Abstract
Background
Poirier–Bienvenu neurodevelopmental syndrome is a neurologic disorder caused by mutations in the CSNK2B gene. It is mostly characterized by early-onset seizures, hypotonia, and mild dysmorphic features. Craniodigital syndrome is a recently described disorder also related to CSNK2B, with a single report in the literature.
Objective
To report two unrelated cases of children harboring CSNK2B variants (NM_001320.6) who presented with distinct diseases.
Case report
Case 1 is a 7-month-old, Caucasian, female patient with chief complaints of severe hypotonia and drug-refractory myoclonic epilepsy, with a likely pathogenic de novo variant c.494A>G (p.His165Arg). Case 2 is a 5-year-old male, Latino patient with craniodigital intellectual disability syndrome subjacent to a de novo, likely pathogenic variant c.94G>T (p.Asp32Tyr). His dysmorphic features included facial dysmorphisms, supernumerary nipples, and left-hand postaxial polydactyly.
Conclusion
This report suggest that the CSNK2B gene may be involved in the physiopathology of neurodevelopmental disorders and variable dysmorphic features.
Keywords: Epilepsy, Hypotonia, Dysmorphic features, Case report
Introduction
Poirier–Bienvenu neurodevelopmental syndrome (POBINDS; OMIM #618732) is a recently described disorder characterized by hypotonia, seizures, and developmental delay [1]. POBINDS is caused by mutations in the CSNK2B gene (located at 6p21.33), which encodes the beta subunit (CK2β) of the casein kinase 2 enzyme (CK2). It has been reported that the CSNK2B gene is neither susceptible to missense mutations (Z = 3.83) nor loss of function (pLi 0.92; observed/expected = 0.08; 95% confidence interval 0.03–0.38). In fact, all cases reported have been subjacent to de novo mutations and caused variants that lead to loss of function [2].
Dysmorphic features other than those originally reported have recently been associated with craniodigital syndrome (CDS), a condition that can be distinguished from POBINDS [3]. However, these dysmorphic features are not fully characterized [4].
Th work aims to detail the clinical manifestations of patients with two CSNK2B variants presenting with distinct phenotypes, one with the phenotype closer to POBINDS and another with the phenotype closer to CDS. To the best of our knowledge, only two cases have described patients with similar dysmorphic features. We also discuss possible pathogenic mechanisms based on a literature review, emphasizing the necessity to better elucidate the clinical phenotype spectrum related to the CSNK2B gene.
Case report
Case #1: Hypotonia and neonatal myoclonic spasm
A 7-month-old, female Caucasian patient presented with neurodevelopmental delay followed by epilepsy with unaltered electroencephalogram (EEG). The patient is the second child of a nonconsanguineous couple, having an 11-year-old healthy brother. No other family member had a medical history of neurological or genetic disorders. Although the pregnancy was uneventful, the patient was delivered by cesarean due to placental detachment with a gestational age of 39 + 1 weeks. The patient was a full-term infant with birth weight of 3.4 kg and APGAR score of 6 and 8 in the first and fifth minutes, respectively. Due to myoclonic movements, hypotonia, and cyanosis, the patient was admitted to the neonatal intensive care unit.
At the age of 23 days, the patient was referred to a Reference Center in Rare Genetic Diseases at the Porto Alegre University Hospital. The epileptiform crisis evolved to recurring focal motor seizures in the upper limbs with simultaneous apnea. The physical evaluation revealed marked global hypotonia and dysmorphic features such as midfacial hypoplasia, bilateral strabismus, tongue protrusion, and dysplastic ears (overfolded helix) as shown in Fig. 1.
Fig. 1.

