Abstract
Background
Infliximab is a human murine chimeric anti‐tumour necrosis factor alpha monoclonal antibody recently approved for the treatment of refractory RA.
Objectives
To assess the efficacy and safety of infliximab for the treatment of rheumatoid arthritis.
Search methods
Electronic databases including Biological Abstracts, CINAHL, Current Contents, Dissertation Abstracts, EBM Reviews, HealthSTAR and MEDLINE were searched from 1966 to March 2002. Rheumatoid arthritis was searched as an exploded MESH heading. Infliximab was searched as a text word as it is not currently indexed . The search was not limited by language, year of publication or type of publication. The specific search strategy is shown below.
Selection criteria
All randomized controlled trials comparing infliximab 1, 3, 5 or 10 mg/kg with methotrexate(MTX) to MTX alone, or without MTX to placebo, with a minimum duration of 6 months and at least 2 infusions were eligible.
Data collection and analysis
Data was extracted by 2 independent reviewers and the methodological quality of the trials was assessed using a validated assessment tool scale. Outcome variables included the ACR core set of disease activity measures for RA clinical trials and radiographic outcome data. Withdrawals and toxicity were also included. End of trial results were pooled. Continuous data were pooled using weighted mean differences and dichotomous data using relative risks.
Main results
Two trials with a total of 529 patients met the inclusion criteria. Patients fulfilling the American Rheumatism Association 1987 RA diagnostic criteria were randomized to receive either infliximab 1mg/kg (with and without MTX), 3mg/kg(with and without MTX) , 10mg/kg of infliximab (with and without MTX) or placebo infusion plus MTX. Infusions were given every 4 or 8 weeks. After 6 months ACR 20, ACR 50 and ACR 70 response rates were significantly improved in all infliximab doses compared to control.
The number needed to treat with infliximab to achieve an ACR 20, 50 or 70 response in patients with refractory RA under specialist care ranged from 2.94‐3.33 for ACR 20, 3.57‐4.76 for ACR 50 and 5.88 ‐12.5 for ACR 70 depending on the dose (3mg/kg or 10mg/kg given either every 4 or 8 weeks).
Total withdrawals and withdrawals due to lack of efficacy were lower for all doses of infliximab versus controls. Withdrawals for adverse events and withdrawals for other reasons were not statistically significantly different for those receiving infliximab from control.
Authors' conclusions
Treatment with infliximab for 6 and 12 months significantly reduces RA disease activity and appeared to have an acceptable safety profile in these trials. Total radiographic scores improved, fewer patients showed radiographic progression, and more patients showed radiographic improvement with infliximab treatment at 12 months compared to controls. However, only 2 trials met the inclusion criteria, and these results are largely driven by the largest trial. The available efficacy and toxicity data is relatively short‐term (6‐12 months). In order to detect rare events that may be associated with infliximab, larger and longer term studies are required.
Plain language summary
Infliximab for rheumatoid arthritis
Infliximab in combination with methotrexate is an effective treatment for rheumatoid arthritis( RA).
Infliximab is a relatively new disease modifying anti‐rheumatic drug that inhibits tumour necrosis factor alpha. Short term (six to twelve month) studies suggest infliximab is well tolerated, and in combination with methotrexate, decreases disease activity in RA. Infliximab 3mg/kg or 10mg/kg, in combination with methotrexate, taken every 4 or 8 weeks for either 6 or 12 months, significantly improved disease activity as measured by tender and swollen joints and ACR response rates. Pain and physical function also improved compared to those taking methotrexate alone. Infliximab significantly reduced radiographic progression at 12 months.
Background
Rheumatoid arthritis (RA) is a systemic inflammatory arthritis associated with significant morbidity, deformity and impaired quality of life. Medications known as disease modifying anti‐rheumatic drugs (DMARDs) are the main stay of treatment and have been shown to reduce disease activity, retard joint erosions and improve patients' quality of life. Unfortunately, patients often fail or are unable to tolerate the currently available DMARDs.
Infliximab (Remicade) and etanercept (Enbrel) are biological agents that have recently been introduced for the treatment of refractory RA. Infliximab is a chimeric anti‐TNF (tumour necrosis factor) alpha monoclonal antibody. Etanercept is a TNF alpha inhibitor. Studies to date indicate they may be revolutionary treatments with greater efficacy than current DMARDs. These agents however are relatively costly.
The goal of this review is to summarize the current data on infliximab's efficacy and safety in treating RA. This data is needed for clinicians to chose the most appropriate treatment for their RA patients.
Objectives
To assess the efficacy and safety of infliximab in the treatment of RA.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) or controlled clinical trails (CCTs) comparing infliximab and methotrexate to methotrexate alone or comparing infliximab alone to placebo were eligible for inclusion. Patients could also be taking other DMARDs or corticosteroids provided they were on stable doses and were randomly allocated to treatment with infliximab or to treatment without infliximab.
Types of participants
Patients at least 16 years of age meeting the ACR 1987 revised criteria (Arnett 1988) for RA. These patients must have evidence of active disease as demonstrated by at least 2 of:
1. Tender joint count 2. Swollen joint count 3. Duration of early morning stiffness >30 minutes. 4. Acute phase reactants such as Westergren erythrocyte sedimentation rate (ESR) or C‐ reactive protein (CRP)
Types of interventions
Treatment with infliximab/methotrexate (MTX) versus MTX or infliximab alone versus placebo were eligible for inclusion. Doses of infliximab eligible for inclusion include 1mg/kg, 3mg/kg, 5 mg/kg and 10 mg/kg with a minimum trial duration of 6 months.
Types of outcome measures
The primary efficacy outcomes included in this review were the response of rheumatoid arthritis to treatment with infliximab as defined by the World Health Organization (WHO), the International League of Associations for Rheumatology (ILAR) core set of disease activity measures and the American College of Rheumatology outcome measures for RA clinical trials (OMERACT 1993, Boers 1994, Felson 1993)
EFFICACY OUTCOMES
1. Tender joint count (TJC) 2. Swollen joint count (SWJ) 3. Patient's assessment of pain using 10 cm visual analogue scale or Likert scale. 4. Patient global assessment of disease activity 5. Physician global assessment of disease activity using 10cm visual analogue scale or Likert scale. 6. Patient's assessment of functional ability as measured by a validated scale such as the Health Assessment Questionnaire (HAQ), which is a standardized, validated scale used in arthritis patients. 7. Acute phase reactants such as ESR or CRP. 8. Radiographic bone changes are accepted as part of the core set of disease activity measures in studies of a minimum of 12 months duration. Bony progression as measured by Sharp scores or Larsen scale were thus included as a primary outcome measure for studies with a minimum duration of 12 months.
Definition of improvement: The issue of statistical versus clinical significance in clinical trials is relevant to clinical care. Based on the set of efficacy measures outlined above the following definition of clinical improvement has been established (Felson 1995, Pincus 1999). An ACR 20 response represents a 20% improvement in tender and swollen joints counts plus a 20% improvement in 3 of the 5 following remaining core measures: patient and physical global assessments, pain, functional status and an acute phase reactant. Results have been calculated to provide an indication of the number needed to treat (NTT) for each dichotomous outcome. The NNT reflects the effort required (or number of patients one would need to treat) to obtain a beneficial outcome with an intervention.
Secondary outcomes included health‐related quality of life (HRQoL) such as the SF‐36.
SAFETY OUTCOMES
Safety outcomes presented include adverse event outcomes and withdrawals.
Search methods for identification of studies
Electronic databases including Biological Abstracts, CINAHL, Current Contents, Dissertation Abstracts, EBM Reviews, HealthSTAR and Medline were searched from 1966 to March 2002. Rheumatoid arthritis was searched as an exploded MESH heading. Infliximab was searched as a text word as it is not currently indexed . The search was not limited by language, year of publication or type of publication. The specific search strategy is shown below.
The Cochrane Clinical Trials registry was searched (Issue 4, 2001). The proceedings of major rheumatology conferences including The American College of Rheumatology (ACR) (1990‐2002), the European League of Rheumatology (EULAR) and the Canadian Rheumatology Association (CRA) were hand searched. The reference lists from standard rheumatology textbooks, comprehensive reviews and identified clinical trials were searched. Content experts and the pharmaceutical companies that manufacture infliximab (Schering in Canada and Centocor in the USA) were contacted. Additional unpublished data from published trials was found by searching the FDA web site. The only unpublished data used in this review was additional information from published trials.
