bupivacaine for both groups (Olofsson 1997).
Seven studies used a variable dose of local anaesthetic for its epidural groups (Alahuhta 1990; Helbo‐Hansen 1988; Jani 1989; Ledan 1993; Lertakyamanee 1999; Mahajan 1992; Vegfors 1992) while three trials used a fixed dose of local anaesthetic for the epidural groups (Erbay 2001; Olofsson 1997; Saito 1998). Of the other seven studies using a variable epidural dose, five described the addition of local anaesthetic into the epidural space to achieve a specified level of anaesthesia (Alahuhta 1990; Helbo‐Hansen 1988; Jani 1989; Ledan 1993; Vegfors 1992), and two studies did not give a reason as to why a variable dose was used (Lertakyamanee 1999; Mahajan 1992). In contrast, most studies used a fixed dose of local anaesthetic for its spinal groups. Only two trials used a range of local anaesthetic doses for its spinal group without stating a reason why (Jani 1989; Mahajan 1992). Most trials used approximately 2.5 ml of 0.5% bupivacaine or its equivalent for spinal anaesthesia. Only two trials differed by using 5% dibucaine (Saito 1998) and 5% lignocaine (Lertakyamanee 1999).
The woman's position during insertion of the spinal needle for anaesthesia was divided equally between the sitting (Alahuhta 1990; Olofsson 1997; Vegfors 1992) and lateral (Helbo‐Hansen 1988; Mahajan 1992; Saito 1998) positions, with three studies in each position. However, four studies made no mention of the woman's position for spinal anaesthesia (Erbay 2001; Jani 1989; Ledan 1993; Lertakyamanee 1999). The majority of women receiving epidural anaesthesia had the needles inserted in the left lateral position (Alahuhta 1990; Mahajan 1992; Olofsson 1997; Saito 1998; Vegfors 1992) with only two studies performing the epidural needle insertion in the sitting position (Helbo‐Hansen 1988; Ledan 1993). Three studies did not mention patient positioning for epidural insertion (Erbay 2001; Jani 1989; Lertakyamanee 1999).
Most of the studies performed the spinal anaesthetic with a small gauge spinal needle (25 gauge or smaller). Three studies did not mention the size of the spinal needle used (Erbay 2001; Jani 1989; Olofsson 1997), Mahajan 1992 used a 22 to 24 gauge needle, and Ledan 1993 used a 24 gauge Sprotte needle. The spinals were inserted most frequently at L3‐4, but three studies did not mention the level used (Erbay 2001; Jani 1989; Vegfors 1992) and one study used L4‐5 as the level of insertion (Saito 1998). For epidural anaesthesia, most studies specified the use of a Tuohy needle but half of the studies did not specify the size of the epidural needle or catheter. When mentioned, most epidurals were performed with the 17 or 18 gauge Tuohy needle (Alahuhta 1990; Ledan 1993; Olofsson 1997; Saito 1998; Vegfors 1992). Three studies did not mention the use of an epidural catheter (Erbay 2001; Lertakyamanee 1999; Mahajan 1992). The level of insertion for both spinal and epidural anaesthetics was comparable for both groups except for Saito 1998 where the spinal was performed at L4‐5 and the epidurals were inserted at L2‐3. Three studies did not specify what levels were used for its spinal or epidural groups (Erbay 2001; Jani 1989; Vegfors 1992). (SeeTable 15 for doses used, level of insertion and use of catheter.)
1. Doses used, level of insertion and use of catheter.
| author | spinal | criteria for anaesth | level of insertion | epidural | criteria for anaesth | level of insertion | catheter used |
| Alahuhta | 2.5 ml 0.5% heavy bupivacaine | T4‐5 | L3‐4 | 0.5% bupivacaine 2+14‐20 ml | additional boluses up to T4‐5 | not mentioned | yes |
| Erbay | 2.5 ml 0.5% heavy bupivacaine | T4‐7 | not mentioned | 10 ml 0.5% heavy bupivacaine | T4‐7 | not mentioned | not mentioned |
| Helbo‐Hansen | 2.6 ml 0.5% plain marcain | T6 | L3‐4 | 3+10 ml + boluses to T6 0.5% plain bupivacaine | boluses to T6 | L2‐3 | yes |
| Jani | 0.5% plain marcain 0.14 mg/kg | not mentioned | not mentioned | Incremental doses of 0.5% plain marcain | not mentioned | not mentioned | yes |
| Ledan | 0.5% heavy marcain 0.08 mg/kg | T6 | L2‐3 | 10 ml 0.5% marcain + 20 ml/hour infusion | T6 | L2‐3 | yes |
| Lertakyamanee | 1.2 ml 5% lignocaine | not mentioned | L3‐4 | 18‐20 ml 2% lignocaine + adrenaline | not mentioned | L3‐4 | not mentioned |
| Mahajan | 1.2‐1.5 ml 1% marcain | ?T5‐6 | L2‐3/L3‐4 | 12‐20 ml 0.5% marcain | ?T5‐6 ‐ unclear if titrated | L2‐3/L3‐4 | not mentioned |
| Olofsson | 2.5 ml 0.5% heavy marcain with/without 10 mcg fentanyl 2 ml heavy 5% dibucaine + tilting | T4 | L3‐4 | 20 ml 0.5% marcain with/without 100 mcg fentanyl | T4 | L3‐4 | yes |
| Saito | 2 ml heavy 5% dibucaine + tilting | tilting to T4 | L4‐5 | 20 ml 2% mepivacaine | T4 | L2‐3 | yes |
| Vegfors | 2.5 ml heavy marcain | height assessed to "desired level of anaesthesia" | not mentioned | 5+17 ml + 5 ml boluses until "desired level of anaesthesia" with mepivacaine 2% + adrenaline | height assessed to "desired level of anaesthesia" | not mentioned | yes |
Outcomes
See 'Additional Tables' for details of individual studies and to compare different studies.
Main outcomes of interest:
Of the main outcomes of interest, four studies reported the number of women failing to achieve adequate anaesthesia to begin surgery (Alahuhta 1990; Helbo‐Hansen 1988; Lertakyamanee 1999; Saito 1998), one study reported the number of women requiring change of anaesthetic technique during the course of surgery (Lertakyamanee 1999), four studies reported the number of women requiring supplemental analgesia during surgery (Alahuhta 1990; Ledan 1993; Olofsson 1997; Vegfors 1992) and two studies reported the number of women unsatisfied with the anaesthetic (Ledan 1993; Lertakyamanee 1999).
Criteria for failure to achieve adequate anaesthesia to begin surgery was specified in the methods section in two studies (Alahuhta 1990; Saito 1998) as the spinal or epidural anaesthetic being unable to achieve a specified dermatomal level of anaesthesia after 30 minutes. The other two studies (Helbo‐Hansen 1988; Lertakyamanee 1999) described the reasons for failure of each technique individually in the results section of each paper. Two of the four studies had their spinal and epidural needles inserted at comparable levels (Helbo‐Hansen 1988; Lertakyamanee 1999). Saito 1998 had its spinal and epidural levels of insertion differing by two segments, and Alahuhta 1990 did not specify what level of insertion its epidural group used. All the spinal local anaesthetics used were fixed doses, but only one study had a fixed dose of epidural local anaesthetic (Saito 1998). However, Saito 1998 used different local anaesthetics for its spinal and epidural groups. Of the three studies that used a variable local anaesthetic dose for its epidural group, only Helbo‐Hansen 1988 specified the reason for doing so. (SeeTable 1 ‐ Failure to achieve adequate anaesthesia to begin surgery.)
2. Failure to achieve adequate anaesthesia to begin surgery.
| study | spinal | epidural |
| Alahuhta | Failure to reach T4‐5 30 minutes after injection 1 failure | Failure to reach T4‐5 30 minutes after injection, LA added to achieve level 8 failures |
| Helbo‐Hansen | 3 failures described in text ‐ T10, sharp despite T5, pain on incision despite T5 | 1 failure to reach T6 bilaterally despite boluses. Unilateral block |
| Lertakyamanee | The randomised technique could be changed at the anaesthetist's consideration but the reasons why were specified. 14 failures | The randomised technique could be changed at the anaesthetist's consideration but the reasons why were specified. 6 failures |
| Saito | Block up to T4, table tilted to achieve level 0 failures | Block up to T4, block tested up to 30 minutes after injection 1 failure |
Change of anaesthetic technique during surgery was reported in one study (Lertakyamanee 1999) but the results were presented on an intention to treat basis. Of the nine patients from the spinal group needing change of anaesthetic technique, five had partial analgesia, one had a high block and three had a block that did not last the operation. Of the six patients from the epidural group needing change of anaesthetic technique, five had partial analgesia, and one had a block that did not last the operation. No other studies reported a change in anaesthetic technique after commencement of surgery. (SeeTable 2 ‐ Need for another anaesthetic technique during the course of surgery.)
3. Need for another anaesthetic technique during the course of surgery.
| study | spinal | operating time | epidural | operating time | reason for change |
| Lertakyamanee | 9 patients to general anaesthesia | 63.1 + 19.3 minutes | 6 patients to general anaesthesia | 69.1 + 19.3 minutes | The randomised technique could be changed at the anaesthetist's consideration but the reasons why were specified. |
Need for supplemental analgesia during surgery was reported in six studies, but in two studies it was reported as "about 1/5ths" of women in both spinal and epidural groups (Lertakyamanee 1999) and "higher anaesthetic requirements" in the epidural group (Erbay 2001). Of the four studies where data were used, Alahuhta 1990 offered pethidine as supplemental analgesia when the visual analogue scale was asked frequently during the course of surgery. Olofsson 1997 rated pain intensity and discomfort using numerical rating scales at defined intervals during surgery, i.e. skin incision, uterine incision, delivery, uterine exteriorization, and peritoneal closure, and pethidine was used as rescue analgesia. Vegfors 1992 used fentanyl if patients complained of discomfort during surgery. Ledan 1993 used a 50/50 mixture of oxygen/nitrous oxide when anaesthetic quality was judged insufficient by the anaesthetist. (SeeTable 3 ‐ Need for additional pain relief during surgery.)
