Abstract
The prevalence of dyslipidaemia has been increasing in the Asia-Pacific region and this is attributed to dietary changes and decreasing physical activity. While there has been substantial progress in dyslipidaemia therapy, its management in the region is hindered by limitations in awareness, adherence and healthcare costs. The Asian Pacific Society of Cardiology (APSC) developed these consensus recommendations to address the need for a unified approach to managing dyslipidaemia. These recommendations are intended to guide general cardiologists and internists in the assessment and treatment of dyslipidaemia and are hoped to pave the way for improving screening, early diagnosis and treatment. The APSC expert panel reviewed and appraised the evidence using the Grading of Recommendations Assessment, Development, and Evaluation system. Consensus recommendations were developed, which were then put to an online vote. The resulting consensus recommendations tackle contemporary issues in the management of dyslipidaemia, familial hypercholesterolaemia and lipoprotein(a) in the Asia-Pacific region.
Keywords: Asia-Pacific, consensus, dyslipidaemia, familial hypercholesterolaemia, lipoprotein(a)
Dyslipidaemia, one of the major risk factors of atherosclerotic cardiovascular disease (ASCVD), is a condition marked by the imbalance of atherogenic and protective lipids, such as triglycerides, LDL cholesterol (LDL-C) and HDL cholesterol (HDL-C). As ASCVD is one of the leading causes of mortality worldwide, effective management of dyslipidaemia is more important than ever. The increasing prevalence of dyslipidaemia in the Asia Pacific is associated with dietary changes and decreasing physical activity.[1] While there has been substantial progress in dyslipidaemia therapy, its management in the region is hindered by limitations in awareness, adherence and healthcare costs.
The Asian Pacific Society of Cardiology (APSC) developed these consensus recommendations to address the need for a unified approach to managing dyslipidaemia. These recommendations are intended to guide general cardiologists and internists in the assessment and treatment of dyslipidaemia. Although there is limited published clinical evidence and a lack of country-specific guidelines on dyslipidaemia management in the region, these recommendations hope to pave the way for improving screening, early diagnosis, and treatment throughout the region.
Methods
The APSC convened an expert consensus panel to review the literature on the assessment of dyslipidaemia, discuss gaps in current management, determine areas where further guidance is needed to and develop consensus recommendations on the use of LDL-C lowering therapies. The 26 experts of the panel are members of the APSC who were nominated by national societies and endorsed by the APSC consensus board or invited international experts. The expert consensus panel comprised cardiologists from Australia, China, Hong Kong, India, Indonesia, Japan, South Korea, Malaysia, Philippines, Singapore, Taiwan, Thailand, UK and US. For the development of these consensus recommendations, the panel agreed to use the APSC ‘CVD’ system for defining high-risk and very-high-risk patients (Table 1).[2]
Table 1: High Thrombotic Risk ‘Coronary–Vascular–Disease’ Algorithm.
| Assessment of High-risk Chronic Coronary Syndrome | ||
|---|---|---|
| C = CORONARY | V = VASCULAR | D = DISEASE |
|
|
|
The presence of any single factor listed would indicate high thrombotic risk in a chronic coronary syndrome patient. Presence of multiple factors would indicate even higher risk of thrombosis in the patient. *Left main PCI, bifurcation PCI, multivessel PCI, more than three stents. †Documented by CT cardiac angiography, severe ischaemia on functional stress test, prior PCI, CABG or bypass. ‡Claudication or prior peripheral intervention, carotid stenosis >50%, mesenteric artery disease, renal artery stenosis. §Ischaemic stroke or transient ischaemic attacks due to atherosclerosis. CABG = coronary artery bypass graft; eGFR = estimated glomerular filtration rate; PCI = percutaneous coronary intervention. Source: Tan et al. 2021.[32] Reproduced with permission from Radcliffe Cardiology.
After a comprehensive literature search using the broad search terms “dyslipidemia” and [“Asia” OR “Asia Pacific”], selected applicable articles were reviewed and appraised using the Grading of Recommendations Assessment, Development, and Evaluation system, as follows:
High (authors have high confidence that the true effect is similar to the estimated effect.
