Abstract
Background
Low muscle strength has been pointed out as a key characteristic of sarcopenia, but the prognostic significance of muscle function next to reduced skeletal muscle mass (SMM) in patients with cancer has been scantily investigated.
Methods
Data on muscle strength by handgrip (HG) dynamometry and total‐body SMM estimated by bioelectrical impedance analysis (BIA) of Italian and German patients with cancer observed prospectively until death or censoring were analysed (N = 1076). Patients were stratified in four risk categories based on low HG (<10th percentiles of age and gender‐specific normative values) and low total‐body SMM according to SMM index cutoffs (<10.75 and <6.75 kg/m2 in men and women, respectively).
Results
During a median follow‐up of 58 months [25th–75th percentile, 37–60], 566 patients had died. Patients presenting low HG in combination or not with low SMM were characterised by shorter median survival (12.7 vs. 27.2 months, respectively; p < 0.001) compared to those with low SMM/normal HG and normal SMM/normal HG (>60 months for both). After adjusting for sex, age, body mass index and percentage of weight loss, disease's stage, performance status and type of cancer, compared to reference category (normal HG and SMM; N = 210) the hazard ratios were: low SMM/normal HG (N = 342), 0.83 [95% confidence interval, CI, 0.67–1.02] (p = 0.073); normal SMM/low HG (N = 158), 1.19 [95% CI, 1.07–1.32] (p = 0.002); low SMM/low HG (N = 366), 1.39 [95% CI, 1.27–1.53] (p < 0.001).
Conclusions
Muscle weakness was found to be a more powerful predictor of survival than BIA‐estimated SMM and should be considered as an additional key feature of sarcopenia in patients with cancer.
Keywords: bioelectric impedance analysis (BIA), cancer, handgrip strength, mortality, prognosis, sarcopenia
Short abstract
Low muscle strength has been pointed out as a key characteristic of sarcopenia, but the prognostic significance of muscle function next to reduced skeletal muscle mass (SMM) in patients with cancer has been scantily investigated. Muscle weakness was found to be a more powerful predictor of survival than SMM and should be considered as additional key feature of sarcopenia in patients with cancer.
1. INTRODUCTION
In the last two decades, there has been growing interest in the prognostic impact of body composition in patients with cancer. Changes in body composition are heterogeneously associated with unintentional weight loss and of multifactorial origin, but mainly caused by a combination of reduced food intake and metabolic processes related not only to the tumor inflammatory burden, but also to anticancer treatments. 1 , 2 , 3 Specifically, reduced skeletal muscle mass (SMM)––namely sarcopenia––has been associated with increased mortality and chemotherapy‐related toxicity independently of body mass index, 4 , 5 , 6 , 7 which is a relevant issue, given the current overweight/obesity epidemic. 8 However, although sarcopenia has received an independent International Classification of Disease‐10 code as a condition characterised by low muscle mass and weakness, 9 literature shows some inconsistencies in its definition. In patients with cancer, the diagnosis of sarcopenia has been always based on the presence of low muscle mass, while in old adults its identification requires the presence of low muscle strength in combination with muscle changes, with poor physical performance as indicative of a severe condition. 10 , 11 , 12 Interestingly, recent studies have addressed and highlighted that also reduced muscle function could have a negative and independent prognostic impact, resulting in increased mortality, dose‐limiting toxicity and other perioperative outcomes (e.g. length of stay, readmission rate), 13 , 14 , 15 , 16 , 17 particularly when reduced strength and lean/muscle mass coexist. 13 , 14 , 15 Its use as alternative phenotypic criterion of malnutrition for indicating reduced muscle mass––rather than other body composition parameters, such as arm muscle circumference, fat‐free mass index and muscle mass by computed tomography (CT)––has been also tested, showing a stronger prediction of mortality in patients with cancer. 18 , 19 , 20 As muscle strength testing could be an informative, low‐cost, well accepted and routinely feasible procedure, 21 further study in this area are warranted.
In this cohort study, we investigated the prognostic role of handgrip strength in addition to body composition in patients with cancer.
2. SUBJECTS AND METHODS
2.1. Participants
Study participants were Italian and German patients with cancer, consecutively included in previous intervention trials (NCT02055833; NCT02065726; NCT02828150) 22 , 23 , 24 and cohort studies 25 , 26 , 27 and prospectively followed at the Fondazione IRCCS Policlinico San Matteo (Pavia, Italy) and the Charité – Universitätsmedizin Berlin (Berlin, Germany). In these studies, common inclusion criteria were: age ≥18 years, solid or haematological neoplastic disease, complete data on most relevant clinical features (anthropometry, cancer type, stage and performance status), exposure (handgrip strength and body composition) and outcome (vital status). We excluded patients with implanted pacemakers or defibrillators, oedema or ascites as these factors can interfere with body composition assessment by bioelectric impedance.
