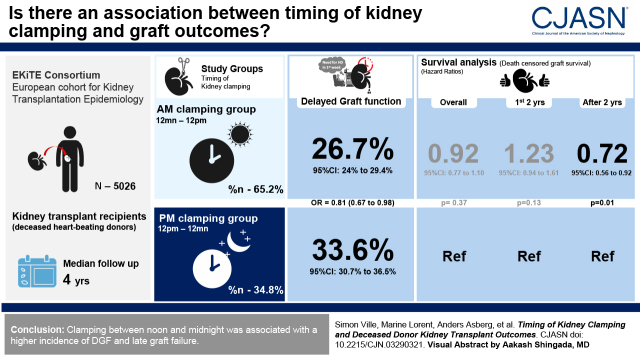
Visual Abstract
Keywords: cadaver organ transplantation, delayed graft function, ischemia-reperfusion, kidney transplantation, organ transplant, constriction, graft survival, kidney
Abstract
Background and objectives
The fact that metabolism and immune function are regulated by an endogenous molecular clock that generates circadian rhythms suggests that the magnitude of ischemia reperfusion, and subsequent inflammation on kidney transplantation, could be affected by the time of the day.
Design, setting, participants, & measurements
We evaluated 5026 individuals who received their first kidney transplant from deceased heart-beating donors. In a cause-specific multivariable analysis, we compared delayed graft function and graft survival according to the time of kidney clamping and declamping. Participants were divided into those clamped between midnight and noon (ante meridiem [am] clamping group; 65%) or clamped between noon and midnight (post meridiem [pm] clamping group; 35%), and, similarly, those who underwent am declamping (25%) or pm declamping (75%).
Results
Delayed graft function occurred among 550 participants (27%) with am clamping and 339 (34%) with pm clamping (adjusted odds ratio, 0.81; 95% confidence interval, 0.67 to 0.98; P=0.03). No significant association was observed between clamping time and overall death-censored graft survival (hazard ratio, 0.92; 95% confidence interval, 0.77 to 1.10; P=0.37). No significant association of declamping time with delayed graft function or graft survival was observed.
Conclusions
Clamping between midnight and noon was associated with a lower incidence of delayed graft function, whereas declamping time was not associated with kidney graft outcomes.
Introduction
Kidney transplantation is the standard of care for kidney failure (1). However, over the last decades, long-term graft survival has improved slowly (2). To date, the best outcomes in kidney transplantation are obtained when using organs from living donors, even if they are immunologically mismatched (3). This suggests that donor organ procurement, organ conservation, and, finally, recipient surgical engraftment may be temporally linked to graft outcome (4). One of the major advances in the past decade has been the use of hypothermic machine perfusion (5). The early phase involves ischemia followed by reperfusion, resulting in metabolic changes associated with activation of innate immunity, which may prime the adaptive alloimmune response (6,7).
Whereas cold ischemia time appears to be a major explicative variable of ischemia reperfusion (6), there are many other potential mechanisms. Recent discoveries have shown that one of the main circadian-clock genes, Period Circadian Regulator 2 (Per2), could be involved in ischemia-reperfusion injury, because its modulation by carbon monoxide could have protective effects on the donor kidney (8). In addition, a link between the molecular clock and tolerance to myocardial ischemia has also been shown (9,10). Recently, a study compared the outcomes of patients exposed to myocardial ischemia-reperfusion injury after on-pump cardiac surgery for aortic valve replacement in the morning versus surgery in the afternoon and showed that ischemia-reperfusion tolerance was better in the afternoon; the authors identified intrinsic circadian variations in the main molecular clock proteins from the myocardial biopsy specimens (11). These studies suggest that the time of day when surgery is performed may be clinically important due to the differential effects of diurnal variations on ischemia reperfusion, and that time of day may, therefore, affect downstream organ transplant survival.
Close links between the circadian system and energy metabolism have emerged that support the idea that metabolic and immune activities occur at predictable times of the day. In mammals, this pacemaker, which is located in the suprachiasmatic nuclei neurons and is synchronized by daylight (12), regulates basic functions, such as sleep-wake and feeding behaviors, BP, and body temperature. In recent years, the importance of circadian control of numerous biologic functions, such as metabolic pathways (13) and the innate and adaptative immune systems, have been recognized (14). Although synchronized by this central clock, peripheral organs, including the kidney (15), possess an intrinsic clock to regulate their function (16).
The sequence of steps involved in kidney organ transplantation from a donor to a recipient is complex and involves multiple pathways influenced by the circadian clocks of both the donor and the recipient. However, it is unknown whether “donor time” (i.e., the time of organ procurement, when the vessels are clamped, and the organ cooled) or the “recipient time” (the time when the vessels are declamped after a period of cold ischemia time) are important for kidney transplant outcomes. In a multicenter cohort, on the basis of observation of the incidence of delayed graft function (DGF) according to the time of clamping, we hypothesize that the circadian clocks (defined as morning [am] and afternoon [pm]) of the donor (clamping time) and of the recipient (declamping time) could affect early and late transplant outcomes.
Materials and Methods
Population
Data were extracted from the Epidemiology in Kidney Transplantation–a European validated database cohort (EKiTE; approved by the Commission Nationale de l'Informatique et des Libertés [CNIL], number 917155), comprising kidney transplant recipients in the transplantation units of Lyon, Montpellier, Nancy, Nantes, Necker, Nice, and Saint-Louis University Hospitals in France, and from the Department of Transplantation Medicine of the Oslo University Hospital in Norway. Data quality was verified with respect to each cohorts’ local procedures. EKiTE cohort participants gave informed consent. The clinical and research activities being reported are consistent with the principles of the Declaration of Istanbul, as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Only adult recipients of a first kidney transplantation from deceased heart-beating donors were included in the study (donors of donation after circulatory death were excluded). Transplants involving multiple organs were excluded. The study was limited to transplantations performed between January 2005 and December 2017 to represent current practices. Clamping was defined by the crossclamping at the time of organ recovery, whereas declamping referred to reperfusion at the time of arterial anastomosis. Participants were classified into the ante meridiem (am) clamping group if the clamping hour was between midnight and noon (am clamping group), or in the post meridiem (pm) clamping group if their clamping hour was between noon and midnight (pm clamping group). The same time intervals were considered to differentiate the am declamping group and the pm declamping group.