Moderate (authors believe that the true effect is probably close to the estimated effect).
Low (true effect might be markedly different from the estimated effect).
Very low (true effect is probably markedly different from the estimated effect).[3]
The authors adjusted the level of evidence if the estimated effect when applied in the Asia-Pacific region might differ from the published evidence because of various factors such as ethnicity, cultural differences and/or healthcare systems and resources.
The available evidence was then discussed during two consensus meetings (May 2020 and December 2020). Consensus recommendations were developed during the meetings, which were then put to an online vote. Each recommendation was voted on by each panel member using a three-point scale (agree, neutral, or disagree). Consensus was reached when 80% of votes for a recommendation were agree or neutral. In the case of non-consensus, the recommendations were further discussed using email, then revised accordingly until the criteria for consensus were fulfilled.
Consensus Recommendations
Dyslipidaemia
Recommendation 1. Patients with chronic coronary syndrome (CCS) should be assessed according to the Coronary–Vascular–Disease (‘CVD’) system (APSC CCS consensus recommendations[2]) and categorised as having high-risk CCS (one risk factor) or very-high-risk CCS (more than one risk factor).
Level of evidence: Low.
Consensus: 96.3% agree, 3.7% neutral, 0% disagree.
In these consensus statements, CCS is defined as the clinically stable phase between the index cardiovascular event and recurrent events in patients with coronary artery disease (CAD).[2] To ensure that these consensus recommendations are aligned with other recommendations by the APSC, these recommendations adopted the ‘CVD’ classification of high-risk and very-high-risk CCS developed by the APSC.[2] The ‘CVD’ system was developed to serve as the backbone of risk classification for the patient with dyslipidaemia. The presence of any single factor listed would indicate high clinical risk in a CCS patient. The presence of factors from multiple (more than one) categories (but not two factors from the same category only) would indicate even higher risk of clinical events in the patient. The assessment table was created through a separate APSC consensus and followed the pattern of levels of total cardiovascular risk presented in the 2019 European Society of Cardiology and European Atherosclerosis Society guidelines for the management of dyslipidaemia.[4]
Some countries in the Asia Pacific have created their own guidelines for the prevention, assessment and management of dyslipidaemia. These guidelines were also taken into consideration during the creation of the consensus recommendations to create a unified approach in the region.
It should be noted that total cardiovascular risk estimation is a part of a continuum. The cut-off points that were used to define high-risk levels are partly based on clinical trial evidence and – by necessity – partly based on clinical judgement. As the categories are based on an ideal setting with unlimited resources and best available evidence, appropriate measures within the local healthcare system should still be considered in clinical practice.[4]
Recommendation 2. High-intensity statins are recommended for all patients with clinically manifest CCS, regardless of risk.
Level of evidence: Moderate.
Level of consensus: 88.9% agree, 11.1% neutral, 0% disagree.
Recommendation 3. LDL-C treatment targets for high-risk CCS are both a reduction from baseline LDL-C of at least 50% and achieving an on-treatment level at least <1.8 mmol/l.
Level of evidence: Low.
Level of consensus: 96.3% agree, 3.7% neutral, 0% disagree.
Recommendation 4. LDL-C treatment targets for very high-risk CCS are a reduction from baseline LDL-C of at least 50% and achieving an on-treatment level at least <1.4 mmol/l.
Level of evidence: Low.
Level of consensus: 96.3% agree, 3.7% neutral, 0% disagree.
Recommendation 5. For high-risk CCS patients already treated with maximally tolerated statins, ezetimibe and/or a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor may be added for those who do not achieve target.
Level of evidence: Moderate.
Level of consensus: 100% agree; 0% neutral; 0% disagree.
Recommendation 6. For very-high-risk CCS, upfront initiation of combination therapy with high-intensity statins and ezetimibe may be considered. A PCSK9 inhibitor may be added for those who do not achieve target within 4 weeks of initial therapy.