2.2. Assessments
The following data were collected at first evaluation:
-
1
Demographic and major clinical information: cancer site, disease's stage (American Joint Committee on Cancer stage groupings) and performance status (Eastern Cooperative Oncology Group [ECOG]). 28
-
2
Anthropometry: body weight (to the nearest 0.1 kg) and height (to the nearest 0.5 cm); in body mass index (BMI; calculated as weight [kg]/height [m]2); and percentage of unintentional weight loss (%WL) occurred in the previous 6 months. 29 Then, patients were graded in 5 BMI‐adjusted WL risk categories using the 5 × 5 matrix proposed by Martin et al. 30 and based on BMI (<20.0, 20.0–21.9, 22.0–24.9, 25.0–27.9 and ≥28.0 kg/m2) and %WL (≤−2.5%, −2.5% to −5.9%, −6.0% to −10.9%, −11.0% to −14.9% and ≥−15.0) strata.
-
3
Body composition: bioelectrical impedance analysis (BIA) was used to estimate total‐body SMM, using the equation of Janssen et al. 31
[height, in sm; Resistance, in Ohms; gender, men = 1 and women = 0; age, in years]
We used the phase‐sensitive device NUTRILAB Akern srl in Italians (accuracy for resistance, 1%) and the multi‐frequency device Nutriguard–M Data Input GmbH in Germans (accuracy for resistance, ±0.5%). The two instruments have been derived from and cross‐validated against one another. Although a significant difference in the measure of 50 KHz‐resistance between the two devices (approximately +9 Ohms for the German technology) has been found, 32 we have estimated that, using the equation of Janssen et al., 31 this would translate in a mean discrepancy in SMM < 0.5 kg. Furthermore, the diagnostic validity of BIA against CT for the assessment of muscle mass (MM) in patients with cancer has been recently reported. 33 Its accuracy in identifying patients with low MM has been also demonstrated in the intensive care setting. 34
Therefore, SMM index (SMMI) was calculated (SMM [kg]/height [m]2) and BIA‐derived low SMMI in men and women was defined by a value <10.75 and <6.75 kg/m2, respectively. 35
-
4
Muscle strength: handgrip strength (HG) was measured by hand dynamometry (DynEx™; Akern/MD Systems in Italians; Jamar, Sammons Preston Rolyan in Germans) in the dominant hand, testing the patient in sitting position with the shoulder adducted and neutrally rotated, the elbow flexed at 90°, and the forearm and wrist in neutral position. The mean of three consecutive trials was used in the analysis. Low HG was defined as a value <10th percentiles of age and gender‐specific normative values. 36
2.3. Outcome ascertainment
Patients were actively followed (up to January 2020) until death or censoring (date of last contact) using the following methods: linkage to municipal registries, in‐office visits, inquiries by mail or telephone to participants or proxy respondents.
2.4. Statistical analysis
Descriptive statistics were provided for continuous (mean and standard deviation or median and interquartile range) and categorical variables (count and percentage). Patients were stratified in four categories by the presence of low SMMI and low HG. Differences between groups were investigated using the one‐way analysis of variance (continuous variables) and the Fisher's exact test (categorical variables). We used the reverse Kaplan–Meier method to calculate median follow‐up and survival curves were provided for SMMI/HG strata. Mortality rates (per 100 person‐year) and median survival, together with their 95% confidence interval (95% CI) were also computed. A multivariable model (Cox's regression) was used to evaluate the independent association of SMMI/HG strata and mortality. The following noncollinear confounders were included: sex, age, ECOG performance status, disease's stage, BMI‐adjusted weight loss risk categories and cancer site. Hazard ratio (HR) and 95% CI were computed. Huber–White's robust standard error was used to account for intra‐centre correlation. The interaction of SMMI and HG was assessed.
Data were analysed using the software STATA 16.1 (Stata Corporation) setting statistical significance to a two‐sided p level of <0.05.
