Abstract
Background:
Type 2 immunity can be modulated by regulatory T cell (Treg) activity. It has been suggested that the deubiquitinase CYLD plays a role in the development or function of Treg cells implying it could be important for normal protective immunity where type 2 responses are prevalent.
Objective:
We sought to investigate the role of CYLD in Treg function and T helper type (Th2) immune responses under steady state conditions and during helminth infection.
Methods:
Foxp3-restricted CYLD conditional knockout mice (cKO) were examined in mouse models of allergen-induced airway inflammation and Nippostrongylus brasiliensis infection. We performed multiplex magnetic bead assays, flow cytometry and qPCR to understand how a lack of CYLD affected cytokine production, homing and suppression in Tregs. Target genes regulated by CYLD were identified and validated by microarray analysis, co-immunoprecipitation, shRNA knockdown, and transfection assays.
Results:
Treg-specific CYLD knockout mice showed severe spontaneous pulmonary inflammation with increased migration of Treg cells into the lung. CYLD-deficient Treg cells furthermore produced high levels of interleukin-4 (IL-4) and failed to suppress allergen-induced lung inflammation. Supporting this, the cKO mice displayed enhanced protection against N. brasiliensis infection by contributing to type 2 immunity. Treg conversion into IL-4 producing cells was due to augmented MAPK and NF-κB signaling. Moreover, Scinderin, a member of the actin-binding gelsolin family, was highly upregulated in CYLD-deficient Treg cells, and controlled IL-4 production through forming complexes with MEK/ERK. Correspondingly, both excessive IL-4 production in vivo and the protective role of CYLD-deficient Treg cells against N. Brasiliensis were reversed by Scinderin ablation.
Conclusions:
Our findings indicate that CYLD controls type 2 immune responses by regulating Treg conversion into Th2-like effector cells, which potentiates parasite resistance.
Capsule Summary
Deubiquitinating enzyme CYLD plays a role in maintaining Treg function on type 2 immunity, which will be beneficial for therapeutic approaches to autoimmune disease or pathogen infection.
Keywords: Helminth infection, Type 2 immunity, Regulatory T cells, CYLD deubiquitinase, Lung inflammation, Interleukin-4, Scinderin, MAPK signaling, NF-κB signaling
Graphical Abstract

Introduction
Regulatory T (Treg) cells play an important role in controlling immune homeostasis by suppressing excessive autoimmunity as well as maintaining immune tolerance.1, 2 The expression of Foxp3, a master transcription factor crucial for Treg generation and function, is indispensable as Foxp3 mutations lead to multi-organ autoimmunity in both mouse and human.3,4 Treg cells are generated in the thymus and migrate into the periphery to become thymus-derived naturally occurring Treg (tTreg) cells. In addition, naïve CD4+ T cells can be differentiated into Foxp3+ Treg cells, to then become peripherally induced Treg(pTreg) cells. Foxp3 expression in Treg cells is quite stable under normal conditions.5 However, these cells lose their Foxp3 expression under certain inflammatory or disease conditions.6 This loss of Foxp3 expression impairs the suppressive function of Treg cells and can contribute to elevate inflammation and effector cytokine production. Recent studies have highlighted the role of ubiquitination in controlling Treg suppressive vs. inflammatory activity. For example, we have demonstrated that VHL, an E3 ubiquitin ligase, controls interferon-γ (IFN-γ) production by Treg cells through regulating HIF-1α ubiquitination7, whereas the deletion of SHARPIN that forms a linear-ubiquitin-chain-assembly complex (LUBAC) resulted in inflammatory interleukin-17 (IL-17) expression.8, 9 Treg cells are also able to exhibit pro-inflammatory immune responses regardless of Foxp3 expression. For example, Treg cells are capable of displaying a T helper type 2 (Th2)-like phenotype without losing Treg activity nor Foxp3 stability.10 The above findings indicate that the expression of pro-inflammatory cytokines such as IL-4 are tightly regulated in Treg cells and this might be important in Th2 immunity, including during infections which are controlled by type 2 cytokine production.
Helminths are multicellular worms which can reside in various tissues. Helminth infection generally promotes type 2 immune responses with increased expression of IL-4, IL-5 and IL-13.11, 12 A number of immune cells in the innate and adaptive immune systems play a role in protective immune reactions against helminth infection.13–16 IL-4 and IL-13 initiates Th2-dependent immune responses after gastrointestinal nematode invasion.17 It has been suggested that Treg cells play a role in controlling immune responses upon helminth invasion. CD4+ Foxp3+ regulatory T cells rapidly expand upon helminth infection18–20 and group 2 innate lymphoid cells (ILC2) activated by IL-33 promote Treg accumulation through ICOSL-ICOS upon N. brasiliensis infection.21 Interestingly, however, Treg cells do not always limit immune responses during infection, and the conversion of Treg cells into Th2-like cells has been shown to promote type 2 immunity against H. polygyrus.22, 23 On the other hand, helminths are able to escape from host immunity by augmenting pTreg differentiation via the production of a TGF-β mimic.24 Thus, the modulation of Treg function might be closely related with anti-helminth immune responses.
Cylindromatosis protein (CYLD), a deubiquitinating enzyme, is encoded by a tumor suppressor gene whose mutation is linked to the development of multiple benign skin tumors.25 It contains several functional domains including the protein interaction domains for TRAF2 and NEMO binding, and a C-terminal deubiquitinase enzymatic domain. CYLD is known as a negative regulator of the NF-κB signaling pathway in several types of immune cells such as T cells,26 B cells,27 and dendritic cells.28 A study using transgenic CYLD ex7/8 mice, which overexpress a short form of CYLD lacking TRAF2 and NEMO binding motifs, showed impaired Treg function,29 with reduced capability to suppress inflammatory immune responses, and the loss of CTLA4 and CD25 expression on their surface. CYLD also can negatively regulate TGF-β signaling which is involved in the generation of pTreg cells.30 However, these findings were either based on in vitro studies or in mice with germline deletion or a truncation mutation of CYLD that modulated CYLD in both Treg cells and conventional T cells, as well as non-T cells, and thus did not definitively address the physiological and intrinsic roles of CYLD in Treg cells. In addition, the observed phenotypes could have been influenced by other cell types, due to the chronic inflammation in these mice. It is therefore, still unclear how and if CYLD plays an intrinsic role in regulating Treg cell development and function during pathogen invasion.
In this study, we investigated the involvement of CYLD in Treg cell regulation by generating Treg-specific CYLD knockout mice. We found that loss of CYLD in Treg cells resulted in enhanced type 2 immunity and protection against helminth infection. CYLD modulated Th2 cytokine production through controlling the expression of an actin-binding protein Scinderin. Our data suggest that CYLD activity in Treg cells maintains their suppressive function and limits host protective immunity against Nippostrongylus brasiliensis infection.
Methods
Mice
Il4−/−, and CD45.1 congenic mice were purchased from Jackson Laboratories. CYLD floxed61 and Scinderin floxed mice37 were kindly provided by Dr. Ting, Icahn School of Medicine at Mount Sinai, and Dr. Glogauer, University of Toronto, respectively. The floxed mice were crossed with Foxp3-cre-YFP62 mice to generate the conditional knockout mice in the animal facility at La Jolla Institute for Immunology. For inducible deletion of CYLD in Treg cells, CYLD floxed mice were crossed with mice expressing CreERT2 fusion gene under control of Foxp3 gene. For the induction of Cre recombinase nuclear translocation, 6-week-old mice were injected intraperitoneally with 100 μl of 10 mg/ml tamoxifen (Sigma T5648) once every day for seven days. All mice were maintained according to the animal protocols approved by the Institutional Animal Care and Use Committee of the La Jolla Institute for Immunology. Six to twelve week-old mice were used for most experiments, and aged mice (8 month-old) were used for histological analysis. Both female and male mice were randomly assigned to each group since there was no difference in observed phenotypes between male and female mice. However, only female heterozygote Foxp3cre/+ mice were used for other experiments.
Cell isolation and sorting
Cells were isolated as described in the previous report.7 Briefly, cells from the spleen and lymph nodes were isolated by grinding through nylon mesh (70 μm). For isolation of cells from lung and intestine, tissues were digested with Collagenase III (1 mg/ml, Worthington) at 37°C for 1 hr. For the purification of naïve T cells and Treg cells, CD4+ T cells were first enriched from the spleen by using a CD4 isolation kit (BioLegend or STEMCELL Technologies). Then, CD4+ CD25−CD44−CD62L+ and CD4+YFP+ (Foxp3+) cells were further sorted by FACS Aria (BD Biosciences) for naïve and Treg cells, respectively. To isolate several kinds of immune cells from WT spleen, total cells were subjected to FACS-sorting for Treg cells (CD4+ YFP+), naïve CD4 T cells (CD4+ YFP− CD44− CD62L+), effector/memory CD4 T cells (CD4+ YFP− CD44+ CD62L−), naïve CD8 T cells (CD8+ CD44− CD62L+), effector/memory CD8 T cells (CD8+ CD44+ CD62L−), B cells (CD3− CD19+), NK cells (NK1.1+ NKp46+), and myeloid cells (CD11c+ CD11b−, CD11c+ CD11b+ and CD11c− CD11b+). The purity of sorted cells was ~99%.