Search Strategy: 1 exp arthritis, rheumatoid/ or *arthritis, juvenile rheumatoid/ or *caplan's syndrome/ or *felty's syndrome/ or *rheumatoid nodule/ or *sjogren's syndrome/ or *spondylitis, ankylosing/ or *still's disease, adult‐onset/ 2 (felty$ adj2 syndrome).tw. 3 (caplan$ adj2 syndrome).tw. 4 rheumatoid nodule.tw. 5 (sjogren$ adj2 syndrome).tw. 6 (sicca adj2 syndrome).tw. 7 still$ disease.tw. 8 (spondylitis adj2 ankylosing).tw. 9 (arthritis adj2 rheumat$).tw. 10 or/1‐9 11 infliximab.tw. 12 remicade.tw. 13 anti‐interleukin$.tw. 14 anti‐tumour necrosis factor$.tw. 15 anti‐tumor necrosis factor.tw. 16 anti‐tnf.tw. 17 *Tumor Necrosis Factor/ 18 *Antibodies, Monoclonal/tu [Therapeutic Use] 19 or/11‐18 20 10 and 19 21 from 20 keep 1‐567
Data collection and analysis
STUDY SELECTION Two reviewers (BB, AC) independently ascertained whether each study met the inclusion criteria outlined in an a priori protocol for the review. Case reports or case series were not included. Consensus was reached by discussion of any disagreement. The reason(s) for exclusion of any study was noted.
QUALITY ASSESSMENT The methodological quality of the included studies was assessed by independent reviewers (BB, AC) on the basis of randomisation, adequate concealment of randomization, degree of blinding, use of intention to treat analysis and description of dropouts and withdrawals. The validated Jadad (Jadad 1996) instrument was used to assess the quality of each study.
DATA ABSTRACTION Two reviewers (BB, AC) independently abstracted data from each study using pre‐determined data abstraction forms. These forms were cross‐referenced. Discrepancies were resolved by a consensus of the two reviewers. The identification of the trials were not masked, as there is no consensus regarding effectiveness of masking. From each selected trial, information regarding study design, population demographics, treatment regime, treatment duration and baseline and end‐of‐study outcome measures were collected. Information on simultaneous DMARD usage, number of previous DMARDs used, consumption/average dose of corticosteroids and disease duration were also collected.
DATA SYNTHESIS AND ANALYSIS The data was analyzed using an intention to treat model whenever possible. Continuous data was analyzed as a weighted mean difference. Dichotomous data were reported as relative risk. Chi square test using n‐1 degrees of freedom and a p value of less than or equal to 0.05 was performed to test homogeneity of the data. A fixed effects model was used to pool studies to calculate a pooled estimate of effect. Where no significant heterogeneity existed, the data were not pooled. The mean and standard deviation (sd) were used when available. When only median and interquartile ranges were reported, the median was used as the mean, and one half of the difference between the 1st and 3rd quartile range was used as the sd. When only the baseline sd was available, it was used as the end of study sd as well. When numerical data was reported only graphically, the value was extracted from the graph.
SENSITIVITY AND SUBGROUP ANALYSES Sensitivity and subgroup analysis were planned to determine the effects of disease duration, previous DMARD treatment , corticosteroid dose and disease severity on the response to infliximab.
Results
Description of studies
Maini 1998 was a 26 week randomized double blind, double dummy, multi‐centre study. One hundred and one patients fulfilling the American Rheumatism Association 1987 RA diagnostic criteria were randomized to infliximab 1mg/kg (with and without MTX), 3mg/kg(with and without MTX) 10mg/kg of infliximab (with and without MTX) or placebo infusion plus MTX. Infusions were given at weeks 2, 6, 10 and 14. Patients randomized to receive MTX received 7.5 mg once weekly. Patients had to have had an incomplete response to at least 6 months treatment with MTX 7.5 to 15mg once weekly to participate and have evidence of active disease on the day of screening. Patients had to have a SJC of at least 6 plus at least 2 of TJC of at least 6, EMS>45 minutes, ESR>28 or CRP> 15mg/dl. All DMARDs except MTX were withdrawn 4 weeks prior to screening. Patients on prednisone had to be on a stable dose for 4 weeks prior to screening and the dose could not exceed 7.5 mg daily. Patients in the study were predominantly white females and rheumatoid factor positive with a disease duration of 7.6 to 11.1 years.
Maini 1999 (ATTRACT) was a 30 week randomized double blind multi‐ centre study. Four hundred and twenty‐eight patients fulfilling the 1987 American Rheumatism Association RA diagnostic criteria were randomized to infliximab 3mg/kg every 8 weeks, 3 mg/kg every 4 weeks, 10mg/kg every 8 weeks, 10mg/kg every 4 weeks or placebo infusion every 4 weeks. All patients had evidence of active disease in spite of at least 3 months of a stable MTX dose of at least 12.5 mg per week . Patients remained on the same dose of MTX they were on prior to randomization. Patients had to have a TJC and SJC of at least 6 plus 2 of EMS > 45 minutes, ESR>28 or CRP> 2mg/dl. Prednisone doses had to be stable for at least 4 weeks prior to screening and the dose could not exceed 10mg daily. DMARDs other than MTX were not permitted within 4 weeks of screening. The enrolled patients were predominantly white females and rheumatoid factor positive with a disease duration of 7.2 to 9.0 years.
Lipsky 2000 is a continuation of Maini 1999 to 54 weeks. Two year ATTRACT data was not incorporated as the study was unblinded after 1 year.
Risk of bias in included studies
Maini 1998 scored 4 of a possible 5 on the Jadad quality assessment tool, scoring top points for randomization and double blinding and losing 1 point as drop‐outs and withdrawals were only completely reported to week 14. Maini 1999 and Lipsky 2000 both scored 5. All 3 studies received a concealment allocation rating of A as they all blinded their randomization of subjects.
Effects of interventions
Three trials (Maini 1998, Maini 1999, Lipsky 2000) met the inclusion criteria. Maini 1998 and Maini 1999 studies followed participants for 26 and 30 weeks respectively which has been treated as 6 months for this review; Lipsky 2000 is a continuation of Maini 1999 to 12 months. Only 2 doses (infliximab 3mg/kg every 4 weeks and 10mg/kg every 4 weeks) were used in all 3 studies; 3mg/kg every 8 weeks and 10mg/kg every 8 weeks were used only in the ATTRACT trial (Maini 1999). Maini 1998 and Maini 1999 were pooled to obtain 6‐month outcomes for TJC, SJC, CRP, ACR 20 and 50, withdrawals and toxicity. The 6‐month values for physician global assessment, patient global assessment, pain, HAQ and ACR 70 were only available for Maini 1999, although they were assessed, results were not reported in Maini 1998. All reported 12‐month data are from Lipsky 2000.
All results have been reported using a fixed effects (FE) model. When significant heterogeneity existed, the data was not pooled. Efficacy outcomes are reported as weighted mean difference (WMD) with 95% confidence interval for continuous data and as relative risk with 95% confidence interval for dichotomous outcomes.
EFFICACY AT 6 MONTHS
ACR 20 response rates were significantly improved in all infliximab doses compared to control. For infliximab 3mg/kg every 8 weeks, 53% achieved an ACR 20 response (compared to 20% of controls) with an absolute treatment benefit (ATB) of 33%(95% CI 20,47) and a NNT of 3.03 people. For 3mg/kg every 4 weeks, 49% achieved an ACR 20 (compared to 19% of controls) with an ATB of 30% (95% CI 18,42) and NNT 3.33. For infliximab 10mg/kg q 8 weeks the ACR 20 was 53% versus 20% in controls with an ATB of 32% (95% CI 19,46) and NNT of 3.13. For infliximab 10mg/kg every 4 weeks, the ACR 20 was 55% versus 19% in controls with an ATB of 36% (95% CI 24,49) and NNT of 2.78. For all ATTRACT doses pooled, the ACR 20 was 54% versus 20% in controls with an ATB of 34% (95% CI 24,44) and NNT of 2.94.
ACR 50 responses were significantly improved in all infliximab doses compared to control. For infliximab 3mg/kg q 8 weeks, 26% achieved an ACR 50 compared to 5% of controls, with an ATB of 21% (95% CI 11,31) and NNT 4.76. For 3mg/kg q 4 weeks, 32% achieved an ACR 50 compared to 4% of controls with an ATB of 28% (95% CI 18,38) and NNT of 3.57. For 10mg/kg q 8 weeks, 30% achieved an ACR 50 versus 5% of controls with an ATB of 25% (95% CI 15,36) and NNT 4. For 10mg/kg q 4 weeks the ACR50 was 28% versus 4% in controls with an ATB of 24% (95% 15,34) and NNT of 4.17. All ATTRACT doses combined yielded an ACR 50 of 28% versus 5% in controls with an ATB of 23% ( 95% CI 17,30) and NNT 4.35.
ACR 70 responses were significantly improved in all infliximab doses compared to control. For infliximab 3mg/kg every 8 weeks, 8% achieved an ACR 70 versus 0% in controls with an ATB of 8% (95% CI 2,14) and NNT of 12.5. For 3mg/kg q 4 weeks, 10% achieved an ACR 70 versus 0% of controls, with an ATB of 10% (95% CI 4,17) and NNT 10. For 10mg/kg q 8 weeks 17% achieved an ACR 70 versus 0% in controls with an ATB of 17% (95% CI 9,25) and NNT 5.88. For 10mg/kg q 4 weeks, 11% achieved an ACR 70 versus 0% of controls, with an ATB of 11% (95% CI 4,18) and NNT 9.09. For all ATTRACT doses combined, 12% achieved an ACR 70 versus 0% of controls with an ATB of 12% (95% CI 8,16) and NNT of 8.33.