3. RESULTS
In total, 1218 patients were enrolled in the study and 1076 were eligible for analyses (Italians, N = 450; Germans, N = 626). Reasons of exclusion were: lost to follow‐up, n = 1; missing data in handgrip strength, n = 141. Italian patients were mainly evaluated at diagnosis, while Germans were evaluated at different stages of the disease's course. The two cohorts presented heterogeneous and different cancer diagnoses but comparable stage. Italians were characterised by better performance status, although they presented lower muscle strength reasonably due to higher age, lower BMI and more frequent unintentional WL. 37 , 38
Reduced SMMI was found in 708 patients and 524 showed muscle weakness. The characteristics (clinical and nutritional) of the study population by low SMMI and HG strata are reported in Table 1. Reduced muscle mass and muscle weakness were associated with all demographic and clinical variables. Particularly, low HG was associated with WL, reduced performance status and advanced disease's stage. Furthermore, the risk of combined low muscle mass and strength was twice higher in male patients.
TABLE 1.
Features of the study cohort by reduced skeletal muscle mass and muscle weakness
| Demographic and clinical characteristic |
Whole cohort (N = 1076) |
Normal SMMI Normal HG (N = 210) |
Low SMMI Normal HG (N = 342) |
Normal SMMI Low HG (N = 158) |
Low SMMI Low HG (N = 366) |
p‐value a |
|---|---|---|---|---|---|---|
| Sex (male), N (%) | 636 (59.1) | 55 (26.2) | 238 (31.8) | 54 (34.2) | 289 (79.0) | <0.001 |
| Age (years), median (IQR) | 64.7 (55.0–72.1) | 64.2 (54.0–70.0) | 66.0 (56.0–72.8) | 61.3 (52.0–68.2) | 65.0 (55.3–74.0) | 0.23 |
| Body mass index (kg/m2), mean (SD) | 23.6 (4.3) | 25.7 (4.4) | 23.9 (4.1) | 24.5 (4.9) | 21.8 (3.5) | <0.001 |
| 6‐month weight loss (%), mean (SD) | 8.6 (9.6) | 5.8 (8.7) | 6.9 (8.8) | 9.2 (8.9) | 11.4 (10.3) | <0.001 |
| Cancer site, N (%) | <0.001 | |||||
| Gastrointestinal | 343 (31.9) | 72 (34.2) | 133 (38.9) | 39 (24.7) | 99 (27.1) | |
| Head and neck | 229 (21.3) | 40 (19.1) | 79 (23.1) | 25 (15.8) | 85 (23.2) | |
| Urogenital | 157 (14.6) | 35 (16.7) | 35 (10.2) | 37 (23.4) | 50 (13.7) | |
| Haematological | 144 (13.4) | 35 (16.7) | 47 (13.7) | 22 (13.9) | 40 (10.9) | |
| Neuroendocrine, adrenal and thyroid | 37 (3.4) | 10 (4.8) | 5 (1.5) | 10 (6.3) | 12 (3.3) | |
| Lung | 75 (7.0) | 8 (3.8) | 22 (6.4) | 11 (7.0) | 34 (9.3) | |
| others | 91 (8.5) | 10 (4.8) | 21 (6.1) | 14 (8.9) | 46 (12.6) | |
| Cancer stage, N (%) | 0.002 | |||||
| I | 138 (12.8) | 30 (14.3) | 51 (14.9) | 10 (6.3) | 47 (12.8) | |
| II | 107 (10.0) | 29 (13.8) | 42 (12.3) | 8 (5.1) | 28 (7.7) | |
| III | 180 (16.7) | 38 (18.1) | 60 (17.5) | 26 (16.5) | 56 (15.3) | |
| IV | 651 (60.5) | 113 (53.8) | 189 (55.3) | 114 (72.2) | 235 (64.2) | |
| ECOG performance status, N (%) | 0.01 | |||||
| 0–1 | 721 (67.0) | 151 (71.9) | 241 (70.5) | 104 (65.8) | 225 (61.5) | |
| 2 | 324 (30.1) | 55 (26.2) | 97 (28.4) | 45 (28.5) | 127 (34.7) | |
| 3 | 31 (2.9) | 4 (1.9) | 4 (1.2) | 9 (5.7) | 14 (3.8) | |
| Skeletal muscle mass index (kg/m2), mean (SD) | <0.001 for all | |||||
| Overall | 8.71 (1.91) | 8.92 (1.87) | 8.39 (1.96) | 9.39 (2.48) | 8.31 (1.47) | |
| Males | 9.59 (1.51) | 11.67 (0.73) | 9.38 (0.87) | 12.12 (1.99) | 8.89 (1.02) | |
| Females | 7.20 (1.26) | 7.95 (0.97) | 6.11 (0.51) | 7.98 (1.19) | 6.11 (0.52) | |
| Handgrip strength (kg), mean (SD) | <0.001 for all | |||||
| Overall | 25.4 (10.3) | 26.9 (8.8) | 32.7 (9.9) | 17.7 (7.5) | 21.1 (7.3) | |
| Males | 30.1 (9.9) | 38.2 (6.7) | 37.9 (7.0) | 25.3 (6.5) | 23.1 (6.7) | |
| Females | 18.6 (6.3) | 22.8 (5.3) | 20.9 (4.3) | 13.8 (4.3 | 13.6 (4.2) |
Abbreviations: ECOG, Eastern Cooperative oncology Group; HG, handgrip strength; IQR, interquartile range; SD, standard deviation; SMMI, skeletal muscle mass index.
