Abstract
Background
Colorectal cancer metastatic to the liver, when technically feasible, is resected with a moderate chance of cure. The most common site of failure after resection is within the remaining liver. With this pattern of clinical failure in mind and in order to enhance survival, chemotherapy has been delivered directly to the liver post resection via the hepatic artery.
Objectives
To assess the effect of post hepatic resection hepatic artery chemotherapy on overall survival. Secondary objectives include adverse events related to the chemotherapy, the risk of intra‐hepatic tumour recurrence and tumour free survival.
Search methods
Randomised trials were sought in MEDLINE; the Cochrane Central Register of Controlled Trials; the Cochrane Hepato‐Biliary Group Controlled Trials Register; and through contact of trial authors and reference lists using key words: Colorectal, cancer, hepatic metastases, hepatic artery, chemotherapy. Searches were performed in December, 2008.
Selection criteria
Trials in which patients having resection of colorectal cancer metastatic to the liver were randomised either to hepatic artery chemotherapy or any alternative treatment.
Data collection and analysis
Survival data were obtained principally from abstraction from survival curves in published studies using the method of Parmar. A study specific log hazard ratio and then combined effect log hazard ratio were calculated, as well as a combined Kaplan‐Meier survival probability curve.
Main results
Seven randomised trials addressed this issue, encompassing 592 patients. No significant advantage was found in the meta‐analysis for hepatic artery chemotherapy measuring overall survival and calculating survival based upon "intention to treat" (lnHR = 0.0848; favouring the control group, 95% confidence interval = ‐0.1189 to 0.2885, or a Hazard Ratio of 1.089, an 8.9% survival advantage for the control group, 95% CI of the HR = 0.887 ‐ 1.334). Adverse events related to the hepatic artery therapy were common, including five therapy related deaths. Intra‐hepatic recurrence was more frequent in the control group (97 patients versus 43 in the HAI group), though denominators are not reported, and additional outcomes could not be subjected to a combined analysis.
Authors' conclusions
Though recurrence in the remaining liver happened less in the hepatic artery chemotherapy group, overall survival was not improved, and even favoured the control group, though not significantly. This added intervention cannot be recommended at this time.
Plain language summary
Chemotherapy delivered via the hepatic artery following surgical resection of liver metastases arising from colorectal cancer does not improve survival.
Patients who die of colorectal cancer usually die from, or at least with, liver metastases. On the other hand, isolated liver metastases can, on occasion, be resected with a chance of cure, if metastatic disease is not present elsewhere. After liver resection is performed for colorectal cancer metastases, the most common site of treatment failure is in the remaining liver. For that reason it has been proposed that chemotherapy be given in the hepatic artery after surgery to treat microscopic disease in the remaining liver. This systematic review assesses the randomised trials that have addressed the effective ness of this additional chemotherapy. Seven studies have been published in this field, and the combined analysis shows that survival is not improved by hepatic artery chemotherapy.
Background
Hepatic resection and/or ablation of metastases to the liver of colorectal cancer are being done with increasing frequency and a moderate chance of cure (Iwatsuki 1999) in highly selected patients. No randomised studies have been performed to assess the relative effectiveness of surgical resection against any alternative therapy, including either cryo or radiofrequency ablation of metastatic disease. Since the most common site of failure after liver resection in many reports is in the remaining liver, i.e., the growth of microscopic disease in the liver not detected at the time of surgery (Bozzetti 1986), chemotherapy has been administered via the hepatic artery after hepatic resection to focus adjuvant treatment on this most likely site of recurrence. Seven randomised controlled trials have been performed testing this hypothesis, the results of which have varied (Kemeny 1999; Kemeny 2002; Lorenz 1998; Lygidakis 1995; Rudroff 1999; Tono 2000; Wagman 1990; ). We performed the first meta‐analysis of reported randomised trials in order to assess the efficacy and morbidity of adjuvant chemotherapy delivered in the hepatic artery after hepatic resection of colorectal tumour metastases.
Objectives
The principal objective of this review is to determine if delivery of chemotherapy via the hepatic artery after resection of cancer of the colon metastatic to the liver confers a survival advantage. Secondary objectives include the risk of recurrence of tumour within the remaining liver and assessment of adverse events related to this therapy. Tumour free survival and the effect of intra‐hepatic chemotherapy on quality of life were also sought.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials in which patients having curative resection of liver metastases, either concurrently with colon resection or at a time distant from the colon resection, are allocated either to a hepatic artery chemotherapy group or a control group (which may or may not include systemic chemotherapy) and in which the patients were followed for a sufficient amount of time to assess survival.