SJC was significantly reduced for all doses of infliximab versus control. Since significant heterogeneity existed for SJC as evidenced by a p value<0.00001, the studies were not combined. Infliximab 3mg/kg every 8 weeks had a WMD of ‐4.00(‐6.39,‐1.61). Infliximab 3mg/kg every 4 weeks had a WMD of ‐14.00(‐18.10,‐9.90) for Maini 1998 and ‐4.00(‐6.21, ‐1.79) for Maini 1999. Infliximab 10mg/kg every 8 weeks had a WMD of ‐6.00(‐8.21,‐3.79). Infliximab 10mg/kg every 4 weeks had a WMD of ‐11.50(‐17.11,‐5.89) for Maini 1998 and ‐7.00(‐9.12,‐4.88) for Maini 1999.
TJC was significantly reduced for all doses of infliximab versus control. Since significant heterogeneity existed for TJC as evidenced by a p value=0.0001, the studies were not combined. For infliximab 3mg/kg every 8 weeks, the WMD was ‐4.00(‐7.32,‐0.68). For infliximab 3mg/kg every 4 weeks, the WMD was ‐23.30(‐31.42,‐15.18) for Maini 1998 and ‐5.00(‐8.51,‐1.49) for Maini 1999. For infliximab 10mg/kg every 8 weeks the WMD was ‐4.00(‐7.31,‐0.69). For infliximab 10mg/kg every 4 weeks the WMD was ‐23.30(‐30.80,‐15.80) for Maini 1998 and ‐7.00(‐10.69,‐3.31) for Maini 1999.
CRP results for both Maini 1998 and Maini 1999, and patient pain, evaluator's global assessment, patient global assessment and HAQ scores from Maini 1999 are presented in the graphs within this review.
Six month radiographic results were not available.
WITHDRAWAL DATA AT 6 MONTHS
Total withdrawals were lower for all doses of infliximab versus controls. For infliximab 3mg/kg every 8 weeks, 17% withdrew versus 36% in controls with absolute reduction in withdrawals of 19% (6,32). For 3mg/kg every 4 weeks, 10% withdrew versus 39% of controls with an absolute reduction of 29%(18,41) For 10mg/kg every 8 weeks, 9% withdrew versus 36% of controls with an absolute reduction of 27%(15,39). For 10mg/kg every 4 weeks,15% withdrew versus 39% in controls with an absolute reduction of 25%(13,36). For all ATTRACT doses combined, 13% withdrew versus 36% of controls with an absolute reduction of 23%(12,34).
Withdrawals due to lack of efficacy were lower for all doses of infliximab versus controls. For infliximab 3mg/kg every 8 weeks, 13% withdrew versus 25% of controls with an absolute reduction of 12%(1,24). For infliximab 3 mg/kg every 4 weeks, 6% withdrew versus 29% of controls with an absolute reduction of 24%(14,33). For 10mg/kg every 8 weeks, 6% withdrew versus 25% of controls with an absolute reduction of 19%(9,30). For 10mg/kg every 4 weeks, 6% withdrew versus 29% of controls with an absolute reduction of 23%(13,33) For all ATTRACT doses pooled, 8% withdrew versus 25% of controls with an absolute reduction of 17%(8,27.)
Withdrawals for adverse events and withdrawals for other reasons were not statistically significantly different from control as the CI for the relative risk crossed 1, and the CI for the absolute treatment effect crossed 0. The specific numbers are listed in the additional tables.
TOXICITY AT 6 MONTHS
Toxicity data were reported as pooled infliximab doses versus control. Infections requiring antibiotics occurred in 31% of infliximab treated patients versus 21% of controls with a RR of infection of 1.48(0.99,2.23) which is not statistically significant. Serious infections occurred in 3.7% of infliximab treated patients versus 5% of controls with a RR of 0.72(0.28,1.84) which is not statistically significant. Neoplasm occurred in less than 1% of infliximab treated patients versus 0% of controls with a RR of 1.78(0.09,34.05) which is not statistically significant. SLE‐like illness occurred in less than 1% of infliximab treated patients versus 0% of controls with a RR of 0.63(0.07,5.93) which is not statistically significant. Death occurred in less than 1% of the infliximab treated patients versus 3% of controls with a RR of 0.22(0.05,0.99) which just achieves statistical significance.
EFFICACY DATA 12 MONTHS
ACR 20 response rates were significantly improved in all infliximab doses compared to control. For infliximab 3mg/kg every 8 weeks, 42% achieved an ACR 20 versus 17% of controls with an absolute treatment benefit (ATB) of 25%(12,38) and NNT 4 people. For 3mg/kg every 4 weeks, 48% achieved an ACR 20 versus 17% of controls with an ATB of 31%(17,44) and NNT 3.23. For 10mg/kg every 8 weeks, 59% achieved an ACR 20 versus 17% of controls with an ATB of 42%(29,55) and NNT 2.38. For 10mg/kg every 4 weeks 59% achieved an ACR 20 versus 17% of controls with an ATB of 42%(29,55) and NNT 2.38. For all ATTRACT doses pooled 52% achieved an ACR 20 versus 17% of controls with an ATB of 35%(25,44) and NNT 2.86.
ACR 50 response rates were significantly improved in all infliximab doses compared to control. For infliximab 3mg/kg every 8 weeks 21% achieved an ACR 50 versus 8% with an ATB of 13%(3,23) and NNT 7.69. For 3mg/kg every 4 weeks , 34% achieved an ACR50 versus 8% of controls with an ATB of 26%(14,37) and NNT 3.85. For 10mg/kg every 8 weeks 39% obtained an ACR 50 compared to 8% of controls with an ATB of 31%(19,43) and NNT 3.23. For 10mg/kg every 4 weeks, 38% obtained an ACR 50 versus 8 % of controls with an ATB of 30%(18,42) and NNT 3.33. For all ATTRACT doses pooled, 33% achieved an ACR 50 versus 8% of controls with an ATB of 25%(17,33) and NNT 4.
ACR 70 response rates were significantly improved in all infliximab doses compared to control. For infliximab 3mg/kg every 8 weeks 10% achieved an ACR 70 versus 2% of controls with an ATB of 8%(1,15) and NNT 12.5. For 3mg/kg every 4 weeks 17% achieved an ACR 70 versus 2% of controls with an ATB 15%(7,24) and NNT 6.67. For 10mg/kg every 8 weeks 25% achieved an ACR 70 versus 2% of controls with an ATB of 23%(13,33) and NNT 4.35. For 10mg/kg every 4 weeks, 19% achieved an ACR 70 versus 2% in controls with an ATB of 16%(7,25) and NNT 6.25. For all ATTRACT doses pooled, 18% achieved an ACR 70 versus 2% of controls with an ATB of 16%(11,21) and NNT 6.35.
Major radiographic progression was significantly reduced in all infliximab doses versus control . The rate of progression was 31% in the control group. For infliximab 3mg/kg every 8 weeks 8% progressed with a ATB of 23%(10,36) and NNT 4.35. For 3mg/kg every 4 weeks 13% progressed with an ATB of 19%(5,32) and NNT 5.26. For infliximab 10mg/kg 1% progressed with an ATB of 30%(18,42) and NNT 3.33. For 10mg/kg every 4 weeks 0% progressed with an ATB of 31%(20,43) and NNT 3.23.
Radiographic improvement was more frequent in all infliximab doses versus control. In the control group, 14% improved. For infliximab 3mg/kg every 8 weeks, 44% improved with an ATB of 30%(15,44) and NNT 3.33. For 3mg/kg every 4 weeks, 48% improved with an ATB of 34%(19,48) and NNT 2.94. For 10mg/kg every 8 weeks, 39% improved with an ATB of 25%(11,39) and NNT 4. For 10mg/kg every 4 weeks 55% improved with an ATB of 40%(26,55) and NNT 2.5.
Total radiographic scores were also significantly reduced for all infliximab doses compared with control. Total radiographic scores were reduced for infliximab 3mg/kg every 8 and 4 weeks, and infliximab 10mg/kg every 8 and every 4 weeks, respectively, with WMD of ‐8.70(‐11.58,‐5.82), ‐16.40(‐19.61, ‐13.19), ‐21.80(‐24.45,‐19.15) and ‐13.70(‐16.38,‐11.02) respectively.
WITHDRAWAL DATA 12 MONTHS Total withdrawals were reduced for all infliximab doses versus controls. For the pooled infliximab dose the RR of withdrawal was 0.42 (0.31,0.56). Withdrawals due to adverse events were not statistically different from placebo with any of the infliximab doses. The RR of withdrawals due to adverse events for the pooled infliximab doses was 0.96(0.43,2.14) Withdrawals due to lack of efficacy were significantly reduced in all infliximab doses with a pooled infliximab dose RR of 0.32(0.22,0.48). Withdrawals for other reasons was not significantly different in all doses of infliximab versus placebo, although the pooled infliximab dose had a significantly lower withdrawal rate with a RR or 0.26(0.08,0.87). Specific rates for individual doses can be found in the additional tables.
TOXICITY DATA 12 MONTHS Toxicity results at 12 months were as follows. Serious adverse events (as defined by the World Health Organization Adverse‐Reaction Terminology) were not significantly different between infliximab and placebo for any infliximab dose. The RR for the pooled infliximab dose was 0.80(0.50,1.29). Serious infections were not significantly different between treatment and placebo for any infliximab dose and the RR for the pooled infliximab dose was 0.76(0.33, 1.73).
Development of an ANA of at least 1:320 tilter was more common in all infliximab doses compared to placebo. The RR for infliximab 3mg/kg every 8 and every 4 weeks, and for infliximab 10mg/kg every 8 and every 4 weeks, and for all ATTRACT doses pooled were 2.59(1.69,3.97), 2.4(1.54,3.72), 2.38(1.53,3.68), 2.04(1.29,3.22)and 2.36(1.57,3.55) respectively. The absolute increase in ANA was 41%(27,56), 36%(21,52), 36%(21,52), 36%(21,51), 27%(11,43) and 35%(24,47) respectively. The NNH was 2.44, 2.78, 2.78, 3.70 and 2.86 respectively.
Anti double stranded DNA antibodies (ds‐DNA) were statistically significanlty higher in all infliximab doses except infliximab 10mg/kg every 4 weeks. The RR for infliximab 3mg/kg every 8 and 4 weeks, infliximab 10mg/kg every 8 and 4 weeks, and for all ATTRACT doses pooled were 18.15(1.07,306.97), 18.78(1.11,317.59), 18.35(1.08,310.43),13.48(0.77,269.05) and 16.65(1.03,269.05) respectively. The absolute increase in ds‐DNA was 10%(4,17),11%(4,17),10%(4,17),7%(1,14)and 10%(6,13) respectively. The number needed to harm(NNH) was 10,9.09,10,14.29 and 10 respectively.
Discussion
