Abstract
Background
Around 16% of adults have symptoms of overactive bladder (urgency with frequency and/or urge incontinence). The prevalence increases with age. Anticholinergic drugs are commonly used to treat this condition.
Objectives
To determine the effects of anticholinergic drugs for the treatment of overactive bladder syndrome.
Search methods
We searched the Cochrane Incontinence Group Specialised Trials Register (searched 14 June 2005) and the reference lists of relevant articles.
Selection criteria
Randomised or quasi‐randomised trials in adults with overactive bladder syndrome that compared an anticholinergic drug with placebo treatment or no treatment.
Data collection and analysis
Two reviewer authors independently assessed eligibility, trial quality and extracted data. Data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005).
Main results
Sixty ‐one trials, 42 with parallel‐group designs and 19 crossover trials were included (11,956 adults). Most trials were described as double‐blind but were variable in other aspects of quality. The crossover trials did not present data in a way that allowed inclusion in the meta‐analysis. Nine medications were tested: darifenacin; emepronium bromide or carrageenate; oxybutynin; propiverine; propantheline; tolterodine; trospium chloride; and solifenacin. One trial included the newer, slow release formulation of tolterodine.
At the end of the treatment period, cure or improvement (relative risk (RR) 1.39, 95%CI 1.28 to 1.51), difference in leakage episodes in 24 hours (weighted mean difference (WMD) ‐0.54; 95% CI ‐0.67 to ‐0.41) and difference in number of voids in 24 hours (WMD ‐0.69; 95%CI ‐0.84 to ‐0.54) were statistically significant favouring medication. Statistically significant but modest sized improvements in quality of life scores were reported in recently completed trials. There was three times the rate of dry mouth in the medication group (RR 3.00 95% CI 2.70 to 3.34) but no statistically significant difference in withdrawal (RR 1.11, 95% CI 0.91 to 1.36). Sensitivity analysis, while limited by small numbers of trials, showed little likelihood that the effects were modified by age, sex, diagnosis, or choice of drug.
Authors' conclusions
The use of anticholinergic drugs by people with overactive bladder syndrome results in statistically significant improvements in symptoms. Recent trials suggest that this is associated with modest improvement in quality of life. Dry mouth is a common side effect of therapy but did not seem to have an effect on the numbers of withdrawals. It is not clear whether any benefits are sustained during long‐term treatment or after treatment stops.
Plain language summary
Anticholinergic drugs in patients with overactive bladder syndrome.
An overactive bladder is a condition in which bladder contracts suddenly without any control, resulting in feeling to urinate and or leakage of urine. This is a common condition in adults and is also called as 'irritable' bladder or detrusor instability, urge or urgency‐frequency syndrome. Overactive bladder becomes more common with advancing age. Anticholinergic drugs mainly by their muscle relaxant action can help adults with symptoms of urinary frequency, urgency and urge incontinence.
The review of trials found that on average people taking anticholinergic medication had about five less trips to the toilet and four less leakage episodes every week, with modest improvements in quality of life. About one in three people taking the drugs reported a dry mouth.
Background
According to the International Continence Society (ICS) "urgency, with or without urge incontinence, usually with frequency and nocturia, can be described as overactive bladder (OAB) syndrome, urge syndrome or urgency‐frequency syndrome. These terms can be used if there is no proven infection or obvious pathology" (Abrams 2002). The symptom complex do not represent and is not specific to a single disease, hence ICS definition can be debatable. Nevertheless, this term (OAB) has been commonly used in the literature.
People with overactive bladder syndrome report urgency (with or without urge incontinence), usually in combination with frequency and or nocturia. To be called overactive bladder syndrome these symptoms should not be caused by metabolic problems such as diabetes, or problems with the urinary tract such as urinary tract infection. However, people with neurological causes of overactive bladder symptoms are not excluded from the diagnosis. Overactive bladder syndrome may also be called urge syndrome or urgency‐frequency syndrome.
Urgency is the sudden and compelling desire to pass urine, which is difficult to defer. Sometimes there is involuntary leakage of urine with the feeling of urgency, and this is called urge urinary incontinence. Urgency and urge urinary incontinence usually result from an involuntary increase in bladder pressure due to detrusor (bladder smooth muscle) over‐activity. If further investigation of urgency/urge incontinence with urodynamics demonstrates spontaneous or provoked detrusor muscle contraction in the filling phase of the test then detrusor overactivity is diagnosed. If there is no defined cause for the overactivity this is called idiopathic (no known cause) detrusor overactivity but if there is a relevant neurological condition then the term neurogenic detrusor overactivity or detrusor hyperreflexia is used.
Frequency is the complaint of needing to void often during the day or at night. In clinical practice a person who voids more than eight times in 24 hours is considered to have frequency. If a person wakes more than once at night from sleep to void this is called nocturia.
Frequency, urgency, urge urinary incontinence, or the combination of these symptoms, are a common problem amongst adults living in the community. In a survey (telephone or face to face interviews) of 16,776 randomly selected adults aged 40 years or more, in six European countries, 16.6% reported overactive bladder symptoms (Milsom 2001). Similarly a telephone survey of 5204 people aged 18 years and over in the USA found that the prevalence of self reported overactive bladder symptoms was 16.6% (Stewart 2001). In a sample of 2369 adults from 11 Asian countries the prevalence of overactive bladder was 44.9% (Moothy 2001). A survey of 2005 adults in Korea found a prevalence of overactive bladder of 30.5% (Choo 2001). In a telephone survey of 819 Hong Kong Chinese aged 10‐90 years, 19% reported frequency and 15% complained of urge and/or urge incontinence (Brieger 1996). It is not clear why there are such marked differences in prevalence between some of the Asian studies and those from the USA or Europe. The differences may be real or due to different definitions. Several large population studies have reported that the prevalence of overactive bladder symptoms increases with age in men and women (Brown 1999; Milsom 2001; Moller 2000; Stewart 2001; Ueda 2000). In people with neurological conditions such as multiple sclerosis, urinary dysfunction appears to be more common than in the neurologically unimpaired population: the most frequently reported problems are frequency and/or urgency (Hennessey 1999).
Research on the amount of bother caused by overactive bladder syndrome, and the effect on quality of life, is now underway. It seems that frequency and/or urgency might can be just as bothersome as actual leakage (Milsom 2001), and overall the effects of overactive bladder symptoms on quality of life are marked (Jackson 1997). It also seems clear that many of the people affected by overactive bladder symptoms do not seek help from health care professionals (Milsom 2001; Ueda 2000).
Treatment of overactive bladder syndrome
The two main treatment options for overactive bladder syndrome are conservative management (for example bladder training, electrical stimulation) and pharmacotherapy or a combination of both. A separate Cochrane review on bladder training is available (Wallace 2004) and the scope of the current review is confined to drug treatment.
While the pathophysiology of the overactive bladder remains to be fully elucidated, the involvement of the autonomic nervous system in detrusor function is recognised (de Groat 1997). The motor nerve supply to the bladder is via the parasympathetic nervous system (via sacral nerves: S2,3,4) (Abrams 1988; Ouslander 1982; Ouslander 1986) which effects detrusor muscle contraction. This is mediated by acetylcholine acting on muscarinic receptors at the level of the bladder. Muscarinic receptors are found in other parts of the body too, for example in the gut, salivary glands and tear ducts. Pharmacotherapy relies on the use of drugs with anticholinergic properties. The rationale for using anticholinergic drugs in the treatment of overactive bladder syndrome is to block the parasympathetic acetylcholine pathway and thus abolish or reduce the intensity of detrusor muscle contraction. Unfortunately, none of the anticholinergic drugs that are available to date are specific to the muscarinic receptors in the bladder. As a result, the drugs can cause side effects by acting in other parts of the body too; these include dry mouth or eyes, constipation or nausea.
For the purpose of this review the term 'anticholinergic medications' refers to all medications with primary anticholinergic properties that are given specifically for bladder symptoms. Medications with secondary anticholinergic effects, for example tricyclic antidepressants have been excluded.
The number of anticholinergic drugs available on the market is increasing and various studies, observational and randomised controlled trials exist which evaluate effectiveness (for example Thuroff 1991; VanKerrebroeck 1998). However, uncertainty still exists as to whether anticholinergic drugs are effective and, if so, which ones and by what route of administration. There is also uncertainty about the role of anticholinergic drugs in different patient groups (for example the elderly, male and female). Despite these uncertainties, anticholinergics are increasingly being used in primary and secondary care settings for the treatment of overactive bladder syndrome, and this has considerable resource implications (Kobelt 1997).
There are many studies of the effects of anticholinergic drugs and four Cochrane reviews will consider them. This review compares anticholinergic drugs with no treatment or placebo treatment. Three other reviews consider: 1) whether different anticholinergic drugs have different effects (Hay‐Smith 2005); 2) whether anticholinergic drugs are better than other active non‐drug therapies (Alhasso 2006); and 3) whether anticholinergic drugs are better than other drug treatments (Dublin 2004). Two meta‐analyses of an anticholinergic drug (tolterodine) versus placebo have been published (Appell 1997; Larsson 1999). However, neither of these publications provides an adequate systematic review of the available trials comparing anticholinergic drugs with placebo or no treatment. Neither publication reports the objectives of the systematic review, a search strategy, inclusion and exclusion criteria for trials or the methods of data extraction and analysis. It appears that these meta‐analyses combine the results of pharmaceutical company funded phase II (Larsson 1999) and phase III (Appell 1997) trials of tolterodine versus placebo. A recent systematic review (Chapple 2005b) assessed the variation in the effects of different anticholinergic drugs. This second‐level question has been addressed in a separate Cochrane review (Hay‐Smith 2005) that focused on the safety and effectiveness of anticholinergics as a generic group.
This is an updated version of the present review. The first version was published in The Cochrane Library in 2002 (Issue 3).
Objectives
To determine the effects of anticholinergic drugs compared with placebo or no treatment in the treatment of overactive bladder syndrome.
The following hypothesis will be addressed.
Anticholinergic drugs are better than placebo or no treatment in the management of overactive bladder syndrome.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials of anticholinergic drugs versus placebo or no treatment of overactive bladder syndrome.
Types of participants
All adult men and women with a symptomatic diagnosis of overactive bladder syndrome or a urodynamic diagnosis of detrusor overactivity (either idiopathic or neurogenic), or both.
Types of interventions
At least one arm of the study had to use an anticholinergic drug and one other was a placebo or no treatment arm. To be included, the drug had to be a muscarinic anticholinergic antagonist given for the purpose of decreasing symptoms of overactive bladder. The group of drugs included: emepronium bromide or carrageenate, darifenacin, dicyclomine chloride, oxybutynin chloride, propantheline bromide, propiverine, tolterodine, and trospium chloride. Terodiline, an anticholinergic drug previously used in the treatment of overactive bladder, was excluded because it has been withdrawn from the market. Trials with intravesical anticholinergic medication administration were excluded in this updated version of the review.
Other drugs with less direct anticholinergic effects were also excluded, (for example smooth muscle relaxants, flavoxate hydrochloride, calcium channel blockers, potassium channel openers, beta‐adrenoceptor agonists, alpha‐adrenoceptor antagonists, prostaglandin synthetase inhibitors, and tricyclic antidepressants).
Types of outcome measures
Both subjective and objective outcome measures were included in this review.
Primary outcomes of interest:
A. Patient observations, e.g. symptom scores, perception of cure or improvement, satisfaction with outcome
B. Quantification of symptoms, e.g. number of leakage episodes, frequency and volume (urinary diary)
Secondary outcomes of interest:
C. Quality of life general (e.g. SF36) and condition‐specific quality of life measures (e.g. Incontinence Impact Questionnaire, King's Health Questionnaire) and psychosocial measures.
D. Socioeconomics direct and indirect costs of interventions (for patients and providers), resource implications of differences in outcome, formal economic analysis (e.g. cost effectiveness, cost utility) and desire or need for further treatment.
E. Other, e.g. adverse events, withdrawal or compliance measures, long‐term follow‐up and any other outcome not pre‐specified but judged important when performing the review.
Search methods for identification of studies
Relevant trials were identified from the Cochrane Incontinence Group's Specialised Register of controlled trials, which is described under the Group's details in The Cochrane Library (Please see the ‘Specialized Register’ section of the Group’s module in The Cochrane Library). In addition, the reference lists of identified trials were searched. The register contains trials identified from MEDLINE, CINAHL, the Cochrane Central Register of Controlled Trials (CENTRAL) and handsearching of journals and conference proceedings. Date of the most recent search of the register: 14 June 2005.
The trials in the Incontinence Group Specialised Trials Register are also contained in CENTRAL.
The Incontinence Group Trials Register was searched using the Group's own keyword system. The search terms used were:
{design.rct* or design.cct*} AND{ TOPIC.URINE.INCON*} OR {TOPIC.URINE.overactivebladder.} AND{{ INTVENT.CHEM.DRUG.ANTICHOLINERGIC*} AND {INTVENT.CHEM.PLACEBO}} OR {relevant.review. anticholinergicVSplacebo} (All searches were of the keyword field of Reference Manager 9.5 N, ISI ResearchSoft).
Additional searches conducted for this review
• We checked the reference lists of identified trials and other relevant articles. We did not impose any language or other limits on any of the searches.
Data collection and analysis
Screening for eligibility
Trials under consideration for inclusion in the review were assessed independently for their appropriateness by two review authors without prior consideration of their results. Any disagreements that were unresolved by discussion were considered by a third person. Excluded studies were listed with reasons for their exclusion.
Assessment of methodological quality
The review authors independently made an assessment of methodological quality using the Incontinence Group's quality assessment tool, which includes evaluation of quality of random allocation and concealment, description of dropouts and withdrawals, analysis by intention to treat, and masking during treatment and at outcome assessment. Disagreements were resolved by discussion with a third person.
Data extraction
Data were independently abstracted by at least two review authors and cross‐checked. Where data were collected but were not reported, or were reported in a form not suitable for inclusion in the formal analysis, further clarification was sought from the trialists. In trials where different doses of the same drug were compared against placebo we used what is considered to be the therapeutic dose (for example solifenacin 5mg, tolterodine 2mg twice a day or 4mg once per day). When two different types of anticholinergics (for example tolterodine and solifenacin) were compared against placebo, the results from the two arms of anticholinergics were recalculated as a weighted average.
Data analysis
In trials with two or more active (anticholinergic drug) treatment arms, the data from the active treatments were combined, where possible for comparison with placebo. Included trial data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005).
We intended, where possible, to calculate standardised effect sizes and 95% confidence intervals (CI): weighted mean differences (WMD) where outcomes were continuous variables and relative risks (RR) where they were binary. A fixed‐effect model was used to calculate the pooled estimates and the 95% CIs. The weighted mean differences were weighted by the inverse of the variance.
We combined data on number of episodes at the end of the treatment period with change from baseline scores for both leakage and micturition outcomes.
Cross‐over trials: in order to use the data from crossover trials in a meta‐analysis ideally it should be presented as the mean and standard deviation of the difference between two treatments for continuous data, or a two‐by‐two table for binary data, as the correlation between measurements on the same individual may be important. We did not attempt to include cross‐over trials in the meta‐analysis using the generic inverse variance method as there were large amounts of data available from the parallel‐group trials. The results from cross‐over trials were discussed narratively.
A priori sensitivity analyses were planned to investigate the effects of age, sex, severity of symptoms, cause of overactive bladder symptoms (that is idiopathic versus neurogenic) and type of medication.
Differences between trials were to be further investigated when it appeared obvious from visual inspection of the results, or statistically significant heterogeneity was found at the 10% probability level using the chi‐squared test or by assessment of the I‐squared statistic (Deeks 2005). If a reason was found then a judgement was to be made as to whether it was reasonable to combine the results. If there was no obvious reason for the heterogeneity, a random‐effects model could have been used. However, in the event this was not necessary.
Results
Description of studies
In this first update of the review (2006, Issue 4) fourteen extra trials were added (Cardozo 2004; Chapple 2004a: Chapple 2004b; Coombes 1996; Dmochowski 2003; Freeman 2003; Goode 2002; Haab 2004; Homma 2003; Khullar 2004; Landis 2004; Takayasu 1990; Zinner 2002; Zinner 2004). A total of sixty‐one trials are now included in the review. Thirty‐eight trials were excluded, including several abstracts with no useable data. The other reasons for exclusion are listed in the table 'Characteristics excluded studies'. Of the 61 trials included in the review, 42 were of a parallel‐group design and 19 of a crossover design.
In the parallel‐arm trials, 7822 participants were randomised to receive anticholinergic drugs and 4134 placebo medication. Three trials did not report the numbers randomised to each group (Chaliha 1998; Davila 2001; Tago 1990); and nine only reported the number after dropouts had been excluded (Abrams 1996; Cardozo 2004; Chapple 2004a; Dorschner 2000; Homma 2003; Junemann 1999; VanKerrebroeck 2001; Khullar 2004; Landis 2004). The crossover trials included 609 participants (63 known to be men and 402 known to be women); six crossover trials did not report the sex of the participants. Sample sizes ranged from 6 (Rosario 1995b) to 1529 (VanKerrebroeck 2001).
Three trials were published in German (Dorschner 2003a; Wehnert 1989; Wehnert 1992); one in Italian (Bono 1982); one in Flemish (Kramer 1987); one in Spanish (SerranoBrambila 2000) and one in Japanese (Takayasu 1990). All information and data were abstracted from the original paper.
Participant characteristics
Parallel‐arm trials
Inclusion or exclusion criteria. or both, were not always well described. People with a urodynamically determined or presumed diagnosis of idiopathic detrusor overactivity were included in 16 trials (Abrams 1998; Abrams 2001; Alloussi 1998; Burgio 1998; Chaliha 1998; Chapple 2004a; Jacquetin 2001; Jonas 1997; Homma 2003; Khullar 2004; Madersbacher 1999; Rentzhog 1998; Ulshofer 2001; VanKerrebroeck 2001; Wein 1978; Zinner 2004), and four trials restricted entry to those with neurogenic detrusor overactivity (Abrams 1996; Stohrer 1991; Stohrer 1999; VanKerrebroeck 1998;). Six trials included people with either idiopathic or neurogenic problems (Chapple 2004a;Chapple 2004b;Drutz 1999; Millard 1999; Tago 1990; Thuroff 1991); in the remaining trials participants had symptoms consistent with overactive bladder syndrome. Mean ages of the participants ranged from 30 years (no standard deviation (SD) given) to 82 years (SD 6.1) and five trials were restricted to older people (55 years and over, Burgio 1998; 60 years and over, Dorschner 2000; 60 years and over, Halaska 1994; 65 years and over, Malone‐Lee 2001; 70 years and over, Szonyi 1995). One trial reported data separately for people younger and older than 65 years of age (Zinner 2002). A common exclusion criterion was evidence of voiding dysfunction or bladder outlet obstruction, although in one trial inclusion was restricted to men with symptoms of bladder overactivity and bladder outlet obstruction (Abrams 2001).
Crossover trials
Six trials included people with urgency or urge incontinence (Bagger 1985; Coombes 1996; Kramer 1987; Wehnert 1989; Wehnert 1992; Zeegers 1987). These people usually had urodynamics performed as well but it was not one of the selection criteria. The other 14 crossover trials required a diagnosis of detrusor overactivity. One study was in spinal cord injured patients with detrusor hyperreflexia uncontrolled by oral anticholinergics (Di Stasi 2001a). Two trials recruited elderly people in institutions (Walter 1982; Zorzitto 1989) and one was confined to postmenopausal women (Tapp 1990). In the other trials the mean age ranged from 44 to 63 years, with four trials not reporting age. There was a variety of exclusion criteria.
Interventions
Parallel‐arm trials
In the majority of parallel‐group trials, intervention was preceded by run‐in periods of varying lengths and treatment with co‐medications was specifically an exclusion criterion, although for seven of the 42 included parallel arm trials this information was not reported (Burgio 1998; Chaliha 1998; Chapple 2004a; Homma 2003; Junemann 1999; Stohrer 1999; Tago 1990). All included trials were placebo controlled, and no trial made a comparison between an anticholinergic drug and no treatment.
Trials compared the following active treatments with placebo:
tolterodine (14 trials, Abrams 1996; Abrams 1998; Abrams 2001; Drutz 1999; Freeman 2003; Jacquetin 2001; Jonas 1997; Junemann 2000; Landis 2004; Malone‐Lee 2001; Millard 1999; Rentzhog 1998; VanKerrebroeck 1998; VanKerrebroeck 2001);
oxybutynin (eight trials, Abrams 1998; Burgio 1998; Drutz 1999; Goode 2002; Madersbacher 1999; Szonyi 1995; Thuroff 1991; Wein 1978);
trospium (eight trials, Alloussi 1998; Cardozo 2000; Chaliha 1998; Junemann 1999; Junemann 2000; Stohrer 1991; Ulshofer 2001; Zinner 2004), propiverine (five trials, Dorschner 2000; Halaska 1994; Madersbacher 1999; Stohrer 1999; Tago 1990);
propiverine (five trials, Dorschner 2000; Halaska 1994; Madersbacher 1999; Stohrer 1999; Tago 1990);
solifenacin (three trials, Chapple 2004a; Chapple 2004b; Cardozo 2004);
propantheline (two trials, Takayasu 1990; Thuroff 1991);
seven trials compared two different anticholinergic drugs with placebo:
Abrams 1998 tolterodine and oxybutynin; Dmochowski 2003 transdermal oxybutinin and oral tolterodine; Drutz 1999 tolterodine and oxybutynin; Homma 2003 extended release tolterodine and immediate release oxybutinin; Junemann 2000 tolterodine and trospium chloride; Madersbacher 1999 oxybutynin and propiverine; Thuroff 1991 oxybutynin and propantheline;
two trials compared different doses of the same anticholinergic medication (Chapple 2004a; Chapple 2004b);
length of treatment in most of the trials was 12 weeks (Abrams 1998; Cardozo 2004; Chapple 2004a; Chapple 2004b; Dmochowski 2003; Drutz 1999; Haab 2004; Homma 2003; Millard 1999; VanKerrebroeck 2001; Zinner 2004) with two thirds of trials choosing treatment periods of two to four weeks in duration. Some of the trials did not permit dose modification during study period (Homma 2003; Zinner 2002). Drug doses are described in the table 'Characteristics of included studies'.