Types of participants
Individuals with cancer of the colon or rectum with metastases to the liver that are candidates for liver resection, i.e., with no evidence of primary or metastatic cancer elsewhere, except for those who are having the primary carcinoma of the colorectum resected simultaneously with the liver resection.
Types of interventions
The test group will have had placement of an hepatic artery catheter at the time of liver resection, but after randomizations, i.e., there are no studies in which an hepatic artery catheter was placed for the possible administration of a placebo with post‐operative randomizations. The control groups were either treated by surgery alone or surgery followed by systemic chemotherapy. The inclusion of systemic chemotherapy in some control groups is a potential confounding variable in this review and results will be reported plus and minus those studies in sensitivity analyses.
Types of outcome measures
Overall Survival probability and Adverse events related to catheter placement or administration of the chemotherapy were the primary endpoints assessed
Intra‐hepatic tumour recurrence, Time to recurrence, Extra‐hepatic tumour recurrence, Time to recurrence and Disease specific survival were secondary endpoints sought in the reported studies
Search methods for identification of studies
MEDLINE search using key words: colorectal, cancer, liver, metastases, resection, chemotherapy, hepatic artery, randomised trial, in various combinations from 1966 to December, 2008. The Cochrane Controlled Trials Register and EMBASE were searched with similar key words and the Cochrane Hepatobiliary Controlled Trial Register also was reviewed. None of the seven study authors were aware of any other published or ongoing studies.
Data collection and analysis
Data were extracted from the studies using the above endpoints. Authors have been contacted in each of the above seven studies requesting updated crude data. Four immediately complied, one demanded first authorship of the review for only contributing his data, another had many questions and one has refused to respond. Fortunately the authors not contributing data have quite detailed survival curves in their published reports.
Meta‐analysis of overall survival: A method has been published for the extraction of summary data from survival curves for the purpose of doing meta‐analyses in time to event survival studies (Parmar 1998). The principal endpoint, duration of survival, is assessed for each study using the log hazard ratio, a statistic that best combines time to event and censoring in survival studies (Parmar 1998). The meta‐analysis is also done using this approach and a summary Kaplan‐Meier Survival curve constructed (Parmar 1998).
Sensitivity analyses: Several of the studies in this review enrolled only a small number of patients (Lygidakis 1995; Rudroff 1999; Tono 2000; Wagman 1990). This meant that in many of the time intervals in which overall mortality was measured there were no events ‐ no deaths. It is recommended that in such situations very small numbers be inserted into the data extraction tables (e.g.0.000001) to avoid division by a zero denominator. This effectively eliminated the smaller studies from consideration. It is further recommended and that concatenating intervals be avoided as a means of assuring that an event ‐ a death ‐ occurred at each measurement point (Parmar 1998). The effect of such concatenation of the time intervals in smaller studies was assessed, which required considerable widening of the intervals in the smaller studies, by comparing this maneuver to the recommended technique of small number insertion, and, in this review, adherence to annual measurement intervals. The top three rows of (Table 1) use uniform yearly intervals for mortality determination in all studies. The last two rows vary the intervals for each study to assure that an event occurs in each interval. This was intended as a sensitivity analyses to assess the robustness of this statistical technique. Further sensitivity analyses were performed to assess the effect of exclusion of studies with either quality concerns or confounding data on the summary log hazard ratio for overall survival. The rationale for these exclusions can be found under the Methodological Quality of included studies section. The analyses can be found in rows two and three in Table 1.
1. Summary & Sensitivity Analyses.
| Included Studies | Summary lnHR | Variance lnHR | Standard Error | 95% Conf. Int. | Significance |
| All Studies | 0.0848 | 0.0108 | 0.1039 | +/‐ 0.2037 | NS |
| 6 Studies, Excluding Lygidakis | 0.1584 | 0.0117 | 0.1082 | +/‐ 0.2037 | NS |
| 4 Studies, Excluding Lygidakis, NKemeny, Tono | 0.2405 | 0.0158 | 0.1257 | +/‐ 0.2463 | NS |
| Concantinating Intervals: 7 Studies, to avoid "no event" intervals | 0.0404 | 0.0102 | 0.1010 | +/‐ 0.1979 | NS |
| Concantinating Intervals: 6 Studies, Excluding Lygidakis; to avoid "no event" intervals | 0.1287 | 0.0110 | 0.1049 | +/‐ 0.2056 | NS |
Heterogeneity was calculated using the general variance based method (Petitti 1994) with and without (Lygidakis 1995). Quantitation of heterogeneity was done using the I square method of (Higgins 2003).