Post-Transplantation Outcomes
The short- and midterm outcomes were the DGF (defined by the need for dialysis in the first week post-transplantation) and the eGFR, estimated using the Modification of Diet in Renal Disease Study equation, at 1-year post-transplantation. Note that the analysis of DGF excluded the preemptive transplant recipients and those on peritoneal dialysis.
Long-term outcomes were the patient and graft survival (defined by the time between transplantation and the first event, defined as return to dialysis, preemptive retransplantation, or death) and the death-censored graft survival (death with a functioning graft was right censored). Finally, we analyzed the time to first biopsy sample–proven acute rejection episode.
Statistical Analyses
The characteristics between the two groups of interest (am clamping versus pm clamping or am declamping versus pm declamping) were compared using chi-squared tests for categoric variables and t tests for continuous variables. Survival curves were obtained using the Kaplan–Meier estimator. To further compare the outcomes and to consider possible confounders, multivariable logistic regression was used for binary outcomes, multivariable linear regression for continuous outcomes, and a cause-specific Cox model for times to event. The following variables were considered in the multivariable models: (1) general features, i.e., center (baseline hazard stratification for the Cox regression) and year of transplantation; (2) donor features, i.e., age, sex, death by cerebrovascular accident, blood group, cytomegalovirus serology, recovered cardiac arrest during stay in intensive care, use of vasoactive drugs during stay in intensive care, and terminal serum creatinine; (3) preservation features, i.e., use of a perfusion machine, preservation solution, and cold ischemia time; and (4) recipient features, i.e., age, sex, body mass index, preemptive transplantation, duration on waiting list, primary diagnosis of kidney failure, anti–class I and anti–class II sensitization, four or more HLA-A/-B/-DR incompatibilities, history of diabetes, history of vascular disease, history of cardiac disease, history of malignancy, cytomegalovirus serology, HIV serology, blood group, and type of induction (depleting or not depleting). The log-linearity assumption was automatically checked: the nonfulfillment of this assumption was defined as when the Bayesian information criterion decreased using natural spline transformation compared with the inclusion of the covariate in its natural scale. In case of violation of this assumption, variables were categorized. Hazard proportionality was checked by plotting log-minus-log survival curves according to the two groups of interest and studying the Schoenfeld residuals. Variables significantly associated with both the outcome and the clamping (or declamping) group in unadjusted regressions were retained (P<0.20) in multivariable models. Statistical analyses were performed using Plug-Stat (www.labcom-risca.com).
Results
Graft Clamping Time and Description of the Cohort
In total, 5026 consecutive kidney transplantations performed between January 2005 and December 2017 met the analysis criteria and were included in the study cohort (see Supplemental Figure 1).
For the whole cohort, the mean donor age was 54 years, with 57% being male, and 59% of deaths related to cerebrovascular accident. Among recipients, the mean age was 55 years, with 64% being male. Before transplantation, 87% of participants were dialyzed, 21% were diabetic, the mean body mass index of participants was 25.6 kg/m2, and 28% and 21% of participants were sensitized against class I and II HLA antigens, respectively. Mean cold ischemic time was 12.6 hours. The median follow-up time was 4.0 (range, 0.0–13.2) years. During follow-up, 561 participants died with a functioning graft and 596 returned to dialysis.
Although we did not observe a clear trend regarding declamping time, the distribution of the DGF incidence according to the clamping time through a 24-hour period revealed clear inflections around midnight and noon (Supplemental Figure 2). As a result, we decided to split the cohort between the am and the pm in further analyses. A total of 5006 participants were analyzed regarding clamping time (20 participants with missing data); there were 3264 participants (65%) in the am clamping group versus 1742 (35%) in the pm clamping group. A total of 5026 participants were analyzed regarding the declamping time: 1253 participants (25%) in the am declamping group versus 3773 (75%) in the pm declamping group.
Table 1 shows the baseline characteristics of the study population stratified by clamping and declamping time.
Table 1.
Characteristics of participants in the Epidemiology in Kidney Transplantation (EKiTE) cohort at the time of kidney transplantation
| Characteristics | All Included Participants | Clamping Afternoon (Noon–Midnight) (n=1742) | Clamping Morning (Midnight–Noon) (n=3264) | Declamping Afternoon (Noon–Midnight) (n=3773) | Declamping Morning (Midnight–Noon) (n=1253) |