Flow cytometry
Cells were stained with the following antibodies; anti-CD4 (RM4–5), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD25 (PC61), anti-ICOS, anti-Nrp-1 (3E12), anti-CTLA4 (UC10–4B9), anti-CD103 (2E7), anti-CD357 (GITR, DTA-1), anti-CD304 (Nrp-1, 3E12), anti-OX40 (OX86), anti-LAP-TGF-β1 (TW7–16B4) and anti-4–1BB (17B5) from BioLegend. Anti-CD124 (mIL4R-M1) and anti-IL-33R (T1/ST2, DJ8) antibodies were purchased from BD Biosciences and MD Biosciences, respectively. Cells were stained with CCL1-human IgG FC fusion proteins (Sino Biological Inc) and further incubated with biotinylated anti-human IgG antibodies (Vector Labs), followed by streptavidin-APC or PE-Cy7 for CCR8 expression, while cells were stained with anti-CCR3 (83103, R&D) and anti-CXCR3 (220803, R&D) antibodies, and further incubated with biotinylated anti-rat IgG2a (RMG2a-62), followed by streptavidin-APC or PE-Cy7 for CCR3 and CXCR3 expression. For CCR4 and CCR7, cells were stained with biotinylated anti-CCR4 (2G12, BioLegend) and anti-CCR7 (4B12, BioLegend) antibodies, followed by streptavidin-APC or PE-Cy7. For intracellular staining, cells were fixed with Fixation/Permeabilization buffer (eBioscience) after surface staining, and were further incubated with anti-Foxp3 (FJK-16s), anti-Helios (22F6), anti-Eomes (Dan11mag), anti-T-bet (eBio4B10 (4B10)), anti-GATA3 (TWAJ) and anti-RORγt (Q31–378) antibodies. To determine the expression of cytokines, cells were stimulated with PMA and ionomycin in the presence of GolgiStop (BD Biosciences) for 3 hrs before staining. Cells were then fixed with Cytofix/Cytoperm buffer (BD Biosciences), followed by staining with anti-IL-4 (11B11), anti-IL-5 (TRFK5) and anti-IFN-γ (XMG1.2) antibodies. Stained cells were analyzed by FACSCanto II or LSRII (BD Biosciences).
Airway inflammation induced by OVA
Lung inflammation was induced as previously described.10,32 The mice including Cyld+/+Foxp3cre (WT donor), Cyldfl/flFoxp3cre (KO donor) and WT B6 mice (recipient), were immunized intraperitoneally with 20μg OVA protein (Sigma) adsorbed to alum adjuvant (ThermoFisher) on day 0 and 7. On day 14, YFP+ Treg cells were sorted from spleens of OVA-immunized WT and KO donor mice and then adoptively transferred i.p. (0.35 × 106/mouse) into OVA-sensitized WT B6 recipients. At 12 hrs after Treg transfer, mice were given intranasal OVA protein (100 μg) for 5 consecutive days. At 48 hrs after the last intranasal OVA challenge, mice were examined to assess airway inflammation. CD45+ B220− CD3− CCR3+ eosinophils, B220/CD3+ lymphocytes and CD45+ B220− CD3− CD11c+ MHCII+ mononuclear cells from lung or BAL fluid were analyzed by flow cytometry.
Parasite infection
Nippostrongylus brasiliensis was amplified by monthly passages in rats. The infective third-stage larvae (L3) worms were purified with a Baermann apparatus and washed by PBS. Mice were injected subcutaneously with 500 L3 worms in PBS. At day7 of infection, mice were analyzed for worm burden in the small intestines and egg number in feces.63
Comparative homing in vivo
YFP+ Treg cells were freshly isolated from spleens of either WT or CYLD cKO mice. Isolated WT and KO Treg cells were labeled with CellTrace Violet (ThermoFisher) and eFluor 670 (eBioscience), respectively, according to manufacturer’s protocol. Both labeled Treg cells (2 × 106 cells) were intravenously co-transferred into B6 WT mice. At 18 or 48 hrs after transfer, Treg cells were isolated from several organs and homing capacity was determined by flow cytometry. The absolute number of migrated Treg cells was calculated, and relative homing ability was calculated by the ratio of WT and KO cells.
Microarray analysis and qRT-PCR
RNAs were isolated from sorted Treg (YFP+) cells with RNeasy Plus Micro Kit (Qiagen). Gene expression was then determined by Mouse Gene 1.0 ST array Chip (Affymetrix). Array was performed by UCSD VA/VMRF microarray core facility. For qRT-PCR, total RNAs were subject to cDNA synthesis by using SuperScriptase IV (Invitrogen). Quantitative PCR was performed on LightCycler 480 (Roche) or CFX96 (Bio-Rad). The expression of mRNA was normalized by β-actin or cyclophilin-A. The primers used in qRT-PCR are listed in Table E1.
In vitro culture of Treg cells
For induction of pTreg cells, naϊve T cells were activated with αCD3/28 antibodies (plate-coated, 2 μg/ml) and αCD28 antibodies (soluble, 1 μg/ml) in the presence of IL-2 (100 U/ml) and TGF-β1 (5 ng/ml) in RPMI 1640 medium containing 10% FBS for 3–4 days. Differentiated pTreg cells were further expanded in the presence of IL-2 and TGF-β1 for 3–4 days. Sorted YFP+ tTreg cells or pTreg cells were activated with αCD3/28 antibodies and IL-2 (100 U/ml) in presence or absence of inhibitors for 48 hrs. MAPK inhibitor (U0126) and NF-κB SN50 inhibitor were purchased from EMD Millipore, and treated as indicated.
Retroviral Transduction
Oligonucleotides (5′-TGCTGTTGACAGTGAGCGATCCGTGTCTCTCAAGGCAAAGTAGTGAA GCCACAGATGTACTTTGCCTTGAGAGACACGGACTGCCTACTGCCTCGGA-3′) for Scinderin were cloned into a retroviral LMP vector expressing mAmetrine fluorescent proteins. For overexpression, murine Scinderin was cloned into a pMIG-IRES-GFP retroviral vector. After viral vectors were transfected into viral packaging cells by using TransIT (Mirus Bio LLC), viral supernatants were harvested. Naïve CD4+ T cells were activated with anti-CD3 and anti-CD28 antibodies in the presence of IL-2 and TGF-β1 for 24 hrs, and were then infected with viral supernatants by centrifugation at 2,000 rpm for 60 min at RT in presence of polybrene (8 μg/ml). Cells were further cultured in presence of IL-2 and TGF-β1 for 4–5 days. Transduced cells (mAmetrine+ YFP+ CD4+) were isolated by FACS-sorting.
In vitro suppression assay
Treg cells (YFP+) were sorted from spleens of WT and CYLD cKO mice. Naïve CD45.1+ CD4+ T cells were labeled with CellTrace Violet (Invitrogen) according to manufacturer’s protocol. Labeled naïve T cells (5 × 104) were activated with αCD3 antibodies (soluble, 2 μg/ml) and irradiated splenic APCs (10 × 104) in the presence or absence of sorted Treg cells (CD45.2+ YFP+) at indicated ratios for 4 days in a round-bottom 96-well plate. Proliferation of labeled naïve T cells was determined by the dilution of CellTrace Violet dye.
Apoptosis of Tregcells
Sorted WT and KO Treg cells were activated with anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml) antibodies in the absence or presence of IL-2 (50 U/ml) for 18 hrs. Activated cells were then stained with annexin V (BioLegend) and propidium iodide (PI) according to manufacturer’s protocol.
Measurement of cytokines and chemokines
The production of inflammatory cytokines and chemokines from culture supernatant was determined by Bio-Plex kit (Bio-Rad) according to manufacturer’s protocol.
Immunoprecipitation and immunoblot
Isolated Treg cells were activated with anti-CD3 and anti-CD28 antibodies as indicated. Cell lysates were prepared in RIPA buffer containing a protease inhibitor cocktail. To overexpress proteins, 293T cells were transfected with appropriate amounts of plasmids using TransIT-LT1 transfection reagents (Mirus Bio LLC). At 48 hrs post transfection, cell lysates were prepared with 1% NP-40 lysis buffer containing protease inhibitor cocktail.99 Then, lysates were diluted to 0.1% NP-40 and incubated with anti-HA magnetic beads (ThermoFisher) or anti-FLAG magnetic beads (Sigma) at 4°C for overnight. The immunocomplexes were collected on a magnetic stand after washing with TBST buffer, and were eluted with the acidic buffer (0.1 M glycine, pH 2.0~3.0). Immunoblot was performed with the following antibodies. Anti-CYLD, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-JNK, anti-JNK, anti-phospho-p38, anti-p38, anti-phospho-p65, and anti-STAT6 antibodies were purchased from Cell Signaling. Anti-IκBα, anti-β-actin, anti-HA and anti-MYC antibodies were from Santa Cruz. Anti-Scinderin (Novus), anti-Foxp3 (eBioscience), and anti-Flag (Sigma) antibodies were used.
Statistical analysis
All data was analyzed by non-parametric Mann-Whitney test or Wilcoxon matched-pairs signed rank test using GraphPad Prism 8.