Hands of patient #2: The patient from the second case report showed fifth short fingers, tapered distal phalanges of fingers, left hand postaxial polydactyly (surgically corrected, pointed by the arrow) and nail hypoplasia in the (A) and (B) panels
The patient underwent a metabolic genetic investigation, which included normal urinary organic acids and quantitative plasma amino acids. Empiric treatment with biotin and pyridoxine failed to result in any improvement of her clinical picture. Neuroimaging study (magnetic resonance imaging, MRI) did not reveal any abnormality and the EEG was unaltered even when it was performed during the myoclonic movements. Due to global hypotonia, further investigation ruled out Prader Willi Syndrome, Pompe disease, and congenital disorders of glycosylation. Karyotype was also unchanged, and the chromosomal microarray revealed a duplication of the 1p31.3 (61236531_61661276), which was considered a variant of uncertain significance.
Laryngeal fiberscopic evaluation revealed an obstructive laryngomalacia. The patient underwent a supraglottoplasty at the age of 1 month. Despite the procedure, the spasms persisted. The patient was then discharged from the hospital for further follow-up in the outpatient clinic with the diagnostic hypothesis of sleep myoclonus.
At the age of 7 months, physical examination revealed severe global development delay (inability to lift the head) due to truncal hypotonia. Her epilepsy had worsened (intensity and duration of the episodes) being considered a pharmacoresistent myoclonic epilepsy triggered by both auditory and visual stimuli.
The exome sequencing revealed a de novo likely pathogenic CSNK2B variant (NM_001320.6). The variant c.494A>G (p.His165Arg) is present in exon 6 out of 7 exons, in a highly conserved amino acid and it is not observed in The Genome Aggregation Database (gnomAD). In silico tools predict the variant to be deleterious (SIFT: Damaging 0; Revel: Deleterious low; CADD Score: 26.1). The variant is found in the Human Gene Mutation Database (HGMD) as a disease-causing mutation (DM). No other pathogenic variation was found in other epilepsy candidate genes.
Case #2: Hypotonia and dysmorphisms
A 5-year-old, male Latin patient was referred for genetic evaluation at the age of 13 months, with the chief complaints of dysmorphisms, hypotonia, and developmental delay.
The patient was born from a nonconsanguineous young couple. He was also delivered by cesarean section due to placental detachment, with a gestational age of 41 weeks. He was a full-term infant with birth weight of 3.4 kg and APGAR score of 9 in the first and fifth minutes.
The patient’s motor development was considered delayed: the patient sustained his head at the age of 6 months, sat unassisted at the age of 8 months, and walked independently at 2.4 years. At 9 months of age, the patient presented with acute episodes of muscle tone loss associated with abnormal eye movements. The EEG performed at 1 year showed disorganized basal activity, which was considered to be related to brain immaturity. The sleep-related graph elements did not show any epileptiform paroxysmal waves.
Physical examination revealed axial hypotonia, microbrachycephaly, low nasal bridge, anteverted nares, malar hypoplasia, high and narrow palate, duplicated upper right incisor, prominent and dysplastic ears, and supernumerary nipples. Examination of the extremities showed short fifth fingers, tapered distal phalanges of fingers, left hand postaxial polydactyly (surgically corrected), and nail hypoplasia, as shown in Fig. 1.
Genetic evaluation included karyotype and chromosomal microarrays, which were unaltered. Exome sequencing identified a de novo missense pathogenic CSNK2B variant (NM_001320.6) in heterozygosis. The variant c.94G>T is present in exon 3 out of 7 exons, with a highly conserved amino acid sequence, which are not described in the gnomAD. In silico tools predicted that the variant is likely deleterious (SIFT: Damaging 0; Revel: Deleterious high; CADD Score: 32). In HGMD, the variant position was already reported, leading to a different amino acid substitution (c.94G>A; p.Asp32Asn) and subjacent to the same phenotype. Sanger sequencing confirmed the presence of the variant in the patient but not in the parents.
