Abstract
Background
Primary biliary cirrhosis is a chronic progressive cholestatic liver disease of presumed autoimmune etiology, characterised by the destruction of small intrahepatic bile ducts and the eventual development of cirrhosis and liver failure. Its progression may be influenced by immunosuppression. Glucocorticosteroids are potent immunosuppressive agents, but they are associated with significant adverse effects, including osteoporosis.
Objectives
To systematically evaluate the beneficial and harmful effects of glucocorticosteroids versus placebo or no intervention for patients with primary biliary cirrhosis.
Search methods
The Cochrane Hepato‐Biliary Controlled Trials Register, The Cochrane Library, MEDLINE, EMBASE, and the full text of the identified studies were searched until June 2004. The search strategy included terms for primary biliary cirrhosis and glucocorticosteroids (including the names of frequently used preparations). Previous research groups and manufacturers were contacted for additional references. No language restrictions were applied.
Selection criteria
Double‐blind, single‐blind, or unblinded randomised clinical trials evaluating any preparation of glucocorticosteroids versus placebo or no intervention in patients with primary biliary cirrhosis diagnosed by abnormal liver function tests and either anti‐mitochondrial antibodies or histology were included. Additional agents were allowed if they were administered to both groups equally.
Data collection and analysis
The quality of the randomised clinical trials was evaluated by methodology components (generation of allocation sequence; allocation concealment; blinding; follow up). Analyses were performed according to the intention‐to‐treat method with missing data being accounted for by imputation.
Main results
Only two underpowered trials (reporting 36 and 40 patients) were identified. These differed markedly in their inclusion criteria and treatment protocols. Both stated that they used placebo. However, allocation concealment was unclear. Only one trial reported any patient deaths. No significant improvement in mortality was identified (odds ratio (OR) 0.42, 95% confidence interval (CI) 0.10 to 1.76). Improvements in serum markers of liver inflammation and liver histology were identified. Potentially prognostically linked markers such as bilirubin and albumin were incompletely reported. Bone mineral density (weighted mean difference ‐2.84%, 95% CI ‐4.16 to ‐1.53) and the number of patients with any adverse event (OR 8.99, 95% CI 2.15 to 37.58) were significantly increased in the glucocorticosteroid group.
Authors' conclusions
There is insufficient data to support or reject the use of glucocorticosteroids for patients with primary biliary cirrhosis. It may be appropriate to consider a large prospective randomised clinical trial on this topic.
Plain language summary
No evidence to support or refute glucocorticosteroids for patients with primary biliary cirrhosis
Primary biliary cirrhosis is a chronic progressive cholestatic liver disease of presumed autoimmune aetiology. The clinical course might be improved by glucocorticosteroids. Only two small randomised clinical trials on this topic were identified. The trials were not large enough in terms of sample size or length of follow up to allow changes in mortality to be adequately evaluated. Glucocorticosteroids were associated with improvement in serum markers of inflammation and liver histology, both of which were of uncertain clinical significance. Glucocorticosteroids were also associated with adverse events, including reduced bone mineral density. Further trials are necessary if the effectiveness of glucocorticosteroids is to be properly evaluated.
Background
Primary biliary cirrhosis (PBC) is a chronic liver disease characterised by the presence of serum autoantibodies (predominantly the highly disease specific anti‐mitochondrial antibody (AMA)) and progressive portal inflammation leading eventually to a varying degree of cholestasis and in a proportion of patients to cirrhosis and the complications of liver failure (Kaplan 1996). Patients with PBC represent a highly heterogeneous group. Presentation with the complications of liver failure is now rare, and many patients present with asymptomatic disease found during investigation of unrelated problems or during 'well person screening' (Prince 2000).
The aetiology of PBC is unknown but may reflect an autoimmune process given the presence of autoantibodies, a T‐lymphocyte predominant hepatic infiltrate, and peripheral up‐regulation of alloreactive T‐lymphocytes (Palmer 1999). Given that the hepatic damage in PBC may be immunologically mediated, a number of immune modulating therapies have been investigated for their effects on prognosis. These have included azathioprine (Christensen 1985), chlorambucil (Hoofnagle 1986), cyclosporine (Wiesner 1990), colchicine (Kaplan 1986; Warnes 1987; Vuoristo 1995; Gong 2004), D‐penicillamine (Epstein 1981; Matloff 1982; Neuberger 1985; Dickson 1985), and methotrexate (Kaplan 1991; Lindor 1995). However, the results of these trials have not lead to a widespread use of any of these drugs (Verma 1999).
Ursodeoxycholic acid is a hydrophilic bile acid that has been shown to increase bile flow in animals and to replace a significant proportion of the circulating bile acid pool in humans following oral administration (Combes 1999b). Ursodeoxycholic acid may therefore decrease the biliary concentration of potentially hepatotoxic bile acids. Ursodeoxycholic acid may also have local immunomodulatory effects. Ursodeoxycholic acid has been evaluated versus placebo or no intervention for PBC in 16 randomised clinical trials (RCTs) (Gluud 1999). Eleven of these RCTs were included in a meta‐analysis (Goulis 1999) and all 16 have been analysed in a Cochrane systematic review (Gluud 2001a). Neither of these reviews have reported a statistically significant effect of ursodeoxycholic acid on mortality, although serum (s)‐bilirubin levels were significantly reduced.
Glucocorticosteroids have widespread immunomodulatory effects including down regulation of lymphocyte immune responses (Cupps 1982). Glucocorticosteroids have been reported to be beneficial to liver function tests in PBC (Mitchison 1992). However, they have not entered widespread clinical use. This may be partly due to concerns regarding the effect of glucocorticosteroids on bone mineral density (Stellon 1985). Early reports of PBC observed a high incidence of osteoporosis and osteomalacia (Ahrens 1950; Atkinson 1956). The majority of patients are now less frankly cholestatic at the time of diagnosis than in these early reports and osteoporosis may be no more common in patients with PBC than in age‐ and sex‐matched controls (Newton 2001). We have been unable to identify meta‐analyses or systematic reviews on glucocorticosteroids for patients with PBC.
Objectives
The objectives are, on the basis of randomised clinical trials, to evaluate the effects of glucocorticosteroids tested versus placebo or no intervention in patients with PBC on mortality, need for liver transplantation, clinical symptoms, liver biochemistry, liver histology, and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
The randomised clinical trials may be unpublished or published as an article, an abstract, or a letter, with no language limitations applied. The randomised clinical trials should use a proper method of randomisation. Thus, trials using quasi‐randomisation (for example alternation) were excluded. Randomised clinical trials were included if they were double‐blind, single‐blind, or unblinded.
Types of participants
Patients with PBC diagnosed by any method encompassing elevated serum activity of alkaline phosphatases (or other markers of intrahepatic cholestasis), and at least one of the following: a positive result for serum mitochondrial antibody or liver biopsy findings diagnostic for or compatible with PBC.
Types of interventions
Administration of peroral, intramuscular, or intravenous glucocorticosteroids of any type including prednisone, prednisolone, budesonide, beclomethasone, etc. tested versus placebo or no intervention. Collateral interventions with other immunosuppressive medication, ursodeoxycholic acid, or other interventions are allowed as long as both intervention arms of the randomised clinical trial receive similar collateral interventions.
Types of outcome measures
The following outcome measures were assessed:
(1) Number of patients dying (primary outcome measure). (2) Number of patients undergoing liver transplantation (primary outcome measure). (3) Number of patients dying or undergoing liver transplantation (primary outcome measure). (4) Pruritus. (5) Other clinical symptoms (jaundice, (bleeding) oesophageal varices, (bleeding) gastric varices, ascites, hepatic encephalopathy, hepato‐renal syndrome, sicca complex, fatigue). (6) Liver biochemistry. (7) Liver biopsy findings. (8) Quality of life. (9) Adverse events (including clinical Addisonianism, biochemical hypoadrenalism, diabetes mellitus, osteoporosis, and infective episodes). The adverse events are defined as any untoward medical occurrence in a patient in either of the two arms of the included randomised clinical trials, which did not necessarily have a causal relationship with the treatment, but did, however, result in a dose reduction, discontinuation of treatment, or registration of the event as an adverse event/side effect. The adverse events are subdivided into non‐serious adverse events and serious adverse events according to the ICH‐GCP guidelines (ICH‐GCP 1997). Serious adverse events are any event that: led to death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or congenital anomaly/birth defect, any important medical event which may have jeopardised the patient or required intervention to prevent it. (10) Health economics: the estimated costs connected with the interventions were weighed against any possible health gains.
Search methods for identification of studies
The Cochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Library, MEDLINE, EMBASE, and full text searches were combined until June 2004 (see Appendix 1). Further trials were sought by reading the reference lists of the identified studies. We wrote to the principal authors of the identified randomised clinical trials and enquired about additional randomised clinical trials they might know of. We also wrote to the pharmaceutical companies involved in the production of non‐generic glucocorticosteroid preparations to obtain unpublished randomised clinical trials.
