Abstract
The 22q11.2 Deletion Syndrome has significant impact on brain and behavior, with about 25% of individuals developing schizophrenia. The condition offers a model for prospective studies on the emergence of psychosis and advancing mechanistic hypotheses on gene-environment interactions, with magnified power for examining genome-phenome association. Here, we highlight findings that build on the International 22q11.2 Brain and Behavior Consortium and relate to several key domains in the study of psychosis-risk and schizophrenia. We examine neurocognition, olfaction and neuroimaging data that indicate similar impairment patterns in this rare syndrome and idiopathic presentation of schizophrenia. We conclude that the converging paradigms, studying psychosis dimensionally in rare and common variants samples, provide complementary approaches that will propel precision medicine in psychiatry.
Introduction
The 22q11.2 Deletion Syndrome (22q11DS), the most common microdeletion disorder [1], has significant impact on brain development and its product – behavior. The neuropsychiatric phenotypic cascade in 22q11DS culminates in about 25% of adolescents and young adults having schizophrenia spectrum disorders. Behavioral aberrations manifested in 22q11DS appear to follow the developmental trajectories of ‘idiopathic’ neuropsychiatric disorders, where common variants are considered part of the genetic architecture. The similarities in the clinical phenotype of psychosis in rare and common variant presentations provide heuristic opportunities for converging research strategies [2,3]. Specifically, 22q11DS offers a model in which mechanistic hypotheses on gene - environment interactions can be tested in a population with relatively high yield of psychosis, which magnifies the power of genome-phenome association. Figure 1 illustrates the chromosomal deletion.
Figure 1.
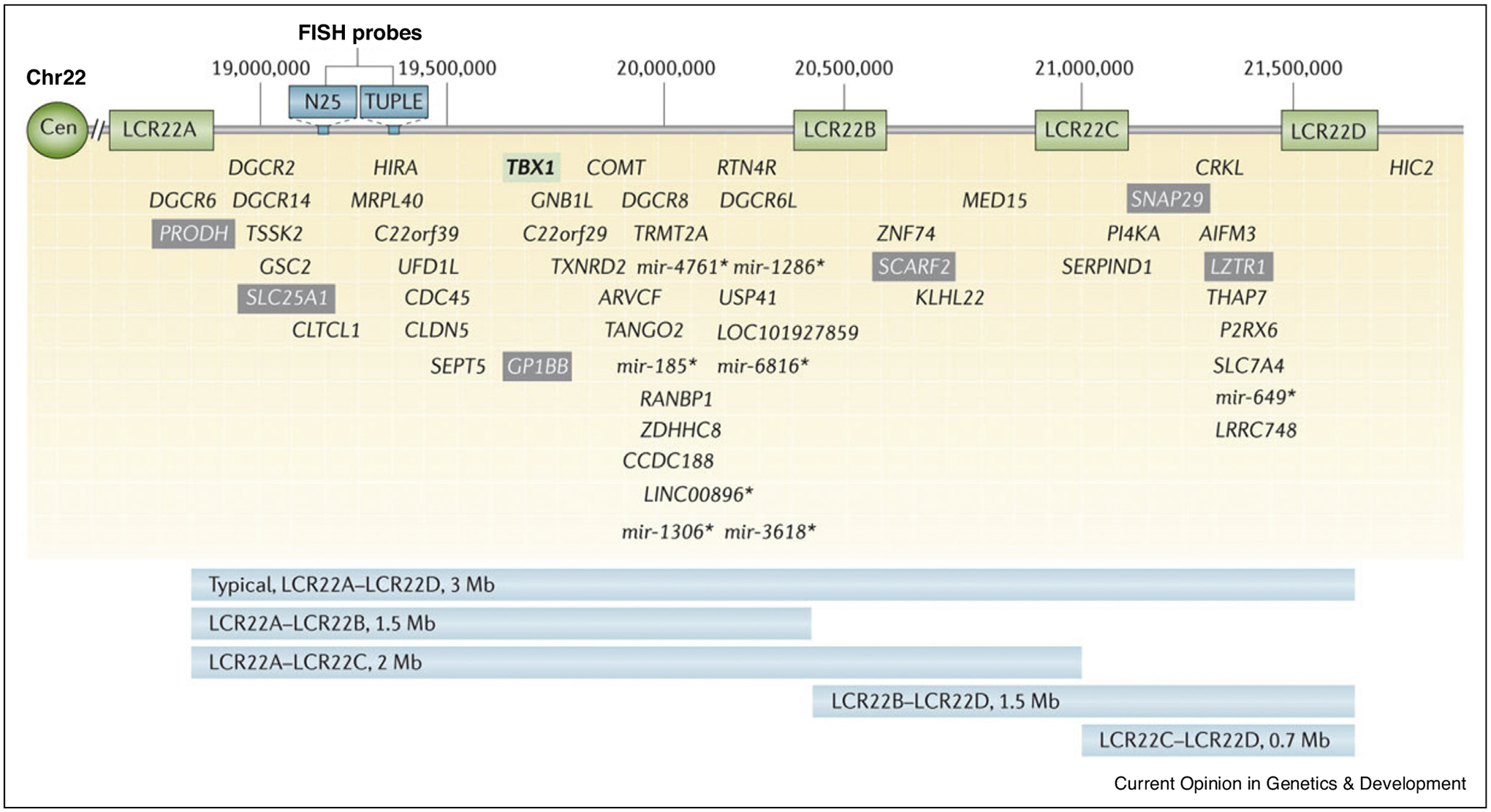
Low copy repeats and genes within the chromosome 22q11.2 deletion. McDonald-McGinn, D.M. et al. (2015) 22q11.2 deletion syndrome Nat. Rev. Dis. Primers doi:10.1038/nrdp.2015.71.
Building on collaborations among 22q11DS investigators, the International 22q11.2 Brain and Behavior Consortium (IBBC) provided a large genomic-phenomic dataset [4], applying categorical and dimensional approaches to study schizophrenia [2] and at risk state [5••]. At risk individuals present with psychotic symptoms that do not meet threshold criteria for schizophrenia spectrum disorders, but have impairment and distress as well as increased risk for future transition into a psychotic disorder.