Results
Description of studies
Seven RCTs have been published from 1990 to December, 2008. These were all found in Medline, with no additional studies disclosed through other data bases or in communications with study authors.
These seven studies incorporated including 592 patients, 302 in the control group and 289 in the hepatic artery chemotherapy group, three from the United States, two from Germany, one from Greece and one from Japan. Details of type and duration of chemotherapy are presented in (Table 2). All seven studies are included in this review. No non‐randomised studies were used in the meta‐analysis. Randomizations or allocation to study group occurred intra‐operatively after determination that a curable hepatic resection was feasible in four studies (Kemeny 1999; Rudroff 1999; Tono 2000; Wagman 1990). Five studies used computerized tomography to assure that metastatic disease was not present outside the liver in the pre‐operative period. The two studies that did not use this technique did not random ize patients until surgery and thus used operative exploration to rule out extra‐hepatic metastases (Rudroff 1999, Tono 2000). One study used a novel technique of randomization, with pre‐operative randomization to an A or B therapy group, but the therapy allocation was determined by operative findings, i.e. there were three strata of therapy pairs depending upon whether the patient had (A) extra‐hepatic metastases, (B) unresectable liver metastases or (C) resectable liver metastases (Wagman 1990). This method accounts for the very small number of participants in the (C) portion of their study relevant to this review. In two other studies using pre‐operative randomization (Kemeny 2002; Lorenz 1998), a number of patients were found during abdominal exploration to be ineligible for hepatic artery catheter chemotherapy, due in most cases to metastases found outside of the liver, or unresectable liver disease. The patients were fortunately followed for survival but not treated with hepatic artery chemotherapy. A number of other participants had difficulties with catheter insertion or function that resulted in no catheter insertion in some cases, misdirected catheter insertion in others and in such cases no chemotherapy was administered. Those found to have tumour at the margin of hepatic resection, suggesting non‐curative resection and of course those that died in the early post‐operative period also never received chemotherapy. Only 234 members of the hepatic artery chemotherapy group (81%) got any chemotherapy in the post‐operative period. One study reported that only 26% of its participants completed all recommended cycles of hepatic artery chemotherapy due to deaths and other adverse events (Table 2) such as appearance of recurrent metastatic disease, toxicity of the chemotherapy, catheter malfunction or sepsis (Kemeny 1999). Many of these problems were encountered in other included studies, and of course most of them could not occur in the control groups.
2. Characteristics of Studies.
| STUDY | Yes or No | Agents | Months | Yes or No | Number | Liver Recurrence | Liver Recurrence |
| Authors | Systemic Chemotherapy in Controls? | HAI Chemo | Duration HAI Chemo | Post Randomization Exclusions | Operative Mortality | HAI | Control |
| Wagman | No | FUdR | 12 | No | 0 | 1 | NS |
| Lygidakis | No | 5FU, Mit. C, Folinic Acid, gamma interferon, Carboplastin, Farmorubicin | 36 | No | 2 | 0 | 8 |
| Lorenz | No | 5FU, Folinic Acid | 6 | Yes | 11 | 23 | 22 |
| Rudroff | No | 5FU, Mit. C | 5 | No | 1 | 3 | 7 |
| NKemeny | Yes | Floxuridine | 4.5 | No | 5 | 7 | 30 |
| MKemeny | No | FUdR + Syst. 5FU | 4 HAI; 8 Systemic | Yes | 2 | 8 | 24 |
| Tono | Yes | 5FU | 1.5 | No | 0 | 1 | 6 |
The therapy delivered through the hepatic artery varied considerably from study to study both in composition and duration (Table 2). They were all similar in several important respects; being all uracil based and all delivered in high concentrations via the hepatic artery. The studies to that degree are therefore all comparable and suitable for meta‐analysis.
Intra‐hepatic tumour recurrence,
Time to recurrence,
Extra‐hepatic tumour recurrence,
Time to recurrence and
Disease specific survival are desirable outcomes to assess but are not reported in the published RCTs in a standard fashion that allowed quantitative compilation of the data. No report presents data that allow a cost comparison or quality of life assessment of various therapies and so these endpoints will not yet be considered, especially in light of the results of the meta‐analysis.