Level of evidence: Moderate.
Level of consensus: 96.3% agree; 3.7% neutral; 0% disagree.
Statins reduce the risk of cardiovascular events and ischaemic strokes, in both primary and secondary prevention populations.[5,6] It is recommended that a high-intensity statin is prescribed up to the highest tolerated dose to reach the goals set for the specific level of risk.[7–9] High-intensity statin therapy is defined as a statin dose that, on average, reduces LDL-C by ≥50%.[4] Of note, the 40 mg dose of rosuvastatin is not approved or recommended in China, South Korea and Japan and the 80 mg dose of atorvastatin is not approved or not recommended in some of these countries.
Several meta-analyses of randomised trials have been performed to evaluate the clinical benefit of statin therapy. The Cholesterol Treatment Trialists’ (CTT) collaboration meta-analysis of individual participant data from randomised trials involving at least 1,000 participants with at least 2 years of follow-up comparing either more versus less intensive statin regimens (five trials; n=39,612; median follow-up 5.1 years) or statin versus placebo or usual care control (21 trials; n=129,526; median follow-up 4.8 years) showed that lowering LDL cholesterol safely reduced the incidence of heart attack, revascularisation and ischaemic stroke, with each 1.0 mmol/l reduction in LDL-C reducing the incidence of major vascular events by over a fifth.[8]
Another meta-analysis by the CTT Collaboration that included individual participant data from 22 trials of statin versus control (n=134,537; mean LDL cholesterol difference at 1 year of 1.08 mmol/l; median follow-up 4.8 years) and five trials of more versus less intensive statin (n=39,612; difference 0.51 mmol/l; median follow-up 5.1 years) reported that with statin therapy, each 1 mmol/l reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1,000 over 5 years in individuals with a 5-year risk of major vascular events of less than 10%.[10]
There is limited evidence on the effectiveness of statins for primary prevention among Asian people, especially in developing countries. However, subgroup analyses with the CTT Collaboration meta-analysis of statin trials suggests that the proportional reduction in risk per mmol/l reduction in LDL-C among Asian participants enrolled in the statin trials was similar to the reduction in risk observed among participants from other countries. A few Asian guidelines recommend the use of statins for primary prevention, but these guidelines come from developed nations and were based on western studies.[11–14] In primary prevention, physicians should consider individual baseline risk, a person’s potential absolute risk reduction, patient preferences, and potential harm, including adverse effects and the need to take a medication daily for an extended period.[15]
The expert panel agreed that there is a need to aggressively treat the high-risk group as lipid treatment targets are often not reached in the region. Non-statin pharmacological options, such as ezetimibe and PCSK9 inhibitors, can also be effective in lowering cardiovascular event rates in high-risk and very-high-risk patients, when used in combination with statins.[16,17] Upfront initiation of combination therapy may be considered early in very-high-risk patients to shorten the time to achieve LDL-C-lowering targets.
Reassessment of lipid levels after 4 weeks of therapy to assess treatment response and the need for uptitration of therapy was agreed on for patients with very-high-risk CCS to avoid treatment inertia and ensure that targets are reached in the shortest time possible.
The recommendations for dyslipidaemia are summarised in Figure 1.
Figure 1: Treatment Algorithm for Dyslipidaemia in Patients with High-risk CCS.