Crossover trials
Some crossover trials were preceded by a week of a placebo washout period. The length of treatment varied from one dose to six weeks with a median length of three weeks. For the one dose study the washout period was three days. Eight of the nineteen trials had no washout period, and it was not clear in three more. The other washout periods varied from one week to one month. Given the short half life of the drugs, a washout period may not be important. Nine of the trials had two arms, seven had three arms and three had four arms. Some arms were the same drug at different doses. Some trials had different combinations of drugs so that they tested, for example, four drugs in a three arm study (Kramer 1987) and some had even more complicated arrangements (Massey 1986).
Eleven of the crossover trials used oxybutynin (Bono 1982; Di Stasi 2001a; Kramer 1987; Moisey 1980; Moore 1990; Murray 1984; Riva 1984; SerranoBrambila 2000; Tapp 1990; Zeegers 1987; Zorzitto 1989)
Seven emepronium ( Bagger 1985; Kramer 1987; Massey 1986; Meyhoff 1983; Murray 1984; Walter 1982; Zeegers 1987)
Two propiverine (Wehnert 1989; Wehnert 1992)
One penthienate( Coombes 1996)
Two darifenacin (Rosario 1995a; Rosario 1995b)
All trials but one (Di Stasi 2001a) used only oral administration of the drugs. Di Stasi used two methods of intravesical administration, one with an electric current, and oral administration.
Outcome measures
Overall, there was a lack of consistency in the types of outcome measures reported by trialists, and a lack of consistency in the way data were reported. Seven trials reported outcomes of interest but without useable data (Chaliha 1998; Di Stasi 2001a; Malone‐Lee 2001; Rosario 1995b; Stohrer 1991; Tago 1990; Wein 1978). Due to deficiencies in data reporting (for example point estimates without any measure of variation), many trials contributed only limited data to the review. The lack of similarity in measures reduced the possibilities for combining results from individual trials.
The primary outcomes of interest in the review were the patient observations (for example perception of cure or improvement) and quantification of symptoms (such as number of leakage episodes). Patient observations were rarely reported and in reading trial reports it does not appear that these data were often collected. Micturition diaries were commonly used to record numbers of leakage episodes and numbers of micturitions over varying lengths of time. In order to combine these data in a meta‐analysis, the number of leakage episodes and number of micturitions in 24 hours were calculated.
Urodynamic measures and adverse events were the two most commonly reported secondary outcomes of interest. A wide range of urodynamic measures were reported. In view of the lack of correlation between urodynamic measures and clinical outcome the data were not abstracted for this review.
Measures of quality of life were reported in seven trials (Chapple 2004a; Dorschner 2000; Dmochowski 2003; Homma 2003; Khullar 2004; VanKerrebroeck 2001; Zinner 2004). Four studies (Chapple 2004a; Homma 2003; Khullar 2004; VanKerrebroeck 2001) used the King's Health Questionnaire (KHQ), two (Dmochowski 2003; Zinner 2004) used the Incontinence Impact Questionnaire (IIQ) and one used the Giessen Complaint Survey and Basle Subjective Wellbeing Study (Dorschner 2000). Chapple (Chapple 2004a) also reported on quality life using the Contilife score. `
In order to use the data from crossover trials in a meta‐analysis it must be presented as the mean and standard deviation of the difference between two treatments for continuous data, or a two‐by‐two table for binary data, as the correlation between measurements on the same individual may be important. Only two of the 19 crossover trials presented data in this way (Kramer 1987; Riva 1984) and then only for one outcome each, so the data could not be used in this way. The data were, therefore, discussed narratively and this is reported in the Results section after the analyses of the parallel‐arm trials. In studies, where medians were reported (Landis 2004; Zinner 2004), data could not be used in the meta‐analysis and were reported in separate data tables.
Further characteristics of the trials are reported in the table 'Characteristics of included studies'.
Risk of bias in included studies
The methodological quality of the published studies was assessed by looking at the methods of generation of random allocation, concealment of allocation, blinding of the trial participants and investigators, completeness of treatment, withdrawals and dropouts and loss to follow up.
Randomisation, allocation concealment and blinding
The method of group allocation was rarely described. Double blinding should adequately conceal group allocation but this is not guaranteed. For the purposes of the review, trials that stated group allocation was 'double‐blind' were coded as having adequate concealment. All trials but two (Wehnert 1989; Wehnert 1992) were double blind and therefore considered to have adequate allocation concealment.
Few trials specifically stated that outcome assessors were blind to group allocation (Burgio 1998; Goode 2002; Homma 2003). Some trials stated the code was broken at the completion of the study and in some it was specified as after the analysis. This would imply that the final measurement was done blinded. A recent study by DuBeau et al suggests that double‐blind designs might not be adequate to blind participants to anticholinergic versus placebo allocation, emphasising the importance of blinded outcome assessors (DuBeau 2000).
Baseline comparability of the groups was not mentioned in seven of the parallel‐group trials (Chaliha 1998; Goode 2002; Halaska 1994; Homma 2003; Junemann 1999; Junemann 2000; Wein 1978). The remaining 34 parallel‐group trials stated that the groups were comparable at baseline, although three trials did not provide supporting data (Abrams 2001; Cardozo 2000; Tago 1990). In 12 trials the evaluation of treatment efficacy was conducted on intention‐to‐treat principles (Abrams 1998; Burgio 1998; Cardozo 2000; Jacquetin 2001; Junemann 1999; Madersbacher 1999; Malone‐Lee 2001; Millard 1999; Szonyi 1995; VanKerrebroeck 2001; Wein 1978; Zinner 2002) and seven trials specifically stated that a per‐protocol analysis was used to assess treatment efficacy (Abrams 1996; Alloussi 1998; Dorschner 2000; Drutz 1999; Junemann 2000; Ulshofer 2001; VanKerrebroeck 1998). Baseline comparability is not an issue of concern for crossover trials.
Withdrawals and dropouts
The description of withdrawals or dropouts was not adequate in 11 trials (Abrams 1996; Bono 1982; Cardozo 2000; Chaliha 1998; Chapple 2004a; Chapple 2004b; Halaska 1994; Junemann 1999; Junemann 2000; Tago 1990; VanKerrebroeck 1998). There were no dropouts from the trials using single oral doses of medication (Di Stasi 2001a; Wein 1978) and in 17 trials the drop out rate was 10% or less (Bagger 1985; Dorschner 2000; Jacquetin 2001; Jonas 1997; Malone‐Lee 2001; Meyhoff 1983; Millard 1999; Murray 1984; Rosario 1995a; SerranoBrambila 2000; Stohrer 1991; Stohrer 1999; Thuroff 1991; Walter 1982; Wehnert 1989; Wehnert 1992; Zinner 2004). In the remainder of trials, drop‐out rates ranged from 12% (Madersbacher 1999; VanKerrebroeck 2001) to 21% (Drutz 1999; Szonyi 1995; Homma 2003) in parallel‐designs and 20% (Riva 1984) to 63% (Kramer 1987) in the crossover trials. Very few parallel design trials included any follow up beyond the initial assessment of outcome. In those trials that did follow up participants this was for short periods of time, that is one week (VanKerrebroeck 2001; Zinner 2002) or two weeks (Abrams 1998; Drutz 1999; Jacquetin 2001; Malone‐Lee 2001; Rentzhog 1998; Ulshofer 2001). Some trials did follow up participants longer term but this was not blinded and by this stage everyone had been offered active treatment. Long term follow up is not relevant for crossover trials: by the nature of their design crossover trials can only address short‐term effects during treatment.
The primary or only reference for nine of the 60 included trials was a conference abstract (Abrams 1996; Abrams 2001; Chaliha 1998; Junemann 1999; Junemann 2000; Murray 1984; Rosario 1995a; Rosario 1995b; Tago 1990) and all of these trials were complete at the time of reporting. Full publications were not found for any of these trials with subsequent searching. All abstracts reported limited details of methods, and few results.
Some of the large multi‐centre and multinational trials were reported in multiple publications. These publications usually presented subsets of the main trial results (for example data from one country) and subsequent publications rarely provided further methodological detail. The most notable example is VanKerrebroeck (VanKerrebroeck 2001); 14 separate reports were identified. Where there were multiple publications of the same trial a primary reference was selected and has been cited throughout the review for simplicity.
Effects of interventions
Primary outcome measures (outcomes 01.01 to 01.05)
A. Patient observations, for example symptom scores, perception of cure or improvement, satisfaction with outcome (outcome 01.01)
Parallel‐arm trials
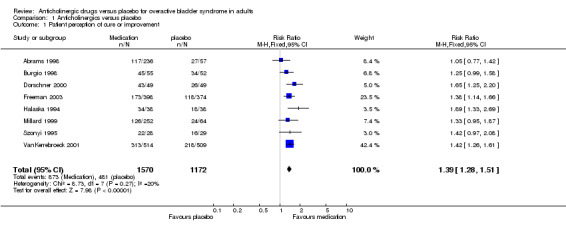
Patients' perception of change in symptoms were reported in eight trials (Abrams 1998; Burgio 1998; Dorschner 2000; Freeman 2003; Halaska 1994; Millard 1999; Szonyi 1995; VanKerrebroeck 2001). Those taking medication were more likely to report cure or improvement in their symptoms than those taking placebo (873 out of 1570, 56% cured or improved in medication group; and 481 out of 1172, 41% cured or improved in placebo group); relative risk (RR) for cure or improvement 1.39 (95% CI 1.28 to 1.51, outcome 01.01). There was no statistically significant heterogeneity.
Some form of patient‐reported outcome was used in eight further trials but these data could not be included in the meta‐analysis because they were not dichotomous or could not be dichotomised by the review authors (Cardozo 2000; Drutz 1999; Rentzhog 1998; Stohrer 1999; Tago 1990; Thuroff 1991; VanKerrebroeck 1998).
Crossover trials
Eight crossover trials reported this outcome (Bagger 1985; Kramer 1987; Moore 1990; SerranoBrambila 2000; Walter 1982; Wehnert 1989; Wehnert 1992; Zeegers 1987), all in a different way. In all trials the patient preference was in favour of anticholinergic drugs.
B. Quantification of symptoms, for example number of leakage episodes, frequency and volume (urinary diary) (outcomes 01.02 to 01.05)
Parallel ‐arm trials
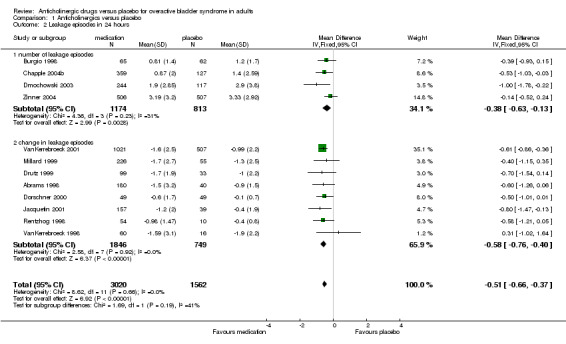
Three trials (Burgio 1998; Chapple 2004b; Dmochowski 2003) reported the number of leakage episodes in 24 hours after treatment. Chapple and Dmochowski also reported change data but this data was not entered in the analysis, only data for average number of leakage episodes in 24 hours after treatment was used. One trial (Zinner 2002) reported number of leakage episodes per week and this was converted to data for 24 hours and combined with the other three trials. Those in the anticholinergic drug groups had approximately 0.38 leakage episodes less per 24 hours than those taking placebo medication (WMD for leakage episodes in 24 hours ‐0.38, 95% CI ‐0.63 to ‐0.13, P=0.003, outcome 01.02).
Ten trials reported the change in number of leakage episodes at the end of treatment, measured over a 24 hour period (Abrams 1998; Chapple 2004b; Dmochowski 2003; Dorschner 2000; Drutz 1999; Jacquetin 2001; Millard 1999; Rentzhog 1998; VanKerrebroeck 1998; VanKerrebroeck 2001). All except one showed greater reduction in leakage episodes in the anticholinergic group (WMD ‐0.58; 95% CI ‐ 0.76 to 0.40 P<0.00001 outcome 01.02). When the two subgroups are combined (number of leakage episodes in 24 hours and change in leakage episodes) the result (WMD ‐0.51; 95% CI ‐0.66 to ‐0.37) shows on average a reduction in leakage episodes of around three to five per week. Zinner reported his result for change in leakage episode as percentages (other data table 1.03) also favouring treatment.
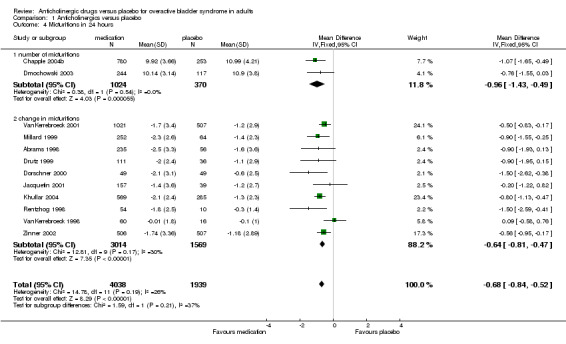
Data on number of micturitions were consistent with this (outcome 01.04). Two trials (Chapple 2004b; Dmochowski 2003) reported on number of micturitions in 24 hours (WMD ‐0.96; 95% CI ‐1.43 to ‐0.49) both studies also reported change data but this was not entered into the analysis. Twelve trials (Abrams 1998; Chapple 2004b; Dmochowski 2003; Dorschner 2000; Drutz 1999; Jacquetin 2001; Khullar 2004; Millard 1999; Rentzhog 1998; VanKerrebroeck 1998; VanKerrebroeck 2001; Zinner 2002) reported change in micturition at end of treatment, measured over 24 hours, the result favoured anticholinergic treatment (WMD ‐0.64 95% CI ‐0.81 to ‐0.47, outcome 01.04). When the two subgroups (micturitions in 24 hours and change in micturitions) are combined this translates to a WMD of ‐0.68 95% CI ‐0.84 to 0.52 i.e. around four to six fewer voids over one week compared with those taking placebo. Two trials (Landis 2004; Zinner 2004) which could not be included in the meta‐analysis as no standard deviations were reported, also found statistically significant differences favouring anticholinergics (other data table 01.05).
Crossover trials
Five crossover trials reported number of leakage episodes in some manner (Bagger 1985; Massey 1986; Meyhoff 1983; Riva 1984; Tapp 1990). In two of these, there was a clear‐cut result in favour of drugs (Massey 1986; Tapp 1990), with a dose response in Massey. Seven trials reported either the number of micturitions or the change in the number of micturitions from baseline (Bagger 1985; Kramer 1987; Massey 1986; Meyhoff 1983; Moore 1990; Riva 1984; Wehnert 1989). In four of these there was a larger decrease during the drug treatment period than during the placebo period, and in three the response, was about the same. In (Massey 1986) there was a clear dose‐response effect. No crossover study used a pad test.
C. Quality of life (outcomes 01.06 to 01.08)
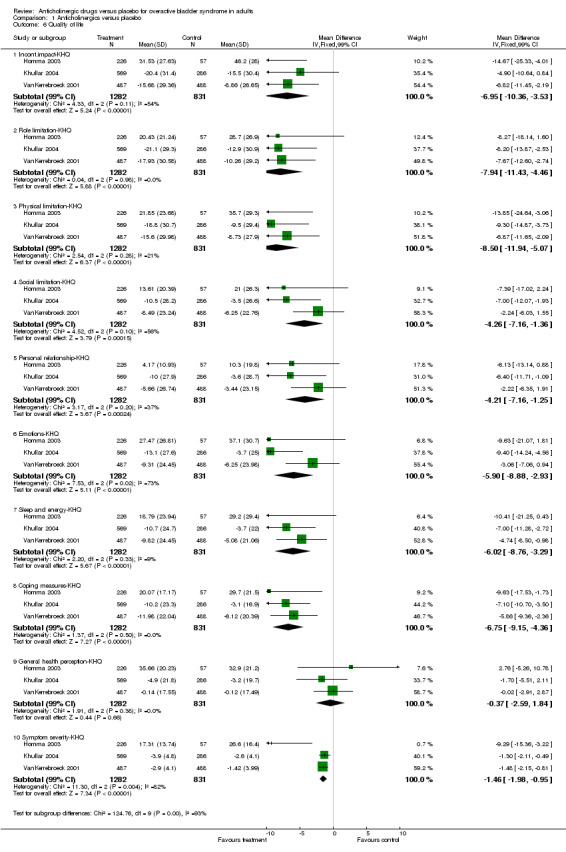
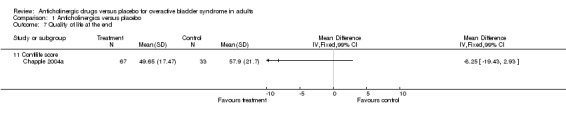
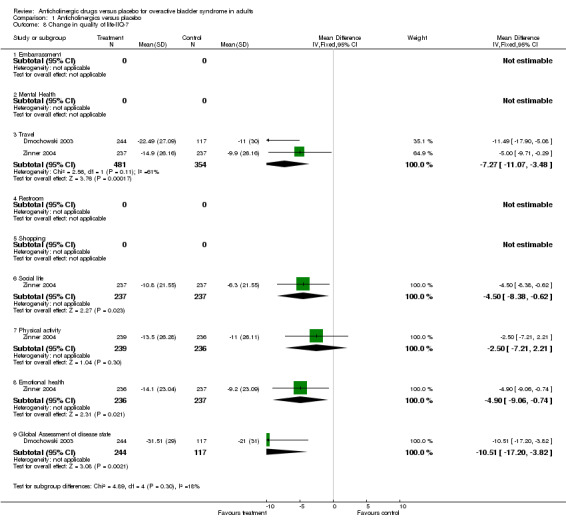
Seven trials reported on condition‐specific quality of life measures(Chapple 2004a; Dmochowski 2003; Dorschner 2000; Homma 2003; Khullar 2004; VanKerrebroeck 2001; Zinner 2002 ). Three trials (Homma 2003; Khullar 2004; VanKerrebroeck 2001) used the King's Health questionnaire; A lower score on the King's Health questionnaire indicates better quality of life. Homma (Homma 2003) reported on quality of life at the end of treatment and Khullar and Vankerrebroeck reported on change in quality of life. When data from all three trials were combined all separate domains apart from general health perception showed statistically significant difference favouring anticholinergic treatment (Outcome 01.06 for example WMD for incontinence impact score ‐6.95; 99% CI ‐10.36 to ‐3.53; P < 0.0000). Due to the fact that multiple domains in quality of life are reported we have chosen to report 99% confidence intervals. Two trials (Dmochowski 2003; Zinner 2004) used the IIQ questionnaire, when combined they showed a statistically significant difference for 'travel', favouring anticholinergics (WMD ‐7.27, 95% CI ‐11.07 to ‐3.48). Zinner found statistically significant differences for social life and emotional health, favouring anticholinergics. Dmochowski found a statistically significant difference for global health assessment of disease state, also favouring medication.
D. Socioeconomics
No trial reported any form of socioeconomic measure.
E. Adverse events (outcomes 01.09 and 01.10)
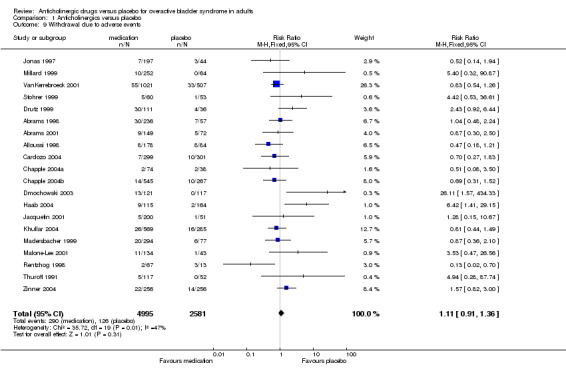
Twenty parallel‐group trials reported the number of people withdrawing due to adverse events (Abrams 1998; Abrams 2001; Alloussi 1998; Cardozo 2004; Chapple 2004a; Chapple 2004b; Dmochowski 2003; Drutz 1999; Haab 2004; Jacquetin 2001; Jonas 1997; Khullar 2004; Madersbacher 1999; Malone‐Lee 2001; Millard 1999; Rentzhog 1998; Stohrer 1999; Thuroff 1991; VanKerrebroeck 2001; Zinner 2002). There was no statistically significant difference in the number of withdrawals due to adverse events between medication and placebo groups (RR for withdrawal 1.11, 95% CI 0.91 to 1.36, outcome 01.10). The results of the meta analysis showed some statistically significant heterogeneity (P = 0.01).The data from a dose‐ranging study of tolterodine (0.5 mg, 1 mg, 2 mg, 4 mg all twice daily versus placebo, Rentzhog 1998) appeared to be different from the other trials, finding significantly more withdrawals in placebo than medication groups (RR 0.13, 95% CI 0.02 to 0.70) Excluding the data from Rentzhog et al did not change the finding of the meta‐analysis much. In contrast, the data from Dmochowski and Haab significantly favoured placebo.
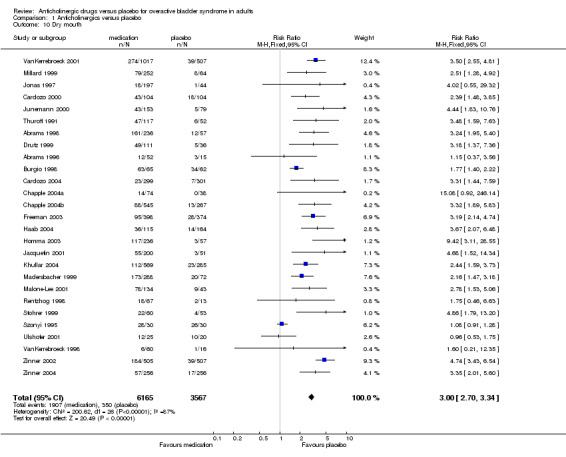
Dry mouth was the most frequently reported side effect and data were available from 27 parallel‐group trials (Abrams 1996; Abrams 1998; Burgio 1998; Cardozo 2000; Cardozo 2004; Chapple 2004a; Chapple 2004b; Drutz 1999; Freeman 2003; Haab 2004; Homma 2003; Jacquetin 2001; Jonas 1997; Junemann 2000; Khullar 2004; Madersbacher 1999; Malone‐Lee 2001; Millard 1999; Rentzhog 1998; Stohrer 1999; Szonyi 1995; Thuroff 1991; Ulshofer 2001; VanKerrebroeck 1998; VanKerrebroeck 2001; Zinner 2002; Zinner 2004). The risk of dry mouth was three times greater in the medication group (1907/6165, 31%, with dry mouth in the medication group versus 350/3567, 9.8%, in the placebo group); the RR for dry mouth was 3.0, (95% CI 2.70 to 3.34, outcome Metaview 01.11). Statistically significant heterogeneity was observed in this comparison (P<0.00001). It was difficult to determine the possible causes of heterogeneity; the possible influence of the type of medication was explored.