Data collection and analysis
The meta‐analysis was performed following the recommendations given by The Cochrane Collaboration and Sacks et al (Sacks 1987).
Patient characteristics, diagnosis, and interventions The following items were recorded from the individual randomised clinical trials: mean (or median) age, sex ratio, stage of PBC, mean duration of PBC, other baseline characteristics including serum (s)‐bilirubin concentration, type, dose and form of glucocorticosteroid intervention, and type of intervention in the control group, other immunosuppressive medication or ursodeoxycholic acid or other collateral interventions.
Selection and data‐extraction All randomised clinical trials considered for inclusion were analysed independently by two of the contributors (MP and CG), who conferred in cases of disagreement. Disagreements were solved by discussion or the third reviewer (EC) functioning as an ombudsman. All randomised clinical trials in this review had pertinent data extracted independently by two contributors (MP and CG), who conferred in the case of disagreement. Disagreements were solved by discussion or the third reviewer (EC) functioning as an ombudsman.
We wrote to all groups having conducted randomised clinical trials on glucocorticosteroids for PBC including a list of identified trials, asking the trialists about any additional trials they might know of. We also asked the trialists to clarify any detail of their randomised clinical trial that was insufficiently described in the published reports.
All identified trials are listed and trials excluded from the meta‐analysis of the review are identified with the reason for exclusion.
We extracted data for the entire time patients were on glucocorticosteroid versus placebo/no intervention in order to secure data from the most unbiased comparisons. However, where possible we also extracted data on mortality and/or liver transplantation from the maximal follow‐up of each randomised clinical trial, including data from patients switched from blinded to open label therapy.
Methodological quality of included randomised clinical trials The methodological quality of the randomised clinical trials was assessed by the following components: generation of the allocation sequence, allocation concealment, blinding, and follow up (Schluz 1995; Moher 1998; Kjaergard 2001).
Generation of the allocation sequence Adequate: by table of random numbers, computer generated random numbers, coin tossing, shuffling or similar. Unclear: if the trial was described as randomised, but the method used for the allocation sequence generation was not described. Inadequate: if a system involving dates, names, or admittance numbers were used for the allocation of patients. Such trials were excluded from the review.
Allocation concealment Adequate: if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes. Unclear: if the trial was described as randomised, but the method used to conceal the allocation was not described. Inadequate: if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised. Such studies were excluded.
Blinding Adequate: if the trial was described as double blind and the method of blinding involved identical placebo or active drugs. Unclear: if the trial was described as double blind, but the method of blinding was not described. Not performed: if the trial was not double blind or the method of blinding was inappropriate.
Follow‐up Adequate: if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals. Unclear: if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated. Inadequate: if the number or reasons for dropouts and withdrawals were not described.
It was intended to perform subgroup analyses, in which trials were grouped according to the adequacy of generation of the allocation sequence, allocation concealment, double‐blinding, and follow‐up (Kjaergard 2001). The major intended subgroup analysis was to stratify the trials into: (1) High‐quality randomised clinical trials, ie, those having adequate generation of the allocation sequence, allocation concealment, blinding, and follow‐up. (2) Intermediate‐quality randomised clinical trials, ie, those being inadequate according to one of these components. (3) Low‐quality randomised clinical trials, ie, those being inadequate according to more than one of these components.
Statistical methods All analyses were performed according to the intention‐to‐treat method, ie, including all randomised patients. The statistical package (RevMan Analyses) provided by The Cochrane Collaboration was used. We examined intervention effects by using both a random‐effects model (DerSimoian 1986) and a fixed‐effect model (DeMets 1987) with the significance level set at P < 0.05. If the results of the two analyses led to the same conclusion, we presented only the results of the fixed‐effect analysis. In case of discrepancies of the two models, we reported the results of both models. We explored the presence of statistical heterogeneity by chi‐squared test with significance set at P < 0.10 and measured the quantity of heterogeneity or inconsistency by I2 (Higgins 2002). Due to the few anticipated trials and the relative large number of outcomes that we assessed, we interpreted significant results with caution. Whenever the random model is used the 95% confidence interval (CI) is suffixed 'random'. In cases where significant heterogeneity is found, the potential causes for heterogeneity will be explored by performing sensitivity analyses.
Sensitivity analyses were planned for major outcome measures (death; death or liver transplantation; symptoms; liver biochemistry; liver histology) taking methodological quality, dose, type and duration of glucocorticosteroid treatment, additional therapy, and PBC severity estimated from the s‐bilirubin level at entry into consideration. We performed sensitivity analyses to assess the impact of patient withdrawal or loss of follow‐up on primary outcomes ('intention‐to‐treat analysis using imputation' (Alderson 2004)). In the 'best‐worst‐case' scenario analyses, we assumed that none of glucocorticosteroid‐group patients but all control dropouts had the primary outcomes. In the 'worst‐best‐case' scenario analyses, we assumed the opposite: all dropouts from the glucocorticosteroid‐group but no controls had primary outcomes. In the reported scenario, we assumed that none of glucocorticosteroid and controls dropouts had primary outcomes, whereas in the likely scenario, we assumed that corticosteroid and placebo dropouts both had the worst outcome.
For secondary outcomes, we adopted 'available case analysis', ie, include data on only those whose results are known, using a denominator the total number of patients who completed the trial for the particular outcome in question. Thus, in the review, the number of patients as the denominator might change according to the secondary outcomes investigated.
Funnel plot asymmetry was planned to be explored by regression analyses (Egger 1997).
Results
Description of studies
Search results The literature search yielded 168 references. MEDLINE identified 122 references. The Cochrane Library identified 118 references, 30 of which were not on MEDLINE. EMBASE identified 24 references, 12 of which were not identified by the previous two methods. The Cochrane Hepato‐Biliary Group Controlled Trials Register identified 22 references, four of which were new (all of which were meeting abstracts). Nine further references were identified from reference lists.
Direct contact to 27 researchers who were known to be active in the study of PBC or senior authors of previous randomised trials of therapy for PBC did not identify any further completed trials. Three authors, however, provided details of potentially relevant randomised clinical trial protocols. However, all three studies were abandoned prior to randomisation. One of these trials was a European multicentre study. The other two were single centre studies based in England and Greece.
Out of these, 140 references were clearly irrelevant to this review and could be excluded on the basis of their titles and abstracts. The 43 remaining references described 16 studies of glucocorticosteroid usage for PBC patients.
The search strategy was repeated in all databases in June 2004. This updated search also corrected for a typographical error that had occurred in the earlier search (in all four databases the misspelt term "Budenoside" had inadvertently been substituted for "Budesonide"). In this updated search 17 additional references were identified in MEDLINE, 38 in The Cochrane Library, five in EMBASE, and no additional references in The Cochrane Hepato‐Biliary Group Controlled Trials Register. Review of the titles and abstracts of the additional references identified that none of these was relevant to the review.
Included studies Two randomised clinical trials of glucocorticosteroid usage met the criteria for this review. These two trials were described in 10 references. The included studies differed markedly in their inclusion criteria (especially with respect to the severity of liver disease in participants) and treatment protocols.
The Newcastle 1989 trial excluded patients with 'early' PBC, defined as Scheuer histological 'stage‐one' disease. A significant number of patients in this trial had advanced PBC, with 14 (39%) out of 36 patients having cirrhosis on initial liver biopsy and 19 (53%) patients having initial s‐bilirubin greater than twice the upper limit of normal (mean s‐bilirubin in all patients 40.0 µmol/L). In contrast the Frankfurt 1999 trial excluded patients with advanced PBC defined as cirrhosis on initial biopsy, varices, ascites, or hepatic encephalopathy. Six of 39 patients had Ludwig 'stage‐one' disease. No data on initial s‐bilirubin levels were reported in this trial.
Patients in the Newcastle 1989 trial received prednisolone (initially 30 mg daily, titrated down to 10 mg daily maintenance over eight weeks) versus placebo and were followed up for a maximum of three years. All patients received intramuscular vitamin D and oral calcium supplements as prophylaxis against osteoporosis. No patients received ursodeoxycholic acid during the trial.
Patients in the Frankfurt 1999 trial received budesonide (3 mg three times daily) versus placebo for two years. All patients also received ursodeoxycholic acid 10‐15mg/kg daily. All patients were either previously untreated with ursodeoxycholic acid (n = 24) or had ursodeoxycholic acid omitted for 10 weeks prior to enrolment (n = 15). Previous use of ursodeoxycholic acid was not reported for one patient. Patients in Frankfurt 1999 did not receive prophylaxis for osteoporosis.
The Newcastle 1989 trial excluded patients over age 70 or those who had had recent glucocorticosteroid therapy. Frankfurt 1999 excluded patients with diabetes, pregnancy, glaucoma, peptic ulceration, or uncontrolled hypertension. Both trials were small (36 and 40 patients respectively). Both trials used similar high‐standard definitions of PBC that included histological confirmation.
Both trial authors responded to requests for additional data. No additional data were available for the Frankfurt 1999 trial. An unpublished medical doctor dissertation gave additional data for the Newcastle 1989 trial.