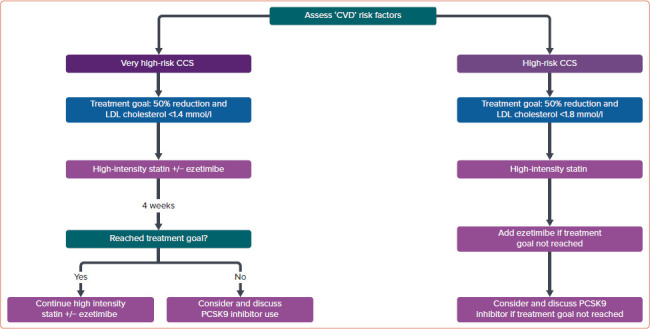
The presence of any single ‘CVD’ factor listed in Table 1 would indicate high thrombotic risk in a patient with CCS. Presence of factors from multiple categories (but not two factors from the same category only) would indicate very high risk. 92.6% agree, 7.4% neutral, 0% disagree. CCS = chronic coronary syndrome; ‘CVD’ = Coronary–Vascular–Disease; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Familial Hypercholesterolaemia
Recommendation 7. Familial hypercholesterolaemia (FH) should be considered in people with:
Severely elevated LDL-C (in adults, >4.9 mmol/l; in children up to age 19 years, >3.9 mmol/l);
LDL-C of >2.6 mmol/l while adherent to a high-intensity statin;
Premature ASCVD (age <55 years for men and <60 years for women);
Elevated LDL-C (in adults >4.1 mmol/l) AND a first-degree relative with premature cardiovascular disease; and
A first-degree relative with FH, tendon xanthoma or arcus cornealis.
Level of evidence: Low.
Level of consensus: 100% agree, 0% neutral, 0% disagree.
Recommendation 8. Clinical criteria can be used to identify and diagnose suspected FH. The choice of criteria used may vary across and within countries.
Level of evidence: Low.
Level of consensus: 96.3% agree, 3.7% neutral, 0% disagree.
Recommendation 9. Confirmation of FH via genetic testing is not necessary for treatment initiation but may be discussed for the purposes of diagnostic confirmation and cascade screening to identify family members with FH.
Level of evidence: Low.
Level of consensus: 100% agree, 0% neutral, 0% disagree.
Recommendation 10. Once an index case is diagnosed, family cascade screening (lipid profile) is recommended.
Level of evidence: Low.
Level of consensus: 96.3% agree, 3.7% neutral, 0% disagree.
Recommendation 11. Patients with FH and ASCVD or another major cardiovascular risk factor are considered to be very high risk. All other patients with FH are considered to be high risk. These patients should be treated with lipid-lowering therapies in accordance with their risk profile.
Level of evidence: Low.
Level of consensus: 96.3% agree, 0% neutral, 3.7% disagree.
FH screening is important as FH is the most common monogenic lipid disorder and the most strongly related to ASCVD. The pooled prevalence of FH from a meta-analysis of 19 studies was 0.40% (95% CI [0.29%–0.52%]), which corresponds to a frequency of 1 in 250 individuals.[18] However, only a small fraction of people with FH are identified and properly treated. If left untreated, FH patients typically develop premature CAD due to lifelong elevation of plasma LDL-C, with the risk of coronary heart disease (CHD) estimated to be increased at least 10-fold (>50% lifetime risk of fatal CAD). The Copenhagen General Population Study showed that the prevalence of CHD among people with definite/probable FH was 33% and only 48% received statins.[19]
While there is a growing awareness of FH worldwide, there are still limited studies about FH in the Asia-Pacific region. These gaps are because of low disease awareness, lack of national screening programs and limited availability of genetic testing.[20] Therefore, data on the prevalence of FH are very limited in Asian countries. The genetic epidemiology of FH in Asian countries may be different from that in European cohorts.[21] The panel acknowledged the various clinical criteria used in the region to diagnose FH. Internationally, the three most widely used diagnostic criteria were developed by the US MEDPED program, the UK Simon Broome Registry Group (SBRG) and the Dutch Lipid Clinic Network (DLCN). Across 16 Asian countries, six used DLCN, five used SBRG, three used MEDPED and 14 used their own criteria.[22] Japan, South Korea and China, in particular, have developed their own diagnostic criteria that are localised for their population. Of note, the cut-off is >4.7 mmol/l in guidelines from Japan and China and might vary between countries according to the distribution of LDL-C levels in the countries.[23,24]
The availability of genetic testing is also variable within and between Asian countries. Hence, the panel has voted to allow individual countries to adopt the clinical criteria most appropriate for their population.