Results
Treg-specific CYLD deficiency leads to allergic-like inflammation
We first examined the expression of CYLD by Treg cells and found that both freshly isolated and in vitro generated Foxp3+ Treg cells had higher levels of CYLD than conventional T cells (Fig 1, A and B), suggesting that CYLD may be essential in Treg cells. Since systemic CYLD KO mice or truncated CYLD mutant mice displayed excessive inflammation due to a lack of suppressive function,29 we examined the effect of Foxp3-specific ablation of CYLD using conditional knockout mice (cKO). Aged CYLD cKO mice had enlarged spleens and lungs (Fig E1, A) with CYLD cKO mice starting to develop inflammation in the lung around 2 months of age, but not in other organs (data not shown). Correspondingly, increased numbers of immune cells were found in bronchoalveolar lavage and lung tissues, including eosinophils, compared with WT control mice (Fig 1, C). In addition, CYLD cKO mice over 8 months old displayed inflammation in the skin reminiscent of atopic dermatitis as well as allergic-like inflammation in the lungs, but not in the liver (Fig 1, D). CD44high CD62Llow effector/memory T cells were greatly increased in CYLD cKO mice compared to control littermates (Fig 1, E and F), indicating that deregulated T cell activation occurred with a CYLD deficiency in Treg cells. Although Foxp3+ T cells were also enriched among CD4+ T cells from CYLD cKO mice (Fig 1, G and H), there was no significant difference in apoptosis in vitro between CYLD-deficient and WT Treg cells (Fig E1, B). We then examined the proliferative capacity of CYLD-deficient Treg cells and found the expression of Ki-67 was significantly increased in Foxp3+ as well as Foxp3− T cells in CYLD cKO mice (Fig E1, C) even though Bcl-2 expression was reduced in CYLD-deficient Treg cells (Fig E1, D). This implies that CYLD-deficient Treg cells are more proliferative under inflammatory conditions. CYLD-deficient Treg cells showed a slight, but not significant reduction in the expression of CTLA4 and CD25 (Fig E1, E) compared with control Treg cells, whereas the expression of LAP-TGF-β1, CD103, GITR, ICOS, OX40, 4–1BB, Nrp-1 and helios, but not IL-33 receptor, was upregulated by CYLD deficiency.
Figure 1. CYLD deficiency in Foxp3+ T cells leads to the lung inflammation.
A and B, Immunoblot for CYLD expression by Treg cells. Lysates were prepared from freshly isolated CD4+ YFP+ Treg and CD4+ YFP− T cells (A). Inducible Treg cells were activated with anti-CD3/CD28 antibodies in the presence of IL-2 and TGF-β1 (B). C, The number of cells was determined from 12 week-old Cyldfl/fl Foxp3cre mice. D, Histology of lung. The lung sections of 8 month-old mice were stained with H&E. E and F, CD44 and CD62L expression from T cells was assessed by flow cytometry. G and H, Foxp3 expression of CD4+ T cells was analyzed by flow cytometry after intracellular staining. I-K, Suppressive function of Treg cells during OVA-induced airway inflammation (I). OVA-immunized mice were sensitized with intranasal injection of OVA proteins in WT B6 mice. Either WT or KO Treg cells were sorted from spleens of OVA-immunized WT or cKO mice, and then transferred into OVA-immunized recipient mice. Number of cells was assessed from BAL fluid (J) and lung histopathology was examined after H&E stain (K). Combined plots for C (n=5~7), F (n=8), H (n=7) and J (n=6~13) are shown. Error bars indicate SEM. ∗ P<0.05, ∗∗ P<0.01, ∗∗∗ P<0.001 (Mann-Whitney test)
To assess whether CYLD-deficient Treg cells are functional, we performed an in vitro suppression assay and found that the suppressive activity of CYLD-deficient Treg cells was comparable to that of WT Treg cells in vitro (Fig E1, F–H), perhaps related to the dominant role of CTLA4, CD25, and TGF-β in suppression in vitro that has previously been reported.31 To further test the suppressor function of Treg cells in vivo, we performed adoptive transfer experiments with antigen-specific Treg cells in an allergen model of lung inflammation.10, 32 We prepared OVA-specific Treg cells from OVA-immunized WT or cKO mice. Isolated OVA-specific Treg cells were then adoptively transferred into WT B6 mice, followed by intranasal injection of OVA protein to induce allergic airway inflammation (Fig 1, I). CYLD-deficient Treg cells, unlike WT Treg cells, failed to inhibit the recruitment of inflammatory immune cells such as eosinophils and lymphocytes into the lung (Fig 1, J), which correlated with pathological changes as revealed by histological examination (Fig 1, K). Thus, these results demonstrate that CYLD-deficiency in Treg cells causes impaired regulatory function in vivo leading to enhanced development of allergic-like inflammation.
CYLD regulates the migratory activity and type 2 cytokine production of Treg cells
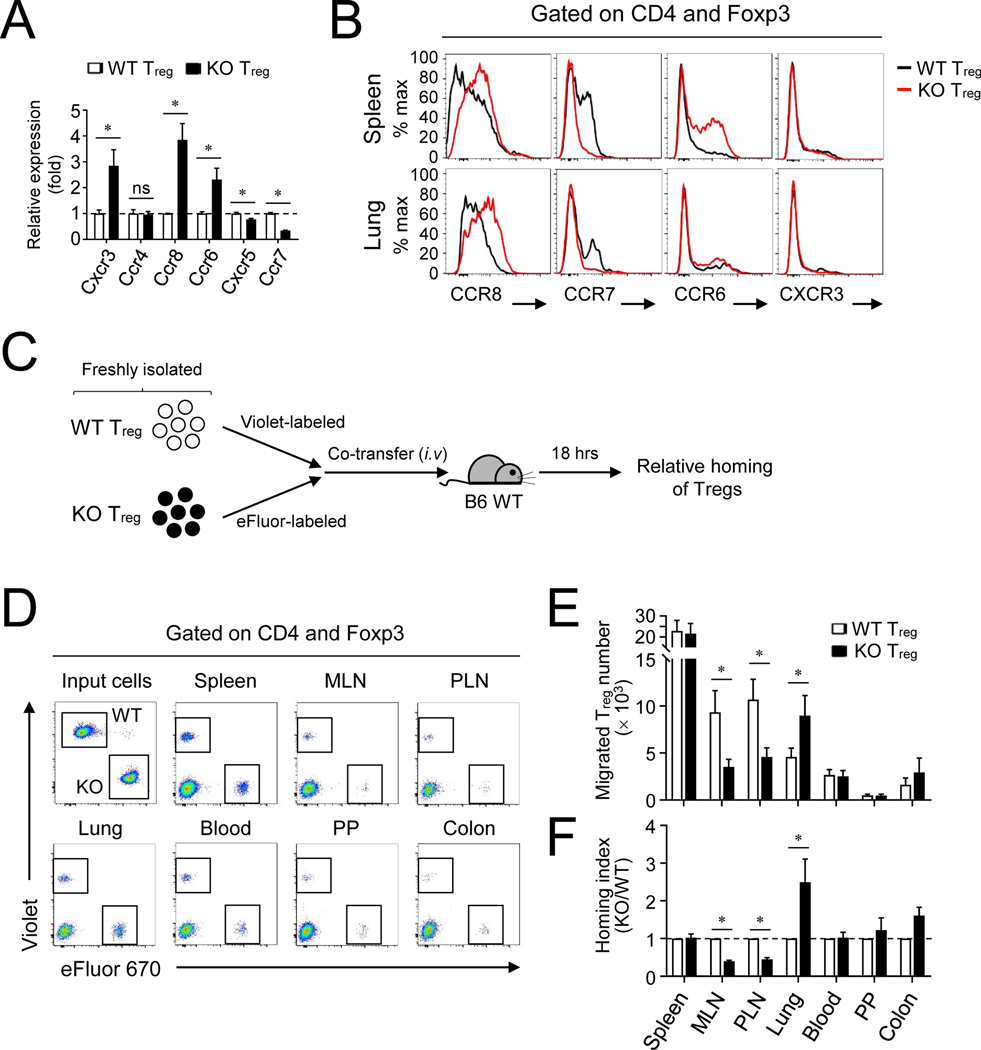
In addition to suppressive function, we tested whether CYLD might regulate other functions in Treg cells such as their homing capacity. To address this hypothesis, we first analyzed the expression of several trafficking receptors in CYLD-deficient Treg cells. mRNA for Th1-associated CXCR3 and Th17-associated CCR6 expression was upregulated, and CCR8 but not CCR4 expression was also upregulated among Th2-associated chemokine receptors in CYLD-deficient Treg cells (Fig 2, A). In addition, the mRNA expression of CCR7, a key homing receptor for migration into secondary lymphoid organs, was reduced, and Tfh-associated CXCR5 mRNA expression was also downregulated. Protein analysis confirmed that the expression of CCR8 was highly increased, whereas CCR7 expression was markedly reduced in Treg cells in both the spleen and lungs (Fig 2, B). To determine whether the differential chemokine receptor expression affects the migratory ability of CYLD-deficient Treg cells, we performed a comparative homing experiment by co-transfer of both WT and CYLD-deficient Treg cells into WT B6 mice (Fig 2, C). At 18 hrs after adoptive co-transfer, CYLD-deficient Treg cells were efficient in migration into the lung among several organs compared to WT Treg cells, whereas they showed decreased homing into the lymph nodes (Fig 2, D–F) correlating with decreased CCR7 expression. The increased homing of CYLD-deficient Treg cells into the lung was prolonged and still seen at 48 hrs after Treg transfer (Fig E2, A and B). Thus, the lack of CYLD promoted both the expansion and the homing capacity of Treg cells resulting in their increased accumulation in the lung.