Excluded studies A total of 14 studies reported in 33 references were excluded for the reasons given in the Table of excluded studies. Eleven of these studies were case series comparing the features of patients before and after steroid therapy with no control group. One study was excluded as it included a small number of patients with PBC along with patients with other liver diseases but it was not possible to obtain data for the PBC patients alone. One study was excluded because it used alternate allocation rather than randomisation. One study was excluded as the patients received either glucocorticosteroids and azathioprine or double placebo and was thus examining the effect of combined immunosuppression rather than glucocorticosteroids.
Risk of bias in included studies
Neither of the included trials reported power calculations to assess sample size. Both trials were considerably underpowered to identify clinically relevant outcomes. Neither trial defined a primary outcome measure a priori. The Newcastle 1989 trial used a post hoc derived composite outcome measure.
Both trials reported adequate methods for generating randomisation schedules (random number tables in the Newcastle 1989 and computerised methods in Frankfurt 1999). The Newcastle 1989 trial randomised patients in pairs (matched by age, menopausal status, and liver disease severity). It was not possible to match two patients and these were randomised separately. Analysis did not, however, use paired statistical tests.
No methods of allocation concealment were described.
Both trials used identical placebos to maintain blinding during the first year. The Newcastle 1989 trial was unblinded after the first year for unstated reasons. Nether trial reported assessments of the success of the blinding. This is especially relevant to the Newcastle 1989 trial where a high incidence of steroid‐related cosmetic effects were reported (see below).
Follow up was adequate in the Newcastle 1989 trial. Follow up was unclear in the Frankfurt 1999 trial where the exact number of patients lost to follow up varies between abstracts which describe no loss to follow up (two abstract reports, but very little detail reported and no mortality data recorded), three losses (one abstract) and one loss (peer reviewed report). The authors attributed these differences to "reclassification" (personal contact 2003). The primary outcomes are assessed using data for all patients in the Frankfurt 1999 trial using the most complete data set (one loss to follow up), with the effect of this loss being examined through intention‐to‐treat analysis using imputation as described above.
Effects of interventions
Number of deaths Eight deaths occurred in the Newcastle 1989 trial; three in steroid‐treated patients (at 26, 33, and 35 months) and five in placebo‐treated patients (at 3, 15, 20, 30, and 36 months). The earliest of these deaths was attributed to deterioration prior to randomisation. All deaths were described as "liver related" although one followed a myocardial infarction (active treatment arm). There were no reported deaths in the Frankfurt 1999 trial. The combined odds ratio for death was 0.45 (95% CI 0.09 to 2.26) during the whole follow up in the reported scenario and worst‐best‐case scenario, and was 0.42 (95% CI 0.10 to 1.76) in the likely and best‐worst‐case scenario. After one year, the odds ratios of death were 0.28 (95% CI 0.01 to 7.40) and 0.30 (95% CI 0.03 to 3.01), respectively. Due to the small number of trials we did not perform any subgroup analysis. Number of liver transplants Only one patient (in the placebo‐treated arm in the Newcastle 1989 trial) underwent liver transplantation around 20 months after inclusion. This patient died shortly afterwards.
Number of deaths and liver transplants As the sole patient undergoing liver transplantation died shortly after surgery, the combined odds ratio for death or transplantation were the same in all analyses as the odds ratios of death alone (see above). Symptoms of liver disease Neither trial reported progression of individual symptoms of liver disease during the trial, nor were standardised methods used to assess any symptoms. The Newcastle 1989 trial reported that itch or fatigue improved during the initial high‐dose phase of treatment in 15 patients in the active treatment arm and none in the placebo arm. This improvement disappeared before the end of the first year of therapy in nine of the steroid‐treated patients. Symptoms during treatment were not reported in the Frankfurt 1999 trial.
Complications of liver disease Complications were more frequent in the Newcastle 1989 trial possibly reflecting the differences in inclusion criteria. Two placebo‐treated patients in the Newcastle 1989 trial are reported as developing jaundice within one year of inclusion, one of whom also developed hepatic encephalopathy. A graph of bilirubin levels in patients in the Newcastle 1989 trial indicates that the number of patients with a s‐bilirubin level greater 50 µmol/L at last recording rose from 5 to 6 in prednisolone‐treated patients and from 4 to 6 in placebo‐treated patients. The number of patients whose bilirubin doubled was three and five, respectively. After three years, two further patients in the placebo‐treated group developed new complications of liver disease (one ascites and one encephalopathy and bleeding).
Two placebo‐treated patients in Frankfurt 1999 trial and three‐placebo treated patients in the Newcastle 1989 trial developed varices. The combined odds ratio of developing any presentation of portal hypertension was 0.13 (95% CI 0.02 to 1.16).
Biochemical variables Both trials reported biochemical variables as percentage change from baseline (except for bilirubin and albumin levels in the Newcastle 1989 trial). As the patients included in these studies differed greatly in their initial disease severity, interpretation and combination of these data may be problematic.
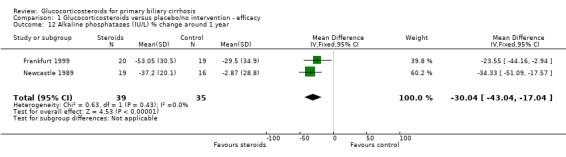
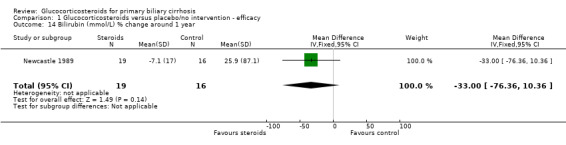
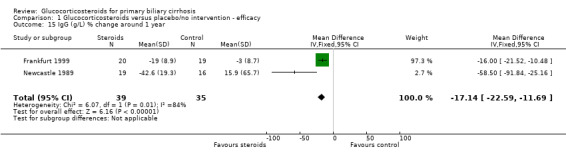
Glucocorticosteroid‐treated patients had a greater proportionate reduction in activity of alkaline phosphatase (WMD ‐30.4%, 95% CL ‐43.0 to ‐17.0), IgG levels (in fixed‐ but not random‐effects models) (‐17.4%, 95% CL ‐22.6 to ‐11.7 (fixed effects); ‐33.9%, 95% CL ‐75.1 to +7.2 (random effects)), and IgM levels (‐23.2%, 95% CL‐36.4 to ‐ 9.7) after one year. Bilirubin and albumin levels were not recorded in the Frankfurt 1999 trial and were not significantly altered by therapy in the Newcastle 1989 trial (both reported as 'non‐significant').
Histological changes A total of 35 patients in the Newcastle 1989 trial underwent repeat liver biopsy after one year. Histology worsened in 1 out of 19 actively treated patients and 6 out of 16 placebo‐treated patients (Fisher's exact test P = 0.02). Interpretation of histology in the Frankfurt 1999 trial is difficult as 13 (33%) patients refused repeat biopsy. Using an unvalidated semi‐quantitative assessment scale, histology worsened more in the placebo‐treated patients (3.5% deterioration versus 30.3% improvement, P < 0.001). The number of patients with improved histology is not reported. It is not possible to provide a combine the histological results in these studies given differing methods of assessment.
Adverse events The frequency of steroid‐related adverse effects differed markedly between the two trials. Nine patients (47%) in the active arm of the Newcastle 1989 trial developed Cushingoid facies and six (32%) developed other problems that were thought to be steroid related (diabetes (n = 2), weight gain (n = 1), duodenal ulcer (n = 1), and furuncles (n = 1)). Seven other non‐hepatic events were recorded in the steroid arm that were not thought to be treatment related. In total, 15 patients developed one or more adverse events (a total of 22 adverse events) reported in the active arm of therapy versus four in the placebo arm. The Frankfurt 1999 trial reported only one patient developing any treatment related adverse event ‐ a single patient who suffered marked suppression of endogenous cortisol secretion and loss of bone mineral density (10.6% at one year). The risk of developing adverse events was significantly increased in the glucocorticosteroid group versus the placebo group (OR 8.99, 95% CI 2.15 to 37.58).
Femoral bone mineral density reduction was greater in steroid patients at one year of follow up than in control patients in both studies. This was significant in fixed but not random effects models. (WMD ‐2.84%, 95% CI ‐4.16 to ‐1.53 (fixed effects); ‐3.01, 95% CI ‐6,86 to ‐0.84 (random effects)). There was, however, considerable heterogeneity between the studies results possibly reflecting the pharmacodynamics of the steroid preparations used.
Quality of life and health economics None of the trials examined specific quality‐of‐life scales or health economics.
Bias detection As only two trials were identified it was not possible to draw meaningful funnel plots on the primary outcome measures or to use other methods to identify trial selection bias.
Discussion
We could only identify two small randomised clinical trials examining the effects of glucocorticosteroids for patients with PBC. There was considerable heterogeneity between these trials in their inclusion criteria, treatment protocols, and methods of outcome assessment. A trend towards improved survival was noted in glucocorticosteroid‐treated patients, but this was based on deaths occurring only in one trial and confidence limits were wide and included unity. Activity of alkaline phosphatases and immunoglobulin levels improved to a greater extent in steroid‐treated patients.