|---|---|---|---|---|---|
| Recipient | |||||
| Age, yr | 56 (14) | 55 (14) | 55 (14) | 56 (14) | 55 (14) |
| Sex, male | 3226 (64) | 1121 (64) | 2105 (64) | 2434 (65) | 804 (64) |
| BMI, kg/m2 | 25.6 (5.0) | 25.7 (5.2) | 25.5 (4.9) | 25.5 (4.9) | 25.6 (5.3) |
| Duration on waiting list, d | 615 (578) | 591 (561) | 628 (588) | 630 (593) | 570 (532) |
| Preemptive transplantation | 671 (13) | 232 (13) | 439 (13) | 495 (13) | 178 (14) |
| Primary diagnosis | |||||
| Diabetes | 438 (11) | 136 (8) | 302 (9) | 358 (9) | 84 (7) |
| GN | 955 (25) | 307 (18) | 648 (20) | 732 (19) | 224 (18) |
| Renovascular disease | 478 (12) | 160 (9) | 318 (10) | 381 (10) | 97 (8) |
| Tubulointerstitial disease | 1404 (36) | 435 (25) | 969 (30) | 1128 (30) | 286 (23) |
| Unknown | 613 (16) | 153 (9) | 460 (14) | 504 (13) | 114 (9) |
| History of diabetes | 1030 (21) | 386 (22) | 644 (20) | 775 (21) | 262 (21) |
| History of vascular disease | 768 (15) | 282 (16) | 486 (15) | 583 (15) | 187 (15) |
| History of cardiac disease | 1363 (27) | 470 (27) | 893 (27) | 1046 (28) | 324 (26) |
| History of malignancy | 509 (10) | 162 (9) | 347 (11) | 404 (11) | 107 (9) |
| Positive CMV serology | 3261 (66) | 1142 (66) | 2119 (65) | 2438 (65) | 834 (67) |
| Positive HIV serology | 46 (1) | 6 (0) | 40 (1) | 38 (1) | 9 (1) |
| HLA sensitization | |||||
| Anti–class I | 1225 (28) | 450 (31) | 775 (27) | 928 (28) | 302 (29) |
| Anti–class II | 882 (21) | 283 (20) | 599 (21) | 701 (21) | 186 (18) |
| Recipient blood group | |||||
| A | 2206 (44) | 762 (44) | 1444 (44) | 1681 (45) | 537 (43) |
| AB | 238 (5) | 91 (5) | 147 (5) | 179 (5) | 60 (5) |
| B | 559 (11) | 195 (11) | 364 (11) | 418 (11) | 142 (11) |
| O | 2001 (40) | 694 (40) | 1307 (40) | 1494 (40) | 513 (41) |
| Donor | |||||
| Age, yr | 54 (17) | 55 (17) | 54 (17) | 55 (17) | 53 (17) |
| Sex, male | 2876 (57) | 991 (57) | 1885 (58) | 2157 (57) | 731 (58) |
| ECD | 2343 (47) | 844 (49) | 1499 (46) | 1806 (48) | 548 (44) |
| Death by CVA | 2897 (59) | 1073 (63) | 1824 (57) | 2169 (59) | 741 (61) |
| Positive donor CMV serology | 2970 (60) | 1055 (61) | 1915 (59) | 2226 (59) | 758 (61) |
| Donor blood group | |||||
| A | 2223 (45) | 767 (44) | 1456 (45) | 1690 (45) | 546 (44) |
| AB | 195 (4) | 77 (4) | 118 (4) | 153 (4) | 42 (3) |
| B | 494 (10) | 171 (10) | 323 (10) | 377 (10) | 117 (9) |
| O | 2080 (42) | 723 (41) | 1357 (42) | 1544 (41) | 543 (43) |
| Intensive care events | |||||
| Cardiac arrest | 1133 (23) | 352 (20) | 781 (24) | 889 (24) | 251 (20) |
| Use of vasoactive drugs | 4206 (90) | 1474 (90) | 2732 (90) | 3179 (90) | 1043 (89) |
| Terminal serum creatinine, mg/dl | |||||
| <1 | 3260 (70) | 1148 (66) | 2112 (65) | 2459 (65) | 812 (65) |
| 1–1.5 | 900 (19) | 290 (17) | 610 (19) | 697 (18) | 208 (17) |
| ≥1.5 | 524 (11) | 178 (10) | 346 (11) | 415 (11) | 113 (9) |
| Transplantation | |||||
| Transplantation year | |||||
| 2005–2008 | 1091 (22) | 443 (25) | 648 (20) | 344 (9) | 749 (60) |
| 2009–2012 | 1713 (34) | 638 (37) | 1075 (33) | 458 (12) | 1265 (101) |
| 2013–2017 | 2202 (44) | 661 (38) | 1541 (47) | 451 (12) | 1759 (140) |
| HLA-A/-B/-DR incompatibilities ≥4 | 673 (14) | 240 (14) | 433 (13) | 532 (14) | 144 (12) |
| Machine perfusion | 677 (20) | 199 (20) | 478 (20) | 555 (20) | 126 (20) |
| Preservation solution | |||||
| Extracellular | 3941 (81) | 1407 (81) | 2534 (78) | 2917 (77) | 1037 (83) |
| Intracellular | 257 (5) | 79 (5) | 178 (5) | 204 (5) | 55 (4) |
| HMP solution | 225 (5) | 62 (4) | 163 (5) | 187 (5) | 41 (3) |
| Other | 447 (9) | 152 (9) | 295 (9) | 363 (10) | 85 (7) |
| Cold ischemia time, h | 12.6 (9.5) | 11.7 (10.5) | 13.1 (8.8) | 13.0 (8.5) | 11.6 (11.8) |
| Depleting induction | 2870 (57) | 1105 (63) | 1765 (54) | 2107 (56) | 773 (62) |
| One-year events | |||||
| BK virus infection | 276 (7) | 87 (7) | 189 (7) | 220 (7) | 57 (7) |
| CMV infection | 570 (15) | 155 (13) | 415 (15) | 470 (15) | 103 (13) |
| Pyelonephritis | 558 (15) | 177 (16) | 381 (15) | 440 (15) | 120 (17) |
| Biopsy sample–proven rejection | 605 (12) | 197 (11) | 408 (13) | 476 (13) | 130 (10) |
Numbers are n (%) for categoric variables and mean (SD) for continuous variables. The following data were not available: recipient BMI, n=136; preemptive transplantation, n=11; duration on waiting list, n=218; primary diagnosis of kidney disease, n=1118; positive recipient CMV serology, n=38; positive recipient HIV serology, n=29; HLA class I sensitization, n=682; HLA class II sensitization, n=710; recipient blood group, n=2; ECD donor status, n=24; donor cardiac arrest in intensive care, n=17; use of vasoactive drugs in intensive care, n=321; donor blood group, n=14; donor terminal creatininemia, n=322; HLA-A/-B/-DR incompatibilities, n=42; machine perfusion, n=1660; preservation solution, n=136; 1-year BK virus infection, n=1143; 1-year CMV infection, n=1143; 1-year pyelonephritis, n=1374. EKiTE, Epidemiology in Kidney Transplantation–a European validated database; BMI, body mass index; CMV, cytomegalovirus; ECD, expanded criteria donor; CVA, cerebrovascular accident; HMP, hypothermic machine perfusion.