4. Need for additional pain relief during surgery.
| study | spinal | epidural | operation duration |
| Alahuhta | 11 patients required pethidine ‐ median 59.5 mg (range 25‐100 mg). VAS asked frequently during course of surgery | 11 patients required pethidine ‐ median 49.1 mg (range 25‐80 mg). VAS asked frequently during course of surgery | not mentioned |
| Ledan | 3 patients required 50/50 mixture of oxygen/nitrous oxide. Given when anaesthetic quality was insufficient | 2 patients required 50/50 mixture of oxygen/nitrous oxide. Given when anaesthetic quality was insufficient | 55 +‐ 11 minutes |
| Olofsson | 8 patients required pethidine. Pain intensity and discomfort was rated using numerical rating scales at defined intervals during surgery, i.e. skin incision, uterine incision, delivery, uterine exteriorization, and peritoneal closure | 10 patients required pethidine. Pain intensity and discomfort was rated using numerical rating scales at defined intervals during surgery, i.e. skin incision, uterine incision, delivery, uterine exteriorization, and peritoneal closure | not mentioned |
| Vegfors | 1 patients required fentanyl, given when patients complained of discomfort | 6 patients required fentanyl, given when patients complained of discomfort | not mentioned |
| DATA NOT USED | |||
| Erbay | "Higher anaesthetic requirement" ?additional epidural boluses | ||
| Lertakyamanee | About "one‐fifths" of patients required additional analgesia | About "one‐fifths" of patients required additional analgesia | |
Two studies had data on satisfaction with the anaesthetic used for surgery (Ledan 1993; Lertakyamanee 1999). In Lertakyamanee 1999, satisfaction scores were asked 24 hours after the operation with a visual analogue scale, but patients who preferred the same anaesthetic technique again were reported as a percentage. Hence, the number of women not satisfied with their anaesthetic technique in each group was assumed to be the women who did not prefer the same technique again. In Ledan 1993, quality of anaesthesia (on a visual analogue scale (VAS) of 0 to 10) was asked the day after the operation. Number of patients preferring the same technique again was reported. (SeeTable 4 ‐ Satisfaction with technique.)
5. Satisfaction with technique.
| study | spinal | epidural |
| Ledan | Quality of anaesthesia (0 to 10) asked the day after the operation. Patients preferring same technique again reported = number of women satisfied | Quality of anaesthesia (0 to 10) asked the day after the operation. Patients preferring same technique again reported = number of women satisfied |
| Lertakyamanee | Satisfaction scores asked 24 hours post operation with VAS, with percentage of patients preferring same technique again reported = number of women satisfied | Satisfaction scores asked 24 hours post operation with VAS, with percentage of patients preferring same technique again reported = number of women satisfied |
Secondary outcomes
Of the secondary outcomes, six studies reported the number of women requiring treatment for hypotension after commencement of the anaesthetic (Alahuhta 1990; Ledan 1993; Lertakyamanee 1999; Mahajan 1992; Saito 1998; Vegfors 1992), one study reported the number of women requiring treatment for nausea and vomiting during the anaesthetic (Alahuhta 1990), one study reported treatment for post dural puncture headache (Ledan 1993), two studies reported the number of women needing treatment for pain postoperatively (Helbo‐Hansen 1988; Ledan 1993), and one study reported postoperative anaesthetic intervention for Horner's syndrome and backache (Alahuhta 1990).
All included studies reported hypotension and its treatment in both spinal and epidural groups. Data from four studies were not used because they did not report number of women requiring treatment for hypotension, as well as not mentioning criteria for treatment of hypotension (Erbay 2001; Helbo‐Hansen 1988; Jani 1989; Olofsson 1997). Data from Olofsson 1997 were not used due to a difference in timing of ephedrine prophylaxis between the spinal and epidural groups. The other six studies reported clear criteria for treatment of hypotension, but only four studies received a fluid preload prior to institution of the blockade (Alahuhta 1990; Ledan 1993; Lertakyamanee 1999; Vegfors 1992). Mahajan 1992 described giving intravenous fluids "within 5‐10 minutes after injection of local anaesthetic", and Saito 1998 was unclear about its timing and amount of intravenous fluids given. (SeeTable 5 ‐ Treatment for hypotension.)
6. Treatment for hypotension.
| study | preload | treatment criteria |
| Alahuhta | 1000 ml balanced electrolyte solution + 10 ml/kg preload | Ephedrine when systolic blood pressure < 100 mmHg or > 30% control |
| Ledan | All patients received 1000 ml of Ringer‐lactate solution and 30 mg subcutaneous ephedrine before having regional anaesthesia | Hypotension (systolic blood pressure < 100 mmHg or a fall of 30% from pre‐anaesthetic level) was treated with 10 mg ephedrine and infusion of Ringer‐lactate solution |
| Lertakyamanee | All patients received 1 litre of Ringer lactate as preload before the blocks were performed, and had a small pillow under the right buttock to prevent supine hypotension | Ephedrine was given if systolic blood pressure decreased more than 20% of baseline |
| Mahajan | 750‐1000 ml of balanced salt solution within 5‐10 minutes after injection of LA | Ephedrine when systolic blood pressure decreased > 25% from pre‐anaesthetic values |
| Saito | IV fluids given "as necessary to maintain normal hemodynamic values" but "patients in both groups were given comparable amounts of intravenous fluids" | Ephedrine (5 mg boluses) was administered to maintain systolic arterial pressure > 90 mmHg |
| Vegfors | All patients received a preload of 1000 ml Ringer lactate and Macrodex 500 ml 25‐30 minutes before institution of blockade | Hypotension (systolic blood pressure < 100 mmHg) was treated with volume infusion and 5‐10 mg ephedrine iv |
| DATA NOT USED | ||
| Erbay | Reported as higher ephedrine requirement in spinal group, but no numbers reported | |
| Helbo‐Hansen | Reported as doses given but not number of patients treated | |
| Jani | More ephedrine used in spinal group ‐ but no numbers reported | |
| Olofsson | All patients received an iv fluid preload but prophylaxis against hypotension was not standardised ie. timing of ephedrine injection different between groups | |
Five studies reported other interventions during the anaesthetic but only one study (Alahuhta 1990) had data that could be analysed. (SeeTable 6 ‐ Any other intervention during surgery.)
7. Any other intervention during surgery.
| study | treatment criteria |
| Alahuhta | Medication was used during surgery to relieve pain, discomfort, anxiety, nausea and to restore blood pressure |
| DATA NOT USED | |
| Erbay | Nausea and vomiting observed more often in spinal group, but no numbers reported |
| Ledan | Nausea, vomiting, bradycardia reported but no numbers or interventions mentioned |
| Lertakyamanee | Nausea, vomiting and unconsciousness reported but no details of treatment |
| Vegfors | Numbers vomited reported but no treatment given |
Treatment for post dural puncture headache was reported in only one study (Ledan 1993). Headache postoperatively was reported in two other studies (Alahuhta 1990; Vegfors 1992) but no treatment details were given. (SeeTable 7 ‐ Treatment for post dural puncture headache.) Data for postoperative interventions were used from three studies: Helbo‐Hansen 1988 reported number of women requiring pethidine postoperatively, where women were reviewed daily and pethidine intramuscularly was given on request; Ledan 1993 reported number of women requesting analgesia postoperatively, where paracetamol 2 gm was given every six hours if requested; and Alahuhta 1990 reported anaesthetic follow up of women who developed Horner's syndrome and backache. Erbay 2001 reported urinary retention as being more often in women receiving spinal anaesthesia, but did not give actual numbers. Olofsson 1997 reported the amount of analgesics consumed postoperatively, but not the number of women needing analgesics. (SeeTable 8 ‐ Post‐operative interventions ‐ unplanned pain relief and Table 9 ‐ Post‐operative interventions ‐ any other intervention.)
8. Treatment for post dural puncture headache.
| study | treatment details |
| Ledan | 1 patient in spinal group received treatment for post dural puncture headache with a "standard analgesic" (antalgique banal) |
| DATA NOT USED | |
| Alahuhta | Post dural puncture headache ‐ headache described but no treatment given |
| Vegfors | Post dural puncture headache ‐ headache described but no treatment given |
9. Postoperative interventions ‐ unplanned pain relief.
| study | treatment criteria |
| Helbo‐Hansen | Postoperatively, patients were reviewed daily and pethidine im was given on request |
| Ledan | Postoperatively, patients were given 2 gm paracetamol every 6 hours if requested |
| DATA NOT USED | |
| Olofsson | Consumption of analgesics reported but not number of women needing postoperative pain relief |
10. Postoperative interventions ‐ any other intervention.
| study | interventions |
| Alahuhta | One patient from the spinal group was reviewed for backache and two patients from the epidural group was reviewed for Horner's syndrome |
| DATA NOT USED | |
| Erbay | Urinary retention ‐ more often in spinal group, but no treatment details or numbers reported |
Other outcomes
None of the included studies reported satisfactory breast feeding postoperatively or time to ambulation post surgery.
Neonatal outcomes
Neonatal intervention was reported in only one study (Vegfors 1992), but seven studies described assessment of neonates after delivery in various ways such as Apgar scores, neurobehavioral status, neurologic and adaptive capacity scoring and umbilical vessel blood sampling. These studies did not, however, describe any neonatal interventions (Erbay 2001; Helbo‐Hansen 1988; Jani 1989; Ledan 1993; Lertakyamanee 1999; Mahajan 1992; Olofsson 1997). (SeeTable 10 ‐ Neonatal interventions.)
