Abstract
With the incremental improvements in long-term kidney transplant survival, there is renewed focus on what causes failure of the transplanted allograft. Over the past decade, our understanding of the injuries that lead to loss of graft function over time has evolved. Chronic allograft injury includes both immune-mediated and nonimmune-mediated injuries, which may involve the organ donor, the recipient, or both. The targets of injury include the kidney tubular epithelium, the endothelium, and the glomerulus. As a response to injury, there are the expected tissue remodeling and repair processes. However, if inflammation persists, which is not uncommon in the transplant setting, the resulting maladaptive response is matrix deposition and/or fibrosis. This ultimately leads to declining graft function and, finally, failure. With our advancing knowledge of the multiple etiologies and mechanisms, enhanced by more recent cohort studies in humans, there is an opportunity to identify those at greater risk to initiate new strategies to ameliorate the process. Although the most recent studies focus on immune-mediated injuries, there is a critical need to identify both markers of injury and mechanisms of injury. In this review, we highlight the findings of recent studies, highlight the potential therapeutic targets, and identify the continued unmet need for understanding the mechanisms of late graft failure.
Keywords: transplantation, renal fibrosis, renal injury, chronic allograft failure, allografts, kidney transplantation series
Introduction
In the last 30 years, kidney transplantation has achieved incredible success, with well over 90% patient and graft survival in the first year after transplant, accompanied by infrequent rejection (1). This success is primarily due to the potency of immunosuppressive therapy and improved recognition of recipient immune risk. However, at 10 years, only about half of deceased donor and 70% of living donor kidney grafts remain functional (1), due to a combination of death with a functioning allograft and death-censored graft loss. In the latter, graft failure results in significant morbidity and a financial cost of returning to dialysis, with an associated higher risk of death. This adds to the ever-increasing demand on transplantable organs, with repeat transplants accounting for approximately 15% of all kidney transplants (2). Thus, understanding the etiology of chronic allograft injury and identifying therapeutic targets are important research priorities in kidney transplantation.
The classic understanding of late allograft failure has evolved from “chronic rejection” to the recognition of specific entities that require proactive clinical monitoring and biopsy diagnosis (3,4). Such studies identify a broad, complex end result that encompasses both immune-mediated and nonimmune-mediated injuries. In this review, we provide an overview of these entities, with emphasis on mechanism.
A Historical Perspective
Prior to the 1990s, kidney graft failure was attributed to the oft-used but misleading term “chronic rejection.” However, this diagnosis did not adequately encompass all causes of chronic graft injury. Moreover, it assumed that there was “rejection,” with the common clinical practice not to perform late biopsies. In 1991, the first Banff Conference on Allograft Pathology defined chronic allograft nephropathy as kidney transplants with significant interstitial fibrosis and tubular atrophy (IF/TA) (5), but this histologic description was nondescriptive of specifying the etiology. By 2005, at the eighth Banff Conference, the term chronic allograft nephropathy was replaced by “interstitial fibrosis and tubular atrophy (IF/TA) without evidence of specific etiology,” a diagnosis to be used when the underlying process could not be identified (6). This descriptive categorization also advocated for specific annotation of morphology that could distinguish entities, such as rejection, hypertension, calcineurin inhibitor toxicity, chronic obstruction, recurrent bacterial infection, and viral nephropathy. The recognition that IF/TA is a final common pathway for many injuries led to a call for improved allograft surveillance, including biomarkers, obtaining a biopsy before chronic injury caused irreversible fibrosis, and improved recognition of changes in the slope of GFR, which may be a late finding in chronic allograft injury (7). Thus, the context (clinical features and timing) of histology became an important advancement in our understanding of late graft failure and would facilitate better insights into disease pathogenesis, biomarkers, and proper trial design.
Mechanistic Insights into Graft Failure
Classically, the causes of chronic allograft injury are divided into immune mediated and nonimmune mediated, with divergent diagnostic and therapeutic implications. The former reflects alloimmune responses to donor tissue, including cellular and antibody-mediated rejection, and infections, such as BK nephropathy and recurrent urinary tract infections, that may begin with innate immune responses with subsequent adaptive alloimmune injury. The nonimmune-mediated, antigen-independent etiologies include calcineurin inhibitor nephrotoxicity, obstruction or reflux disease, recurrence of native kidney disease, and secondary causes, such as diabetic nephropathy. Rather than providing detail on each entity, the reader is encouraged to consult these recent publications (8–10).