Fourteen trials compared tolterodine with placebo (Abrams 1996; Abrams 1998; Drutz 1999; Freeman 2003; Jacquetin 2001; Jonas 1997; Junemann 2000; Khullar 2004; Malone‐Lee 2001; Millard 1999; Rentzhog 1998; VanKerrebroeck 2001; VanKerrebroeck 1998; Zinner 2002). The risk of dry mouth was three times higher in the tolterodine group (1184/3951, 29%, in tolterodine group versus 178/2091, 8.5%, in the placebo group); RR for dry mouth 3.37 (95% CI 2.90 to 3.90).
Seven trials made comparisons of oxybutynin and placebo (Abrams 1998; Burgio 1998; Drutz 1999; Homma 2003; Madersbacher 1999; Szonyi 1995; Thuroff 1991). The risk of dry mouth was more than twice as great in the oxybutynin group (RR for dry mouth 2.41, 95% CI 2.02 to 2.87). However, statistically significant heterogeneity was observed amongst the oxybutynin trials (P<0.00001). Two trials in the elderly had very high rates of dry mouth in the placebo arm (Burgio 1998; Szonyi 1995), perhaps as a consequence of polypharmacy. When these two trials were excluded from the pooled analysis the risk of dry mouth was three times greater in the oxybutynin groups (266/434, 61% in oxybutynin group versus 48/284, 17%, in placebo group); RR for dry mouth 3.23 (95% CI 2.48 to 4.20) and the test for heterogeneity was no longer significant (P = 0.43).
Three trials (Chapple 2004a; Chapple 2004b; Cardozo 2004) compared solifenancin and placebo (RR for dry mouth 3.62, 95%CI 2.29 to 5.74).
Four trials compared trospium and placebo (Cardozo 2000; Junemann 2000; Ulshofer 2001; Zinner 2004 ). The risk of dry mouth was twice as great in the trospium group (RR for dry mouth 2.66, 95% CI 1.98 to 3.55) but statistically significant heterogeneity was observed in this comparison (P = 0.0065). Both Cardozo et al (Cardozo 2000) and Junemann et al (Junemann 2000) found significantly higher rates of dry mouth in the trospium groups but Ulshofer et al (Ulshofer 2001) found similar rates (approximately 50%) in both medication and placebo groups. The drug dose in the two former trials was 20 mg trospium twice daily, while the latter used 15 mg three times a day; otherwise the trials were very similar with regard to method and study population. Ulshofer et al (2001) also stated that trial participants were asked specific questions about side effects, including dry mouth, and it is possible this yielded high positive rates of reporting.
Propiverine was compared with placebo in two trials (Madersbacher 1999; Stohrer 1999) and propantheline with placebo in a single trial (Thuroff 1991). All three trials found significantly higher rates of dry mouth in the medication groups.
Adverse events were poorly recorded in the crossover trials, many of which did not provide numbers and only one study (Kramer 1987) presented the results in such a way that they could be combined with others. Adverse events were reported for 17 trials and in most trials there were more adverse events while on anticholinergic drugs than while on placebo. A few had about the same number. In the crossover trials, dry mouth data were reported in 17 trials (Bagger 1985; Bono 1982; Kramer 1987; Massey 1986; Meyhoff 1983; Moisey 1980; Moore 1990; Murray 1984; Riva 1984; Rosario 1995a; SerranoBrambila 2000; Tapp 1990; Walter 1982; Wehnert 1989; Wehnert 1992; Zeegers 1987; Zorzitto 1989). In eight of these, anticholinergics resulted in more people suffering from dry mouth, in six the numbers were about the same, and in three it was not clear which treatment was worse. Apart from one trial (Kramer 1987) it was not clear whether those who suffered from dry mouth in the drug and placebo periods were the same or different people.
Some form of compliance measure (for example pill counting) was reported in six trials (Jacquetin 2001; Jonas 1997; Malone‐Lee 2001; Szonyi 1995; Ulshofer 2001; VanKerrebroeck 2001).
Sensitivity analysis (data not shown) Despite clinical heterogeneity of the included trials (for example populations and medication) sensitivity analyses did not suggest that the findings were significantly modified by age, sex or diagnosis (neurogenic or idiopathic detrusor overactivity). The same applied to the type of medication, except for dry mouth as discussed above.
Discussion
This review is the first of a series of Cochrane reviews of drug therapy for overactive bladder syndrome and it should be viewed in that context. Other reviews have considered or will consider: (1) whether different anticholinergic drugs have different effects (Hay‐Smith 2005); (2) whether anticholinergic drugs are better than other active (non‐drug) therapies (Alhasso 2006); and (3) whether anticholinergic drugs are better than other drug treatments (Dublin 2004).
Principal findings
In contrast to many other treatments for lower urinary tract dysfunction, there are a relatively large number of trials comparing anticholinergic drugs with placebo medication. Adults with overactive bladder were more likely to report cure or improvement when taking active anticholinergic treatment. However, it appears that treatment has a large placebo response with 41% of people in the placebo groups reporting cure or improvement in symptoms. The additional benefit of active treatment was about 15% more improved or cured (equivalent to a number needed to treat of seven). The difference between the groups in micturitions and leakage episodes after treatment is statistically significant. All the trials included in the formal analysis showed a reduction in micturitions and leakage episodes in both active treatment and placebo groups. The difference in improvement between anticholinergic and placebo medication groups was on average about four less leakage episodes and five less voids per week in favour of anticholinergics. While the difference between the groups in micturitions and leakage episodes after treatment is statistically significant (the confidence intervals are tight), the issue is their clinical significance.
Although the data are still limited, this update contains more information about quality of life (for example Chapple 2004b; Khullar 2004). Where data are available, they favour the medication group and are highly statistically significant. The key issue, then, is whether the differences are clinically important and useful to patients. Pleil and colleagues (Pleil 2005) related changes in the King's Health Questionnaire to patients perceptions of benefit: firstly if they perceived a benefit from treatment and if so, secondly, whether they perceived it as 'a little' or 'much' benefit. Generally speaking, our estimates lie somewhat below the change scores of patients who described their benefit as 'a little' but with overlapping confidence intervals. This suggests that the changes observed in leakage episodes and voiding frequency do have a broader impact to improve health, but on average the benefits are modest.
The drugs do, however, have adverse effects. While there was no significant difference in the number of people withdrawing from the active and placebo treatment arms due to adverse events the confidence interval is compatible with up to a third withdrawing due to medication (RR 1.11, 95% CI 0.91 to 1.36). Dry mouth was a side effect reported by many trial participants. This was not surprising as dry mouth is the most widely experienced side effect of anticholinergic therapy. The risk of dry mouth in the active treatment groups increased three fold, depending on the type of medication. Two trials in elderly populations, both comparing oxybutynin chloride with placebo, seemed to report high rates of dry mouth compared with trials in other populations. Elderly people are often taking a number of medications and the higher rates of dry mouth in both active treatment and placebo groups might be related to other concurrent therapy. Aside from this, there were no obvious differences in the outcomes for elderly patients.
Limitations
In general, the reported methods of the trials were of moderate to high quality. Overall the reporting of many trials was poor with many not even reporting the age, sex or concurrent medications of the participants. This may be important as age and polypharmacy are independent predictors of dry mouth, the main adverse side effect of anticholinergics. However, the methods of group allocation were rarely given in sufficient detail to be sure that group allocation was adequately concealed. Only one trial blinded outcome assessors. The reporting of group allocation of dropouts and/or reasons for withdrawal was adequate in less than half the trials. Crossover trials were usually poorly reported. In order to be included in a meta‐analysis the results from crossover trials for continuous variables must be reported as a difference in means together with either the standard deviation, standard error or 95% confidence interval of that difference. This does coincide with what is of clinical interest, but can become complex when there are more than two treatments. Dichotomous outcomes should be reported so that it is clear whether the events on each treatment occurred in the same or different people. Only two of the nineteen crossover trials reported outcomes in this manner, but they were not consistent in the outcomes that were reported.
In view of the substantial number of trials it is disappointing that it was not possible to combine more data. There was considerable variation in outcomes chosen to be reported, and also variation in how the same outcome was measured and reported. Relatively few trials sought the patient's opinion on satisfaction with, and acceptability of, treatment and these are important factors in the choice of management. Only seven relatively recent trials addressed quality of life, and none reported socioeconomic outcomes. These areas need to be addressed in future research. In view of the lack of validated 'normal' values and the lack of correlation between urodynamic findings and clinical outcome, the emphasis on urodynamic measures should be re‐evaluated, and we have chosen to remove these data from this update.
None of the included trials reported results for those with frequency and/or urgency alone separately from those with urge incontinence. A number of trials included men and women, but did not report outcome separately by sex so it was difficult to investigate possible differences in effects between the sexes.
We decided to combine the data from all the active anticholinergic treatment arms in trials where there was more than one drug or more than one dose of the same drug being compared. It is possible that some medications are more effective than others but that this was not seen in the formal comparisons. However, direct comparisons of different anticholinergic therapies, and comparisons of different doses of the same therapy, will be investigated in a separate review (Hay‐Smith 2005). One of the limitations of the review is that many trials do not contribute to the meta‐analyses. Those that do may or may not be a biased selection of the population of primary trials. In addition, despite comprehensive searching not all trials may have been located. Data were combined from primary trials recruiting on different criteria or using different drugs in various doses. These factors may influence the estimates of effect in this review. Examination of the graphs shows, however, that the effects are generally consistent across the included trials that did contribute. It proved difficult to explore the reasons on the few occasions where heterogeneity was observed. The best way to address these concerns would be to perform an individual patient data meta‐analysis. Many more trials would then contribute to the analysis. In addition the effect of confounders (such as age, cause of overactive bladder) could be investigated.
In general, many of the included trials had the characteristics of explanatory rather than pragmatic trials. Explanatory trials, also known as efficacy trials, address the question 'can this therapy work?'. Efficacy studies tend to have strict inclusion and exclusion criteria, a comparison of therapy versus placebo, short‐term outcomes, measure surrogate rather than patient centred outcomes (for example urodynamics rather than quality of life) and take place in centres of clinical excellence. Their results are commonly used to support applications for drug regulatory approval. In contrast, pragmatic trials, also known as effectiveness trials, address the question 'does this therapy work?'. Effectiveness studies are characterised by large, more heterogeneous samples, comparisons with standard care, less restrictive inclusion/exclusion criteria, and long‐term patient‐centred outcomes (Roland 1998). Their results inform the choice of management in everyday clinical settings.
All the included trials were of short duration and measured outcome at the end of treatment. Therefore outcome was measured when the effects of treatment were likely to be at their maximum. Anticholinergics are unlikely to cure overactive bladder syndrome, so the long term effects of treatment are of most clinical interest. Some trials have continued with open‐label follow up. The interpretation of these is difficult, not only because of the use of active treatment by those originally allocated placebo but also because of the number of overlapping pooled analyses published, based on different numbers of primary studies. In a follow up of a single study, it was found that 71% of patients completed 12 months of open label follow up on extended release tolterodine (van Kerrebroeck 2001). In contrast, Lawrence et al (2000) audited a pharmaceutical database in the USA and reported that less than a third of people continued to fill out prescriptions for either immediate release tolterodine or immediate release oxybutynin six months after the first prescription, although the use of oxybutynin was discontinued faster than tolterodine (Lawrence 2000). This may represent the differences between people prescribed anticholinergics in a trial versus a more typical care setting.
Pharmaceutical companies are continuing to develop anticholinergic drugs. Some drugs are no longer in widespread clinical use (for example emepronium ) and others have been withdrawn from the market (for example terodiline). Alternative delivery systems (such as transdermal, slow‐release preparations) and new drugs (for example darifenacin) are becoming available, which may be more effective or have fewer side effects, or both due to adverse effects.
It is worth noting that 21 of the 61 trials declared pharmaceutical company support (Abrams 1998; Bagger 1985; Cardozo 2000; Chapple 2004b; Coombes 1996;Davila 2001; Dorschner 2000; Drutz 1999; Homma 2003; Jacquetin 2001; Meyhoff 1983; Moisey 1980; Moore 1990; Rentzhog 1998; Szonyi 1995; Tapp 1990; Thuroff 1991; Ulshofer 2001; VanKerrebroeck 1998; VanKerrebroeck 2001; Walter 1982). This support ranged from the supply of active and placebo tablets (in blinded packaging) through to full funding and data analysis. None of the remaining trials made any statement about the absence or presence of company involvement. Two trials were funded by grants from health research bodies (Burgio 1998; Zorzitto 1989). In other settings, meta‐analyses comparing findings from drug company funded studies with non‐drug company funded trials have found that the outcomes of company funded studies are more favourable to the new treatment, although this is not always the case. In general, in this review, the trials supported by companies were well reported and appeared to be of better methodological quality. Their limitations are that, for understandable reasons, they addressed issues of efficacy and safety rather than clinical and cost effectiveness.
This review provides clear evidence of efficacy and of the likelihood of adverse effects, particularly dry mouth. Newer trials suggest that the positive effects are translated into improved quality of life while medication continues, at least on average. However, there is very little evidence about the long‐term effects of medication. This applies to fixed length courses of treatment and to continued treatment for an indefinite period of time, both during treatment and after it has stopped. Addressing these issues requires a shift in the research agenda to more pragmatic trial designs.
Authors' conclusions
Implications for practice.
The administration of anticholinergic drugs for overactive bladder syndrome results in statistically significant differences compared to placebo medication. Those receiving anticholinergic therapy were more likely to report cure or improvement of symptoms, and a reduction in leakage episodes (about four per week) and voids (about five per week). Evidence from more recently reported trials indicates modestly improved quality of life too. There was a marked placebo response, however. When counseling those with overactive bladder syndrome, these benefits need to be balanced with the risk of side effects, notably dry mouth. Depending on the type of medication being offered, the risk of dry mouth is increased by three times.
The only long‐term follow up comes from open‐label studies, with anticholinergic therapy offered to all trial participants regardless of their original allocation to active or placebo treatment. The short duration of most trials and the lack of long‐term follow up gives little information about the long‐term effects and acceptability of anticholinergic therapy.
Cost‐effectiveness was also not addressed.
Implications for research.
To allow the results of trials to be considered together most usefully, future research in overactive bladder syndrome should incorporate standardised, validated outcome and quality of life measures that are relevant to those with an overactive bladder. Particular attention needs to be paid to the patient perception of change and satisfaction with outcome, quality of life, and economic outcomes. The current emphasis on urodynamic measures needs to be redressed. Reporting of the methods of group allocation and the description of dropouts needs to be improved; outcome assessors should be masked to group allocation. The reporting of crossover trials needs to be dramatically improved if they are to add anything to the body of knowledge about these drugs.
As anticholinergic drugs are unlikely to be curative, sustained success is likely to depend on people continuing to take them. Trials are needed to assess the long‐term usefulness of these drugs.
Both oxybutynin and tolterodine have been compared with placebo in a number of trials. For these drugs it seems that priority should be given to research in subgroups that might benefit most, or who have previously been excluded from trials. In addition, all drugs need to be tested in large pragmatic trials. Nearly all the included trials used oral administration of medication. Further research would also be useful to investigate whether the magnitude of effect changes with different delivery systems (for example skin patches or slow‐release preparations) would also be useful. Another Cochrane review comparing anticholinergics with each other will address these questions (Hay‐Smith 2005). Intravesical administration has potential as it delivers the drug directly to the desired site of action, thus eliminating some troublesome anticholinergic side effects but would only be clinically useful if intravesical administration could be made easier. In our view, placebo‐controlled trials should be confined to testing the short‐term efficacy and safety of new anticholinergic therapies.
The reliability and hence value of this review would be greatly enhanced if data from more trials could be incorporated in an individual patient data meta‐analysis.
Feedback
Study characteristics (pages 27?28) for reference
Summary
Dear Authors > > With regards to the above reference, we believe that the study > characteristics quoted on pages 27?28 do not appear to correlate to > those in the published paper. Whereas the study cited in the review > investigated only solifenacin, with tolterodine as a comparator, the > characteristics quoted include details about another anticholinergic > compound, darifenacin, implying that this compound was included for > comparison in the study. > It > seems possible that a second paper by Chapple et al, reporting on the > efficacy of darifenacin, has been included in the description of the > study characteristics by mistake (this second paper, entitled ?A > pooled analysis of three phase III studies to investigate the > efficacy, tolerability and safety of darifenacin, a muscarinic M3 > selective receptor antagonist, in the treatment of overactive bladder? > (BJU 2005;95(7):993‐1001), discusses two doses of darifenacin (7.5 mg, > n=337 and 15 mg, n=334) versus placebo (n=388)). > > We would therefore like to request a review of the text on pages 27 ? > 28, > and a potential amendment to the study characteristics as necessary. > In addition, as quality of life (QoL) data is not discussed in either > of the above papers by Chapple et al, statements such as ?QoL data > reported favours solifenacin? in the table of study characteristics > are potentially misleading to the reader. > We look forward to your review of the text and modification of the > study characteristics table.
Reply
the info in characteristics of studies table has now been changed
Contributors
Nabi Ghulam, Peter Herbison, June Cody
What's new
| Date | Event | Description |
|---|---|---|
| 16 September 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 22 August 2006 | New citation required and conclusions have changed | Substantive amendment. 14 trials added |
Acknowledgements
The authors would like to thank the following: Birgit Moehrer (BM) for help with assessment of trials published in German, Italian and Flemish. Fernanda Teixeira (FT) for help with trials published in Spanish. Dr Life Rentzhog and Professor Wolfgang Dorschner for providing unpublished data. Atsuo Kondo (AK) for translation of a study reported in Japanese. Sylvia Anton (SA) for translation of a study reported in German. U Azman, P Collin, S Radley, D Richmond, C Chapple who were the authors of a previous protocol for this review.
Data and analyses
Comparison 1. Anticholinergics versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient perception of cure or improvement | 8 | 2742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.28, 1.51] |
| 2 Leakage episodes in 24 hours | 12 | 4582 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.66, ‐0.37] |
| 2.1 number of leakage episodes | 4 | 1987 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.63, ‐0.13] |
| 2.2 change in leakage episodes | 8 | 2595 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.76, ‐0.40] |
| 3 Leakage episodes in 24 hours | Other data | No numeric data | ||
| 4 Micturitions in 24 hours | 12 | 5977 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐0.84, ‐0.52] |
| 4.1 number of micturitions | 2 | 1394 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐1.43, ‐0.49] |
| 4.2 change in micturitions | 10 | 4583 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.81, ‐0.47] |
| 5 Micturition in 24 hours | Other data | No numeric data | ||
| 6 Quality of life | 3 | Mean Difference (IV, Fixed, 99% CI) | Subtotals only | |
| 6.1 Incont.impact‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐6.95 [‐10.36, ‐3.53] |
| 6.2 Role limitation‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐7.94 [‐11.43, ‐4.46] |
| 6.3 Physical limitation‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐8.50 [‐11.94, ‐5.07] |
| 6.4 Social limitation‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐4.26 [‐7.16, ‐1.36] |
| 6.5 Personal relationship‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐4.21 [‐7.16, ‐1.25] |
| 6.6 Emotions‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐5.90 [‐8.88, ‐2.93] |
| 6.7 Sleep and energy‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐6.02 [‐8.76, ‐3.29] |
| 6.8 Coping measures‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐6.75 [‐9.15, ‐4.36] |
| 6.9 General health perception‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐0.37 [‐2.59, 1.84] |
| 6.10 Symptom severity‐KHQ | 3 | 2113 | Mean Difference (IV, Fixed, 99% CI) | ‐1.46 [‐1.98, ‐0.95] |
| 7 Quality of life at the end | 1 | Mean Difference (IV, Fixed, 99% CI) | Totals not selected | |
| 7.11 Contilife score | 1 | Mean Difference (IV, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in quality of life‐IIQ‐7 | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Embarrassment | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Mental Health | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Travel | 2 | 835 | Mean Difference (IV, Fixed, 95% CI) | ‐7.27 [‐11.07, ‐3.48] |
| 8.4 Restroom | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.5 Shopping | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 Social life | 1 | 474 | Mean Difference (IV, Fixed, 95% CI) | ‐4.50 [‐8.38, ‐0.62] |
| 8.7 Physical activity | 1 | 475 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐7.21, 2.21] |
| 8.8 Emotional health | 1 | 473 | Mean Difference (IV, Fixed, 95% CI) | ‐4.9 [‐9.06, ‐0.74] |
| 8.9 Global Assessment of disease state | 1 | 361 | Mean Difference (IV, Fixed, 95% CI) | ‐10.51 [‐17.20, ‐3.82] |
| 9 Withdrawal due to adverse events | 20 | 7576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.36] |
| 10 Dry mouth | 27 | 9732 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [2.70, 3.34] |
1.1. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 1 Patient perception of cure or improvement.
1.2. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 2 Leakage episodes in 24 hours.
1.3. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 3 Leakage episodes in 24 hours.
| Leakage episodes in 24 hours | |
|---|---|
| Study | |
| Zinner 2004 | Treatment group: decrease of 59% from baseline at 12 weeks Placebo Group: decrease of 44% from baseline at 12 weeks |
1.4. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 4 Micturitions in 24 hours.
1.5. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 5 Micturition in 24 hours.
| Micturition in 24 hours | |
|---|---|
| Study | |
| Landis 2004 | Treatment group: median of 9.85 voids (range 2.28 to 51.28) Placebo group: median of 10.57 voids (range 2 to 37.42) |
| Zinner 2004 | Treatment group: decrease of 2.37 voids from baseline at 12 weeks Placebo group: decrease of 1.29 voids from baseline at 12 weeks |
1.6. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 6 Quality of life.
1.7. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 7 Quality of life at the end.
1.8. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 8 Change in quality of life‐IIQ‐7.
1.9. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 9 Withdrawal due to adverse events.