11. Neonatal interventions.
| study | treatment criteria |
| Vegfors | Neonates deemed to require oxygen treatment were determined by the neonatologists. No other criteria was described. |
| DATA NOT USED | |
| Erbay | Apgar scores measured only. |
| Helbo‐Hansen | Umbilical vessel blood sampling and Apgar scores performed. No interventions. |
| Jani | Umbilical vessel blood sampling and Neurological and Adaptive Capacity Scores performed. No interventions. |
| Ledan | Umbilical vessel blood sampling and Apgar scores performed. No interventions. |
| Lertakyamanee | Umbilical vessel blood sampling, Apgar and Neurological and Adaptive Capacity Scores performed. No interventions. |
| Mahajan | Umbilical vessel blood sampling, Apgar and Neurological and Adaptive Capacity Scores performed. No interventions. |
| Olofsson | Umbilical vessel blood sampling, Apgar and Neurological and Adaptive Capacity Scores performed. No interventions. |
Time to surgery
Time taken for commencement of surgery was reported in four studies; Alahuhta 1990; Erbay 2001 and Ledan 1993 measured the time taken for anaesthesia to reach a specified dermatome after injection, and Lertakyamanee 1999 measured the time taken from start of anaesthetic to start of surgery. Three studies measured time taken from incision to delivery of the neonate (Helbo‐Hansen 1988; Mahajan 1992; Vegfors 1992). Olofsson 1997 reported median times from injection to surgery, and Saito 1998 mentioned time from induction to surgery without giving a mean or standard deviation. (SeeTable 11 ‐ Time for surgery to commence.)
12. Time for surgery to commence.
| study | assessment criteria |
| Alahuhta | Onset times for analgesia to reach T6 was recorded. |
| Erbay | Time for block to reach T4 was measured. |
| Ledan | Onset times for analgesia to reach T6 was recorded. |
| Lertakyamanee | Time taken from start of anaesthetic to start of surgery was recorded. |
| DATA NOT USED | |
| Helbo‐Hansen | Induction of anaesthesia to delivery and skin incision to delivery times only. |
| Mahajan | Induction of anaesthesia to delivery, skin incision to delivery and uterine incision to delivery times recorded, not injection to skin incision times. |
| Olofsson | Median (not mean) time taken from injection to surgery. |
| Saito | Time from induction of anaesthesia to surgery given without mean or standard deviation. |
| Vegfors | Induction of anaesthesia to delivery time only. |
Risk of bias in included studies
Methodological quality of the studies were assessed as described in Clarke 2003 (see 'Quality assessment of included studies' in 'Methods of the review' section).
Method of allocation and allocation concealment
All the included trials specified that the women were randomised to receive either spinal or epidural anaesthesia, but only two studies described method of randomisation (Lertakyamanee 1999; Mahajan 1992). None of the trials clearly described allocation concealment.
Documentation of exclusions
Two studies had women excluded after treatment allocation. Vegfors 1992 had three women in the epidural group excluded because "they did not fulfil the inclusion criteria". Helbo‐Hansen 1988 had one woman in the spinal group excluded due to spontaneous labour. Excluded women were included in the intention to treat analysis.
Blinding of outcome assessment
Blinding of outcome assessors was clearly not met in three trials (Alahuhta 1990; Ledan 1993; Lertakyamanee 1999). There was no mention of blinding in the other trials.
Losses to follow up
Follow up of patients was adequate in all trials included, with no losses to follow up.
Effects of interventions
Ten trials (751 women) met our inclusion criteria.
Primary outcomes
No difference between spinal and epidural anaesthesia was seen for any of the primary outcomes. However, confidence intervals were often wide, with insufficient data to be able to detect differences, should they exist. A random effects model has been used throughout.
The relative risk (RR) for failure to achieve adequate anaesthesia to begin surgery was 0.98, 95% confidence interval (CI) 0.23 to 4.24; four studies.
Across five studies, the RR for need for additional pain relief during surgery was 0.88, 95% CI 0.59 to 1.32. Criteria for additional intraoperative analgesia was fairly consistent amongst the studies analysed, with Alahuhta 1990 and Olofsson 1997 using visual analogue scales and numerical rating scales respectively and Vegfors 1992 giving rescue analgesia whenever women complained of discomfort. The dose of local anaesthetic as well as any additives in either spinal or epidural groups could have made a difference to this outcome, but subgroup analyses of the data showed that having bupivacaine or lignocaine, or adding fentanyl to bupivacaine did not seem to make a difference. The epidural technique in Alahuhta 1990; Ledan 1993; and Vegfors 1992 included 'topping up' via the epidural catheter to achieve a specified level of anaesthesia prior to start of surgery, and this accounted for the variable anaesthetic dose. Only Olofsson 1997 used a fixed epidural dose, but half of the women received fentanyl in the epidural group.
Need for another anaesthetic techniqueduring surgery was only measured in one study; the relative risk was 1.53, 95% CI 0.56 to 4.15. Lertakyamanee 1999 measured failure of the anaesthetic technique and therefore change to general anaesthesia intraoperatively, but there were no defined criteria for failure in the methods section of the study. However, each failure and its reason why was defined in the results section of the study. Of note was the author's comment that the success rate of spinal anaesthesia could have been better if bupivacaine instead of lignocaine was used (there were three failures due to the operation outlasting the block). Also of note was the comment that the success rate of epidural anaesthesia in order for surgery to begin could have been improved if the surgeons had waited longer for the anaesthetic to take full effect.
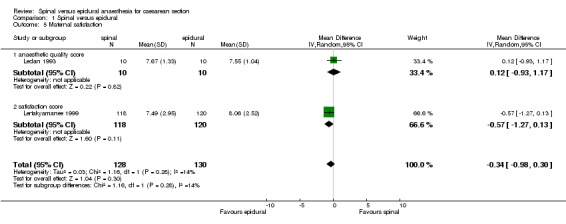
Maternal satisfaction was assessed by Lertakyamanee 1999 and Ledan 1993 as the percentage of women who preferred the same anaesthetic technique again when asked 24 hours postoperatively. From this percentage, we derived the number of women unsatisfied with the technique they were randomised to receive, which showed no difference between the spinal and the epidural groups (RR 1.00, 95% CI 0.71 to 1.41). Both trials also measured satisfaction on a visual analogue scale (VAS) of 0 to 10, with 10 representing the highest level of satisfaction. Pooled scores also showed no difference (WMD ‐0.34 points, 95% CI ‐0.98 to 0.30) with mean scores for each group being 7 or 8 points out of 10.
Other outcomes
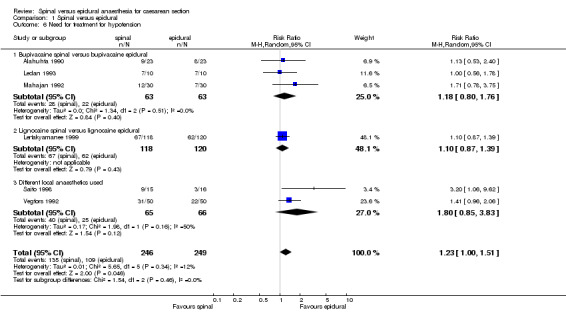
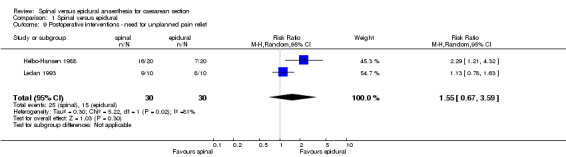
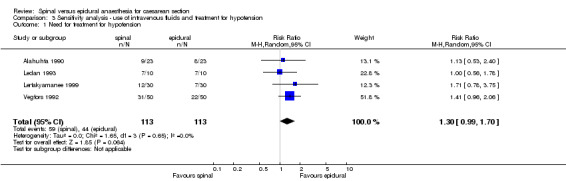
The secondary outcome of needing treatment for hypotension was well described in the six included studies (Alahuhta 1990; Ledan 1993; Lertakyamanee 1999; Mahajan 1992; Saito 1998; Vegfors 1992), including set criteria and method of treatment. The difference in incidence of women needing treatment for hypotension was just statistically significant, with more women in the spinal group needing treatment for hypotension compared with the epidural group (RR 1.23, 95% CI 1.00 to 1.51). This result lost its statistical significance (RR 1.30, 95% CI 0.99 to 1.70) in the sensitivity analysis (MetaView comparison 03/01), which excluded studies that were unclear about fluid preload (Mahajan 1992; Saito 1998). No difference was seen in need for postoperative pain relief in women receiving spinal anaesthesia or epidural for caesarean section; RR 1.55, 95% CI 0.67 to 3.59 (two studies). Data were used from Helbo‐Hansen 1988, which used only 2.6 ml of 0.5% plain bupivacaine, and Ledan 1993, which used 0.08 mg/kg of 0.5% hyperbaric bupivacaine, which may have contributed to the significant statistical heterogeneity of this result. None of the other studies reported postoperative pain relief. Only one other study (Olofsson 1997) used an opioid (fentanyl) in both spinal and epidural groups that could have contributed to postoperative analgesia, but the study did not report any postoperative interventions for pain relief.
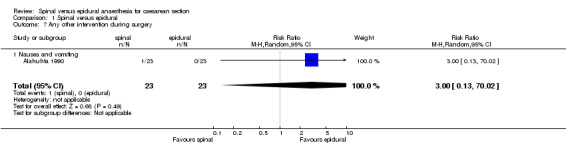
Other secondary outcomes with data able to be analysed were nausea and vomiting, and postoperative interventions for back pain and Horner's syndrome. The numbers reported are too small for any conclusions to be made. In studies unable to be fully analysed, Lertakyamanee 1999 and Vegfors 1992 reported the number of patients vomiting but no treatment details were given. Erbay 2001 observed that nausea and vomiting occurred "more often" in the spinal group, but provided no numbers. Ledan 1993 reported that the frequency of bradycardia and nausea did not differ between the two groups, but gave no details of treatment.