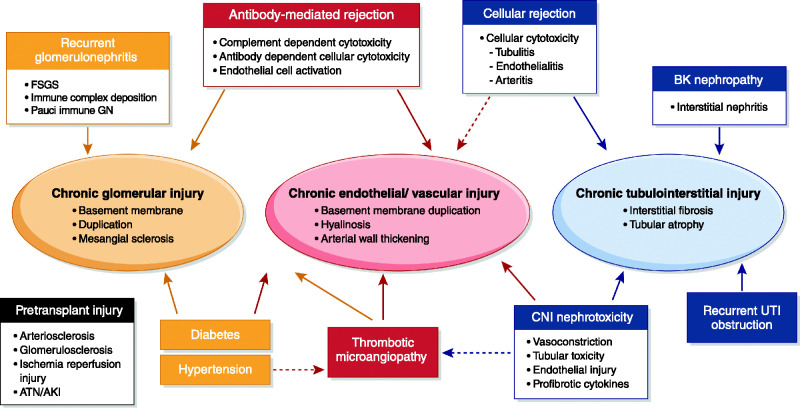
We suggest an alternative paradigm, where injuries are oriented around the kidney compartment directly affected (Figure 1). This approach demonstrates the interactive nature of injury among the compartments. For example, endothelial injury in the peritubular capillaries leads to tubular interstitial injury and fibrosis and demonstrates the multiple clinical entities that may present with injury in that compartment. Likewise, both immune and nonimmune injuries may affect each compartment.
Figure 1.
Schema of the potential sites of injury associated with late allograft failure. There may be “fixed” injury as a consequence of the donation and transplant process (“pretransplant injury”). Three target sites of injury within the kidney are shown, with defined histologic characteristics. The entities that contribute to these sites directly (solid arrows) are shown aligned to their injury at that site. There may also be “crosstalk” of disease processes between compartments (hashed arrows) as well as cumulative injury from multiple entities directly (solid arrows) or indirectly (hashed arrows). ATN, acute tubular necrosis; CNI, calcineurin inhibitor; UTI, urinary tract infection.
Underscoring the potential contributions of these insults to the allograft is the recognition that there may be fixed “deficits,” at the start of transplantation, on the basis reduced nephron mass. Such suboptimal donor tissue derives in part from older donors, with reduced baseline function and kidney reserve. In such allografts, there is reduced capacity to respond to injury, with accumulation of senescent cells with associated inflammatory response leading to impaired organ function (11). This is reflected by a higher Kidney Donor Risk Index. This index was developed by assessing post-transplant outcomes data from the Scientific Registry of Transplant Recipients, taking into account 14 variables in the deceased donor at transplantation (12), expressed as the relative rate of graft failure of the donor kidney. Although higher–Kidney Donor Risk Index kidneys still are associated with better survival of the recipient than remaining on the waiting list (13), older age or size mismatch between donor and recipient may lead to hyperfiltration and progressive loss of kidney function (14), even in the absence of other intervening injuries over time. This is further aggravated by brain death and ischemia-reperfusion injury, resulting in a proinflammatory environment in the allograft that may worsen rejection risk as well (reviewed in ref. 15).
Targets of Injury: The Interstitium
Studies on late allograft failure have focused on profibrogenic pathways in the kidney as potential therapeutic targets. Such investigations are derived in part from studies of acute injury models where maladaptive repair of tubular epithelium following injury is associated with persistent structural and metabolic abnormalities (16). Recognizing the regenerative capacity of the kidney epithelium, reports indicate that within the environment of repeated stress, injury, or under duress of calcineurin inhibitors (17), tubular epithelial cells, as well as T cells and macrophages, express profibrotic growth factors (18). This results in downstream activation of mesenchymal cells, including pericytes, fibroblasts, and fibrocytes, which themselves may now produce matrix. Key in this pathway were reports of TGF-β as well as enhanced expression of microRNA21, a profibrogenic signal seen in other organs, resulting in loss of epithelial phenotype (19). Although it has been debated whether epithelial (or endothelial) cells may undergo myofibroblast transformation, more recent studies using fate tracing of cells in mice determined that epithelial mesenchymal transformation was unlikely. Rather, pericytes, the cells surrounding the vasculature in the kidney, were associated with α-smooth muscle actin–positive fibroblasts (reviewed in ref. 20).
Additional support comes from gene expression studies of mouse and human kidney allografts with IF/TA with upregulation of connective tissue growth factor and TGF-β, matrix metalloproteinases, collagens, and α-smooth muscle actin (21–23). In human kidney allograft biopsies, investigators have identified signatures in the whole-kidney cortex associated with progressive fibrosis detected in the early course of transplantation that are predictive of later injury (24,25). Such studies indicate the importance of IF/TA in the progression of late allograft injury. Recent examination of mouse kidneys following acute kidney ischemia with single-cell RNA sequencing demonstrates a “failed-repair proximal tubule cell state,” which is also demonstrated in human kidney allografts over time (26). These transcriptional studies indicate regional variation of cells to ischemic injury and suggest that the dynamic nature of the repair response may be a new target for mitigating graft failure.
In spite of such results, the prevalence of IF/TA is debated. In a large prospective cohort of kidney and kidney/pancreas transplant recipients monitored closely with surveillance biopsy, 66% of transplants demonstrated atrophy and fibrosis by 5 years post-transplantation (27). More recent studies indicate that only 17% of transplants have moderate to severe IF/TA detected by 5 years (28), and stability of GFR over time and less frequent graft loss (29). The success of this cohort was attributed in part to more effective immunosuppression with tacrolimus, with less subclinical and clinical inflammation as well as the notion that tacrolimus may be “less fibrogenic” compared with the use of cyclosporin (27). Regardless of the prevalence of IF/TA, there is agreement that the extent of tubular atrophy and interstitial scarring correlates to allograft failure (4).