One‐way analysis of variance (continuous variables) or Fisher's exact test (categorical variables).
After a median follow‐up of 58 months [25th–75th percentile, 37–60], 566 patients had died (mortality rate, 23.0 per 100 person‐year [95% CI, 21.2–25.0]). At univariable analysis both low SMMI and low HG were associated with higher mortality risk: HR = 1.13 [95% CI, 1.04–1.22] (p = 0.004) and HR = 1.80 [95% CI, 1.56–2.07] (p < 0.001), respectively. A significant qualitative interaction between these two factors was found (p < 0.001), with a lower mortality rate in patients with normal HG and low SMMI than in those with normal HG and normal SMMI, and vice versa a higher mortality in patients with low HG and low SMMI than in those with low HG and normal SMMI (Figure 1). This results in an increased risk of death associated with low HG (HR = 1.41, 95% CI 1.28–1.55, p < 0.001) in patients with normal SMMI and an even higher risk in patients with low SMMI (HR = 2.02, 95% CI 1.75–2.34, p < 0.001). Adjustment for major confounders yielded consistent findings, with an HR associated with a low HG was 1.19 [95% CI 1.07–1.32, p = 0.002] in patients normal SMMI and of 1.39 [95% CI 1.27–1.53, p = 0.001] in patients with low SMMI (Figure 2).
FIGURE 1.
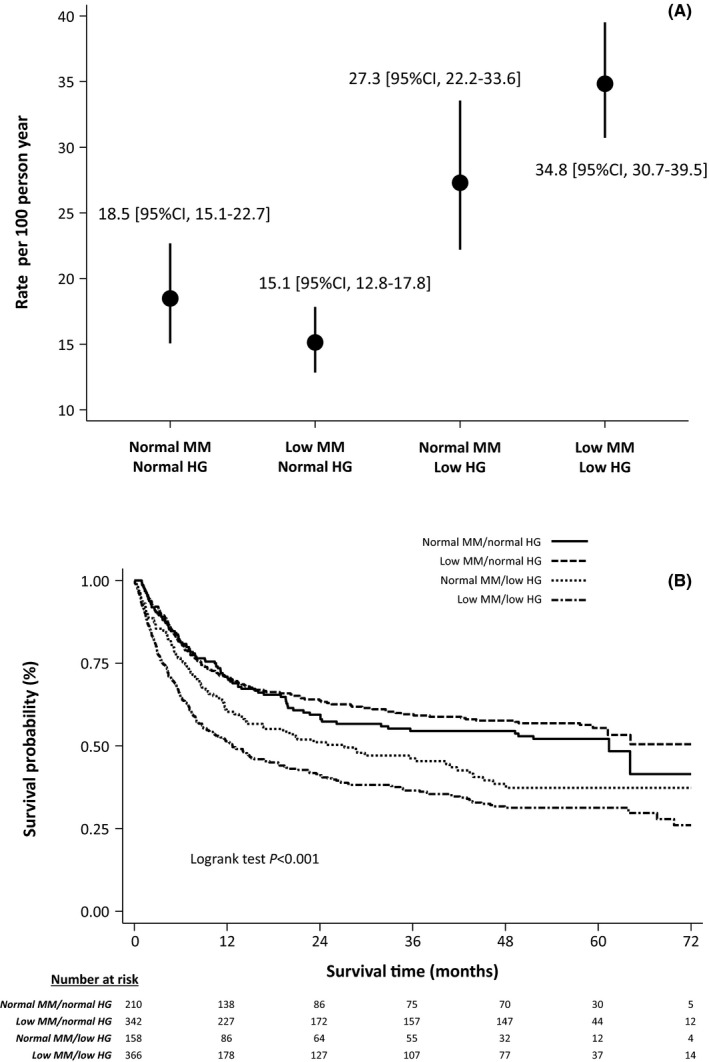
Mortality rates (A) and cumulative survival curves (B) across reduced skeletal muscle mass and muscle weakness strata
FIGURE 2.