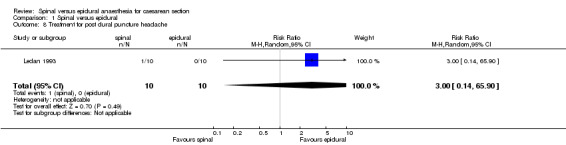
Post dural puncture headache requiring treatment with paracetamol was reported from Ledan 1993 but the numbers were too small to make any conclusions. No studies reported on the need for blood patches after post dural puncture headache.
Urinary retention was reported to have occurred more frequently in the spinal group in Erbay 2001, but the actual number of women who suffered this complication was not mentioned.
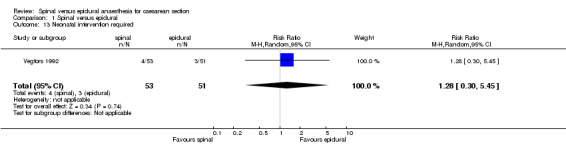
In the one study where data were available, the number of neonates "deemed to require oxygen treatment were determined by the neonatologists" (Vegfors 1992). Unfortunately, no criteria were described for this intervention.
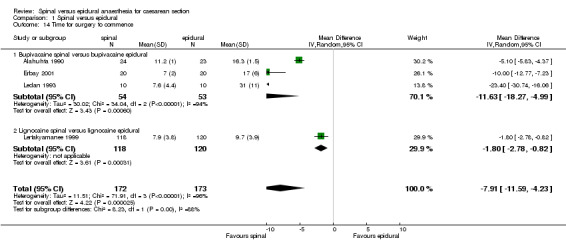
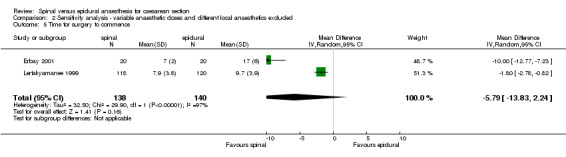
As would be expected, the time taken to achieve adequate anaesthesia for surgery was faster for the spinal group (weighted mean difference (WMD) 7.91 minutes less (95% CI ‐11.59 to ‐4.23; four studies), although there was statistically significant heterogeneity. The use of lignocaine (Lertakyamanee 1999) or bupivacaine (Alahuhta 1990; Erbay 2001; Ledan 1993) still showed faster times for the spinal group when subgroup analyses were performed. The pooled result lost its statistical significance in a sensitivity analysis omitting the two potentially confounded studies (WMD 5.79 minutes less, 95% CI ‐13.83 to 2.24). Although each of the two studies included in this sensitivity analysis showed faster times for the spinal groups, the random effects model gives wide confidence intervals, making the pooled result no longer statistically significant. Alahuhta 1990, Erbay 2001 and Ledan 1993 measured time for the block to reach a specified dermatome, whereas Lertakyamanee 1999 measured time from start of anaesthetic to start of surgery. Several trials measured time from injection to delivery, as well as time from incision to delivery, as this was more relevant to neonatal outcome. Time for preparation and insertion of the spinal versus epidural techniques was not taken into account by any of the studies.
For summary of analyses see MetaView: Tables and Figures.
Discussion
The trend towards spinal anaesthesia as the regional technique of choice for caesarean sections in the US (Hawkins 1999) and UK (Scott 1995) is thought to be due to several reasons, with rapidity of anaesthetic onset, quality of anaesthesia and ease of performance of block amongst them. This review confirms that spinal anaesthesia achieved faster onset of anaesthesia than epidural anaesthesia, but required more treatment for hypotension.
It was not possible to determine which technique women generally preferred. The ways of measuring satisfaction are usually fairly 'blunt' tools and analysing scores, with means and standard deviations as though they were normally distributed, may not be valid. It was also not possible to detect a difference between spinal and epidural failure rates. However, the absolute failure rates for both spinal and epidural groups were typically about 10% in the studies, which seems very high. A recent UK survey deemed a 1.3% failure rate for elective regional anaesthesia and a 4.9% rate for emergency regional anaesthesia as "unacceptably high" (Jenkins 2003). The reasons for the high failure rates in the studies included in this review are not clear, although the type of anaesthetic used (lignocaine) may have contributed to the high failure rate seen in Lertakyamanee 1999.
The practice of adding opioids to spinal or epidural solutions has become more common (Cousins 1998). However, there was not enough information available in the studies included in this review to be able to investigate how the addition of opioids might affect outcomes such as postoperative need for analgesia. Future studies should be designed to address this important question.
Authors' conclusions
Implications for practice.
Both spinal and epidural techniques are shown to provide effective anaesthesia for caesarean section, although failure rates for both techniques were relatively high. Both techniques are associated with moderate degrees of maternal satisfaction with respect to choosing the same technique again. Women receiving spinal anaesthesia are more likely to require treatment for hypotension.
Implications for research.
Future trials might include assessment of time to ambulation post surgery, as well as breastfeeding ability, as these outcomes have practical consequences to the woman undergoing caesarean section, particularly if the operation was elective or planned. Measuring the time taken from injection to achieving anaesthesia for surgery did not include time taken for additional tasks in the preparation for, and performance of, spinal or epidural blockade. Future trials might include a total preparation time from start of the anaesthetic procedure to adequate anaesthesia for surgery. The effects of adding opioids to spinal or epidural solutions require further investigation.
Many trials gave doses of additional analgesia or treatment for hypotension rather than the number of women needing treatment. Neonatal assessments, but not interventions, were reported in many studies. Future trials might include the number of women and their neonates needing treatment as outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 7 March 2012 | Amended | Linked Table 15 to text. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 4 October 2010 | Amended | Contact details edited. |
| 20 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Lynn Hampson for assistance with searching for studies from the Cochrane Pregnancy and Childbirth Group Trials Register. We also thank Sonja Henderson and members of the Cochrane Pregnancy and Childbirth Group Consumer panel for their valuable feedback on the draft protocol. We also thank Richmal Oates‐Whitehead for assistance with translation.
Data and analyses
Comparison 1. Spinal versus epidural.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to achieve adequate anaesthesia to begin surgery | 4 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.23, 4.24] |
| 1.1 Bupivacaine spinal versus bupivacaine epidural | 2 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.04, 12.08] |
| 1.2 Lignocaine spinal versus lignocaine epidural | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [0.94, 5.97] |
| 1.3 Different local anaesthetics used | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 8.08] |
| 2 Need for another anaesthetic technique during surgery | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.56, 4.15] |
| 3 Need for additional pain relief during surgery | 5 | 504 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.59, 1.32] |
| 3.1 Bupivacaine spinal versus bupivacaine epidural | 3 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.61, 1.50] |
| 3.2 Lignocaine spinal versus lignocaine epidural | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.56, 4.15] |
| 3.3 Spinal plus fentanyl versus epidural plus fentanyl | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.17, 1.45] |
| 3.4 Different local anaesthetics used | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.33] |
| 4 Women unsatisfied with anaesthetic | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.71, 1.41] |
| 4.1 Bupivacaine spinal versus bupivacaine epidural | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.02, 7.32] |
| 4.2 Lignocaine spinal versus lignocaine epidural | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.43] |
| 5 Maternal satisfaction | 2 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.98, 0.30] |
| 5.1 anaesthetic quality score | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.93, 1.17] |
| 5.2 satisfaction score | 1 | 238 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.27, 0.13] |
| 6 Need for treatment for hypotension | 6 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.00, 1.51] |
| 6.1 Bupivacaine spinal versus bupivacaine epidural | 3 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.80, 1.76] |
| 6.2 Lignocaine spinal versus lignocaine epidural | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.87, 1.39] |
| 6.3 Different local anaesthetics used | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.85, 3.83] |
| 7 Any other intervention during surgery | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.02] |
| 7.1 Nausea and vomiting | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.02] |
| 8 Treatment for post dural puncture headache | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 65.90] |
| 9 Postoperative interventions ‐ need for unplanned pain relief | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.67, 3.59] |
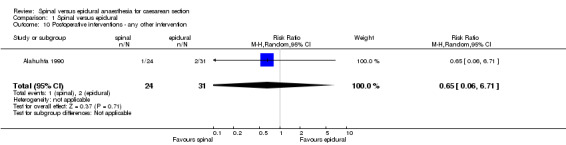
| 10 Postoperative interventions ‐ any other intervention | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.06, 6.71] |
| 11 Women unable to breastfeed satisfactorily | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Time to ambulation post‐surgery | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Neonatal intervention required | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.30, 5.45] |
| 14 Time for surgery to commence | 4 | 345 | Mean Difference (IV, Random, 95% CI) | ‐7.91 [‐11.59, ‐4.23] |
| 14.1 Bupivacaine spinal versus bupivacaine epidural | 3 | 107 | Mean Difference (IV, Random, 95% CI) | ‐11.63 [‐18.27, ‐4.99] |
| 14.2 Lignocaine spinal versus lignocaine epidural | 1 | 238 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐2.78, ‐0.82] |
1.1. Analysis.
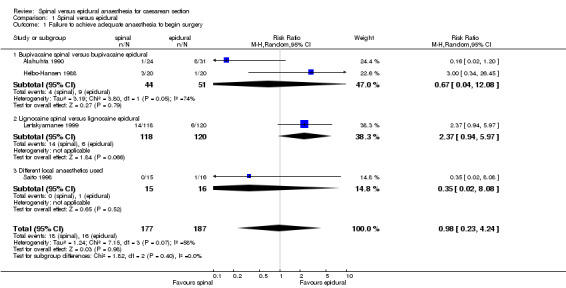
Comparison 1 Spinal versus epidural, Outcome 1 Failure to achieve adequate anaesthesia to begin surgery.