Incidence of DGF
A total of 1365 participants were excluded because they were receiving peritoneal dialysis or not dialyzed before transplantation, and 568 participants had missing data. Among the remaining 3073 participants, 889 events were observed: 339 (34%) in the pm clamping group and 550 (27%) in the am clamping group. When adjusted for possible cofounders (n=2879), kidney procurement between midnight and noon was significantly associated with a lower DGF (odds ratio [OR], 0.81; 95% confidence interval [95% CI], 0.67 to 0.98; P=0.03) compared with participants who received a clamped kidney between noon and midnight (Table 2).
Table 2.
Association of clamping time with delayed graft function
| Characteristics | N (%) | Model Comparing am to pm Clamping (for Midnight–Noon, n=1932; for Noon–Midnight, n=947) |
Model Comparing 6-h Time Periods of Clamping (for Midnight–6 am, n=1135; for 6 am–Noon, n=797; for Noon–6 pm, n=360; for 6 pm–Midnight, n=587) |
Model Using 6 pm–Midnight as Reference (for Midnight–6 pm, n=2292; for 6 pm–Midnight, n=587) |
|||
|---|---|---|---|---|---|---|---|
| Adjusted Odds Ratio (95% Confidence Interval) | P Value | Adjusted Odds Ratio (95% Confidence Interval) | P Value | Adjusted Odds Ratio (95% Confidence Interval) | P Value | ||
| Time of clamping | 0.03 | ||||||
| Noon–midnight | 328 (35) | 1 (ref) | |||||
| Midnight–noon | 526 (27) | 0.81 (0.67 to 0.98) | |||||
| 6 pm–midnight | 214 (36) | 1 (ref) | 0.15 | ||||
| Midnight–6 am | 325 (29) | 0.77 (0.60 to 0.98) | |||||
| 6 am–noon | 201 (25) | 0.77 (0.59 to 1.00) | |||||
| Noon–6 pm | 114 (32) | 0.85 (0.62 to 1.15) | |||||
| 6 pm–midnight | 214 (36) | 1 (ref) | 0.03 | ||||
| Midnight–6 pm | 640 (28) | 0.78 (0.63 to 0.97) | |||||
| Donor age, yr | 1.01 (1.00 to 1.02) | <0.001 | 1.01 (1.00 to 1.02) | <0.001 | 1.01 (1.00 to 1.02) | <0.001 | |
| Terminal donor serum creatinine (per 0.01 mg/dl) | 1.01 (1.00 to 1.01) | <0.001 | 1.01 (1.00 to 1.01) | <0.001 | 1.01 (1.00 to 1.01) | <0.001 | |
| Cause donor death | 0.19 | 0.2 | 0.21 | ||||
| Other | 345 (28) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| CVA | 509 (31) | 1.14 (0.94 to 1.38) | 1.13 (0.93 to 1.38) | 1.13 (0.93 to 1.38) | |||
| Donor CMV serology | 0.22 | 0.24 | 0.24 | ||||
| Negative | 386 (32) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Positive | 468 (28) | 0.89 (0.74 to 1.07) | 0.90 (0.75 to 1.08) | 0.90 (0.75 to 1.08) | |||
| Sex | 0.001 | <0.001 | 0.002 | ||||
| Female | 268 (26) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Male | 586 (32) | 1.37 (1.13 to 1.65) | 1.35 (1.12 to 1.64) | 1.36 (1.12 to 1.64) | |||
| Recipient BMI, kg/m2 | 1.07 (1.05 to 1.09) | <0.001 | 1.07 (1.05 to 1.10) | <0.001 | 1.07 (1.05 to 1.10) | <0.001 | |
| History of diabetes | 0.3 | 0.3 | 0.30 | ||||
| No | 644 (28) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Yes | 210 (34) | 0.89 (0.71 to 1.11) | 0.89 (0.71 to 1.11) | 0.89 (0.71 to 1.11) | |||
| History of vascular disease | 0.02 | 0.02 | 0.02 | ||||
| No | 653 (28) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Yes | 201 (40) | 1.32 (1.05 to 1.65) | 1.32 (1.05 to 1.65) | 1.31 (1.05 to 1.65) | |||
| History of cardiac disease | 0.001 | 0.001 | 0.001 | ||||
| No | 522 (26) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Yes | 332 (39) | 1.39 (1.14 to 1.69) | 1.38 (1.14 to 1.69) | 1.39 (1.14 to 1.69) | |||
| Recipient blood group | 0.03 | 0.02 | 0.07 | ||||
| A | 330 (27) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| AB | 45 (34) | 1.29 (1.24 to 0.98) | 1.31 (0.84 to 1.99) | 1.31 (0.85 to 2.00) | |||
| B | 97 (29) | 1.15 (0.85 to 1.54) | 1.16 (0.86 to 1.56) | 1.16 (0.86 to 1.56) | |||
| O | 382 (32) | 1.33 (1.10 to 1.62) | 1.33 (1.09 to 1.62) | 1.33 (1.10 to 1.62) | |||
| HLA-A/-B/-DR incompatibilities ≥4 | 0.04 | 0.04 | 0.02 | ||||
| No | 403 (27) | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Yes | 451 (32) | 1.30 (1.02 to 1.66) | 1.30 (1.02 to 1.66) | 1.24 (1.04 to 1.48) | |||
| Cold ischemia time, h | 1.05 (1.02 to 1.06) | <0.001 | 1.05 (1.03 to 1.06) | <0.001 | 1.05 (1.03 to 1.06) | <0.001 | |
Total n=2879. ref, reference; CVA, cerebrovascular accident; CMV, cytomegalovirus; BMI, body mass index.
To identify a narrower risk window, we repeated the analysis by splitting the day into four (6-hour periods). The 6 pm to midnight time period tended to be the most associated with DGF, with an incidence of 35% in this group versus 27% in the other participants (OR, 0.78; 95% CI, 0.63 to 0.97; P=0.03; Table 2).
No significant difference was observed when comparing the am declamping group to the pm declamping afternoon group: an incidence of 29% was observed in both (OR, 0.92; 95% CI, 0.74 to 1.15; P=0.48; Supplemental Table 1).
