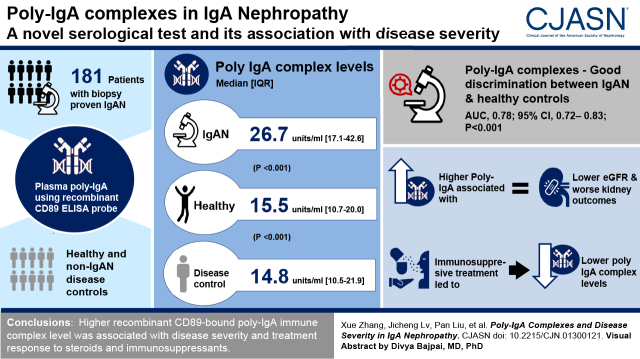
Visual Abstract
Keywords: IgA nephropathy, glomerulonephritis, immune complexes, Fc receptors
Abstract
Background and objectives
Poly-IgA immune complex formation and glomerular deposition play a key role in IgA nephropathy. Our study sought to develop a new methodology for one-step serologic detection of poly-IgA levels.
Design, setting, participants, & measurements
A novel ELISA method using recombinant CD89 as a “capturing” probe was established for detecting poly-IgA immune complex in plasma. We applied semiquantitative measurements of these poly-IgA indices in patients recruited at Peking University First Hospital who had IgA nephropathy or other kidney disease types, as compared with healthy controls. The longitudinal trend of the poly-IgA index and the association with pathologic parameters and treatment responses were evaluated. Finally, we analyzed the molecular composition of poly-IgA complexes in patients by mass spectrometry.
Results
Recombinant CD89–mounted ELISA plates specifically captured plasma poly-IgA. The levels of poly-IgA immune complex (26.7 [interquartile range (IQR) 17.1–42.6] U/ml) in IgA nephropathy were significantly higher than those in healthy controls (15.5 [IQR 10.7–20.0] U/ml; P<0.001) or in controls with non-IgA nephropathy disease (14.8 [IQR 10.5–21.9] U/ml; P<0.001). Higher levels of poly-IgA immune complex were associated with lower eGFR and worse kidney outcome. Accuracy parameters and concordant statistics showed good discrimination between IgA nephropathy and healthy controls based on poly-IgA index levels (area under the curve [AUC], 0.78; 95% confidence interval [95% CI], 0.72 to 0.83; P<0.001), significantly outperforming galactose-deficient IgA1 levels (AUC, 0.70; P=0.05). Corticosteroid and immunosuppressant treatments lowered poly-IgA indices. After a recombinant CD89–directed workflow in conjunction with mass spectrometry, we also analyzed the molecular composition of IgA immune complex in patients with IgA nephropathy.
Conclusions
Higher level of recombinant CD89–bound poly-IgA immune complex was associated with the severity of the disease and with treatment response to steroids and immunosuppressants.
Introduction
IgA nephropathy is the most common form of primary GN worldwide. The disease typically follows a slow, but relentless, clinical course that could progress to kidney failure in 30%–40% of patients within 20–30 years (1). IgA nephropathy is characterized by glomerular deposits of IgA immune complexes comprised of IgA1, complement C3, and variable amounts of IgG and/or IgM (2,3). Local immune reactivity to IgA immune complexes stimulates proliferation of mesangial cells, synthesis of extracellular matrix, and infiltration of inflammatory cells in the glomerulus (1,4). The observations of recurrent IgA deposition in kidney grafts in transplant recipients with IgA nephropathy strongly suggest that an extrarenal origin of IgA immune complexes is pathogenic (5,6). Further evidence of circulating immune complexes in individual patients with IgA nephropathy who share the same Ig classes with IgA extracted from the kidney also suggests a plasma origin of IgA in kidney deposits (7).
Mounting evidence shows that circulatory poly-IgA–containing immune complexes are pathogenic (8–17), which is further supported by the observation of patients with IgA nephropathy having higher plasma levels of poly-IgA complexes than healthy controls (8,13,17). Among individual patients with IgA nephropathy, plasma levels of poly-IgA complexes correlate with the phases of clinical activity (9), and with the degree of hematuria and proteinuria (16). It was further demonstrated that high poly-IgA complexes levels are, in part, attributable to overproduction of IgA (18), and that poly-IgA complexes purified from plasma of patients promote proliferation of cultured human mesangial cells (12,15,19), indicating a causal role of circulating poly-IgA complexes in pathogenesis.