1.2. Analysis.
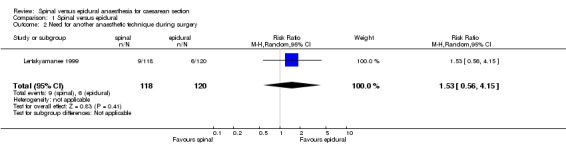
Comparison 1 Spinal versus epidural, Outcome 2 Need for another anaesthetic technique during surgery.
1.3. Analysis.
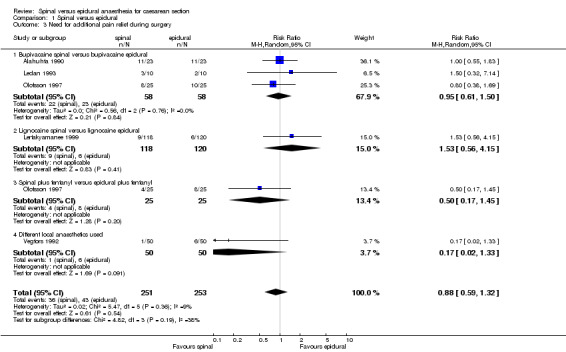
Comparison 1 Spinal versus epidural, Outcome 3 Need for additional pain relief during surgery.
1.4. Analysis.
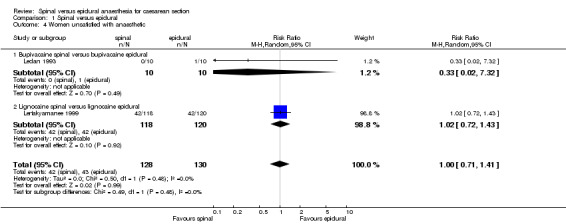
Comparison 1 Spinal versus epidural, Outcome 4 Women unsatisfied with anaesthetic.
1.5. Analysis.
Comparison 1 Spinal versus epidural, Outcome 5 Maternal satisfaction.
1.6. Analysis.
Comparison 1 Spinal versus epidural, Outcome 6 Need for treatment for hypotension.
1.7. Analysis.
Comparison 1 Spinal versus epidural, Outcome 7 Any other intervention during surgery.
1.8. Analysis.
Comparison 1 Spinal versus epidural, Outcome 8 Treatment for post dural puncture headache.
1.9. Analysis.
Comparison 1 Spinal versus epidural, Outcome 9 Postoperative interventions ‐ need for unplanned pain relief.
1.10. Analysis.
Comparison 1 Spinal versus epidural, Outcome 10 Postoperative interventions ‐ any other intervention.
1.13. Analysis.
Comparison 1 Spinal versus epidural, Outcome 13 Neonatal intervention required.
1.14. Analysis.
Comparison 1 Spinal versus epidural, Outcome 14 Time for surgery to commence.
Comparison 2. Sensitivity analysis ‐ variable anaesthetic doses and different local anaesthetics excluded.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to achieve adequate anaesthesia to begin surgery | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [0.94, 5.97] |
| 2 Need for another anaesthetic technique during surgery | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.56, 4.15] |
| 3 Women unsatisfied with anaesthetic | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.43] |
| 4 Treatment for hypotension after commencement of anaesthetic | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.87, 1.39] |
| 5 Time for surgery to commence | 2 | 278 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐13.83, 2.24] |
2.1. Analysis.
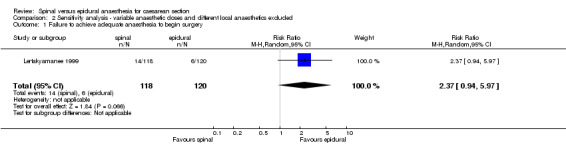
Comparison 2 Sensitivity analysis ‐ variable anaesthetic doses and different local anaesthetics excluded, Outcome 1 Failure to achieve adequate anaesthesia to begin surgery.
2.2. Analysis.
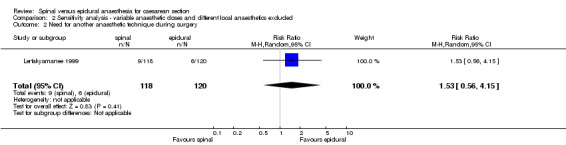
Comparison 2 Sensitivity analysis ‐ variable anaesthetic doses and different local anaesthetics excluded, Outcome 2 Need for another anaesthetic technique during surgery.
2.3. Analysis.
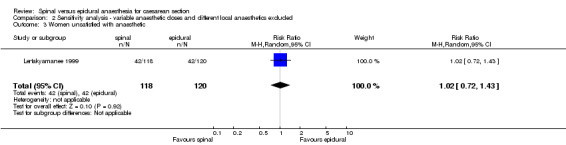
Comparison 2 Sensitivity analysis ‐ variable anaesthetic doses and different local anaesthetics excluded, Outcome 3 Women unsatisfied with anaesthetic.
2.4. Analysis.
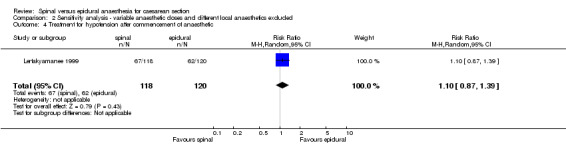
Comparison 2 Sensitivity analysis ‐ variable anaesthetic doses and different local anaesthetics excluded, Outcome 4 Treatment for hypotension after commencement of anaesthetic.
2.5. Analysis.
Comparison 2 Sensitivity analysis ‐ variable anaesthetic doses and different local anaesthetics excluded, Outcome 5 Time for surgery to commence.
Comparison 3. Sensitivity analysis ‐ use of intravenous fluids and treatment for hypotension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for treatment for hypotension | 4 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.99, 1.70] |
3.1. Analysis.
Comparison 3 Sensitivity analysis ‐ use of intravenous fluids and treatment for hypotension, Outcome 1 Need for treatment for hypotension.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alahuhta 1990.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal or epidural anaesthesia, no other details of methodology given. Allocation concealment: No details given. Documentation of exclusions: No exclusions. Blinding of outcome assessment: No blinding of outcome assessor ‐ "The patients were closely and continuously observed by the anaesthetists performing the blockade". Losses to follow up: None. |
|
| Participants | Inclusion criteria:
55 women undergoing elective caesarean section at university hospital in Finland. Inclusion criteria were term gestation with no medical or obstetric complications. Exclusions: None mentioned. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
2.5 ml of 0.5% bupivacaine containing 8% glucose injected via a 25 G needle at L3‐4 in the sitting position. Women placed supine immediately after injection with wedge under right hip. Women excluded from study if analgesia did not reach T4‐5 30 minutes after injection. Epidural anaesthesia: 0.5% bupivacaine, 2 ml test dose followed by 14‐20 ml main dose via epidural catheter inserted with 18 G Tuohy needle in the left lateral position. Bupivacaine added until analgesia reached T4‐5. All women received 1000 ml of a balanced electrolyte solution before institution of regional blockade. A further 10 ml/kg was infused during onset of the blockade. All women asked frequently about pain during surgery and pain severity was recorded on a standard visual analogue scale. Medication was used during surgery to relieve pain, discomfort, anxiety and nausea, and to restore blood pressure. |
|
| Outcomes | Number of women failing to achieve adequate anaesthesia to begin surgery was recorded. Adequate anaesthesia being defined as "a T4‐5 level of analgesia 30 minutes after injection….". Number of women receiving additional analgesia during surgery was recorded. Degree of pain measured by standard visual analogue scale, pethidine iv given for analgesia. Number of women requiring treatment for hypotension was recorded. Hypotension defined as systolic blood pressure < 100 mmHg or fall of > 30% of control value. This was treated by increasing the infusion of balanced electrolyte solution and/or giving 5 mg iv bolus(es) of ephedrine. Other interventions for amnesia, nausea and bradycardia were mentioned. Onset times for analgesia to reach T6 were recorded. Postoperative follow up for post‐dural puncture headache was recorded but no treatment was mentioned. Follow up for Horner's syndrome and backache was mentioned. |
|
| Notes | Finland. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Erbay 2001.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal, epidural or combined spinal/epidural anaesthesia. No other details of methodology given. Allocation concealment: No details given. Documentation of exclusions: No exclusions. Blinding of outcome assessment: Not mentioned. Losses to follow up: None. |
|
| Participants | Inclusion criteria:
60 healthy women scheduled for caesarean section at a university hospital in Turkey. Exclusion criteria: None mentioned. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
2.5 ml of 0.5% hyperbaric bupivacaine. Epidural anaesthesia: 10 ml of 0.5% hyperbaric bupivacaine. |
|
| Outcomes | Time for block to reach T4 was measured.
Maternal satisfaction was assessed with no details as to how this was done.
Maternal hypotension was recorded and reported as ephedrine requirement in each group, not number of women treated.
Additional anaesthetic requirements and quality of anaesthesia was reported but with no further details.
Nausea, vomiting and urinary retention was observed as "more often in the spinal group".