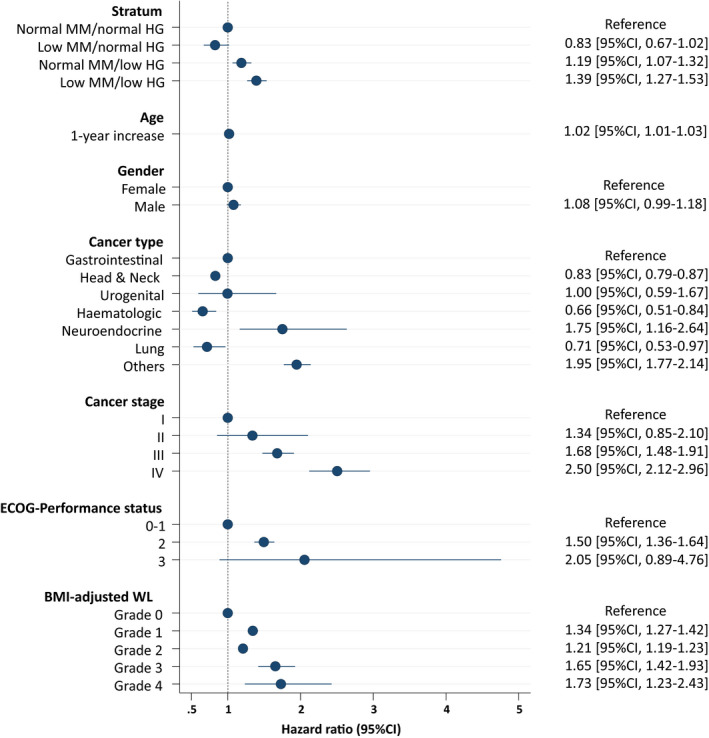
Predictors of mortality (risk estimates by Cox's regression) in the study cohort
4. DISCUSSION
The present study showed that muscle weakness is a strong independent prognostic factor in patients with cancer, particularly when it coexists with reduced BIA‐estimated SMM (additive effect).
Our results are consistent with recent findings supporting not only the independent negative impact of low muscle strength in different cancer types, but also its additional predictive value of worse outcomes (e.g. perioperative outcome and survival) when combined with reduced lean body mass as estimated by BIA or assessed by CT. 13 , 14 , 15 , 16 , 17 , 39 Burtin et al. found that muscle weakness alone predicted survival in patients with lung cancer and that it significantly worsened prognosis when associated with reduced fat‐free mass, particularly in patients with a good performance status. 14 Sarcopenia defined by reduced muscle mass (by CT) and strength was also found to predict higher rates of 90‐day morbidity than isolated weakness or low muscle mass after liver resection for malignant tumors. 13 Muscle weakness has been also proposed as a reliable phenotypic criterion of malnutrition, substitute of reduced muscle mass or arm muscle circumference or fat‐free mass index, showing a stronger prediction of mortality in patients with cancer. 18 , 19 , 20 Indeed, low SMM is a risk factor and not an absolute determinant of worse outcomes (e.g. dose‐limiting toxicity, mortality.) in this patient's population, 40 even when assessed by CT. We cannot exclude that the additional evaluation of muscle weakness would improve risk assessment and discrimination of patients, thus resulting in a more powerful trigger for guiding tailored and timely interventions consisting of adequate nutritional care and exercise. Further studies in this area are warranted, but it is noteworthy that muscle weakness, with reduced performance, likely denotes refractory cachexia when combined with other nutritional features such as low BMI, WL or reduced muscle mass frequently secondary to variable degree of anorexia and systemic inflammation. 1 , 2 , 41 However, recent studies have shown that upper body strength alone did not discriminate between patients with or without cachexia and those with and without muscle wasting, as the incidence of both may be heterogeneous in different cancer types. 42 , 43 On the other hand, although the relationship between muscle mass and muscle function is complex, 44 it is interesting that muscle dysfunction predicted worse outcome, even in presence of normal muscle mass and independently of multiple confounders. Reduced functional capacity in patients with cancer may be present even in absence of muscle catabolism, likely being an early sign of systemic inflammation, not yet activated within the muscle. 45 , 46 Nonetheless, low grip strength has been associated with higher case‐fatality rates in different chronic diseases. 47 Interestingly, in patients with cancer, anabolic agents have been proven to increase muscle mass but have failed in improving muscle function and performance as well as survival, 44 while studies addressing the role of exercise interventions––namely prehabilitation, which may include nutrition––have shown not only an increase in strength and performance, but also improved outcome such as lower post‐operative complications, increased treatment‐tolerance, reduced symptoms burden and hospitalisation rates, 48 , 49 , 50 , 51 , 52 , 53 thus supporting the importance of physical functioning. As grip strength is an inexpensive, simple and routinely feasible measurement also in patients with cancer, 21 it is reasonable to argue for its implementation in the screening and diagnostic approach of sarcopenia in this patient's population. This proposal is consistent with the operational algorithm for case‐finding and confirmation recently implemented for