Allograft Inflammation Is an Important Mediator of Chronic Graft Injury
The presence of inflammation has been associated with higher risk of graft failure. Surveillance biopsy studies associated subclinical cellular rejection with progression of IF/TA (30,31). In the absence of randomized control trials of treatment and with a disconnect between severity of inflammation and outcomes (32), treatment of subclinical inflammation remains debated (reviewed in ref. 18).
Although such studies focused on inflammation in nonatrophic and nonscarred kidney tissue, investigators soon demonstrated the negative effect of inflammation in areas of IF/TA, an area of the biopsy not originally part of the Banff scoring schema. Specifically, total inflammation (total I; “ti”), including inflammation in both scarred and unscarred tissue, was identified as an independent risk factor for graft failure and added as a component score in 2008 (33). Further work from two large cohort studies identified that inflammation in areas of scarring and atrophy (“i-IFTA”) was an independent risk factor for allograft failure in both for-cause biopsies (34,35) and surveillance biopsies (26,36), leading to the incorporation of i-IFTA into the Banff schema (37). These findings were independently validated by other clinical cohorts, with correlation of these lesions to prior T cell–mediated rejection (38,39) or underimmunosuppression (38). Not surprisingly, i-IFTA is associated with the extent of inflammation and severity of IF/TA. Importantly, i-IFTA is not specific and may be seen in BK virus nephropathy, recurrent glomerular disease, antibody-mediated rejection, and pyelonephritis. This has led to considerable disagreement of the specificity of this finding and whether it truly represents a form of chronic active T cell–mediated rejection. In the latter diagnostic category, i-IFTA must be at a threshold level of moderate severity accompanied by moderate tubulitis. Interestingly, gene expression of allografts with i-IFTA lesions was more frequently associated with antibody-mediated rejection gene transcripts and eventual graft loss (40). Thus, questions remain about appropriate therapy (if any) and the effect of those treatments, including the role of molecular tissue analysis.
Calcineurin Inhibitors: Friends and Foes.
The introduction of calcineurin inhibitors in kidney transplantation had an immediate effect by reducing allograft rejection and improving graft survival. However, their use was associated with nephrotoxicity both with acute and with long-term exposure. The mechanisms of calcineurin inhibitor nephrotoxicity are complex and associated with vascular (arteriolar), tubular, and glomerular dysfunction (41). Strongly implicated has been the activation of the renin-angiotensin system, with effects on kidney vasculature as well as on juxtaglomerular cells (42). Additionally, adverse remodeling has been implicated through direct effects on the epithelium and interstitium, with release of reactive oxygen species (17), mitochondrial dysfunction and HMGB1 release by tubular epithelial cells, and apoptosis (43). In vitro, cyclosporin treatment of proximal tubular epithelial cells results in a loss of epithelial phenotype (44,45) with endoplasmic reticulum stress (46). Such studies suggest that intervention in some of these signaling pathways may mitigate the nephrotoxicity, supporting tissue repair and correction of metabolic maladaptation. In spite of significant advances in transplantation and better mechanistic insights, there are no specific therapies that ameliorate calcineurin inhibitor nephrotoxicity. Furthermore, minimization or avoidance of these agents has resulted in cellular and antibody-mediated allograft rejection (47). Indeed, minimization of these agents to avoid toxicity may provoke subclinical and clinical alloimmune activation (7). Effective management strategies that limit calcineurin inhibitor injury while facilitating therapeutic levels are an unmet need in the transplant recipient.
Viral Nephropathy with BK Virus.
BK polyomavirus nephropathy can cause significant allograft injury, leading to graft failure (48). BK virus, with a tropism for uroepithelium, undergoes replication under the immunosuppressive milieu resulting in a marked inflammatory response, leading to tubular epithelial cell injury and, ultimately, IF/TA (49). In spite of our knowledge of this entity for 2 decades, there are no approved antiviral treatments, with empirical therapies either untested or unproven by randomized trials. The mainstay of intervention includes proactive monitoring for viral DNA with immunosuppressive reduction upon detection of BK viremia and/or viruria.
The progressive fibrosis and inflammation are not unlike that of other injuries and are unremitting in the context of continued viral replication. Histopathology is unable to distinguish alloimmune versus antiviral inflammation. Indeed, molecular analysis of allograft biopsies with BK nephropathy indicated similar gene expression to that of acute T cell–mediated rejection (50). Moreover, BK virus nephropathy was associated with a profibrogenic milieu, although whether this was due directly to viral invasion or related to the antiviral immune response is not clear. Recent studies quantifying gene expression in formalin-fixed kidney biopsy sections and the NanoString 800-gene panel to detect immune response and BK viral genes have also demonstrated an overlap of transcripts in BK virus nephropathy and T cell–mediated rejection, with excellent diagnostic performance of BK-specific genes to detect viral pathology. However, these transcript profiles were unable to predict outcomes in the BK virus nephropathy cohort, such as disease resolution, persistence, or subsequent T cell–mediated rejection. Further evaluation of the predictive value of these gene expression targets will require a larger cohort with discrete outcomes and a platform for therapeutic development.