Risk of bias in included studies
Methodological quality needs to be considered in this review, though difficult to assess. Technique and timing of randomization is one area where this can be considered. For instance, hepatic resection is not recommended if metastatic disease is found outside the liver. In most studies randomization was delayed until abdominal exploration was complete in the operating room to assure patient eligibility. When randomizations occurred before surgery and yet 100% of the patients had a successful resection, one might be concerned about quality (Lygidakis 1995). Only two RCTs specified the technique of randomizations, the drawing of cards (Lygidakis 1995; Tono 2000). Blinding of neither surgeon nor patient was done in any of these studies. No mention of outcome assessment by blinded investigators was made in any publication. No hepatic artery catheters were inserted for the administration of placebo.
Sensitivity analyses were conducted to assess several issues related to quality, potential confounding therapy and validity of this statistical method. The first relates to quality and the discussion of randomizations in the previous paragraph, operative findings and the delivery of therapy. Pre‐operative imaging for the detection of metastatic disease is often imprecise and underestimates the actual disease burden. For that reason many patients in whom resection of hepatic metastases from colorectal cancer is planned do not get the procedure because additional metastases are discovered during surgery that makes the disease incurable by any operation. It is also not uncommon for catheter insertion in patients intended to get hepatic artery chemotherapy to be difficult and even unsuccessful. For that reason 4 of 7 studies randomised patients only during surgery ‐ creating at least a little problem with informed consent ‐ in order to appropriately assess their eligibility for the study (Kemeny 1999; Rudroff 1999; Tono 2000; Wagman 1990). Two additional studies randomised pre‐operatively but then during surgery found many randomised patients were ineligible for the therapy. "As treated" and "Intention to Treat" became, in this situation, very disparate numbers (Kemeny 2002; Lorenz 1998). Only one study randomised pre‐operatively and yet treated all patients as randomised, suggesting a problem with quality of assessment or allocation (Lygidakis 1995). When tables describe analysis of 6 studies only, it means with the exclusion of this study.
The second, relating to confounders: there are two studies that treated all members of the control group with systemic chemotherapy, feeling that this was the "best standard care", though no data exist from randomised studies to support this plan. When tables describe analyses of 4 studies only it is with the exclusion of the study above (Lygidakis 1995) and the two studies using systemic chemotherapy in controls (Kemeny 1999; Tono 2000). The only study that showed a significantly improved survival delivered a massive range of chemotherapy for an unusually long period of time without reported adverse effect, which further separates it from other publications (Lygidakis 1995). The same study had an unusual relationship of randomizations and surgical outcome that also suggested it was one to segregate in sensitivity analysis.
The third relates to the statistical technique: The concantination of time intervals to insure that an event, a death, occurred in as many studies as possible in each measurement interval had a minor effect on the summary statistics (Table 1), when compared to the maintanence of uniform time itnervals and insertion of very samll event numbers as recommended by (Parmar 1998).
Effects of interventions
The log hazard ratio (lnHR) for overall survival all studies combined is greater than zero, and therefore the meta‐analysis shows diminished all cause mortality risk for the control group compared to the hepatic artery chemotherapy (HAI) group. The sensitivity analyses further strengthen this finding, increasing the lnHR in all but one case, and in each case remaining greater than zero (Table 1). That is, exclusion of studies from analysis that had concerns over quality or confounding increased the survival probability of the control group over the hepatic artery chemotherapy group. The range of survival benefit varies in these analyses from 4% to 27% in the control group. It is 8.9% in the full analysis. However, none of these analyses demonstrate a statistically significant difference between the control group and the HAI group. The 95% confidence intervals in each case cross zero.
The summary survival probability curve is shown in (Figure 1). Once again in the overall survival curve no overall survival advantage is shown for hepatic artery chemotherapy. The reliability of the comparison of the survival curves diminishes to the right of the curve as the number of patients being assessed declines markedly and the standard error increases from 0.03 in year two to 0.23 in year seven, reflecting the decline in number of patients at risk in each time interval from 592 at year 0 to 30 at year 7.
1.