The findings buttressed the utility of the convergent approach, where standard paradigms are applied to common and rare syndromic presentations as means to elucidate mechanisms underlying psychosis.
Here, we highlight findings, published in 2018–2020, related to several key domains that examine brain-behavior parameters in 22q11DS. Several papers build upon previously published research of the IBBC and extend the efforts to new directions reinforcing integration of rare and common variants approaches in the study of psychosis. We begin with the neurocognitive findings, which examine specific domains related to psychosis in 22q11DS. Olfaction is specifically examined because it models a prevalent behavioral deficit strongly linked to embryonic development of midline structures that are impacted in 22q11DS. We then summarize neuroimaging findings building on the IBBC and conclude by suggesting potential future directions that can advance the field.
Neurocognition
Developmental delay and cognitive impairments across multiple domains characterize individuals with 22q11DS. Standardized testing of intellectual abilities, usually focusing on obtaining a measure of an ‘intelligence quotient’ (IQ), have been applied in clinical and research settings. The global availability of IQ measures enabled the IBBC to examine longitudinal data in relation to the emergence of psychosis [6]. Decline in verbal IQ was found to presage psychosis. The association between IQ and deletion size was examined by Zhao et al. [7], who reported that individuals with the smaller, 1.5 Mb AB deletion had modestly higher IQ scores than those with the larger, 3 Mb AD deletion (see Figure 1). The polygenic risk scores (PRSs) for schizophrenia and IQ were evaluated in an IBBC sample of 962 participants [5••]. It was found that the PRSs were associated both with schizophrenia and baseline IQ. PRS for schizophrenia was also associated with verbal IQ decline. Thus, there appears to be a shared genetic basis for schizophrenia and cognitive phenotypes such as IQ and encourage the future use of PRSs for parsing heterogeneity in risk for and course of psychosis.