Figure 2. CYLD−/− Treg cells preferentially migrate into the lung.
A and B, Expression of several key chemokine receptors from KO Treg cells at mRNA (A) and protein (B) levels. Treg cells from spleen (A and B) and lung (B) were analyzed. C-F, A schematic procedure of comparative Treg homing in vivo (C). After splenic WT and KO Treg cells were labeled with CellTrace Violet and eFluor 670 respectively, both cells were co-transferred into WT B6 mice. At 18 hrs after adoptive transfer, migration of Treg cells into several organs was determined by flow cytometry (D). The absolute number of migrated cells (E) and the relative homing index was normalized by the ratio between the numbers of KO and WT cells (F). Representative of at least three independent experiments are shown. Combined plots for A (n=4), E (n=6) and F (n=6) are shown. Error bars indicate SEM. ∗ P<0.05 (Mann-Whitney test)
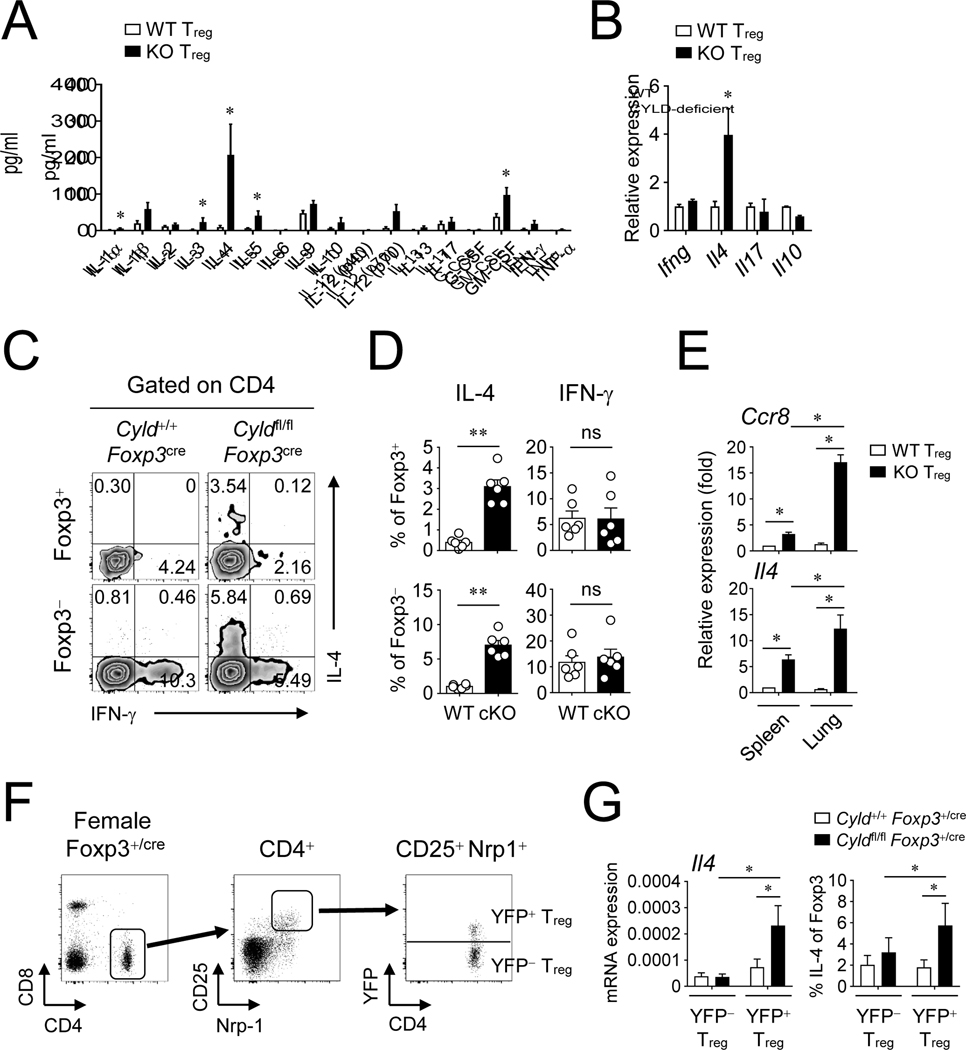
Given the lack of suppression in vivo (Fig 1, I–K), but relatively normal expression of suppressive molecules (Fig E1, E), we then examined if CYLD-deficient Treg cells lost regulatory activity by gaining the ability to produce inflammatory cytokines after activation. Strikingly, CYLD-deficient Treg cells strongly produced IL-4 protein compared to WT Treg cells that had minimal expression of IL-4 (Fig 3, A), and other cytokines were also elevated in CYLD-deficient Treg cells albeit to a lesser extent. The mRNA level of Il4 was also markedly increased by the CYLD deficiency, whereas that of Ifng, Il17, or Il10 was not changed (Fig 3, B). This was further confirmed by intracellular cytokine staining, with IL-4 expression markedly increased in Foxp3+ cells T cells from CYLD cKO mice (Fig 3, C and D). Although IL-5 production was increased in CYLD-deficient Treg cells in vitro (Fig 3, A), Foxp3+ T cells minimally produced IL-5 in the lungs of CYLD cKO mice (Fig E3, A and B). Interestingly, Foxp3− T cells in the CYLD cKO mice also expressed higher levels of IL-4 (Fig 3, C and D), and IL-5 (Fig E3), suggesting conventional T cells also became dysregulated toward type 2 immunity because of the deficiency of CYLD in Treg cells. We also found that CYLD-deficient Treg cells in the lung expressed higher levels of CCR8 as well as IL-4 compared to those in the spleen (Fig 3, E). There was a possibility that the upregulated IL-4 expression in CYLD-deficient Treg cells might be due to the increased Th2-dominant immune responses in CYLD cKO mice and not be intrinsic to the CYLD deficiency. To address this issue, we examined Cyldfl/fl Foxp3+/cre heterozygote female mice in which both WT and KO Treg cells are present (Fig 3, F). As expected, we did not observe any inflammation in these mice and the proportion of effector/memory T cells was very similar to control WT mice (Fig E4, A and B). Interestingly, the expression of GITR and ICOS was not altered by CYLD-deficient Treg cells in Cyldfl/flFoxp3+/cre heterozygote female mice unlike Cyldfl/flFoxp3cre mice, implying that the expression of GITR and ICOS might be upregulated under inflammatory conditions (Fig E4, C and D). Nevertheless, CYLD-deficient Treg cells still produced higher levels of IL-4 even in the presence of WT Treg cells (Fig 3, G) suggesting that CYLD directly restricted production of IL-4 in Treg cells.
Figure 3. CYLD controls IL-4 expression by Treg cells.