One-Year Kidney Function after Transplantation (eGFR Using Modification of Diet in Renal Disease Equation)
A total of 597 participants were excluded due to a follow-up period <1-year post-transplantation, 348 participants were excluded because of death or graft failure before 1 year post-transplantation, and 1066 participants had missing data. Among the 3015 participants, the overall mean 1-year eGFR was 51 ml/min per 1.73 m2: the mean was 51 ml/min per 1.73 m2 in the am clamping group versus 50 ml/min per 1.73 m2 in the pm clamping group. When adjusted for possible confounders (n=2648), no significant difference was observed (OR, 1.21; 95% CI, −0.14 to 2.57; P=0.08; Supplemental Table 2). Also, there was no significant difference between the am and the pm declamping group (52 versus 51 ml/min per 1.73 m2; adjusted OR, −0.19; 95% CI, −1.73 to 1.34; P=0.81; Supplemental Table 3).
Survival Analysis
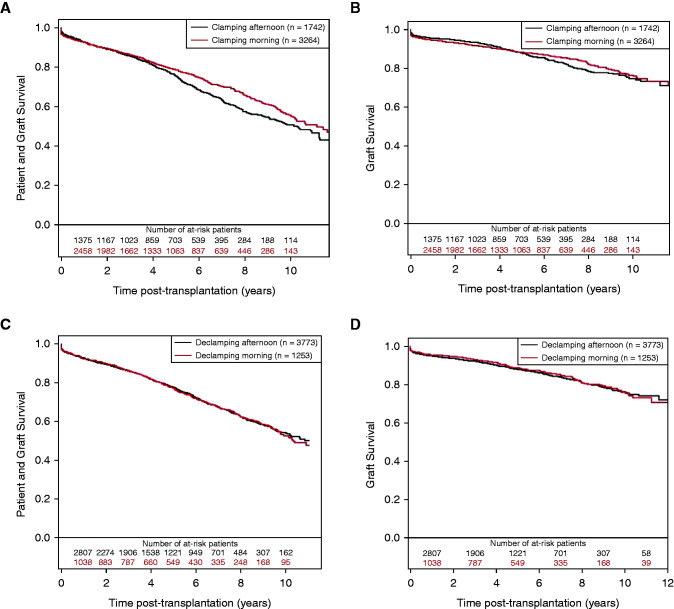
The patient and graft survival and death-censored graft survival according to clamping time are presented in Figure 1, A and B, respectively. Among the 5006 participants, survival was 55% (95% CI, 52% to 59%; n=663) at 10 years post-transplantation in the am clamping group versus 51% (95% CI, 47% to 55%; n=470) in the pm clamping group. In the multivariable analysis (Table 3), the corresponding confounder-adjusted hazard ratio (HR) of the am clamping versus pm clamping group was 0.91 (95% CI, 0.80 to 1.03; P=0.13). When the 6 pm–midnight group was used as reference, a significant association was observed (HR, 0.83; 95% CI, 0.73 to 0.95; P=0.006). When deaths were censored, the overall confounder-adjusted HR of the am clamping versus pm clamping group was 0.92 (95% CI, 0.77 to 1.10; P=0.37); when the 6 pm–midnight group was used as reference, a trend was observed (HR, 0.83; 95% CI, 0.69 to 1.01; P=0.06). Remarkably, within 2 years after transplantation, the HR was 1.23 (95% CI, 0.94 to 1.61; P=0.13) and 0.97 (95% CI, 0.72 to 1.30; P=0.84) with the pm clamping group or 6 pm–midnight clamping group as reference, respectively. After 2 years, a significant association between clamping time and graft survival was observed, with an HR of 0.72 (95% CI, 0.56 to 0.92; P=0.01) and 0.73 (95% CI, 0.57 to 0.95; P=0.02), respectively, with a significant interaction for association with time from transplant (P=0.03).
Figure 1.
Crude survival curves estimated by the Kaplan–Meier estimator. (A and B) Clamping in the morning versus clamping in the afternoon (n=5006). (C and D) Declamping in the morning versus declamping in the afternoon (n=5026). (A and C) Patient and graft survival; (B and D) death-censored graft survival.
Table 3.
Associations of clamping time with patient and graft survival and death-censored graft survival
| Time of Clamping | N (%) | Model Comparing am with pm Clamping (for Midnight–Noon, n=3094; for Noon–Midnight, n=1678) |
Model Using 6 pm–Midnight as Reference (for 6 pm–Midnight, n=1139; for Midnight–6 PM, n=3663) |
||
|---|---|---|---|---|---|
| Adjusted Hazard Ratio (95% Confidence Interval) | P Value | Adjusted Hazard Ratio (95% Confidence Interval) | P Value | ||
| Patient and graft survival | |||||
| Noon–midnight | 458 (27) | 1 (ref) | 0.13 | ||
| Midnight–noon | 625 (20) | 0.91 (0.80 to 1.03) | |||
| 6 pm–midnight | 372 (33) | 1 (ref) | 0.006 | ||
| Midnight–6 pm | 711 (20) | 0.83 (0.73 to 0.95) | |||
| Death-censored graft survival | |||||
| Noon–midnight | 209 (12) | 1 (ref) | 0.37 | ||
| Midnight–noon | 333 (11) | 0.92 (0.77 to 1.10) | |||
| 6 pm–midnight | 164 (14) | 1 (ref) | 0.06 | ||
| Midnight–6 pm | 378 (10) | 0.83 (0.69 to 1.01) | |||
Total N=4772. ref, reference.
Patient and graft survival curves and death-censored graft survival curves regarding the declamping time are presented in Figure 1, C and D, respectively. Multivariable analysis showed no significant association between declamping time and patient and graft survival curves and death-censored graft (HRs of 1.04 [95% CI, 0.91 to 1.19; P=0.56] and 1.07 [95% CI, 0.88 to 1.30; P=0.47], respectively; Supplemental Tables 4 and 5).