Discussion
After the initial report of POBINDS [1], two subsequent papers documented the medical history of three unrelated patients with marked impaired global development and complex partial seizures before 2 months of age [5, 6]. The refractory seizures observed in these patients included focal evolving to generalized tonic–clonic episodes, and epileptic encephalopathy. Epilepsy in POBINDS is also characterized by unaltered EEG and MRI. Being drug resistant, tonic–clonic seizures are the most frequent presentation [2, 7, 8]. The absence of a specific imaging pattern corroborated in both of our cases to a delayed diagnosis, as exemplified in Case #1 where sleep myoclonus was one of the differential diagnosis owing to the unaltered EEG.
Few in vivo studies have demonstrated the interaction of CK2 and voltage channels that could explain the epilepsy seen in these patients. Potassium channels are the most diverse group of the ion channel family. These channels are not only involved in shaping the action potential, but also in neuronal excitability and plasticity. One study using transfected Chinese hamster ovary cells expressing Kv3.1 (a potassium channel with a very high threshold) demonstrated the importance of its phosphorylation by CK2. When CK2 was inhibited, there was a decrease in the phosphorylation of the Kv3.1 channels, leading to the decline in its electric potentials [9, 10]. Although speculative, it might be related to the etiology of seizures found in these patients.
The most striking clinical feature of Case #2 was the dysmorphic features that were recently associated with CDS, which could be distinguished from POBINDS [3]. A recently published paper reported the case of a young male Japanese patient with a deletion of 6p21.33 (which encompasses the CSNK2B gene). The patient displayed very similar features to patient #2, including large low-set ears, downslanted palpebral fissures, flared eyebrows, wide-base nose, and flat philtrum with thin upper lip [11] (for further details, see [8] and Table 1). Collectively, these cases corroborate the loss of function as plausible mechanisms described for both POBINDS and CDS.
Table 1.
Comparison of the clinical phenotype of patients described in this paper with those in [11]
| Clinical Features | Case 1 | Case 2 | [11] |
|---|---|---|---|
| Genotype | c.494A>G (p.His165Arg)/– | c.94G>T(p.Asp32Tyr)/– | 6p.21.33 deletion/– |
| Perinatal history | Placental detachment | Placental detachment | Uneventful |
| Hypotonia | Yes | Yes | Yes |
| ID/DD | Yes | Yes | Yes |
| Gross motor development delay | Yes | Yes | Yes |
| Development of speech and language | Yes | No | Yes |
| Facial dysmorphisms | Midfacial hypoplasia, bilateral strabismus, lingual protrusion, and over-folded helices | Microbrachycephaly, low nasal root, anteverted nostrils, malar hypoplasia, high and narrow palate, duplicated upper right incisor, prominent and dysplastic ears | Relative macrocephaly. Large low-set ears, downslanting palpebral fissures, flared eyebrows, wide-base nose, and flat philtrum with thin upper lip |
| Other dysmorphisms | No | Supernumerary nipples, short fifth fingers, distal tapering of fingers, left hand postaxial polydactyly, and nail hypoplasia | ND |
| Age at seizure onset | Newborn | 9 months | ND |
| Seizure types | Myoclonic spasms | Atonic Seizures | ND |
| Familiar recurrence | No | No | No |
| EEG | Normal | Disorganized base activity | ND |
| Brain image | Normal | Normal | Incomplete hippocampal folding |
| Other | Obstructive laryngomalacia | No | Asthma attacks and food allergies |
CK2 is a heterotrimeric enzyme consisting of two catalytic CK2α or CK2α′ subunits and two regulatory β subunits. It also contains several distinct domains. The three main domains are Asp/Glu-enriched (acidic), zinc-binding (where the dimerization of β subunit occurs), and α-subunit-interaction domains [12, 13]. Even though it is considered a regulatory subunit, CK2β is highly conserved. Previous studies have demonstrated that if this regulatory subunit is excessively synthesized, there is a trend to the formation of of CK2β dimers, which are, in turn, a prerequisite for the formation and correct function of the whole enzyme [12, 14].