Postoperative complications was recorded (see above). Neonates were assessed but no interventions were mentioned. |
|
| Notes | Turkey. Study in abstract form only. Results not given in numerical format. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Helbo‐Hansen 1988.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal or epidural anaesthesia. No other details of methodology given. Allocation concealment: No details given. Documentation of exclusions: One patient in spinal group excluded due to spontaneous labour. Included in intention‐to treat analysis. Blinding of outcome assessment: Not mentioned. Losses to follow up: None. |
|
| Participants | Inclusion criteria:
40 healthy women without maternal or fetal complications booked for elective caesarean section in a university hospital in Denmark. Exclusion criteria: None mentioned. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
2.6 ml plain bupivacaine 0.5% injected in left lateral position through 26 gauge spinal needle at L3‐4 interspace. Women then placed supine with 15 degrees left lateral tilt within 2 minutes after injection. Epidural anaesthesia: With epidural catheter via Tuohy needle at L2‐3 interspace, inserted in sitting position. Test dose of 3 ml 0.5% bupivacaine, followed by initial dose of 10 ml injected 5 minutes later. Women were then placed in the left lateral position after another 5 minutes. Further boluses of 0.5% bupivacaine were given until anaesthetic level had reached T6 bilaterally. Women then turned supine with 15 degrees left lateral tilt for operation. Ephedrine 5‐10 mg was given if systolic blood pressure fell below 100 mmHg or > 30% of baseline. All women received a fluid preload and were given prophylactic ephedrine, but this was not standardised between the spinal and epidural groups. All women were offered iv pethidine during operation if they showed signs of discomfort. Postoperatively, patients were reviewed daily and pethidine im was given on request. |
|
| Outcomes | Number of women failing to achieve adequate anaesthesia to begin surgery and the reasons why were recorded. Treatment for hypotension was recorded as doses used in each group and not number of women treated. Pain experienced during the operation was recorded using a visual analogue scale. There was no record of iv pethidine use due to "excellent analgesia" during surgery. Postoperatively, the number of women receiving pethidine im for analgesia were recorded. Neonates were assessed but no interventions were mentioned. |
|
| Notes | Denmark. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Jani 1989.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal or epidural anaesthesia. No other details of methodology given. Allocation concealment: No details given. Documentation of exclusions: No exclusions. Blinding of outcome assessment: Not mentioned. Losses to follow up: None. |
|
| Participants | Inclusion criteria:
41 healthy women with uncomplicated pregnancies scheduled to undergo elective caesarean section under regional anaesthesia. Exclusion criteria: None mentioned. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
0.5% plain bupivacaine (median dose 0.14 mg/kg). Epidural anaesthesia: Incremental doses of plain 0.5% bupivacaine (median dose 1.7 mg/kg). |
|
| Outcomes | Incidence of hypotension (systolic blood pressure < 100 mmHg) and ephedrine requirement was recorded but not number of women treated. Neonates were assessed but no interventions were mentioned. |
|
| Notes | UK. Study in abstract form only. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ledan 1993.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal or epidural anaesthesia by drawing lots. Allocation concealment: No details given. Documentation of exclusions: No exclusions. Blinding of outcome assessment: No blinding of intraoperative outcomes ‐ the anaesthetist evaluated anaesthetic quality and gave appropriate treatment. Losses to follow up: None. |
|
| Participants | Inclusion criteria:
20 women (ASA 1) after normal full‐term pregnancy wanting a regional anaesthetic. Exclusion criteria: Contra‐indications to regional anaesthesia. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
24 gauge Sprotte needle inserted at L2‐3. 0.08 mg/cm of 0.5% hyperbaric bupivacaine injected in 30 seconds. Women placed in left lateral 15 degree tilt after induction of anaesthesia. Epidural anaesthesia: 17 G Tuohy needle inserted in sitting position at L2‐3. 10 ml 0.5% plain bupivacaine injected followed 5 minutes later by 20 ml/hour infusion until T6 level achieved. Women placed in left lateral 15 degree tilt after induction of anaesthesia. All women received 1000 ml of Ringer‐lactate solution and 30 mg subcutaneous ephedrine before having regional anaesthesia. Hypotension (systolic blood pressure < 100 mmHg or a fall of 30% from pre‐anaesthetic level) was treated with 10 mg ephedrine and infusion of Ringer‐lactate solution. The anaesthetist evaluated anaesthetic quality and gave a 50/50 mixture of oxygen and nitrous oxide when anaesthetic quality was deemed insufficient. Perioperative complications were noted ‐ nausea, vomiting and bradycardia but no interventions were described. |
|
| Outcomes | Number of women receiving additional interventions for pain relief during surgery was recorded. A 50/50 mixture of oxygen/nitrous oxide was given if anaesthetic quality was deemed insufficient by the anaesthetist. Number of women needing treatment for hypotension was recorded. Onset time for analgesia to reach T6 was recorded. Postoperatively, the number of women receiving paracetamol for analgesia was recorded. Number of women who were satisfied with the anaesthetic technique was also recorded. Neonates were assessed but no interventions were mentioned. |
|
| Notes | France. Study translated from French to English. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lertakyamanee 1999.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal or epidural anaesthesia with random number table. Allocation concealment: No details given. Documentation of exclusions: No exclusions. Blinding of outcome assessment: No blinding of intraoperative outcomes ‐ "the attending anesthesiologist decided to give other treatment as clinically indicated". Losses to follow up: None. |
|
| Participants | Inclusion criteria:
238 normal pregnancies at term scheduled to have elective or emergency caesarean sections during office hours. Exclusion criteria: Women with abruptio placenta, bleeding placenta praevia, fetal distress, diabetes mellitus, moderate to severe hypertension of pregnancy, severe cardiac or respiratory disease, multiple pregnancy, and coagulopathy. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
25 G spinal needle inserted at L3‐4. 1.2 ml 5% lignocaine injected. Epidural anaesthesia: Tuohy needle at L3‐4. 18‐20 ml of 2% lignocaine with adrenaline 1:200,000. All patients received 1 litre of Ringer lactate as preload before the blocks were performed, and had a small pillow under the right buttock to prevent supine hypotension. Ephedrine was given if systolic blood pressure decreased more than 20% of baseline. The attending anaesthetist "decided to give other treatment as clinically indicated, e.g., vasopressor, sedatives, intravenous fluid, blood, etc". The randomised technique could be changed at the anaesthetist's consideration but the reasons why were specified. |
|
| Outcomes | Number of women failing to achieve adequate anaesthesia to begin surgery and number of women requiring change of the anaesthetic technique during surgery were recorded, as well as reasons for the above. Number of women who were satisfied with the anaesthetic technique was also recorded. Number of women requiring treatment for hypotension was recorded. Time taken from start of anaesthetic to start of surgery was recorded. Neonates were assessed but no interventions were mentioned. |
|
| Notes | Thailand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mahajan 1992.
| Methods | Treatment allocation:
Women were allocated to receive general, epidural or spinal anaesthesia according to a random chart. Allocation concealment: No details given. Documentation of exclusions: No exclusions. Blinding of outcome assessment: Not mentioned. Losses to follow up: None. |
|
| Participants | Inclusion criteria:
99 healthy parturients (ASA 1) presenting for elective caesarean section.
Exclusion criteria:
None mentioned. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
22‐24 gauge needle inserted in the lateral position at L2‐3 or L3‐4. 1.2‐1.5 ml of 1% bupivacaine injected slowly. Epidural anaesthesia: Tuohy needle, lateral position, L2‐3 or L3‐4, loss of resistance to air, 12‐20 ml 0.5% bupivacaine injected slowly. Women were then placed supine with a wedge under the right hip. Women in the epidural and spinal groups were infused with 750‐1000 ml of balanced salt solution within 5‐10 minutes after injection of local anaesthetic. |
|
| Outcomes | Number of women requiring treatment for hypotension was recorded. Ephedrine was given if systolic blood pressure decreased > 25% from pre‐anaesthetic values. Neonates were assessed but no interventions were mentioned. |
|
| Notes | India. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Olofsson 1997.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal or epidural anaesthesia. No other details of methodology given. Allocation concealment: No details given. Blinding with regard to the addition of fentanyl to the local anaesthetic was performed by substituting fentanyl with saline in the control group. Documentation of exclusions: No exclusions. Blinding of outcome assessment: Not mentioned. Losses to follow up: None. |
|
| Participants | Inclusion criteria:
100 women scheduled for elective caesarean section. All were ASA 1 with uncomplicated pregnancies. Exclusion criteria: None mentioned. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
Performed at the L3‐4 interspace, inserted in the sitting position. 2.5 ml 0.5% hyperbaric bupivacaine injected with or without 10 mcg fentanyl. Women were then placed supine with a wedge under the right hip. Epidural anaesthesia: 18 G Tuohy needle inserted at the L3‐4 interspace in the left lateral position. An epidural catheter was threaded 3 cm into epidural space. 20 ml 0.5% bupivacaine with or without 100 mcg fentanyl was injected via the catheter into the epidural space. Women then turned into the tilted supine position (as above) following the injection. Pain intensity and discomfort was rated using numerical rating scales at defined intervals during surgery, i.e.. skin incision, uterine incision, delivery, uterine exteriorization, and peritoneal closure. Discomfort described as non‐painful sensations during surgery such as pressure and traction. Rescue analgesic medication during surgery was iv fentanyl 50 mcg x 2 followed by nitrous oxide followed by general anaesthesia. For discomfort and anxiety, diazepam in iterative doses of 2.5 mg iv was used followed by general anaesthesia. All women received an iv fluid preload but prophylaxis against hypotension was not standardised i.e.. timing of ephedrine injection different between groups. |
|
| Outcomes | Number of women requiring additional pain relief during surgery was recorded. Number of women requiring iv diazepam for discomfort was recorded. Number of women receiving ephedrine for hypotension was recorded, but data not used due to lack of standardization. Consumption of analgesics post‐op was recorded as doses used but not number of women treated. Neonates were assessed but no interventions were mentioned. |
|
| Notes | Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Saito 1998.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal or epidural anaesthesia. No other details of methodology given. Allocation concealment: No details given. Documentation of exclusions: No exclusions. Blinding of outcome assessment: Not mentioned. Losses to follow up: None. |
|
| Participants | Inclusion criteria:
31 women undergoing caesarean delivery.