The panel recommended opportunistic and cascade screening of FH. Index cases can be detected by opportunistic or targeted systematic screening in primary care, guided by severely elevated LDL-C levels, persistently elevated LDL-C despite statin therapy; or a family history of ASCVD, FH, tendon xanthoma or arcus cornealis.
FH testing was found to be cost-effective in a genetic screening programme that showed 3.3 years of life gained for each new diagnosed case.[25] A more recent study determined that cascade testing from index patients with both clinically defined definite and possible FH using DNA testing for the family mutation when it can be identified, or LDL-cholesterol levels when it cannot, was the most cost-effective strategy with an incremental cost-effectiveness ratio of £3,666 per quality-adjusted life-year gained.[26] Nonetheless, because of wide disparity in healthcare resources across the Asia-Pacific region, genetic testing may be unavailable or cost-prohibitive in some areas. In these circumstances, clinical evaluation and lipid testing should be emphasised.
The Copenhagen General Population Study found that CHD was increased 13-fold among patients with definite/probable FH not receiving statins, while the risk remained 10-fold higher among persons treated with a statin.[19] This suggests that high-intensity statin therapy is needed in many FH patients. A study of 70 patients with heterozygous FH treated with high-dose statins and ezetimibe found that the regimen improved total cholesterol (p<0.05), LDL-C (p<0.05), triglycerides (p<0.05) and apolipoprotein-B (p<0.05) in comparison to statin monotherapy over a 12-month follow-up period.[27] Furthermore, a study of 50 patients with homozygous FH found that patients receiving ezetimibe plus atorvastatin or simvastatin (40 mg or 80 mg for either drug) significantly reduced LDL-C levels compared with those receiving 80 mg of either statin as monotherapy (−20.7% versus −6.7%, p=0.007).[28] The study also found that the addition of ezetimibe was safe and well tolerated.
Lipoprotein(a)
Recommendation 12. Resources permitting, lipoprotein(a) measurement should be performed at least once in each adult person’s lifetime, especially those with family history of premature ASCVD. Those with very high inherited lipoprotein(a) levels >430 nmol/l (>180 mg/dl) may have a lifetime risk of ASCVD equivalent to the risk associated with heterozygous FH.
Level of evidence: Low.
Level of consensus: 92.6% agree, 7.4% neutral, 0% disagree.
Recommendation 13. Lipoprotein(a) measurement should be considered in selected patients with a family history of premature cardiovascular disease.
Level of evidence: Low.
Level of consensus: 92.6% agree, 7.4% neutral, 0% disagree.
Recommendation 14. As lipoprotein(a) is a risk enhancer, measurement may be considered for people who are borderline between high- and very-high risk.
Level of evidence: Low.
Consensus: 92.6% agree, 7.4% neutral, 0% disagree.
The INTERHEART study demonstrated how lipoprotein(a) can be used for the risk assessment of acute MI in ethnically diverse populations. South Asian people with elevated lipoprotein(a) concentrations had the highest odds for acute MI (OR 2.14; 95% CI [1.59–2.89]; p<0.001) and the highest population-attributable risk (10%) of ASCVD (adjusted for age, sex, apolipoprotein-A, and apolipoprotein-B).[29,30] This was followed by Southeast Asian people, with an OR of 1.83 (95% CI [1.17–2.88]; p=0.009).
The panel agreed on the contemporary need to include lipoprotein(a) in the consensus recommendations. It is also acknowledged that there is a lack of evidence regarding lipoprotein(a) in the region and that it will be more useful as a risk modifier rather than a treatment target.
Lipoprotein(a) kits are widely available in the West and in developed Asia-Pacific regions, such as Australia, New Zealand, Singapore, Japan and South Korea. However, the availability and the cost of testing are prohibitive elsewhere in the Asia-Pacific region, and lipoprotein(a) testing is often not reimbursed by national insurers.