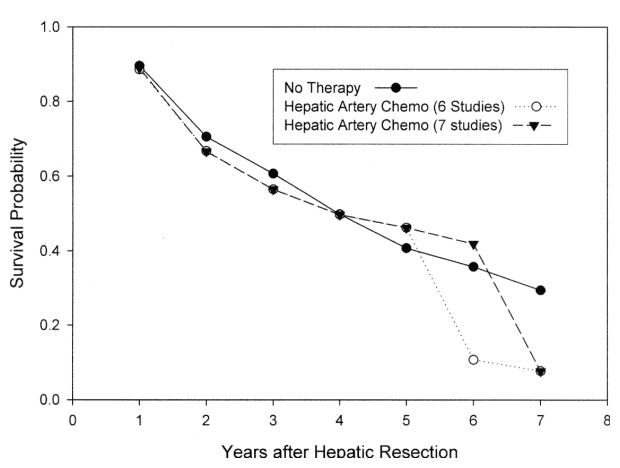
Meta‐analysis of Randomized Studies of Hepatic Artery Chemotherapy After Hepatic Resection for Metastatic Colorectal Cancer. At no point is the divergence of these curves statistically significant. (6 studies) is a sensitivity analysis performed in the absence of one study (see text).
On the other hand, 43 patients developed recurrence within the remaining liver of colorectal cancer in the HAI group and 97 patients developed recurrence in the control group (Table 2). A rate for this finding, and thus test for statistical significance, cannot be calculated from the data presented because of uncertainty about the denominators, as the time of recurrence varies or is not in some cases specified.
Appropriate analyses (Table 3) demonstrated that, measuring overall survival in individual studies, only one showed a statistically significant result, favouring hepatic artery chemotherapy (Lygidakis 1995). Many of the studies claimed in publication that their results were statistically significant, using inappropriate techniques of analysis; only one (Lorenz 1998) used hazard ratio in calculating effect. Significance was unearthed by individual study authors choosing arbitrary points in follow up, by analysing patients "as treated" or perhaps not accounting for right censoring of subjects, an integral part of time to event assessments.
3. Individual Study Analyses of Overall Survival.
| STUDY | Minimum ‐ Months | Maximum ‐ Months | Randomization | Log Hazard Ratio | Variance of lnHR | Standard Error lnHR | 95% confidence inter | Number Subjects | HAI as treated |
| Authors | Follow‐up | Follow‐up | When How | lnHR | HAI Control | ||||
| Wagman | 44.3 | 81.6 | IO NS | ‐0.6293 | 1.0989 | 1.0483 | +/‐ 2.0547 | 6 5 | 5 |
| Lygidakis | 62 | 98 | PO Cards | ‐0.8059 | 0.1418 | 0.3766 | +/‐ 0.7383 | 20 20 | 19 |
| Tono | 49.5 | 70 | IO Cards | ‐0.8004 | 1.0340 | 1.0169 | +/‐ 1.9931 | 9 10 | 9 |
| NKemeny | 16 | 95 | IO NS | ‐0.0510 | 0.0486 | 0.2205 | +/‐ 0.4322 | 74 82 | 68 |
| MKemeny | 30 | 113 | PO NS | 0.3382 | 0.0476 | 0.2182 | +/‐ 0.4277 | 53 56 | 30 |
| Rudroff | 145 | 162 | IO NS | 0.2885 | 0.1614 | 0.4017 | +/‐ 0.7873 | 14 16 | 13 |
| Lorenz | 0 | 69 | PO NS | 0.1988 | 0.0279 | 0.1670 | +/‐ 0.3274 | 113 113 | 84 |
Other endpoints are more problematic. In the published studies sufficient data are not presented to perform similar analyses for tumour free survival and hepatic recurrence free survival. Nor are adverse events routinely described. As stated above, attempts to obtain individual patient data have only been partially successful, and only from the smallest studies.
Adverse events in the overall intervention group were common, including toxicity of the chemotherapy that limited the number of cycles subjects could tolerate, as mentioned above. In addition catheter sepsis, venous thrombosis and catheter dislodgment were also reported. Five deaths were directly attributed to the therapy.
Disease free survival was often reported, but in a manner that usually made time to event impossible to calculate, much less use in a combined analysis.
Quality of Life was not discussed nor data presented in any study.
Heterogeneity was calculated including all studies (chi square = 8.918; six degrees of freedom, p > 0.10. When one study was excluded (Lygidakis 1995), the calculation of heterogeneity was chi square = 3.75; five degrees of freedom, p > 0.7. In both situations statistically significant heterogeneity was not detected. The I square for all studies was 33% (33% of the observed variation across studies due to heterogeneity), whereas for the studies excluding (Lygidakis 1995), the I square was 0% (Higgins 2003).