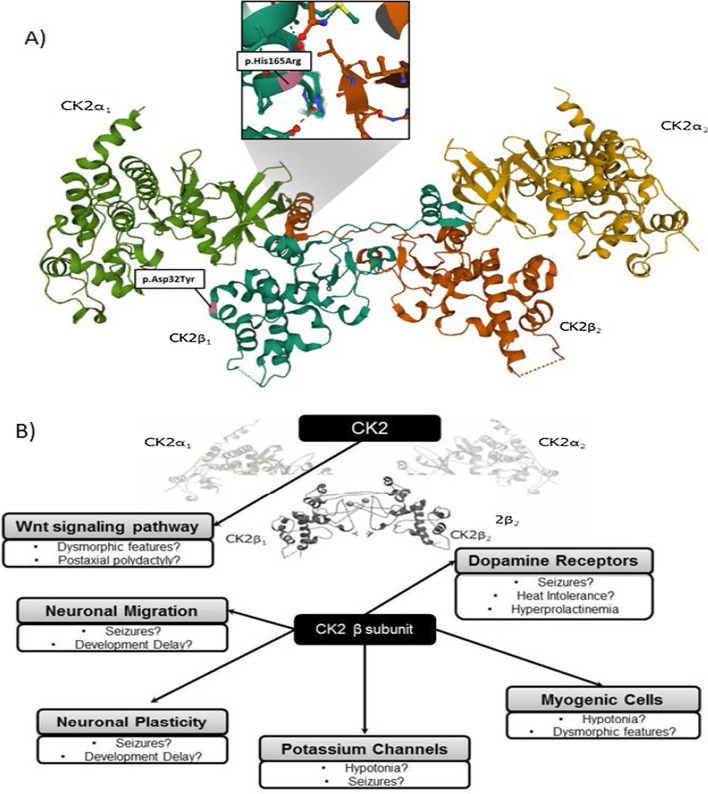
It is believed that the zinc-binding domain might be a hotspot for pathogenic variants and that the α-subunit-interaction domain might be the one related to refractory myoclonic epilepsy [2, 15]. The closest zinc binding site in the variant-related protein from patient#1 is a cysteine in position 137. However, p.His165Arg, according to crystal structure, is in close interaction with the complementary beta subunit, as seen in Fig. 2. The dimers of CK2β are located at the core of the tetrameric CK2 complexes and we hypothesize that symptoms observed in patient #1 might be due to alterations in this beta-beta interaction, subsequently leading to a disruption in the CK2β dimerization.
Fig. 2.
Hypothetical association among the patients’ phenotypes to the CSK2B variants. In A, the Ribbon diagram illustrates the high-resolution structure of tetrameric CK2 and the location of the variants. p.His165Arg is located in close interaction between CK2β1 and CK2β2 and p.Asp32Tyr to the alfa1 catalytic subunit. In the crystal structure of full-length symmetric CK2 (α2β2) holoenzyme (PDB ID: 4MD7) (B), the β subunit interacts with different systems. Gray boxes contain previously described roles of CK2 and white boxes detail the putative association with the clinical manifestation of the reported patients.
Two de novo missense mutations (p.Asp32Asn and p.Asp32His) in the same CSNK2B codon, which is mutated in patient #2, have recently been associated with CDS [3, 7]. Due to the modification of the CK2 structure, the closest CK2 catalytic subunit would be position 32, which could exert a more severe dysmorphic phenotype. One study using whole transcriptome and whole phosphoproteome profiling demonstrated that variants in this protein amino acid position caused an up-regulation of CSNK2B gene expression at transcript and protein levels, resulting in impaired cross talk between α and β subunits of CK2 [3]. Both hypotheses, however, must be further demonstrated by functional studies. A definite association between phenotype and genotype in CSNK2B gene-related epilepsy has still not been described [2].