Both trials reported adequate methods to generate the allocation sequence, but otherwise the quality was low. Both trials were markedly underpowered to assess important outcomes such as death and development of complications of liver disease by virtue of their size and short follow up. Neither trial reported adequate allocation concealment. This may bias assessment of outcomes (Schluz 1995; Moher 1998; Kjaergard 2001). Reflecting the year of publication, neither trial used CONSORT statements for the report (Moher 2001) to demonstrate that there was not significant referral or selection bias. Symptoms and adverse events were not recorded systematically making interpretation of these trials unreliable. Although measures were taken to blind the trials with placebo, it is questionable if blinding was maintained. This may bias assessment of outcomes (Schluz 1995; Kjaergard 2001). Further, the Newcastle 1989 trial introduced post hoc composite outcome measures. This introduces outcome measure bias and makes the interpretation of results difficult (Chan 2004). Finally, we are unable to exclude publication bias (Gluud 1998).
There is not enough data to adequately examine the effects of glucocorticosteroids on prognosis. Data on s‐bilirubin and s‐albumin, which may both be related to prognosis (Dickson 1989), were only reported in one trial. Prothrombin time data were not reported in either trial. It is controversial whether use of prognostic markers as proxies of predicted survival in short term studies is valid (Kilmurry 1996; Poupon 1999; Gluud 2001b). There have been no studies of the reliability of such markers specifically in patients treated with glucocorticosteroids.
The significance of the biochemical and histological changes identified is uncertain, since these variables may reflect changes immunological activity but have little if any proven relationship to prognosis.
Neither study used validated assessments of symptoms.
Cosmetic side‐effects were common in patients treated with prednisolone in the Newcastle 1989 study. These were less common with in the Frankfurt 1999 trial, possibly reflecting higher first pass metabolism with budesonide or that patients with milder disease are less likely to have portosystemic shunts that allow oral medication to avoid hepatic processing. Both studies reported increased bone mineral density loss in steroid‐treated patients.
Authors' conclusions
Implications for practice.
There is currently insufficient data available to allow firm conclusions on the clinical importance of glucocorticosteroids for PBC to be drawn. Adverse effects may be frequent.
Implications for research.
Further randomised clinical trials are necessary if the potential effectiveness of glucocorticosteroids for PBC patients is to be assessed. In any such trial there will need to be careful consideration of the inclusion criteria and prophylactic therapy for osteoporosis, including an independent data monitoring and safety committee. Future trials should be reported following the CONSORT recommendations.
What's new
| Date | Event | Description |
|---|---|---|
| 23 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Thanks to all the staff at The Cochrane Hepato‐Biliary Group, especially Dimitrinka Nikolova, for their assistance with identification and access to references. We are grateful to all the authors contacted for providing additional study data. We thank Ronald Koretz and Bodil Als‐Nielson for their helpful comments.
Appendices
Appendix 1. Search Strategies
| Database | Search strategy | Period searched |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | #1 = RCT and 'primary biliary cirrhosis' and corticosteroid*, #2 = RCT and 'primary biliary cirrhosis' and glucocortico*, #3 = RCT and 'primary biliary cirrhosis' and predniso*, #4 = RCT and 'primary biliary cirrhosis' and budesonid*, and #5 = RCT and 'primary biliary cirrhosis' and becl?met?ason. #1 = randomised controlledl trial and 'primary biliary cirrhosis' and corticosteroid*, #2 = randomised controlled trial and 'primary biliary cirrhosis' and glucocortico*, #3 = randomised controlled trial and 'primary biliary cirrhosis' and predniso*, #4 = randomised controlled trial and 'primary biliary cirrhosis' and budesonid*, and #5 = randomised controlled trial and 'primary biliary cirrhosis' and becl?met?ason. #1 = randomised controlledl trial and 'primary biliary cirrhosis' and corticosteroid*, #2 = randomised controlled trial and 'primary biliary cirrhosis' and glucocortico*, #3 = randomised controlled trial and 'primary biliary cirrhosis' and predniso*, #4 = randomised controlled trial and 'primary biliary cirrhosis' and budenosid, and #5 = randomised controlled trial and 'primary biliary cirrhosis' and becl?met?ason. | Inception to June 2004 |
| The Cochrane Library | #1 LIVER‐CIRRHOSIS‐BILIARY*:ME #2 (PRIMARY and (BILIARY and CIRRHOSIS)) or PBC) #3 (((((((((GLUCOCORTICO* or CORTICO*) or PREDNISO*) or METHYLPREDNISO*) or BUDESONID) or BECLMETASON) or BETAMETASON) or HYDROCORTISON) or IMMUNOSUPPRES*) OR (URSODEOXYCHOLIC and ACID)) #4 ADRENAL‐CORTEX‐HORMONES*:ME #5 (#3 or #4) #6 (#1 and #5) #7 (#2 and #5) #8 (#6 or #7) #9 ((RANDOM* or PLACEBO*) OR (DOUBLE and BLIND*)) #10 (#8 and #9) | Inception to June 2004 |
| MEDLINE | #1 explode "Liver‐Cirrhosis‐Biliary"/ all subheadings #2 "primary" #3 "biliary" #4 "cirrhosis" #5 pbc #6 "primary biliary cirrhosis" or pbc #7 glucocortico* #8 cortico* #9 predniso* #10 methylpredniso* #11 budesonid? #12 becl?met?ason? #13 betamet?ason? #14 hydrocortison? #15 immunosuppres* #16 "ursodeoxycholic" #17 "acid" #18 glucocortico* or cortico* or predniso* or methylpredniso* or budenosid? or becl?met?ason? or betamet?ason? or hydrocortison? or immunosuppres* or "ursodeoxycholic acid" #19 explode "Adrenal‐Cortex‐Hormones"/ all subheadings #20 #18 or #19 #21 #1 and #20 #22 #6 and #20 #23 #21 or #22 #24 random* #25 placebo* #26 double #27 blind* #28 random* or placebo* or double blind* #29 #23 and #28 | Inception until JUne 2004 |
| EMBASE | #1 explode 'primary‐biliary‐cirrhosis/ all subheadings #2 explode 'glucocorticoid'/ all subheadings #3 explode 'steroid'/ all subheadings #4 exact{PREDNISONE} #5 predn?so* #6 bud?s?nid* #7 explode 'beclometasone‐dipropionate'/ all subheadings #8 becl?me?a* #9 (#1) and (#2 or #3 or #4 or #5 or #6 or #7 or #8) #10 medication #11 treatment #12 medication #13 medication or treatment #14 random* #15 double‐blind* #16 placebo* #17 altern* #18 #9 and #13 and (random* or double‐blind* or placebo* or altern*) | Inception until June 2004 |
Data and analyses
Comparison 1. Glucocorticosteroids versus placebo/no intervention ‐ efficacy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 1‐year follow up ‐ reported scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.40] |
| 1.2 1‐year follow up ‐ likely scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 3.01] |
| 1.3 1‐year follow up ‐ worst‐best scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.40] |
| 1.4 1‐year follow up ‐ best‐worst scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 3.01] |
| 1.5 Whole of follow up ‐ reported scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.26] |
| 1.6 Whole of follow up ‐ likely scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.10, 1.76] |
| 1.7 Whole of follow up ‐ worst‐best scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.26] |
| 1.8 whole of follow up ‐ best‐worst scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.10, 1.76] |
| 2 Liver transplanation | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Reported scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.40] |
| 2.2 Likely scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 3.01] |
| 2.3 Worst‐best scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.40] |
| 2.4 Best‐worst scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 3.01] |
| 3 Mortality or liver transplantation | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 1‐year follow up ‐ reported scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.40] |
| 3.2 1‐year follow up ‐ likely scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 3.01] |
| 3.3 1‐year follow up ‐ worst‐best scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.40] |
| 3.4 1‐year follow up ‐ best‐worst scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 3.01] |
| 3.5 Whole of follow up ‐ reported scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.26] |
| 3.6 Whole of follow up ‐ likely scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.10, 1.76] |
| 3.7 Whole of follow up ‐ worst‐best scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.26] |
| 3.8 Whole of follow up ‐ best‐worst scenario | 2 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.26] |
| 4 Liver‐related mortality | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 1‐year follow up | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.40] |
| 4.2 Whole of follow up | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.26] |
| 5 No. developing jaundice | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.56] |
| 6 No. with a doubling of s‐bilirubin | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.26] |
| 7 No. developing ascites | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 7.40] |
| 8 No. developing portal hypertension | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 1.16] |
| 9 No. developing hepatic encephalopathy | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.56] |
| 10 Dichotomous histology variables | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 No with worsening of histology | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.88] |
| 10.2 No with new cirrhosis on biopsy | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Albumin (g/L) % change around 1 year | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 3.51 [‐2.74, 9.76] |
| 12 Alkaline phosphatases (IU/L) % change around 1 year | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐30.04 [‐43.04, ‐17.04] |
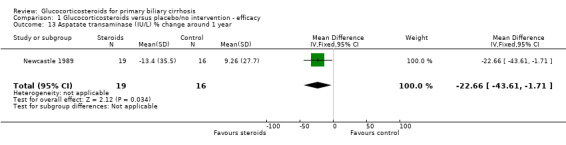
| 13 Aspatate transaminase (IU/L) % change around 1 year | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐22.66 [‐43.61, ‐1.71] |
| 14 Bilirubin (mmol/L) % change around 1 year | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐33.0 [‐76.36, 10.36] |
| 15 IgG (g/L) % change around 1 year | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐17.14 [‐22.59, ‐11.69] |
| 16 IgM (g/L) % change around 1 year | 2 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐23.02 [‐36.35, ‐9.68] |
1.1. Analysis.

Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 1 Mortality.