Rheumatoid arthritis is a common inflammatory polyarthritis that causes significant morbidity and mortality. Drugs known as disease modifying anti‐rheumatic drugs have been shown to reduce symptoms, improve quality of life and retard radiographic progression. Unfortunately many patients are unable to tolerate standard DMARDs or fail to maintain a treatment response long‐term. Recently tumour necrosis factor alpha has been implicated in the pathogenesis of RA. Infliximab, a human mouse chimeric anti‐tumour necrosis alpha monoclonal antibody was recently approved for the treatment of Crohn's disease and refractory RA in Canada . Since it has a different mechanism of action than standard DMARD therapy, it may allow control of RA refractory to standard treatment.
This meta‐analysis confirms the effectiveness of infliximab when measured with components of the ACR core set of disease activity measures for RA clinical trials and with radiographic outcomes. The withdrawal and toxicity profile appear acceptable at the present time. However, these results are largely driven by ATTRACT, the largest of the 2 trials.
While infliximab is commonly given as 3 mg/kg and 10mg/kg, the dosing interval is usually every 8 weeks as opposed to every 4 weeks. We have included the 4 week data as this dose was common to both studies and could be pooled (Maini 1998, Maini 1999). As additional studies are completed, further information on the more standard 8 week dosing interval should be available and this information will then be incorporated.
An extensive literature search revealed 2 positive studies that satisfied the inclusion criteria. It was impractical to perform funnel plot analysis to look for publication bias with only 2 studies.
The studies included in this review were not powered to examine secondary outcomes (such as cancer and TB) and hence failure to find a significant difference could reflect this lack of power. Additional studies are needed to determine secondary outcomes, especially adverse events.
Authors' conclusions
Implications for practice.
Infliximab has been shown to be an efficacious DMARD for the treatment of rheumatoid arthritis. Although long term studies are lacking, current data suggest that it is well tolerated . On‐going post marketing surveillance will be required to determine the incidence of adverse events and sustainability of treatment response. The reports of disseminated or extrapulmonary tuberculosis, invasive fungal infections and other opportunistic infections with an anti‐TNF treatment requires on‐going surveillance to determine the true incidence of TB with infliximab treatment and to watch for potentially rare adverse events (Keane 2001; Product Label 2001).
Implications for research.
Further long‐term studies are needed to provide information on rare adverse events and sustainability of treatment response. Studies comparing infliximab to more conventional DMARDs in early RA are needed to determine the most appropriate treatment for new‐onset RA. Comparison of infliximab to conventional combination DMARD therapy would also be extremely interesting, especially from a radiographic outcome perspective. Such studies would assist clinicians in choosing the most appropriate therapy for their RA patients. Comparing infliximab treatment with standard DMARDS in people not known to be DMARD refractory in early RA would also be useful.
What's new
| Date | Event | Description |
|---|---|---|
| 19 September 2008 | Amended | Converted to new review format. C044‐R |
Acknowledgements
This work was funded by the Arthritis Society. We would like to thank Jessie McGowan for her assistance with the search strategy and David Moher, Margaret Sampson and Nick Barrowman for their instruction in systematic review methodology.
Data and analyses
Comparison 1. Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TJC‐end of study Rx; (Maini 1998 PBO only to 8/52 and values obtained by graphical extraction) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infliximab 3mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐7.32, ‐0.68] |
| 1.2 Infliximab 3mg/kg q4 weeks | 2 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐7.88 [‐11.10, ‐4.66] |
| 1.3 Infliximab 10mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐7.31, ‐0.69] |
| 1.4 Infliximab 10mg/kg q 4 weeks | 2 | 197 | Mean Difference (IV, Fixed, 95% CI) | ‐10.18 [‐13.49, ‐6.87] |
| 2 SJC‐ end of study | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infliximab 3 mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐6.39, ‐1.61] |
| 2.2 Inflixmab 3mg/kg q 4 weeks | 2 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐6.26 [‐8.20, ‐4.31] |
| 2.3 Infliximab 10mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐8.21, ‐3.79] |
| 2.4 Infliximab 10mg/kg q 4 weeks Maini 1998 PBO only reported to 12 weeks | 2 | 197 | Mean Difference (IV, Fixed, 95% CI) | ‐7.56 [‐9.55, ‐5.58] |
| 3 Patient Pain (VAS 0‐10cm)‐ end of study | 1 | 692 | Mean Difference (IV, Fixed, 95% CI) | ‐2.38 [‐2.71, ‐2.05] |
| 3.1 Infliximab 3mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐2.75, ‐1.45] |
| 3.2 Infliximab 3mg/kg q 4 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.08, ‐1.72] |
| 3.3 Infliximab 10 mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐3.44, ‐2.16] |
| 3.4 Infliximab 10mg/kg q 4 weeks | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐2.87, ‐1.53] |
| 4 Evaluator's Global Assessment ‐ end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Infliximab 3mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐2.97, ‐1.83] |
| 4.2 Infliximab 3mg/kg q 4 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐2.97, ‐1.83] |
| 4.3 Infliximab 10 mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐2.4 [‐2.92, ‐1.88] |
| 4.4 Infliximab 10mg/kg q 4 weeks | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐2.50 [‐3.03, ‐1.97] |
| 5 PGA ‐ end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infliximab 3 mg/kg/q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐1.9 [‐2.59, ‐1.21] |
| 5.2 Inflixmab 3mg/kg q 4 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐3.16, ‐1.84] |
| 5.3 Infliximab 10mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.51, ‐1.09] |
| 5.4 Infliximab 10 mg/kg q 4 weeks | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐2.87, ‐1.53] |
| 6 HAQ‐ end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infliximab 3mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.21, 0.21] |
| 6.2 Infliximab 3mg/kq q 4 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.41, 0.01] |
| 6.3 Infliximab 10mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.42, 0.02] |
| 6.4 Infliximab 10mg/kg q 4 weeks | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.43, 0.03] |
| 6.5 Infliximab combined | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.26, 0.06] |
| 7 CRP (graphical extraction for Maini 1998) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Infliximab 3 mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.05, ‐0.95] |
| 7.2 Infliximab 3mg/kg q 4 weeks | 2 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐1.81 [‐2.26, ‐1.37] |
| 7.3 Infliximab 10mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.17, ‐1.23] |
| 7.4 Infliximab 10 mg/kg q 4 weeks | 2 | 197 | Mean Difference (IV, Fixed, 95% CI) | ‐1.87 [‐2.30, ‐1.45] |
| 8 ACR 20 response rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.61 [1.66, 4.13] |
| 8.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.67, 4.11] |
| 8.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [1.64, 4.08] |
| 8.4 MTX and Infliximab 10mg/kg q 4 weeks | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [1.90, 4.60] |
| 8.5 Infliximab combined | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.73, 4.04] |
| 9 ACR 50 response rate | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.63 [2.02, 15.66] |
| 9.2 Infliximab 3mg/kg q 4 weeks/MTX versus Placebo/MTX | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.28 [2.81, 18.83] |
| 9.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.57 [2.39, 18.05] |
| 9.4 Infliximab 10mg/kg q 4 weeks | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.55 [2.50, 17.10] |
| 9.5 Infliximab combined | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [2.30, 16.09] |
| 10 ACR 70 response rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.34 [0.89, 264.59] |
| 10.2 Infliximab 3mg/kg q 4 weeks/MTX versus Placebo/MTX | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.44 [1.15, 328.85] |
| 10.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 31.35 [1.91, 515.94] |
| 10.4 Infliximab 10mg/kg q 4 weeks/MTX versus Placebo/MTX | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 20.62 [1.22, 348.73] |
| 10.5 Infliximab combined(ATTRACT) | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 21.14 [1.31, 340.46] |
1.1. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 1 TJC‐end of study Rx; (Maini 1998 PBO only to 8/52 and values obtained by graphical extraction).
1.2. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 2 SJC‐ end of study.
1.3. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 3 Patient Pain (VAS 0‐10cm)‐ end of study.
1.4. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 4 Evaluator's Global Assessment ‐ end of study.
1.5. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 5 PGA ‐ end of study.
1.6. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 6 HAQ‐ end of study.
1.7. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 7 CRP (graphical extraction for Maini 1998).
1.8. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 8 ACR 20 response rate.
1.9. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 9 ACR 50 response rate.
1.10. Analysis.