an old age population by expert panels, 12 , 54 according to which the evaluation of muscle strength and/or performance should come to the forefront, as some methodologies used in the assessment of body composition––such as BIA and Dual‐Energy X‐ray Absorptiometry––do not directly measure MM. 55 Indeed, the complexity of sarcopenia in oncology must be acknowledged. To address this issue we have adjusted for multiple confounders, including age, and used age and gender‐specific threshold values. Nowadays, despite the recognised prognostic value of reduced MM in patients with cancer, the evaluation of body composition is still not part of routine care and it is reasonably affected by the availability of technologies and local research interests. Most literature is based on the use of CT, which provides a more accurate assessment of this body compartment and it is not influenced by hydration such as BIA, but it could be performed only at established time points as a standard of care and still lacks of definite threshold values. Furthermore, also age‐specific cut‐offs for SMM assessed by CT are not available. On the other hand, BIA is non‐invasive, inexpensive and, as bedside procedure, can be performed whenever a major event occurs or the conditions of the patient change. In our study we have excluded patients with fluid retention, in order to limit assessment bias, but we recognise the use of BIA as a limitation as more standardisation of its use is needed also to support the inter‐changeability of the different devices. However, our study likely reflects the daily practice as we have analysed data of patients assessed at different moments of their disease's course.
Therefore, a lot of work still needs to be done in this area and confirmatory studies combining the evaluation of muscle weakness with the use of more accurate technologies in the evaluation of MM are clearly warranted. These should consider multiple relevant outcomes, including treatment tolerance. Nonetheless, the evaluation of physical performance and its prognostic value needs to be addressed. In our study measures of this domain (e.g. gait speed) were not available but they could provide a more comprehensive assessment of sarcopenia.
In conclusion, muscle strength was found to be a relevant predictor of survival both in the absence and in the presence of compromised BIA‐estimated SMM and, as an easy measurement to perform, it should be considered as additional key feature of sarcopenia also in patients with cancer. Confirmatory studies are needed to support an improvement in the operational definition of sarcopenia in this patient's population.
ETHICS STATEMENT
The use of follow‐up data for the present study has been approved by Institutional Ethics Committees. We obtained written informed consent from every patient.
CONFLICT OF INTEREST
All the authors have no relationship to disclose.
ACKNOWLEDGMENTS
The authors gratefully thank all the healthcare professionals and the caregivers for their assistance with recruitment and data collection. None received compensation beyond their normal salaries.
Cereda E, Tancredi R, Klersy C, et al. Muscle weakness as an additional criterion for grading sarcopenia‐related prognosis in patients with cancer. Cancer Med. 2022;11:308–316. doi: 10.1002/cam4.4362
Abstract (preliminary draft) presented at the ESPEN 2020 Virtual Congress c/o MCI Geneva (19–21 September 2020).
Funding information
The study was funded by Alleanza Contro il Cancro (RCR‐2020‐23670066), the Fondazione IRCCS Policlinico San Matteo, the Charité – Universitätsmedizin Berlin and by an unconditioned grant from Nutricia Italia S.p.a.
DATA AVAILABILITY STATEMENT
Data described in the manuscript, code book and analytic code will not be made available because a specific note was not included in the informed consent at the time of protocol approval and recruitment. Therefore, data sharing was not approved by the Ethics Committees.