1.10. Analysis.
Comparison 1 Anticholinergics versus placebo, Outcome 10 Dry mouth.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abrams 1996.
| Methods | RCT. Placebo controlled, parallel design. Phase II. Double‐blind. Masking of assessors not stated. PP analysis. Multicentre. | |
| Participants | 82 patients. Inclusion criteria: Objective signs of neurological disease and urinary frequency or incontinence and urodynamically proven detrusor hyperreflexia. Exclusion criteria: Treatment within preceding 14 days with other anticholinergic drugs. | |
| Interventions | Group 1: placebo (n=15) Group 2: tolterodine 0.5mg bid (n=12) Group 3: tolterodine 1mg bid (n=14) Group 4: tolterodine 2mg bid (n=16) Group 5: tolterodine 4mg bid (n=10) 14 day treatment period. 2 week runin. | |
| Outcomes | Number of leakage episodes, frequency of micturition, volume voided. Urodynamic parameters. Adverse events. Laboratory tests. ECG. Blood pressure. | |
| Notes | Abstract. Dose reduction permitted within first week. Dropouts not stated. Incomplete subjective data. No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Abrams 1998.
| Methods | RCT. Placebo controlled, parallel design. Phase III. Randomised 2:2:1 Double‐blind. Masking of assessors not stated. ITT analysis. Multicentre (42) Multinational (3). | |
| Participants | 293 male and female patients. Inclusion criteria: At least 18 years old with urodynamically confirmed OB, increased frequency of micturition (at least 8/24 hours) UI (at least 1/24 hours) and/or urgency Exclusion criteria: Clinically significant SI, detrusor hyperreflexia, hepatic, renal or haemotological disorders, symptomatic or recurrent UTI, BOO, bladder training or electrostimulation therapy, indwelling catheter or self catheterisation, pregnant or breastfeeding or women not using reliable contraception. | |
| Interventions | Group 1: placebo (n=57) Group 2: tolterodine 2mg bid (n=118) Group 3: oxybutynin 5mg tid (n=118) 12 week treatment period. 1 week runin. | |
| Outcomes | Symptom questionnaire ( 6 point rating severity scale) Number of leakage episodes, frequency of micturition, volume voided. Adverse events. Laboratory tests. Blood pressure. | |
| Notes | Dose reduction to prevent withdrawal. Two week follow up. 37 dropouts (Group 1: 7, Group 2: 10, Group 3: 20) Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Abrams 2001.
| Methods | RCT. Placebo controlled, parallel design. Randomised 2:1. Double‐blind. Masking of assessors not stated. Multicentre. Multinational. | |
| Participants | 221 male patients. Inclusion criteria: Men over 40 years with urodynamically verified overactive bladder and mild, moderate or severe BOO. Exclusion criteria: Concurrent treatment with 5alpha‐reductase inhibitors or alpha‐adrenergic antagonists, baseline postvoid residual >40% maximum cystometric capacity, prior prostate or bladder surgery. | |
| Interventions | Group 1: tolterodine 2mg bid (n=149) Group 2: placebo (n=72) 12 week treatment period. | |
| Outcomes | Urodynamic parameters. Adverse events. | |
| Notes | Abstract. 28 dropouts (Group 1: 16, Group 2: 12). No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Alloussi 1998.
| Methods | RCT. Placebo controlled, parallel design. Randomised 2:1. Double‐blind. Masking of assessors not stated. PP analysis. Multicentre (46) Multinational (3). | |
| Participants | 309 male and female patients. Inclusion criteria: At least 18 years old with written, informed consent, confirmed DO by medical history and urodynamics, for mixed incontinence, motor component to be dominant and accompanied by at least one unstable contraction at minimum 10cm H2O with simultaneous urge or urge incontinence. Maximum cystometric capacity <350ml. Exclusion criteria: Pregnant or breastfeeding women, urological or gynaecological surgeries <3 months previous, neurogenic detrusor hyperactivity, exclusive stress incontinence, closed‐angle glaucoma, untreated tachycardiac dysrhythmia, gastrointestinal stenoses, myasthenia gravis, UTI, allergies and/or intolerance towards atropine, oxybutynin, trospium chloride, or tablet adjuvants. Patients treated with anticholinergics, tri‐ or tetracyclic antidepressants, calcium antagonists started >3 months before study, or beta‐sympathomimetics within 7 days before first urodynamic measurement, antihistamines, amantadine, quinidine and disopyramide disallowed. | |
| Interventions | Group 1: trospium chloride 20mg bid (n=210) Group 2: placebo (n=99) 3 week treatment period. 7 day run‐in. | |
| Outcomes | Assessment of patient improvement. Micturition diaries for 2 days during study. Urodynamic parameters. Adverse events. Laboratory tests. | |
| Notes | 47 dropouts ( Group 1: 32, Group 2: 15). No follow up. Micturition diaries done inconsistently and only a few available at end of study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bagger 1985.
| Methods | RCT. Placebo controlled, cross‐over design. Double‐blind. Masking of assessors not stated. ITT analysis. | |
| Participants | 18 female patients. Inclusion criteria: Urge incontinence as defined by the International Continence Society. Exclusion criteria: Recent cystitis, pregnancy, vaginal vault prolapse and stress incontinence. Major neurological disorders. Intake of drugs with presumed effect on bladder function. | |
| Interventions | Treatment 1: placebo Treatment 2: emepronium carrageenate 500mg per day Treatment 3: emepronium carrageenate 1000mg per day. 2 week period for each treatment. Washout?? | |
| Outcomes | Number of leakage episodes, frequency of micturition, volume voided. Adverse events. Laboratory tests. | |
| Notes | 1 dropout (Treatment 2). No follow up. Data not in useable form for this review. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bono 1982.
| Methods | RCT. Placebo controlled, cross‐over design. Assessors masked. ITT analysis. | |
| Participants | 16 male and female patients Inclusion criteria: Non‐pregnant with cystometric diagnosis or symptoms of bladder pain, hesitancy, dysuria, urgency, urge incontinence, stress incontinence, frequency, nocturia. Exclusion criteria: UTI or other urinary pathology. | |
| Interventions | Treatment 1: oxybutynin 5mg tid Treatment 2: placebo 2 x 10 day treatments. | |
| Outcomes | Urodynamic parameters. Adverse events. | |
| Notes | Translated from Italian. 1 dropout No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Burgio 1998.
| Methods | RCT. Placebo controlled, parallel design. Stratified by type (urge, mixed) and whether incontinence mild, moderate or severe. Randomisation within each stratum. Assessors masked. ITT analysis. | |
| Participants | 197 female patients. Inclusion criteria: community dwelling, ambulatory and at least 55 years of age. Urge incontinence at least twice per week and for at least 3 months. Urge incontinence to be the predominant pattern (number of urge accidents to exceed number of stress accidents). Urodynamic evidence of bladder dysfunction. Exclusion criteria: continual leakage, postvoid residual >200 ml, uterine prolapse, narrow‐angle glaucoma, unstable angina, decompensated congestive heart failure, history of malignant arrhythmias, or impaired mental status (MMSE score <20) | |
| Interventions | Group 1: pelvic floor muscle training/behavioral training with or without biofeedback (n=65) Group 2: oxybutynin 2.5‐5mg tid (n=67) Group 3: placebo (n=65) 8 week treatment period. | |
| Outcomes | Patient satisfaction and perceptions of treatment. Leakage episodes. Adverse events. | |
| Notes | Dose reduction allowed to prevent withdrawal. 28 dropouts (Group 1: 4, Group 2: 12, Group 3: 12) No follow up. Support from health research grant declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cardozo 2000.
| Methods | RCT. Placebo controlled, parallel design. Phase III. Double‐blind. Masking of assessors not stated. ITT and PP analyses. Multicentre (16) Multinational (3). | |
| Participants | 208 male and female patients. Inclusion criteria: 18‐70 years old, normal body weight, vital signs and age‐appropriate ECG findings. Confirmed DI. Exclusion criteria: SI confirmed by medical history, closed‐angle glaucoma, tachydysrhythmias, mechanical stenoses of the gastrointestinal tract or urinary outlet obstruction, myastheenia gravis, allergies, and other severe diseases. Concomitant treatment with other anticholinergics, antidepressants, alpha‐blockers and beta‐sympathomimetics not allowed. | |
| Interventions | Group 1: trospium chloride 20mg bid (n = 104) Group 2: placebo (n = 104) 3 week treatment period. 7 day runin. | |
| Outcomes | Four‐point score for improvement. Four‐point scale for medication acceptability. Urodynamic parameters. Adverse events. Laboratory tests. ECG. Physical examination, vital signs. | |
| Notes | Dropouts not stated. No follow up. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Cardozo 2004.
| Methods | RCT. Placebo controlled, parallel design. Phase III. Double‐blind. Masking of assessors not stated. PP analysis. Multicentre. | |
| Participants | 907 male and female patients. Inclusion criteria: More than 18 years old, Average urinary frequency of more than 8 or more times in 24 hours and atleast 3 episodes of urgency and /or 3 episodes of urinary incontinence during 3 day micturition diary. Normal body weight, vital signs and age‐appropriate ECG findings. Confirmed DI. Exclusion criteria: Neurogenic bladder, Outlet obstruction, Urinary retention, Bladder stone, stress Incontinence, UTI.Int.cystitis, Pelvic radiation, Diabetic neuropathy,use of concomitant anticholinergics. | |
| Interventions | Group 1: solifenacin 5 mg (n = 286) Group 2: solifenacin 10 mg (n = 290) Group 3: placebo (n= 281) 12 week treatment period. 2 week run in. | |
| Outcomes | Change in baseline in mean number of micturition per 24 hours. Change from baseline in mean number of urgency episodes, Change in urge incontinence and voulme voided per micturition. Safety assessment Adverse events. Laboratory tests. ECG. Blood pressure. | |
| Notes | Primary reasons for discontinuation mentioned Loss to follow up mentioned Power calculation done, Qaulity of life reported subsequently favours solifenacin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chaliha 1998.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. Multicentre. | |
| Participants | 76 participants. Inclusion criteria: Low compliance bladder, urodynamically confirmed DO. | |
| Interventions | Group 1: trospium chloride 10mg bid Group 2: trospium chloride 20mg bid Group 3: trospium chloride 40mg bid Group 4: placebo 21 day treatment period. | |
| Outcomes | Tolerability score. Urodynamic parameters. Adverse events. Biochemical analyses. | |
| Notes | Abstract. Numbers for each group not stated. Dropouts not stated. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chapple 2004a.
| Methods | RCT. Placebo controlled, parallel design. Phase II. Double‐blind. Masking of assessors not stated. Multicentre. | |
| Participants | 225 male and female patients. Inclusion criteria: Age between 18‐80 years; Idiopathic DI; >8 voids/24 hours for 3 days; 3 episodes of incontinence or urgency during three day of mict.diary Exclusion criteria: Neurogenic bladder, Outlet obstruction, Urinary retention, Bladder stone, stress Incont, UTI.Int.cystitis,Pelvic radiation, Diabetic neuropathy,use of concomitant anticholinergics. | |
| Interventions | Group 1: solifenacin 2.5 mg (n = 40) Group 2: solifenacin 5 mg (n = 37) Group 3: solifenacin 10 mg (n=33) Group 4: solifenacin 20mg (n=34) Group 5 : placebo (n=36) Group 6: tolterodine 2mg (n=37) 12 week treatment period. 2 week run‐in. | |
| Outcomes | Change in baseline in mean number of micturition per 24 hours. Change from baseline in mean number of urgency episodes, Change in urge incontinence and volume voided per micturition. Total sum score of Contilife domains and overall Contilife score Adverse events. Laboratory tests. ECG. Blood pressure. | |
| Notes | Loss to follow up or failure to complete study (Gr.1 5; Gr.2 3, Gr.3 7, Gr.4 7 Gr.5 6 and Gr.6 5) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chapple 2004b.
| Methods | RCT. Placebo controlled, parallel design. Phase II. Double‐blind. Masking of assessors not stated. Multicentre. | |
| Participants | 1081 male and female patients. Inclusion criteria: Age more than18 years; DI more than 3 months; >8 voids/24 hours for 3 days; 5‐50 episodes of incontinence or urgency during 1 week,urgency 1 episode/24 hours Exclusion criteria: BladderOutlet obstruction symptoms stabilized over 6 months, pregnancy,Post void residual >200ml; contra indications to anticholinergics, Hypersensitivity to drug | |
| Interventions | Group 1: solifenacin 5 mg (n = 266) Group 2: solifenacin 10 mg (n = 264) Group 3: tolterodine 2mg (n= 250) Group 4: placebo (n= 253) 12 week treatment period. 2 week run in. | |
| Outcomes | Using electronic diaries, Change in baseline in mean number of micturition per 24 hours. Change from baseline in mean number of urgency episodes, Change in urge incontinence and volume voided per micturition. Adverse events. Laboratory tests. ECG. Blood pressure. | |
| Notes | Women committed to use of contraceptives during pregnancy were recruited Bladder training not allowed during study, Concomitant use of drugs modifying liver enzymes not permitted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Coombes 1996.
| Methods | RCT. Placebo controlled, Cross over design. Double‐blind Masking of assessors stated. PP analysis. | |
| Participants | 20 male and female patients. Inclusion criteria: Age >18,symptomatic urgency or incontinenceDI on videourodynamicNo neurological diseaseNo stress incontinence Exclusion criteria: Gi obstructionAllergy to drug, pregnancy. | |
| Interventions | Group 1: penthienate 5 mg TDS (n = 19) Group 2: placebo (n=19) Group 3: propantheline (n=23) 4week treatment period with 5 days wash out period 1 week run in. | |
| Outcomes | Continence and urodynamic characteristics Adverse events. Laboratory tests. ECG. Blood pressure. | |
| Notes | Dose reduction allowed No urodynamics in second phase Data could not be used as it has been reported as median. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Davila 2001.
| Methods | RCT. Placebo controlled, parallel design. Phase III. Double‐blind. Masking of assessors not stated. | |
| Participants | 520 male and female patients. Inclusion criteria: At least 10 urge leakages per week; at least 8 voids per day; average urinary volume not >350 ml. | |
| Interventions | Group 1: placebo (n=?) Group 2: transdermal oxybutynin 1.3mg/day 2x week (n=?) Group 3: transdermal oxybutynin 2.6mg/day 2x week (n=?) Group 4: transdermal oxybutynin 3.9mg/day 2x week (n=?) 12 week treatment period. | |
| Outcomes | Number of leakage episodes, frequency of micturition, volume voided. QOL scores (IIQ, UDI, SF36) Adverse events. Laboratory tests. | |
| Notes | Abstract. Numbers for each group not stated. 73 dropouts (group not stated) No follow up. Data not in useable form for this review. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Di Stasi 2001a.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Masking of assessors not stated. | |
| Participants | 10 patients with hyperreflexia unresponsive to standard oral and intravesical oxybutynin regimens. Inclusion criteria: Unacceptable detrusor activity suppression (clinical and urodynamic) by oral and intravesical passive diffusion of oxybutynin, intolerable systemic side effects from oral oxybutynin, bladder capacity at least 120ml, no vesicoureteral reflux. One month washout of anticholinergic medications. No UTIs. | |
| Interventions | Treatment 1: placebo Treatment 2: oral oxybutynin (5mg single dose) Treatment 3: control intravesical with passive diffusion Treatment 4: intravesical oxybutynin 5mg/100ml with passive diffusion Treatment 5: control intravesical with electromotive administration Treatment 6: intravesical oxybutynin 5mg/100ml with electromotive drug administration. 6 x 8 hour sessions at weekly intervals. | |
| Outcomes | Urinary leakage, bladder volume. Urodynamic hyperreflexic episodes. Laboratory tests. | |
| Notes | Outcomes and population different from other studies. No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dmochowski 2003.
| Methods | CT. Placebo controlled, parallel design. Double‐blind, Masking of participants present Power calculation present | |
| Participants | 361 male and female patients. Inclusion criteria: >18 yearBeneficial response to pre study treatment Exclusion criteria: Lower urinary tract surgery in last 6 monthsInt.cystitisUrethral syndrome, Painful bladder syndrome, Overflow incontinence | |
| Interventions | Group 1: oxybutinin transdermal 3.9mg (121) Group2: oxybutinin 4 mg (n=123) placebo (n=117) 12 weeks study period | |
| Outcomes | Change in baseline of incontinence episodes/day; daily urinary frequency; urinary volume per void Quality of life | |
| Notes | Method of randomisation not described 41 patients did not complete treatment Quality of life data reported as median and hence not used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dorschner 2000.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. Multicentre, Multinational (2). | |
| Participants | 107 patients (male and female) Mean age 67. Inclusion criteria: Over 60 years of age; >7 micturitions/24 hours, >0 incontinence episodes/24 hours, <300ml volume/micturition Exclusion criteria: Acute UTI's, bladder emptying disorders, residual urine >20% voided volume, micturition volume >300ml. Renal insufficiency, concomitant medication interfering with the study drug, clinically relevant variations in laboratory parameters prior to study. Patients suffering from serious, life‐threatening cardiovascular diseases. | |
| Interventions | Group 1: placebo (n=49) Group 2: propiverine 15mg tid (n=49) 4 week treatment period. 2 week run‐in. | |
| Outcomes | Urge score (Gaudenz). Number of leakage episodes, frequency of micturition, bladder volume. Quality of Life (Giessen Complaint Survey and Basle Subjective Wellbeing Study). Uroflow. Residuals. Adverse events. ECG (standard and 24hr long term). Laboratory tests. | |
| Notes | 9 dropouts (Group not stated). No follow up. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Drutz 1999.
| Methods | RCT. Placebo and comparator controlled, parallel design. Randomised 1:2:2. Double‐blind. Masking of assessors not stated. PP analysis. Multicentre (25). Multinational (2). | |
| Participants | 277 patients (male and female) Mean age 64. Inclusion criteria: At least 18 years old, understood and signed informed consent. Females to be post menopausal, surgically sterile or using adequate contraception. Cystometric evidence of detrusor overactivity plus urinary frequency (at least 8/day) and either UI (at least 1/24 hours) and/or urgency. Exclusion criteria: Clinically evaluated stress incontinence, hepatic or renal disease, diseases that made patient unsuitable for study, recurrent UTI, interstitial cystitis, uninvestigated haematuria or haematuria secondary to malignant disease, indwelling catheter or intermittent catheterization, treatment with any investigational drug in 2 months pre entry, previous treatment with tolterodine, electrostimulation or bladder training within 14 days of entry, treatment with any anticholinergic drug or urge incontinence drug within 14 days, unstable dosage of any treatment with anticholinergic, adverse effects or initiation of such treatment during study, previously serious adverse effects on oxybutynin, average total voided/24 hours >3000ml, clinically significant voiding difficulty with risk of urinary retention (residual volume >200ml or flow rate <10 ml/s). | |
| Interventions | Group 1: placebo (n=56) Group 2: tolterodine 2mg bid (n=109) Group 3: oxybutynin 5mg tid (n=112) 12 week treatment period. | |
| Outcomes | Number of leakage episodes, frequency of micturition and volume voided. Adverse events. Laboratory tests. Blood pressure. | |
| Notes | Dose reduction permitted within first 2 weeks only as alternative to withdrawal. 57 dropouts (Group 1: 8, Group 2: 14, Group 3: 35) 36% placebo, 36% tolterodine and 63% oxybutynin patients were excluded from the analysis. No follow up. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Freeman 2003.
| Methods | RCT.Multi‐institutional and multinational. Placebo controlled,double blind,parallel design. | |
| Participants | 772 male and female patients inclusion criteria: >18 years. Urinary freq. more than 8 times in 24 hours. Urge incontinence 5 times in a week. Exclusion criteria: Lower urinary tract surgery in last 6 monthsInt.cystitisUrethral syndrome, Painful bladder syndrome, Overflow incontinence Total urinary volume of more than 3 litres. Significant hepatic and renal insufficiency. Pregnant women | |
| Interventions | Group 1: tolterodine ER 4 mg once a day Group2: placebo. | |
| Outcomes | No. of incontinence episodes No. of micturitions in 24 hours Volume voided per micturition | |
| Notes | The data is reported in mean and range and hence can not be analysed by meta analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Goode 2002.
| Methods | RCT. Placebo controlled, parallel‐design study. | |
| Participants | 197 women were randomised into three arm study Inclusion criteria: Age > 55 years; History of urge or mixed urinary incontinence, Ambulatory patient, DI on urodynamics Exclusion criteria: Neurogenic bladderOutlet obstructionUrinary retentionBladder stone, stress Incont, UTI.Int.cystitisPelvic radiation, Diabetic neuropathy,use of concomitant anticholinergics. | |
| Interventions | Group1: behaviour treatment Group2 : oxybutinin 2.5 mg Group 3: placebo. | |
| Outcomes | Incontinence episodes Cystometeric capacity. | |
| Notes | Main comparison is behavioural treatment and not sufficient data is reported. Urodynamic data is combined from another paper by same authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Haab 2004.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Description of randomisation present Concealment of allocation present Masking of participants present Power calculation present | |
| Participants | 561 male and female patients. Inclusion criteria: >18 years but <88, OAB symptoms for more than 6 months, urge incontinence 0‐5 episodes/week, ability to fill up micturition charts Exclusion criteria: Contra indications to anticholin; Stress Incont; BOO with residual more than 200 ml; Hypersensitivity to medications. | |
| Interventions | Group 1: placebo (n=164) Group 2: darifenacin 3.75mg tid (n=53) Group 3: darifenacin 7.5mg (n=229) Group 4: darifenacin 15 mg (n= 115) 12 week treatment period. 2 week run in. | |
| Outcomes | Number of incontinence episodes per week, frequency of micturition, frequency of urgency, Nocturnal awakening, incontinence episodes resulting in change of pads, volume voided. Adverse events. Laboratory tests. Blood pressure. | |
| Notes | 8 patients reported side effects (Group 1= 1, Gr.2= 6 Gr.4=1) One patient had serious av heart block, Data reported in median with no SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Halaska 1994.
| Methods | RCT. Placebo controlled, parallel group. Double‐blind. Masking of assessors not stated. | |
| Participants | 93 patients (male and female) Inclusion criteria: Over 60 years old, with urgency or urge incontinence, micturition volume 100‐300ml, residual volume >20% of micturition volume at visit 1. | |
| Interventions | Group 1: placebo (n=47) Group 2: propiverine 15mg tid (n=46) 28 day treatment period. 14 day run‐in. | |
| Outcomes | Number of leakage episodes, volume voided, urgency. Uroflows, urological investigations, psychometric evaluations. Laboratory tests. Gaudenz questionnaire. | |
| Notes | Abstract. No follow up. Dropouts not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Homma 2003.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. | |
| Participants | 608 patients (male and females) inclusion criteria: >20 years. Urinary freq more than 8 times in 24 hours. Urge incontinence 5 times in a week,symptoms of overactive bladder more than 6 months Exclusion criteria: stress urinary incontinence, urinary volume of more than 3 l, average volume per void of more than 200 ml, Significant hepatic and renal insufficiency. Pregnant women. | |
| Interventions | Group 1: placebo (n= 122) Group 2: tolterodine ER 4 mg (n=239) oxybutinin IR 3 mg (n= 244) 12 weeks treatment period. 1‐2 weeks wash out/ run in period. | |
| Outcomes | Number of leakage episodes, frequency of micturition, volume voided. Adverse events. patient quality of life. Laboratory tests. Blood pressure. Compliance by pill count. | |
| Notes | Quality of life reported in a separate publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jacquetin 2001.