While IQ measures are helpful for gauging the extent of intellectual deficits in 22q11DS, they are limited in the ability to gain insight on specific neurocognitive systems involved. Understanding behavioral deficits related to brain systems can offer clues on underlying brain circuitry and stimulate mechanistically driven investigations in humans and animal models. An increasing number of studies have incorporated neuropsychological testing, and these were evaluated in the only meta-analysis to date [8•], which reviewed 43 articles (282 effects). Results demonstrated robust deficits in specific domains of executive functions, language and memory, and the effect sizes were influenced by several factors, including age, sex, and clinical status.
Language has been a specific focus in the clinical evaluation of children with 22q11DS [9], and the relation between language impairment and subsequent emergence of psychosis features has been evaluated with standard instruments [10]. Poorer language performance in childhood was associated with an independent evaluation of psychosis features about a decade later. Furthermore, steeper age-related decline was associated with psychosis across language measures, which was only marginally the case for IQ. Longitudinal systematic evaluations of individuals with 22q11DS are essential for parsing the heterogeneity in the clinical course and for uncovering informative neuromarkers.
Across neuropsychiatric disorders, standard neuropsychological batteries are being replaced by more precise computerized assessments that are based on cognitive and affective neuroscience and informed by neuroimaging. Such efforts have been productive in psychosis research and more recently in 22q11DS. A computerized neuroscience-based battery, developed with functional neuroimaging probes [11], was administered in large-scale genetic studies of schizophrenia and in 22q11DS [12]. The profile of dysfunction is more significant in 22q11DS than in non-deleted individuals with psychosis spectrum features. However, within the 22q11DS sample, those with psychosis features were distinguishable from their non-psychosis counterparts by greater deficits in executive functions, complex cognition and social cognition [13].
Supporting the importance of executive function, Morrison et al. [14] reported that attention, a central component of executive function, was specifically associated with psychosis in a collaborative sample of 236 children, adolescents and adults with 22q11DS and 106 typically developing controls. A longitudinal study from the IBBC demonstrated that attention performance combined with attention deficits hyperactivity disorders were strong predictors of psychosis features, although not for schizophrenia [15]. Notably, the sample was young and has yet to reach the age of emergence of schizophrenia, commonly late adolescence or early adulthood. Similarly, inattention symptoms were strongly associated with psychosis features including positive, negative, and disorganized symptoms in a sample of 137 youth with 22q11DS [16].
Social cognition has been investigated in non-deleted schizophrenia and clinical risk youth [17]. Face processing is an aspect of social cognition, and a study examining face processing with eye tracking in youths with 22q11DS showed impaired processing [18]. However, association with psychosis features requires longitudinal study and remains to be established. Impaired performance on social cognition tasks is related to clinical presentation and course of idiopathic schizophrenia and its measurement can contribute to longitudinal research in 22q11DS.
The22q11.2deletionaffectsmultipleneurodevelopmental pathways, including midline features early in embryogenesis. Olfactory functioning, measured predominately by odor identification and discrimination, provides a window to examine midline structures and pathways and have been investigated in idiopathic schizophrenia and in psychosis spectrum [19]. It has not been well established in 22q11DS. In the first study to evaluate relationships among olfactory deficits, neurocognition and psychosis-spectrum symptoms [20], both odor identification and odor discrimination were significantly impaired in 22q11DS compared to healthy controls. Olfactory deficits were not related to neurocognitive performance, but impairment in odor discrimination was correlated with higher severity of negative symptoms and overall psychosis-spectrum symptoms. In a recent meta-analysis [21], olfactory test results were compared between individuals with 22q11DS (n = 194) and typically developing comparisons (n = 466). Patients exhibited marked olfactory dysfunction (Cohen d = −1.11) that was homogeneous across tests. Olfactory deficits were not moderated by age or sex and were of a magnitude consistent with observed neuropsychological impairments.
Olfactory deficits are clinically relevant in idiopathic psychosis – deficits in odor identification are correlated with severity of negative symptoms associated with psychosis and these deficits are predictive of transition to a psychotic disorder and functional outcome [22]. In patients with psychosis, abnormalities exist at all levels of the ascending olfactory system: nasal cavities, olfactory bulbs, and primary olfactory cortex in the medial temporal lobe. Recent studies in 22q11DS [20,21] and individuals at clinical high-risk for psychosis [22] converge with previous work in psychosis identifying robust deficits in olfactory function. These findings support the hypothesis that midline structural anomalies are byproducts of an early developmental disturbance and are relevant for psychosis. While the specific mechanisms responsible for the olfactory deficit in 22q11DS and psychosis have not been identified, dopaminergic dysregulation and alterations in brain regions relevant to both olfaction and psychosis risk are likely involved.