Captions for Tables Table 01 HAI Hepatic Artery Chemotherapy Group 5FU 5 Fluorouracil FUdR Fluorouracil deoxyribose Mit. C Mitomycin C
Table 02 A lnHR >0 favours the Control Group <0 favours Hepatic Artery Chemotherapy IO Intra‐operative randomizations PO Pre‐operative randomizations
All Analyses except Lygidakis are not statistically significant
Table 03 lnHR >0 favours the control group for overall survival NS Not statistically significant
Discussion
This systematic review provides little support for the addition of hepatic artery chemotherapy after hepatic resection of metastatic colorectal cancer. Though hepatic recurrence was seen less, the significance of this observation could not be properly assessed in the published studies because of the methods of data presentation. Overall survival was not enhanced, and in fact was 9% worse. The survival advantage in the control group did not reach statistical significance, so a potential survival advantage of the intervention . The sensitivity analyses further strengthened the conclusion, in no case demonstrating an advantage to the intervention group, ranging from 4% to 27% survival advantage in controls in the various analyses. The hepatic artery chemotherapy was not without risk as well, frequently associated with morbidity and occasionally therapy related death in 1.7% of those randomised to intra‐hepatic artery chemotherapy.
In several individual publications calculation of statistical significance was made based upon patients "as treated" rather than allocation according to the original randomizations, an error that inevitably selected out from analysis those hepatic artery chemotherapy patients who were destined to have the worst outcome. The sensitivity analyses were undertaken to segregate studies of questionable quality, those in which significant confounding therapies may have been applied, i.e., systemic chemotherapy in the control group, and to assess the variability in the method of log hazard ratio calculation when time intervals were varied. In addition, when data were presented in a manner that allowed additional approaches, log hazard ratios were calculated by alternate methods to assess the robustness of the method used most frequently, abstraction from survival curves. In each of these cases there was little variation in the overall results, (data not shown).
This review covers 592 patients. A larger population might have disclosed a benefit to HAI, since the confidence intervals cross zero (or 1.0 for the hazard ratio). But this is very unlikely since the trend is clearly in favour of the control group for overall survival, and the statistical power of this sample size to detect as little as a 5% difference in overall survival is greater than 0.8 ( http://calculators.stat.ucla.edu/powercalc/ ) . Another lingering issue is how the control group should be treated. Ethical considerations mandate best standard care, and probably eliminate the possibility of a true placebo controlled trial in which hepatic artery catheters are inserted for sham therapy. Systemic chemotherapy is where opinions differed in the reported trials, though the benefit of systemic chemotherapy in this setting has not been established. Until that time, an unblinded no treatment control after hepatic resection is probably the best comparison group.
In order to construct a survival curve of the combined studies, a correction was necessary in the publication by Parmar (Parmar 1998). In equation #23 the second term on the right should be an estimate of the conditional probability of surviving from t e‐1 to t e, or S c (t e )/S c (t e‐1 ). A reasonable estimate of this would be the weighted sum of conditional probabilities from each study, or k Σ [ S c (t e )/S c (t e‐1 ) ] x w ci (t e ) i=1 Thus the entire correct equation should be k S c (t e ) = S c (t e‐1 ) x Σ [S c (t e )/S c (t e‐1 )] x w ci (t e ) i=1
where te = the last measured time interval Sc = the survival probability at time interval t in the control group wci = weight assigned to each study in the control group i = individual study summed, from i=1 to i=kth study. If this correction is not used, there is a precipitous fall in all survivals, much steeper than in any of the individual studies (Freels 2004).
Authors' conclusions
Implications for practice.
Without evidence of improved survival hepatic artery infusion chemotherapy after resection of colorectal cancer metastatic to the liver cannot be recommended.
Implications for research.
This intervention seemed to be effective in diminishing hepatic recurrence. More precise definition of failure patterns may disclose additional therapy that could improve survival. Also liver metastases are now frequently being treated not by resection but ablation. This has diminished the morbidity associated with hepatic resection, and expanded the number of patients whose liver metastases can be treated. Randomised trials are needed to assess the relative effectiveness of liver resection compared to many alternative therapies in patients with surgically resectable metastases from colorectal cancer and no signs of metastatic disease elsewhere. A study in which the control group gets either no treatment or systemic chemotherapy or regional chemotherapy may never be done because historic comparisons have shown survival from liver resection in selected patients and not with these other therapies (Iwatsuki 1999). A direct comparison of resection to ablation is much more feasible. The development of adjuvant therapy added to ablation that might increase survival may also be the next priority.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2009 | New search has been performed | Searches revealed no new trials |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 31 July 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
The old title for the protocol 'Adjuvant chemotherapy for patients having resection or ablation of colorectal cancer metastatic to the liver', was changed to the present after suggestion from one of the peer reviewers.