Targets of Injury: The Endothelium
The allograft endothelium is the interface between the recipient and donor and serves as a target for innate and adaptive immunity. The injured endothelium responds by increased vasoconstriction, inflammation, and hypercoagulation (51), as well as upregulation of adhesion molecules and class 2 MHC expression, increasing graft immunogenicity. Furthermore, endothelial cell apoptotic death may release apoptotic exosome-like vesicles, which, in turn, promote further endothelial dysfunction, autoantibody production, and complement deposition (52). Additionally, endothelial cell activation leads to recruitment of inflammatory cells and proliferation of vascular smooth muscle cells, resulting in neointima formation.
Recently, Valenzuela and Reed (53) demonstrated that ligation and crosslinking of HLA class 1 molecules induce endothelial cell activation and proliferation, with cytoskeletal alterations leading to vascular smooth muscle proliferation. Long term, this results in vascular remodeling and matrix deposition with intimal hyperplasia and arteriopathy (53). As such, chronic antibody-mediated rejection may be characterized by peritubular capillary injury, ultimately resulting in basement membrane duplication or laminations. Injury may also occur in the glomerular capillaries, leading to transplant glomerulopathy, a diagnosis associated with progressive allograft dysfunction, proteinuria, and graft loss (54). Recent studies utilizing archetype analysis have identified clusters of histology and clinical features, with five variants with differing outcomes (55,56). Archetypes with more advanced tubulointerstitial inflammation, vascular lesions, and microcirculation changes or high levels of proteinuria and more advanced cg lesions had the poorest graft survivals. Such analyses allow more specific phenotypic identification of subgroups of perhaps treatable patients. Similarly, the Deterioration of Kidney Allograft Function study, which created a prospective and cross-sectional cohort from seven transplant centers in North America using central pathology and central HLA antibody detection, used cluster analysis of clinical features and histology scores to identify grafts that are higher risk of failure after biopsy and least likely to respond to any therapeutic maneuver (4).
Chronic active antibody-mediated rejection has developed into one of the most difficult entities to manage clinically, with no prescribed and effective therapy, although many agents are used empirically. The frequency varies depending on the patient population, ranging from 3.5% in conventional recipients to as high as 50% in HLA-incompatible transplant kidneys, preceded commonly by microvascular injury (57). After diagnosed, median graft survival is 3.25 years, with a three-fold higher risk of graft failure (58). Risk factors for graft failure include proteinuria and reduced allograft function, as well as class 2 HLA donor-specific antibody (DSA) and de novo DSA compared with preformed/preexisting donor antibody (reviewed in ref. 59). Recent international consensus guidelines for treatment of chronic active antibody-mediated rejection with de novo DSA include optimizing immunosuppression with supportive care, such as reintroduction of steroids (if on a steroid-free regimen), maintaining trough tacrolimus levels >5 ng/ml, and optimizing medical management with a focus on BP, blood glucose, and dyslipidemia (60). Past studies with complement inhibition and proteasome inhibitors have had no effect on outcomes, and newer trials are underway, including the use of neutralizing IL-6 antibody, targeting natural killer and plasma cells (reviewed in ref. 61). As noted earlier, identifying treatable phenotypes is critical to define effective therapeutics.
Thrombotic microangiopathy of the glomerulus is another important endothelial injury that can lead to graft loss. Etiologies include primary genetic syndromes with complement activation as well as secondary causes, including antidonor HLA antibody, calcineurin inhibitor, and viral infections such as HIV, CMV, and parvovirus. With the therapeutic potential to inhibit complement activation, more attention has been paid in the recognition of this lesion and its etiology (62). Regardless of the cause, the presence of microcirculation occlusion with platelet thrombi, complement activation, and terminal membrane attack complexes causing endothelial cell death is a highly inflammatory microenvironment leading to progressive kidney damage with graft loss.
Target of Injury: The Glomerulus
Entities that cause chronic glomerular injury include recurrent glomerular disease, secondary diseases, and microvascular injury to the endothelium, such as thrombotic microangiopathy and chronic active antibody-mediated rejection as discussed above. Although primarily glomerular disease accounts for approximately 25% of kidney failure, recurrent GN following kidney transplant may account for up to 15% of graft failures. The diagnosis and management of recurrent GN depend on the underlying disease and are beyond the scope of this review. Notably, the natural history and presentation of recurrent GN in an allograft are often modified by global post-transplant immunosuppression. This limits the ability to extrapolate native kidney disease protocols to post-transplant recurrence, highlighting the need for development of transplant-specific responses to recurrent disease.