Minor physical anomalies (MPAs)
MPAs are phenotypic abnormalities of aberrant development and are evident in 22q11DS and psychosis. MPAs include subtle abnormalities of morphological structures in the face and head. Such abnormalities likely represent a disruption of early embryologic development of facial morphology that overlaps with the development of the olfactory system. Consistent with this intertwining of neurodevelopment, individuals with facial dysmorpho-genesis such as cleft lip/palate, show abnormalities of brain structure in association with cognitive deficits. Craniofacial anomalies are frequent in 22q11DS [23] and seem to be the predominant physical abnormalities differentiating those with psychosis from other groups. The application of anthropometric and 3D laser facial scanning techniques have revealed subtle facial dysmorphology [24] and higher frequency of malformations of the limbs, face, and eyes in adults with idiopathic psychosis [25,26]. This evidence suggests a relationship between abnormal craniofacial development — which undergoes initial morphogenesis during the first trimester in humans — and the forebrain — which also undergoes initial differentiation during the first three months of pregnancy. It appears that the topography of craniofacial dysmorphology may reflect subtle disruption to a critical trajectory of embryonic-fetal craniofacial growth, particularly along the midline, and is likely related to the development of psychosis.
The presence of olfactory deficits and dysmorphic facial features in patients with 22q11DS implicates, at least in part, a genetic etiology manifested in utero and affecting subsequent development. Importantly, measures of midline structural morphology and behavioral performance associated with these structures provide an opportunity to better understand the pattern of relative contribution of genetic and embryonic developmental markers relevant for emergent psychosis.
Neuroimaging
Neuroimaging studies of individuals with 22q11DS aim to characterize brain correlates of deletion-related cognitive and clinical phenotypes, and to accelerate the discovery of imaging biomarkers of psychosis risk in general. As is the case for many other clinical conditions, nearly every brain region has been implicated by at least one neuroimaging study of 22q11DS, as summarized by recent review [27–29]. Discordant results from early studies are partly explained by relatively small sample sizes leading to decreased statistical reliability [29], and large multi-site and meta-analytic studies have begun to clarify patterns of neuroanatomical alterations in 22q11DS [30•,31,32••,33]. The largest studies to date show generalized reductions in cortical surface area coupled with increased cortical thickness overall but focal thinning in temporal and cingulate regions [32••], as well as decreased volume and alterations in the shape of multiple subcortical structures [33]. Individuals with 22q11DS and psychosis appear to manifest a distinct neuroanatomical signature that is similar to the pattern observed in idiopathic schizophrenia [32••], suggesting a convergent underlying biological mechanism for the development of psychosis.
Future work will deploy multimodal imaging-genetics approaches to characterize the maturational trajectories of individuals with 22q11DS who transition to psychosis. Widespread disruptions related to brain connectivity are suggested by white matter microstructural alterations in diffusion MRI [34] and dysconnectivity in resting-state fMRI networks [35–38]. Revealing the potential to move beyond case-control comparisons to more individualized genetic associations, the size of the deleted chromosomal region correlated with the extent of the neuroanatomical insult [32••,33], and preliminary evidence suggests that the spatial expression pattern of deleted genes in postmortem human brains may be predictive of the pattern of neuroimaging alterations [39]. A recent longitudinal study reported an accelerated decline during adolescence in the volume of hippocampal subfields for individuals with 22q11DS and psychotic symptoms [40•], but a major gap remains for data to capture the period prior to the onset of psychotic symptoms in individuals who subsequently develop schizophrenia. A technically challenging but relatively untapped resource is the use of clinically acquired brain scans for research purposes [41], which may offer insights into sub-populations often excluded from prospective studies and represent a retrospective source of data on individuals who go on to develop psychopathology.