Acknowledgements
None
Data and analyses
Comparison 1. Overall Survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 7 | 591 | Hazard Ratio (Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 2 Sensitivity analysis excluding for quality | 5 | 395 | Hazard Ratio (Fixed, 95% CI) | 1.25 [0.98, 1.60] |
| 3 Sensitivity analysis excluding confounders | 4 | 376 | Hazard Ratio (Fixed, 95% CI) | 1.27 [1.00, 1.63] |
1.1. Analysis.
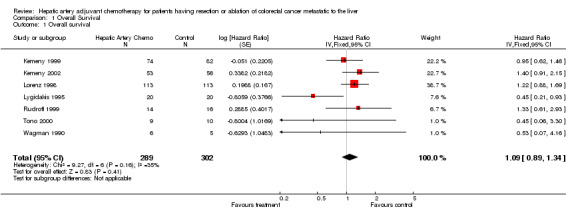
Comparison 1 Overall Survival, Outcome 1 Overall survival.
1.2. Analysis.
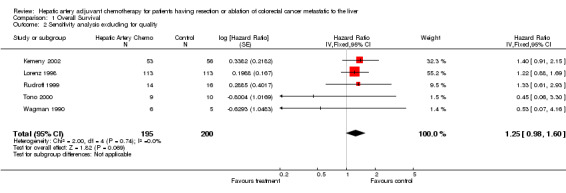
Comparison 1 Overall Survival, Outcome 2 Sensitivity analysis excluding for quality.
1.3. Analysis.
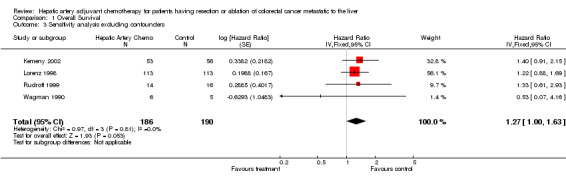
Comparison 1 Overall Survival, Outcome 3 Sensitivity analysis excluding confounders.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kemeny 1999.
| Methods | RCT | |
| Participants | Colorectal cancer (CRC) metastatic to the liver eligible for resection | |
| Interventions | LR (liver resection) + hepatic artery chemotherapy vs no hepatic artery therapy. Both groups received systemic chemotherapy | |
| Outcomes | Overall survival (OS), Disease free survival (DFS), Hepatic resection FS, toxicity, surgical & catheter related adverse events, | |
| Notes | Actuarial analysis supported the use of hepatic artery injection of adjuvant chemotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | |
| Blinding? All outcomes | High risk | |
| Blinding? All outcomes | High risk | |
| Incomplete outcome data addressed? All outcomes | High risk | |
Kemeny 2002.
| Methods | RCT | |
| Participants | Colorectal cancer (CRC) metastatic to the liver eligible for resection | |
| Interventions | LR (liver resection) + hepatic artery chemotherapy (HAI) | |
| Outcomes | Overall survival (OS), Disease free survival (DFS), Hepatic resection FS, toxicity, | |
| Notes | Hepatic resection significant improved by HAI | |
Lorenz 1998.
| Methods | RCT | |
| Participants | Colorectal Cancer (CRC) metastastatic to the liver, eligible for liver resection (LR) based upon pre‐operative imaging | |
| Interventions | LR + hepatic artery chemotherapy (HAI) versus LR alone | |
| Outcomes | Overall Survival (OS) | |
| Notes | Study terminated due to diminished probability of determined benefit | |
Lygidakis 1995.
| Methods | RCT | |
| Participants | Colorectal cancer (CRC) metastatic to the liver eligible for resection | |
| Interventions | LR (liver resection) + hepatic artery chemotherapy (HAI) | |
| Outcomes | Overall survival (OS), Disease free survival (DFS), Hepatic resection FS, toxicity, | |
| Notes | marked benefit in Hepatic resection with multi‐modality HAI | |
Rudroff 1999.
| Methods | RCT | |
| Participants | Colorectal cancer (CRC) metastatic to the liver eligible for resection | |
| Interventions | LR (liver resection) + hepatic artery chemotherapy (HAI) | |
| Outcomes | Overall survival (OS), Disease free survival (DFS), Hepatic resection FS, | |
| Notes | HAI not warranted | |
Tono 2000.