The 2018 Cholesterol Clinical Practice Guideline has recognised elevated lipoprotein(a) as an ASCVD risk enhancer.[31] Among patients with enhanced risk because of elevated lipoprotein(a) levels, the initiation or intensification of statin therapy may be considered. The current management strategies for persons with elevated lipoprotein(a) include cascade screening as well as aggressive prevention and control of all modifiable risk factors.[32] In particular, this should emphasise more intensive lowering of LDL-C as the initial therapeutic action. Currently available treatments have not been shown to lower ASCVD risk via lipoprotein(a) lowering per se. While PCSK9 inhibitors lower lipoprotein(a) by 30%, and this may explain some of their benefit, most of their benefit is because of their effect on LDL-C.[33]
New therapies are under development that potently and specifically lower lipoprotein(a) levels. Lp(a)HORIZON (NCT04023552) is a large phase 3 cardiovascular outcome trial underway that is evaluatating whether lowering lipoprotein(a) with one of these newer agents will reduce the risk of major cardiovascular events. Increasing screening for elevated lipoprotein(a) to identify individuals who may benefit from these therapies will allow more rapid integration of these therapies into clinical practice in the future. Mendelian randomisation implies that lipoprotein(a) plays a causal role in both ASCVD and aortic stenosis. If so, lowering lipoprotein(a) may be favourable.[34]
Conclusion
These consensus recommendations aim to provide a comprehensive guide on the management of dyslipidaemia, in patients in the Asia-Pacific region. The 14 recommendations presented in this paper aim to guide clinicians based on the most updated evidence. However, given the varied clinical situations and healthcare resources present in the region, these recommendations should not replace clinical judgement. The management of dyslipidaemia should be managed on an individual basis, accounting for an individual’s baseline risk, clinical characteristics and comorbidities, as well as patient concerns and preferences. Clinicians should also be aware of the challenges that may limit the applicability of these consensus recommendations, such as the availability and affordability of specific drugs, interventions and other technologies, differences in each country’s healthcare resources and currently accepted standards of care along with cultural factors.
Acknowledgments
Medical writing support was provided by Agnes Agustin and Ivan Olegario of MIMS Pte Ltd.
References
- 1.Lin C-F, Chang Y-H, Chien S-C et al. Epidemiology of dyslipidemia in the Asia Pacific region. Int J Gerontol. 2018;12:2–6. doi: 10.1016/j.ijge.2018.02.010. [DOI] [Google Scholar]
- 2.Tan JWC, Chew DP, Brieger D et al. 2020 Asian Pacific Society of Cardiology consensus recommendations on antithrombotic management for high-risk chronic coronary syndrome. Eur Cardiol. 2021;16:e26. doi: 10.15420/ecr.2020.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balshem H, Helfand M, Schünemann HJ et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Mach F, Baigent C, Catapano AL et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–88. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists' (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tramacere I, Boncoraglio GB, Banzi R et al. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: a systematic review and network meta-analysis. BMC Med. 2019;17:67. doi: 10.1186/s12916-019-1298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugts JJ, Yetgin T, Hoeks SE et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baigent C, Blackwell L, Emberson J et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills EJ, Rachlis B, Wu P et al. Primary prevention of cardiovascular mortality and events with statin treatments. J Am Coll Cardiol. 2008;52:1769–81. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Cholesterol Treatment Trialists' (CTT) Collaboratio. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 11.Cheung BMY, Cheng CH, Lau CP 2016 consensus statement on prevention of atherosclerotic cardiovascular disease in the Hong Kong population. Hong Kong Med J. 2017. pp. 23191–201. [DOI] [PubMed]