REFERENCES
- 1. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489‐495. [DOI] [PubMed] [Google Scholar]
- 2. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11‐48. [DOI] [PubMed] [Google Scholar]
- 3. Peixoto da Silva S, Santos JMO, Costa e Silva MP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. 2020;11(3):619‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9(7):629‐635. [DOI] [PubMed] [Google Scholar]
- 5. Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15(22):6973‐6979. [DOI] [PubMed] [Google Scholar]
- 6. Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non‐small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91(4):1133S‐1137S. [DOI] [PubMed] [Google Scholar]
- 7. Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539‐1547. [DOI] [PubMed] [Google Scholar]
- 8. Donini LM, Busetto L, Bauer JM, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr. 2019;39(8):2368‐2388. [DOI] [PubMed] [Google Scholar]
- 9. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berardi G, Antonelli G, Colasanti M, et al. Association of sarcopenia and body composition with short‐term outcomes after liver resection for malignant tumors. JAMA Surg. 2020;155(11):e203336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burtin C, Bezuidenhout J, Sanders KJC, et al. Handgrip weakness, low fat‐free mass, and overall survival in non‐small cell lung cancer treated with curative‐intent radiotherapy. J Cachexia Sarcopenia Muscle. 2020;11(2):424‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang DD, Cai HY, Wang WB, et al. Measurement of muscle quantity/quality has additional predictive value for postoperative complications and long‐term survival after gastrectomy for gastric cancer in patients with probable sarcopenia as defined by the new EWGSOP2 consensus: analysis from a large‐scale prospective study. Nutrition. 2021;16(86):111156. [DOI] [PubMed] [Google Scholar]
- 16. Hagens ERC, Feenstra ML, van Egmond MA, et al. Influence of body composition and muscle strength on outcomes after multimodal oesophageal cancer treatment. J Cachexia Sarcopenia Muscle. 2020;11(3):756‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Botsen D, Ordan MA, Barbe C, et al. Dynapenia could predict chemotherapy‐induced dose‐limiting neurotoxicity in digestive cancer patients. BMC Cancer. 2018;18(1):955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Contreras‐Bolívar V, Sánchez‐Torralvo FJ, Ruiz‐Vico M, et al. GLIM criteria using hand grip strength adequately predict six‐month mortality in cancer inpatients. Nutrients. 2019;11(9):2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Groot LM, Lee G, Ackerie A, van der Meij BS. Malnutrition screening and assessment in the cancer care ambulatory setting: mortality predictability and validity of the Patient‐Generated Subjective Global Assessment Short form (PG‐SGA SF) and the GLIM criteria. Nutrients. 2020;12(8):2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou LP, Yu DY, Ma BW, et al. Feasibility of substituting handgrip strength for muscle mass as a constituent standard in the Global Leadership Initiative on Malnutrition for diagnosing malnutrition in patients with gastrointestinal cancers. Nutrition. 2021;84:111044. [DOI] [PubMed] [Google Scholar]
- 21. Ordan MA, Mazza C, Barbe C, et al. Feasibility of systematic handgrip strength testing in digestive cancer patients treated with chemotherapy: the FIGHTDIGO study. Cancer. 2018;124(7):1501‐1506. [DOI] [PubMed] [Google Scholar]
- 22. Cereda E, Cappello S, Colombo S, et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol. 2018;126(1):81‐88. [DOI] [PubMed] [Google Scholar]
- 23. Cereda E, Turri A, Klersy C, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019;8(16):6923‐6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caccialanza R, Cereda E, Caraccia M, et al. Early 7‐day supplemental parenteral nutrition improves body composition and muscle strength in hypophagic cancer patients at nutritional risk. Support Care Cancer. 2019;27(7):2497‐2506. [DOI] [PubMed] [Google Scholar]
- 25. Norman K, Stobäus N, Zocher D, et al. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am J Clin Nutr. 2010;92(3):612‐619. [DOI] [PubMed] [Google Scholar]
- 26. Norman K, Wirth R, Neubauer M, Eckardt R, Stobäus N. The bioimpedance phase angle predicts low muscle strength, impaired quality of life, and increased mortality in old patients with cancer. J Am Med Dir Assoc. 2015;16(2):173.e17‐e22. [DOI] [PubMed] [Google Scholar]
- 27. Caccialanza R, Cereda E, Klersy C, et al. Bioelectrical impedance vector analysis‐derived phase angle predicts survival in patients with systemic immunoglobulin light‐chain amyloidosis. Amyloid. 2020;27:168‐173. [DOI] [PubMed] [Google Scholar]
- 28. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649‐655. [PubMed] [Google Scholar]
- 29. World Health Organization . Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1‐452. [PubMed] [Google Scholar]
- 30. Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol. 2015;33(1):90‐99. [DOI] [PubMed] [Google Scholar]
- 31. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985). 2000;89(2):465‐471. [DOI] [PubMed] [Google Scholar]