Comparison 1 Efficacy at 6 months (Infliximab/MTX versus Placebo/MTX ), Outcome 10 ACR 70 response rate.
Comparison 2. Withdrawals at 6 months ( Infliximab/MTX versus Placebo/MTX).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 MTX and Infliximab 3mg/kg q 8 weeks vs MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.82] |
| 1.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.49] |
| 1.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.12, 0.52] |
| 1.4 MTX and infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.22, 0.64] |
| 1.5 All Infliximab(ATTRACT) | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.25, 0.54] |
| 2 Lack of Efficacy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 MTX and infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.26, 0.99] |
| 2.2 MTX and infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.10, 0.48] |
| 2.3 MTX and infliximab 10 mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.09, 0.58] |
| 2.4 MTX and infliximab 10 mg/kg q 4 weeks versus MTX(PBO) | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.49] |
| 2.5 All Infliximab(ATTRACT) | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.19, 0.53] |
| 3 Adverse Events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 MTX and infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.12, 1.64] |
| 3.2 MTX and infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.18, 1.93] |
| 3.3 MTX and infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.62] |
| 3.4 MTX and infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.40, 2.86] |
| 4 Other | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 MTX and Infliximab 3 mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.22] |
| 4.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 2 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.79] |
| 4.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.76] |
| 4.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.04, 3.41] |
2.1. Analysis.