1.2. Analysis.
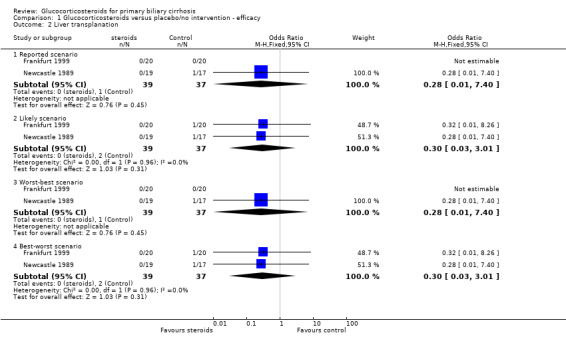
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 2 Liver transplanation.
1.3. Analysis.
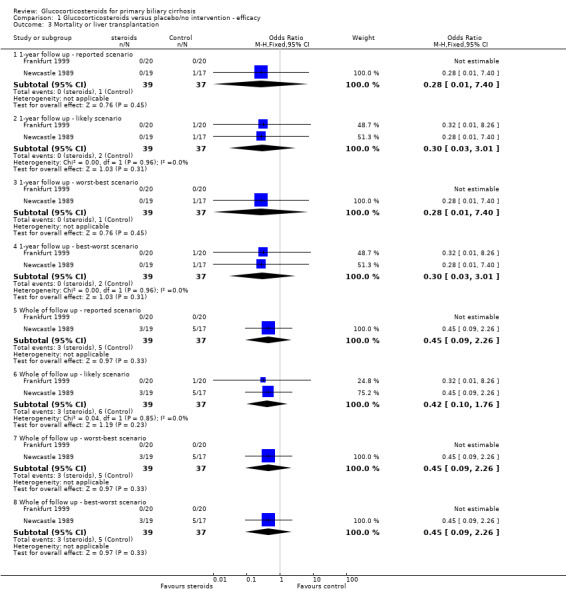
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 3 Mortality or liver transplantation.
1.4. Analysis.
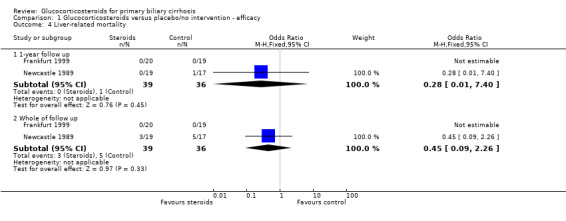
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 4 Liver‐related mortality.
1.5. Analysis.
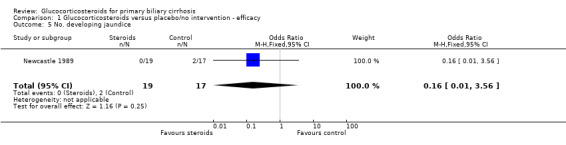
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 5 No. developing jaundice.
1.6. Analysis.
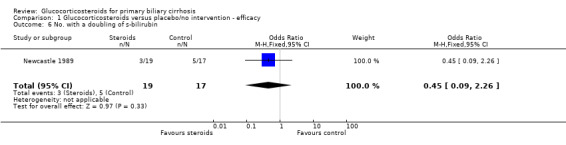
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 6 No. with a doubling of s‐bilirubin.
1.7. Analysis.
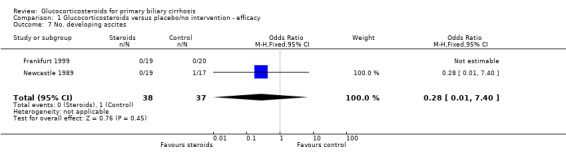
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 7 No. developing ascites.
1.8. Analysis.

Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 8 No. developing portal hypertension.
1.9. Analysis.
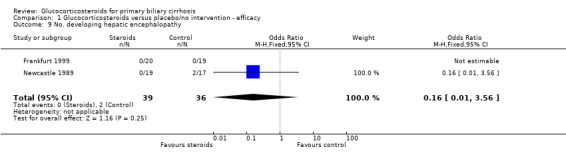
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 9 No. developing hepatic encephalopathy.
1.10. Analysis.
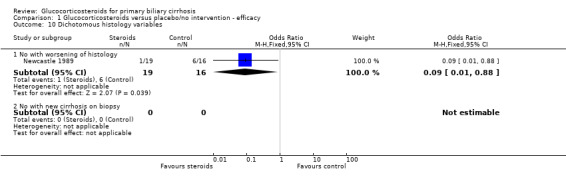
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 10 Dichotomous histology variables.
1.11. Analysis.

Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 11 Albumin (g/L) % change around 1 year.
1.12. Analysis.
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 12 Alkaline phosphatases (IU/L) % change around 1 year.
1.13. Analysis.
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 13 Aspatate transaminase (IU/L) % change around 1 year.
1.14. Analysis.
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 14 Bilirubin (mmol/L) % change around 1 year.
1.15. Analysis.
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 15 IgG (g/L) % change around 1 year.
1.16. Analysis.
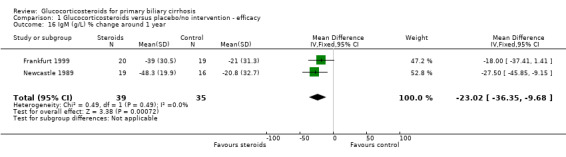
Comparison 1 Glucocorticosteroids versus placebo/no intervention ‐ efficacy, Outcome 16 IgM (g/L) % change around 1 year.
Comparison 2. Glucocorticosteroids versus placebo/no intervention ‐ adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No. with fragility fracture | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Bone mineral density | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐2.84 [‐4.16, ‐1.53] |
| 3 No. clincally Cushingoid | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 31.67 [1.67, 601.97] |
| 4 No. with weight gain over 2.5 kg | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.11, 74.42] |
| 5 Any reported adverse event | 2 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.99 [2.15, 37.58] |
2.1. Analysis.
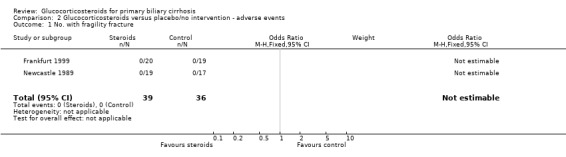
Comparison 2 Glucocorticosteroids versus placebo/no intervention ‐ adverse events, Outcome 1 No. with fragility fracture.
2.2. Analysis.
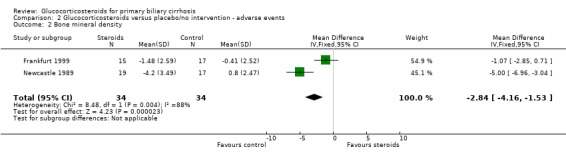
Comparison 2 Glucocorticosteroids versus placebo/no intervention ‐ adverse events, Outcome 2 Bone mineral density.
2.3. Analysis.
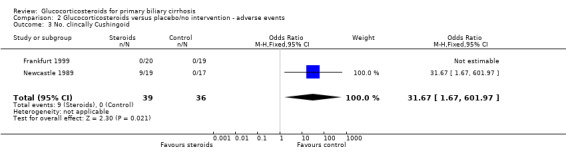
Comparison 2 Glucocorticosteroids versus placebo/no intervention ‐ adverse events, Outcome 3 No. clincally Cushingoid.
2.4. Analysis.

Comparison 2 Glucocorticosteroids versus placebo/no intervention ‐ adverse events, Outcome 4 No. with weight gain over 2.5 kg.