| Methods | RCT. Placebo controlled, parallel group. Phase III. Double‐blind. Masking of assessors not stated. Multicentre, Multinational (4). | |
| Participants | 251 male and female patients. Inclusion criteria: Over 18 years old with urodynamically verified detrusor overactivity, symptoms of urinary frequency and either urge incontinence or urgency or both. Exclusion criteria: SI, hepatic or renal disease, symptomatic or recurrent UTI, interstitial cystitis, haematuria, clinically significant voiding difficulty, patients receiving bladder training, electrostimulation, or having an indwelling catheter or intermittent catheterisation; pregnant or breastfeeding women or women of childbearing age who were not using reliable contraception. | |
| Interventions | Group 1: placebo (n=51) Group 2: tolterodine 1mg bid (n=97) Group 3: tolterodine 2mg bid (n=103) 4 week treatment period. 2 week run‐in. | |
| Outcomes | Number of leakage episodes, frequency of micturition, volume voided. Adverse events. Laboratory tests. Blood pressure. Compliance by pill count. | |
| Notes | 6 dropouts (Group 1: 1, Group 2: 3, Group 3: 2) 2 week follow up. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Jonas 1997.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. Multicentre (58), Multinational(3). | |
| Participants | 242 patients (male and female) Mean age 58. Inclusion criteria: At least 18 years old with detrusor overactivity and evidence of frequency in combination with UI, urinary urgency or both. Exclusion criteria: Significant stress incontinence, hepatic or renal disease, any condition contraindicating anticholinergic therapy, recurrent UTI's, interstitial cystitis, uninvestigated haematuria or clinically significant voiding difficulty with risk of urinary retention. Patients on any anticholinergic treatment or using an indwelling catheter or who had electrostimulation or bladder training in the 14 days prior to inclusion visit. | |
| Interventions | Group 1: placebo (n=44) Group 2: tolterodine 1mg bid (n=99) Group 3: tolterodine 2mg bid (n=99) 4 week treatment period. 2 week run‐in. | |
| Outcomes | Urodynamic parameters. Adverse events. Laboratory tests. Blood pressure. | |
| Notes | 10 dropouts (Group 1: 3, Group 2: 4, Group 3: 3) No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Junemann 1999.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. ITT and PP analyses. Multicentre (2). | |
| Participants | 175 patients with urge syndrome. Inclusion and exclusion criteria not stated. | |
| Interventions | Group 1: trospium chloride 40mg qid (n=56) Group 2: trospium chloride 2 x 40mg qid (n=56) Group 3: placebo (n=58) 3 week treatment period. | |
| Outcomes | Frequency of micturition. Urodynamic parameters. Adverse events. | |
| Notes | Abstract. Dropouts not stated. No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Junemann 2000.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. ITT analysis. Multicentre. Multinational (3). | |
| Participants | 234 patients Inclusion criteria: Urge syndrome (motor urge, sensory urge and combined motor urge and stress incontinence) verified by urodynamics. | |
| Interventions | Group 1: trospium chloride 2 x 20mg daily (n=76) Group 2: tolterodine 2 x 2mg daily (n = 77) Group 3: placebo (n=79) 3 week treatment period. 10 day run‐in. | |
| Outcomes | Frequency of micturition. Adverse events. Laboratory tests. Physical examinations. | |
| Notes | Abstract. Dropouts not stated. No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Khullar 2004.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Randomisation provided by permuted blocks Multicentre(101). | |
| Participants | Inclusion criteria: >18 years age; 8 mic.episodes/24 hours; 5 episodes of urge incont. /week, urgency Exclusion criteria: Stress incontinence, total urine volume> 3L Renal pathology, UTI, Bladder outflow obstruction, PregnancyAny contraindication to anticholinergics | |
| Interventions | Group 1: Tolteradine 4mg Group 2: placebo (n = 104) 8 week treatment period. 7 day run‐in. | |
| Outcomes | Mict. Dairies used to assess any change in urge incont.episodes, micturition frequency, Change in bladder perception using a validated 6‐point rating scale (1 no prob 2, very minor prob 3, minor prob 4, Moderate prob 5, Severe prob 6, Many severe prob) Tolerability assessed Adverse events Quality of life | |
| Notes | Analysis was performed on an intent to treat basis Power calculation done (80%0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kramer 1987.
| Methods | RCT. Crossover design. Double‐blind. Masking of assessors not stated. Multicentre (2). | |
| Participants | 60 male and female patients. Inclusion criteria: Weight 56‐85kg, frequency, urgency or urge incontinence confirmed by symptoms or urodynamics. Exclusion criteria: Kidney, liver or circulatory disease, UTI, use of anticholinergics, glaucoma, Parkinson's disease. | |
| Interventions | Group 1: Treatment 1: emepronium 200mg tid; Treatment 2: oxybutynin 5mg tid; Treatment 3: placebo (n=30) Group 2: Treatment 1: emepronium 200mg tid; Treatment 2: flavoxate 200mg tid; Treatment 3: placebo (n=30) Treatment consisted of two active drugs and placebo taken at random during consecutive 3 week periods. | |
| Outcomes | Subjective outcomes. Urodynamic parameters. Adverse events. | |
| Notes | Translated from Flemish. 19 dropouts ( Group 1: 10, Group 2: 9) No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Landis 2004.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. Multicentre. Multinational. | |
| Participants | 1,529 patients were randomised inclusion criteria: >18 years. Urinary freq. more than 8 times in 24 hours. Urge incontinence 5 times in a week. Exclusion criteria: Lower urinary tract surgery in last 6 monthsInt.cystitisUrethral syndrome, Painful bladder syndrome, Overflow incontinence Total urinary volume of more than 3 litres. Significant hepatic and renal insufficiency. Pregnant women | |
| Interventions | Tolteradine ER Tolteradine IR Placebo. | |
| Outcomes | Number of incontinence episodes per week, frequency of micturition, frequency of urgency, Nocturnal awakening, incontinence episodes resulting in change of pads, volume voided. Adverse events. Laboratory tests. Blood pressure. | |
| Notes | Data reported in median and hence suitable for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Madersbacher 1999.
| Methods | RCT. Placebo controlled, parallel design. Randomised 2:2:1. Double‐blind. Masking of assessors not stated. ITT analysis. Multicentre (32). Multinational (2). | |
| Participants | 366 male and female patients Inclusion criteria: History of urgency or urge incontinence, maximum cystometric bladder capacity at least 300 ml, at least 18 years old and body weight at least 45kg. Exclusion criteria: Detrusor hyperreflexia, postoperative (bladder) incontinence, intravesical obstruction, post void residual urine >15% maximum cystometric bladder capacity, acute UTIs, angina pectoris, glaucoma, megacolon, clinically relevant cardiac, renal or hepatic dysfunctions, tachy/dysrhythmias, frequency or nocturia due to heart or renal insufficiency, or overt cerebral sclerosis. Use of other spasmolytics or anticholinergics, Beta‐sympathomimetics, calcium antagonists, dopamine agonists, prolactin inhibitors, prostaglandin synthesis inhibitors, striated muscle relaxants or medication for Parkinsonism. | |
| Interventions | Group 1: propiverine 15mg tid (n=149) Group 2: oxybutynin 5mg bid (n=145) Group 3: placebo (n=72) 4 week treatment period. 7 day run‐in. | |
| Outcomes | Frequency of micturition, urgency. Urodynamic parameters. Clinical symptoms and overall assessment documented by physicians. Incontinence questionnaire (Gaudenz). | |
| Notes | 42 dropouts ( Group 1: 19, Group 2: 16, Group 3: 7) No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Malone‐Lee 2001.
| Methods | RCT. Placebo controlled, parallel design. Randomised 3:3:2. Double‐blind. Masking of assessors not stated. ITT analysis. Multicentre (26). Multinational (3). | |
| Participants | 177 male and female patients. Inclusion criteria: At least 65 years old with 8 or more voids per 24 hours and/or urge incontinence at least 1 per 24 hours. Exclusion criteria: "standard". | |
| Interventions | Group 1: placebo (n=43) Group 2: tolterodine 1mg bid (n=61) Group 3: tolterodine 2mg bid (n=73) 4 week treatment period. 14 day run‐in. | |
| Outcomes | Number of leakage episodes, frequency of micturition, volume voided. Adverse events. Laboratory tests. ECG. Compliance by pill count. | |
| Notes | 12 dropouts (Group 1: 1, Group 2: 4, Group 3: 7) Two week follow up for adverse events. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Massey 1986.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Masking of assessors not stated. | |
| Participants | 72 female patients. Inclusion criteria: Cystometrically proven detrusor instability. Exclusion criteria: Bacteriuria or concomitant medication likely to affect urinary tract such as beta blockers or diuretics. | |
| Interventions | Treatment order randomised into groups between placebo (n=67) and low dose emepronium (1200mg/day), medium dose emepronium (1600mg/day) or high dose emepronium (2000mg/day) All patients took 2.5 tablets qid at all stages. The titration period was 1 week at 1600mg/day. No washout. Each treatment period was 28 days. | |
| Outcomes | Number of leakage episodes, frequency of micturition, voided volume. Urodynamic parameters. Adverse events. Residuals. Laboratory tests. | |
| Notes | 24 dropouts. No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Meyhoff 1983.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Masking of assessors not stated. | |
| Participants | 19 female patients Inclusion criteria: Cystometric evidence of detrusor instability; absent or minimal bladder suspension defect; max urinary flow above 15ml/s; residual <50ml; MSU <100,000 colonies/ml; no bladder stone or tumour by cystoscopy. Exclusion criteria: Severe heart failure or glaucoma. Neurological disease or detrusor sphincter dyssynergia. Drugs taken during study affecting autonomous nervous system or smooth muscles. | |
| Interventions | Treatment 1: emepronium bromide 200mg qid Treatment 2: flavoxate chloride 200mg qid Treatment 3: placebo Each treatment period 14 days with 7 day washout between. | |
| Outcomes | Patient drug preferences. Number of leakage episodes, frequency of micturition, nocturia. Adverse events. | |
| Notes | Data not in useable form for this review. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Millard 1999.
| Methods | RCT. Placebo controlled, parallel design. Phase III. Randomised 1:2:2. Double‐blind. Masking of assessors not stated. ITT analysis. Multicentre. Multinational (2). | |
| Participants | 316 patients (male and female) Inclusion criteria: At least 18 years old, with cystometrically proved detrusor overactivity (idiopathic or hyper‐reflexia or contractions with an amplitude at least 10cm H2O); at least 8 voids/24 hours; at least 1 incontinent episode/24 hours and/or urinary urgency, Premenopausal women required to use adequate contraception. Excusion criteria: SI (cough test), clinically significant voiding difficulty, recurrent UTIs, interstitial cystitis, uninvestigated haematuria or any bladder cancer, indwelling catheter or self catheterisation, hepatic or renal disease, narrow angle glaucoma, electrostimulation or bladder training or anticholinergic drug initiated 14 days before or any time during study, unstable dose of any treatment with anticholinergic side effects, average total voided volume >3000ml/24 hours, treatment with any other investigational drug during or 2 months pre study. | |
| Interventions | Group 1: placebo (n=64) Group 2: tolterodine 1mg bid (n=123) Group 3: tolterodine 2mg bid (n=129) 12 week treatment period. 2 week run‐in. | |
| Outcomes | Cured incontinence and complete cure. Patient rating of bladder condition (6 point Likert). Leakage episodes, frequency of micturition. Achievement of normal voiding frequency (< 8/day) Adverse events. Laboratory tests. ECG. Blood pressure. Compliance by pill count. | |
| Notes | No dose reductions permitted. 25 dropouts ( Group 1: 3, Group 2: 7; Group 3: 15) No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Moisey 1980.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Masking of assessors not stated. | |
| Participants | 30 male and female patients. Inclusion criteria: Proven detrusor instability, either idiopathic or neurogenic. | |
| Interventions | Treatment 1: placebo Treatment 2: oxybutynin 5mg tid Patients were divided into three groups each reported separately: Group 1: idiopathic group (n=12) Group 2: Post‐obstructive group (n=4) Group 3: neurogenic group (n=7) Consecutive 28 day courses. | |
| Outcomes | Subjective assessment. Urodynamic parameters. Adverse events. | |
| Notes | 7 dropouts. No follow up. Patients studied included those with established neurological disease and others who had failed to respond to other treatments. Data not in useable form for this review. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Moore 1990.
| Methods | RCT. Placebo controlled crossover study. Stratified according to severity of detrusor contractions. Double‐blind. Masking of assessors not stated. | |
| Participants | 53 female patients. Inclusion criteria: Involuntary detrusor contractions >30cm H20 during filling phase. Exclusion criteria: Coexisting stress incontinence, low compliance bladder, bacterial or interstitial cystitis, >75 years old, previous oxybutynin treatment, neurological or other urological disorders. | |
| Interventions | Treatment 1: oxybutynin 3mg tid (n=28) Treatment 2: placebo (n=25) 2 x 4 week treatment period with 4 week washout between treatment periods. | |
| Outcomes | Symptomatic improvement recorded for urgency and urge incontinence. Frequency of micturition. Voided volume. Urodynamic parameters. Adverse events. | |
| Notes | Follow up at 2 monthly intervals until no more than 8 voids/day or 18 months. Low dose of oxybutynin. Basic bladder training advice given during study. Data not in useable form for this review. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Murray 1984.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Assessors masked. ITT unclear. Single centre. | |
| Participants | 25 patients. Inclusion criteria: Urodynamically proven DI. | |
| Interventions | Treatment 1: placebo Treatment 2: oxybutynin 5mg tid Treatment 3: cetiprin 400mg tid 28 day treatment with each drug with 14 day washout between treatment periods. | |
| Outcomes | Subjective assessment after each treatment. Fluid/volume chart in last week of treatment. Adverse events. | |
| Notes | Abstract. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rentzhog 1998.
| Methods | RCT. Placebo controlled, parallel design. Phase II. Double‐blind. Masking of assessors not stated. PP analysis. Multicentre (17). Multinational (2). | |
| Participants | 81 male and female patients. Inclusion criteria: Aged 18‐75 years old with symptoms of urinary urgency, increased frequency and/or urge incontinence. Urodynamically confirmed detrusor instability. Insignificant bacteriuria and normal laboratory tests. No evidence of bladder outlet obstruction Exclusion criteria: Stress incontinence or detrusor hyperreflexia, clinically significant cardiac, hepatic, renal or haematological disorders, patients with contraindications to antimuscarinic agents, pregnant or lactating women, women of childbearing age who were not using reliable contraception. | |
| Interventions | Group 1: placebo (n=13) Group 2: tolterodine 0.5mg bid (n=21) Group 3: tolterodine 1mg bid (n=16) Group 4: tolterodine 2mg bid (n=14) Group 5: tolterodine 4mg bid(n=16) 2 week treatment period. 3 week run‐in. | |
| Outcomes | Symptomatic improvement (VAS). Number of leakage episodes, frequency of micturition. Number of pads used. Urodynamic parameters. Adverse events. ECG. | |
| Notes | Dose reduction allowed to next lowest level. 16 dropouts ( Group 1: 3, Group 2: 4, Group 3: 1, Group 4: 3, Group 5: 5) Two week telephone follow up. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Riva 1984.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Masking of assessors not stated. Single centre. | |
| Participants | 30 female patients. Inclusion criteria: Bladder instability or 'sensorial' urge incontinence. Exclusion criteria: Moderate or high blood pressure, glaucoma, heart or liver disease. Study included both patients treated previously and unsuccessfully with different drugs and untreated cases. | |
| Interventions | Treatment 1: oxybutynin chloride 5mg tid Treatment 2: placebo 20 day treatment period with 10 day washout between treatment periods. | |
| Outcomes | Leakage episodes, frequency of micturition, urgency, nocturia. Urodynamic parameters. Adverse events. Laboratory tests. | |
| Notes | 6 dropouts. Dose reductions permitted. Followup unclear. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rosario 1995a.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Assessors masked. | |
| Participants | 18 patients. Inclusion criteria: Not clear. Exclusion criteria: Not clear. | |
| Interventions | Treatment 1: placebo Treatment 2: darifenacin 10mg Treatment 3: darifenacin 2.5mg Single oral dose for each treatment, one hour prior to cystometry, with 3 day minimum washout before the next treatment. | |
| Outcomes | Urodynamic parameters. Salivary flow. Laboratory tests. | |
| Notes | Abstract. No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rosario 1995b.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Assessors masked. Single centre. | |
| Participants | 6 patients (1 male, 5 female) Inclusion criteria: urge incontinence; demonstrated on videocystometrography to have detrusor instability. | |
| Interventions | Treatment 1: placebo Treatment 2: darifenacin 5mg 8 hourly. Each treatment period of seven days (6 days full dose and one day single dose) followed by ambulatory monitoring before crossover to the other treatment. | |
| Outcomes | Ambulatory urodynamic parameters. Laboratory tests. | |
| Notes | Abstract. 1 dropout. No follow up. Very small sample size. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
SerranoBrambila 2000.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Masking of assessors not stated. | |
| Participants | 44 female patients. Inclusion criteria: Unstable bladder. | |
| Interventions | Treatment 1: oxybutynin 5mg tid (n=22) Treatment 2: placebo (n=22) 2 x 6 week treatments with one week washout between treatment periods. | |
| Outcomes | Symptom questionnaire (VAS). Leakage episodes, frequency of micturition. Residuals. Urodynamic parameters. Adverse events. Laboratory tests. | |
| Notes | Translated from Spanish. 7 dropouts (treatment 1: 5, treatment 2: 2) No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Stohrer 1991.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. Multicentre (3). | |
| Participants | 61 male and female patients. Inclusion criteria: Spinal cord injury with consecutive detrusor hyperreflexia. Exclusion criteria: Urinary infections, mechanical obstruction of lower urinary tract, congestive glaucoma, known allergy to atropine, N‐butylscopolamine bromide or trospium chloride, trachyarrhythmias, renal, hepatic or cardiovascular insufficiency, body weight exceeding 90kg, anticholinergic treatment within 14 days of study start. | |
| Interventions | Group 1: trospium chloride 20mg bid (n=29) Group 2: placebo (n=32) 3 week treatment period. 2 week run‐in. | |
| Outcomes | Urodynamic parameters. Laboratory tests. Adverse events. | |
| Notes | 6 dropouts (Group 1: 2, Group 2: 4) No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Stohrer 1999.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. Multicentre. | |
| Participants | 113 male and female in‐patients Inclusion criteria: Over 18 years old with detrusor hyperreflexia and suprasacral spinal cord injury. Exclusion criteria: Pregnancy, cardiac, hepatic and renal dysfunctions, intestinal and urogenital obstructions, narrow angle glaucoma, severe psychotics and acute urinary tract infections. | |
| Interventions | Group 1: propiverine 15mg tid (n=60) Group 2: placebo (n=53) 14 day treatment period. | |
| Outcomes | Patient assessment of improvement. Micturition variables. Urodynamic parameters. Adverse events. Laboratory tests. Physician assessment of efficacy. | |
| Notes | 11 dropouts ( Group 1: 8, Group 2: 3) No follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Szonyi 1995.
| Methods | RCT. Placebo controlled, parallel group. Double‐blind. Masking of assessors not stated. | |
| Participants | 60 male and female patients. Mean age 82 years. Inclusion criteria: Over 70 years old with symptoms of urinary frequency, urgency and urge incontinence. Patients had to be mobile, able to attend outpatient department, able to keep a diary chart and able to give consent. Exclusion criteria: Urinary infections, severe hepatic or renal disease, glaucoma or uncontrolled diabetes, patients on concomitant anticholinergic therapy with imipramine or propantheline. | |
| Interventions | Group 1: oxybutynin 2.5mg bid plus bladder training (n=28) Group 2: placebo plus bladder training (n=29) 6 week treatment period. 2 week run‐in. | |
| Outcomes | Subjective evaluation of symptoms on Likert scale. Micturition variables. Adverse events. Laboratory tests. Compliance by pill count. | |
| Notes | Frail elderly (over 70 years old) living independently. Dose titration. No follow up. 13 dropouts (Group 1: 8, Group 2: 5) Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Tago 1990.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. Multicentre (41). | |
| Participants | 142 patients. Inclusion criteria: Neurogenic bladder and unstable bladder with uninhibited detrusor contraction, pollakisuria and UI. | |
| Interventions | Group 1: propiverine 20mg od (n=?) Group 2: placebo (n=?) 2 week treatment period. | |
| Outcomes | Subjective symptoms, global improvement rating, global utility rating. Urodynamic parameters. Adverse events | |
| Notes | Abstract. Group numbers not reported. Dropouts not reported. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Takayasu 1990.
| Methods | RCT. Placebo controlled, Parallel design. Double‐blind, multicentre. Masking of assessors not stated. | |
| Participants | 142 male and female recruited Inclusion criteria: >20 years age with OAB symptoms Exclusion criteria: BOO with residual urine of more than 10 ml; UTI; Glaucoma; Drug allergy,Severe heart and renal disease Lacttating women | |
| Interventions | Group 1: propiverine hydrochloride 10 mg (n=141) Group 2: placebo (n=141) 3 days run in 2 weeks treatment period. | |
| Outcomes | No. of micturition, urgency and urinary incontinence using urinary charts Cystometry and PVR measurement Adverse events Blood tests and urine analysis. | |
| Notes | Translated from Japanese 15 patients dropped out Data can not be used as it is not reported as mean with SD Reporting is done as %age reduction in symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tapp 1990.