A, Production of inflammatory cytokines by KO Treg cells. Sorted YFP+ Treg cells were activated with anti-CD3 and anti-CD28 antibodies for 48 hrs. The amount of cytokines from culture was examined by Bio-Plex. B, The mRNA expression of key cytokines was assessed by qPCR. C and D, IL-4 and IFN-γ expression in both Foxp3+ and Foxp3− T cells was measured by flow cytometry. Cells were activated with PMA and ionomycin for 3 hrs before intracellular staining of Foxp3, IL-4 and IFN-γ. CD4+ T cells in the lung were analyzed by flow cytometry. E, The expression of Ccr8 and Il4 by Treg cells. YFP+ Treg cells were sorted from spleen and lung. Relative expression was determined by qPCR. F and G, IL-4 expression in female Cyldfl/fl Foxp3+/cre mice. Both YFP+ and YFP− Treg cells were sorted from splenic CD4+ CD25high Nrp-1high cells (F). The expression was assessed by qPCR and flow cytometry (G). Representative of at least two independent experiments are shown. Combined plots for A (n=3~5), B (n=4), D (n=6), E (n=4), and G (n=3~6) are shown. Error bars indicate SEM. ∗ P<0.05, ∗∗ P<0.01; ns, non-significant (Mann-Whitney test or Wilcoxon test)
CYLD-deficient Treg cells limit parasite infection by producing IL-4
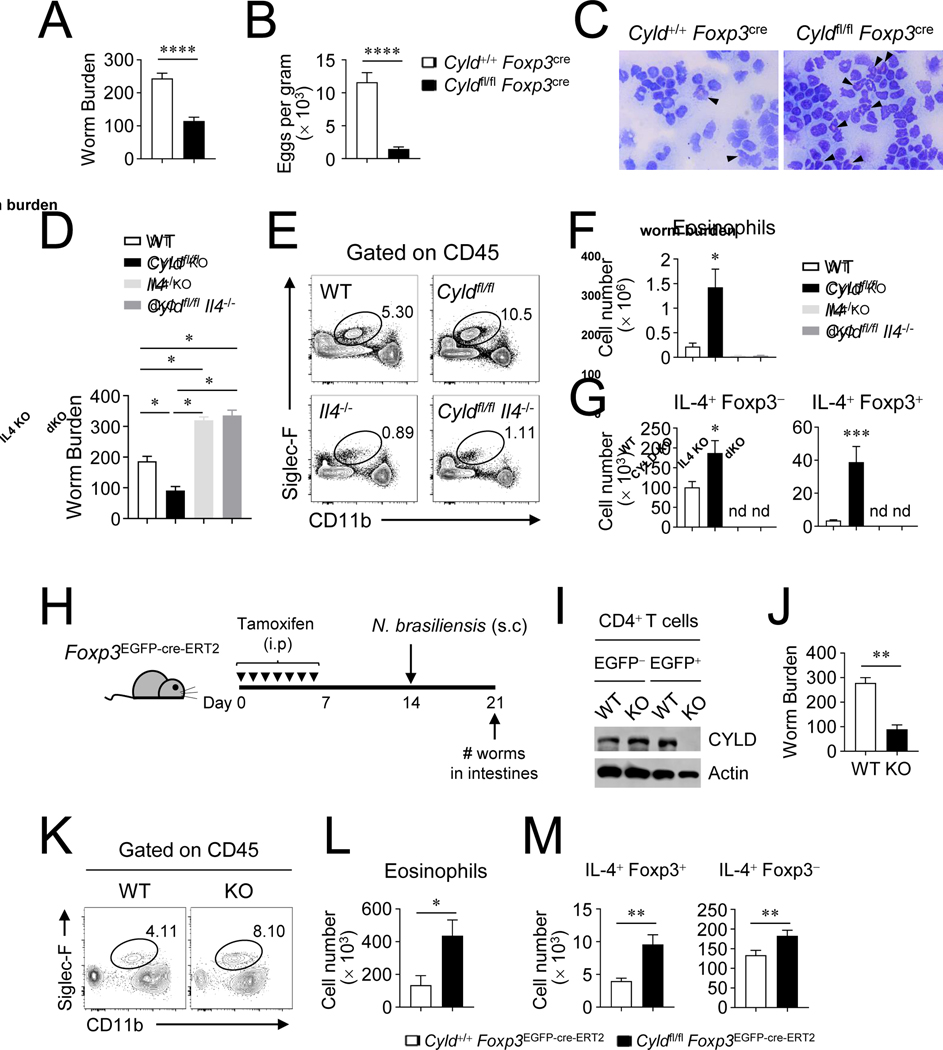
Besides the induction of harmful allergic inflammation, IL-4 production by Treg cells may be beneficial for certain Th2 immune responses such as protection from parasite infection. To test this possibility, both WT and CYLD cKO mice were infected with N. brasiliensis and we examined whether there was any difference in pathogen clearance. CYLD cKO mice had much lower worm numbers in the intestines and decreased egg numbers in feces than control WT mice (Fig 4, A and B), and the recruitment of eosinophils into the peritoneum was greatly increased in CYLD cKO mice compared to WT mice at 7 days after helminth infection (Fig 4, C). The numbers of IL-4+ Treg cells and conventional Th2 cells were increased, and this was maintained over 2 weeks post infection, in CYLD cKO mice (Fig E5). To further test whether the protective immunity was dependent on IL-4, we examined N. brasiliensis-infected CYLD/IL4 dKO mice, and found that CYLD/IL4 dKO mice failed to reduce the number of worms in the intestines unlike CYLD cKO mice (Fig 4, D). CYLD/IL-4 dKO and IL-4 KO mice were also unable to limit the intestinal worm burden as well as to recruit CD45+ CD11blow Siglec-F+ eosinophils into the peritoneum (Fig 4, E and F), likely due to the absence of IL-4-expressing cells (Fig 4, G). Furthermore, we investigated the protective role of CYLD during helminth invasion by utilization of another system with inducible conditional knockout of CYLD using Foxp3-EGFP-cre-ERT2 mice, to rule out any effect of the spontaneous inflammatory environment in CYLD cKO mice. The mice were injected with tamoxifen for 7 days, and infected with the helminth (Fig 4, H). First, we verified the deletion of CYLD from Foxp3+ Treg cells after tamoxifen treatment (Fig 4, I). We found the worm number in the intestines was greatly reduced in Cyldfl/fl Foxp3-EGFP-cre-ERT2 mice (Fig 4, J), and CD45+ CD11blow Siglec-F+ eosinophils were increased in the peritoneum (Fig 4, K and L). Importantly, this was accompanied by elevated numbers of IL-4 producing Foxp3+ Treg cells as well as conventional Th2 cells (Fig 4, M). These results demonstrate that Treg function regulated by CYLD is critical for Th2 and anti-parasite immunity.
Figure 4. CYLD-deficient Treg cells elevate protective immunity against N. brasiliensis infection.
A-C, N. brasiliensis infection in Cyldfl/flFoxp3cre mice. The number of worms in the intestines (A) and the egg number in feces (B) was examined at day 7 post infection. C, Histology of peritoneal cavity. Cytospins of cells from peritoneal cavity were stained with modified Giemsa Stain. Eosinophils were indicated by arrowheads. D-G, N. brasiliensis infection in Il4−/−Cyldfl/fl Foxp3cre mice. D, The number of worms in the intestines at day 7 after infection. E and F, The absolute number of peritoneal CD45+ CD11blow Siglec-F+ eosinophils. G, The absolute number of IL-4+ Foxp3+ and IL-4+ Foxp3− T cells from mesenteric lymph nodes. H-M, A schematic procedure of N. brasiliensis infection from tamoxifen-treated Cyldfl/fl Foxp3EGFP-cre-ERT2 mice (H). I, Immunoblot of EGFP+ and EGFP− CD4+ T cells after tamoxifen-treatment. J, The number of worms in the intestines was measured. K and L, The number of eosinophils from peritoneal cavity. M, The absolute number of IL-4+ T cells from mesenteric lymph nodes. Combined plots for A (n=18~19), B (n=12), D (n=4~5), F (n=4~5), G (n=4~9), J (n=5~6), L (n=5~6), and M (n=5~6) are shown. Error bars indicate SEM. ∗ P<0.05, ∗∗ P<0.01, ∗∗∗ P<0.001, ∗∗∗∗ P<0.0001; nd, non-detected (Mann-Whitney test or Wilcoxon test)
CYLD restrains MAPK signaling pathway by regulating Scinderin expression in Tregcells
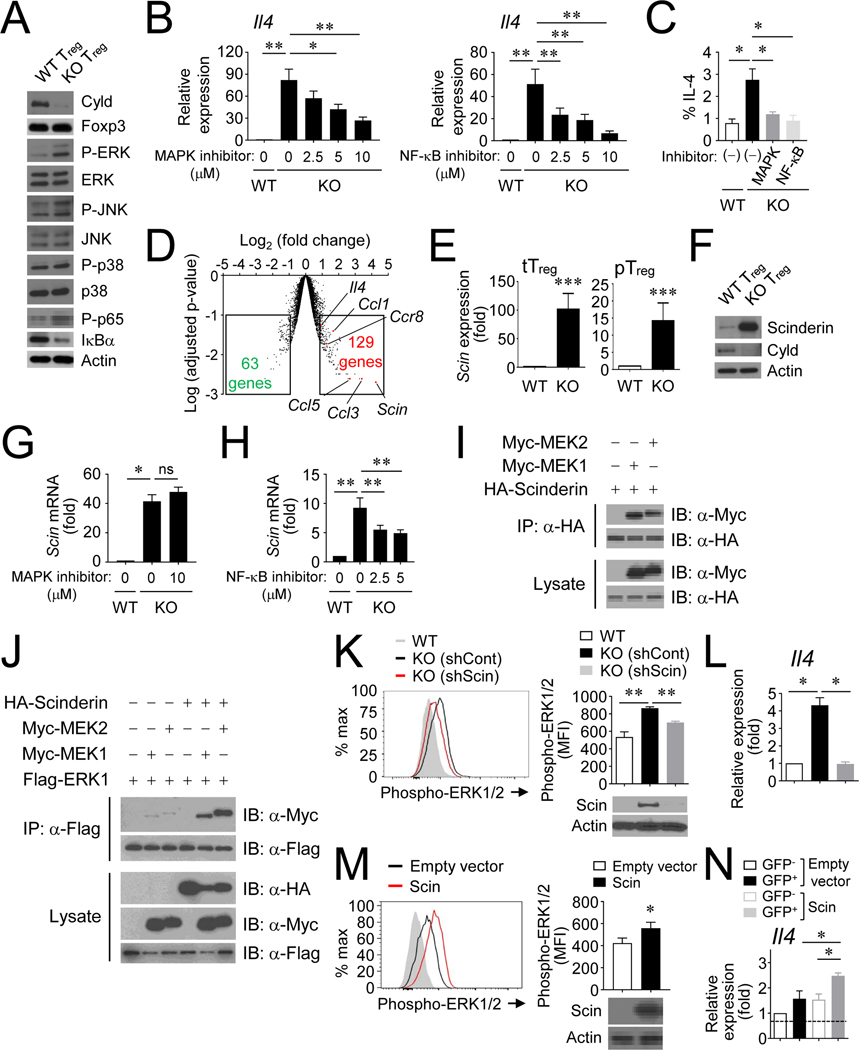
It has been shown that CYLD acts as a negative regulator of the NF-κB signaling pathway by regulating IKKγ and TRAF2.33, 34 In keeping with this, CYLD-deficient Treg cells showed a reduction of IκBα as well as increased phosphorylation of p65/RelA compared to WT Treg cells (Fig 5, A). Interestingly, MAPK signaling was also strongly activated in CYLD-deficient Treg cells as the phosphorylation of ERK was increased (Fig 5, A). To examine whether this might explain increased IL-4 production by CYLD-deficient Treg cells, we utilized both U0126, an inhibitor of MEK1/2 that blocks the ERK signaling pathway, and SN50, an inhibitor of the NF-κB signaling pathway. Both inhibitors were effective in suppressing IL-4 expression in CYLD-deficient Treg (Fig 5, B and C).