Conclusions
In this review we have examined 22q11.2DS as a window for unveiling potential mechanistic pathways from genes to psychosis. Individuals with the chromosome 22q11.2 deletion have a significant risk for psychosis that shares multiple clinical features with idiopathic schizophrenia, as was established by the IBBC. Increasingly, other pertinent measures developed and applied to examine non-deleted individuals along the psychosis dimension are being applied to this rare CNV and this include measures of neurocognitive performance, olfaction, facial morphometry and brain imaging parameters. The continued and future application of this multimodal approach will provide the data required for rare and common variants in understanding pathways of risk and resilience to psychosis.
Acknowledgements
Funding was received from NIH grants: U01 MH101719 (REG), U01 MH119738 (REG), R01 MH119185 (DRR), R01 MH120174 (DRR), NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (DRR), K08MH120564 (AAB), U01 MH119738 (DMM), U01 MH101722 (DMM), R01 MH119219 (REG/RCG), R01 MH117014 (RCG).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE et al. : 22q.2 deletion syndrome. Nat Rev Dis Prim 2015, 1:15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassett AS, Lowther C, Merico D, Costain G, Chow EWC, Van Amelsvoort T, McDonald-McGinn D, Gur RE, Swillen A, Van Den Bree M et al. : Rare genome-wide copy number variation and expression of schizophrenia in 22q.2 deletion syndrome. Am J Psychiatry 2017. 10.1176/appi.ajp.2017.16121417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang SX, Moore TM, Calkins ME, Yi JJ, Savitt A, Kohler CG, Souders MC, Zackai EH, McDonald-McGinn DM, Emanuel BS et al. : The psychosis spectrum in 22q.2 deletion syndrome is comparable to that of nondeleted youths. Biol Psychiatry 2017, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gur RE, Bassett AS, McDonald-Mcginn DM, Bearden CE, Chow E, Emanuel BS, Owen M, Swillen A, Van Den Bree M, Vermeesch J et al. : A neurogenetic model for the study of schizophrenia spectrum disorders: the international 22q.2 deletion syndrome brain behavior consortium. Mol Psychiatry 2017. 10.1038/mp.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.••.Davies R, Fiksinski A, Breetvelt E, Williams NM, Hooper SR, Monfueuga T, Bassett AS, Owen MJ, Gur RE, Morrow BE et al. : Using common genetic variation to examine phenotypic expression and risk prediction in 22q.2 deletion syndrome. Nat Med 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]; This IBBC study examined the shared genetic basis between schizophrenia and schizophrenia-related phenotypes in a cohort of 962 individuals with 22qDS. They evaluated individual risk prediction in 22qDS for these phenotypes with two polygenic scores, derived for schizophrenia and intelligence. Polygenic scores were not only associated with schizophrenia and baseline intelligence quotient (IQ), respectively, but schizophrenia polygenic score was also significantly associated with cognitive (verbal IQ) decline and nominally associated with sub-threshold psychosis. These data show a shared genetic basis for schizophrenia and schizophrenia-related phenotypes in 22qDS and idiopathic cases and highlight the potential of polygenic scores for risk stratification.
- 6.Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, Armando M, Vicari S, Shashi V, Hooper SR et al. : Cognitive decline preceding the onset of psychosis in patients with 22q.2 deletion syndrome. JAMA Psychiatry 2015, 72:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Guo T, Fiksinski A, Breetvelt E, McDonald-McGinn DM, Crowley TB, Diacou A, Schneider M, Eliez S, Swillen A et al. : Variance of IQ is partially dependent on deletion type among 1,427 22q11.2 deletion syndrome subjects. Am J Med Genet Part A 2018, 176:2172–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.•.Moberg PJ, Richman MJ, Roalf DR, Morse CL, Graefe AC, Brennan L, Vickers K, Tsering W, Kamath V, Turetsky BI et al. : Neurocognitive functioning in patients with 22q.2 deletion syndrome: a meta-analytic review. Behav Genet 2018, 48 [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first systematic review of cross-sectional studies comparing neuropsychological performance of individuals with 22q.2DS with age-matched healthy typically developing and sibling comparison subjects. Potential moderators were analyzed. Analyses included 43 articles (282 effects) that met inclusion criteria. Very large and heterogeneous effects were seen for global cognition and in neuropsychological domains of intellectual functioning, achievement, and executive function. These deficits are influenced by several factors, including type of comparison group utilized, age, sex, and clinical status. These findings highlight the clinical relevance of characterizing cognitive functioning in 22q.2DS and the importance of considering demographic and clinical moderators in future analyses.