| Methods | RCT. Placebo controlled crossover design. Double‐blind. Masking of assessors not stated. | |
| Participants | 37 neurologically normal postmenopausal women. Inclusion criteria: Systolic detrusor instability, provoked detrusor instability, low compliance bladder or a combination of these. | |
| Interventions | Treatment 1: oxybutynin 5mg four times daily Treatment 2: placebo 2 week treatment period with 2 week washout between treatment periods. | |
| Outcomes | Visual analogue symptom score for urgency, UI, SI and enuresis. Urodynamic parameters. Adverse events. | |
| Notes | 6 dropouts. 6 month follow up for drug compliance. General health questionnaire at study entry. Data not in useable form for this review. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Thuroff 1991.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. Multicentre. Multinational (2). | |
| Participants | 169 male and female patients. Inclusion criteria: At least 15 years old with symptoms of frequency, urgency and/or incontinence. Cystometry findings related to either idiopathic (unstable detrusor) or neurogenic origins (detrusor hyperreflexia). Exclusion criteria: No drugs affecting lower urinary tract function to be taken. Antihypertensive medication allowed if regularly taken at consistent dosage. Minor tranquillizers allowed if taken for sleep only. Pregnancy, congestive heart failure, severe renal/liver disease, myasthenia gravis, unable to swallow/uncooperative patient, hiatal hernia/reflux oesophagitis, gastro. tract obstruction, urinary tract obstruction, residual >50ml, untreated UTI, hyperreflexia without urge, lower urinary tract pathological conditions. | |
| Interventions | Group 1: oxybutynin 5mg tid (n=63) Group 2: propantheline 15mg tid (n=54) Group 3: placebo (n=52) 4 week treatment period. 1 week run‐in. | |
| Outcomes | Urinary symptoms (VAS). Frequency of micturition. Urodynamic parameters. Urine analysis. Laboratory tests. Adverse events. | |
| Notes | 15 dropouts (Group 1: 4, Group 2: 6, Group 3: 5) No follow up. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ulshofer 2001.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. PP analysis. Multicentre. | |
| Participants | 46 male and female patients. Mean age 52 years. Inclusion criteria: 18‐75 years old with cysometric bladder capacity >300ml, early primary urge to void (at 60% capacity) and involuntary loss of urine during filling stage of cystometry with detectable detrusor contractions. Exclusion criteria: Alcohol or drug abuse, signs and symptoms of other forms of incontinence, bodyweight >90kg, myasthenia gravis, narrow angle glaucoma, UTI, pregnancy, psychiatric disease, and pre and/or co‐medication with other antimuscarinic drugs, amantadine, quinidine, tricyclic antidepressants, antihistamines, disopyramide and beta‐adrenoceptor agonists. | |
| Interventions | Group 1: trospium chloride 15mg tid (n=25) Group 2: placebo (n=21) 28 day treatment period. | |
| Outcomes | Urodynamic parameters. Laboratory tests. Adverse events. Comliance by pill count. | |
| Notes | 7 dropouts (Group 1: 3, Group 2: 4) No follow up. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
VanKerrebroeck 1998.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. PP analysis. Multicentre (14) Multinational (4). | |
| Participants | 90 male and female patients with objective evidence of neurological diseases or injuries that would affect the lower urinary tract or its nervous supply. Inclusion criteria: 18‐75 years old with symptoms of urgency, increased frequency of micturition/self catheterization and/or urge incontinence. Urodynamically proven detrusor hyperreflexia. Insignificant bacteriuria and normal laboratory tests. Exclusion criteria: Stress incontinence, cardiac, hepatic, renal or hematological disorders, bladder outlet obstruction, poor general or mental health, contraindications to antimuscarinic agents and patients already receiving therapy for urinary incontinence. Pregnant or lactating women and women of childbearing age not using reliable contraception. | |
| Interventions | Group 1: placebo (n=19) Group 2: tolterodine 0.5mg bid (n=20) Group 3: tolterodine 1mg bid (n=16) Group 4: tolterodine 2mg bid (n=18) Group 5: tolterodine 4mg bid (n=17) 2 week treatment period. 1 week run‐in preceded by 2 week washout if necessary. | |
| Outcomes | Subjective urinary symptoms (VAS). Number of leakage episodes, frequency of micturition, volume voided. Urodynamic parameters. Pad test. Adverse events. Laboratory tests. Blood pressure. ECG. | |
| Notes | Two week telephone follow up. No dropouts. Almost half patients using self catheterisation. Dose reduction permitted. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
VanKerrebroeck 2001.
| Methods | RCT. Placebo controlled, parallel design. Randomised 1:1:1. Double‐blind. Masking of assessors not stated. ITT analysis. Multicentre (167) Multinational. | |
| Participants | 1529 male and female patients. Inclusion criteria: At least 18 years of age with urinary frequency (8 or more /24 hours), urge incontinence (at least 5/week) and symptoms of overactive bladder for at least 6 months. Exclusion criteria: Stress incontinence, total daily urine >3000ml, any contraindications to antimuscarinic treatment, significant hepatic or renal disease, symptomatic or recurrent UTI, interstitial cystitis, hematuria or BOO, current electrostimulation or bladder training therapy, indwelling catheter or intermittent self catheterisation. Pregnant or breastfeeding women, and women of childbearing potential not using adequate contraception. Other treatments for overactive bladder are not allowed apart from oestrogen started >2 months before randomisation. No treatment by any other investigational drug allowed. | |
| Interventions | Group 1: tolterodine extended release 4mg qid (n=507) Group 2: tolterodine immediate release 2mg bid (n=514) Group 3 : placebo (n=508) 12 week treatment period. 1 to 2 week run‐in. | |
| Outcomes | Number of leakage episodes, frequency of micturition, volume voided. Adverse events. Laboratory tests. Some subgroups with pad tests and ECG. QoL Health Measures. Compliance by pill count. | |
| Notes | No dose reductions permitted. 187 dropouts (no group data) One week follow up. Comany support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Walter 1982.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Masking of assessors not stated. | |
| Participants | 20 male and female patients Inclusion criteria: Symptoms of urinary incontinence, frequency and uninhibited bladder contractions during cystometry. Exclusion criteria: >100 ml residual urine, maximum flow rate <10ml/s, bacterial cystitis, treated with other anticholinergic drugs or anti‐Parkinsonism preparations. | |
| Interventions | Treatment 1: emepronium bromide 200mg tid. Treatment 2: placebo. 2 x 4 week treatment period. Washout not stated. | |
| Outcomes | Urinary symptom questionnaire. Patient preference for treatment. Urodynamic parameters. Adverse events. Laboratory tests. | |
| Notes | Urodynamic parameters not detailed as no change. Small numbers in study. Adverse events unclear. 1 withdrawal. No follow up. Data not in useable form for this review. Company support declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wehnert 1989.
| Methods | RCT. Placebo controlled parallel design. Masking of assessors not stated. | |
| Participants | 46 patients. Inclusion criteria: Urgency, urge incontinence. Exclusion criteria: Other urological and gynaecological pathology. | |
| Interventions | Treatment 1: propiverine 45mg od (n=25) Treatment 2: placebo (n=25) Treatment 3: flavoxate 300mg od (n=21) 3 x 4 week treatments. | |
| Outcomes | Symptom scale (5 point). Frequency of micturition, nocturia. Urodynamic parameters. | |
| Notes | Translated from German. No dropouts. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wehnert 1992.
| Methods | RCT. Placebo controlled, crossover design. Blinding not stated. Masking of assessors not stated. Single centre. | |
| Participants | 10 patients. Inclusion criteria: Urgency and urge incontinence. Exclusion criteria: Inflammatory bladder changes, bladder tumours, leucoplasia of bladder or trigone, other urological and gynaecological pathology or inflammation, outflow obstruction, unstable urethra, other use of anticholinergics or spasmolytics. | |
| Interventions | Treatment 1: oxybutynin 5mg tid (n=10) Treatment 2: propiverine 15mg tid (n=10) Treatment 3: placebo 3 x 3 week treatment period, no washout. | |
| Outcomes | VAS for effects and side effects. Frequency of micturition. Urodynamic parameters. Residuals. Laboratory tests. | |
| Notes | Translated from German. No dropouts. No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wein 1978.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind. Masking of assessors not stated. ITT analysis. | |
| Participants | 106 male patients. Inclusion criteria: Transurethral resection either of the prostrate or a superficial bladder lesion within 3 days prior to study. Complaints of bladder discomfort and urinary urgency accociated with cystometric finding of a low volume uninhibited bladder contraction. Exclusion criteria: Neurogenic bladder dysfunction, UTI, medication with a cholinergic, anticholinergic, antispasmodic, sympathomimetic or sympathetic blocking agent, gastrointestinal obstruction, hyperthyroidism and/or cardiovascular disease. | |
| Interventions | Group 1: placebo (n=53) Group 2: oxybutynin 10mg increased to 20mg half way through study (n=53) Route of administration unclear. 6 hour treatment period. | |
| Outcomes | Urinary tract discomfort. Urodynamic parameters. Subjective and objective evaluation by authors. | |
| Notes | No dropouts. No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zeegers 1987.
| Methods | RCT. Placebo controlled, cross over design. Double‐blind. Masking of assessors not stated. Multicentre. | |
| Participants | 60 male and female patients, no age or sex restriction. Inclusion criteria: 56‐85 kg, frequent voiding, urgency or urge incontinence, may include patients with neurogenic bladder. Exclusion criteria: Kidney, liver or cardiovascular pathology, obstruction or infection, ongoing anticholinergic therapy, glaucoma or Parkinson's disease. | |
| Interventions | Group 1 (n=30): Treatment 1: flavoxate 200mg tid; Treatment 2: emepronium bromide 200mg bid; Treatment 3: placebo Group 2 (n=30): Treatment 1: oxybutynin 5mg tid; Treatment 2: emepronium bromide 200mg bid; Treatment 3: placebo. Each person received 3 weeks of one treatment before crossover to the next treatment. All 60 patients thus tested placebo and emepronium; 30 tested flavoxate and 30 tested oxybutynin. | |
| Outcomes | Independent patient and physician subjective scores. Number of leakage episodes, frequency, urgency and enuresis. Urodynamic parameters. Residual urine. Adverse events. | |
| Notes | 19 dropouts ( group not stated). No follow up. Data not in useable form for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zinner 2002.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind, multicentre, multinational Description of randomisation present Concealment of allocation present Masking of participants present | |
| Participants | 1015 patients randomised Inclusion criteria: >18 years, urinary frequency more than 8/24 hours urge incontinence>5 episodes/weekOAB >6 months, ability to fill up micturition charts. Exclusion criteria: Stress urinary incont, Urine volume of more than 3L, Hepatic or renal failure, UTI, Hematuria, Interstitial cystitis, Pregnancy. | |
| Interventions | Group 1: tolterodine 4mg (507) Group 2: placebo (508). | |
| Outcomes | Changes in micturition charts from baseline, incontinence episodes/week; Mean number of micturition/24 hours, Mean volume voided per micturition. | |
| Notes | Data reported as below 65 years and above 65 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zinner 2004.
| Methods | RCT. Placebo controlled, parallel design. Double‐blind, multicentre, multinational Description of randomisation present Concealment of allocation present Masking of participants present. | |
| Participants | 523 male and female patients Inclusion criteria: Cystometric evidence of detrusor instability; absent or minimal bladder suspension defect; max urinary flow above 15ml/s; residual <50ml; MSU <100,000 colonies/ml; no bladder stone or tumour by cystoscopy. Exclusion criteria: Severe heart failure or glaucoma. Neurological disease or detrusor sphincter dyssynergia. Drugs taken during study affecting autonomous nervous system or smooth muscles. | |
| Interventions | Group1: trospium 20mg (n=262) Group 2: placebo (n= 261). | |
| Outcomes | Change in no. of micturition episodes in 24 hours, Change in episodes of urge incontinence, Efficacy and tolerability of drugs Urgency severity scale Incontinence impact questionnaires | |
| Notes | Intention to treat analysis done No standard deviation given in outcome results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zorzitto 1989.
| Methods | RCT. Placebo controlled, crossover design. Double‐blind. Masking of assessors not stated. Multicentre. | |
| Participants | 24 male and female patients. Average age 73.9 years. Inclusion criteria: Established incontinence, age over 60 years, ability to empty bladder (residual <50ml), detrusor instability (confirmed urodynamically), no contra‐indications to anticholinergic medication, signed informed consent. Exclusion criteria: Significant urinary infection, obstruction, bladder tumour or atonic bladder. Patients assessed by history, physical examination, urinalysis, urine culture, cystoscopy and urodynamic studies, and when indicated, determination of renal function. Assessment included particular attention to mental status, mobility, ability to toilet, diagnoses and ability to cooperate. | |
| Interventions | Treatment 1: oxybutynin 5mg bid Treatment 2: placebo Treatment period 8 days with 6 day washout between next treatment period. | |
| Outcomes | Number of leakages over 5 days. Incontinence recorded using a bedside electronic monitor. Only one incontinent event recorded for each toileting interval, regardless of the number of such events occurring within that interval. Voiding record (Ten scheduled toileting intervals daily) Adverse events. | |
| Notes | Fourteen of the patients were co‐operative and reliable; nine were sometimes confused and unreliable, one patient confused and unreliable. 6 dropouts. No follow up. Data not in useable form for this review. Support from health research grant declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
bid = twice per day BOO = bladder outlet obstruction cm = centimetre DO = detrusor overactivity ECG = electrocardiogram H2O = water IIQ = incontinence impact questionnaire ITT = intention to treat kg = kilogram mg = milligram ml = millilitre ml/s = millilitre per second MMSE = mini mental state exam OB = overactive bladder od = once per day PP = per protocol qid = four times per day QOL = quality of life RCT = randomised control trial SF36 = standard form 36 SI = stress incontinence tid = three times per day UDI = urogenital distress inventory UI = urinary incontinence UTI = urinary tract infection VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alloussi 1998a | No usable data in abstract |
| Andersen 1987 | No usable data in abstract |
| Appell 1997 | A meta‐analysis of 4 studies. Trials not reported separately. Not all trials placebo‐controlled. |
| Baert 1995 | Abstract. Means reported for incontinent episodes and voids per day but no meausures of variability, need more information from authors |
| Brocklehurst 1972 | A study in the elderly with no evidence presented that the participants had overactive bladders. |
| Burgio 1994 | No usable data in abstract |
| Cardozo 2005 | Outcome reported was warning time which was not an inclusion criteria of the review |
| Chapple 2001 | No usable data in abstract |
| Chapple 2002 | No usable data in abstract |
| Chapple 2003 | No usable data in abstract |
| Chapple 2004 | No usable data in abstract |
| Chapple 2005a | A pooled analysis of three studies. Trials not reported separately |
| Coombes 1994 | This study has two parallel designs with one side randomised and other non‐randomised |
| Davila 2001a | Comparison of oral + placebo versus transdermal + placebo |
| Di Stasi 2001b | RCT. Excluded as intravesical administration. |
| Dorschner 2003b | The extracted data reported in percentage and hence not usable for analysis |
| Gerstenberg 1986 | Terodiline has been withdrawn from therapeutic useworldwide |
| Goode 2004 | Data reported as percentage improvement. No medians or means reported. |
| Griebenow 1994 | A randomised trial of propiverine versus placebo. Only outcome dysrhythmia on ECG. |
| Kirschner‐Hermanns | A study in the elderly with no evidence presented that the participants had overactive bladders |
| Kuipers 2002 | No usable data in abstract |
| Larsson 1999 | A meta‐analysis of 4 studies Trials not reported separately Some studies published separately |
| Norton 1994 | Terodiline withdrwan from therapeutic use worldwide |
| Ouslander 1995 | A study in the elderly with no evidence presented that the participants had overactive bladders |
| Robinson 1983 | Emepronium bromide with flavoxate hydrochloride versus placebo. Excluded as active therapy includes flavoxate |
| Rosario 1999 | Crossover trial with darifenacin versus placebo. Principle outcomes are comparison of ambulatory versus conventional cystometry |
| Rudy 2004 | No usable data in abstract |
| Smith 2002 | No usable data in abstract |
| Staskin 2004 | This study reported on somnolence and sleepiness in patients with overactive bladder |
| Takayasu 1990 1 | The data reported as percentage and hence not usable for analysis |
| Tapp 1987 | Terodiline has been withdrawn from therapeutic useworldwide |
| Tapp 1989 | Terodiline has been withdrawn from therapeutic useworldwide |
| Terodiline 1993 | Terodiline has been withdrawn from therapeutic useworldwide |
| Viayna 2004 | RCT. Healthy male volunteers with no mention of overactive bladder symptoms or urinary incontinence at baseline. Aim of study to evaluate safety and tolerability of an antimuscarinic. |
| Wagg 2003 | This randomised trail compares urodynamic findings in retrospect from a previous report with hypothesis that anticholinergics change at the onset and end of urinary flow with anticholinergics |
| Wein 1999 | A meta‐analysis of 2 studies Trials not reported separately |
| Whitehead 1967 | A study in the elderly with no evidence presented that the participants had overactive bladders |
| Williams 1981 | A study in the elderly with no evidence presented that the participants had overactive bladders |
Contributions of authors
For the first update of this review (Issue 4, 2006) GN and JC screened the trials, carried out quality assessment and data abstraction. GN and JC interpreted the data and amended the results and discussion. JHS and PH helped with interpretation and approved the final review.
GE took principle responsibility for trial screening, assessment, data extraction and data entry. PH (cross‐over studies) and JHS (parallel‐group designs) independently screened, assessed and extracted data from the trials and checked the data entry. JHS and PH interpreted the data and wrote the discussion, with assistance from KM.
Sources of support
Internal sources
University of Otago, New Zealand.
External sources
Health Research Council of Aotearoa New Zealand, New Zealand.
Declarations of interest
Gaye Ellis was the study co‐ordinator for one of the centres in a multi centre/multinational trial included in the review. None declared for Peter Herbison and Jean Hay‐Smith, Nabi Ghulam and June Cody.
Edited (no change to conclusions)
References
References to studies included in this review
Abrams 1996 {published data only}
- Abrams P, Jackson S, Mattiasson A, Krishnan K, Haendler L. A randomised, double‐blind, placebo controlled, dose ranging study of the safety and efficacy of tolterodine in patients with hyperreflexia. Proceedings of the International Continence Society 26th Annual Meeting. 1996:276‐7.
Abrams 1998 {published data only}
- Abrams P, Freeman R, Anderstrom C, Mattiasson A. Tolterodine, a new antimuscarinic agent: as effective but better tolerated than oxybutynin in patients with an overactive bladder. British Journal of Urology 1998;81:801‐10. [DOI] [PubMed] [Google Scholar]
- Abrams P, Freeman RN, Anderstrom C, Mattiasson A. Efficacy and tolerability of tolterodine vs. oxybutynin and placebo in patients with detrusor instability. Journal of Urology Annual Meeting. 1997; Vol. 157(4):103.
Abrams 2001 {published data only}
- Abrams P, Kaplan S, Millard R. Tolterodine treatment is safe in men with bladder outlet obstruction (BOO) and symptomatic detrusor overactivity (DO). Proceedings of the International Continence Society, 32nd Annual Meeting,Seoul. 2001.
Alloussi 1998 {published data only}
- Alloussi S, Laval K U, Ballering‐Bruhl B, Grobe‐Freese M, Bulitta M, Schafer M. Trospium chloride (Spasmo‐lyt) in patients with motor urge syndrome (detrusor instability): a double‐blind, randomised, multicentre, placebo‐controlled study. Journal of Clinical Research 1998;1:439‐51. [Google Scholar]
Bagger 1985 {published data only}
- Bagger P, Fischer‐Rasmussen W, Hansen RI. Emepronium carregeenate: clinical effects and urinary excretion in treatment of female urge incontinence. Scandanavian Journal of Urology and Nephrology 1985;19:31‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bagger PV, Fischer‐Rasmussen W. [Emepronium carragenate in the treatment of female urinary motor‐urge incontinence]. Proceedings of the 14th Annual Meeting of the International Continence Society; 1984 Sep 13‐15; Innsbruck. 1984:304.