Acute Rejection Occurrence
The cumulative probability of an acute rejection episode at 5 years post-transplantation in participants transplanted with an organ removed between midnight and noon was 20% (95% CI, 19% to 22%; n=528) versus 17% (95% CI, 15% to 19%; n=253) between noon and midnight. The corresponding confounder-adjusted HR (n=4661) for the morning versus afternoon clamping time groups was 0.92 (95% CI, 0.79 to 1.08; P=0.32; Supplemental Table 6). Also, the comparison between the am declamping and the pm declamping groups showed no significant association: 16% (95% CI, 14% to 18%; n=170) versus 20% (95% CI, 19% to 22%; n=614), with an adjusted HR of 1.02 (95% CI, 0.86 to 1.22; P=0.79; Supplemental Table 7).
Discussion
In this large, prospective, European cohort of kidney transplant recipients, a significant association between the donor time and the incidence of DGF was observed. No association with the overall death-censored graft survival was identified. However, when the 6 pm–midnight period was used as the reference, a significant association between kidney clamping time and the patient- and death-censored graft survival was observed. Furthermore, there was a significant association between the clamping time and the death-censored graft survival after 2 years of follow-up. There was no association between clamping time and the incidence of acute rejection or 1-year graft function. When am and pm groups were compared for declamping time, we observed no association on any transplantation outcomes.
Regarding declamping time, our study confirms data previously published. Assuming that graft outcomes could worsen due to the limitation in human and material resources at night, others have compared graft outcomes and surgical complication rates depending on the time of transplantation. These studies reported similar global outcomes, whatever the time of the day (17–19), and even less pure technical graft failure at nighttime (19). Likewise, numerous studies have refuted the assumption of a “weekend effect” in the setting of kidney transplantation (20,21). Thus, changes in human and medical factors related to the time of the day, or the day of the week, are unlikely to affect graft outcome.
The association between clamping time and kidney graft outcome had never previously been analyzed. Our results are consistent with the growing evidence about the importance of circadian regulation in health and disease (22,23). Indeed, the circadian clock operates in every cell, and large transcriptome studies revealed that >80% of mRNA expression levels show circadian variations (24). Interestingly, in our cohort, only the donor time seems to be associated with kidney graft outcomes.
All well-known risk factors for DGF (male sex, obesity, prolonged cold ischemia time) (25) were associated with the risk of DGF in our cohort; although, notably, no association was observed with induction treatment, which has been however recently reported (26,27). These factors are associated with poorer tolerance to ischemia reperfusion. We can assume that the association that we observed is related to similar mechanisms. Circadian oscillation of the heart tolerance to ischemia-reperfusin has been clearly demonstrated (11). Regarding the kidney, while local kidney circadian-clock control of fluid and electrolyte balance and BP have been shown (28), the circadian rhythmicity of AKI is unclear (29), even if a body of evidence suggests this is the case. Indeed, in an experimental model, exposure to bright blue light (a powerful extrinsic regulator of the circadian clock) before ischemia reperfusion significantly reduced AKI (30) and, as already mentioned, a protective effect of carbon monoxide on kidney ischemia-reperfusion injury occurs through modulation of Per2 (8). Although circadian oscillations persist in isolated peripheral tissues incubated at physiologic temperature (16), this is unlikely to be the case in the graft during cold ischemia, as investigated in lung (31).
Although we did not observe an association between kidney clamping time and death-censored graft survival when we compared am and pm groups, when the 6–12 pm group (the group with the highest incidence of DGF) was used as the reference, a trend was observed with death-censored graft survival and a significant association was observed with patient and graft survival. When we considered the period after 2 years post-transplantation, we observed an association between the kidney clamping time, divided in am and pm group, and the graft survival after 2 years. Studies exploring outcomes of mate kidney transplants (from the same deceased donor) found that graft loss related to DGF occurs mainly during the first year (32). Similarly, assessment of progression of interstitial fibrosis, depending on DGF occurrence, concluded that DGF is an initial and time-limited process (33). Because the association we observed starts after 2 years post-transplantation, this suggests other causes, such as immune processes. Recent studies in mice (34,35) have, remarkably, demonstrated that leukocytes infiltrate tissues in a manner dependent on the time of day, mainly due to cell-autonomous circadian oscillatory expression of adhesion molecules on leukocytes on one side and by endothelial cells on the other side. Alloreactive T-cell priming by graft passenger leukocytes (36–39) and graft infiltration by recipient leukocytes (40,41) are the first steps in the alloimmune response. In view of the above, one may speculate that there is a high-risk window during the day where the donor organ contains more passenger leukocytes and the endothelial wall is more porous to recipient leukocytes. If this speculation is correct, it would have a profound effect on the strength and nature of the alloimmune response and, consequently, on long-term graft outcomes.
The main limitation of our work is that we do not have mechanistic evidence to support these assumptions. Indeed, we only have clinical data, and further work will be required to establish a causal relationship. Although our results are based on recipients from a large, multicenter cohort under recent immunosuppressive maintenance therapy (allowing an accurate comparison by considering the main classic confounding factors) and long-term follow-up (enabling us to model the time-dependent effect of clamping time), it is possible that other hidden confounding factors have not been considered. For instance, human factors, data concerning the resuscitation of the donor, and the organ procurement surgery (intraoperative complication, use of drugs) should be considered. Also, we did not consider the donor’s length of stay in intensive care. Some studies have shown a circadian arrhythmia in this environment (42), but these findings were mainly based on the dosage of melatonin and its urinary derivative, which reflects a disturbance of the central clock in the suprachiasmatic nuclei but does not necessarily imply a disturbance of the molecular clock at the level of the organs or cells (16). Evidence is even more scarce regarding how brain death could affect on this.
If confirmed, our results could change organ procurement practices. Indeed, a recent study analyzing the time from brain death to organ procurement found that longer procurement delay was associated with noninferior kidney allograft outcomes (43). Therefore, it would be conceivable to adapt the time of organ procurement to the most favorable time window.
Overall, our study identified an association between clamping time and DGF occurrence and long-term graft survival. Further investigation is required to confirm these findings and to establish a causal relationship.