- 9.Hamsho N, Antshel KM, Eckert TL, Kates WR: Childhood predictors of written expression in late adolescents with 22q.2 deletion syndrome: a longitudinal study. J Intellect Disabil Res 2017, 61:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solot CB, Moore TM, Crowley TB, Gerdes M, Moss E, McGinn DE, Emanuel BS, Zackai EH, Gallagher S, Calkins ME et al. : Early language measures associated with later psychosis features in 22q.2 deletion syndrome. Am J Med Genet Part B Neuropsychiatr Genet 2020, 183:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE: A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods 2010, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gur RE, Yi JJ, McDonald-Mcginn DM, Tang SX, Calkins ME, Whinna D, Souders MC, Savitt A, Zackai EH, Moberg PJ et al. : Neurocognitive development in 22q.2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Mol Psychiatry 2014, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger R, Yi J, Calkins M, Guri Y, McDonald-McGinn DM, Emanuel BS, Zackai EH, Ruparel K, Carmel M, Michaelovsky E et al. : Neurocognitive profile in psychotic versus nonpsychotic individuals with 22q.2 deletion syndrome. Eur Neuropsychopharmacol 2016, 26. [DOI] [PubMed] [Google Scholar]
- 14.Morrison S, Chawner SJRA, van Amelsvoort TAMJ, Swillen A, Vingerhoets C, Vergaelen E, Linden DEJ, Linden S, Owen MJ, van den Bree MBM: Cognitive deficits in childhood, adolescence and adulthood in 22q.2 deletion syndrome and association with psychopathology. Transl Psychiatry 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niarchou M, Calkins ME, Moore TM, Tang SX, McDonald-McGinn DM, Zackai EH, Emanuel BS, Gur RC, Gur RE: Attention deficit hyperactivity disorder symptoms and psychosis in 22q.2 deletion syndrome. Schizophr Bull 2018, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niarchou M, Moore TM, Tang SX, Calkins ME, McDonald-McGuinn DM, Zackai EH, Emanuel BS, Gur RC, Gur RE: The dimensional structure of psychopathology in 22q.2 deletion syndrome. J Psychiatr Res 2017, 92. [DOI] [PubMed] [Google Scholar]
- 17.Kimoto S, Makinodan M, Kishimoto T: Neurobiology and treatment of social cognition in schizophrenia: bridging the bed-bench gap. Neurobiol Dis 2019, 131 104315. [DOI] [PubMed] [Google Scholar]
- 18.Zaharia A, Schneider M, Glaser B, Franchini M, Menghetti S, Schaer M, Debbané M, Eliez S: Face processing in 22q.2 deletion syndrome: atypical development and visual scanning alterations. J Neurodev Disord 2018, 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moberg PJ, Arnold SE, Doty RL, Gur RE, Balderston CC, Roalf DR, Gur RC, Kohler CG, Kanes SJ, Siegel SJ et al. : Olfactory functioning in schizophrenia: Relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol 2006, 28. [DOI] [PubMed] [Google Scholar]
- 20.Tang SX, Moberg PJ, Yi JJ, Wiemken AS, Dress EM, Moore TM, Calkins ME, McDonald-McGinn DM, Zackai EH, Emanuel BS et al. : Olfactory deficits and psychosis-spectrum symptoms in 22q.2 deletion syndrome. Schizophr Res 2018, 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moberg PJ, Turetsky BI, Moberg EA, Kohler CG, Tang SX, Gur RC, Gur RE, Roalf DR: Meta-analysis of olfactory dysfunction in 22q.2 deletion syndrome. Psychiatry Res 2020, 285. [DOI] [PubMed] [Google Scholar]
- 22.Turetsky BI, Moberg PJ, Quarmley M, Dress E, Calkins ME, Ruparel K, Prabhakaran K, Gur RE, Roalf DR: Structural anomalies of the peripheral olfactory system in psychosis high-risk subjects. Schizophr Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer LD, Butcher NJ, Boot E, Hodgkinson KA, Heung T, Chow EWC, Guna A, Crowley TB, Zackai E, McDonald-McGinn DM: Elucidating the diagnostic odyssey of 22q. 2 deletion syndrome. Am J Med Genet Part A 2018, 176:936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waddington JL, Russell V: The Cavan-Monaghan First Episode Psychosis Study (CAMFEPS): arbitrary diagnostic boundaries across the gene-environment interface and within evolving models of care. Ir J Psychol Med 2019, 36:293–303. [DOI] [PubMed] [Google Scholar]
