Abstract
Under the new US heart allocation policy, transplant centers listed significantly more candidates at high priority statuses (Status 1 and 2) with mechanical circulatory support devices than expected. We determined whether the practice change was widespread or concentrated among certain transplant centers. Using data from the Scientific Registry of Transplant Recipients, we used mixed-effect logistic regression to compare the observed listings of adult, heart-alone transplant candidates post-policy (December 2018 to February 2020) to seasonally matched pre-policy cohort (December 2016 to February 2018). US transplant centers (N = 96) listed similar number of candidates in each policy period (4472 vs. 4498) but listed significantly more at high priority status (25.5% vs. 7.0%, p < .001) than expected. Adjusted for candidate characteristics, 91 of 96 (94.8%) centers listed significantly more candidates at high-priority status than expected, with the unexpected increase varying from 4.8% to 50.4% (interquartile range [IQR]: 14.0%–23.3%). Centers in OPOs with highest Status 1A transplant rate pre-policy were significantly more likely to utilize high-priority status under the new policy (OR: 9.73, p = .01). The new heart allocation policy was associated with widespread and significantly variable changes in transplant center practice that may undermine the effectiveness of the new system.
Keywords: cardiology, ethics, heart transplantation, organ allocation, organ Procurement and Transplantation Network (OPTN), public policy
1 |. INTRODUCTION
In October 2018, the Organ Procurement and Transplant Network (OPTN) implemented a new heart allocation policy designed to improve compliance with the US Department of Health and Human Services Final Rule on organ transplantation.1,2 In addition to increasing the geographic sharing of donor hearts, the policy expanded the number of therapy-based Status levels from 3 to 6, intending to improve candidate risk stratification, reduce exception requests, and accommodate changing mechanical circulatory support (MCS) practices.3 The Status tier changes were intended to decompress the highest priority status level by splitting Status 1A into Status 1–3 and adding strict hemodynamic requirements. The OPTN further enhanced the priority of Status 1 and 2 by assigning these tiers a larger first geographic allocation circle compared to Status 3 (500 nautical miles compared to 250 nautical miles). If treatment practices remained stable, only 6% of candidates were expected to be initially listed at Status 1 or Status 2.4 However, transplant centers used exception requests, extracorporeal membrane oxygenation (ECMO), and intra-aortic balloon pumps (IABPs) at much higher rates than anticipated,4–7 listing over 25% of candidates at Status 1 or Status 2.
It is unknown if this practice shift was uniform or concentrated among a subgroup of centers. Past heart allocation policy changes have had widely variable effects across the country. The first expansion of geographic sharing of donor hearts in 2006 was associated with marked regional differences in left ventricular assist device (LVAD) use as a bridge to transplant.8 Under the old heart allocation system, transplant centers in large urban areas and competitive Organ Procurement Organizations (OPOs) were more likely to use IABPs or high-dose inotropes when their candidates did not meet cardiogenic shock hemodynamic criteria.9 Substantial between-center variation in listing practice under the new heart allocation policy without meaningful difference in candidate characteristics would suggest concerning disconnection between medical severity and access to transplantation.
The new heart allocation system significantly expanded the geographic sharing of donor hearts, effectively giving the new Status 1 and 2 levels even higher priority. Therefore, understanding why and how centers are changing their practices in response to the new heart allocation policy is critical to ensure the broad and fair distribution of donor hearts. In this observational cohort study, we aimed to (1) confirm that the high Status 1 and Status 2 listing rates have persisted over time, (2) estimate each center’s expected and observed high-priority status listing rate, and (3) identify local OPO characteristics associated with between-center variation in policy response.
2 |. METHODS
2.1 |. Data source and study period
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight to the activities of the OPTN and SRTR contractors. Since this was a secondary analysis of a prospectively obtained de-identified cohort, this study was deemed exempt by the University of Chicago Institutional Review Board.
We identified all adult, active, heart-only candidates newly added to the waitlist between December 1, 2016 and February 28, 2020 in the SRTR dataset. We constructed a study population from two seasonally matched cohorts from before and after the implementation of the new policy. The pre-policy cohort consists of all qualifying candidates listed between December 1, 2016 and February 28, 2018. The post-policy cohort consists of qualifying candidates listed between December 1, 2018 and February 28, 2020 (Figure S1). We excluded candidates listed inactive, for multiorgan transplant, or by a small transplant center with fewer than 10 candidate listings per year in either the pre-policy or post-policy periods.
2.2 |. Classification of status 1–6 and primary outcome
The primary outcome of this study was the probability of high-priority status listing, defined as initial listing at Status 1 or Status 2. The new allocation system divides the old Status 1A group into Status 1, 2, and 3. We designated only Status 1 and 2 as high-priority because these statuses have larger geographic priority than Status 3 (500 nautical miles compared to 250 nautical miles) and because of previously described increases in ECMO and IABP utilization (Status 1 and 2 qualifying therapies) and decreases in high-dose inotrope use (Status 3 qualifying therapy).4–7 Candidates in the post-policy cohort had their official status assignment at the time of listing directly extracted from the SRTR dataset. To determine the expected distribution of candidates under the new allocation policy, we classified pre-policy candidates into Status 1–6 using the treatments and hemodynamic values recorded in their listing justification forms. Importantly, we retrospectively applied the hemodynamic portion of the cardiogenic shock criteria to the pre-policy cohort. Pre-policy candidates were reclassified into Status 1–4 if they received qualifying therapeutic intervention and met necessary hemodynamic requirements (if applicable). Candidates originally assigned to Status 1A by exception requests are reclassified into Status 3. This reclassification method was previously employed in OPTN’s simulations and several other observational studies.4,9–11 Full details of our reclassification procedure are available in the Supplemental Material.
2.3 |. Candidate-level variables
To account for changes in candidate-level characteristics between policy periods that might explain changes in transplant center practices, we collected available demographic, medical and socioeconomic data at the time of initial listing. Demographic and medical covariates were weight, height, sex, body mass index (BMI), cardiac diagnosis, blood type, functional status, renal function, history of diabetes, smoking, cardiac index, and pulmonary capillary wedge pressure (PCWP). Functional status was recorded on Karnofsky 11-point performance status scale, which has been validated in heart failure patients. Socioeconomic variables were race, work history, education level, and insurance type.
2.4 |. Center-level variables
We investigated three potential transplant center and OPO characteristics for association with listing behavior in the post-policy period: transplant center volume, the level of competition in the OPO, and Status 1A transplant rate in the pre-policy period. We measured local competition for organs by the number of transplant centers in an OPO. An OPO was considered competitive if it consisted of three or more transplant centers, a level of competition previously associated with more aggressive treatment practices.9 Finally, a high rate of transplantation at Status 1A candidates in an OPO indicates that candidates listed at high-priority status had greater access to deceased donor organs. We hypothesized that high Status 1A transplant rate in the pre-policy period would predict larger practice changes in post-policy period as centers sought to maintain high transplant rate for their most prioritized candidates.
2.5 |. Statistical analysis
As candidate-level transplant data are clustered within transplant centers, we analyzed estimated the probability of high-status listing using a mixed-effects logistic regression model with a center-level random intercept and random policy effect. This model structure allows the pre-policy probability of high-status listing and post-policy probability of high-status listing to vary at the center level.12,13 We fitted three nested mixed-effects models: (1) policy effect alone, (2) policy effect and candidate level variables, and (3) policy effect, candidate level variables, and center-level variables (described above). Using the empirical Bayes estimate from Model 2 for the random intercept and pre-post policy effects, we used Center for Medicare and Medicaid services methodology to compute the case-mix adjusted probability of high-priority status listing at each transplant center in the pre- and post-policy periods, generating standard errors associated with each point estimate via bootstrapping.14–17 See the Supplemental Material for detailed statistical methods.
We compared center listing volumes in the two cohorts using a mixed-effect Poisson regression. Chi-square test was performed to assess whether the distribution of candidates across status assignments differed across policy periods. All analyses were conducted using R version 3.6.1 (The R Foundation for Statistical Computing) and Stata version 16 (Stata-Corp, LLC). All statistical testing was two-sided with a p-value threshold of <.05.
2.6 |. Sensitivity analyses
We performed two sensitivity analyses. First, we computed the odds ratio of high-priority status listing during the first and second half of the post-policy study period, using seasonally matched pre-policy cohorts as reference, to determine whether the impact of the allocation policy changed over time. Then, we applied our mixed-effect model to examine the relationship between policy period and probability of exception requests in high priority statuses (i.e., Status 1A pre-policy, Status 1 and 2 post-policy), adjusting for candidate characteristics.
3 |. RESULTS
3.1 |. Candidate characteristics
A total of 12 904 active, adult heart-only candidates were listed from December 1, 2016 and February 28, 2020. We excluded 399 candidates for inactive listing, three candidates for missing data, 519 candidates listed at low volume centers, and 178 multiorgan candidates. There were 4472 patients in the seasonally matched pre-policy period (December 1, 2016 to February 28, 2018) compared with 4498 in the post-policy period (December 1, 2018 to February 28, 2020). The number of candidates listed at each transplant center did not differ significantly between policy period on average (rate ratio = 1.00, 95% CI: 0.99–1.00). Table 1 compares candidate characteristics between the pre-policy and post-policy cohort. Overall, candidate characteristics remained largely unchanged across policy periods. Candidates listed in the post-policy period had a slightly lower cardiac index (absolute difference, −0.03 L/min/m2; 95% CI: −0.05 to −0.01 L/min/m2), and worse functional status (absolute difference in % with severe impairment, +3.3%; 95% CI: 1.2%–5.4%). Mean PCWP was comparable between the two policy periods (absolute difference, −0.2 mm Hg; 95% CI: −0.58 to 0.15 mm Hg).
TABLE 1.
US heart transplant candidates by policy period
| December 2016 to February 2018 (pre-policy) n = 4472 |
December 2018 to February 2020 (post-policy) n = 4498 |
Differencea (confidence interval) | |
|---|---|---|---|
| Age at listing (SD) | 53 (13) | 53 (13) | −0.4 (−1 to 0.2) |
| Female (%) | 1156 (26) | 1200 (27) | 0.8 (−1.0% to 2.6%) |
| Race | |||
| White | 2818 (63) | 2723 (61) | −2.5% (−4.5% to −0.5%) |
| Black | 1072 (24) | 1156 (26) | 1.7% (−0.1% to 3.5%) |
| Hispanic | 380 (8) | 430 (10) | 1.1% (−0.1% to 2.3%) |
| Other | 202 (5) | 189 (4) | −0.3% (−1.1% to 0.5%) |
| Smoking history (%) | 1992 (45) | 1870 (42) | −3% (−5.0% to −1.0%) |
| Working for income (%) | 870 (20) | 1039 (24) | 3.6% (1.9% to 5.3%) |
| Education status (%) | |||
| College | 2566 (57) | 2480 (55) | −2.2% (−4.3% to −0.1%) |
| High school | 1642 (37) | 1618 (36) | −0.7% (−2.7% to 1.3%) |
| Less than high school or other | 264 (6) | 400 (9) | 3% (1.9% to 4.1%) |
| BMI (kg/m2) | 27.86 (4.94) | 28.10(5.20) | 0.24 (0.02 to 0.46) |
| Blood type (%) | |||
| A | 1692 (38) | 1676 (37) | −0.6% (−2.6% to 1.4%) |
| AB | 213 (5) | 204 (5) | −0.2% (−1.1% to 0.7%) |
| B | 638 (14) | 656 (15) | 0.3% (−1.2% to 1.8%) |
| O | 1929 (43) | 1962 (44) | 0.5% (−1.6% to 2.6%) |
| Diagnosis (%) | |||
| Dilated cardiomyopathy, non-ischemic | 1947 (44) | 1940 (43) | −0.4% (−2.5% to 1.7%) |
| Ischemic cardiomyopathy | 1354 (30) | 1239 (28) | −2.7% (−4.6% to −0.8%) |
| Restrictive cardiomyopathy | 618 (14) | 700 (16) | 1.7% (0.2% to 3.2%) |
| Other | 553 (12) | 619 (14) | 1.4% (0.0% to 2.8%) |
| Creatinine | 66 (35) | 67 (41) | 0.8 (−0.8 to 2.4) |
| Diabetes | |||
| History of diabetes | 1300 (29) | 1278 (28) | −0.7% (−2.6% to 1.2%) |
| No history of diabetes | 3172 (71) | 3171 (70) | −0.4% (−2.3% to 1.5%) |
| Unknown | 0 (0) | 49 (1) | 1.1% (0.8% to 1.4%) |
| Functional status | |||
| Limited impairment, 70%−100% | 1096 (25) | 975 (22) | −2.8% (−4.5% to −1.1%) |
| Moderate impairment, 50%−60% | 1065 (24) | 923 (21) | −3.3% (−5.0% to −1.6%) |
| Severe impairment, ≤40% | 2212 (49) | 2373 (53) | 3.3% (1.2% to 5.4%) |
| Unknown | 99 (2) | 227 (5) | 2.8% (2.0% to 3.6%) |
| Cardiac index, mL/kg/m2 | 2.18 (1) | 2.15 (1) | −0.03 (−0.05 to −0.01) |
| PCWP, mm Hg | 18 (9) | 18 (9) | −0.2 (−0.6 to 0.2) |
| Payor | |||
| Medicaid | 609 (14) | 615 (14) | 0.1% (−1.3% to 1.5%) |
| Medicare | 1493 (33) | 1364 (30) | −3.1% (−5.0% to −1.2%) |
| Other | 175 (4) | 273 (6) | 2.2% (1.3% to 3.1%) |
| Private | 2195 (49) | 2246 (50) | 0.9% (−1.2% to 3.0%) |
| Pertinent justification at listing | |||
| ECMO | 75 (2) | 112 (2) | 0.8% (0.2% to 1.4%) |
| IABP | 206 (5) | 462 (10) | 5.7% (4.6% to 6.8%) |
| LVAD | 1229 (27) | 1105 (25) | −2.9% (−4.7% to −1.1%) |
| Other MCS | 107 (2) | 139 (3) | 0.7% (0.0% to 1.4%) |
| High-dose inotropes | 445 (10) | 210 (5) | −5.3% (−6.4% to −4.2%) |
| Low-dose inotropes | 1077 (24) | 247 (5) | −18.6% (−20% to −17%) |
| Exception requests | 141 (3) | 852 (19) | 15.8% (14% - 17%) |
| None | 1192 (27) | 1371 (30) | 3.8% (1.9% - 5.7%) |
| 90-day waitlist outcomes | |||
| Mortality | 137 (3) | 68 (2) | −1.6% (−2.2% to −1%) |
| Transplantation | 1539 (34) | 2226 (50) | +15.1% (13% to 17%) |
Note: Values are mean (standard deviation) or n (%). Difference in proportions and associated 95% confidence interval are reported for categorical variables.
Abbreviation: PWCP, pulmonary wedge capillary pressure.
Raw mean difference and associated 95% confidence interval are reported for continuous variables.
3.2 |. Distribution of priority statuses in the pre- and post-policy periods
Trends in expected and observed Status 1–6 listings during the transition to the new heart allocation policy are depicted in Figure 1A. After applying the new status justification to candidates listed in pre-policy period, the expected status distribution was 140 Status 1, 173 Status 2, 796 Status 3, 2020 Status 4, and 1343 Status 6. The observed status distribution after implementing the new policy was 206 Status 1, 938 Status 2, 580 Status 3, 1836 Status 4, and 938 Status 6 (p < .001 compared to expected). There were more high-priority listings than expected, with +1.4% more in Status 1 (95% CI: 0.7%–2.2%) and +17% more in Status 2 (95% CI: 15.7%–18.3%). In contrast, there were −4.91% fewer Status 3 (95% CI: −6.39% to −3.42%), −4.35% Status 4 (95% CI: −6.4% to −2.3%), and −9.2% Status 6 listings (95% CI: −11% to −7.4%). Status 5 is for multiorgan candidates who were excluded from the analysis.
FIGURE 1.
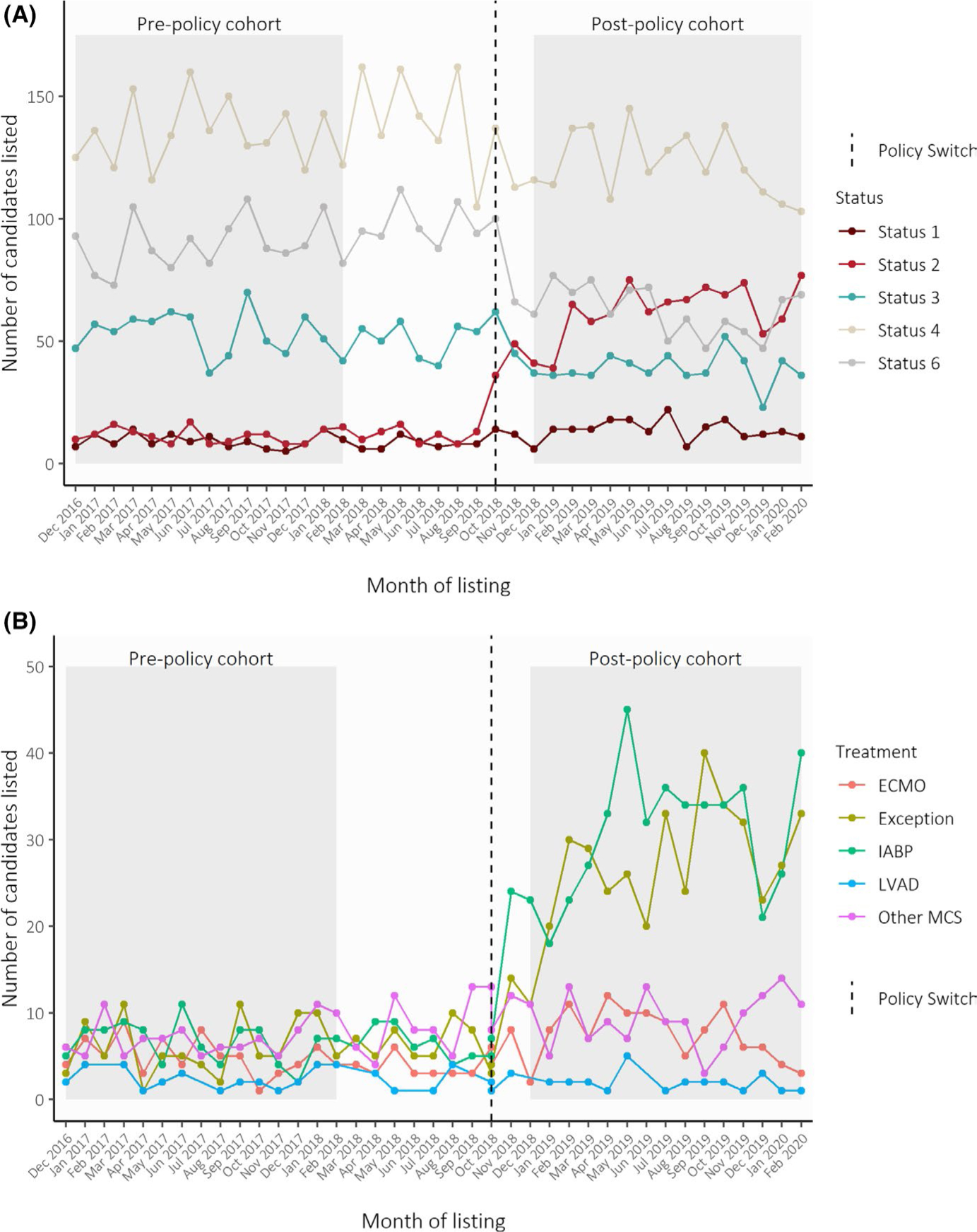
(A) Trends in expected and observed priority statuses during transition to the new heart allocation policy. Trends in the number of adult heart transplant candidates listed each month, as stratified by Status at initial listing. Colors correspond with observed status assignment in the post-policy period (after October 2018), and reclassified expected status assignment in the pre-policy period (before October 2018). Status 5 is for multiorgan transplant candidates, which were excluded from the analyses. Dashed line represents October 2018, when the new allocation policy was implemented. (B) Trends in high-priority status MCS justifications and exceptions during transition to the new heart allocation policy. Trends in the number of adult heart transplant candidates listed at high-priority statuses in each month, as stratified by treatment at initial listing. Prior to the implementation of the new allocation policy in October 2018, colors correspond to the treatments candidates reclassified as Status 1 and 2 or qualified for Status 1A through exception requests. After October 2018, colors correspond to the treatments used to justify Status 1 and Status 2 listings. Dashed line represents October 2018, when the new allocation policy was implemented. ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assistive device (with device malfunction to qualify for Status 1 or 2); Other MCS, other mechanical circulatory support
3.3 |. Justification for high-status listing in the post-policy period
Among the 313 candidates who met Status 1 or Status 2 criteria in the pre-policy cohort, centers listed 75 with ECMO, 99 with IABP, 32 with LVAD with device malfunction, and 107 with other MCS. There were 91 Status 1A exception requests. Among the 1144 candidates listed Status 1 or 2 in the post-policy cohort, centers listed 112 with ECMO, 462 with IABP, 25 with LVAD with device malfunction, and 139 with other MCS. There were 64 Status 1 exception requests and 342 Status 2 exception requests (Figure 1B). Transplant centers used ECMO 1.48 times more often (95% CI: 1.10–2.02), IABP 4.6 times more often (95% CI: 3.73–5.82), and exception requests 4.4 times more often (95% CI: 3.52–5.63) than expected.
3.4 |. Between-center variation in high-priority status listing across policy periods
In total, 7% of candidates met high-priority status criteria (Status 1 and 2) in the pre-policy cohort compared to the 25% candidates listed at high-priority in the post-policy cohort. Accounting for center-effects with the mixed-effects logistic regression, the odds of listing at high-priority status were fivefold higher in the post-policy period than expected (OR: 5.23, 95% CI: 4.26–6.42). After controlling for candidate level variables, the odds was 6-fold higher (adjusted OR: 6.35, 95% CI: 5.08–7.94) (Table 2, Tables S2 and S3).
TABLE 2.
Predictors of high-priority status listing in mixed effect logistic regression
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Listed in post-policy era | 5.23 (4.26, 6.42) | 6.35 (5.08, 7.94) | |
| Listed in post-policy era by Pre-policy Status 1A transplant rate quartilea | |||
| <70% | 5.53 (3.89, 7.86) | ||
| 70%−75% | 6.35 (4.13, 9.78) | ||
| 75%−82% | 4.86 (3.27, 7.20) | ||
| >82% | 9.73b (6.67, 14.19) | ||
Note: Values are odds ratios (95% CI), variance (95% CI), or n. Candidate-level variable coefficients and full model results, including the variances and covariances of the random effects can be found in Tables S2–S4.
Model 1: Adjusted for policy period only.
Model 2: Adjusted for policy period and candidate variables.
Model 3: Adjusted for policy period, candidate variables, and OPO-level Status 1A transplant rate as quartiles, number of transplant centers in the OPO, and total number of heart transplants performed by the center in each policy periods.
The cut-offs correspond to the pre-policy Status 1A transplant quartiles (i.e., centers with Status 1A transplant rate lower than 70% are in the first quartile, centers with transplant rate >82% are in the highest quartile).
OR is significantly different from that of being listed in the post-policy era at a center with <70% of Status 1A candidates receiving a transplant in the pre-policy period, p < .01.
After case-mix adjustment, the expected pre-policy high-priority status listing rate varied from 2.1% to 25.9% (interquartile range [IQR]: 4.9% to 9.9%) across centers. The observed high-status listing rates in the post-policy period varied from 7.6% to 62.4% (IQR: 20.0%–32.5%). Ninety-one (94.8%) centers listed significantly more patients at high-priority status than expected (Figure 2). The observed rate of high-priority status listing exceeded expectations by substantially different rates between transplant centers, ranging from +4.8% to +50.4% (IQR: 14.0%–23.3%). A total of 88 centers (92%) listed 10% more candidates at high status than expected and 39 centers (41%) listed 20% more candidates at high status than expected.
FIGURE 2.
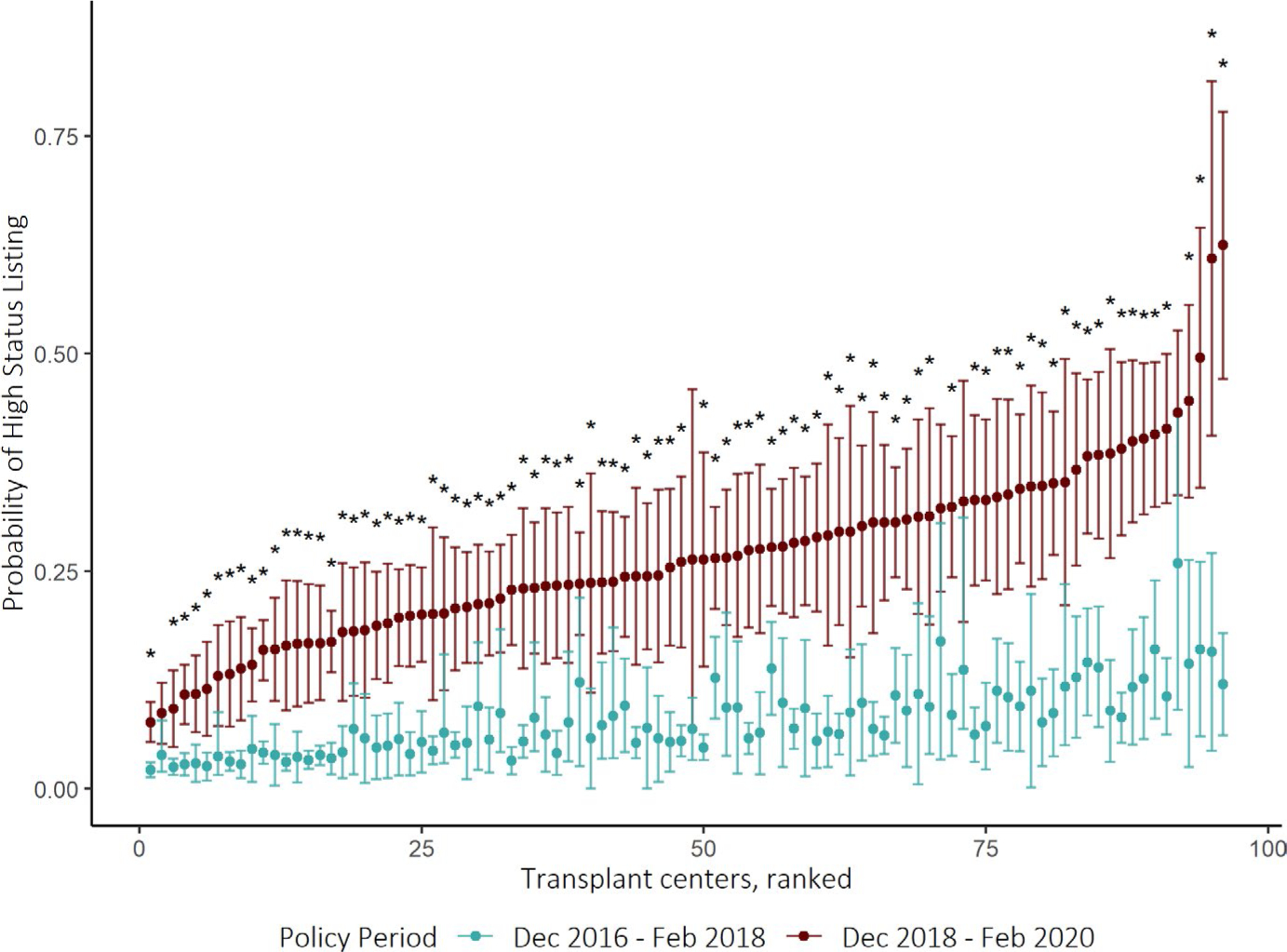
Between-center variation in high-priority status listing after implementation of the new heart allocation policy. Caterpillar plot showing the estimated probability of being listed at high-priority (Status 1 or Status 2) at each transplant center, adjusting for candidate characteristics. Colors correspond to the predicted rate of being listed in high-priority status based on status reclassification of pre-policy candidates (green) and the rate based on observed utilization of high-priority statuses after the policy change (red). The expected rates (green) represent the counterfactual scenario in which center practices did not change in response to the new heart allocation policy. The 95% CIs were constructed via bootstrapping. Transplant centers were ranked based on predicted probability of high-priority listing after policy update. Asterisks indicate centers with statistically significant change in the estimated probability of listing in Status 1 or 2 before and after implementing the new policy. The observed rate significantly exceeded the expected rate in 91 out of 96 centers (94.8%)
3.5 |. Association of center and OPO level variables and the change in high-status listing
Figure 3 compares the geographic variation in the rate of high-priority status listing before and after implementing the new heart allocation policy. After adjusting for candidate-level characteristics and weighting by listing volume at constituent transplant centers, the rate of high-priority status listing increased in all OPOs. While the magnitude of the increase differs substantially across the country, there is no obvious geographical pattern (Figure S3). Areas of the greatest rates of high-priority status listing in the post-policy period not only include densely populated urban areas but also many OPOs with large geographic size and low population density.
FIGURE 3.
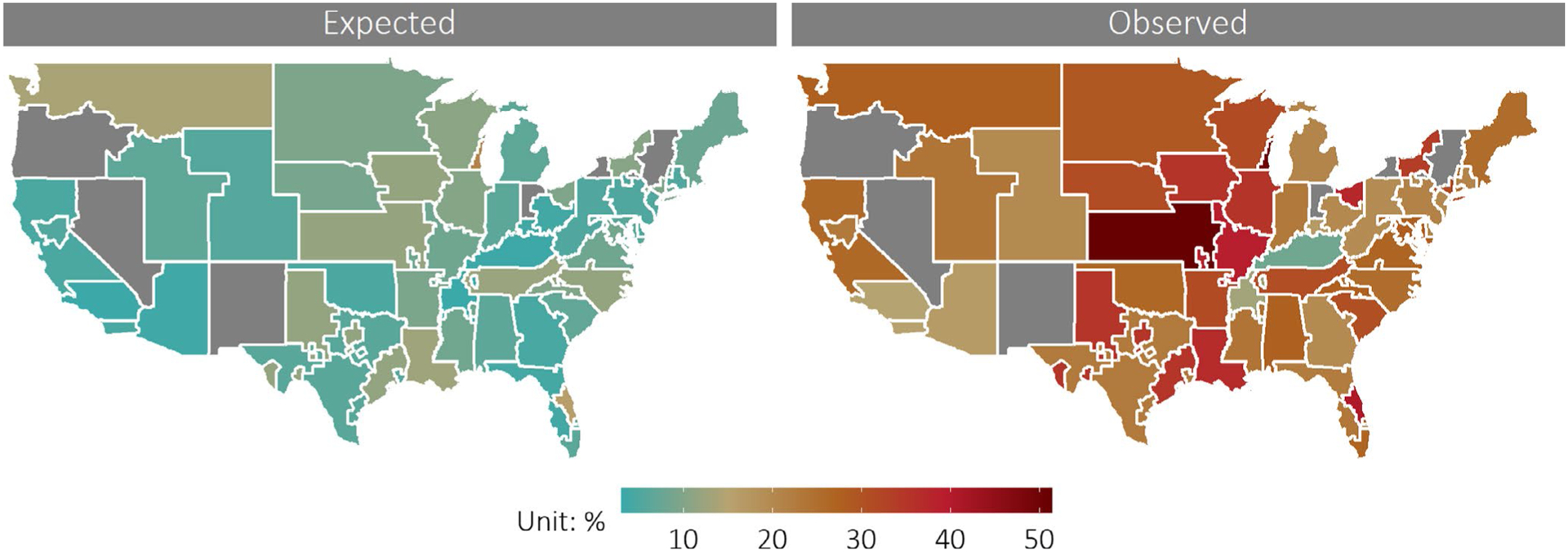
Geographical variation in high-priority status listing after implementation of the new heart allocation policy. National variation in the rate of being listed at high priority (Status 1 or Status 2), estimated from multilevel logistic regression model adjusted for candidate level characteristics. Rates are aggregated at the Organ Procurement Organization (OPO) level, the first local level of organ allocation in the United States. Colors correspond to the estimated probability the average candidate is listed at high priority status (Status 1 or 2). Map on the left displays the expected rate of high-priority listing in each OPO, generated from applying the new allocation scheme to candidates listed between December 2016 and February 2018. Map on the right displays the case-mix adjusted rates of Status 1 and 2 listing observed at each OPO from December 2018 to February 2020, after the new allocation scheme was implemented
Among the three center-level predictors tested, only Status 1A transplant rate in the pre-policy period was significantly associated with greater chance of high-priority status listing than expected (Table 2 and Table S4). The odds of high-priority status listing was 9.73 times (OR: 9.73; 95% CI: 6.67–14.19) higher for transplant centers in OPOs with a high pre-policy Status 1A transplant rate (>82%), in comparison to 5.53 times for those in OPOs with low (<72%) of Status 1A transplant rates (OR: 5.53; 95% CI: 3.89–7.86) (p = .01 for interaction).
3.6 |. Sensitivity analyses
The relationship between policy change and high-priority status listing was not sensitive to passage of time since policy implementation. In the first half of the post-policy study period (December 1, 2018 to July 16, 2019), the odds of high-priority status listing was 5.17 times (95% CI: 3.99–6.69) greater than in seasonally matched pre-policy study period (December 1, 2016 to July 16, 2017). In the second half of the post-policy study period (July 17, 2019 to February 28, 2020), the odds ratio was 7.92 (95% CI: 5.85–10.70).
The effect of policy period on the probability of exception request mirrored its impact on the overall utilization of high-priority statuses. After accounting for candidate characteristics and center-level effects, transplant centers are 5.81 times more likely to utilize exception request at the time of initial listing to qualify for high priority statuses under the new policy (95% CI: 3.73–9.04). The probability of using exception request to qualify for high-priority status increased significantly in 47 out of 96 transplant centers (Figure S4). The high-priority exceptions increased by substantially different rates between transplant centers, ranging from +2.1% to +33% (IQR: 3.5%–14.5%).
4 |. DISCUSSION
In this study of 8970 adult heart transplant candidates from 96 transplant centers in the United States, the number of high-priority status (Status 1 or 2) listings was higher than expected after implementation of the new heart allocation policy. The odds of high-priority listing was more than five times greater than expected in the post-policy period, without accompanying explanatory changes in candidate characteristics. Transplant centers all over the country listed more candidates than expected at high-priority status, mainly by using more IABP support and exception requests than anticipated. This practice change was especially pronounced for transplant centers in OPOs with a high Status 1A transplant rate before the policy change, suggesting that the combination of the new status tiers and expanded geographic sharing of donor hearts prompted these centers to dramatically modify their listing practices to continue transplanting high-priority candidates at the same rate. The impact of the policy change on high-priority status listing was not a temporary “bolus effect,” but rather was driven by persistent and increasing trends towards more aggressive treatment practices.
Two of the policy’s major intended effects were to alleviate the crowding of candidates “with disparate risks in the most urgent status” and reduce the number of exception requests. Our study suggests that the policy has had limited success in achieving these goals.3 Whereas the previous highest priority Status 1A accounted for 45% of all heart transplant candidates listed in 2016, the new highest priority status (Status 1) only accounts for 4.6% of candidates after the policy update. However, our study shows that “crowding” did not disappear with the new policy. It became a shared phenomenon between Status 1 and 2 and attributable to more ECMO, IABP, and exception requests than expected. In particular, the increase in the number of exception requests varied substantially across transplant centers unaccompanied by changes in candidate characteristics. This observation suggests a mismatch between the requirements for high-priority listing and the perceived urgency of transplantation by the listing physicians. On one hand, exceptions may be serving an essential role in identifying very urgent candidates whose level of urgency is not adequately measured by hemodynamic measures required by the new allocation policy. On the other hand, the large number of exception requests could undermine the gatekeeping effect of cardiogenic shock hemodynamic requirements by allowing less urgent candidates access to the expanded geographic priority of Status 1 and 2. The motivations underlying the rise in exception requests are not definitively known and are likely center-specific. Furthermore, data regarding the auditing process of exception requests in terms of percentage of acceptances or denials are not publicly available at time. Either scenario is concerning for the effectiveness of the new status tiers, indicating a limited ability to identify the most urgent heart transplant candidates. Future studies examining reasons for exception request will be critical in understanding the increasing popularity of its usage. The development of evidence-based objective criteria for high-priority listing could reduce the volume of exception requests.
Our results have several important implications for future policy. First, it suggests that the higher rates of aggressive treatment represent deliberate practice changes, rather than artifacts of data collection and codification. Second, although the need to “treat to the priority” has always existed for transplant centers in highly competitive environments, our study suggests that all transplant centers—even those in traditionally non-competitive environments—have started to more aggressively elevate the priority status of their candidates under the new allocation scheme.9,18 It is likely that high-priority status listing became more valuable under the new allocation scheme due to the expansion in the geographic sharing of donor hearts.3 The priority for a donor organ is no longer based on OPO affiliation but instead relies on the physical distance between the transplant center and donor hospital. With procurement areas now overlapping for many more centers, the competition for scarce donor hearts may have increased for many transplant centers even though the total number of donors or candidates remained constant within an OPO.19
Finally, while it is undeniable that listing practices were different from expectations under the new allocation policy, it is impossible to judge the motivation behind an individual physician’s listing and treatment decision based on registry data. In an allocation system based primarily on therapy, the increased use of exception requests could simultaneously indicate greater extent of “gaming” or effective advocacy for patients denied access to transplantation by the unvalidated, and perhaps excessively strict, hemodynamic requirements.20 Similarly, given the decreased likelihood of expedited heart transplant of a patient stable with an LVAD at Status 4, centers may inevitably be motivated to utilize temporary mechanical support strategies in favor of direct transplant. This may be especially true in patients with blood type (Type O) or a larger BMI, in whom a bridge to transplant strategy in a timely manner becomes a challenge without an exception, use of 30-day time or LVAD-related complication to allow for an upgrade to Status 3.
Refining the criteria for high-priority status extensions is another area where the allocation policy could be potentially improved. For example, centers could be required to transition patients supported with temporary MCS to durable LVADs after a short interval if medically possible. These potential policy improvements should be the focus of future research.
To be clear, our results do not imply that the new heart allocation policy was a failure. Indeed, the recently demonstrated reduction in waitlist mortality implies the policy was an improvement over the status quo.21 Nonetheless, the marked variation between transplant centers in response to the new allocation policy observed in our study has significant implications for the future of the US organ allocation system and re-enforces the fundamental limitations of a primarily therapy-based allocation system.11 While more follow-up is required to closely evaluate the impact of these center practice changes on the critical outcomes of wait-list and post-transplant mortality, work must continue to build an objective, score-based allocation system resistant to changes in treatment practices.22,23 Such a system is vital to direct donor hearts to the most urgent candidates and save the most lives.
4.1 |. Limitations
Our study has a few limitations. First, we had to retrospectively classify candidates into pre-policy candidates into Status 1–6 using treatments and hemodynamics at listing. However, our previously published approach follows the methodology of the OPTN in simulation studies of the policy.9–11,24 Second, our study only controlled for observed candidate characteristics. This means that while the absolute number and observed characteristics of candidates in the two policy periods was similar, the pool of candidates being listed post-policy may have been different in some unmeasured way. We also cannot account for unobserved longitudinal trends in practice patterns or heart transplant candidate characteristics that are unrelated to the new allocation policy. Unobserved changes in the candidates’ clinical or social needs over time could explain some of the increased between-center variation in listing practices. Third, the pre-specified center-level variables we tested for association with high-priority status listing may fail to capture important competitive forces experienced by centers, especially given the transition to the new distance-based donor heart distribution system. Finally, candidates can be initially listed at a low priority status and move up after receiving additional therapy. Since we only examined the initial status given to newly listed candidates, we did not capture waitlist dynamics in the current study. This should be examined in future work.
5 |. CONCLUSION
Under the new heart allocation policy, almost all US transplant centers listed more candidates at high priority status than expected, but there was substantial variability between centers. Centers in OPOs with a high Status 1A transplant rate in the pre-policy period changed their practices more than average, potentially continuing to transplant high-priority candidates at high rates. The widespread higher than expected utilization of high-priority listing statuses could prevent fair ranking of candidates based on the urgency of need, and compromise the fair and efficient distribution of scarce donor hearts.
Supplementary Material
ACKNOWLEDGMENTS
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Funding information
William F. Parker was supported by a NIH grant K08 HL150291. Matthew M. Churpek is supported by an R01 from NIGMS (NIGMS (R01 GM123193), has a patent pending (ARCD. P0535US. P2) for risk stratification algorithms for hospitalized patients, and has received research support from EarlySense (Tel Aviv, Israel).
Abbreviations:
- ECMO
extracorporeal membrane oxygenation
- IABP
intra-aortic balloon pump
- LVAD
left ventricular assist device
- MCS
mechanical circulatory support
- OPTN
Organ Procurement and Transplant Network
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the Scientific Registry of Transplant Recipients (SRTR). Restrictions apply to the availability of these data, which were used under license for this study.
REFERENCES
- 1.OPTN. Organ procurement and transplantation network policies. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_06. Accessed January 22, 2020.
- 2.E-CFR: Title 42: Public Health. Vol Title 42: Public Health. http://www.ecfr.gov/cgi-bin/text-idx?SID=bb60e0a7222f4086a88c31211cac77d1&mc=true&node=pt42.1.121&rgn=div5#se42.1.121_18. Accessed December 23, 2015. [Google Scholar]
- 3.Proposal to Modify the Adult Heart Allocation System. https://optn.transplant.hrsa.gov/media/2006/thoracic_brief_201612.pdf. Accessed March 12, 2016.
- 4.Parker WF, Chung K, Anderson AS, Siegler M, Huang ES, Churpek MM. Practice changes at U.S. transplant centers after the new adult heart allocation policy. J Am Coll Cardiol. 2020;75(23):2906–2916. 10.1016/j.jacc.2020.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huckaby LV, Seese LM, Mathier MA, Hickey GW, Arman K. Intra-aortic balloon pump Bridging to heart transplantation. Circulation. 2020;13(8):e006971. 10.1161/CIRCHEARTFAILURE.120.006971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varshney AS, Berg DD, Katz JN, et al. Use of temporary mechanical circulatory support for management of cardiogenic shock before and after the united network for organ sharing donor heart allocation system changes. JAMA Cardiol. 2020;5(6):703–708. 10.1001/jamacardio.2020.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanff TC, Harhay MO, Kimmel SE, et al. Trends in mechanical support use as a bridge to adult heart transplant under new allocation rules. JAMA Cardiol. 2020;5(6):728–729. 10.1001/jamacardio.2020.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze PC, Kitada S, Clerkin K, Jin Z, Mancini DM. Regional differences in recipient waitlist time and pre- and post-transplant mortality after the 2006 United Network for Organ Sharing policy changes in the donor heart allocation algorithm. JACC Heart Fail. 2014;2(2):166–177. 10.1016/j.jchf.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker WF, Anderson AS, Hedeker D, et al. Geographic variation in the treatment of U.S. Adult heart transplant candidates. J Am Coll Cardiol. 2018;71(16):1715–1725. 10.1016/j.jacc.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker WF, Garrity ER, Fedson S, Churpek MM. Potential impact of a shock requirement on adult heart allocation. J Heart Lung Transplant. 2017;36(9):1013–1016. 10.1016/j.healun.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker WF, Anderson AS, Gibbons RD, et al. Association of transplant center with survival benefit among adults undergoing heart transplant in the United States. JAMA. 2019;322(18):1789–1798. 10.1001/jama.2019.15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedeker D, Gibbons RD. Mixed-effects regression models for binary outcomes. Longitudinal Data Analysis. Wiley Series in Probability and Statistics. Hoboken, NJ: John Wiley & Sons, Ltd; 2006. 10.1002/0470036486.ch9. [DOI] [Google Scholar]
- 13.Hedeker D A mixed-effects multinomial logistic regression model. Statist Med. 2003;22(9):1433–1446. 10.1002/sim.1522. [DOI] [PubMed] [Google Scholar]
- 14.Shahian DM, Torchiana DF, Shemin RJ, Rawn JD, Normand S-LT. Massachusetts cardiac surgery report card: implications of statistical methodology. Ann Thorac Surg. 2005;80(6):2106–2113. 10.1016/j.athoracsur.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 15.Shahian DM, Normand S-LT. Comparison of “risk-adjusted” hospital outcomes. Circulation. 2008;117(15):1955–1963. 10.1161/CIRCULATIONAHA.107.747873. [DOI] [PubMed] [Google Scholar]
- 16.Efron B Bootstrap methods: another look at the Jackknife. Ann Statist. 1979;7(1):1–26. 10.1214/aos/1176344552. [DOI] [Google Scholar]
- 17.Readmission Measures Methodology. https://www.qualitynet.org/inpatient/measures/readmission/methodology. Accessed October 19, 2020.
- 18.Stevenson LW, Kormos RL, Young JB, Kirklin JK, Hunt SA. Major advantages and critical challenge for the proposed United States heart allocation system. J Heart Lung Transplant. 2016;35(5):547–549. 10.1016/j.healun.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Goff RR, Uccellini K, Lindblad K, et al. A change of heart: preliminary results of the US 2018 adult heart allocation revision. Am J Transplant. 2020;20(10):2781–2790. 10.1111/ajt.16010. [DOI] [PubMed] [Google Scholar]
- 20.Prateeti K, Drazner MH. The blurred line between gaming and patient advocacy. Circulation. 2019;140(25):2048–2050. 10.1161/CIRCULATIONAHA.119.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilic A, Mathier MA, Hickey GW, et al. Evolving trends in adult heart transplant with the 2018 heart allocation policy change. JAMA Cardiol. 2021;6(2):159–167. 10.1001/jamacardio.2020.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jasseron C, Legeai C, Jacquelinet C, et al. Prediction of waitlist mortality in adult heart transplant candidates: the candidate risk score. Transplant. 2017;101(9):2175–2182. 10.1097/TP.0000000000001724. [DOI] [PubMed] [Google Scholar]
- 23.Hong KN, Iribarne A, Worku B, et al. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg. 2011;92(2):520–527. 10.1016/j.athoracsur.2011.02.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modify adult heart allocation 2016 2nd round - OPTN. https://optn.transplant.hrsa.gov/governance/public-comment/modify-adult-heart-allocation-2016-2nd-round/. Accessed December 14, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Scientific Registry of Transplant Recipients (SRTR). Restrictions apply to the availability of these data, which were used under license for this study.