Figure 5. CYLD controls MAPK signaling pathway by regulation of Scinderin expression.
A, Immunoblot analysis of phospho-MAPKs, IκBα and phospho-p65 from splenic YFP+ Treg cells. B and C, IL-4 expression in Treg cells treated with inhibitors of MAPK or NF-κB pathways. IL-4 expression was measured by qPCR (B) and flow cytometry (C). Both inhibitors were used at 10 μM for IL-4 production. D, A volcano plot of microarray in CYLD-deficient vs. –sufficient Treg cells. E and F, The expression of Scinderin by Treg cells was determined at the mRNA (E) and protein (F) levels. G and H, Scinderin expression in Treg cells treated with inhibitors. I, Scinderin interacts with MEK1/2. 293T cells were transfected with expression vectors for HA-Scinderin, MYC-MEK1 or MYC-MEK2. The immunoprecipitates with anti-HA antibodies were analyzed by immunoblotting with the indicated antibodies. J, The interaction of ERK and MEK was promoted by Scinderin overexpression. 293T cells were transfected with expression vectors, and lysates were immunoprecipitated with anti-FLAG antibodies. K and L, The effect of shRNA knockdown for Scinderin in CYLD KO Treg cells. M and N, The effect of overexpression of Scinderin in WT CD4+ T cells by retroviral transduction. Sorted mAmetrine+ YFP+ cells or GFP+cells were activated with anti-CD3/CD28 for 15 min. Phosphorylation of ERK1/2 was determined by flow cytometry (I and K) and IL-4 expression was examined by qPCR (J and L).The efficiency of overexpression or knockdown was assessed by immunoblot. Combined plots for B (n=6~8), C (n=4), E (n=7~8), G (n=4), H (n=8), K (n=5), L (n=4), M (n=6), and N (n=4) are shown. Error bars indicate SEM. ∗ P<0.05, ∗∗ P<0.01, ∗∗∗ P<0.001 (Mann-Whitney test or Wilcoxon test)
To further understand how CYLD functioned in Treg, we performed a genome-wide microarray analysis. CYLD deficiency influenced the expression of gene families for cytokine/chemokines, cell cycle/apoptosis, and transcriptional regulation (Fig E6, A). The expression of inflammatory chemokines and cytokines such as Ccl3, Ccl5, Ccl1, Xcl1, Cxcl10, Cxcl11 and Il4 were elevated in cKO Treg cells (Fig E6, B). The expression of many genes engaged in cell division/apoptosis were regulated by CYLD (Fig E6, A); Cdkn1a, Mki67, Plk1, Cdk1, Aurka, Nek2 and Cdc25c were upregulated, while Dapk1 and Myc were downregulated in CYLD-deficient Treg cells. We also noted that a number of transcription factors were affected by the CYLD deficiency (Fig E6, C): Eomes and Tbx21 were upregulated, while Foxp3 and RORC were downregulated. It is possible that there is a discrepancy between mRNA and protein levels. Indeed, CYLD did not affect the protein expression levels of Eomes, Tbet and RORγt (Fig E6, D). IL4/STAT6 signaling, upstream of the master transcription factor GATA3, is critical for the differentiation of conventional Th2 cells. The expression of GATA3 was not changed by the CYLD deficiency in Treg cells (Fig E6, C and D). Moreover, shRNA against GATA3 showed a minor effect on IL-4 production in CYLD-deficient Treg cells (data not shown). Additionally, CYLD-deficient Treg cells had reduced expression of IL-4Rα, and unchanged STAT6 expression compared to WT Treg cells (Fig E6, E and F), implying that increased production of IL-4 by these Treg was primarily dependent on enhanced NF-κB and MAPK signaling rather than greater responsiveness to extrinsic signaling from IL-4.
Among the CYLD-regulated genes, Scinderin, a member of the gelsolin protein family,35,36 was most upregulated in Treg cells (Fig 5, D). We verified the robust expression of Scinderin in CYLD-deficient Treg cells at both the mRNA and protein levels (Fig 5, E and F). The expression of Scinderin was regulated by NF-κB signaling, since an inhibitor of NF-κB, but not an inhibitor of the MAPK cascade, was able to reduce Scinderin expression in CYLD-deficient Treg cells (Fig 5, G and H). The differential expression of Scinderin was also examined in various immune cells in WT mice: Scinderin was highly expressed by Treg cells, effector/memory CD8 T cells, and CD11c+ cells compared to other immune cells including naïve CD4/CD8 T cells, B cells, and NK cells (Fig E7, A). Scinderin plays a role in osteoclast differentiation by controlling cytoskeletal remodeling37 and has been reported to regulate NF-κB and p38 MAPK signaling during osteoclast differentiation.38, 39 We therefore examined whether Scinderin played a role in the MAPK activation as we observed in CYLD-deficient Treg cells. We first tested several MAP3Ks as well as MAP2Ks/MAPKs to determine whether they could interact with Scinderin. Among the tested molecules, MEK1 and MEK2 were able to bind to Scinderin (Fig 5, I). Notably, we identified that the interaction between MEK and ERK was remarkably enhanced in the presence of Scinderin (Fig 5, J). Moreover, the knockdown of Scinderin by shRNA was able to reduce the phosphorylation of ERK1/2 (Fig 5, K), suggesting Scinderin expression is important for MAPK activation. Most interestingly, the expression of IL-4 in CYLD-deficient Treg cells was significantly diminished by Scinderin knockdown (Fig 5, L), indicating that Scinderin elicits IL-4 expression by enhancing the activation of the MAPK signaling pathway. In addition, we assessed if overexpression of Scinderin affects ERK1/2 phosphorylation or IL-4 production in WT cells. By using retroviral transduction, the forced overexpression of Scinderin was able to facilitate ERK1/2 phosphorylation (Fig 5, M) and increase IL-4 expression (Fig 5, N). These results demonstrate that Scinderin itself has the ability to increase MAPK activation even in WT T cells to promote IL-4 production, and the deregulated IL-4 production seen in CYLD-deficient Treg cells was likely related to excessive activity of Scinderin and MEK/ERK.
Scinderin negatively mediates CYLD function in Treg cells
To further verify the physiological role of Scinderin in Treg cells, we generated double knockout Cyldfl/fl Scinfl/fl Foxp3cre (dKO) mice. Scinderin ablation in CYLD cKO mice reduced the proportion of CD44high CD62Llow memory/effector T cells that accumulated (Fig 6, A and B), compared to in CYLD cKO mice. In addition, consistent with our results of shRNA knockdown of Scinderin, the increased IL-4 and CCR8 expression in CYLD-deficient Treg cells was notably downregulated in CYLD/Scinderin dKO Treg cells (Fig 6, C). Moreover, the increased number of IL-4+ Treg cells as well as conventional Th2 cells seen in the lungs of CYLD cKO mice was largely eliminated by Scinderin ablation (Fig 6, D and E) further supporting that Scinderin is responsible for IL-4 production by CYLD KO Treg cells. Spontaneous lung inflammation caused by the CYLD cKO deficiency was also ameliorated by Scinderin deletion (Fig 6, F and G). Accordingly, Scinderin deletion was able to abolish the enhanced protective immunity against N. brasiliensis infection (Fig 6, H) accompanied by the reduced recruitment of eosinophils in the peritoneum (Fig 6, I). Of note, Scinderin cKO mice were comparable to WT mice in conventional T cell activation (Fig E7, B and C), Foxp3 expression (Fig E7, D and E) and homing capacity (Fig E7, F and G). Collectively, the data suggest that CYLD deficiency induces Scinderin expression, which is critical in regulating IL-4 production in Treg cells.
Figure 6. Scinderin accounts for defective function of CYLD−/− Treg cells.