- 25.Babović SS, Srdić-Galić B, Žigić S, Mladenović-Silađ Ð, Gajić Z, Bošković K: Craniofacial measures and minor physical anomalies in patients with schizophrenia in a cohort of Serbian population. Srp Arh Celok Lek 2019, 147:348–356. [Google Scholar]
- 26.Mobile RZ, Roussenq AC, de Araujo MR, Sbalqueiro R, Maciel JVB, de Lima AAS: The characteristics of palate and upper dental arch can be an anatomical marker for men with schizophrenia? Case-control study: palate can be a marker for schizophrenia?. Spec Care Dent 2020, 40:412–417. [DOI] [PubMed] [Google Scholar]
- 27.Padula MC, Scariati E, Schaer M, Eliez S: A Mini review on the contribution of the anterior cingulate cortex in the risk of psychosis in 22q.2 deletion syndrome. Front Psychiatry 2018, 9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Rourke L, Murphy KC: Recent developments in understanding the relationship between 22q.2 deletion syndrome and psychosis. Curr Opin Psychiatry 2019, 32:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF et al. : Towards reproducible brain-wide association studies. bioRxiv 2020. [Google Scholar]
- 30.•.Rogdaki M, Gudbrandsen M, McCutcheon RA, Blackmore CE, Brugger S, Ecker C, Craig MC, Daly E, Murphy DGM, Howes O: Magnitude and heterogeneity of brain structural abnormalities in 22q.2 deletion syndrome: a meta-analysis. Mol Psychiatry 2020, 25:1704–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]; This meta-analytic study investigated differences in mean brain volume and structural variability within regions between 22q11.2DS and typically developing controls through 1 May 2019. They found decreased volume in 22q11.2DS compared with control for: total brain, gray matter and white matter. There was also overall decreased volume in frontal, temporal, and parietal lobes, cerebellum and hippocampus. Increased variability in 22q11.2DS individuals was found only for the hippocampus and lateral ventricles. The results support convergence of structural abnormalities in 22q11.2DS and schizophrenia. Increased variability in hippocampus may underlie some of the heterogeneity in the neuropsychiatric phenotype.
- 31.Scarpazza C, Lattanzi GM, Antoniades M, Di Fabio F, Sartori G, Eickhoff SB, McGuire P, Tognin S: Systematic review and multimodal meta-analysis of magnetic resonance imaging findings in 22q.2 deletion syndrome: Is more evidence needed? Neurosci Biobehav Rev 2019, 107:143–153. [DOI] [PubMed] [Google Scholar]
- 32.••.Sun D, Ching CRK, Lin A, Forsyth JK, Kushan L, Vajdi A, Jalbrzikowski M, Hansen L, Villalon-Reina JE, Qu X et al. : Large-scale mapping of cortical alterations in 22q.2 deletion syndrome: convergence with idiopathic psychosis and effects of deletion size. Mol Psychiatry 2020, 25:1822–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study capitalized on the ENIGMA (Enhancing Neuro Imaging Genetics Through Meta-Analysis) 22q11.2 Working Group, representing the largest analysis of brain structural alterations in 22q11DS to date. The imaging data were collected from 10 centers worldwide, including 474 participants with 22q11DS and 315 typically developing, matched controls. 22q11DS individuals showed thicker cortical gray matter overall, but lower focal thickness in temporal and cingulate cortex. Cortical surface area (SA) was pervasively lower in 22q11DS. Cases versus controls were classified with 93.8% accuracy based on these neuroanatomic patterns. Comparison of 22q11DS-psychosis to idiopathic schizophrenia revealed convergence of affected brain regions, particularly in fronto-temporal cortex. Finally, cortical SA was significantly greater in 22q11DS cases with smaller 1.5 Mb deletions, relative to those with typical 3 Mb deletions. Thus, there is a robust neuroanatomic signature of 22q11DS, and deletion size impacts brain structure. Psychotic illness in this highly penetrant deletion was associated with similar neuroanatomic abnormalities to idiopathic schizophrenia. These findings highlight the homogeneity of this single genetic etiology, and support the suitability of 22q11DS as a biological model of schizophrenia.