In vivo, circulating IgA immune complexes are catabolized primarily by the mononuclear phagocyte system through the IgA Fcα receptor I (FcαRI/CD89) (2). CD89 is a type I transmembrane glycoprotein expressed on the surface of myeloid cells, including monocytes/macrophages, dendritic cells, Kupffer cells, neutrophils, and eosinophils (20–22). This IgA-specific receptor binds both IgA1 and IgA2 molecules through its amino terminus Ig domain that interacts with the Cα2/Cα3 junction of IgA (23–26). In the context of IgA nephropathy, prior studies focused on the role of soluble CD89 in IgA immune complexes and whether soluble CD89 levels in blood were associated with IgA nephropathy. It was suggested that, upon binding to receptor CD89, polymeric IgA complexes induce proteolytic shedding of soluble CD89, forming new circulatory soluble CD89-IgA complexes (27–29). However, it remains inconclusive as to whether such soluble CD89-IgA complexes are specific or relevant to the development of IgA nephropathy (30–32). Herein, we exploited the canonic function of CD89 as the monocytic receptor responsible for clearing IgA-pathogen or poly-IgA complexes from circulation. Through a special avidity effect analogous to IgG hexamer binding complement C1q (33), CD89 exhibits higher affinity to poly-IgA than to mono-IgA, allowing phagocytes to selectively capture poly-IgA complexes. We devised a recombinant CD89 affinity probe as an ex vivo tool to measure poly-IgA levels in patients’ plasma.
Materials and Methods
See the Supplemental Appendix for the full methods.
Human Subjects
In this study, all patients’ complete clinical data—including age, sex, systolic/diastolic BP, baseline serum creatinine, eGFR, and 24-hour urine protein excretion—were collected from the medical records at the time of kidney biopsy. All kidney biopsy specimens were reviewed and graded by an independent pathologist who was blind to the participants’ clinical data. The Oxford classification (including crescent scores) was used for the evaluation of pathologic lesions. At the same time, 143 patients with other kidney diseases were enrolled as disease controls, including 24 patients with lupus nephritis, 52 with membranous nephropathy, 15 with FSGS, 11 with minimal change disease, six with ANCA-associated GN, 23 with diabetic nephropathy, and 12 with tubulointerstitial nephritis. All of the patients serving as disease controls were diagnosed by review of their kidney biopsy specimens. In addition, 85 age-, sex-, and geographically matched healthy individuals were enrolled as healthy controls. All participants provided written informed consent before inclusion in the study. The research was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committees.
Measuring Recombinant CD89–Captured Poly-IgA Complexes in Plasma by ELISA
High-binding MaxiSorp 96-well plates (Nalge-Nunc, Rochester, NY) were coated overnight with 10 μg/ml recombinant CD89 protein in carbonate sodium buffer (pH 9.6) at 4°C. Duplicates of two-fold dilutions of plasma samples (1:1000 in blocking buffer) were added to the wells and incubated for 3 hours at 37°C. Mouse anti-human IgA mAb (Abcam, Cambridge, United Kingdom) was used as the detection antibody. Plates were then developed with the 3,3′,5,5′-tetramethylbenzidine liquid substrate system and reactions were stopped with 1 M sulfuric acid. The results for total CD89- captured poly-IgA complexes were expressed as units per milliliter.
Statistical Analysis
Quantitative variables were expressed as the mean and SD for normally distributed variables, or the median and interquartile range (IQR) for non-normally distributed variables. Categoric data were summarized as percentages and absolute ratios. Differences in means for continuous variables between two groups and among multiple groups were compared using the t test. Non-normally distributed data were compared by using the nonparametric Mann–Whitney U test. P<0.05 (two-sided) was considered statistically significant. Kaplan–Meier survival analysis was performed on the basis of predefined end point parameters. The differences between curves were analyzed using a log-rank test. All statistical tests were performed using SPSS version 22.0 (Chicago, IL). The illustrations were generated using GraphPad Prism version 9.0 for Windows (GraphPad Software, San Diego, CA).