All were ASA 1 or 2. None were in labour but 3 women had ruptured membranes. Exclusion criteria: None mentioned. Number lost to follow up: None. |
|
| Interventions | Spinal anaesthesia:
25 G needle inserted in the lateral position at L4‐5, 2 ml hyperbaric 5% dibucaine injected. The operating room table was then tilted to achieve block to T4. Epidural anaesthesia: 17 G Tuohy needle inserted in the lateral position with the paramedian approach at L2‐3 using loss of resistance to air. 20 ml 2% mepivacaine subsequently injected as a bolus through the catheter without a test dose. Sensory block to cold and pinprick was evaluated 5, 10, 15 and 30 minutes after induction of anaesthesia. IV fluids were given "as necessary to maintain normal hemodynamic values" but "patients in both groups were given comparable amounts of intravenous fluids". Ephedrine (5 mg boluses) was administered to maintain systolic arterial pressure > 90 mmHg. |
|
| Outcomes | Number of women failing to achieve adequate anaesthesia to begin surgery (block up to T4) was recorded. Number of women requiring treatment (ephedrine) for hypotension was recorded. Time taken for surgery to commence was reported but not analysed because the standard deviation was not reported. |
|
| Notes | Japan. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Vegfors 1992.
| Methods | Treatment allocation:
Women randomly assigned to receive spinal or epidural anaesthesia. No other details of methodology given. Allocation concealment: No details given. Documentation of exclusions: Three women in epidural group excluded because "they did not fulfil the inclusion criteria". No other details given. Included in intention‐to‐treat analysis. Blinding of outcome assessment: Not mentioned. Losses to follow up: Three women. |
|
| Participants | Inclusion criteria:
100 women requesting regional anaesthesia for elective caesarean section at a university hospital in Sweden. Inclusion criteria were normal pregnancy at term and no known maternal disease. Exclusion criteria: Three women in epidural group excluded because "they did not fulfil the inclusion criteria". No other details given. Number lost to follow up: Three (see above). |
|
| Interventions | Spinal anaesthesia:
2.5 ml of bupivacaine 5 mg/ml in 8% glucose, performed in the sitting position with a 25 gauge Quincke needle. Women placed supine with left lateral tilt within 1 minute of injection. Height of block tested with temperature discrimination 10 minutes after injection. Epidural anaesthesia: Epidural catheter inserted with women in the lateral position through a 17 gauge needle. Mepivacaine 20 mg/ml with adrenaline 5 mg/ml injected via catheter, test dose of 5 ml first, followed 5 minutes later by main dose of 17 ml as a bolus injection. 5 ml supplements given if desired level of anaesthesia not reached after 30 mins. Women turned supine with left lateral tilt after injection of main dose. Height of block tested with temperature discrimination 30 minutes after injection of main dose. All patients received a preload of 1000 ml Ringer lactate and Macrodex 500 ml 25‐30 minutes before institution of blockade. Hypotension (systolic blood pressure < 100 mmHg) was treated with volume infusion and 5‐10 mg ephedrine iv. |
|
| Outcomes | Number of women requiring additional analgesia during surgery was recorded. IV fentanyl supplements were given when women complained about perioperative discomfort. Number of women requiring treatment for hypotension was recorded. Number of neonates requiring supplemental oxygen after delivery was recorded. Neonates deemed to require oxygen treatment were determined by the neonatologists. No other criteria were described. |
|
| Notes | Sweden. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
G: gauge IM: intramuscular IV: intravenous mins: minutes
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albani 1998 | Intervention not standardised ‐ morphine used intraoperatively in spinal group but not in epidural group. |
| Bernstein 1988 | No outcomes looked for were measured (studied effects of anaesthesia on body temperature). |
| Datta 1983 | Unclear if study was randomised. |
| Douvier 1992 | Doses of local anaesthetics used in spinal and epidural groups not stated. |
| Hagnevik 1988 | Combined spinal‐epidural technique used for spinal group. |
| Lam 1994 | Doses of local anaesthetics used in spinal and epidural groups not stated. |
| Mission 1999 | Number of women randomised to each group not specified. |
| Morgan 2000 | Intervention not standardised ‐ spinal morphine used from beginning of surgery but epidural morphine used only after delivery of fetus. |
| Norman 1998 | Combined spinal‐epidural technique used for spinal group. |
| Ratcliffe 1993 | Women allowed to choose between epidural or spinal anaesthesia for operation. |
| Robson 1992 | Combined spinal‐epidural technique used for spinal group. |
| Russell 1995 | Observational prospective study, not randomised. |
| Sarvela 2002 | Combined spinal‐epidural technique used for spinal group. |
| Valli 1994 | Combined spinal‐epidural technique used for spinal group. |
| Zakowski 1990 | Intervention not standardised ‐ spinal morphine used from beginning of surgery but epidural morphine used only after delivery of fetus. |
Contributions of authors
Planning of review: Allan Cyna, Kar Ng Writing protocol: Kar Ng, Allan Cyna Analysis and writing review: Kar Ng, Jacqueline Parsons, Allan Cyna, Philippa Middleton
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Alahuhta 1990 {published data only}
- Alahuhta S, Kangas‐Saarela T, Hillmen A, Edstrom H. Visceral pain during caesarean section under spinal and epidural anaesthesia with bupivacaine. Acta Anaesthesiologica Scandinavica 1990;34:95‐8. [DOI] [PubMed] [Google Scholar]
Erbay 2001 {published data only}
- Erbay H, Gurses E, Tomatir E, Sungurtekin H, Gonullu M. The comparison of low‐dose combined spinal‐epidural, spinal, and epidural anaesthesia in caesarean section. European Journal of Anaesthesiology 2001;18(Suppl 21):109‐10. [Google Scholar]
Helbo‐Hansen 1988 {published data only}
- Helbo‐Hansen S, Bang U, Garcia R, Olesen A, Kjeldsen L. Subarachnoid versus epidural bupivacaine 0.5% for caesarean section. Acta Anaesthesiologica Scandinavica 1988;32:473‐6. [DOI] [PubMed] [Google Scholar]
Jani 1989 {published data only}
- Jani K, McEvedy B, Harris S, Dossetor J. Neonatal outcome after spinal or epidural bupivacaine 0.5% for elective cesarean section. Anesthesiology 1989;71(3A):A866. [Google Scholar]
- Jani K, McEvedy B, Harris S, Samaan A. Maternal and neonatal bupivacaine concentrations after spinal and extradural anaesthesia for caesarean section. British Journal of Anaesthesia 1989;62(2):226P‐7P. [Google Scholar]
Ledan 1993 {published data only}
- Ledan C, Collet D, Vincelot A, Debord J, Lachatre G, Feiss P. Pharmacokinetics of epidural or intrathecal bupivacaine for elective caesarean section [Pharmacocinetique de la bupivacaine administree par voie peridurale ou sous‐arachnoidienne pour cesarienne reglee]. Annales Francaises d' Anesthesie et de Reanimation 1993;12:552‐9. [DOI] [PubMed] [Google Scholar]
Lertakyamanee 1999 {published data only}
- Kolatat T, Somboonnanonda A, Lertakyamanee J, Chinachot T, Tritrakarn T, Muangkasem J. Effects of general and regional anesthesia on the neonate (a prospective randomized trial). Journal of the Medical Association of Thailand 1999;82(1):40‐5. [PubMed] [Google Scholar]
- Lertakyamanee J, Chinachoti T, Tritrakarn T, Mugangkasem J, Somboonnanonda A, Kolatat T. Comparison of general and regional anesthesia for cesarean section: success rate, blood loss and satisfaction from a randomized trial. Journal of the Medical Association of Thailand 1999;82(7):672‐80. [PubMed] [Google Scholar]
Mahajan 1992 {published data only}
- Mahajan J, Mahajan R, Singh M, Anand N. Anaesthetic technique for elective caesarean section and neurobehavioural status of newborns. International Journal of Obstetric Anesthesia 1992;2:89‐93. [DOI] [PubMed] [Google Scholar]
Olofsson 1997 {published data only}
- Olofsson C, Ekblom A, Skoldefors E, Waglund B, Irestedt L. Anesthetic quality during cesarean section following subarachnoid or epidural administration of bupivacain with or without fentanyl. Acta Anaesthesiologica Scandinavica 1997;41:332‐8. [DOI] [PubMed] [Google Scholar]
Saito 1998 {published data only}
- Saito T, Sessler D, Fujita K, Ooi Y, Jeffrey R. Thermoregulatory effects of spinal and epidural anesthesia during cesarean delivery. Regional Anesthesia and Pain Medicine 1998;23(4):418‐23. [DOI] [PubMed] [Google Scholar]
Vegfors 1992 {published data only}
- Vegfors M, Cederholm I, Gupta A, Lindgren R, Berg G. Spinal or epidural anaesthesia for elective caesarean section?. International Journal of Obstetric Anesthesia 1992;1:141‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albani 1998 {published data only}