Unmet Needs and Future Directions in Chronic Kidney Injury
Biomarkers
The diversity of pathologic and clinical entities in late graft failure, spanning the many conditions that we have discussed, has made therapeutic discovery a nightmare. Critical to this discussion is the timing of detection of chronic injury. Although serum creatinine is used to monitor allograft function and detect injury, it lacks specificity and sensitivity for early allograft injury. Indeed, as noted in native kidney diseases, considerable nephron damage and loss can occur before a significant change in creatinine is detected. Thus, by the time there is detection of functional change, biopsy reveals more advanced processes, often with limited reversibility and diminished opportunity to mitigate chronic injury. There is a clear need for biomarkers to detect the onset and etiology of chronic allograft injury for the entities we have discussed. For example, surveillance biopsies at specified time points post-transplant may detect “subclinical” rejection, but benefits of therapeutic implementation are not certain. Moreover, the cost and time-consuming and invasive nature make their routine implementation challenging in most transplant centers. Gene expression profiles may be useful in terms of predicting graft failure and progression as already noted, and further clinical validation awaits (25). Other alternatives include detection of donor-derived cellfree DNA to detect allograft injury (63) or gene transcripts in PBMCs associated with subclinical inflammation (64). Such assays have potential to guide patient management, and with the long-term goal of extending allograft survival, need to be more carefully studied prior to implementation.
Recent reports suggest that a multicomposite measure called the integrated risk prediction score (iBOX) might provide risk prediction of graft failure. Based on data derived from both surveillance and medically indicated biopsies in a large cohort of kidney transplants, machine learning algorithms have identified independent risk factors for graft loss. These include allograft function, proteinuria, the level of donor-specific HLA antibody, and the histopathologic variables of severity of IF/TA, microvascular injury, inflammation, and tubulitis (56). This multiparameter risk score has been extensively validated with predictable accuracy. As such, the iBOX may assist in risk assessment of patient’s clinical course and is under consideration in clinical trial design as a surrogate end point of late graft failure.
Therapeutic Management of Fibrosis
There has been intense investigation into antifibrotic treatments, primarily in native kidney diseases, with some work in preclinical transplant models. With the recognition that fibrosis is perpetuated by immune injury, removing the insult is of primary importance to ameliorate graft injury and subsequent failure. To this end, an initial approach to limit fibrosis and graft failure would be on prevention; such strategies would limit alloimmune and innate injuries, including improved matching using molecular typing between donor and recipient (65), improved donor selection with an understanding of donor quality, and organ preservation methods that mitigate innate injury. Preemptive approaches include detection and management of cellular and humoral rejection prior to clinical detection, the tailoring of immunosuppressive therapies on the basis of immune risk profiles, and addressing medication nonadherence. The final aspect is antifibrotics, a topic that is worthy of an entire review (66). These therapies, however, may work best as “anticipatory” and used concurrently with immunosuppression prior to advanced disease. An example is the use of SGLT2 inhibitors in diabetic glucose management in kidney transplant recipients (67). These agents demonstrate renoprotective effects in native kidneys (68) and may have relevance to improved long-term kidney function in transplantation as well.
The frequency of late graft failure improves slowly over time, with substantial gains in reducing patient death with a functioning graft. However, the incremental improvements in long-term function continue to be a challenge. The etiologies are multifactorial, but our better understanding of the pathways to failure demonstrates common themes. These include better detection prior to clinical manifestation and opportunities for improved therapeutics.
Disclosures
E. Langewisch is a primary investigator and has received grant funding from CareDx and Vitaeris/CSL-Behring. R.B. Mannon has received grant funding from Mallinckrodt and Vitaeris/CSL Behring and honoraria from Vitaeris.
Funding
R.B. Mannon is funded by National Institute of Diabetes and Digestive and Kidney Diseases grant U01 DK115997 and US Department of Veterans Affairs grant I01 BX003272.
Acknowledgments
The views expressed are those of the authors and in no way should be seen as an official policy of any funding agency or source.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.OPTN/SRTR : OPTN/SRTR 2018 Annual Data Report: Introduction. Am J Transplant 20[Suppl s1]: 11–19, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Schold JD, Augustine JJ, Huml AM, O’Toole J, Sedor JR, Poggio ED: Modest rates and wide variation in timely access to repeat kidney transplantation in the United States. Am J Transplant 20: 769–778, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Matas AJ, Leduc R, Rush D, Cecka JM, Connett J, Fieberg A, Halloran P, Hunsicker L, Cosio F, Grande J, Mannon R, Gourishankar S, Gaston R, Kasiske B: Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: Preliminary data from the DeKAF study. Am J Transplant 10: 315–323, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, Croker BP, Droz D, Dunnill MS, Halloran PF, Häyry P, Jennette JC, Keown PA, Marcussen N, Mihatsch MJ, Morozumi K, Myers BD, Nast CC, Olsen S, Racusen LC, Ramos EL, Rosen S, Sachs DH, Salomon DR, Sanfilippo F, Verani R, von Willebrand E, Yamaguchi Y: International standardization of criteria for the histologic diagnosis of renal allograft rejection: The Banff working classification of kidney transplant pathology. Kidney Int 44: 411–422, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ’05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Stegall MD, Gaston RS, Cosio FG, Matas A: Through a glass darkly: Seeking clarity in preventing late kidney transplant failure. J Am Soc Nephrol 26: 20–29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riella LV, Djamali A, Pascual J: Chronic allograft injury: Mechanisms and potential treatment targets. Transplant Rev (Orlando) 31: 1–9, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Van Loon E, Bernards J, Van Craenenbroeck AH, Naesens M: The causes of kidney allograft failure: More than alloimmunity. A viewpoint article. Transplantation 104: e46–e56, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Naesens M, Anglicheau D: Precision transplant medicine: Biomarkers to the rescue. J Am Soc Nephrol 29: 24–34, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tullius SG, Rabb H: Improving the supply and quality of deceased-donor organs for transplantation. N Engl J Med 378: 1920–1929, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The Kidney Donor Risk Index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Massie AB, Luo X, Chow EKH, Alejo JL, Desai NM, Segev DL: Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant 14: 2310–2316, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Brenner BM, Milford EL: Nephron underdosing: A programmed cause of chronic renal allograft failure. Am J Kidney Dis 21[Suppl 2]: 66–72, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Mannon EC, Wood KJ, Mannon RB: Solid Organ Transplantation: Rejection, Immunosuppression, and Tolerance. In: Clinical Immunology Rich RR, London, Elsevier Inc., 2020 [Google Scholar]
- 16.Ferenbach DA, Bonventre JV: Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djamali A, Reese S, Hafez O, Vidyasagar A, Jacobson L, Swain W, Kolehmainen C, Huang L, Wilson NA, Torrealba JR: Nox2 is a mediator of chronic CsA nephrotoxicity. Am J Transplant 12: 1997–2007, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanhove T, Goldschmeding R, Kuypers D: Kidney fibrosis: Origins and interventions. Transplantation 101: 713–726, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Zmijewska A, Zhi D, Mannon RB: Cyclosporine-mediated allograft fibrosis is associated with micro-RNA-21 through AKT signaling. Transpl Int 28: 232–245, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Humphreys BD: Mechanisms of renal fibrosis. Annu Rev Physiol 80: 309–326, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Cheng O, Thuillier R, Sampson E, Schultz G, Ruiz P, Zhang X, Yuen PS, Mannon RB: Connective tissue growth factor is a biomarker and mediator of kidney allograft fibrosis. Am J Transplant 6: 2292–2306, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Mas V, Maluf D, Archer K, Yanek K, Mas L, King A, Gibney E, Massey D, Cotterell A, Fisher R, Posner M: Establishing the molecular pathways involved in chronic allograft nephropathy for testing new noninvasive diagnostic markers. Transplantation 83: 448–457, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Vitalone MJ, O’Connell PJ, Wavamunno M, Fung CL, Chapman JR, Nankivell BJ: Transcriptome changes of chronic tubulointerstitial damage in early kidney transplantation. Transplantation 89: 537–547, 2010 [DOI] [PubMed] [Google Scholar]
- 24.O’Connell PJ, Zhang W, Menon MC, Yi Z, Schröppel B, Gallon L, Luan Y, Rosales IA, Ge Y, Losic B, Xi C, Woytovich C, Keung KL, Wei C, Greene I, Overbey J, Bagiella E, Najafian N, Samaniego M, Djamali A, Alexander SI, Nankivell BJ, Chapman JR, Smith RN, Colvin R, Murphy B: Biopsy transcriptome expression profiling to identify kidney transplants at risk of chronic injury: A multicentre, prospective study. Lancet 388: 983–993, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD: Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol 21: 1987–1997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD: Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci U S A 117: 15874–15883, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nankivell BJ, Borrows RJ, Fung CLS, O’Connell PJ, Allen RDM, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Stegall MD, Park WD, Larson TS, Gloor JM, Cornell LD, Sethi S, Dean PG, Prieto M, Amer H, Textor S, Schwab T, Cosio FG: The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant 11: 698–707, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Park WD, Larson TS, Griffin MD, Stegall MD: Identification and characterization of kidney transplants with good glomerular filtration rate at 1 year but subsequent progressive loss of renal function. Transplantation 94: 931–939, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heilman RL, Devarapalli Y, Chakkera HA, Mekeel KL, Moss AA, Mulligan DC, Mazur MJ, Hamawi K, Williams JW, Reddy KS: Impact of subclinical inflammation on the development of interstitial fibrosis and tubular atrophy in kidney transplant recipients. Am J Transplant 10: 563–570, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Thierry A, Thervet E, Vuiblet V, Goujon JM, Machet MC, Noel LH, Rioux-Leclercq N, Comoz F, Cordonnier C, François A, Marcellin L, Girardot-Seguin S, Touchard G: Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. Am J Transplant 11: 2153–2161, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Kurtkoti J, Sakhuja V, Sud K, Minz M, Nada R, Kohli HS, Gupta KL, Joshi K, Jha V: The utility of 1- and 3-month protocol biopsies on renal allograft function: A randomized controlled study. Am J Transplant 8: 317–323, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Mengel M, Reeve J, Bunnag S, Einecke G, Jhangri GS, Sis B, Famulski K, Guembes-Hidalgo L, Halloran PF: Scoring total inflammation is superior to the current Banff inflammation score in predicting outcome and the degree of molecular disturbance in renal allografts. Am J Transplant 9: 1859–1867, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Mannon RB, Matas AJ, Grande J, Leduc R, Connett J, Kasiske B, Cecka JM, Gaston RS, Cosio F, Gourishankar S, Halloran PF, Hunsicker L, Rush D; DeKAF Investigators : Inflammation in areas of tubular atrophy in kidney allograft biopsies: A potent predictor of allograft failure. Am J Transplant 10: 2066–2073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matas AJ, Helgeson ES, Gaston R, Cosio F, Mannon R, Kasiske BL, Hunsicker L, Gourishankar S, Rush D, Michael Cecka J, Connett J, Grande JP: Inflammation in areas of fibrosis: The DeKAF prospective cohort. Am J Transplant 20: 2509–2521, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Naesens M, Lerut E, Emonds MP, Herelixka A, Evenepoel P, Claes K, Bammens B, Sprangers B, Meijers B, Jochmans I, Monbaliu D, Pirenne J, Kuypers DR: Proteinuria as a noninvasive marker for renal allograft histology and failure: An observational cohort study. J Am Soc Nephrol 27: 281–292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, Duong van Huyen JP, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, Mengel M: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefaucheur C, Gosset C, Rabant M, Viglietti D, Verine J, Aubert O, Louis K, Glotz D, Legendre C, Duong Van Huyen JP, Loupy A: T cell-mediated rejection is a major determinant of inflammation in scarred areas in kidney allografts. Am J Transplant 18: 377–390, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Nankivell BJ, Shingde M, Keung KL, Fung CL, Borrows RJ, O’Connell PJ, Chapman JR: The causes, significance and consequences of inflammatory fibrosis in kidney transplantation: The Banff i-IFTA lesion. Am J Transplant 18: 364–376, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Halloran P, Chang J, Famulski KS: In kidney allografts inflammation in scarred areas is not a reflection of chronic active T cell-mediated rejection. Transplantation 102: S268–S269, 2018 [Google Scholar]
- 41.Naesens M, Kuypers DR, Sarwal M: Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Khanna A, Plummer M, Bromberek C, Bresnahan B, Hariharan S: Expression of TGF-beta and fibrogenic genes in transplant recipients with tacrolimus and cyclosporine nephrotoxicity. Kidney Int 62: 2257–2263, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Yang CW, Faulkner GR, Wahba IM, Christianson TA, Bagby GC, Jin DC, Abboud HE, Andoh TF, Bennett WM: Expression of apoptosis-related genes in chronic cyclosporine nephrotoxicity in mice. Am J Transplant 2: 391–399, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Strutz F, Zeisberg M: Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 17: 2992–2998, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Pallet N, Rabant M, Xu-Dubois YC, Lecorre D, Mucchielli MH, Imbeaud S, Agier N, Hertig A, Thervet E, Legendre C, Beaune P, Anglicheau D: Response of human renal tubular cells to cyclosporine and sirolimus: A toxicogenomic study. Toxicol Appl Pharmacol 229: 184–196, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Pallet N, Bouvier N, Bendjallabah A, Rabant M, Flinois JP, Hertig A, Legendre C, Beaune P, Thervet E, Anglicheau D: Cyclosporine-induced endoplasmic reticulum stress triggers tubular phenotypic changes and death. Am J Transplant 8: 2283–2296, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Karpe KM, Talaulikar GS, Walters GD: Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst Rev 7: CD006750, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirsch HH, Randhawa PS; AST Infectious Diseases Community of Practice : BK polyomavirus in solid organ transplantation-guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 33: e13528, 2019 [DOI] [PubMed] [Google Scholar]