2.5. Analysis.
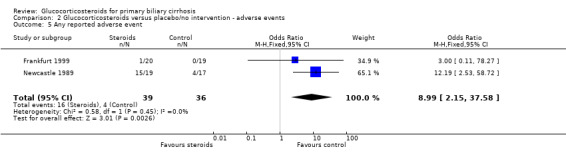
Comparison 2 Glucocorticosteroids versus placebo/no intervention ‐ adverse events, Outcome 5 Any reported adverse event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Frankfurt 1999.
| Methods | Sample size: no justification or power calculation. Generation of allocation schedule: use of computerised random number generator (Rancode). Adequate. Allocation concealment: unclear. Blinding: placebo (details of placebo being sought). Intention‐to‐treat: no (exclusion of one patient in active arm who withdrew after three weeks). Interim analyses: no. |
|
| Participants | Patients with PBC (n = 40) from Germany. PBC defined biochemically and histologically (no further details stated, further details sought from authors). Ultrasonography and ERCP was used to exclude extrahepatic obstruction. Patients (n = 15) previously using ursodeoxycholic acid had this stopped for about 10 weeks prior to study entry. Exclusion criteria were: stage IV disease (Ludwig criteria), oesophageal varices, ascites, hepatic encephalopathy, diabetes mellitus, glaucoma, history of peptic ulceration, hypertension, pregnancy. refusal to use corticosteroids, age < 03 years, PBC treatment within the previous 4 weeks, and alcohol or drug abuse. |
|
| Interventions | Experimental: budesonide 3 mg three times daily
plus
ursodeoxycholic acid 10‐15 mg/kg/day in three divided doses. Control: placebo plus ursodeoxycholic acid 10‐15 mg/kg/day in three divided doses. Duration: two years followed by two months washout. |
|
| Outcomes | Mortality. Serum biochemistry. Bone mineral density. Histology. Liver biochemistry. Liver histology. Autoantibodies. Immunoglobulins. Adrenal biochemistry. Bone mineral density. | |
| Notes | No deaths reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Newcastle 1989.
| Methods | Sample size: no justification or power calculation. Generation of allocation schedule: random number table in pair‐wise fashion. Adequate. Allocation concealment: unclear. Blinding: double blind for one year. Single blind ( patient blinded only for subsequent two years). Details of placebo appearance sought from authors. Intention‐to‐treat: yes. Interim analyses: after one year. |
|
| Participants | Patients with PBC (n = 36) from England. PBC defined as all four of typical clinical features, cholestatic liver blood tests, positive AMA, compatible or diagnostic histology. Exclusion criteria were: age over 70 years, mild histological disease (stage 1 Scheuer), treatment with other disease altering medication within four months of most recent biopsy. |
|
| Interventions | Experimental: prednisolone initially 30 mg/day then reduced by 5 mg/day every two weeks until a maintenance dose of 10 mg/day was reached.
Intramuscular vitamin D
(100,000 units on alternate months). Calcium hydroxyapatite (4.8 g/day). Control: placebo tablets administered in identical fashion to prednisolone. Vitamin D and calcium as for experimental group. Duration: three years. |
|
| Outcomes | Mortality. Serum biochemistry. Bone mineral density. Histology. Adverse events Symptoms. Liver biochemistry. Liver histology. Immunoglobulins. Bone histology. Biochemical markers of bone turnover. Bone mineral density. Combined hepatic assessment score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beukers 1988 | Not a randomised clinical trial. This is a report of two consecutive uncontrolled studies. In the first study ciclosporine monotherapy was assessed. In the second study the effect of ciclosporine and prednisolone was assessed. Therefore it is not possible to determine the additive effect of prednisolone. |
| Carman 1955 | This is an uncontrolled case series of patients treated with glucocorticosteroids for PBC. |
| Fracchia 2000 | Not a randomised clinical trial. Response to intervention with prednisolone and ursodeoxycholic acid for nine patients with PBC compared to that of 14 healthy controls and 14 patients with non‐cirrhotic hepatitis C. |
| Hempfling 2003 | Not a randomised clinical trial. Uncontrolled prospective evaluation of budesonide in 19 patients with PBC concurrently taking ursodeoxycholic acid. |
| Leuschner 1996 | Not a randomised clinical trial. It is a placebo controlled quasi‐randomised study where allocation was made by alternation. |
| Mayo 1951 | A single uncontrolled case report of cortisone usage in a patient with PBC. |
| Mayo 1972 | This is a four‐armed randomised clinical trial comparing prednisolone versus azathioprine versus combination therapy with prednisolone plus azathioprine versus placebo. Ths trial combined the results of patients with a variety of chronic liver diseases (n = 82), including PBC (n = 7). It was not possible to obtain the results for the PBC patients alone. |
| Mayo 2000 | Not a randomised clinical trial. This is a before and after study (n = 22 patients) of budesonide usage in patients with liver function tests that did not normalise on ursodeoxycholic acid monotherapy. |
| Okamota 1999 | Not a randomised clinical trial but an uncontrolled study examining before and after changes in immunological markers in three patients treated with prednisolone. |
| Taal 1985 | Not a randomised study. Longitudinal study of the effects of penicillamine on cryoglobulin levels in PBC. |
| van Berkum 1990 | Not a randomised clinical trial. Retrospective analysis of patients allocated to glucocorticosteroids by clinical decision. No placebo group. |
| Wilson 1958 | This is a non‐randomised retrospective review of progression of disease in 14 patients. |
| Wolfhagen 1994 | This is a retrospective case report of steroid usage in seven patients. There is no control group. |
| Wolfhagen 1998 | This is a placebo controlled, double‐blind randomised trial of the effect of prednisolone and azathioprine plus ursodeoxycholic acid versus double placebo plus ursodeoxycholic acid. Therefore, the effect being measured is that of combination immunosuppression rather than of glucocorticosteroids alone. The trial contains a nested randomised clinical trial of etidronate in active arm. |
PBC = primary biliary cirrhosis
Contributions of authors
MP co‐wrote the search strategies, selected trials, co‐extracted the data, inputted the data, contacted other researchers for additional data, and drafted the review text.
EC acted as ombudsman for disagreements between the other reviewers, validated data extraction, and edited the review text.
CG devised the systematic review, co‐wrote the search strategies, co‐selected the trials, co‐extracted the data, and edited the review text.
Sources of support
Internal sources
Copenhagen Hospital Corporation, Denmark.
External sources
Medical Research Council, UK.
Danish Medical Research Council's Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Frankfurt 1999 {published data only (unpublished sought but not used)}
- Leuschner M, Maier KP, Schitling J, Stefan S, Herrmann G, Dahm HH, et al. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double‐blind trial. Gastroenterology 1999;117(10):918‐25. [DOI] [PubMed] [Google Scholar]
- Leuschner M, Maier KP, Schitling J, Stefan S, Herrmann G, Dahm HH, et al. Ursodeoxycholic acid (UDCA) and budesonide (BUD) in the treatment of primary biliary cirrhosis (PBC). A prospective double‐blind trial. Hepatology 1999;30(4 Pt 2):471A. [DOI] [PubMed] [Google Scholar]
- Leuschner M, Maier KP, Schitling J, Strahl R, Herrmann G, Dahm HH, et al. Ursodeoxycholic acid (UDCA) and budesonide (BUD) is superior to UDCA monotherapy in primary biliary cirrhosis (PBC). Journal of Hepatology 1999;30(1):GS4/20. [Google Scholar]
- Leuschner U, Maier KP, Guldutuna S, Parte‐Peterhans S, Leuschner M. Ursodeoxycholic acid in combination with prednisolone or budenoside in therapy of primary biliary cirrhosis. Falk Symposium 93. Dodecht: Kluwer, 1997:299‐302.
- Leuscner U, Maier P, Schitling J, Strahl R, Herrmann G, Leuschner M. Ursodeoxycholic acid and budenoside in the treatment of primary biliary cirrhosis. XV International Bile Acid Meeting Bile Acids and Cholestasis. 1998; Vol. Falk Symposium 108:60‐1.