Results
Construction and Validation of Recombinant CD89 Probe for Detecting Poly-IgA Immune Complexes from Plasma Samples
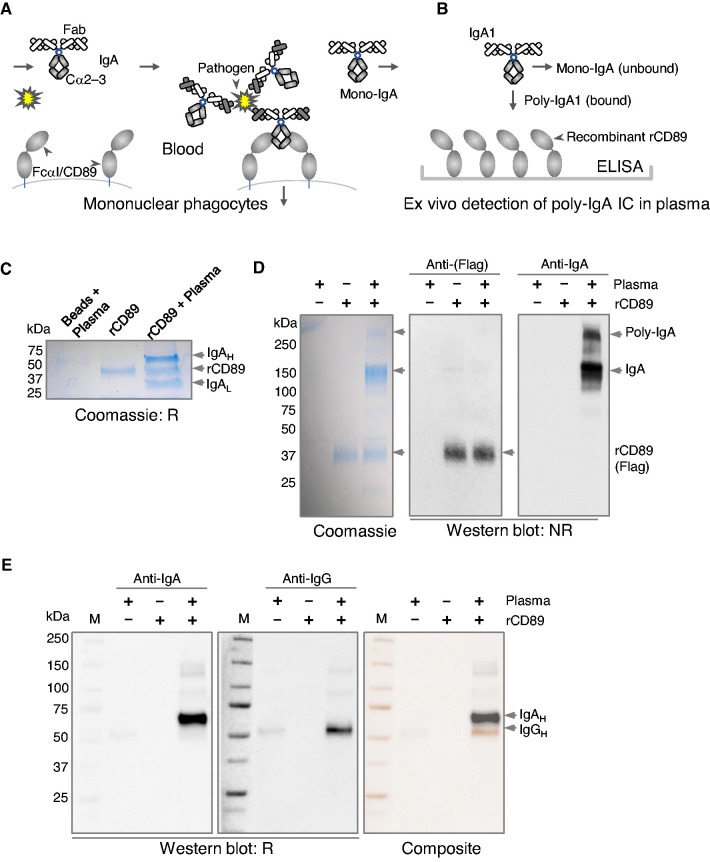
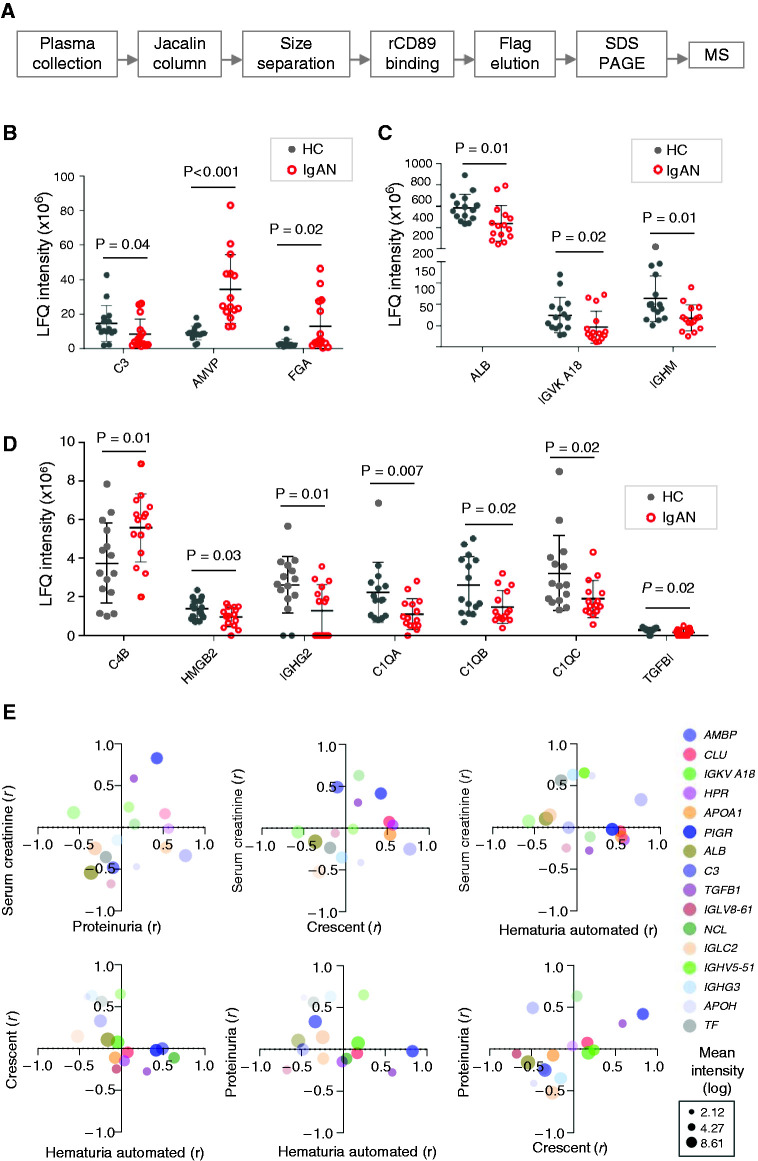
The overall design involved the construction of an ex vivo CD89 affinity trap for isolating poly-IgA complexes from plasma (schematics in Figure 1, A and B). