Abstract
Background & Aims:
Research shows that sensitivity to certain alcohol responses conveys risk for problem drinking. This study aimed to determine if high-risk adolescent drinkers infuse more alcohol and experience greater alcohol-induced stimulation and wanting and less sedation than low-risk adolescent drinkers.
Design and participants:
Ninety-two low- (n=38) and high-risk (n=54) adolescent drinkers, as determined by Alcohol Use Disorders Identification Test scores of <6 or ≥6, respectively, participated in the Dresden Longitudinal Study on Alcohol Use in Young Adults in which intravenous alcohol self-administration was examined in a mixed within- and between-subjects design.
Setting:
Technische Universität Dresden. Dresden, Germany.
Measurements:
Predictors were drinking status (high- v. low-risk), time, and their interactions. Outcomes were arterial blood alcohol concentration (aBAC); alcohol-induced stimulation, sedation, and wanting assessed at baseline, 10 (alcohol prime), 45, 65, 85, 105, 125, and 145 minutes. Covariates were family history of alcohol use disorder, sex, and aBAC.
Results:
The alcohol prime dose produced similar sharp increases in stimulation and sedation in high- and low-risk drinkers (time ps <.001; group-x-time ps > .05). During self-administration, high-risk drinkers reached higher aBACs (p = .028) at a faster rate (group-x-time p <.001), and experienced further increases in stimulation (group-x-time p =.005) but with similar sedation (group-x-time p = .794) than in low-risk drinkers. High-risk drinkers also exhibited greater tonic alcohol wanting (group p = .003) throughout the session.
Conclusions:
High-risk adolescent drinkers appear to have heightened sensitivity to alcohol-induced stimulation and tonic high levels of wanting compared with low-risk adolescent drinkers.
Keywords: Acute alcohol effects, stimulation, sedation, wanting, adolescent drinking, intravenous alcohol self-administration
Alcohol misuse is the seventh leading cause of death worldwide, accounting for approximately 2.8 million deaths annually (1). Several risk factors that increase vulnerability for alcohol misuse include male sex (2–4), a family history of alcohol use disorder (5), and earlier initiation of alcohol use (6), particularly binge drinking (7). Individual differences in subjective alcohol responses also affect the risk for alcohol use disorder (AUD) as a blunted response to the sedating and impairing effects of alcohol (8, 9), and sensitivity to the stimulating and rewarding effects of alcohol (10–12) both predict future binge drinking and AUD symptoms.
Since 1984, the United States National Minimum Drinking Age Act has effectively restricted U.S. laboratory and longitudinal work on subjective alcohol responses to individuals over the age of 21. Although this legislation has improved public safety (13), it has limited research directly measuring acute alcohol effects and their associations with drinking behavior and related problems in adolescence. Research shows that the global median age of alcohol use initiation is between 16 and 19 years (14) and that an onset of drinking by age 14 is associated with excessive drinking in adulthood (7). Investigating how adolescents respond to alcohol may be crucial to understanding how drinking behaviors progress, particularly as binge drinking patterns may start to emerge during this developmental period (15–17).
To our knowledge, the first published study to investigate acute alcohol effects among youth examined 8–15-year-old alcohol naïve boys and showed that a moderate oral alcohol dose (.05 ml/kg) produced subjective effects opposite of baseline mood (i.e., from feeling tired to energized) (18). Due to ethical concerns in administering alcohol to children and the minimum drinking age of 21 in the United States, the youngest drinkers directly examined on responses to alcohol have been older adolescents from Canada and European countries with lower minimum drinking ages, i.e., 18 or 19 years of age. This work showed that an intoxicating intravenous (i.v.) dose of alcohol increased adolescents’ subjective stimulation during the rising limb of the blood alcohol curve, with concurrent smaller increases in sedation and craving (19, 20). Among older adolescents, alcohol craving mediated the relationship between alcohol-induced stimulation and sedation with alcohol self-administration (21). These studies have established that adolescents can be safely assessed on acute alcohol responses in a controlled laboratory setting. However, such studies have primarily focused on moderate and heavy drinking adolescents, so it is unclear whether positive-like alcohol responses are unique to youth engaging in early-age regular or heavy drinking or if they would also be evident in lighter drinking youth.
This issue is relevant for translational behavioral science as adolescent rodents show high sensitivity to alcohol’s locomotor and rewarding effects (22–24) and human studies have shown that sensitivity to alcohol’s positive effects are more pronounced in heavier than lighter young adult drinkers aged 21–35 years (10, 11, 25, 26). Heavy drinking at young ages is a risk factor for future development of AUD (27, 28) and, as such, examining acute alcohol responses in high- and low-risk adolescent drinkers would be valuable in elucidating if high or low sensitivity to alcohol may be present early in the developmental transition of establishing drinking patterns. Thus, the present study examined acute subjective responses to i.v. alcohol in a laboratory setting among high- and low-risk adolescent drinkers. We hypothesized that high-risk drinkers would infuse more alcohol, report greater alcohol-induced stimulation and wanting, and report lower alcohol-induced sedation as compared to low-risk drinkers.
Method
Design
This mixed within- and between-subjects study design was conducted from June 2010 to March 2012 as part of the larger Dresden Longitudinal Study on Alcohol Use in Young Adults (D-LAYA; Clinicaltrials.gov Registration #: NCT01063166) (29, 30). Following screening and eligibility determination, participants underwent a 4- to 5-hour study visit consisting of 30 minutes of health and safety screenings, 45 minutes of preparation for i.v. alcohol self-administration (e.g., i.v. placement, acclamation period), and the 145 minute i.v. alcohol phase with a fixed priming dose followed by self-administration. The remaining time was allotted for the alcohol elimination phase before the participant was allowed to leave the laboratory. The procedures were approved by the ethics committee at the Technische Universität Dresden and complied with the Declaration of Helsinki.
The sample size was supported by power analyses for D-LAYA with >90% power for detecting differences in aBAC, and a post-hoc power analysis indicated ≥ 76% power to detect group-by-time interaction effects for the main outcomes of interest (aBAC and stimulation).
Participants
There were 101 native German 18- and 19-year-olds enrolled in the D-LAYA (see (30) for full study recruitment procedures). Data from nine participants were excluded due to computer malfunction (n=5), adverse reaction (n=1), withdrawn consent (n=2), and not following directions (n=1). Thus, the final sample size was N=92. For the present paper, data were culled from the first of two laboratory-based i.v. alcohol sessions in order to limit learned expectancies from the first to second session. All study surveys, measures, and interviews were delivered in the participants’ native German language.
Screening
Study candidates attended an in-person screening session to sign informed consent and undergo assessment for eligibility. Inclusion criteria included age 18 or 19 years at the time of testing; consuming two or more standard alcoholic beverages (e.g., 14g of alcohol/serving) per week and binge drinking (e.g., consuming 5+ and 4+ standard alcoholic beverages on an occasion for men and women, respectively) at least once in the past two months; abstaining from drugs ≥ one week, alcohol > 24 hours, and tobacco ≥ four hours without adverse withdrawal symptoms at the arrival of the study visit.
A physician performed health screening and a physical examination. Participants were excluded if they had a current physical or mental disorder requiring treatment, liver or pancreatic disease, laboratory results indicating the presence of such disorders, viral hepatitis, HIV infection, past or current alcohol or substance dependence (except tobacco), use of any prescription medications in the past two weeks that could interact with alcohol, or history of adverse reaction to alcohol. A urine drug screen test (Nal von Minden Multi-12TF test, Moers, Germany) confirmed no recent use of stimulants, barbiturates, benzodiazepines, opioids, and tricyclic anti-depressants. Females were excluded if they had premenstrual dysphoric disorder, were pregnant (Alere medical pregnancy test, Koeln, Germany) or had intent to become pregnant, were breast-feeding, or were currently taking hormonal contraceptive medications.
The 10-item Alcohol Use Disorder Identification Test (AUDIT) (31) was given during screening and, based on prior research in adolescents (32), was used to identify participants as high- (AUDIT score ≥6; n=54) or low-risk (AUDIT score <6; n=38) drinkers. Participants also completed the 45-day Timeline Follow-Back (TLFB) (33, 34) interview to provide an estimate of recent alcohol drinking frequency and quantity, and a modified version of the family tree questionnaire (35) to provide information on family history of AUD. Family history positive was defined as having at least one first-degree biological relative with alcohol dependence and family history negative was defined as having no first- or second-degree biological relatives with alcohol dependence. It was not possible to ascertain identification of a family history of AUD for five participants.
Procedure
For the study session, each participant arrived to the laboratory at 1:00 pm and completed drug, pregnancy, and health screenings. A breath alcohol monitor (Draeger Alcotest 6810 med breathalyzer, Lübeck Germany) verified that all participants had a breath alcohol concentration of 0.0g/dL. The session took place in a small testing room with the participant seated in a comfortable chair facing an 81 cm video monitor at a distance of 1.5 m. Thirty minutes after arrival, the study physician inserted an 18G intravenous line into the antecubital fossa vein in the participant’s non-dominant arm.
At 2:15pm, after completion of baseline subjective measures (see Measures below), alcohol was infused using two volumetric infusion pumps (Infusomat fms, BBraun, Melsungen, Germany) and the Computer-assisted Alcohol Infusion System (CAIS) (36) software (for full description of protocol see (36, 37)). Each participant was prompted to self-administer a priming dose of alcohol with four button presses (one every 2.5 minutes) that resulted in .03g/dL arterial blood alcohol concentration (aBAC) over 10 minutes. The button was then deactivated for 15 minutes to allow aBAC to decrease to .015g/dL. During this time, to decrease potential negative effects of boredom, the participant watched a pre-selected television program.
Twenty-five minutes after the onset of the prime dose, the alcohol self-administration portion commenced. The participant was told that s/he could self-administer alcohol as if they were attending a party with alcohol freely available. Each button press produced a .0075g/dL linear increase in aBAC over 2.5 minutes, followed by a decrease in aBAC by .001g/dL per minute. The self-administration phase lasted two hours. The CAIS software prevented self-administration exceeding the safety limit of .12g/dL. Subjective ratings and breathalyzer readings were repeated at 10 minutes (after the priming dose), and every 20 minutes after beginning the free-access i.v. alcohol self-administration (45, 65, 85, 105, 125, and 145 minutes). During the free-access phase, while not completing measures, the participant was allowed to continue watching television shows. The session ended at approximately 4:40pm, after which the i.v. line was removed and the participant was given a full meal. After the participant’s aBAC fell below .045g/dL, s/he was escorted home by friend or livery service. The participant also had the option of walking home after their aBAC was below .02g/dL.
Measures
Subjective Ratings.
The participant was prompted on the video monitor (Presentation software, Neurobehavioral Systems, Albany, CA) to provide subjective ratings throughout the session. The main measures were 100-point visual analog scales, with 0 = “not at all” and 100 = “extremely” for stimulation (stimulation, “right now, I am experiencing stimulating alcohol effects, e.g., cheerful, excited, full of energy, zest for action…”), sedation (sedierung, “right now I am experiencing sedating alcohol effects, e.g., relaxed, tired, sluggish…”), and alcohol wanting (alkohol fehl, “I would like to consume more alcohol right now.”). These items were extracted from longer surveys (38) and chosen as there are no reliable and valid multi-dimensional alcohol response scales available in the German language.
Arterial Blood Alcohol Content (aBAC).
The current study used the CAIS predicted aBAC values that coincided with the presentation of the subjective measures. Breath alcohol concentration (BrAC) levels assessed by the Alcotest 6810 med breathalyzer validated the aBAC predictions (39). The BrAC measurements were entered into the CAIS program every 20 minutes to adjust the pharmacokinetic model calculating future infusion rates (see (36, 37)).
Data Analysis
The high- and low-risk drinker groups were compared on demographic and drinking variables using chi-square and t-tests, as appropriate. The primary outcomes of the study were aBAC during the free-access phase and subjective alcohol effects throughout the session. A series of generalized estimating equation (GEE) (40, 41) models examined the main effects of group, linear and quadratic time (treated as continuous), and their interactions on the study outcomes. Quadratic models were used as prior research shows non-linear associations (e.g., quadratic) between blood alcohol content (BAC) and acute alcohol effects (10, 11, 19). Model fit was confirmed using the quasi-likelihood under the independence model criterion (QIC; (42, 43)) with quadratic models fitting the data better than linear models for all study outcomes (see Table S1). The intercepts of the quadratic models were set to the 45-minute time point, as it was the first assessment period after the commencement of the self-administration phase and coincided with the expected rising BAC limb. These models allowed us to test main effects and interactions of group, time, and acceleration/deceleration in the rate of change over time (i.e., the time2 term) for all study outcomes. All GEE models utilized the exchangeable correlation matrix to account for the correlated nature of the outcome variables. Furthermore, sex and family history of alcohol problems were included as time-invariant covariates, with the models predicting alcohol responses also including aBAC as a time-varying covariate.
GEE models also tested the effects of group, time, and their interaction on alcohol responses at the prime dose by including only the baseline and prime dose (e.g., 10-minute) time points. Stata version 15 (44) was used for all analyses. The analyses were not pre-registered and the results should be considered exploratory.
Results
Sample Descriptive Statistics
The mean age of the overall sample was 18.92 (± 0.39 SD) years, 55% were male, and 41% had a positive family history of AUD. Self-reported education level at the time of testing was advanced with approximately 58% reporting an education level that qualified them for university (Fachabitur, Abitur, or University), 21% were not/not yet qualified for university (Hauptschule, Realschule, or Berufsschule), and 22% did not report their education level. Table 1 depicts the main demographics and drinking variables for the high- and low-risk subgroups. The groups did not differ in age, family history of alcohol problems, or education, but they did differ on sex ratio with more males in the high- than low-risk group (68% vs. 37%). As expected, the high-risk group (vs. low-risk) reported heavier drinking on all alcohol variables (ps < .05; see Table 1).
Table 1.
Demographic and Drinking Characteristics for the Low- and High-Risk Adolescent Drinker Subgroups
| Low-Risk Drinkers (n=38) |
High-Risk Drinkers (n=54) |
|
|---|---|---|
|
| ||
|
Background Characteristics
| ||
| Age (years) | 18.85 (0.39) | 18.97 (0.38) |
| Sex (% male) | 37% | 68%* |
| Family History (% FH+a) | 47% | 37% |
| Education (% university eligibleb,c) | 61% | 56% |
|
Drinking Characteristics | ||
| AUDIT Score | 4.05 (0.98) | 9.33 (4.06) ** |
| % Drinking Days | 22% | 38% ** |
| % Binge Drinking Days | 5% | 15%** |
| Drinks per Drinking Day | 3.18 (2.68) | 4.71 (2.31) ** |
| Drinks on Heavy Drinking Days | 6.42 (2.10) | 7.82 (2.47) * |
| Maximum Drinks | 6.84 (3.81) | 11.59 (5.28) ** |
| Age at First Drink | 14.66 (1.17) | 14.52 (1.28) |
Note: Values are mean (SD) or percent, as indicated.
p<.01.
p<.001. FH+= family history positive for alcohol use disorder. % Drinking Days = percentage of drinking days over the past 45 days. % Binge Drinking Days = percentage of binge drinking days over the past 45 days.
=Family history of an alcohol use disorder could not be determined for n=5.
=versus all other categories.
=education data not available for 20 participants. AUDIT=Alcohol Use Disorder Identification Test.
Responses to the Alcohol Infusion: Prime Dose and Free-Access Periods
Figure 1 shows mean aBAC and acute alcohol effects throughout the session. The alcohol prime infusion increased alcohol stimulation, wanting, and sedation from pre-infusion levels (Time: ps < .001; see Table 2) and these increases were similar between the groups (Group-x-Time: ps > .05). High-risk drinkers reported higher tonic alcohol wanting at the beginning of the session (Group p = .019), that maintained throughout the session versus low-risk drinkers (Group p = .003 see Model 1 in Table S2), reflecting a baseline difference carried forward throughout the session (see Figure 1).
Figure 1.
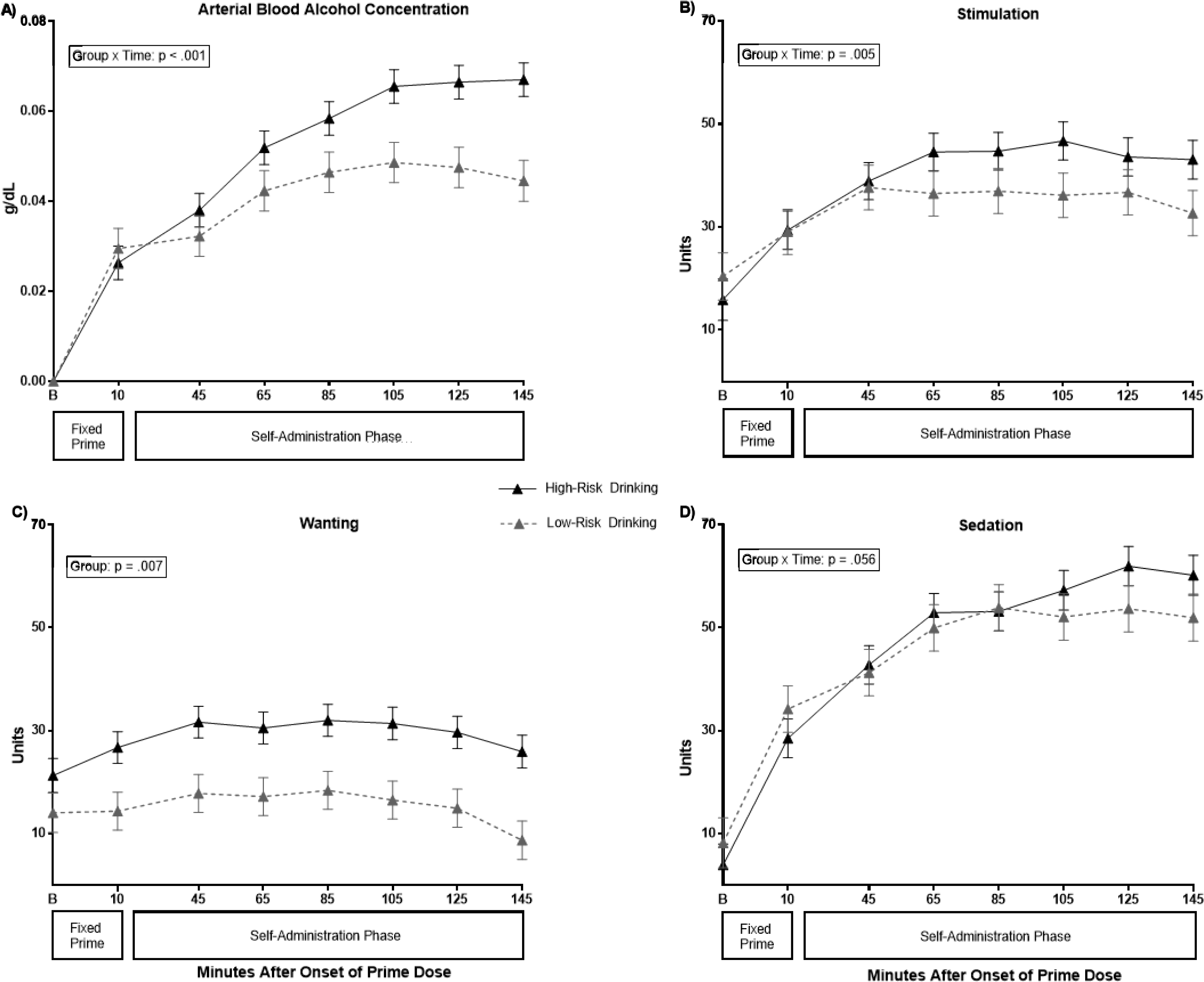
In-Session Levels of Arterial Blood Alcohol Concentration (aBAC) and Acute Subjective Alcohol Effects in High- and Low-Risk Adolescent Drinkers
Note: Raw means and standard error of the means are reported. Stimulation, Wanting, & Sedation were assessed from single item scales (0–100) and presented in German. B=baseline measurement time point. Self-Administration Phase started at 25 minutes. All significant effects are at the 45-minute time point.
Table 2.
Wald Tests and Effect Sizes for the Alcohol Prime Dose on Subjective Alcohol Responses
| Stimulation | Sedation | Wanting | ||||
| Wald χ2 (df) | p | Wald χ2 (df) | p | Wald χ2 (df) | p | |
|
| ||||||
| Group | 0.03 (1) | .855 | 1.14 (1) | .286 | 5.54 (1) | .019 |
| Time | 36.38 (1) | <.001 | 69.12 (1) | <.001 | 17.70 (1) | <.001 |
| Group-x-Time | 0.91 (1) | .339 | 0.00 | .988 | 1.93 (1) | .165 |
|
| ||||||
| Effect Sizes |
||||||
| B (SE) | p | B (SE) | p | B (SE) | p | |
|
| ||||||
| Group | −0.86 (4.57) | .851 | −3.94 (3.69) | .285 | 9.45 (4.01) | .019 |
| Time | 19.51 (3.12) | <.001 | 26.32 (3.09) | <.001 | 8.36 (1.86) | <.001 |
| FH+ | 2.76 (4.45) | .536 | −0.06 (3.60) | .986 | −5.62 (3.91) | .150 |
| Female | 1.33 (4.59) | .773 | 0.25 (3.69) | .945 | −2.62 (4.02) | .515 |
Note: χ2 = chi-square. df = degrees of freedom. SE = standard error. Group: 0=low-risk drinkers, 1=high-risk drinkers. Group-x-Time=drinking group by time interaction. FH+: 0=negative family history for AUD, 1=a positive family history of AUD. Female: 0=male, 1=female. Bold = p<.05.
During the early free-access period (e.g., 45 minutes), high-risk drinkers self-administered alcohol at a faster rate than low-risk drinkers (Group x Time: p < .001; see Figure 1A and Table 3), and they infused more alcohol throughout the session (aBAC group-x-time p <.001; Table S2 Model 3). The peak aBAC in high-risk drinkers was .088g/dL, significantly higher than the peak aBAC of .057g/dL in low-risk drinkers (ANOVA F[df]= 5.73 [3], p = .001; group p = .028) when controlling for sex (p = .006) and family history (p = .449).
Table 3.
GEE Results from Quadratic Growth Curve Models.
| aBAC | Stimulation | Wanting | Sedation | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| B (SE) | p | B (SE) | p | B (SE) | p | B (SE) | p | |
|
| ||||||||
| Group | 0.0052 (0.0051) | .304 | 3.79 (4.99) | .447 | 11.59 (4.32) | .007 | −0.17 (5.11) | .973 |
| Time | 0.0041 (0.0004) | <.001 | 1.59 (0.37) | <.001 | 0.46 (0.29) | .107 | 3.63 (0.39) | <.001 |
| Group-x-Time | 0.0019 (0.0005) | <.001 | 1.27 (0.450 | .005 | 0.47 (0.34) | .171 | 0.89 (0.47) | .056 |
| Time2 | −0.0003 (0.0001) | <.001 | −0.18 (0.05) | .001 | −0.14 (0.04) | .001 | −0.29 (0.06) | <.001 |
| Group-x-Time2 | >−0.000 (0.0001) | .680 | −0.08 (0.07) | .250 | −0.01 (0.05) | .896 | −0.02 (0.07) | .794 |
| Female | −0.0136 (0.0001) | .005 | −2.27 (4.83) | .638 | −2.28 (0.03) | .588 | 2.68 (4.94) | .588 |
| FH+ | −0.0004 (0.0047) | .926 | 0.89 (4.65) | .849 | −5.66 (4.07) | .164 | 0.33 (4.76) | .945 |
| aBAC | - | - | 0.27 (0.04) | <.001 | 0.17 (0.03) | <.001 | 0.12 (0.04) | .002 |
Note: aBAC = arterial blood alcohol concentration. SE=standard error. Group: 0=low-risk drinkers, 1=high-risk drinkers. Group-x-Time=drinking group by time interaction. Time2=quadratic time term. Group x Time2=drinking group by quadratic time interaction. FH+: 0=negative family history for AUD, 1=a positive family history of AUD Bold = p<.05. Time was divided by a factor of 10 for easier interpretability. Intercept was set to 45-minute time point.
For subjective responses, alcohol infusion during the early self-administration period was associated with faster increases and more stimulation in the high- versus low-risk group (Group x Time: p = .005; see Table 3 and Figure 1B). Alcohol-induced stimulation plateaued after 105 minutes and began to decrease in both groups at the latter time points of the session (Time2 p = .001). As stated earlier, the high-risk group reported higher tonic wanting prior to alcohol availability and this was maintained during free-access (Group: p = .007; see Tables 2, S2 Model 1, and Figure 1C). Alcohol wanting began to decrease in both groups during the latter time points in the session (Time2: p =.001).
Alcohol infusion was associated with increases in self-reported sedation for both groups (Time: p < .001). Sedation levels gradually increased to an apex and then began to decrease towards the latter time points of the session (Time2 p < .001), particularly in low-risk drinkers (Group x Time: p = .056; see Table 2 and Figure 1D).
Discussion
This study was the first to compare alcohol responses between high- and low-risk adolescent drinkers as determined by AUDIT scores and validated by different drinking patterns. High-risk adolescent drinkers infused more alcohol during the self-administration period and endorsed greater alcohol-induced stimulation and stronger tonic reward drive (wanting) for alcohol than did low-risk drinkers, even when controlling for group differences in demographic variables and aBAC. These results are consistent with previous research using i.v. alcohol administration in heavy drinking older adolescents (e.g., 19–21 years) and adults (19, 21, 45). The results also extend prior work by demonstrating that not all adolescent drinkers show heightened alcohol stimulation and tonic wanting. Rather, low-risk lighter drinking adolescents experienced less stimulation from alcohol and relatively low tonic alcohol wanting compared to their high-risk counterparts. Finally, contrary to our prediction, high-risk adolescents did not experience lower alcohol sedation than their low-risk peers. This is in contrast to prior research in adult drinkers showing lower alcohol sedation in heavier versus lighter drinkers (10, 12, 46). We may speculate that the discrepancy may be due to early-age drinkers not yet developing tolerance to alcohol’s sedative effects, as such neuroadaptations develop over time and may take several or more years of chronic excessive drinking to manifest.
Another important finding in the current study was the high tonic alcohol wanting among high-risk adolescents, which is consistent with research showing that high-risk young adult social drinkers want alcohol at levels commensurate with heavy drinkers and higher than in low-risk drinkers (47). This finding may relate to the incentive salience model of addiction, which is based on animal models and posits that excessive substance use can lead to the hypersensitization of brain pathways responsible for reward motivation, turning “ordinary wanting” into “excessive craving” (48 p.247, 49–51). While the model focuses on neurobiological processes underlying addiction, and the youth in the present study were heavy drinkers but not yet with AUD, there does appear to be some consistency with the present study findings of heightened early-age tonic alcohol wanting that theoretically could predispose such youth to future exacerbations in drinking and developing AUD. This finding suggests that the emergence of tonic alcohol wanting may be evident earlier in the lifespan than previously thought, and warrants continued investigation.
Both adolescent groups demonstrated sensitivity to a low dose of alcohol, as evidenced by sharp increases in both stimulation and sedation shortly after the 1–2 drink equivalent dose (0.03 g/dL). Adolescent drinkers, regardless of their early-age drinking patterns, may exhibit heightened sensitivity to the effects of alcohol, consistent with the animal literature (22–24). Next, consistent with previous research showing a positive within-person (e.g., participant-specific score change) association between alcohol-induced stimulation and greater alcohol self-administration (21), high-risk youth self-administered more alcohol to an extent that produced further increases in stimulation; whereas low-risk adolescents “applied the brakes” and infused less alcohol, with stimulation likewise plateauing. Despite high-risk adolescents infusing more alcohol, their reported sedation was similar, or marginally higher, relative to their low-risk counterparts. Collectively, high-risk adolescents may not have detected (or may have ignored) sedative-like effects to inhibit further alcohol infusion as their concomitant heightened stimulation-like internal state may have led to infusion of more alcohol. These effects, in combination, may put high-risk adolescent drinkers at future risk for excessive consumption habits.
The current study had several strengths including the use of controlled i.v. alcohol administration that reduces variance in aBAC, assessment of alcohol responses during both a fixed low dose and self-administration, and the recruitment of a sample of exclusively young 18- and 19-year-old drinkers. As most alcohol response studies have examined persons aged 21 and older, the advantage of examining adolescents in this study, with their relatively short alcohol use histories, allowed a unique examination of acute alcohol effects during the early adoption phase of drinking behaviors.
There are several limitations worth noting. First, although this study used single-item visual analog scales of subjective alcohol responses, the items were extracted from a well-validated survey (38) and reduced boredom or complacency that adolescents might experience if given lengthier surveys. Second, the sex ratio differed between groups with more males in the high-risk versus low-risk group. However, this distribution is consistent with sex differences in high-risk drinking, particularly during middle and late adolescence (52, 53), and sex was included as a covariate in analyses. Third, because of the time lag, the acute alcohol effects from the prime dose to the first self-administration assessment cannot be assumed to be consistent. The intercept of the GEE models was set to the 45-minute time point as that was the first available time point to capture acute effects during rising aBAC. Last, participants were aware they were infusing alcohol so the potential confound of expectancy effects on the main dependent variables cannot be ruled out. However, we are confident that our results are primarily due to the effects of alcohol as the pattern and magnitude of results is similar to work in young adult drinkers using double-blinded, placebo-controlled, alcohol administration procedures (10, 54, 55).
In conclusion, the present study showed that 18–19-year-old adolescents at high risk for future AUD, due to their early-age heavy drinking patterns, showed a different pattern of response to alcohol than their low-risk peers. High-risk adolescents administered more alcohol and at a faster rate when given free access, reported higher alcohol-induced stimulation regardless of BAC level, and experienced stronger tonic alcohol wanting prior to alcohol infusion that was maintained after alcohol self-administration. These results extend prior findings in young adult heavy drinkers to a developmental epoch marked by early adoption of excessive drinking. As the work in young adult drinkers indicates that alcohol stimulation and wanting may be risk factors for the development of AUD in young adults (12, 56), this alcohol response phenotype may likewise manifest earlier in the lifespan when drinking patterns and neuroadaptations to alcohol start to emerge. The findings suggest continued investigation in adolescent drinkers to further characterize early risk factors and the role of alcohol responses in the vulnerability to problematic drinking.
Supplementary Material
Acknowledgements:
Appreciation is extended to Patrick McNamara for data collection and database assistance and Dingcai Cao for verifying the analytic procedures. United States Funding: NIH R01-AA013746 (AK) & 3R01AA013746-14S1, P60 AA007611, and 1U01 AA017900-0. German Funding: BMBF 01ZX1311H.
Footnotes
Declarations of Interest: None
Clinical Trial Registration: #NCT01063166. Prospective Assessment of Adolescent Drinking Trajectories with Computer-Assisted Self-Administration of Ethanol (CASE)
Reference
- 1.Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2018;392(10152):1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of Studies on Alcohol. 1997;58(5):464–73. [DOI] [PubMed] [Google Scholar]
- 3.Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD. Extreme Binge Drinking Among 12th-Grade Students in the United States: Prevalence and Predictors. JAMA Pediatrics. 2013;167(11):1019–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace JM Jr, Bachman JG, O’Malley PM, Schulenberg JE, Cooper SM, Johnston LD. Gender and ethnic differences in smoking, drinking and illicit drug use among American 8th, 10th and 12th grade students, 1976–2000. Addiction. 2003;98(2):225–34. [DOI] [PubMed] [Google Scholar]
- 5.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of Alcohol Abuse: Cross-Fostering Analysis of Adopted Men. Archives of General Psychiatry. 1981;38(8):861–8. [DOI] [PubMed] [Google Scholar]
- 6.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at First Alcohol Use: A Risk Factor for the Development of Alcohol Disorders. American Journal of Psychiatry. 2000;157(5):745–50. [DOI] [PubMed] [Google Scholar]
- 7.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–10. [DOI] [PubMed] [Google Scholar]
- 8.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. The American Journal of Psychiatry. 1994;151(2):184–9. [DOI] [PubMed] [Google Scholar]
- 9.Schuckit MA, Smith TL. An 8-Year Follow-up of 450 Sons of Alcoholic and Control Subjects. JAMA Psychiatry. 1996;53(3):202–10. [DOI] [PubMed] [Google Scholar]
- 10.King AC, de Wit H, McNamara PJ, Cao D. Rewarding, Stimulant, and Sedative Alcohol Responses and Relationship to Future Binge Drinking. Archives of General Psychiatry. 2011;68(4):389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King AC, Hasin D, O’Connor SJ, McNamara PJ, Cao D. A Prospective 5-Year Re-examination of Alcohol Response in Heavy Drinkers Progressing in Alcohol Use Disorder. Biological Psychiatry. 2016;79(6):489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King AC, McNamara PJ, Hasin DS, Cao D. Alcohol Challenge Responses Predict Future Alcohol Use Disorder Symptoms: A 6-Year Prospective Study. Biological Psychiatry. 2014;75(10):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voas RB, Tippetts AS, Fell JC. Assessing the effectiveness of minimum legal drinking age and zero tolerance laws in the United States. Accident Analysis & Prevention. 2003;35(4):579–87. [DOI] [PubMed] [Google Scholar]
- 14.Degenhardt L, Chiu W-T, Sampson N, Kessler RC, Anthony JC, Angermeyer M, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5(7):e141–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.French MT, Maclean JC. Underage alcohol use, delinquency, and criminal activity. Health Economics. 2006;15(12):1261–81. [DOI] [PubMed] [Google Scholar]
- 16.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, Correlates, Disability, and Comorbidity of DSM-IV Alcohol Abuse and Dependence in the United States: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2007;64(7):830–42. [DOI] [PubMed] [Google Scholar]
- 17.Swendsen J, Burstein M, Case B, Conway KP, Dierker L, He J, et al. Use and Abuse of Alcohol and Illicit Drugs in US Adolescents: Results of the National Comorbidity Survey–Adolescent Supplement. Archives of General Psychiatry. 2012;69(4):390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behar D, Berg CJ, Rapoport JL, Nelson W, Linnoila M, Cohen M, et al. Behavioral and Physiological Effects of Ethanol in High-Risk and Control Children: A Pilot Study. Alcoholism: Clinical and Experimental Research. 1983;7(4):404–10. [DOI] [PubMed] [Google Scholar]
- 19.Hendershot CS, Wardell JD, Strang NM, Markovich MSD, Claus ED, Ramchandani VA. Application of an alcohol clamp paradigm to examine inhibitory control, subjective responses, and acute tolerance in late adolescence. Experimental and Clinical Psychopharmacology. 2015;23(3):147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jünger E, Javadi A-H, Wiers CE, Sommer C, Garbusow M, Bernhardt N, et al. Acute alcohol effects on explicit and implicit motivation to drink alcohol in socially drinking adolescents. Journal of Psychopharmacology. 2017;31(7):893–905. [DOI] [PubMed] [Google Scholar]
- 21.Wardell JD, Ramchandani VA, Hendershot CS. A multilevel structural equation model of within- and between-person associations among subjective responses to alcohol, craving, and laboratory alcohol self-administration. Journal of Abnormal Psychology. 2015;124(4):1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but Not Adult Rats Exhibit Ethanol-Mediated Appetitive Second-Order Conditioning. Alcoholism: Clinical and Experimental Research. 2008;32(11):2016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spear LP. Adolescents and alcohol: Acute sensitivities, enhanced intake, and later consequences. Neurotoxicology and Teratology. 2014;41:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varlinskaya EI, Spear LP, Spear NE. Acute Effects of Ethanol on Behavior of Adolescent Rats: Role of Social Context. Alcoholism: Clinical and Experimental Research. 2001;25(3):377–85. [PubMed] [Google Scholar]
- 25.Bujarski S, Hutchison KE, Prause N, Ray LA. Functional significance of subjective response to alcohol across levels of alcohol exposure. Addiction Biology. 2017;22(1):235–45. [DOI] [PubMed] [Google Scholar]
- 26.Bujarski S, Hutchison KE, Roche DJ, Ray LA. Factor structure of subjective responses to alcohol in light and heavy drinkers. Alcoholism: clinical and experimental research. 2015;39(7):1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merline A, Jager J, Schulenberg JE. Adolescent risk factors for adult alcohol use and abuse: stability and change of predictive value across early and middle adulthood. Addiction. 2008;103(s1):84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wells JE, Horwood LJ, Fergusson DM. Drinking patterns in mid-adolescence and psychosocial outcomes in late adolescence and early adulthood. Addiction. 2004;99(12):1529–41. [DOI] [PubMed] [Google Scholar]
- 29.Gan G, Guevara A, Marxen M, Neumann M, Jünger E, Kobiella A, et al. Alcohol-Induced Impairment of Inhibitory Control Is Linked to Attenuated Brain Responses in Right Fronto-Temporal Cortex. Biological Psychiatry. 2014;76(9):698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jünger E, Gan G, Mick I, Seipt C, Markovic A, Sommer C, et al. Adolescent Women Induce Lower Blood Alcohol Levels Than Men in a Laboratory Alcohol Self-Administration Experiment. Alcoholism: Clinical and Experimental Research. 2016;40(8):1769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babor TF, Higgins-Biddle J, Saunders J, Monteiro M. The alcohol use disorders identification test. Guidlines for use in primary care. Second ed: World Health Organization - Department of Mental Health and Substance Dependence; 2001. [Google Scholar]
- 32.Rumpf HJ, Wohlert T, Freyer-Adam J, Grothues J, Bischof G. Screening Questionnaires for Problem Drinking in Adolescents: Performance of AUDIT, AUDIT-C, CRAFFT and POSIT. European Addiction Research. 2013;19(3):121–7. [DOI] [PubMed] [Google Scholar]
- 33.Sobell LC, Agrawal S, Sobell MB, Leo GI, Young LJ, Cunningham JA, et al. Comparison of a quick drinking screen with the timeline followback for individuals with alcohol problems. Journal of Studies on Alcohol. 2003;64(6):858–61. [DOI] [PubMed] [Google Scholar]
- 34.Sobell LC, Sobell MB. Timeline Follow-Back. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. p. 41–72. [Google Scholar]
- 35.Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug and Alcohol Dependence. 1985;15(1):61–7. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann U, Mick I, Plawecki M, O’Connor S. S27. 1THE COMPUTER-ASSISTED INFUSION SYSTEM (CAIS) FOR EXPERIMENTAL ETHANOL ADMINISTRATION IN HUMANS. Alcohol and Alcoholism. 2013;48(suppl_1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O’Connor S. Development and Pilot Validation of Computer-Assisted Self-Infusion of Ethanol (CASE): A New Method to Study Alcohol Self-Administration in Humans. Alcoholism: Clinical and Experimental Research. 2008;32(7):1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and Validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research. 1993;17(1):140–6. [DOI] [PubMed] [Google Scholar]
- 39.Lindberg L, Brauer S, Wollmer P, Goldberg L, Jones AW, Olsson SG. Breath alcohol concentration determined with a new analyzer using free exhalation predicts almost precisely the arterial blood alcohol concentration. Forensic Science International. 2007;168(2):200–7. [DOI] [PubMed] [Google Scholar]
- 40.Twisk JWR. Longitudinal Data Analysis. A Comparison Between Generalized Estimating Equations and Random Coefficient Analysis. European Journal of Epidemiology. 2004;19(8):769–76. [DOI] [PubMed] [Google Scholar]
- 41.Zeger SL, Liang K-Y. Longitudinal Data Analysis for Discrete and Continuous Outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 42.Cui J QIC Program and Model Selection in GEE Analyses. The Stata Journal. 2007;7(2):209–20. [Google Scholar]
- 43.Cui J, Qian G. Selection of Working Correlation Structure and Best Model in GEE Analyses of Longitudinal Data. Communications in Statistics - Simulation and Computation. 2007;36(5):987–96. [Google Scholar]
- 44.StataCorp. STATA Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 45.Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA. Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. American Journal of Psychiatry. 2017;174(11):1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinn PD, Fromme K. Subjective Response to Alcohol Challenge: A Quantitative Review. Alcoholism: Clinical and Experimental Research. 2011;35(10):1759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sloan ME, Gowin JL, Janakiraman R, Ester CD, Stoddard J, Stangl B, et al. High-risk social drinkers and heavy drinkers display similar rates of alcohol consumption. Addiction Biology. 2020;25(2):e12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–91. [DOI] [PubMed] [Google Scholar]
- 49.Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist. 2016;71(8):670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–14. [DOI] [PubMed] [Google Scholar]
- 51.Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1507):3137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen P, Jacobson KC. Developmental Trajectories of Substance Use From Early Adolescence to Young Adulthood: Gender and Racial/Ethnic Differences. Journal of Adolescent Health. 2012;50(2):154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patrick ME, Schulenberg JE. Prevalence and predictors of adolescent alcohol use and binge drinking in the United States. Alcohol Res. 2013;35(2):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilman JM, Smith AR, Ramchandani VA, Momenan R, Hommer DW. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addiction Biology. 2012;17(2):465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, et al. Effects of Family History of Alcohol Dependence on the Subjective Response to Alcohol Using the Intravenous Alcohol Clamp. Alcoholism: Clinical and Experimental Research. 2013;37(12):2011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bujarski S, Ray LA. Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: An examination of Koob’s allostatic model in humans. Drug and Alcohol Dependence. 2014;140:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