Bono 1982 {published data only}
- Bono AV, Marconi AM, Gianneo E. Oxybutynin for Unstable Bladder. A preliminary placebo controlled trial [L'ossibutinina cloridrato nella vescica instabile pura (Italian)]. Urologia 1982;49:764‐8. [Google Scholar]
Burgio 1998 {published data only}
- Burgio KL, Locher JL, Goode PS. Combined behavioral and drug therapy for urge incontinence in older women. Journal of the American Geriatrics Society 2000;48:370‐4. [DOI] [PubMed] [Google Scholar]
- Burgio KL, Locher JL, Goode PS, Hardin M, McDowell BJ, Dombrowski M, et al. Behavioral vs drug treatment for urge urinary incontinence in older women. A randomized controlled trial. JAMA 1998;280(23):1995‐2000. [DOI] [PubMed] [Google Scholar]
- Burgio KL, Locher JL, Roth DL, Goode PS. Psychological improvements associated with behavioral and drug treatment of urge incontinence in older women. Journal of Gerontology: Psychological Sciences 2001;56B(1):46‐51. [DOI] [PubMed] [Google Scholar]
Cardozo 2000 {published data only}
- Cardozo L, Chapple CR, Toozs‐Hobson P, Grosse‐Freese M, Bulitta M, Lehmacher W, et al. Efficacy of trospium chloride in patients with detrusor instability: a placebo‐controlled, randomised, double‐blind, multicentre clinical trial. British Journal of Urology International 2000;85:659‐64. [DOI] [PubMed] [Google Scholar]
Cardozo 2004 {published data only}
- Cardozo L, Lisec M, Millard R, Vierssen Trip O, Kuzmin I, Drogendijk TE, et al. Randomized, double‐blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. Journal of Urology 2004;172(5(Pt 1 of 2)):1919‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kelleher CJ, Cardozo L, Chapple CR, Haab F, Ridder AM. Improved quality of life in patients with overactive bladder symptoms treated with solifenacin. BJU International 2005;95(1):81‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chaliha 1998 {published data only}
- Chaliha C, Halaska M, Stanton S. Trospium chloride for the treatment of detrusor instability: a placebo‐controlled dose‐finding study. British Journal of Obstetrics and Gynaecology 1998;105(Suppl 17):276. [Google Scholar]
Chapple 2004a {published data only}
- Chapple CR, Arano P, Bosch JHR, Ridder D, Kramer G, Ridder AM. YM905 appears effective and well tolerated in patients with symptomatic idiopathic detrusor overactivity in a European placebo‐ and tolterodine‐controlled, phase‐II, dose‐finding study (Abstract). Neurourology and Urodynamics 2002;21(4):381‐2. [MEDLINE: ] [Google Scholar]
- Chapple CR, Arano P, Bosch JL, Ridder D, Kramer AE, Ridder AM. Solifenacin appears effective and well tolerated in patients with symptomatic idiopathic detrusor overactivity in a placebo‐ and tolterodine‐controlled phase 2 dose‐finding study. BJU International 2004;93(1):71‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kelleher CJ, Cardozo L, Chapple CR, Haab F, Ridder AM. Improved quality of life in patients with overactive bladder symptoms treated with solifenacin. BJU International 2005;95(1):81‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chapple 2004b {published data only}
- Chapple CR, Rechberger T, Al Shukri S, Meffan P, Everaert K, Huang M, et al. Randomized, double‐blind placebo‐ and tolterodine‐controlled trial of the once‐daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU International 2004;93(3):303‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coombes 1996 {published data only}
- Coombes GM, Millard RJ. A randomised double blind placebo controlled trial of penthienate in the treatment of detrusor instability (Abstract). Proceedings of the International Continence Society 24th Annual Meeting; 1994 Aug 30 ‐ Sept 2; Prague, Czech Republic; 1994 104. [MEDLINE: ]
- Coombes GM, Millard RJ. Urinary urge incontinence: randomised crossover trials of penthienate versus placebo and propantheline. Medical Journal of Australia 1996;165(9):473‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davila 2001 {published data only}
- Davila GW, Sanders S, for the Transdermal Oxybutynin Study Group. Transdermal oxybutynin: A multi‐center, prospective, randomized, double‐blind, placebo‐controlled study in adults with urge urinary incontinence. International Urogynecology Journal 2001;12(Suppl 3):S43. [Google Scholar]
- Davila GW, Sanders SW, Lyttle S, Gittelman MC, Zinner N, Saltzstein DR et al for the Transdermal Oxybutynin Study Group. Transdermal oxybutynin is safe, effective, and improves quality of life in patients with overactive bladder. Neurourology and Urodynamics 2001;20(4):426‐7. [Google Scholar]
Di Stasi 2001a {published data only}
- Stasi SM, Giannantoni A, Vespasiani G, Navarra P, Capelli G, Massoud R, et al. Intravesical electromotive administration of oxybutynin in patients with detrusor hyperreflexia unresponsive to standard anticholinergic regimens. Journal of Urology 2001;165:491‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dmochowski 2003 {published data only}
- Dmochowski RR, Sand PK, Zinner NR, Gittelman MC, Davila GW, Sanders SW, et al. Comparative efficacy and safety of transdermal oxybutynin and oral tolterodine versus placebo in previously treated patients with urge and mixed urinary incontinence. Urology 2003;62(2):237‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dorschner 2000 {published and unpublished data}
- Dorschner W, Stolzenburg JU, Griebenow R, Halaska M, Schubert G, Murtz G, et al. Efficacy and cardiac safety of propiverine in elderly patients ‐ a double‐blind, placebo‐controlled clinical study. European Urology 2000;37:702‐8. [DOI] [PubMed] [Google Scholar]
Drutz 1999 {published data only}
- Drutz H, Appell RA. Clinical efficacy and safety of Tolterodine vs Oxybutynin and placebo in patients with unstable bladder. Acta Obstetrica et Gynecologica Scandinavica 1997;Suppl 167(5):24. [Google Scholar]
- Drutz HP, Appell RA. Enhanced tolerability of Tolterodine compared to Oxybutynin in a controlled clinical study. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8(1):S14. [DOI] [PubMed] [Google Scholar]
- Drutz HP, Appell RA, Gleason D, Klimberg I, Radomski S. Clinical efficacy and safety of tolterodine compared to oxybutynin and placebo in patients with overactive bladder. International Urogynecology Journal 1999;10:283‐9. [DOI] [PubMed] [Google Scholar]
Freeman 2003 {published data only}
- Freeman R, Hill S, Millard R, Slack M, Sutherst J, Tolterodine Study Group. Reduced perception of urgency in treatment of overactive bladder with extended‐release tolterodine. Obstetrics and Gynecology 2003;102(3):605‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goode 2002 {published data only}
- Goode PS, Burgio KL, Locher JL, Umlauf MG, Lloyd LK, Roth DL. Urodynamic changes associated with behavioral and drug treatment of urge incontinence in older women. Journal of the American Geriatrics Society 2002;50(5):808‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haab 2004 {published data only}
- Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well‐tolerated once‐daily treatment for overactive bladder. European Urology 2004;45(4):420‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Halaska 1994 {published data only}
- Halaska M, Dorschner W, Frank M. Treatment of urgency and incontinence in elderly patients with propiverine hydrochloride. Neurourology and Urodynamics 1994;13(4):428‐30. [Google Scholar]
Homma 2003 {published data only}
- Homma Y, Kawabe K. Health‐related quality of life of Japanese patients with overactive bladder treated with extended‐release tolterodine or immediate‐release oxybutynin: a randomized, placebo‐controlled trial. World Journal of Urology 2004;22(4):251‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Homma Y, Paick JS, Lee JG, Kawabe K, Japanese and Korean Tolterodine Study Group. Clinical efficacy and tolerability of extended‐release tolterodine and immediate‐release oxybutynin in Japanese and Korean patients with an overactive bladder: a randomized, placebo‐controlled trial. BJU International 2003;92(7):741‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jacquetin 2001 {published data only}
- Jacquetin B, Wyndaele JJ. Tolterodine reduces the number of incontinence episodes in patients with an overactive bladder. European Journal of Obstetrics and Gynecology and Reproductive Biology 2001;98:97‐102. [DOI] [PubMed] [Google Scholar]
- Jacquetin B, Wyndaele JJ. Tolterodine reduces the number of incontinence episodes in patients with detrusor overactivity. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8(1):S30. [Google Scholar]
Jonas 1997 {published data only}
- Jonas U, Hofner K, Madersbacher H. Efficacy and safety of two doses of tolterodine versus placebo in patients with detrusor overactivity and symptoms of frequency, urge incontinence, and urgency: urodynamic evaluation. World Journal of Urology 1997;15:144‐51. [DOI] [PubMed] [Google Scholar]
- Jonas U, Hofner K, Madersbacher H, Holmdahl TH. Efficacy and safety of two doses of Tolterodine compared to placebo in patients with detrusor overactivity. Neurourology and Urodynamics 1997;16(5):477‐8. [DOI] [PubMed] [Google Scholar]
Junemann 1999 {published data only}
- Junemann KP, Fusgen I. Placebo‐controlled, randomised, double‐blind, multicentre clinical trial on the efficacy and tolerability of 1 x 40 mg and 2 x 40 mg trospium chloride (Spasmo‐lyt) daily for 3 weeks in patients with urge‐syndrome. Neurourology and Urodynamics 1999;18(4):375‐6. [Google Scholar]
Junemann 2000 {published data only}
- Junemann KP, Al‐Shukri S. Efficacy and tolerability of trospium chloride and tolterodine in 234 patients with urge‐syndrome: a double‐blind, placebo‐controlled, multicentre clinical trial. Neurourology and Urodynamics 2000;19(4):488‐90. [Google Scholar]
Khullar 2004 {published data only}
- Khullar V, Hill S, Laval KU, Schiotz HA, Jonas U, Versi E. Treatment of urge‐predominant mixed urinary incontinence with tolterodine extended release: a randomized, placebo‐controlled trial. Urology 2004;64(2):269‐74; discussion 274‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kramer 1987 {published data only}
- Kramer AEJL, Zeegers AGM, Kiesswetter H, Jonas U. Drug treatment of urgency: a double blind trial of cetiprin®, dridase®, urispas® and placebo [Medicamenteuze behandeling van overmatige mictiedrang: Dubbel‐blind onderzoek van cetiprin®, dridase®, urispas® en placebo (Flemish)]. TGO tijdschrift voor Therapie, Geneesmiddel, en Onderzoek 1987;12(7):256‐61. [Google Scholar]
Landis 2004 {published data only}
- Landis JR, Kaplan S, Swift S, Versi E. Efficacy of antimuscarinic therapy for overactive bladder with varying degrees of incontinence severity. Journal of Urology 2004;171(2 (Pt 1)):752‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Madersbacher 1999 {published data only}
- Halaska M, Madersbacher H, Voigt R, Hofner K, Martan A. Propiverine in patients with urgency and urge incontinence ‐ a placebo controlled, multicenter study comparing its tolerability and efficacy with oxybutynin. International Urogynaecology Journal and Pelvic Floor Dysfunction 2000;11(Suppl 1):46. [Google Scholar]
- Madersbacher H, Halaska M, Voigt R, Alloussi S, Hofner K. A placebo‐controlled, multicentre study comparing the tolerability and efficacy of propiverine and oxybutynin in patients with urgency and urge incontinence. British Journal of Urology International 1999;84:646‐51. [DOI] [PubMed] [Google Scholar]
- Madersbacher H, Halaska M, Voigt R, Alloussi S, Hofner K. A urodynamically controlled multicenter study in patients with urge incontinence: tolerability and efficacy of propiverine hydrochloride in comparison to oxybutynin. Proceedings of the International Continence Society 27th Annual Meeting. 1997:153‐4.
Malone‐Lee 2001 {published data only}
- Malone‐Lee JG, Walsh B, Maugourd MF and the Tolterodine in the Elderly Study Group. The safety and clinical efficacy of two doses of Tolerodine, compared to placebo in elderly patients. Proceedings of the International Continence Society. 1997:155‐6.
- Malone‐Lee JG, Walsh JB, Maugourd M‐F, and the Tolterodine in the Elderly Study Group. Tolterodine: A safe and effective treatment for older patients with overactive bladder. Journal of the American Geriatric Society 2001;49:700‐5. [DOI] [PubMed] [Google Scholar]
Massey 1986 {published data only}
- Massey JA, Abrams P. Dose titration in clinical trials. An example using emepronium carrageenate in detrusor instability. British Journal of Urology 1986;58:125‐8. [DOI] [PubMed] [Google Scholar]
- Massey JA, Abrams PH. Dose‐titrated emepronium carrageenate for detrusor instability. Proceedings of the International Continence Society 14th Annual Meeting. 1984:109‐10.
Meyhoff 1983 {published data only}
- Meyhoff H H, Gerstenberg TC, Nordling J. Placebo ‐ the drug of choice in female motor urge incontinence?. British Journal of Urology 1983;55:34‐7. [DOI] [PubMed] [Google Scholar]
Millard 1999 {published data only}
- Millard R, Tuttle J, Moore K, Susset J, Clarke B, Dwyer P, Davis B. Clinical efficacy and safety of tolterodine compared to placebo in detrusor overactivity. Journal of Urology 1999;161:1551‐5. [PubMed] [Google Scholar]
- Moore K, Millard R, Dwyer P, Tuttle J. A randomized controlled multicentre trial of Tolterodine in detrusor instability/hyperreflexia. International Urogynecology Journal and Pelvic Floor Dysfunction 1997;8(1):S129. [Google Scholar]
- Rosamilia A, Dwyer PL, Clarke B, Moore K, Millard RP, Tuttle J. The clinical efficacy and safety of two doses of Tolterodine in detrusor instability. Acta Obstetrica et Gynecologica Scandinavica 1997;167(5):24. [Google Scholar]
Moisey 1980 {published data only}
- Bary PR, Moisey CU, Stephenson TP, Brendler CB. The urodynamic and subjective results of treatment of detrusor instability with oxybutynin chloride. Progress in Clinical & Biological Research 1981;78:313‐9. [PubMed] [Google Scholar]
- Moisey CU, Stephenson TP, Brendler CB. The urodynamic and subjective results of treatment of detrusor instability with oxybutynin chloride. British Journal of Urology 1980;52:472‐5. [DOI] [PubMed] [Google Scholar]
Moore 1990 {published data only}
- Moore KH, Hay DM, Imrie AE, Watson A, Goldstein M. Oxybutynin hydrochloride (3mg) in the treatment of women with idiopathic detrusor instabilty. British Journal of Urology 1990;66:479‐85. [DOI] [PubMed] [Google Scholar]
- Moore KH, Hay DM, Imrie AH, Cockin J. Randomized double blind placebo‐controlled trial of oxybutynin in females with detrusor instability. Proceedings of the Annual Meeting of the International Continence Society. 1987:137‐8.
- Moore KH, Sutherst JR. Response to treatment of detrusor instability in relation to psychoneurotic status. British Journal of Urology 1990;66:486‐90. [DOI] [PubMed] [Google Scholar]
Murray 1984 {published data only}
- Murray KHA, Patterson JR, Stephenson TP. A double‐blind three way cross over placebo controlled trial of cystrin and cetiprin novum. Proceedings of the 14th annual Meeting of the International Continence Society. 1984:454.
Rentzhog 1998 {published data only}
- Rentzhog SL, Stanton L, Cardozo L, Nelson E, Fall M, Abrams P. Efficacy and safety of tolterodine in patients with detrusor instability: a dose‐ranging study. British Journal of Urology 1998;81:42‐8. [DOI] [PubMed] [Google Scholar]
Riva 1984 {published data only}
- Riva D, Casolati E. Oxybutynin chloride in the treatment of female idiopathic bladder instability. Results from double blind treatment. Clinical and Experimental Obstetrics and Gynecology 1984;XI(1‐2):37‐42. [PubMed] [Google Scholar]
Rosario 1995a {published data only}
- Rosario DJ, Leaker BR, Noble JG, Milroy E, Chapple CR. A double‐blind placebo controlled crossover study of the effects of single doses of darifenacin on cystometric parameters in patients with detrusor instability. Proceedings of the 25th Annual Meeting of the International Continence Society. 1995:223.
Rosario 1995b {published data only}
- Rosario DJ, Leaker BR, Smith DJ, Chapple CR. A pilot study of the effects of multiple doses of the M3 muscarinic receptor agonist darifenacin on ambulatory parameters of detrusor activity in patients with detrusor instability (Abstract 36). Neurourology and Urodynamics 1995;14(5):464‐5. [Google Scholar]
SerranoBrambila 2000 {published data only}
- Serrano Brambila EA, Avila RGQ, Monterrubio JLL, Aranda JM. Evaluation of the effectiveness and tolerance of oxybutynin in the treatment of unstable bladder [Evaluación de la efectividad y tolerancia de la oxibutinina en la tratamiento de la vejiga inestable en la mujer (Spanish)]. Ginecologia y Obstetricia de México 2000;68:174‐81. [PubMed] [Google Scholar]
Stohrer 1991 {published data only}
- Stohrer M, Bauer P, Giannetti BM, Richter R, Burgdorfer H, Murtz G. Effect of trospium chloride on urodynamic parameters in patients with detrusor hyperreflexia due to spinal cord injuries. Urologia Internationalis 1991;47:138‐43. [DOI] [PubMed] [Google Scholar]
Stohrer 1999 {published data only}
- Stohrer M, Madersbacher H, Richter R, Wehnert J, Dreikorn K. Efficacy and safety of propiverine in SCI‐patients suffering from detrusor hyperreflexia ‐ A double‐blind, placebo‐controlled clnical trial. Spinal Cord 1999;37:196‐200. [DOI] [PubMed] [Google Scholar]
Szonyi 1995 {published data only}
- Collas DM, Szonyi G, Ding YY, Malone‐Lee JG. Oxybutynin with bladder retraining for detrusor instability in the elderly ‐ a placebo controlled trial. Age and Ageing 1994;23(Suppl. 2):9. [DOI] [PubMed] [Google Scholar]
- Szonyi G, Collas DM, Ding YY, Malone‐Lee JG. Oxybutynin with bladder retraining for detrusor instability in elderly people: A randomised controlled trial. Age and Ageing 1995;24:287‐91. [DOI] [PubMed] [Google Scholar]
Tago 1990 {published data only}
- Tago K, Ueno A, Takayasu H. Clinical study of propiverine hydrochloride for the treatment of patients with urinary frequency and incontinence. Neurourology and Urodynamics 1990;9(4):337‐8. [Google Scholar]
Takayasu 1990 {published data only}
- Takayasu H, Ueno A, Tsutida S, Koiso K, Kurita K, Kawabe K, et al. Clinical effects of propiverine hydrochloride in the treatment of urinary frequency and incontinence associated with detrusor overactivity: a double blind, parallel, placebo controlled multicenter study. Igaku No Ayumi (Progress in Medicine) 1990;153:459‐71. [MEDLINE: ] [Google Scholar]
Tapp 1990 {published data only}
- Cardozo LD, Cooper D, Versi E. Oxybutynin chloride in the management of idiopathic detrusor instability (Abstract 88). Neurourology and Urodynamics 1987;6(3):256‐7. [Google Scholar]
- Tapp AJS, Cardozo LD, Versi E, Cooper D. The treatment of detrusor instability in post‐menopausal women with oxybutynin chloride : a double blind placebo controlled study. British Journal of Obstetrics and Gynaecology 1990;97(June):521‐6. [DOI] [PubMed] [Google Scholar]
Thuroff 1991 {published data only}
- Thuroff JW, Bunke B, Ebner A, Faber P, Geeter P, Hannappel J, et al. Randomized, double‐blind multicenter trial on treatment of frequency, urgency and incontinence related to detrusor hyperactivity: oxybutynin vs. propantheline vs. placebo. Neurourology and Urodynamics 1990;9(4):337‐8. [DOI] [PubMed] [Google Scholar]
- Thuroff JW, Bunke B, Ebner A, Faber P, Geeter P, Hannappel J, et al. Randomized, double‐blind, multicenter trial on treatment of frequency, urgency and incontinence related to detrusor hyperactivity: oxybutynin versus propantheline versus placebo. Journal of Urology 1991;145(April):813‐7. [DOI] [PubMed] [Google Scholar]
Ulshofer 2001 {published data only}
- Ulshofer B, Bihr AM, Bodeker RH, Schwantes U, Jahn HP. Randomised, double‐blind, placebo‐controlled study on the efficacy and tolerance of trospium chloride in patients with motor urge incontinence. Clinical Drug Investigation 2001;21(8):563‐9. [Google Scholar]
- Ulshofer B, Schwantes U, Bodeker RH, Bihr AM, Jahn HP. Efficacy and tolerance of trospium chloride in patients with motor urge incontinence ‐ results of a randomised double‐blind placebo‐controlled study. Proceedings of the International Consultation on Incontinence (ICI), Paris. 2001:5.
VanKerrebroeck 1998 {published data only}
- Messelink EJ, Soler JM, Madersbacher H, Thuroff JW, Amarenco G, Kerrebroeck PEV. Urodynamic aspects of the efficacy of tolterodine, a new anti muscarine drug in the treatment of detrusor hyperreflexia. Proceedings of the International Continence Society 25th Annual Meeting. 1995:95‐6.
- Kerrebroeck PEVA, Amarenco G, Thuroff JW, Madersbacher HG, Lock MTWT, Messelink EJ, Soler JM. Dose‐ranging study ot tolterodine in patients with detrusor hyperreflexia. Neurourology and Urodynamics 1998;17:499‐512. [DOI] [PubMed] [Google Scholar]
VanKerrebroeck 2001 {published data only}
- Chancellor M, Freedman S, Mitcheson HD, Antoci J, Primus G, Wein A. Tolterodine, an effective and well tolerated treatment for urge incontinence and other overactive bladder symptoms. Clinical Drug Investigation 2000 Feb;19(2):83‐91. [Google Scholar]
- Garely A, on behalf of the Tolterodine Study Group. Once‐daily tolterodine treatment significantly decreases perception of urgency and urge incontinence episodes in patients with overactive bladder. International Urology Journal 2001;12(Suppl):18. [Google Scholar]
- Kelleher CJ, Pleil AM, Okano GJ, Reese PR. Long‐term health‐related quality of life of patients with overactive bladder receiving tolterodine. Neurourology and Urodynamics 2001;20(4):504‐6. [Google Scholar]
- Kelleher CJ, Pleil AM, Okano GJ, Reese PR. Long‐term health‐related quality of patients with overactive bladder receiving Tolterodine. Proceedings of the International Continence Society Annual Meeting, Seoul. 2001:No. 82.
- Kelleher CJ, Pleil AM, Reese PR. Health related quality of life of patients with overactive bladder receiving tolterodine once‐daily. Neurourology and Urodynamics 2000;19(4):519‐21. [Google Scholar]
- Kelleher CJ, Reese PR, Pleil AM, Okano GJ. Health‐related quality of life of patients receiving extended‐release tolterodine for overactive bladder. American Journal of Managed Care 2002;8 Suppl 19:608‐15. [MEDLINE: ] [PubMed] [Google Scholar]
- Kelleher CJ, on behalf of the Tolterodine Study Group. Health‐related quality of life of female patients receiving once‐daily tolterodine treatment for overactive bladder. International Urogynecology Journal 2000;11(Suppl 1):94. [Google Scholar]
- Kreder KJ. Antimuscarinic therapy: relationship between efficacy and side effects in responders and non‐responders. Journal of Urology 2001;165, May(5 Suppl):S165. [Google Scholar]
- Kreder KJ. Clinical effectiveness of antimuscarinic therapy: the relationship between efficacy and tolerability. Proceedings of the International Continence Society Annual Meeting, Seoul. 2001:No. 140.
- Mallett V, on behalf of the Tolterodine Study Group. Health‐related quality of life of female patients receiving once‐daily tolterodine treatment for overactive bladder. International Urogynecology Journal 2001;12(Suppl 1):4. [Google Scholar]
- Swift S. Efficacy and tolerability of once‐daily Tolterodine for women with overactive bladder. Proceedings of the International Continence Society Annual Meeting, Seoul. 2001:No. 329.
- Swift S. Once‐daily tolterodine is effective and well tolerated in women with overactive bladder. 2nd International Consultation on Incontinence, Paris. July 2001:Abstract 57.
- Swift SE. Once‐daily (OD) tolterodine treatment significantly decreases perception of urgency and urge incontinence episodes in patients with overactive bladder (OAB). International Urogynecology Journal 2000;11(Suppl. 1):15. [Google Scholar]
- Swift SE. Once‐daily administration of extended‐release tolterodine is effective and well‐tolerated in patients with oveactive bladder. Proceedings of the XVI FIGO World Congress of Obstetrics & Gynecology; 3‐8 Sept; Washington DC. 2000; Vol. Book 1:40.