A hypothesis for the POBINDS-related “brain-centered symptoms” could be raised after preclinical research on the CK2 function in animal cell models. The CK2 subunit appears to be constitutively active, being a target for dopamine D1 receptors signaling pathway [1, 16]. The resulting alterations in the dopaminergic system (as per increased D1 receptor signaling and/or dopamine production) has also been found in patients with juvenile myoclonic epilepsy [8], possibly contributing to understanding of myoclonic epilepsy, as well as the excessive sweating observed in the patient in case #1.
Additionally, a study using GN11 cells as a model of immature migrating neurons, demonstrated that CK2β was involved in cell migration and microfilament/microtubule organization in neurons, thus playing a role in neurodevelopmental steps such as neural differentiation, neuritogenesis, and synaptic plasticity [15, 17]. In line with these findings, studies using β subunit CK2 knockout cell lines (KOβ) pointed out that the β subunit may serve as an anchorage point for other kinases, being involved in the myogenic commitment of C2C12 (myogenic cell lines in murines) by regulating MyoD expression independently from the catalytic subunits [15, 17]. In a C2C12 model, CK2β contributes to a variety of cell cycle processes such as proliferation and cellular transport in vitro. Collectively, these findings might contribute to the elucidation of the hypotonia presented by both patients. CK2 has also been implicated in organogenesis, playing a role in heart embryogenesis and limb bud differentiation via the Wnt signaling pathway. Further studies are warranted to determine the association of this role to the limb dysmorphisms found in the patient from the second case [4].
Conclusion
To date, 16 patients have been reported in the literature with POBINDS and 2 patients have been reported with craniodigital intellectual disability syndrome related to mutations in the CSNK2B gene. The present cases may assist in the diagnosis of POBINS when patients present with refractory epilepsy, hypotonia, and dysmorphic features. Further investigation is warranted to better understand the interaction of CK2 and the neuronal and neuromuscular systems. We suggest that screening of CSNK2B could be included in the most common gene panels for epilepsy.
Acknowledgements
Not applicable.
Abbreviations
- ACMG
American College of Medical Genetics
- CDS
Craniodigital syndrome
- CMA
Chromosomal microarray
- CK2
Casein kinase 2 enzyme
- CK2β
Casein kinase 2 enzyme beta subunit.
- EEG
Electroencephalogram
- gnomAD
The Genome Aggregation Database
- HGMD
Human Gene Mutation Database
- LOF
Loss of function
- POBINDS
Poirier–Bienvenu neurodevelopmental syndrome
Authors’ contributions
CFMS and MJRD collected the clinical data. CFMS and MVMBW conceptualized the manuscript and drafted its first version. BMO and FK performed the genetic analysis of the patients. CFMS, BMO, FK, MJRD, and AP participated in critical analysis and review of the manuscript. All authors read and approved the final manuscript. Camilla Patti Hissamura provided language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data was retrieved from patients medical files from Hospital de Clinicas de Porto Alegre (Porto Alegre – RS, Brazil), Hospital Moinhos de Vento (Porto Alegre – RS, Brazil), Laboratorio Mendelics (Sao Paulo- SP Brazil) with consent.
Declarations
Ethics approval and consent to participate
All procedures followed were in accordance with the ethical standards of the Ethical Committee from the Hospital de Clínicas de Porto Alegre – Porto Alegre; RS; Brazil and Laboratório Mendelics – Sao Paulo; SP – Brazil and with the Helsinki Declaration of 2013, as revised in 2000.
Consent for publication
Written informed consent was obtained from the patients' legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
All authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Poirier K, Hubert L, Viot G, et al. CSNK2B splice site mutations in patients cause intellectual disability with or without myoclonic epilepsy. Hum Mutat. 2017;38:932–941. doi: 10.1002/humu.23270. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Gao K, Cai S, et al. Germline de novo variants in CSNK2B in Chinese patients with epilepsy. Sci Rep. 2019;9:17909. doi: 10.1038/s41598-019-53484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asif M, Kaygusuz E, Shinawi M, et al. De novo pathogenic variants in CSNK2B cause a new intellectual disability-craniodigital syndrome distinguished from Poirier-Bienvenu neurodevelopmental syndrome. Eur J Hum Genet. 2020;28:129–131. [Google Scholar]
- 4.Lou DY, Dominguez I, Toselli P, et al. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol Cell Biol. 2008;28:131–139. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakashima M, Tohyama J, Nakagawa E, et al. Identification of de novo CSNK2A1 and CSNK2B variants in cases of global developmental delay with seizures. J Hum Genet. 2019;64:313–322. doi: 10.1038/s10038-018-0559-z. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi Y, Uehara T, Suzuki H, et al. Truncating mutation in CSNK2B and myoclonic epilepsy. Hum Mutat. 2017;38:1611–1612. doi: 10.1002/humu.23307. [DOI] [PubMed] [Google Scholar]
- 7.Ernst ME, Baugh EH, Thomas A, et al. CSNK2B: a broad spectrum of neurodevelopmental disability and epilepsy severity. Epilepsia. 2021;62:e103–e109. doi: 10.1111/epi.16931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvam P, Jain A, Cheema A, et al. Poirier-Bienvenu neurodevelopmental syndrome: a report of a patient with a pathogenic variant in CSNK2B with abnormal linear growth. Am J Med Genet A. 2021;185:539–543. doi: 10.1002/ajmg.a.61960. [DOI] [PubMed] [Google Scholar]
- 9.Macica CM, Kaczmarek LK. Casein kinase 2 determines the voltage dependence of the Kv3.1 channel in auditory neurons and transfected cells. J Neurosci. 2001;21:1160–1168. doi: 10.1523/JNEUROSCI.21-04-01160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller C. An overview of the potassium channel family. Genome Biol. 2000;1:reviews0004.1–reviews0004.5. doi: 10.1186/gb-2000-1-4-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohashi I, Kuroda Y, Enomoto Y, et al. 6p21.33 Deletion encompassing CSNK2B is associated with relative macrocephaly, facial dysmorphism, and mild intellectual disability. Clin Dysmorphol. 2021;30:139–141. doi: 10.1097/MCD.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 12.Niefind K, Guerra B, Ermakowa I, Issinger O-G. Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J. 2001;20:5320–5331. doi: 10.1093/emboj/20.19.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raaf J, Brunstein E, Issinger O-G, Niefind K. The interaction of CK2α and CK2β, the subunits of protein kinase CK2, requires CK2β in a preformed conformation and is enthalpically driven. Protein Sci Publ Protein Soc. 2008;17:2180–2186. doi: 10.1110/ps.037770.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham KC, Litchfield DW. The regulatory β subunit of protein kinase CK2 mediates formation of tetrameric CK2 complexes*. J Biol Chem. 2000;275:5003–5010. doi: 10.1074/jbc.275.7.5003. [DOI] [PubMed] [Google Scholar]
- 15.Lettieri A, Borgo C, Zanieri L, et al. Protein kinase CK2 subunits differentially perturb the adhesion and migration of GN11 cells: a model of immature migrating neurons. Int J Mol Sci. 2019 doi: 10.3390/ijms20235951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole A, Poore T, Bandhakavi S, et al. A global view of CK2 function and regulation. Mol Cell Biochem. 2005;274:163–170. doi: 10.1007/s11010-005-2945-z. [DOI] [PubMed] [Google Scholar]
- 17.Der Vartanian A, Audfray A, Al Jaam B, et al. Protein O-fucosyltransferase 1 expression impacts myogenic C2C12 cell commitment via the notch signaling pathway. Mol Cell Biol. 2015;35:391–405. doi: 10.1128/MCB.00890-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data was retrieved from patients medical files from Hospital de Clinicas de Porto Alegre (Porto Alegre – RS, Brazil), Hospital Moinhos de Vento (Porto Alegre – RS, Brazil), Laboratorio Mendelics (Sao Paulo- SP Brazil) with consent.