Newcastle 1989 {published and unpublished data}
- Combes B. Prednisolone for primary biliary cirrhosis ‐ good news, bad news. Hepatology 1989;10(4):511‐3. [DOI] [PubMed] [Google Scholar]
- Mitchison HC, Bassendine MF, Malcolm AJ, Watson AJ, Record CO, James OF. A pilot double blind controlled trial of prednisolone treatment in primary biliary cirrhosis (PBC) [EASL abstract]. Journal of Hepatology 1986;3(Supp 1):S28. [Google Scholar]
- Mitchison HC, Bassendine MF, Malcolm AJ, Watson AJ, Record CO, James OF. A pilot, double‐blind, controlled 1‐year trial of prednisolone treatment in primary biliary cirrhosis: hepatic improvement but greater bone loss. Hepatology 1989;10(4):420‐9. [DOI] [PubMed] [Google Scholar]
- Mitchison HC, Bassendine MF, Watson AJ, Record CO, James OF. Double blind placebo‐controlled trial of prednisolone treatment in primary biliary cirrhosis (PBC): a 3 year update [EASL abstract]. Journal of Hepatology 1989;9:4. [DOI] [PubMed] [Google Scholar]
- Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three‐year results. Journal of Hepatology 1992;15(3):336‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Beukers 1988 {published data only}
- Beukers R, Schalm SW. Effect of cyclosporin and cyclosporin and prenisolone in primary biliary cirrhosis. Transplant Proceedings 1988;20(Suppl 4):340‐3. [PubMed] [Google Scholar]
- Beukers R, Schalm SW. Immunosuppressive therapy for primary biliary cirrhosis. Journal of Hepatology 1992;14(1):1‐6. [DOI] [PubMed] [Google Scholar]
Carman 1955 {published data only}
- Carman CT, Giansiruca JE. Effect of steroid theraapy on the clinical and laboraoty features in primary biliary cirrhosis. Gastroenterology 1955;28(2):193‐215. [PubMed] [Google Scholar]
Fracchia 2000 {published data only}
- Fracchia M, Secreto P, Tabone M, Zaffino C, Pera A, Galatola G. Serum interferon gamma in primary bilary cirrhosis: effect of ursodeoxycholic acid and prednisolone therapy alone and in combination. EJGH 2000;121:463‐88. [DOI] [PubMed] [Google Scholar]
Hempfling 2003 {published data only}
- Elias E. Ursodeoxycholic acid and budesonide for PBC. Unexpected risks and benefits. Hepatology 2003;38:541. [Google Scholar]
- Hempfling W, Grunhage, F, Dilger K, Reichel C, Beuers U, Sauerbrach T. Pharmacokinetics and pharmacodynamic action of budesonide in early stage primary biliary cirrhosis. Hepatology. 2002; Vol. 36, issue 2:422A. [DOI] [PubMed]
- Hempfling W, Grunhage, F, Dilger K, Reichel C, Beuers U, Sauerbrach T. Pharmacokinetics and pharmodynamic action of budesonide in early and late stage PBC. Hepatology 2003;38:196‐202. [DOI] [PubMed] [Google Scholar]
- Hempfling W, Grunhage, F, Dilger K, Reichel C, Beuers U, Sauerbrach T. Portal vein thrombosis in patients with late stage primary biliary cirrhosis treated with budesonide. Hepatology 2002;36(2):423A. [DOI] [PubMed] [Google Scholar]
Leuschner 1996 {published data only}
- Leuschner M. Ursodeoxycholic acid in combination with prednisolone. Progress in Hepatopharmacology 1997;2(1):79‐90. [Google Scholar]
- Leuschner M, Guldutuna S, You T, Hubner K, Bhatti S, Leuschner U. Ursodeoxycholic acid and prednisolone versus ursodeoxycholic acid and placebo in the treatment of early stages of primary biliary cirrhosis. Journal of Hepatology 1996;25(1):49‐57. [DOI] [PubMed] [Google Scholar]
- Leuschner U, Maier KP, Guldutuna S, Parte‐Peterhans S, Leuschner M. Ursodeoxycholic acid in combination with prednisolone or budenoside in therapy of primary biliary cirrhosis. Falk Symposium 93 1997;1:299‐302. [Google Scholar]
Mayo 1951 {published data only}
- Butt HR, Comfort MW, Power MH, Mason HL. Observations on the effect of cortisone acetate in two pateints with hepatic disease. Journal of Laboratory and Clincal Medicine 1951;37:870‐84. [PubMed] [Google Scholar]
Mayo 1972 {published data only}
- Baggenstoss AH, Soloway RD, nSummerskill WHJ, Elveback LR, Schoenfield LJ. Chronic acitve liver disease. Range of histologic lesions and thier response to treatment and evolution. Human Pathology 1972;3(2):183‐98. [DOI] [PubMed] [Google Scholar]
- Uribe M, Wolf AM, Summerskill WHJ. Steroid side effect during therapy of chronic liver disease; what to expect. Gastroenterology 1976;71(5):932. [Google Scholar]
Mayo 2000 {published data only}
- Angulo P, Jorgenesen RA, Keach JC, Dickson ER, Smith C, Lindor KD. Oral budesonide in the treatment of patients with a suboptimal response to UDCA. Hepatology 2000;31:318‐23. [DOI] [PubMed] [Google Scholar]
- Angulo P, Smith C, Jorgenesen RA, Keach JC, Dickson ER, Lindor KD. Budesonide in the treatment of patients with a suboptimal response to UDCA. Hepatology 1999;30(4 Pt 2):471A. [DOI] [PubMed] [Google Scholar]
Okamota 1999 {published data only}
- Okamoto R, Yamamoto K, Yabushita K, Matsumura S, Snimda N, Okano N, Tsuli T. Prednisolone treatment changes T‐cell reptiore in primary biliary cirrhosis. Clonal analysis of T cell receptor. Hepatology 1999;30(4 Pt 2):471A. [Google Scholar]
Taal 1985 {published data only}
- Taal BG, Schalm, SW. Cryoglobulins in primary biliary cirrhosis: prevalence and modulation by immunosuppressive therapy. Zeitschrift für Gastroenterologie 1985;23:228‐34. [PubMed] [Google Scholar]
van Berkum 1990 {published data only}
- Berkum FNR, Beukers R, Birkenhager JC, Kooij PPM, Schalm SW, Pols HAP. Bone mass in women with primary biliary cirrhosis. The relation with histological stage and use of glucocorticoids. Gastroenterology 1990;99:1134‐9. [DOI] [PubMed] [Google Scholar]
Wilson 1958 {published data only}
- Howat HT, Ralston AJ, Varley H, Wilson JAC. The late results of long term treatment of primary biliary cirrhosis by corticosteroids. Revue Internationale d Hepatologie 1966;16:227‐38. [PubMed] [Google Scholar]
- Wilson JAC, Short IA, Varley H, Howat HT. The biochemical changes induced by corticosteroids in xanthomatous biliary cirrhosis. Gastroenterolgia 1958;89:343‐5. [DOI] [PubMed] [Google Scholar]
Wolfhagen 1994 {published data only}
- Wolfhagen FH, Buuren HR, Schalm SW. Combined treatment with ursodeoxycholic acid and prednisone in primary biliary cirrhosis. Netherlands Journal of Medicine 1993;43:A9. [PubMed] [Google Scholar]
- Wolfhagen FH, Buuren HR, Schalm SW. Combined treatment with ursodeoxycholic acid and prednisone in primary biliary cirrhosis. Netherlands Journal of Medicine 1994;44(3):84‐9. [PubMed] [Google Scholar]
Wolfhagen 1998 {published data only}
- Lim AG, Wolfhagen FHJ, Verma A, Buuren HR, Jazrawi RP, Levy JH, et al. Soluble intercellular adhesion molecule‐1 in primary biliary cirrhosis: effect of ursodeoxycholic acid and immunosuppressive therapy. EJHG 1997;9(2):155‐61. [DOI] [PubMed] [Google Scholar]
- Lim AG, Wolfhagen FHJ, Verma A, Buuren HR, Jazrawi RP, Levy JH, et al. Soluble intercellular adhesion molecule‐1 in primary biliary cirrhosis: effect of ursodeoxycholic acid, prenisolone and azathioprine. Falk Symposium 86. 1995.
- Burren H, Wolfhagen FHJ, Lim AG, Verma A, Jazrawi RP, Northfield TC, et al. A randomized placebo‐controlled trial with prednisone/azathioprine in addition to ursodeoxycholic acid in primary biliary cirrhosis. Falk Symposium 75. 1995.