- Albani A, Renghi A, Ciarlo M, Avallone V, Toscano M. Epidural anaesthesia versus spinal anaesthesia in caesarean section. A prospective clinical trial [Anestesia peridurale versus anestesia subarachnoidea nel parto cesareo. Studio clinico prospettico]. Minerva Anestesiologica 1998;64:387‐91. [PubMed] [Google Scholar]
Bernstein 1988 {published data only}
- Bernstein J, Ramanathan S, Ramabadran K, Parker F, Turndorf H. Body temperature changes with epidural and intrathecal morphine. Anesthesiology 1988;69(3A):A688. [Google Scholar]
Datta 1983 {published data only}
- Datta S, Carr D, Lambert D, Morrison J, Naulty J, Fischer J, et al. Anesthesia for cesarean delivery: relationship of maternal and fetal plasma B‐endorphin concentrations to different types of anesthesia. Anesthesiology 1983;59(3):A418. [Google Scholar]
Douvier 1992 {published data only}
- Douvier S, Ferrut O, Lancon JP, Feldman JP. Epidural vs spinal anesthesia effects on neonates for elective cesarean section. Journal of Perinatal Medicine 1992;20(1):132. [Google Scholar]
Hagnevik 1988 {published data only}
- Hagnevik K, Irestedt L, Lundell B, Skoldefors E. Cardiac function and sympathoadrenal activity in the newborn after cesarean section under spinal and epidural anesthesia. Acta Anaesthesiologica Scandinavica 1988;32:234‐8. [DOI] [PubMed] [Google Scholar]
Lam 1994 {published data only}
- Lam F, Broome I, Matthews P. A comparison of postoperative analgesia following spinal or epidural anaesthesia for caesarean section. Anaesthesia 1994;49:65‐7. [DOI] [PubMed] [Google Scholar]
Mission 1999 {published data only}
- Mission J, Storme B, Bolandard F, Tubert V, Schoeffler P. Regional anaesthesia for caesarean section: a prospective and randomized study. British Journal of Anaesthesia 1999;82 Suppl:160. [Google Scholar]
Morgan 2000 {published data only}
- Morgan P, Halpern S, Evers J. A comparison of maternal satisfaction between epidural and spinal anaesthesia for elective caesarean section. Canadian Journal of Anaesthesia 1998;45:A60. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Halpern S, Lam‐McCulloch J. Comparison of maternal satisfaction between epidural and spinal anesthesia for elective cesarean section. Canadian Journal of Anaesthesia 2000;47(10):956‐61. [DOI] [PubMed] [Google Scholar]
Norman 1998 {published data only}
- Norman B, Yentis S. Analgesia produced by epidural diamorphine is better following caesarean section under spinal anaesthesia than under epidural anaesthesia. International Journal of Obstetric Anesthesia 1998;7:98‐102. [DOI] [PubMed] [Google Scholar]
Ratcliffe 1993 {published data only}
- Ratcliffe F, Evans J. Neonatal wellbeing after elective caesarean delivery with general, spinal, and epidural anaesthesia. European Journal of Anaesthesiology 1993;10:175‐81. [PubMed] [Google Scholar]
Robson 1992 {published data only}
- Robson S, Boys R, Rodeck C, Morgan B. Maternal and fetal haemodynamic effects of spinal and extradural anaesthesia for elective caesarean section. British Journal of Anaesthesia 1992;68:54‐9. [DOI] [PubMed] [Google Scholar]
Russell 1995 {published data only}
- Russell I. Levels of anaesthesia and intraoperative pain at caesarean section under regional block. International Journal of Obstetric Anesthesia 1995;4:71‐7. [DOI] [PubMed] [Google Scholar]
Sarvela 2002 {published data only}
- Sarvela J, Halonen P, Soikkeli A, Korttila K. A double‐blinded, randomised comparison of intrathecal and epidural morphine for elective cesarean delivery. Anesthesia and Analgesia 2002;95:436‐40. [DOI] [PubMed] [Google Scholar]
Valli 1994 {published data only}
- Valli J, Pirhonen J, Aantaa R, Erkkola R, Kanto J. The effects of regional anaesthesia for caesarean section on maternal and fetal blood flow velocities measured by Doppler ultrasound. Acta Anaesthesiologica Scandinavica 1994;38:165‐9. [DOI] [PubMed] [Google Scholar]
Zakowski 1990 {published data only}
- Zakowski M, Ramanathan S, Khoo P, Turndorf H. Plasma histamine with intraspinal morphine in cesarean section. Anesthesia and Analgesia 1990;70:S448. [Google Scholar]
References to studies awaiting assessment
Hernandez‐Granados {unpublished data only}
- Hernandez‐Granados J, Delgado M. Spinal versus epidural anesthesia for cesarean section: a cost effectiveness study. Personal communication 2001.
Huang 1993 {published data only}
- Huang JS, Yung‐YI I, Tung CC, Chou P. Comparison between the effects of epidural and spinal anaesthesia for selective cesarean section. Chung Hua I Hsueh Tsa Chih Taipei 1993;51(1):40‐7. [PubMed] [Google Scholar]
Additional references
Behaque 2002
- Behague DP, Victora CG, Barros FC. Consumer demand for caesarean sections in Brazil: informed decision making, patient choice, or social inequality? A population based cohort study linking ethnographic and epidemiological methods. BMJ 2002;324:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bridenbaugh 1998
- Bridenbaugh PO, Greene NM, Brull SJ. Spinal (subarachnoid) neural blockade. In: Cousins MJ, Bridenbaugh PO editor(s). Neural blockade in clinical anaesthesia and management of pain. 3rd Edition. Philadelphia: Lippincott‐Raven, 1998:203‐42. [ISBN 0‐397‐51159‐0] [Google Scholar]
Brown 1999
- Brown DL. Spinal, epidural and caudal anesthesia; anatomy, physiology and technique. In: Chestnut DH editor(s). Obstetric anesthesia. St. Louis: Mosby, 1999:187‐208. [Google Scholar]
Chamberlain 1999
- Chamberlain G, Steer P. ABC of labour care: operative delivery. BMJ 1999;318(7193):1260‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.2 [updated March 2003]. In: Review Manager (RevMan) [Computer program]. Version 4.2. The Cochrane Collaboration. Oxford: Update Software; 2003. Oxford, UK.
Cousins 1998
- Cousins MJ, Veering BT. Epidural neural blockade. In: Cousins MJ, Bridenbaugh PO editor(s). Neural blockade in clinical anesthesia and management of pain. Philadelphia: Lippincott‐Raven, 1998:243‐322. [ISBN 0‐397‐51159‐0] [Google Scholar]
Ezri 2001
- Ezri T, Szmuck P, Evron S, Geva D, Hagay Z, Katz J. Difficult airway in obstetric anesthesia: a review. Obstetrical and Gynecological Survey 2001;56(10):631‐41. [DOI] [PubMed] [Google Scholar]
Hawkins 1997
- Hawkins JL, Koonin LM, Palmer SK, Gibbs CP. Anesthesia‐related deaths during obstetric delivery in the United States, 1979‐1990. Anesthesiology 1997;86(2):277‐84. [DOI] [PubMed] [Google Scholar]
Hawkins 1999
- Hawkins JL, Beaty BR, Gibbs CP. Update on obstetric anesthesia practices in the US. Anesthesiology 1999;90(4AS):53A. [Google Scholar]
Hibbard 1996
- Hibbard BM, Anderson MM, Drife JO, Tighe JR, Gordon G, Willatts S, et al. Deaths associated with anaesthesia. In: Rubery E, Bourdillon P editor(s). Report on confidential enquiries into maternal deaths in the United Kingdom 1991‐1993. Norwich: HMSO, 1996:87‐102. [ISBN 0‐11‐321983‐0] [Google Scholar]
Jenkins 2003
- Jenkins J, Khan MM. Anaesthesia for Caesarean section: a survey in a UK region from 1992 to 2002. Anaesthesia 2003;58(11):1114‐8. [DOI] [PubMed] [Google Scholar]
MacKay 1996
- MacKay RSF. Epidural anesthesia. In: Zundert AV, Ostheimer GW editor(s). Pain relief and anesthesia in obstetrics. New York: Churchill Livingstone, 1996:441‐50. [ISBN 0‐443‐04474‐0] [Google Scholar]
Reisner 1987
- Reisner L. Anesthesia for cesarean delivery. Clinical Obstetrics and Gynecology 1987;30(3):539‐51. [DOI] [PubMed] [Google Scholar]
Reisner 1999
- Reisner L, Lin D. Anesthesia for cesarean section. In: Chestnut D editor(s). Obstetric anesthesia‐principles and practice. 2nd Edition. St. Louis: Mosby, 1999:465‐92. [ISBN 0‐3230‐0383‐4] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2. Oxford, UK: The Cochrane Collaboration, 2003.
Riley 1995
- Riley ET, Cohen SE, Macario A, Desai JB, Ratner EF. Spinal versus epidural anesthesia for cesarean section; a comparison of time efficiency, costs, charges and complications. Anesthesia and Analgesia 1995;80(4):709‐12. [DOI] [PubMed] [Google Scholar]
Rout 2000
- Rout C. Regional anesthesia for cesarean section. In: Birnbach D, Gatt S, Datta S editor(s). Textbook of obstetric anesthesia. Philadelphia: Churchill Livingstone, 2000:245‐66. [ISBN 0‐443‐06560‐8] [Google Scholar]
Scott 1995
- Scott DB, Tunstall ME. Serious complications associated with epidural/spinal blockade in obstetrics: a two‐year prospective study. International Journal of Obstetric Anesthesia 1995;4:133‐9. [DOI] [PubMed] [Google Scholar]
Sinatra 1999
- Sinatra RS, Ayoub CM. Postoperative analgesia: epidural and spinal techniques. In: Chestnut DH editor(s). Obstetric anesthesia: principles and practice. 2nd Edition. St. Louis: Mosby, 1999:521‐55. [ISBN 0‐3230‐0383‐4] [Google Scholar]
Stamer 1999
- Stamer UM, Grond S, Schneck H, Wulf H. Surveys on the use of regional anesthesia in obstetrics. Current Opinion in Anaesthesiology 1999;12(5):565‐71. [DOI] [PubMed] [Google Scholar]
Weeks 1999
- Weeks SK. Postpartum headache. In: Chestnut DH editor(s). Obstetric anaesthesia. St. Louis: Mosby, 1999:621‐38. [Google Scholar]
Weir 2000
- Weir EC. The sharp end of the dural puncture. BMJ 2000;320:127. [PMC free article] [PubMed] [Google Scholar]