- 49.Nickeleit V, Singh HK, Dadhania D, Cornea V, El-Husseini A, Castellanos A, Davis VG, Waid T, Seshan SV: The 2018 Banff Working Group classification of definitive polyomavirus nephropathy: A multicenter validation study in the modern era [published online ahead of print July 11, 2020]. Am J Transplant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mannon RB, Hoffmann SC, Kampen RL, Cheng OC, Kleiner DE, Ryschkewitsch C, Curfman B, Major E, Hale DA, Kirk AD: Molecular evaluation of BK polyomavirus nephropathy. Am J Transplant 5: 2883–2893, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Cardinal H, Dieudé M, Hébert MJ: Endothelial dysfunction in kidney transplantation. Front Immunol 9: 1130, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, Hamelin K, Qi S, Pallet N, Béland C, Dhahri W, Cailhier JF, Rousseau M, Duchez AC, Lévesque T, Lau A, Rondeau C, Gingras D, Muruve D, Rivard A, Cardinal H, Perreault C, Desjardins M, Boilard É, Thibault P, Hébert MJ: The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med 7: 318ra200, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Valenzuela NM, Reed EF: Antibodies to HLA molecules mimic agonistic stimulation to trigger vascular cell changes and induce allograft injury. Curr Transplant Rep 2: 222–232, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell LD, Clahsen-van Groningen MC, Demetris AJ, Dragun D, Duong van Huyen JP, Farris AB, Fogo AB, Gibson IW, Glotz D, Gueguen J, Kikic Z, Kozakowski N, Kraus E, Lefaucheur C, Liapis H, Mannon RB, Montgomery RA, Nankivell BJ, Nickeleit V, Nickerson P, Rabant M, Racusen L, Randhawa P, Robin B, Rosales IA, Sapir-Pichhadze R, Schinstock CA, Seron D, Singh HK, Smith RN, Stegall MD, Zeevi A, Solez K, Colvin RB, Mengel M: The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant 20: 2318–2331, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aubert O, Higgins S, Bouatou Y, Yoo D, Raynaud M, Viglietti D, Rabant M, Hidalgo L, Glotz D, Legendre C, Delahousse M, Shah N, Sis B, Campbell P, Mengel M, Jouven X, Duong Van Huyen JP, Lefaucheur C, Loupy A: Archetype analysis identifies distinct profiles in renal transplant recipients with transplant glomerulopathy associated with allograft survival. J Am Soc Nephrol 30: 625–639, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loupy A, Aubert O, Orandi BJ, Naesens M, Bouatou Y, Raynaud M, Divard G, Jackson AM, Viglietti D, Giral M, Kamar N, Thaunat O, Morelon E, Delahousse M, Kuypers D, Hertig A, Rondeau E, Bailly E, Eskandary F, Böhmig G, Gupta G, Glotz D, Legendre C, Montgomery RA, Stegall MD, Empana JP, Jouven X, Segev DL, Lefaucheur C: Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 366: l4923, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagnasco SM, Zachary AA, Racusen LC, Arend LJ, Carter-Monroe N, Alachkar N, Nazarian SM, Lonze BE, Montgomery RA, Kraus ES: Time course of pathologic changes in kidney allografts of positive crossmatch HLA-incompatible transplant recipients. Transplantation 97: 440–445, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Kovács G, Devercelli G, Zelei T, Hirji I, Vokó Z, Keown PA: Association between transplant glomerulopathy and graft outcomes following kidney transplantation: A meta-analysis. PLoS One 15: e0231646, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schinstock CA, Stegall M, Cosio F: New insights regarding chronic antibody-mediated rejection and its progression to transplant glomerulopathy. Curr Opin Nephrol Hypertens 23: 611–618, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Schinstock CA, Mannon RB, Budde K, Chong AS, Haas M, Knechtle S, Lefaucheur C, Montgomery RA, Nickerson P, Tullius SG, Ahn C, Askar M, Crespo M, Chadban SJ, Feng S, Jordan SC, Man K, Mengel M, Morris RE, O’Doherty I, Ozdemir BH, Seron D, Tambur AR, Tanabe K, Taupin JL, O’Connell PJ: Recommended treatment for antibody-mediated rejection after kidney transplantation: The 2019 expert consensus from the Transplantion Society Working Group. Transplantation 104: 911–922, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Böhmig GA, Eskandary F, Doberer K, Halloran PF: The therapeutic challenge of late antibody-mediated kidney allograft rejection. Transpl Int 32: 775–788, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brocklebank V, Wood KM, Kavanagh D: Thrombotic microangiopathy and the kidney. Clin J Am Soc Nephrol 13: 300–317, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bloom RD, Bromberg JS, Poggio ED, Bunnapradist S, Langone AJ, Sood P, Matas AJ, Mehta S, Mannon RB, Sharfuddin A, Fischbach B, Narayanan M, Jordan SC, Cohen D, Weir MR, Hiller D, Prasad P, Woodward RN, Grskovic M, Sninsky JJ, Yee JP, Brennan DC; Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators : Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol 28: 2221–2232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedewald JJ, Kurian SM, Heilman RL, Whisenant TC, Poggio ED, Marsh C, Baliga P, Odim J, Brown MM, Ikle DN, Armstrong BD, Charette JI, Brietigam SS, Sustento-Reodica N, Zhao L, Kandpal M, Salomon DR, Abecassis MM; Clinical Trials in Organ Transplantation 08 (CTOT-08) : Development and clinical validity of a novel blood-based molecular biomarker for subclinical acute rejection following kidney transplant. Am J Transplant 19: 98–109, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiebe C, Nickerson PW: More precise donor-recipient matching: The role of eplet matching. Curr Opin Nephrol Hypertens 29: 630–635, 2020 [DOI] [PubMed] [Google Scholar]
- 66.Isaka Y: Targeting TGF-β signaling in kidney fibrosis. Int J Mol Sci 19: 2532, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halden TAS, Kvitne KE, Midtvedt K, Rajakumar L, Robertsen I, Brox J, Bollerslev J, Hartmann A, Åsberg A, Jenssen T: Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care 42: 1067–1074, 2019 [DOI] [PubMed] [Google Scholar]
- 68.Hesp AC, Schaub JA, Prasad PV, Vallon V, Laverman GD, Bjornstad P, van Raalte DH: The role of renal hypoxia in the pathogenesis of diabetic kidney disease: A promising target for newer renoprotective agents including SGLT2 inhibitors?. Kidney Int 98: 579–589, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]