Comparison 2 Withdrawals at 6 months ( Infliximab/MTX versus Placebo/MTX), Outcome 1 Total.
2.2. Analysis.

Comparison 2 Withdrawals at 6 months ( Infliximab/MTX versus Placebo/MTX), Outcome 2 Lack of Efficacy.
2.3. Analysis.

Comparison 2 Withdrawals at 6 months ( Infliximab/MTX versus Placebo/MTX), Outcome 3 Adverse Events.
2.4. Analysis.

Comparison 2 Withdrawals at 6 months ( Infliximab/MTX versus Placebo/MTX), Outcome 4 Other.
Comparison 3. Toxicity at 6 months (Infliximab/MTX versus Placebo/MTX).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infections Requring Antibiotics | 2 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.99, 2.23] |
| 2 Serious Infections | 2 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.28, 1.84] |
| 3 Neoplasm | 2 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.09, 34.05] |
| 4 "SLE" | 2 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.07, 5.93] |
| 5 Death | 2 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.99] |
3.1. Analysis.

Comparison 3 Toxicity at 6 months (Infliximab/MTX versus Placebo/MTX), Outcome 1 Infections Requring Antibiotics.
3.2. Analysis.

Comparison 3 Toxicity at 6 months (Infliximab/MTX versus Placebo/MTX), Outcome 2 Serious Infections.
3.3. Analysis.

Comparison 3 Toxicity at 6 months (Infliximab/MTX versus Placebo/MTX), Outcome 3 Neoplasm.
3.4. Analysis.

Comparison 3 Toxicity at 6 months (Infliximab/MTX versus Placebo/MTX), Outcome 4 "SLE".
3.5. Analysis.

Comparison 3 Toxicity at 6 months (Infliximab/MTX versus Placebo/MTX), Outcome 5 Death.
Comparison 4. Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TJC | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infliximab 3mg/kg q 8 weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Infliximab 3mg/kg q 4weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Infliximab 10mg/kg q 8 weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Infliximab 10mg/kg q 4 weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 SJC | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infliximab 3mg/kg q 8 weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Infliximab 3mg/kg q 4weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Infliximab 10mg/kg q 8 weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Infliximab 10mg/kg q 4 weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Infliximab Combined | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Pain (VAS 0‐10 cm) | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Evaluators Global Assessment | 1 | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Infliximab 3mg/kg q 4 weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Infliximab 10mg/kg q 4 weeks | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 HAQ‐end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Infliximab 3mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.31, 0.11] |
| 5.2 Infliximab 3mg/kg q 4 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.41, 0.01] |
| 5.3 Infliximab 10mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.52, ‐0.08] |
| 5.4 Infliximab 10 mg/kg q 4 weeks | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.41, 0.01] |
| 5.5 Infliximab combined | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.36, ‐0.04] |
| 6 Patient Global Assessment‐ end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Infliximab 3mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.56, ‐0.04] |
| 6.2 Infliximab 3 mg/kg q 4 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.9 [‐1.67, ‐0.13] |
| 6.3 infliximab 10mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.49, 0.09] |
| 6.4 Infliximab 10 mg/kg q 4 weeks | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.58, ‐0.02] |
| 6.5 Infliximab combined | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.41, ‐0.19] |
| 7 CRP (graphical extraction) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Infliximab 3mg/kg q 8 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.96, ‐0.44] |
| 7.2 Infliximab 3mg/kg q 4 weeks | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.14, ‐0.46] |
| 7.3 Infliximab 10mg/kg q 8 weeks | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐2.34, ‐0.86] |
| 7.4 Infliximab 10 mg/kg q 4 weeks | 1 | 169 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.42, ‐0.98] |
| 8 ACR 20 Response Rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.45, 4.15] |
| 8.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.80 [1.68, 4.66] |
| 8.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.44 [2.10, 5.63] |
| 8.4 MTX and Infliximab 10mg/kg q 4weeks versus MTX(PBO) | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.48 [2.12, 5.70] |
| 8.5 Infliximab combined | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [1.89, 4.87] |
| 9 ACR 50 Response Rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.16, 5.98] |
| 9.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [1.96, 9.16] |
| 9.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [2.30, 10.48] |
| 9.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.81 [2.24, 10.32] |
| 9.5 Infliximab combined | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [2.00, 8.57] |
| 10 ACR 70 Response Rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.60 [1.02, 20.70] |
| 10.2 MTX and Infliximab 3mg/kg q 4weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [1.81, 32.56] |
| 10.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.13 [2.70, 45.89] |
| 10.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.15 [1.92, 34.54] |
| 10.5 Infliximab combined | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.89 [1.97, 31.66] |
| 11 Total Radiographic Score (0 to 440); Inc worse | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐8.70 [‐11.58, ‐5.82] |
| 11.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐16.40 [‐19.61, ‐13.19] |
| 11.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐21.80 [‐24.45, ‐19.15] |
| 11.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐13.70 [‐16.38, ‐11.02] |
| 12 Major Radiographic Progression | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.12, 0.63] |
| 12.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.20, 0.83] |
| 12.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.30] |
| 12.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.38] |
| 13 Radiographic Improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.60, 6.01] |
| 13.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.41 [1.77, 6.54] |
| 13.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.42, 5.40] |
| 13.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [2.04, 7.39] |
4.1. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 1 TJC.
4.2. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 2 SJC.
4.3. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 3 Pain (VAS 0‐10 cm).
4.4. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 4 Evaluators Global Assessment.
4.5. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 5 HAQ‐end of study.
4.6. Analysis.
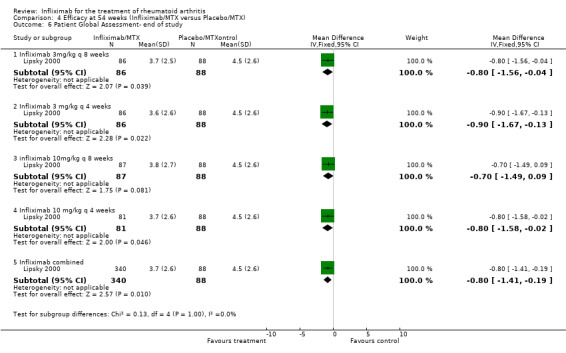
Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 6 Patient Global Assessment‐ end of study.
4.7. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 7 CRP (graphical extraction).
4.8. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 8 ACR 20 Response Rate.
4.9. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 9 ACR 50 Response Rate.
4.10. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 10 ACR 70 Response Rate.
4.11. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 11 Total Radiographic Score (0 to 440); Inc worse.
4.12. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 12 Major Radiographic Progression.
4.13. Analysis.

Comparison 4 Efficacy at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 13 Radiographic Improvement.
Comparison 5. Withdrawals at 54 weeks (infliximab/MTX versus Placebo/MTX).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Withdrawals | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 MTX and infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.80] |
| 1.2 MTX and infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.30, 0.72] |
| 1.3 MTX and infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.16, 0.49] |
| 1.4 MTX and infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.64] |
| 1.5 All infliximab/MTX versus Placebo/MTX | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.31, 0.56] |
| 2 Withdrawals Secondary to Adverse Events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 MTX and infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.21] |
| 2.2 MTX and infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.51, 3.37] |
| 2.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.18, 1.90] |
| 2.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.47, 3.27] |
| 2.5 All Infliximab/MTX versus Placebo/MTX | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.43, 2.14] |
| 3 Withdrawals Due to Lack Of Efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.33, 0.90] |
| 3.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.17, 0.61] |
| 3.3 MTX and Infliximab 10 mg/kg q 8 weeks versus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.08, 0.43] |
| 3.4 MTX and Infliximab 10 mg/kg q 4 weeks versus MTX(PBO) | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.11, 0.51] |
| 3.5 All Infliximab/MTX versus Placebo/MTX | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.22, 0.48] |
| 4 Withdrawals For Other Reasons | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.72] |
| 4.2 MTX and Infliximab 3 mg/kg q 4weeks versus MTX(PBO) | 1 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.72] |
| 4.3 MTX and Infliximab 10mg/kg q 8 weeks verus MTX(PBO) | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.03] |
| 4.4 MTX and Infliximab 10mg/kg q 4weeks versus MTX(PBO) | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.82] |
| 4.5 All Infliximab/MTX versus Placebo/MTX | 1 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.08, 0.87] |
5.1. Analysis.

Comparison 5 Withdrawals at 54 weeks (infliximab/MTX versus Placebo/MTX), Outcome 1 Total Withdrawals.
5.2. Analysis.

Comparison 5 Withdrawals at 54 weeks (infliximab/MTX versus Placebo/MTX), Outcome 2 Withdrawals Secondary to Adverse Events.
5.3. Analysis.

Comparison 5 Withdrawals at 54 weeks (infliximab/MTX versus Placebo/MTX), Outcome 3 Withdrawals Due to Lack Of Efficacy.
5.4. Analysis.

Comparison 5 Withdrawals at 54 weeks (infliximab/MTX versus Placebo/MTX), Outcome 4 Withdrawals For Other Reasons.
Comparison 6. Toxicity at 54 weeks (Infliximab/MTX versus Placebo/MTX).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious Adverse Events as defined by WHO Adverse‐Reaction Terminology | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.27, 1.13] |
| 1.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.41, 1.46] |
| 1.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.69] |
| 1.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.52, 1.72] |
| 1.5 All Infliximab/MTX versus Placebo/MTX | 1 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.50, 1.29] |
| 2 Serious Infections | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.34] |
| 2.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.30, 2.45] |
| 2.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.36, 2.70] |
| 2.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.32, 2.59] |
| 2.5 All Infliximab/MTX versus Placebo/MTX | 1 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.33, 1.73] |
| 3 Serious Infusion Reactions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX)PBO) | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 ANA( of at least 1:320) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.69, 3.97] |
| 4.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.54, 3.72] |
| 4.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.53, 3.68] |
| 4.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.29, 3.22] |
| 4.5 All Infliximab/MTX versus Placebo/MTX | 1 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.57, 3.55] |
| 5 Anti‐ds‐DNA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 MTX and Infliximab 3mg/kg q 8 weeks versus MTX(PBO) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.15 [1.07, 306.95] |
| 5.2 MTX and Infliximab 3mg/kg q 4 weeks versus MTX(PBO) | 1 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.78 [1.11, 317.57] |
| 5.3 MTX and Infliximab 10mg/kg q 8 weeks versus MTX(PBO) | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.35 [1.09, 310.41] |
| 5.4 MTX and Infliximab 10mg/kg q 4 weeks versus MTX(PBO) | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.48 [0.77, 235.40] |
| 5.5 All Infliximab/MTX versus Placebo/MTX | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.65 [1.03, 269.03] |
6.1. Analysis.

Comparison 6 Toxicity at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 1 Serious Adverse Events as defined by WHO Adverse‐Reaction Terminology.
6.2. Analysis.

Comparison 6 Toxicity at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 2 Serious Infections.
6.3. Analysis.

Comparison 6 Toxicity at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 3 Serious Infusion Reactions.
6.4. Analysis.

Comparison 6 Toxicity at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 4 ANA( of at least 1:320).
6.5. Analysis.

Comparison 6 Toxicity at 54 weeks (Infliximab/MTX versus Placebo/MTX), Outcome 5 Anti‐ds‐DNA.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lipsky 2000.
| Methods | Randomized double blind; continuation of ATTRACT | |
| Participants | As per ATTRACT | |
| Interventions | As per ATTRACT | |
| Outcomes | As per ATTRACT plus radiographic data | |
| Notes | As per ATTRACT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Maini 1998.
| Methods | Randomized double blind study | |
| Participants | 101 pts; med 101pts;med age 47‐56.3y;dis dur 7.6‐14.3y; RF(+) 66.7‐99.3% | |
| Interventions | Infliximab 1,3 or 10 mg/kg(w and w/0 MTX) at week 2,6,10 and 14. | |
| Outcomes | SJC; TJC; Pain; EMS; pt/MD global ass; CRP; ESR; mHAQ | |
| Notes | 28.6‐66.7% on PDZ; prev med # DMARDS 2.0‐3.0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Maini 1999.
| Methods | Randomized double blind study | |
| Participants | 428 pts median age 51‐56y; dis duration 7.2‐9.0 y RF(+) 77‐84% | |
| Interventions | Infliximab 3mg/kg or 10mg/kg q 4 or 8 weeks plus MTX | |
| Outcomes | SJC; TJC; Pain; pt/MD global ass; CRP; ACR 20, 50,70; HAQ | |
| Notes | 46‐53% on PDZ; 45‐54% stage III or IV; Prev med # DMARDS 2.5‐2.8 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
ACR=American College of Rheumatology response rates; CRP= C‐reactive protein; dis=disease; EMS= early morning stiffness; ESR=erythrocyte sedimentation rate; HAQ=health assessment questionnaire; med=median; mHAQ= modified HAQ; MTX= methotrextate; pain=patient's pain score; PDZ= prednisone; prev med# DMARDS = previous median number disease modifying anti‐rheumatic drugs, excluding methotrextae; pt/MD global ass= patient global assessment, and physician global assessment; pts= patients; RF= rheumatoid factor; SJC= swollen joint count; TJC= tender joint count; w=with; w/o=without; y=year.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ATTRACT‐2 year data | Study unblinded after 1 year. |
| Brennan 1997 | 1. Single infusion 2. Data contained in other studies |
| Elliott 1993 | Not RCT |
| Elliott 1994 | Not RCT |
| Elliott 1994(RCT) | 1. Single infusion 2. 4 week duration |
| Elliott 1997 | Not RCT |
| Kalden‐Nemeth 1997 | Study duration only 4 weeks |
| Kavanaugh 2000 | 1. RCT portion only 12 weeks 2. Trial extension past 12 weeks not RCT |
| Oshima 1999 | Not RCT |
| Perkins 1998 | 1. Single infusion 2. Only 4 week duration 3. ? duplicated data |
Contributions of authors
BB and AC extracted and analyzed the data and selected trials of the initial review and preparation of the initial manuscript.
AB and MH contributed data, updated of the selection of the reference list, updated the analyses and updated the interpretation of results.
BB and MJ wrote the manuscript, contributed data extraction, updated the analyses and interpretation of results.
AC, GW and PT contributed methodological expertise and commented on drafts.
Sources of support
Internal sources
Institute of Population Health, Canada.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Lipsky 2000 {published and unpublished data}
- Lipsky PE, Heijde DMFM, Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Infliximab and methotrexate in the treatment of rheumatoid arthritis. The New England Journal of Medicine 2000;343:1594‐1602. [DOI] [PubMed] [Google Scholar]
Maini 1998 {published and unpublished data}
- Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, MacFarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JN, Schaible TF, Feldmann M. Therapeutic efficacy of multiple intravenous infusions of anti‐tumor necrosis factor alpha monoclonal antibody combined with low‐dose weekly methotrextae in rheumatoid arthritis. Arthritis and Rheumatism 1998;41(9):1552‐1563. [DOI] [PubMed] [Google Scholar]
Maini 1999 {published and unpublished data}
- Maini R, Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomized phase III trial. Lancet 1999;354:1932‐1939. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ATTRACT‐2 year data {unpublished data only}
Brennan 1997 {published data only}
- Brennan FM, Browne KA, Green PA, Jaspar JM, Maini RN, Feldmann M. Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti‐tumour necrosis factor‐alpha (cA2) therapy. British Journal of Rheumatology 1997;36:643‐650. [DOI] [PubMed] [Google Scholar]
Elliott 1993 {published data only}
- Elliott MJ, Maini RN, Feldmann M, Long‐Fox A, Charles P, Katsikis P, Brennan FM, Walker J, Bijl H, Ghrayeb J, Woody JN. Treatment of rheumatoid arthritis with chimeric monoclonal antibody to tumour necrosis factor alpha. Arthritis and Rheumatism 1993;36(12):1681‐1690. [DOI] [PubMed] [Google Scholar]
Elliott 1994 {published data only}
- Elliott MJ, Maini RN, Feldmann M, Long‐Fox A, Charles P, Bijl H, Woody JN. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet 1994;344:1125‐1127. [DOI] [PubMed] [Google Scholar]
Elliott 1994(RCT) {published data only}
- Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, MacFarlane JD, Bijl H, Woody JN. Randomized double‐blind comparision of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 1994;344:1105‐1110. [DOI] [PubMed] [Google Scholar]
Elliott 1997 {published data only}
- Elliott MJ, Woo P, Charles P, Long‐Fox A, Woody JN, Maini RN. Suppression of fever and the acute‐phase response in a patient with juvenile chronic arthritis treated with monoclonal antibody to tumour necrosis factor‐ alpha (cA2). British Journal of Rheumatology 1997;36:589‐593. [DOI] [PubMed] [Google Scholar]
Kalden‐Nemeth 1997 {published data only}
- Kalden‐Nemeth D, Grebmeiser J, Antoni C, Manger B, Wolf F, Kalden JR. NMR monitoring of rheumatoid arthritis patients receiving anti‐TNF alpha monoclonal antibody therapy. Rheumatology International 1997;16:249‐255. [DOI] [PubMed] [Google Scholar]
Kavanaugh 2000 {published data only}
- Kavanaugh A, Clair EW, McCune WJ, Braakman T, Lipsky P. Chimeric anti‐tumour necrosis factor‐alpha monoclonal antibody treatment of patients with rheumatoid arthritis receiving methotrexate therapy. Journal of Rheumatology 2000;27:841‐850. [PubMed] [Google Scholar]
Oshima 1999 {published data only}
- Oshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Ishida H, Shimizu M, Suemura M, McCloskey R, Kishimoto T. Long‐term floow‐up of the changes in circulating cytokines, soluble cytokine receptors, and white blood cell subset counts in patients with rheumatoid arthritis (RA) after monoclonal anti‐TNF alpha therapy. journal of Clinical Immunology 1999;19:305‐313. [DOI] [PubMed] [Google Scholar]
Perkins 1998 {published data only}
- Perkins DJ, Clair EW, Misukonis MA, Weinberg JB. Reduction of NOS2 overexpression in rheumatoid arthritis patients treated with anti‐tumour necrosis factor alpha monoclonal antibody (cA2). Arthritis and Rheumatism 1998;41(12):2205‐2210. [DOI] [PubMed] [Google Scholar]
Additional references
Arnett 1988
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31:315‐24. [DOI] [PubMed] [Google Scholar]
Boers 1994
- Boers M, Tugwell P, Felson DT. World health organization and international league of associations for rheumatology core endpoints for symptom modifying antirheumatic drugs in rheumatoid arthritis clinical trials. J Rheumatol 1994;21:86‐9. [PubMed] [Google Scholar]
Felson 1993
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith D, et al. The American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1993;36:729‐40. [DOI] [PubMed] [Google Scholar]
Felson 1995
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith D, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1995;38:727‐35. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A, Moore A, Carrol D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Keane 2001
- Keane J, Gershon S, Wise RP, Mirabile‐Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha‐neutralizing agent. N Engl J Med 2001;345(15):1098‐1104. [DOI] [PubMed] [Google Scholar]
OMERACT 1993
- OMERACT. Conference on Outcome Measures in Rheumatoid Arthritis Clinical Trials. J Rheumatol 1993;20:526‐91. [PubMed] [Google Scholar]
Pincus 1999
- Pincus T, Stein CM. ACR20: clinical or statistical significance?. Arthritis Rheum 1999;42(8):1572‐6. [DOI] [PubMed] [Google Scholar]
Product Label 2001
- [Remicade Product Label]. 2001.