Disclosures
D. Glotz reports receiving honoraria from AstraZeneca, CSL Behring, and Hansa; receiving research funding from CSL Behring; and serving as a scientific advisor or member of Hansa. C. Lefaucheur reports having consultancy agreements with CSL Behring and Hansa Biopharma. C. Legendre reports receiving honoraria from Alexion, Astellas, Novartis, and Sandoz; having consultancy agreements with CSL Behring and Hansa Medical; and serving on a speakers bureau for, and as a scientific advisor or member of, Hansa Medical. M. Le Quintrec reports receiving honoraria from Alexion, Novartis, and Sanofi. A. Sicard reports serving as a scientific advisor or member of Astellas and having consultancy agreements with Astellas and Novartis. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We wish to thank the following members of the clinical research assistant team: S. Le Floch, A. Petit, J. Posson, C. Scellier, V. Eschbach, K. Zurbonsen, C. Dagot, F. M’Raiagh, V. Godel, X. Longy, and P. Przednowed.
We acknowledge Roche Pharma, Novartis, Astellas, and Sanofi laboratories for supporting the DIVAT cohort and the CENTAURE Foundation (http://www.fondation-centaure.org).
S. Ville, M. Giral, and M. Lorent participated in research design; S. Ville, M. Giral, M. Lorent, and D. Jacobi wrote the article; S. Ville, M. Lorent, C. Legendre, E. Morelon, F. Buron, V. Garrigue, M. Ladrière, S. Girerd, L. Albano, A. Sicard, D. Glotz, and C. Lefaucheur performed the research; and S. Ville, M. Giral, M. Lorent, and C. Kerleau participated in data analysis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: Anna V. Reisæter, Pål-Dag Line, Anders Åsberg, Gilles Blancho, Julien Branchereau, Diego Cantarovich, Agnès Chapelet, Jacques Dantal, Clément Deltombe, Lucile Figueres, Claire Garandeau, Magali Giral, Caroline Gourraud-Vercel, Maryvonne Hourmant, Georges Karam, Clarisse Kerleau, Aurélie Meurette, Simon Ville, Christine Kandell, Anne Moreau, Karine Renaudin, Florent Delbos, Alexandre Walencik, Anne Devis, Christophe Masset, Delphine Kervella, Lucile Amrouche, Dany Anglicheau, Olivier Aubert, Lynda Bererhi, Christophe Legendre, Alexandre Loupy, Frank Martinez, Rébecca Sberro-Soussan, Anne Scemla, Julien Zuber, Arnaud Méjean, Marc Olivier Timsit, Pascal Eschwege, Luc Frimat, Sophie Girerd, Jacques Hubert, Marc Ladriere, Emmanuelle Laurain, Louis Leblanc, Pierre Lecoanet, Jean-Louis Lemelle, Lionel Badet, Maria Brunet, Fanny Buron, Rémi Cahen, Sameh Daoud, Coralie Fournie, Arnaud Grégoire, Alice Koenig, Charlène Lévi, Emmanuel Morelon, Claire Pouteil-Noble, Thomas Rimmelé, Olivier Thaunat, Sylvie Delmas, Moglie Le Quintrec, Vincent Pernin, Jean-Emmanuel Serre, Denis Glotz, Carmen Lefaucheur, Laetitia Albano, and Etienne Sicard
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03290321/-/DCSupplemental.
Supplemental Figure 1. Flow chart.
Supplemental Figure 2. Incidence (percentage) of delayed graft function according to the clamping (A) and declamping (B) time of the kidney transplant. Each cross corresponds to a 2-hour period.
Supplemental Table 1. Multivariable logistic model regarding the risk of delayed graft function (DGF) comparing am to pm declamping.
Supplemental Table 2. Multivariable linear model studying the 1-year kidney function after transplantation comparing am to pm clamping.
Supplemental Table 3. Multivariable linear model studying the 1-year kidney function after transplantation comparing am to pm declamping.
Supplemental Table 4. Multivariable Cox model studying the risk of death or graft failure comparing am to pm declamping.
Supplemental Table 5. Multivariable Cox model studying the risk of graft failure comparing am to pm declamping.
Supplemental Table 6. Multivariable Cox model studying the risk of acute rejection comparing am to pm clamping.
Supplemental Table 7. Multivariable Cox model studying the risk of acute rejection comparing am to pm declamping.
References
- 1.Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J: Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 11: 2093–2109, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Lamb KE, Lodhi S, Meier-Kriesche H-U: Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant 11: 450–462, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Terasaki PI, Cecka JM, Gjertson DW, Takemoto S: High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med 333: 333–336, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2012 Annual Data Report: Kidney. Am J Transplant 14: 11–44, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Moers C, Smits JM, Maathuis M-HJ, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJH, Squifflet J-P, van Heurn E, Kirste GR, Rahmel A, Leuvenink HGD, Paul A, Pirenne J, Ploeg RJ: Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 360: 7–19, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Debout A, Foucher Y, Trébern-Launay K, Legendre C, Kreis H, Mourad G, Garrigue V, Morelon E, Buron F, Rostaing L, Kamar N, Kessler M, Ladrière M, Poignas A, Blidi A, Soulillou J-P, Giral M, Dantan E: Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int 87: 343–349, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Ponticelli CE: The impact of cold ischemia time on renal transplant outcome. Kidney Int 87: 272–275, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Correa-Costa M, Gallo D, Csizmadia E, Gomperts E, Lieberum J-L, Hauser CJ, Ji X, Wang B, Câmara NOS, Robson SC, Otterbein LE: Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc Natl Acad Sci U S A 115: E2302–E2310, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK: Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 18: 774–782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow C-W, Dyck JRB, Young ME: Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock. Circ Res 106: 546–550, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme J-L, Lefebvre P, Staels B: Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: A single-centre propensity-matched cohort study and a randomised study. Lancet 391: 59–69, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Inouye ST, Kawamura H: Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A 76: 5962–5966, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panda S: Circadian physiology of metabolism. Science 354: 1008–1015, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Man K, Loudon A, Chawla A: Immunity around the clock. Science 354: 999–1003, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crislip GR, Masten SH, Gumz ML: Recent advances in understanding the circadian clock in renal physiology. Curr Opin Physiol 5: 38–44, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-K, Oh WJ, Yoo OJ, Menaker M, Takahashi JS: PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 