- 33.Ching CRK, Gutman BA, Sun D, Villalon Reina J, Ragothaman A, Isaev D, Zavaliangos-Petropulu A, Lin A, Jonas RK, Kushan L et al. : Mapping subcortical brain alterations in 22q.2 deletion syndrome: effects of deletion size and convergence with idiopathic neuropsychiatric illness. Am J Psychiatry 2020, 177:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villalón-Reina JE, Martínez K, Qu X, Ching CRK, Nir TM, Kothapalli D, Corbin C, Sun D, Lin A, Forsyth JK et al. : Altered white matter microstructure in 22q.2 deletion syndrome: a multisite diffusion tensor imaging study. Mol Psychiatry 2019. 10.1038/s41380-019-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zöller D, Sandini C, Karahanoğlu FI, Padula MC, Schaer M, Eliez S, Van De Ville D: Large-scale brain network dynamics provide a measure of psychosis and anxiety in 22q.2 deletion syndrome. Biol Psychiatry Cogn Neurosci Neuroimaging 2019, 4:881–892. [DOI] [PubMed] [Google Scholar]
- 36.Zhan L, Jenkins LM, Zhang A, Conte G, Forbes A, Harvey D, Angkustsiri K, Goodrich-Hunsaker NJ, Durdle C, Lee A et al. : Baseline connectome modular abnormalities in the childhood phase of a longitudinal study on individuals with chromosome 22q.2 deletion syndrome. Hum Brain Mapp 2018, 39:232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattiaccio LM, Coman IL, Thompson CA, Fremont WP, Antshel KM, Kates WR: Frontal dysconnectivity in 22q.2 deletion syndrome: an atlas-based functional connectivity analysis. Behav Brain Funct 2018, 14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mancini V, Zöller D, Schneider M, Schaer M, Eliez S: Abnormal development and dysconnectivity of distinct thalamic nuclei in patients with 22q.2 deletion syndrome experiencing auditory hallucinations. Biol Psychiatry Cogn Neurosci Neuroimaging 2020, 5:875–890. [DOI] [PubMed] [Google Scholar]
- 39.Seidlitz J, Nadig A, Liu S, Bethlehem RAI, Vértes PE, Morgan SE, Váša F, Romero-Garcia R, Lalonde FM, Clasen LS et al. : Transcriptomic and cellular decoding of regional brain vulnerability to neurogenetic disorders. Nat Commun 2020, 11:3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.•.Mancini V, Sandini C, Padula MC, Zöller D, Schneider M, Schaer M, Eliez S: Positive psychotic symptoms are associated with divergent developmental trajectories of hippocampal volume during late adolescence in patients with 22qDS. Mol Psychiatry 2019. 10.1038/s41380-019-0443-z [DOI] [PubMed] [Google Scholar]; Low hippocampal volume is a consistent finding in schizophrenia and this study examined MRIs acquired from 140 patients with 22q11DS (53 with psychotic symptoms) and 135 healthy controls aged 6 to 35 years and with up to 5 time points per participant. Individuals with 22q11DS had lower volume of all subfields except for CA2/3. No divergent trajectories in hippocampal development were found. Patients with psychotic symptoms compared to those without psychosis had lower volume during late adolescence, starting in CA1 and spreading to other subfields. Thus, patients with 22q11DS and psychotic symptoms undergo a further decrease in volume during adolescence.
- 41.Brudfors M, Balbastre Y, Nachev P, Ashburner J: A tool for super-resolving multimodal clinical MRI. arXiv [eessIV] 2019. [Google Scholar]