- Swift SE. Overactive bladder in females: treatment with once‐daily Tolterodine. International Urogynecology Journal 2001;12(Suppl 3):71. [Google Scholar]
- Kerrebroeck P, Kreder K, Jonas U, Zinner N. Tolterodine once‐daily: superior efficacy and tolerability in the treatment of overactive bladder. Urology 2001;57:414‐21. [DOI] [PubMed] [Google Scholar]
- Kerrebroeck PEV. Long‐term (12 months) efficacy and tolerability of tolterodine once‐daily in the treatment of overactive bladder. Neurourology and Urodynamics 2001;20(4):401‐2. [Google Scholar]
- Kerrebroeck PEVA, for the Tolterodine Study Group. Long‐term tolerability and efficacy of once‐daily (OD) Tolterodine in the treatment of overactive bladder (OB). International Urogynecology Journal 2001;12(Suppl 3):49. [Google Scholar]
- Kerrebroeck PEVA, on behalf of the Tolterodine Study Group. Significant decreases in perception of urgency and urge incontinence episodes with once‐daily tolterodine treatment in patients with overactive bladder. Neurourology and Urodynamics 2000;19(4):493‐4. [Google Scholar]
Walter 1982 {published data only}
- Walter S, Hansen J, Hansen L, Maegaard E, Meyhoff HH, Nordling J. Urinary incontinence in old age. A controlled clinical trial of Emepronium Bromide. British Journal of Urology 1982;54:249‐51. [DOI] [PubMed] [Google Scholar]
Wehnert 1989 {published data only}
- Wehnert VJ, Sage S. Comparative study of the effect of Mictonorm (Propiverine hydrochloride) and Spasuret (Flavoxate hydrochloride) on the bladder detrusor muscle [Vergleichende untersuchungen zur wirkung von Mictonorm (Propiverin hydrodhlorid) und Spasuret (Flavoxat hydrochlorid) auf den detrusor vesicae (German)]. Zentralblatt für Urologie und Nephrologie 1989;82:259‐63. [PubMed] [Google Scholar]
Wehnert 1992 {published data only}
- Wehnert VJ, Sage S. Treatment of bladder unstability and urge incontinence with Propiverine Hydrochloride (Mictonorm®) and Oxybutynin chloride (Dridase®) ‐ a randomised crossover study [Therapie der blaseninstabilität und urge‐inkontinenz mit Propiverin hydrochlorid (Mictonorm®) und Oxybutinin chlorid (Dridase®) ‐ eine randomisierte cross‐over‐vergleichsstudie (German)]. Aktuelle Urologie 1992;23:7‐11. [Google Scholar]
Wein 1978 {published data only}
- Wein AJ, Hanno PM, Raezer DM, Benson GS. Effect of oxybutynin chloride on bladder spasm following transurethral surgery. Urology 1978;XII(2):184‐6. [DOI] [PubMed] [Google Scholar]
Zeegers 1987 {published data only}
- Zeegers AGM, Kiesswetter H, Kramer AEJL, Jonas U. Conservative therapy of frequency, urgency and urge incontinence: a double‐blind clinical trial of flavoxate hydrochloride, oxybutynin chloride, emepronium bromide and placebo. World Journal of Urology 1987;5:57‐61. [Google Scholar]
Zinner 2002 {published data only}
- Zinner NR, Mattiasson A, Stanton SL. Efficacy, safety, and tolerability of extended‐release once‐daily tolterodine treatment for overactive bladder in older versus younger patients. Journal of the American Geriatrics Society 2002;50(5):799‐807. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zinner 2004 {published data only}
- Zinner N, Gittelman M, Harris R, Susset J, Kanelos A, Auerbach S, Trospium Study Group. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. Journal of Urology 2004;171(6 (Pt 1)):2311‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zorzitto 1989 {published data only}
- Zorzitto ML, Holliday PJ, Jewett MAS, Herschorn S, Fernie GR. Oxybutynin chloride for geriatric urinary dysfunction: a double‐blind placebo‐controlled study. Age and Ageing 1989;18:195‐200. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alloussi 1998a {published data only}
- Alloussi S, Laval K‐U, Mertins B. Efficacy and tolerability of trospium chloride (spasmo‐lyt) versus placebo in patients with motor urge‐syndrome. Proceedings of the International Continence Society (ICS), 28th Annual Meeting; 1998 Sept 14‐17; Jerusalem, Israel; 1998 109‐10. [MEDLINE: ]
Andersen 1987 {published data only}
- Andersen JR, Lose G, Norgaard M, Stimpel H, Andersen JT. Terodiline, Emepronium bromide or placebo for treatment of female detrusor overactivity? A randomized, double‐blind cross‐over study. Proceedings of the International Continence Society (ICS), 17th Annual Meeting; 1987 Sept 2‐5; Bristol,UK; 1987 1‐2. [MEDLINE: ]
Appell 1997 {published data only}
- Appell RA. Clinical efficacy and safety of tolterodine in the treatment of overactive bladder: A pooled analysis. Urology 1997;50(Suppl 6A):90‐6. [DOI] [PubMed] [Google Scholar]
Baert 1995 {published data only}
- Baert L, Leuven G, Dijkman B, the Darifenacin study group. [Darifenacin, a novel M3 muscarinic receptor antagonist in detrusor overactivity [Read by title abstract]]. Proceedings of the 25th Annual Meeting of the International Continence Society, Sydney, Australia. 1995.
Brocklehurst 1972 {published data only}
- Brocklehurst JC, Armitage P, Jouher AJ. Emepronium bromide in urinary incontinence. Age and Ageing 1972;1:152‐7. [DOI] [PubMed] [Google Scholar]
Burgio 1994 {published data only}
- Burgio KL, Locher JL, Goode PS, Hardin JM, McDowell BJ, Candib D. Behavior vs drug therapy for urge incontinence in older women (Abstract). Proceedings of the American Urogynecology Society, 15th Annual Scientific Meeting; 1994 Sept 21‐24; Toronto, Ontario. 1994:48 (Abstract 26).
Cardozo 2005 {published data only}
- Cardozo L, Dixon A. Increased warning time with darifenacin: a new concept in the management of urinary urgency. Journal of Urology 2005;173(4):1214‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chapple 2001 {published data only}
- Chapple CR, Madersbacher H, Dreikorn K, Dorschner W, Murtz G. Urodynamics and frequency/volume chart ‐ do they correlate? Treatment analysis of Propiverine in comparison to Oxybutynin and Placebo in urge incontinence (Abstract). Proceedings of the International Continence Society (ICS), 31st Annual Meeting; 2001 Sept 18‐21; Seoul, Korea. 2001:Abstract 224. [MEDLINE: ]
Chapple 2002 {published data only}
- Chapple CR, Arano P, Bosch JHR, Ridder D, Kramer G, Ridder AM. YM905 appears effective and well tolerated in patients with symptomatic idiopathic detrusor overactivity in a European placebo‐ and tolterodine‐controlled, phase‐II, dose‐finding study (Abstract). Neurourology and Urodynamics 2002;21(4):381‐2. [MEDLINE: ] [Google Scholar]
Chapple 2003 {published data only}
- Chapple C, Rechberger T, Al‐Shukri S, Meffan P, Everaert K, Ridder A. Results of a randomized phase 3 study comparing solifenacin succinate with tolterodine and placebo in patients with symptomatic overactive bladder (Abstract). Neurourology and Urodynamics 2003;22(5):534‐5. [MEDLINE: ] [Google Scholar]
Chapple 2004 {published data only}
- Chapple C. Fesoterodine a new effective and well‐tolerated antimuscarinic for the treatment of urgency‐frequency syndrome: results of a phase 2 controlled study (Abstract). Neurourology and Urodynamics 2004;23(5/6):598‐9. [MEDLINE: ] [Google Scholar]
Chapple 2005a {published data only}
- Chapple C, Steers W, Norton P, Millard R, Kralidis G, Glavind K, et al. A pooled analysis of three phase III studies to investigate the efficacy, tolerability and safety of darifenacin, a muscarinic M3 selective receptor antagonist, in the treatment of overactive bladder. British Journal of Urology International 2005;95(7):993‐1001. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coombes 1994 {published data only}
- Coombes GM, Millard RJ. A randomised double blind placebo controlled trial of penthienate in the treatment of detrusor instability (Abstract). Proceedings of the International Continence Society 24th Annual Meeting; 1994 Aug 30 ‐ Sept 2; Prague, Czech Republic; 1994 104. [MEDLINE: ]
Davila 2001a {published data only}
- Davila GW, Daugherty CA, Sanders SW, for The Transdermal Oxybutynin Study Group. A short‐term, multicenter, randomized double‐blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinence. Journal of Urology 2001;166(July):140‐5. [PubMed] [Google Scholar]
Di Stasi 2001b {published data only}
- Stasi SV, Giannantoni A, Navarra P, Capelli G, Storti L, Porena M, Stephen RL. Intravesical oxybutynin: mode of action assessed by passive diffusion and electromotive administration with pharmacokinetics of oxybutynin and n‐desethyl oxybutynin. Journal of Urology 2001;166:2232‐6. [PubMed] [Google Scholar]
Dorschner 2003b {published data only}
- Dorschner W, Stolzenburg JU, Griebenow R, Halaska M, Brunjes R, Frank M, et al. [The elderly patient with urge incontinence or urge‐stress incontinence ‐ efficacy and cardiac safety of propiverine]. [German]. Aktuelle Urologie 2003;34(2):102‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gerstenberg 1986 {published data only}
- Gerstenberg TC, Klarskov P, Ramirez D, Hald T. Terodiline in the treatment of women with urgency and motor urge incontinence. A clinical and urodynamic double‐blind cross‐over study. British Journal of Urology 1986;58(2):129‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goode 2004 {published data only}
- Goode PS. Behavioral and drug therapy for urinary incontinence. Urology 2004;63(Suppl 3A):58‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Griebenow 1994 {published data only}
- Griebenow R, Wieners F, Lange Th. Possible induction of cardiac dysrhythmia in elderly patients under therapy with propiverine hydrochloride. Neurourology and Urodynamics. 1994; Vol. 13:430‐2.
Kirschner‐Hermanns {published data only}
- Kirschner‐Hermanns R, DuBeau C, Resnick NM. Oxybutynin for incontinence in institutionalized elderly ‐ a re‐evaluation. Proceedings of the International Continence Society 27th Annual Meeting. 1997:149‐50.
Kuipers 2002 {published data only}
- Kuipers M, Tran DD, Krauwinkel W, Abila B, Mulder H. Absolute bioavailability of YM905 in healthy male volunteers: A single‐dose, randomized, two‐period crossover study (Abstract). Proceedings of the International Continence Society (ICS), 32nd Annual Meeting; 2002 Aug 28‐30; Heidelberg, Germany. 2002:296‐7. [MEDLINE: ]
Larsson 1999 {published data only}
- Larsson G, Hallen B, Nilvebrant L. Tolterodine in the treatment of overactive bladder: analysis of the pooled phase II efficacy and safety data. Urology 1999;53(5):990‐8. [DOI] [PubMed] [Google Scholar]
Norton 1994 {published data only}
- Norton P, Karram M, Wall LL, Rosenzweig B, Benson JT, Fantl JA. Randomized double‐blind trial of terodiline in the treatment of urge incontinence in women. Obstetrics & Gynecology 1994;84(3):386‐91. [MEDLINE: ] [PubMed] [Google Scholar]
Ouslander 1995 {published data only}
- Ouslander JG, Schelle JF, Uman G, Fingold S, Nigam JG, Tuico E. Does oxybutynin add to the effectiveness of prompted voiding for urinary incontinence among nursing home residents?. Journal of the American Geriatric Society 1995;43:610‐7. [DOI] [PubMed] [Google Scholar]
Robinson 1983 {published data only}
- Robinson JM, Brocklehurst JC. Emepronium bromide and flavoxate hydrochloride in the treatment of urinary incontinence associated with detrusor instability in elderly women. British Journal of Urology 1983;55:371‐6. [DOI] [PubMed] [Google Scholar]
Rosario 1999 {published data only}
- Rosario DJ, Smith DJ, Radley SC, Chapple CR. Pharmacodynamics of anticholinergic agents measured by ambulatory urodynamic monitoring: a study of methodology. Neurourology and Urodynamics 1999;18:223‐34. [DOI] [PubMed] [Google Scholar]
Rudy 2004 {published data only}
- Rudy D, Cline K, Goldberg K, Harris R. A multicenter, randomized, placebo‐controlled trial of trospium chloride in overactive bladder patients (Abstract). Neurourology & Urodynamics 2004;23(5/6):600‐1. [MEDLINE: ] [Google Scholar]
Smith 2002 {published data only}
- Smith N, Grimes I, Ridge S, Tempel D, Uchida T, Yamanouchi USA. YM905 is effective and safe as treatment of overactive bladder in women and men: results from phase II study (Abstract). Proceedings of the International Continence Society (ICS), 32nd Annual Meeting; 2002 Aug 28‐30; Heidelberg, Germany. 2002:138‐9. [MEDLINE: ]
Staskin 2004 {published data only}
- Staskin DR, Harnett MD. Effect of trospium chloride on somnolence and sleepiness in patients with overactive bladder. Current Urology Reports 2004;5(6):423‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Takayasu 1990 1 {published data only}
- Takayasu H, Ueno A, Tsutida S, Koiso K, Kurita K, Kawabe K, et al. Clinical effects of propiverine hydrochloride in the treatment of urinary frequency and incontinence associated with detrusor overactivity: a double blind, parallel, placebo controlled multicenter study. Igaku No Ayumi (Progress in Medicine) 1990;153:459‐71. [MEDLINE: ] [Google Scholar]
Tapp 1987 {published data only}
- Tapp A, Fall M, Norgaard J, Massey A, Choa R, Carr T, et al. A dose‐titrated, multicentre study of terodiline in the treatment of detrusor instability. Neurourololgy and Urodynamics. 6 1987; Vol. 6, issue 3:254‐5. [MEDLINE: ]
Tapp 1989 {published data only}
- Tapp A, Fall M, Norgaard J, Massey A, Choa R, Carr T, Korhonen M, Abrams P. Terodiline: a dose titrated, multicenter study of the treatment of idiopathic detrusor instability in women. Journal of Urology 1989;142(4):1027‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Terodiline 1993 {published data only}
- Terodiline in the Elderly American Multicenter Study Group. Effects of terodiline on urinary incontinence among older non‐ institutionalized women. Journal of the American Geriatrics Society 1993;41(9):915‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Viayna 2004 {published data only}
- Viayna C, Alvarez D, Lopez R, Sanagustin J, Lagunas C. A randomised, placebo‐controlled, dose‐rising study in healthy males volunteers to evaluate safety, tolerability, pharmacokinetics and pharmacodynamics of single oral doses of SVT‐40776, a novel selective M3 antimuscarinic (Abstract). Proceedings of the International Continence Society (34th Annual Meeting) and the International UroGynecological Association; 2004 Aug 23‐27;Paris;. 2004:Abstract number 270. [MEDLINE: ]
Wagg 2003 {published data only}
- Wagg A, Malone‐Lee J. Pressure‐flow variables in patients treated with tolterodine for detrusor overactivity. BJU International 2003;92(9):969‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wein 1999 {published data only}
- Wein AJ, Appell RA. A comparison of the efficacy response profile of tolterodine and oxybutynin. International Urogynaecology Journal. 1999; Vol. 10 (Suppl 1):150.
- Wein AJ, Appell RA. A comparison of the efficacy response profile of tolterodine and oxybutynin [Read by title abstract]. Proceedings of the 29th Annual Meeting of the International Continence Society; 1999 Aug 23‐26; Denver, Colorado. 1999:252‐3.
Whitehead 1967 {published data only}
- Whitehead JA. Urinary incontinence in the aged. Propantheline bromide as an adjunct to treatment. Geriatrics 1967;22(1):154‐8. [PubMed] [Google Scholar]
Williams 1981 {published data only}
- Whitehead JA. Urinary incontinence in the aged. Propantheline bromide as an adjunct to treatment. Geriatrics 1967;22(1):154‐8. [PubMed] [Google Scholar]
- Williams AJ, Prematalake JKTG, Palmer RL. A trial of emepronium bromide for the treatment of urinary incontinence in the elderly mentally ill. Pharmatherapeutica 1981;2(8):539‐42. [PubMed] [Google Scholar]
Additional references
Abrams 1988
- Abrams PH, Blaivas JG, Stanton SL, Andersen JT. Standardisation of terminology of lower urinary tract function. Neurourology and Urodynamics 1988;7(5):403‐27. [Google Scholar]
Abrams 2002
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub‐committee of the International Continence Society. Neurourology and Urodynamics 2002;21(2):167‐78. [DOI] [PubMed] [Google Scholar]
Alhasso 2006
- Alhasso AA, McKinley J, Patrick K, Stewart L. Anticholinergic drugs versus non‐drug active therapies for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2006, Issue 4. [Art. No.: CD003193. DOI: 10.1002/14651858.CD003193.pub3] [DOI] [PubMed] [Google Scholar]
Brieger 1996
- Brieger GM, Yip SK, Hin LY, Chung TK. The prevalence of urinary dysfunction in Hong Kong Chinese women. Obstetrics and Gynecology 1996;88(6):1041‐4. [DOI] [PubMed] [Google Scholar]
Brown 1999
- Brown JS, Grady D, Ouslander JG, Herzog AR, Varner RE, Posner SF. Prevalence of urinary incontinence and associated risk factors in postmenopausal women. Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstetrics and Gynecology 1999;94(1):66‐70. [DOI] [PubMed] [Google Scholar]
Chapple 2005b
- Chapple C, Khullar V, Gabriel Z, Dooley JA. The effects of antimuscarinic treatments in overactive bladder: a systematic review and meta‐analysis. European Urology 2005;48(1):5‐26. [DOI] [PubMed] [Google Scholar]
Choo 2001
- Choo M‐S, Lee YS, Kim HY, Lee JB, Kwon DD, Lee T, et al. The prevalence of overactive bladder in Korea. Proceedings of the International Continence Society. Seoul, 2001.
de Groat 1997
- Groat WC. A neurologic basis for the overactive bladder. Urology 1997;50(Suppl 6A):36‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Higgins JPT, Altman DG, editors. Analysing and presenting results. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [update May 2005]; Section 8. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
Dorschner 2003a
- Dorschner W, Stolzenburg JU, Griebenow R, Halaska M, Brunjes R, Frank M, et al. [The elderly patient with urge incontinence or urge‐stress incontinence ‐ efficacy and cardiac safety of propiverine]. [German]. Aktuelle Urologie 2003;34(2):102‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DuBeau 2000
- DuBeau CE, Miller KL, Bergmann M, Resnick NM. Urge incontinence outcomes in RCTs depend on assumed and not actual drug assignment [Abstract]. Neurourology and Urodynamics 2000;19(4):492. [Google Scholar]
Dublin 2004
- Dublin N, Alhasso AA, Stewart L. Anticholinergic drugs versus other medications for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2004, Issue 2. [Art. No.: CD003190. DOI: 10.1002/14651858.CD003190.pub2] [DOI] [PubMed] [Google Scholar]
Hay‐Smith 2005
- Hay‐Smith J, Herbison P, Ellis G, Morris A. Which anticholinergic drug for overactive bladder symptoms in adults?. Cochrane Database of Systematic Reviews 2005, Issue 3. [Art. No.: CD005429. DOI: 10.1002/14651858.CD005429] [DOI] [PubMed] [Google Scholar]
Hennessey 1999
- Hennessey A, Robertson NP, Swingler R, Compston DA. Urinary, faecal and sexual dysfunction in patients with multiple sclerosis. Journal of Neurology 1999;246(11):1027‐32. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd, 2005. [Google Scholar]
Jackson 1997
- Jackson S. The patient with an overactive bladder ‐ symptoms and quality of life issues. Urology 1997;50(Suppl. 6A):18‐22. [DOI] [PubMed] [Google Scholar]
Kobelt 1997
- Kobelt G. Economic considerations and outcome measurement in urge incontinence. Urology 1997;50(6A suppl):100‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lawrence 2000
- Lawrence M, Guay DR, Benson SR, Anderson MJ. Immediate‐release oxybutynin versus tolterodine in detrusor overactivity: a population analysis. Pharmacotherapy 2000;20(4):470‐5. [DOI] [PubMed] [Google Scholar]
Milsom 2001
- Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population‐based prevalence study. BJU International 2001;87(9):760‐6. [DOI] [PubMed] [Google Scholar]
Moller 2000
- Moller LA, Lose G, Jorgensen T. The prevalence and bothersomeness of lower urinary tract symptoms in women 40‐60 years of age. Acta Obstetrica et Gynecologica Scandinavica 2000;79(4):298‐305. [DOI] [PubMed] [Google Scholar]
Moothy 2001
- Moothy P, Lapitan MC, Lim PHC. Prevalence of overactive bladder in Asian males: an epidemiologic survey. Proceedings of the International Continence Society. Seoul, 2001.
Ouslander 1982
- Ouslander JG, Kane RL, Abrass IB. Urinary incontinence in elderly nursing home patients. JAMA 1982;248(10):1194‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Ouslander 1986
- Ouslander JG, Hepps K, Raz S, Su HL. Genitourinary dysfunction in a geriatric outpatient population. Journal of the American Geriatric Society 1986;34(7):507‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pleil 2005
- Pleil AM, Coyne KS, Reese PR, Jumadilova Z. The Validation of Patient‐Rated Global Assessments of Treatment Benefit, Satisfaction, and Willingness to Continue‐The BSW. Value in Health 2005;8:S25‐34. [DOI] [PubMed] [Google Scholar]
Roland 1998
- Roland M, Torgerson DJ. What are pragmatic trials?. BMJ 1998;316:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2001
- Stewart WF, Corey R, Herzog AR, Wein A, Norton PA, Payne C, et al. Prevalence of overactive bladder in women: results from the Noble Program. International Urogynecology Journal. 2001; Vol. 12 (3):66.
Ueda 2000
- Ueda T, Tamaki M, Kageyama S, Yoshimura N, Yoshida O. Urinary incontinence among community‐dwelling people aged 40 years or older in Japan: prevalence, risk factors, knowledge and self‐perception. International Journal of Urology 2000;7(3):95‐103. [DOI] [PubMed] [Google Scholar]
van Kerrebroeck 2001
- Kerrebroeck PHEV, for the Tolterodine study group. Long‐term tolerability and efficacy of once‐daily (OD) tolterodine in the treatment of overactive bladder (OAB). International Urogynecology Journal. 2001; Vol. 12 (Suppl 3):549.
Wallace 2004
- Wallace S, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2004, Issue 1. [Art. No.: CD001308. DOI: 10.1002/14651858.CD001308.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