- 12.Kinoshita M, Yokote K, Arai H et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25:846–984. doi: 10.5551/jat.GL2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee E-J, Kim HC, Kim JH et al. 2018 Guidelines for the management of dyslipidemia in Korea. Korean J Intern Med. 2019;34:1171. doi: 10.3904/kjim.2019.188.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minisytry of Health Singapore. Lipids: MOH Clinical Practice Guidelines 2/2016. Singapore: Ministry of Health 2016
- 15.Byrne P, Cullinan J, Smith A, Smith SM. Statins for the primary prevention of cardiovascular disease: an overview of systematic reviews. BMJ Open. 2019;9:e023085. doi: 10.1136/bmjopen-2018-023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papademetriou V, Stavropoulos K, Papadopoulos C et al. Role of PCSK9 inhibitors in high risk patients with dyslipidemia: focus on familial hypercholesterolemia. Curr Pharm Des. 2018;24:3647–53. doi: 10.2174/1381612824666181010124657. [DOI] [PubMed] [Google Scholar]
- 17.Choi JY, Na JO. Pharmacological strategies beyond statins: Ezetimibe and PCSK9 inhibitors. J Lipid Atheroscler. 2019;8:183–91. doi: 10.12997/jla.2019.8.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akioyamen LE, Genest J, Shan SD et al. Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open. 2017;7:e016461. doi: 10.1136/bmjopen-2017-016461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97:3956–64. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 20.Jackson CL, Zordok M, Kullo IJ. Familial hypercholesterolemia in Southeast and East Asia. Am J Prev Cardiol. 2021;6:100157. doi: 10.1016/j.ajpc.2021.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livy A, Lye S. Familial hypercholesterolemia in Asia: a review. Journal OMICS Research. 2011;1:22–31. [Google Scholar]
- 22.Zhou M, Zhao D. Familial hypercholesterolemia in Asian populations. J Atheroscler Thromb. 2016;23:539–49. doi: 10.5551/jat.34405. [DOI] [PubMed] [Google Scholar]
- 23.Harada-Shiba M, Arai H, Ishigaki Y et al. Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J Atheroscler Thromb. 2018;25:751–70. doi: 10.5551/jat.CR003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atherosclerosis and Coronary Heart Disease Group of the Chinese Society of Cardiology of Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology. Chinese expert consensus on screening, diagnosis and treatment of familial hypercholesterolemia. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:99–103 [in Chinese]. doi: 10.3760/cma.j.issn.0253-3758.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Wonderling D, Umans-Eckenhausen M, Marks D et al. Cost-effectiveness analysis of the genetic screening program for familial hypercholesterolemia in the Netherlands. Semin Vasc Med. 2004;4:97–104. doi: 10.1055/s-2004-822992. [DOI] [PubMed] [Google Scholar]
- 26.Nherera L, Marks D, Minhas R et al. Probabilistic cost-effectiveness analysis of cascade screening for familial hypercholesterolaemia using alternative diagnostic and identification strategies. Heart. 2011;97:1175–81. doi: 10.1136/hrt.2010.213975. [DOI] [PubMed] [Google Scholar]
- 27.Pitsavos C, Skoumas I, Tousoulis D et al. The impact of ezetimibe and high-dose of statin treatment on LDL levels in patients with heterozygous familial hypercholesterolemia. Int J Cardiol. 2009;134:280–1. doi: 10.1016/j.ijcard.2007.12.065. [DOI] [PubMed] [Google Scholar]
- 28.Gagné C, Gaudet D, Bruckert E, Group ES. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–75. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- 29.Paré G, Çaku A, McQueen M et al. Lipoprotein(a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation. 2019;139:1472–82. doi: 10.1161/CIRCULATIONAHA.118.034311. [DOI] [PubMed] [Google Scholar]
- 30.Enas EA, Varkey B, Dharmarajan TS et al. Lipoprotein(a): An independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J. 2019;71:99–112. doi: 10.1016/j.ihj.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grundy SM, Stone NJ, Bailey AL et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afshar M, Thanassoulis G. Lipoprotein(a): new insights from modern genomics. Curr Opin Lipidol. 2017;28:170–6. doi: 10.1097/MOL.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 33.Bittner VA, Szarek M, Aylward PE et al. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–44. doi: 10.1016/j.jacc.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 34.Schnitzler JG, Ali L, Groenen AG et al. Lipoprotein(a) as orchestrator of calcific aortic valve stenosis. Biomolecules. 2019;9:760. doi: 10.3390/biom9120760. [DOI] [PMC free article] [PubMed] [Google Scholar]