- Wolfhagen FH, Buuren HR, Ouden JW, Hop WC, Leeuwen JP, Schalm SW, et al. Cyclical etidronate in the prevention of bone loss in corticosteroid‐treated primary biliary cirrhosis. A prospective, controlled pilot study. Journal of Hepatology 1997;26(2):325‐30. [DOI] [PubMed] [Google Scholar]
- Wolfhagen FH, Hoogstraten HJ, Buuren HR, Berge Henegouwen GP, Kate FJ, Hop WC, et al. Triple therapy with ursodeoxycholic acid, prednisone and azathioprine in primary biliary cirrhosis: a 1‐year randomized, placebo‐controlled study. Journal of Hepatology 1998;29(5):736‐42. [DOI] [PubMed] [Google Scholar]
- Wolfhagen FHJ, Lim AG, Verma A, Buuren HR, Jazrawi RP, Northfield TC, et al. Soluble ICAM‐1 in primary biliary cirrhosis (PBC) during combined treatment with ursodeoxycholic acid, prednisone and azathioprine [abstract]. Netherlands Journal of Medicine 1994;47:A29‐30. [Google Scholar]
- Wolfhagen FHJ, Buuren HR, Berge Henegouwen GP, Hattum J, Ouden JW, Kerbert MJ, et al. A randomized placebo‐controlled trial with prednisone/azathioprine in addition to ursodeoxycholic acid in primary biliary cirrhosis. Journal of Hepatology 1994;21(Supp 1):S49. [Google Scholar]
- Wolfhagen FHJ, Buuren HR, Berge Henegouwen GP, Hattum J, Ouden JW, Kerbert MJ, et al. Prednisone/azathioprine treatment in primary biliary cirrhosis (PBC). A randomized, placebo‐controlled trial [abstract]. Netherlands Journal of Medicine 1995;46:A10. [Google Scholar]
- Hoogstraten HJF, Wolfhagen FHJ, Berge Henegouwen GP, Schalm SW, Kate FJW, Hop WCJ, et al. Combined bile acid‐immunocuppressive therapy for primary biliary cirrhosis. Results of a 1‐year multi‐centre, placebo controlled trial. Hepatology 1996;24:168A. [Google Scholar]
- Hoogstraten HJF, Wolfhagen FHJ, Berge Henegouwen GP, Schalm SW, Kate FJW, Hop WCJ, et al. Combined bile acid‐immunosuppresive therapy for primary biliary cirrhosis. Results of a l‐year multi centre, placebo controlled trial [abstract]. EJGH 1996;8(12):A41. [Google Scholar]
Additional references
Ahrens 1950
- Ahrens E, Payne M, Kunkel H, Eisenmenger E, Blondheim S. Primary biliary cihrrosis. Medicine 1950;29:299‐366. [DOI] [PubMed] [Google Scholar]
Alderson 2004
- Alderson P, Green S, Higgins JPT, editors. Cochrane Reviewers' Handbook 4.2.1. Chichester, UK: John Wiley & Sons, Ltd, 2004. [Google Scholar]
Atkinson 1956
- Atkinson M, Nordin BEC, Sherlock S. Bone disease in obstructive jaundice. Quarterly Journal of Medicine 1956;25:299‐312. [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [DOI] [PubMed] [Google Scholar]
Christensen 1985
- Christensen E, Neuberger J, Crowe J, Altman DG, Popper H, Portmann B, et al. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology 1985;89(5):1084‐91. [DOI] [PubMed] [Google Scholar]
Combes 1999b
- Combes B, Carithers R L, Maddrey WC, Munoz S, Garcia Tsao G, Bonner GF, et al. Biliary bile acids in primary biliary cirrhosis: effect of ursodeoxycholic acid. Hepatology 1999;29(6):1649‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cupps 1982
- Cupps TR, Fauci AS. Coritcosteroid‐mediated immunregulation in man. Immunological reviews 1982;65:133‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods of combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6:341‐8. [DOI] [PubMed] [Google Scholar]
DerSimoian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dickson 1985
- Dickson ER, Fleming TR, Wiesner RH, Baldus WP, Fleming CR, Ludwig J, et al. Trial of penicillamine in advanced primary biliary cirrhosis. New England Journal of Medicine 1985;312:1011‐5. [DOI] [PubMed] [Google Scholar]
Dickson 1989
- Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology 1989;10:1‐7. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 1981
- Epstein O, Jain S, Lee RG, Cook DG, Boss AM, Scheuer PJ, et al. D‐penicillamine treatment improves survival in primary biliary cirrhosis. Lancet 1981;1(8233):1275‐7. [DOI] [PubMed] [Google Scholar]
Gluud 1998
- Gluud C. 'Negative trials' are positive!. Journal of Hepatology 1998;28:731‐3. [DOI] [PubMed] [Google Scholar]
Gluud 1999
- Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis (Protocol for a Cochrane Review). The Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI] [PubMed] [Google Scholar]
Gluud 2001a
- Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis (Cochrane Review). The Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI] [PubMed] [Google Scholar]
Gluud 2001b
- Gluud, C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis: lesson for the future?. Jounal of Hepatology 2001;34:787‐8. [DOI] [PubMed] [Google Scholar]
Gong 2004
- Gong Y, Gluud C. Colchicine for primary biliary cirrhosis (Cochrane Review). The Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI] [PubMed] [Google Scholar]
Goulis 1999
- Goulis J, Lerandro G, Burroughs A. Randomised controlled trials of ursodeoxycholic‐acid therapy for primary biliary cirrhosis: a meta‐analysis. Lancet 1999;354:1053‐60. [DOI] [PubMed] [Google Scholar]
Hoofnagle 1986
- Hoofnagle JH, Davis GL, Schafer DF, Peters M, Avigan MI, Pappas SC, et al. Randomized trial of chlorambucil for primary biliary cirrhosis. Gastroenterology 1986;91:1327‐34. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. Code of Federal Regulations & International Conference on Harmonization Guidelines. Media: Parexel Barnett, 1997. [Google Scholar]
Kaplan 1986
- Kaplan MM, Alling DW, Zimmerman HJ, Wolfe HJ, Sepersky RA, Hirsch GS, et al. A prospective trial of colchicine for primary biliary cirrhosis. New England Journal of Medicine 1986;315:1448‐54. [DOI] [PubMed] [Google Scholar]
Kaplan 1991
- Kaplan MM, Knox TA. Treatment of primary biliary cirrhosis with low‐dose weekly methotrexate. Gastroenterology 1991;101:1332‐8. [DOI] [PubMed] [Google Scholar]
Kaplan 1996
- Kaplan M. Primary biliary cirrhosis. New England Journal of Medicine 1996;335:1570‐80. [DOI] [PubMed] [Google Scholar]
Kilmurry 1996
- Kilmurry MR, Heathcote EJ, Cauch‐Dudek K, O'Rourke K, Bailey RJ, Blendis LM. Is the Mayo model for predicting survival useful after the introduction of ursodeoxycholic acid treatment for primary biliary cirrhosis?. Hepatology 1996;23:1148‐53. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lindor 1995
- Lindor KD, Janes CH, Crippin JS, Jorgensen RA, Dickson ER. Bone disease in primary biliary cirrhosis: does ursodeoxycholic acid make a difference?. Hepatology 1995;21:389‐92. [PubMed] [Google Scholar]
Matloff 1982
- Matloff DS, Alpert E, Resnick RH, Kaplan MM. A prospective trial of D‐penicillamine in primary biliary cirrhosis. New England Journal of Medicine 1982;306(6):319‐26. [DOI] [PubMed] [Google Scholar]
Mitchison 1992
- Mitchison HC, Palmer JM, Bassendine MF, Watson AJ, Record CO, James OF. A controlled trial of prednisolone treatment in primary biliary cirrhosis. Three‐year results. Jouranl of Hepatology 1993;15:336‐44. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher B, Schulz KF, Altam DG. The consort statement revised recommendations for improving the quality of reports of parallel group controlled trials. Lancet 2001;357:1191‐4. [PubMed] [Google Scholar]
Neuberger 1985
- Neuberger J, Christensen E, Portmann B, Caballeria J, Rodes J, Ranek L, et al. Double blind controlled trial of D‐penicillamine in patients with primary biliary cirrhosis. Gut 1985;26(2):114‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 2001
- Newton J, Francis R, Prince M, James O, Bassendine M, Rawlings D, Jones D. Osteoporosis in primary biliary cirrhosis revisited. Gut 2001;48:282‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 1999
- Palmer JM, Diamond AG, Yeaman SJ, Bassendine MF, Jones DEJ. T cell responses to the putative dominant autoepitope in primary biliary cirrhosis (PBC). Clinical and Experimental Iimmunology 1999;116:133‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poupon 1999
- Poupon, RE, Bonnand AM, Chretien Y, Poupon R. Ten‐year survival in ursodeoxycholic acid‐treated patients with primary biliary cirrhosis. The UDCA‐PBC Study Group. Hepatology 1999;29:1668‐71. [DOI] [PubMed] [Google Scholar]
Prince 2000
- Prince M, Jones D, Metcalf J, Craig W, James O. Symptom development and prognosis of initially asymptomatic PBC. Hepatology 2000;32(4 Pt 2):171A. [Google Scholar]
Sacks 1987
- Sacks HS, Berrier J, Reitman D, Angona‐Berk VA, Chalmers TC. Meta‐analysis of randomised controlled trials. New England Journal of Medicine 1987;19:450‐5. [DOI] [PubMed] [Google Scholar]
Schluz 1995
- Schulz KF, Chalmers I, Haeys RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Stellon 1985
- Stellon AJ, Davies A, Compston J, Williams R. Osteoporosis in chronic liver disease. Quarterly Journal of Medicine 1985;223:783‐90. [PubMed] [Google Scholar]
Verma 1999
- Verma A, Jazrawi RP, Ahmed HA, Northfield TC. Prescribing habits in primary biliary cirrhosis: a national survey. European Journal of Gastroenterology and Hepatology 1999;11(8):817‐20. [DOI] [PubMed] [Google Scholar]
Vuoristo 1995
- Vuoristo M, Farkkila M, Karvonen AL, Leino R, Lehtola J, Makinen J, et al. A placebo‐controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid [see comments]. Gastroenterology 1995;108:1470‐8. [DOI] [PubMed] [Google Scholar]
Warnes 1987
- Warnes TW, Smith A, Lee FI, Haboubi NY, Johnson PJ, Hunt L. A controlled trial of colchicine in primary biliary cirrhosis: trial design and preliminary report. Journal of Hepatology 1987;5:1‐7. [DOI] [PubMed] [Google Scholar]
Wiesner 1990
- Wiesner RH, Ludwig J, Lindor KD, Jorgensen RA, Baldus WP, Homburger HA, et al. A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. New England Journal of Medicine 1990;322(20):1419‐24. [DOI] [PubMed] [Google Scholar]