Analysis of Cyldfl/fl Scinfl/fl Foxp3cre mice. A and B, The expression of CD62L and CD44 of splenic CD4+ T cells was assessed by flow cytometry. C-E, The expression of IL-4 was determined qPCR (C) and flow cytometry (D and E). Splenic YFP+ Treg cells were sorted for qPCR. For flow cytometry, lung CD4+ T cells were activated with PMA and ionomycin for 3 hrs prior to intracellular staining of IL-4 and Foxp3 (D) and the absolute number of IL-4+ Foxp3+ and Foxp3− T cells were calculated (E). F and G, histological analysis of lungs from Cyldfl/fl Scinfl/fl Foxp3cre double KO mice. The lung sections of 2 month-old mice were stained with H&E (F) and the clinical scores were assessed (G). H and I, N. Brasiliensis infection in Cyldfl/fl Scinfl/fl Foxp3cre double KO mouse. H, The number of worms in the intestines was examined at day 7 after infection. I, The recruitment of eosinophils to the peritoneum was assessed. Representative of at least two independent experiments are shown. Combined plots for B (n=6), C (n=4~6), E (n=3~5), G (n=3~6), H (n=9~11) and I (n=9~11) are shown. Error bars indicate SEM. ∗ P<0.05, ∗∗ P<0.01, ∗∗∗∗ P<0.0001 (Mann-Whitney test)
Discussion
The present study demonstrates the critical role of CYLD in the maintenance of Treg cell function by controlling IL-4 production. Our study also reveals that CYLD regulates the differential migration capacity of Treg cells by modulating the expression of trafficking receptors. We further show increased anti-parasite immunity driven by dysregulation of Treg by CYLD deletion. Importantly, we show that CYLD controls the expression of Scinderin, which in turn activates the MAPK pathway and downstream IL-4 production, suggesting a novel mechanism by which CYLD acts as a key regulator for Treg function in maintaining immune tolerance and immunity during infection.
A previous study demonstrated that CYLD can impact the suppressive function of Treg cells, but was complicated as it used germline transgenic targeting of CYLD through mutation of TRAF2 and NEMO binding motifs.29 This would have directly affected non-Treg cells as well as Treg, and also might have produced a situation where CYLD function was biased as opposed to eliminated. The weak suppressive activity of Treg cells in this mouse with a CYLDex7/8 mutation was explained by lower expression of CTLA-4 and CD25. Another study also suggested that CYLD might function by restraining differentiation of conventional naive T cells into Treg by regulating Smad7 and p38 signaling downstream of TGF-β.30 While these observations imply that CYLD may have several different roles in Treg development and function, our present study with conditional knockout of CYLD only in Foxp3+ Treg suggests an alternative role where dysregulation of IL-4 production is important to Treg function. We found that CYLD-deficient Treg cells promoted type 2 immune responses and were impaired in their suppressive activity in vivo, even though they exhibited functional suppressive activity in vitro. Our data showed CYLD-deficient Treg cells still maintained CD25, CTLA4, and TGF-β expression, as well as high levels of GITR and ICOS, all of which may contribute to the regulatory function in vitro.40, 41 We also observed that GITR and ICOS expression were not significantly changed under non-inflammatory conditions in female heterozygote Cyldfl/fl Foxp3cre/+ mice. Thus, it is possible that the environment in the aforementioned CYLDex7/8 transgenic mice may have affected Treg development and function42 and explained the difference with our results. Regardless, our data suggest that Treg conversion into IL-4 producing cells is intrinsic even in a non-inflammatory environment, and this conversion eliminates suppression and promotes a type 2 immune response regardless of expression of Treg surface antigens normally involved in suppressive activity. While TGF-β and p38 signaling promotes Foxp3 expression,30 our findings indicate that differential signaling involving either of these molecules might not explain our observations since both Foxp3 transcription and p38 phosphorylation were not altered in CYLD-deficient Treg cells. Nevertheless, we cannot exclude the possibility that CYLD regulates the intracellular crosstalk among various signaling pathways including NF-κB, MAPK, TGF-β, and TCR signaling.
The homing ability of Treg cells is one of the key aspects of their function, because they are able to suppress target cells once they migrate into and localize in a specific tissue site. For example, Treg cells that are enriched in human breast cancer exhibited CCR8-dependent suppression, correlating with clinical outcome.43 However, the present study demonstrates that deregulated expression of homing receptors in Treg cells can lead to either enhanced inflammation or anti-parasite immunity, rather than a suppressed immune response. Specific deletion of CYLD in Foxp3+ Treg cells caused inflammation in the lungs correlating with their increased migratory activity into the lungs. CYLD-deficient Treg cells shared the skin/lung homing receptor expression of CCR8, but not CCR4, with conventional Th2 cells.44 Thus, the increased homing of CYLD-deficient Treg cells is causal to the excessive IL-4 production and type 2 immunity in the lungs.
IL-4 produced by CYLD-deficient Treg cells also appeared to impact conventional Th2 cells expressing type 2 cytokines such as IL-4 and IL-5, providing another mechanism to further boost type 2 immunity against helminth infection. However, CYLD-deficient Treg cells seemed not to employ the IL-4/STAT6 signaling pathway for their conversion to Th2-like cells or IL-4 that controls cell homeostasis by intracellular signaling through p38 or TGFβR,45, 46 since IL-4R expression was downregulated on their surface, whereas conventional T cells showed a high level of IL-4R expression. Furthermore, the expression of GATA3, a master transcription factor for Th2 cells,47 was unaffected by CYLD-deficiency and its expression differed from that of conventional Th2 cells. Our results then imply that the increased IL-4 production might be due to a mechanism of action of CYLD in Treg cells distinct from that controlling IL-4 in conventional Th2 cells. In line with this notion, Scinderin expression was upregulated by the CYLD deficiency, leading to an enhancement of the MAPK signaling pathway that induces IL-4 expression.
Scinderin has actin-severing activity, which promotes actin depolymerization like other gelsolin family proteins, and plays a role in osteoclast differentiation and cytoskeletal remodeling37 and can regulate NF-κB and p38 MAPK signaling during osteoclast differentiation.38, 39 It has been suggested that Scinderin plays a role in lymphocytes,48 and serves as a potential biomarker or target for both acute and chronic asthma.49 It is also evident that Treg cells showed higher levels of Scinderin expression compared to conventional T cells 50, though the function of Scinderin has not been explored. Interestingly, actin rearrangement is associated with competitive survival by promoting cell polarity.51, 52 Moreover, actin dynamics play a pivotal role in the formation of immunological synapses between TCR and pMHC molecules,53 as well as intracellular Ca++/ERK phosphorylation54, 55 during TCR signaling indirectly implying Scinderin might participate in these processes. The noteworthy finding of the present study is that Scinderin was able to regulate the intracellular signal transduction cascade by promoting a protein-protein interaction network beyond the regulation of actin remodeling (Fig E8). Scinderin promoted a MAPK signaling cascade required for IL-4 expression in Tregs, while Scinderin expression was regulated by enhanced NF-κB activity that resulted from the CYLD-deficiency. Our data suggests that Scinderin may serve as an adapter protein to recruit several protein components in order to increase the efficiency of signaling and interaction between ERK and MEK in the MAPK signaling pathway.
Our results together with those from prior studies imply that CYLD might provide a potential target for therapeutic intervention of inflammatory diseases or infections. Since it is been well-known that CYLD mutation in humans causes several types of skin tumors25, 56 and CYLD also has an anti-inflammatory function in IBD patients,57, 58 our findings suggest that CYLD may regulate immune tolerance by regulating Treg function in humans. It has been reported that Tregs failed to suppress Th2 cells in asthma patients59 and Tregs undergo Th2-reprogramming in human food-allergic subjects.23 Human IL-4 producing Tregs shared a phenotype with Th2 cells.60 These Th2-like Tregs showed higher chemotactic activity compared to other Treg subsets, and conventional effector T cells, with increased expression of CCR4 and CCR8, and were enriched in skin tumors. As our preliminary data shows that CYLD knockdown is able to upregulate IL-4 and Scinderin expression in human Treg cells (data not shown), this indicates that CYLD may play an important role in Treg cells in humans. This also suggests that it would be interesting to address the role of CYLD in Treg plasticity in type 2 immunity in humans in the future.
Supplementary Material
Key Messages.
Treg-specific CYLD knockout mice develop a type 2-based lung inflammation while they are resistant against helminth infection
CYLD-deficient Treg cells produce IL-4 via activation of MAPK and NF-κB signaling pathways
CYLD controls Scinderin expression, which promotes MEK/ERK complex formation required for IL-4 production
Acknowledgements
We thank Drs. Ting and Glogauer for providing CYLD-floxed and Scinderin-floxed mice, respectively, J. Greenbaum for help with statistical analysis, and members of Liu laboratory for help and discussion.
Supported by NIH grants R01AI140130 and R21AI12258. LZ, RY, QW, XZ and YCL were supported by the Center for Life Sciences, Tsinghua University.