- 32. Dittmar M, Reber H. Validation of different bioimpedance analyzers for predicting cell mass against whole‐body counting of potassium (40K) as a reference method. Am J Hum Biol. 2004;16(6):697‐703. [DOI] [PubMed] [Google Scholar]
- 33. Mueller TC, Reik L, Prokopchuk O, Friess H, Martignoni ME. Measurement of body mass by bioelectrical impedance analysis and computed tomography in cancer patients with malnutrition ‐ a cross‐sectional observational study. Medicine (Baltimore). 2020;99(50):e23642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Looijaard WGPM, Stapel SN, Dekker IM, et al. Identifying critically ill patients with low muscle mass: agreement between bioelectrical impedance analysis and computed tomography. Clin Nutr. 2020;39(6):1809‐1817. [DOI] [PubMed] [Google Scholar]
- 35. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413‐421. [DOI] [PubMed] [Google Scholar]
- 36. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12):e113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cereda E, Caraccia M, Klersy C, et al. Validation of a new prognostic body composition parameter in cancer patients. Clin Nutr. 2021;40(2):615‐623. [DOI] [PubMed] [Google Scholar]
- 38. Cereda E, Pedrazzoli P, Lobascio F, et al. The prognostic impact of BIA‐derived fat‐free mass index in patients with cancer. Clin Nutr. 2021;40(6):3901‐3907. [DOI] [PubMed] [Google Scholar]
- 39. Otten L, Stobäus N, Franz K, et al. Impact of sarcopenia on 1‐year mortality in older patients with cancer. Age Ageing. 2019;48(3):413‐418. [DOI] [PubMed] [Google Scholar]
- 40. Carneiro IP, Mazurak VC, Prado CM. Clinical implications of sarcopenic obesity in cancer. Curr Oncol Rep. 2016;18(10):62. [DOI] [PubMed] [Google Scholar]
- 41. Cederholm T, Barazzoni R, Austin P, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49‐64. [DOI] [PubMed] [Google Scholar]
- 42. Moreau J, Ordan MA, Barbe C, et al. Correlation between muscle mass and handgrip strength in digestive cancer patients undergoing chemotherapy. Cancer Med. 2019;8(8):3677‐3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson LJ, Lee J, Mallen MC, et al. Evaluation of physical function and its association with body composition, quality of life and biomarkers in cancer cachexia patients. Clin Nutr. 2021;40(3):978‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramage MI, Skipworth RJE. The relationship between muscle mass and function in cancer cachexia: smoke and mirrors? Curr Opin Support Palliat Care. 2018;12(4):439‐444. [DOI] [PubMed] [Google Scholar]
- 45. Op den Kamp CM, Langen RC, Minnaard R, et al. Pre‐cachexia in patients with stages I‐III non‐small cell lung cancer: systemic inflammation and functional impairment without activation of skeletal muscle ubiquitin proteasome system. Lung Cancer. 2012;76(1):112‐117. [DOI] [PubMed] [Google Scholar]
- 46. Norman K, Stobäus N, Kulka K, Schulzke J. Effect of inflammation on handgrip strength in the non‐critically ill is independent from age, gender and body composition. Eur J Clin Nutr. 2014;68(2):155‐158. [DOI] [PubMed] [Google Scholar]
- 47. Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266‐273. [DOI] [PubMed] [Google Scholar]
- 48. Waterland JL, McCourt O, Edbrooke L, et al. Efficacy of prehabilitation including exercise on postoperative outcomes following abdominal cancer surgery: a systematic review and meta‐analysis. Front Surg. 2021;19(8):628848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Waart H, Stuiver MM, van Harten WH, et al. Effect of low‐intensity physical activity and moderate‐ to high‐intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918‐1927. [DOI] [PubMed] [Google Scholar]
- 50. Padilha CS, Marinello PC, Galvão DA, et al. Evaluation of resistance training to improve muscular strength and body composition in cancer patients undergoing neoadjuvant and adjuvant therapy: a meta‐analysis. J Cancer Surviv. 2017;11(3): 339‐349. [DOI] [PubMed] [Google Scholar]
- 51. Squires RW, Shultz AM, Herrmann J. Exercise training and cardiovascular health in cancer patients. Curr Oncol Rep. 2018;20(3):27. [DOI] [PubMed] [Google Scholar]
- 52. Mijwel S, Bolam KA, Gerrevall J, Foukakis T, Wengström Y, Rundqvist H. Effects of exercise on chemotherapy completion and hospitalization rates: the OptiTrain breast cancer trial. Oncologist. 2020;25(1):23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mijwel S, Backman M, Bolam KA, et al. Adding high‐intensity interval training to conventional training modalities: optimizing health‐related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat. 2018;168(1):79‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bhasin S, Travison TG, Manini TM, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc. 2020;68(7): 1410‐1418. [DOI] [PubMed] [Google Scholar]
- 55. Cawthon PM, Orwoll ES, Peters KE, et al.; Osteoporotic FRACTURES IN Men (MrOS) Study Research Group . Strong relation between muscle mass determined by D3‐creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book and analytic code will not be made available because a specific note was not included in the informed consent at the time of protocol approval and recruitment. Therefore, data sharing was not approved by the Ethics Committees.