| Methods | RCT | |
| Participants | Colorectal cancer (CRC) metastatic to the liver eligible for resection | |
| Interventions | LR (liver resection) + hepatic artery chemotherapy (HAI) | |
| Outcomes | Overall survival (OS), Disease free survival (DFS), Hepatic resection FS, toxicity | |
| Notes | HAI provides significant disease free survival benefit | |
Wagman 1990.
| Methods | RCT | |
| Participants | Colorectal cancer (CRC) metastatic to the liver eligible for resection (LR) | |
| Interventions | LR + hepatic artery chemotherapy (HAI) | |
| Outcomes | Overall survival Time to Failure | |
| Notes | Extended Disease free survival (DFS) with HAI | |
Contributions of authors
Dr. Freels provided statistical expertise in the meta‐analysis, including correction of a key equation used for combined survival curve construction. Dr. Nelson conceived the project, searched the field, abstracted the data, performed the statistical analyses, wrote the text of the review and is primarily responsible for its conclusions.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Kemeny 1999 {published data only}
- Kemeny N, Huang Y, Cohen AM, Shi W, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer.. N Eng J Med 1999;341(27):2039‐2048. [DOI] [PubMed] [Google Scholar]
- Kemeny N, Huang Y, Cohen AM, Shi W, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer.. N Eng J Med 1999;341(27):2039‐2048. [DOI] [PubMed] [Google Scholar]
Kemeny 2002 {published data only}
- Kemeny MM, Adak S, Gray B, MacDonald JS, et al. Combined Modality treatment for resectable metastatic colorectal carcinoma to the liver: Surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy ‐ an intergroup study. J. Clin. Oncol. 2002;20:1499‐1505. [DOI] [PubMed] [Google Scholar]
Lorenz 1998 {published data only}
- Lorenz M, Mueller HH, Schramm H, Gassel HJ, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5‐fluorouracil and folinic acid for live metastases of colorectal cancer. Ann Surg 1998;228(6):756‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lygidakis 1995 {published data only}
- Lygidakis NJ, Ziras N, Parissis J. Resection versus resection combined with adjuvant pre‐ and post‐operative chemotherapy‐‐immunotherapy for metastatic colorectal liver cancer. A new look at an old problem.. Hepatogastroenterol 1995;42:155‐161. [PubMed] [Google Scholar]
Rudroff 1999 {published data only}
- Rudroff C, Altendorgf‐Hoffmann, Stangl R, Scheele J. Prospective randomized trial on adjuvant hepatic artery infusion chemotherapy after R0 resection of colorectal liver metastases. Langenbeck's Arch Surg 1999;384:243‐249. [DOI] [PubMed] [Google Scholar]
Tono 2000 {published and unpublished data}
- Tono T, Hasuike Y, Ohzato H, Takasuka Y, Kikkawa N. Limited but definite efficacy of prophylactic hepatic arterial infusion chemotherapy after curative resection of colorectal liver metastases.. Cancer 2000;88(7):1549‐1556. [PubMed] [Google Scholar]
Wagman 1990 {published data only}
- Wagman LD, Kemeny MM, Leong L, Terz JJ, et al. A prosepctive randomized evaluation of the treatment of colorectal cancer metastatic to the liver.. J Clin Oncol 1990;8(11):1885‐1893. [DOI] [PubMed] [Google Scholar]
Additional references
Bozzetti 1986
- Bozzetti F, Bignami P, Morabito A, Doci R, Gennari L. Patterns of failure following surgical resection of colorectal cancer liver metastases. Ann Surg 1987;205(3):264‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freels 2004
- Freels S. Statistics in Medicine in press.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. Brit Med J 6/9/2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iwatsuki 1999
- Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, Geller DA, Gayowski TJ, Fung JJ, Starzl TE. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system.. J Amer. Col. Surg. 1999;189(3):291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemeny 1999
- Kemeny N, Huang Y, Cohen AM, Shi W, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer.. N Eng J Med 1999;341(27):2039‐2048. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat in Med 1998;17:2815‐2834. [DOI] [PubMed] [Google Scholar]
Petitti 1994
- Petitti, Diana, B. Meta‐analysis, decision analysis and cost effectiveness analysis. Meta‐analysis, decision analysis and cost effectiveness analysis. first. Oxford University Press, 1994. [isbn 0‐19‐507334‐7] [Google Scholar]