Abbreviations
- Foxp3
Forkhead box P3
- Treg
Regulatory T cells
- CYLD
Cylindromatosis
- cKO mouse
Conditional knockout mouse
- YFP
Yellow fluorescent protein
- IL-4
Interleukin-4
- CCR8
C-C motif chemokine receptor 8
- OVA
Ovalbumin
- BAL fluid
Bronchoalveolar lavage fluid
- MAPK
Mitogen-activated kinase
- NF-κB
Nuclear factor kappa B
- ERK
Extracellular receptor kinase
- MEK
Mitogen-activated protein kinase kinase
- Scin
Scinderin
Footnotes
Disclosure: The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 2005; 6:345–52. [DOI] [PubMed] [Google Scholar]
- 2.Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol 2003; 15:690–6. [DOI] [PubMed] [Google Scholar]
- 3.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 2001; 27:20–1. [DOI] [PubMed] [Google Scholar]
- 4.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001; 27:18–20. [DOI] [PubMed] [Google Scholar]
- 5.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, et al. Stability of the regulatory T cell lineage in vivo. Science 2010; 329:1667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 2009; 10:1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JH, Elly C, Park Y, Liu YC. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1alpha to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity 2015; 42:1062–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park Y, Jin HS, Lopez J, Elly C, Kim G, Murai M, et al. TSC1 regulates the balance between effector and regulatory T cells. J Clin Invest 2013; 123:5165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park Y, Jin HS, Lopez J, Lee J, Liao L, Elly C, et al. SHARPIN controls regulatory T cells by negatively modulating the T cell antigen receptor complex. Nat Immunol 2016; 17:286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin HS, Park Y, Elly C, Liu YC. Itch expression by Treg cells controls Th2 inflammatory responses. J Clin Invest 2013; 123:4923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama T, Hirahara K, Onodera A, Endo Y, Hosokawa H, Shinoda K, et al. Th2 Cells in Health and Disease. Annu Rev Immunol 2017; 35:53–84. [DOI] [PubMed] [Google Scholar]
- 12.Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 2016; 138:666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014; 41:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med 2011; 208:1863–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b(+) dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity 2013; 39:733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halim TYF, Rana BMJ, Walker JA, Kerscher B, Knolle MD, Jolin HE, et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity 2018; 48:1195–207 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med 1999; 189:1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finney CA, Taylor MD, Wilson MS, Maizels RM. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. Eur J Immunol 2007; 37:1874–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McSorley HJ, Harcus YM, Murray J, Taylor MD, Maizels RM. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J Immunol 2008; 181:6456–66. [DOI] [PubMed] [Google Scholar]
- 20.Blankenhaus B, Klemm U, Eschbach ML, Sparwasser T, Huehn J, Kuhl AA, et al. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J Immunol 2011; 186:4295–305. [DOI] [PubMed] [Google Scholar]
- 21.Molofsky AB, Van Gool F, Liang HE, Van Dyken SJ, Nussbaum JC, Lee J, et al. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity 2015; 43:161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelly VS, Coomes SM, Kannan Y, Gialitakis M, Entwistle LJ, Perez-Lloret J, et al. Interleukin 4 promotes the development of ex-Foxp3 Th2 cells during immunity to intestinal helminths. J Exp Med 2017; 214:1809–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 2015; 42:512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston CJC, Smyth DJ, Kodali RB, White MPJ, Harcus Y, Filbey KJ, et al. A structurally distinct TGF-beta mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat Commun 2017; 8:1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet 2000; 25:160–5. [DOI] [PubMed] [Google Scholar]
- 26.Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med 2007; 204:1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hovelmeyer N, Wunderlich FT, Massoumi R, Jakobsen CG, Song J, Worns MA, et al. Regulation of B cell homeostasis and activation by the tumor suppressor gene CYLD. J Exp Med 2007; 204:2615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srokowski CC, Masri J, Hovelmeyer N, Krembel AK, Tertilt C, Strand D, et al. Naturally occurring short splice variant of CYLD positively regulates dendritic cell function. Blood 2009; 113:5891–5. [DOI] [PubMed] [Google Scholar]
- 29.Reissig S, Hovelmeyer N, Weigmann B, Nikolaev A, Kalt B, Wunderlich TF, et al. The tumor suppressor CYLD controls the function of murine regulatory T cells. J Immunol 2012; 189:4770–6. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Thornton AM, Kinney MC, Ma CA, Spinner JJ, Fuss IJ, et al. The deubiquitinase CYLD targets Smad7 protein to regulate transforming growth factor beta (TGF-beta) signaling and the development of regulatory T cells. J Biol Chem 2011; 286:40520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 2009; 30:636–45. [DOI] [PubMed] [Google Scholar]
- 32.Sawant DV, Sehra S, Nguyen ET, Jadhav R, Englert K, Shinnakasu R, et al. Bcl6 controls the Th2 inflammatory activity of regulatory T cells by repressing Gata3 function. J Immunol 2012; 189:4759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature 2003; 424:801–5. [DOI] [PubMed] [Google Scholar]
- 34.Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 2003; 424:793–6. [DOI] [PubMed] [Google Scholar]
- 35.Kwiatkowski DJ. Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol 1999; 11:103–8. [DOI] [PubMed] [Google Scholar]
- 36.Li GH, Arora PD, Chen Y, McCulloch CA, Liu P. Multifunctional roles of gelsolin in health and diseases. Med Res Rev 2012; 32:999–1025. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Wang Y, Viniegra A, Sima C, McCulloch CA, Glogauer M. Adseverin plays a role in osteoclast differentiation and periodontal disease-mediated bone loss. FASEB J 2015; 29:2281–91. [DOI] [PubMed] [Google Scholar]
- 38.Nurminsky D, Magee C, Faverman L, Nurminskaya M. Regulation of chondrocyte differentiation by actin-severing protein adseverin. Dev Biol 2007; 302:427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song MK, Lee ZH, Kim HH. Adseverin mediates RANKL-induced osteoclastogenesis by regulating NFATc1. Exp Mol Med 2015; 47:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodman WA, Cooper KD, McCormick TS. Regulation generation: the suppressive functions of human regulatory T cells. Crit Rev Immunol 2012; 32:65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Layman AA, Oliver PM. Ubiquitin Ligases and Deubiquitinating Enzymes in CD4+ T Cell Effector Fate Choice and Function. J Immunol 2016; 196:3975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A 2008; 105:19396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, et al. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity 2016; 45:1122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikhak Z, Fukui M, Farsidjani A, Medoff BD, Tager AM, Luster AD. Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. J Allergy Clin Immunol 2009; 123:67–73 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunt AE, Williams LM, Lali FV, Foxwell BM. IL-4 regulation of p38 MAPK signalling is dependent on cell type. Cytokine 2002; 18:295–303. [DOI] [PubMed] [Google Scholar]
- 46.Macey MR, Sturgill JL, Morales JK, Falanga YT, Morales J, Norton SK, et al. IL-4 and TGF-beta 1 counterbalance one another while regulating mast cell homeostasis. J Immunol 2010; 184:4688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 1997; 89:587–96. [DOI] [PubMed] [Google Scholar]
- 48.Robbens J, Louahed J, De Pestel K, Van Colen I, Ampe C, Vandekerckhove J, et al. Murine adseverin (D5), a novel member of the gelsolin family, and murine adseverin are induced by interleukin-9 in T-helper lymphocytes. Mol Cell Biol 1998; 18:4589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Valentin E, Crahay C, Garbacki N, Hennuy B, Gueders M, Noel A, et al. New asthma biomarkers: lessons from murine models of acute and chronic asthma. Am J Physiol Lung Cell Mol Physiol 2009; 296:L185–97. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Nishio J, van Maurik A, Mathis D, Benoist C. Differential response of regulatory and conventional CD4(+) lymphocytes to CD3 engagement: clues to a possible mechanism of anti-CD3 action? J Immunol 2013; 191:3694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, et al. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J Cell Sci 2010; 123:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levayer R, Hauert B, Moreno E. Cell mixing induced by myc is required for competitive tissue invasion and destruction. Nature 2015; 524:476–80. [DOI] [PubMed] [Google Scholar]
- 53.Huppa JB, Axmann M, Mortelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature 2010; 463:963–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity 2001; 14:315–29. [DOI] [PubMed] [Google Scholar]
- 55.Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol 2014; 15:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ 2010; 17:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinu I, Mahasirimongkol S, Liu Q, Yanai H, Sharaf Eldin N, Kreiter E, et al. SNP-SNP interactions discovered by logic regression explain Crohn’s disease genetics. PLoS One 2012; 7:e43035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cleynen I, Vazeille E, Artieda M, Verspaget HW, Szczypiorska M, Bringer MA, et al. Genetic and microbial factors modulating the ubiquitin proteasome system in inflammatory bowel disease. Gut 2014; 63:1265–74. [DOI] [PubMed] [Google Scholar]
- 59.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol 2007; 119:1258–66. [DOI] [PubMed] [Google Scholar]
- 60.Halim L, Romano M, McGregor R, Correa I, Pavlidis P, Grageda N, et al. An Atlas of Human Regulatory T Helper-like Cells Reveals Features of Th2-like Tregs that Support a Tumorigenic Environment. Cell Rep 2017; 20:757–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Legarda D, Justus SJ, Ang RL, Rikhi N, Li W, Moran TM, et al. CYLD Proteolysis Protects Macrophages from TNF-Mediated Auto-necroptosis Induced by LPS and Licensed by Type I IFN. Cell Rep 2016; 15:2449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008; 28:546–58. [DOI] [PubMed] [Google Scholar]
- 63.Knott ML, Matthaei KI, Giacomin PR, Wang H, Foster PS, Dent LA. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol 2007; 37:1367–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.