101: 5339–5346, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fechner G, Pezold C, Hauser S, Gerhardt T, Müller SC: Kidney’s nightshift, kidney’s nightmare? Comparison of daylight and nighttime kidney transplantation: Impact on complications and graft survival. Transplant Proc 40: 1341–1344, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kienzl-Wagner K, Schneiderbauer S, Bösmüller C, Schneeberger S, Pratschke J, Ollinger R: Nighttime procedures are not associated with adverse outcomes in kidney transplantation. Transpl Int 26: 879–885, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Brunschot DMDÖ, Hoitsma AJ, van der Jagt MFP, d’Ancona FC, Donders RART, van Laarhoven CJHM, Hilbrands LB, Warlé MC: Nighttime kidney transplantation is associated with less pure technical graft failure. World J Urol 34: 955–961, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baid-Agrawal S, Martus P, Feldman H, Kramer H: Weekend versus weekday transplant surgery and outcomes after kidney transplantation in the USA: A retrospective national database analysis. BMJ Open 6: e010482, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson BM, Mytton JL, Evison F, Ferro CJ, Sharif A: Outcomes after weekend admission for deceased donor kidney transplantation: A population cohort study. Transplantation 101: 2244–2252, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Panda S: The arrival of circadian medicine. Nat Rev Endocrinol 15: 67–69, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Seifalian A, Hart A: Circadian rhythms: Will it revolutionise the management of diseases? J Lifestyle Med 9: 1–11, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, Ngotho M, Kariuki T, Dkhissi-Benyahya O, Cooper HM, Panda S: Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359: eaao0318, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schröppel B, Legendre C: Delayed kidney graft function: From mechanism to translation. Kidney Int 86: 251–258, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Masset C, Boucquemont J, Garandeau C, Buron F, Morelon E, Girerd S, Ladrière M, Mourad G, Garrigue V, Cassuto E, Albano L, Foucher Y, Dantal J; DIVAT Consortium : Induction therapy in elderly kidney transplant recipients with low immunological risk. Transplantation 104: 613–622, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Butler T, Hayde N: Impact of induction therapy on delayed graft function following kidney transplantation in mated kidneys. Transplant Proc 49: 1739–1742, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, Bonny O, Gachon F, Gomez RA, Sequeira-Lopez MLS, Firsov D: Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J Am Soc Nephrol 25: 1430–1439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ninni S, Seunes C, Ortmans S, Mouton S, Modine T, Koussa M, Jegou B, Edme J-L, Staels B, Montaigne D, Coisne A: Peri-operative acute kidney injury upon cardiac surgery time-of-day. Int J Cardiol 272: 54–59, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Yuan D, Collage RD, Huang H, Zhang X, Kautza BC, Lewis AJ, Zuckerbraun BS, Tsung A, Angus DC, Rosengart MR: Blue light reduces organ injury from ischemia and reperfusion. Proc Natl Acad Sci U S A 113: 5239–5244, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham PS, Maidstone R, Durrington HJ, Venkateswaran RV, Cypel M, Keshavjee S, Gibbs JE, Loudon AS, Chow C-W, Ray DW, Blaikley JF: Incidence of primary graft dysfunction after lung transplantation is altered by timing of allograft implantation. Thorax 74: 413–416, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill J, Dong J, Rose C, Gill JS: The risk of allograft failure and the survival benefit of kidney transplantation are complicated by delayed graft function. Kidney Int 89: 1331–1336, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Heilman RL, Smith ML, Smith BH, Qaqish I, Khamash H, Singer AL, Kaplan B, Reddy KS: Progression of interstitial fibrosis during the first year after deceased donor kidney transplantation among patients with and without delayed graft function. Clin J Am Soc Nephrol 11: 2225–2232, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, Uhl O, Yao L, Sander LE, Chen C-S, Kraus K, de Juan A, Hergenhan SM, Ehlers M, Koletzko B, Haas R, Solbach W, Oster H, Scheiermann C: Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 46: 120–132, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He W, Holtkamp S, Hergenhan SM, Kraus K, de Juan A, Weber J, Bradfield P, Grenier JMP, Pelletier J, Druzd D, Chen C-S, Ince LM, Bierschenk S, Pick R, Sperandio M, Aurrand-Lions M, Scheiermann C: Circadian expression of migratory factors establishes lineage-specific signatures that guide the homing of leukocyte subsets to tissues. Immunity 49: 1175–1190.e7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, Anderson D, Cowan S, Price K, Naemura J, Emswiler J, Greene J, Turk LA, Bajorath J, Townsend R, Hagerty D, Linsley PS, Peach RJ: Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant 5: 443–453, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Lechler RI, Batchelor JR: Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med 155: 31–41, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper IG, Ali JM, Harper SJF, Wlodek E, Alsughayyir J, Negus MC, Qureshi MS, Motalleb-Zadeh R, Saeb-Parsy K, Bolton EM, Bradley JA, Clatworthy MR, Conlon TM, Pettigrew GJ: Augmentation of recipient adaptive alloimmunity by donor passenger lymphocytes within the transplant. Cell Rep 15: 1214–1227, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan MLG, Gibson GA, Watkins SC, Larregina AT, Morelli AE: Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest 126: 2805–2820, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR: Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: An alternative mechanism of allorecognition. Nat Med 8: 233–239, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Zhuang Q, Liu Q, Divito SJ, Zeng Q, Yatim KM, Hughes AD, Rojas-Canales DM, Nakao A, Shufesky WJ, Williams AL, Humar R, Hoffman RA, Shlomchik WD, Oberbarnscheidt MH, Lakkis FG, Morelli AE: Graft-infiltrating host dendritic cells play a key role in organ transplant rejection. Nat Commun 7: 12623, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oldham MA, Lee HB, Desan PH: Circadian rhythm disruption in the critically ill: An opportunity for improving outcomes. Crit Care Med 44: 207–217, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Eerola V, Helanterä I, But A, Lempinen M, Mäkisalo H, Nordin A, Isoniemi H, Sallinen V: The association of time to organ procurement on short- and long-term outcomes in kidney transplantation. Clin J Am Soc Nephrol 16: 427–436, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.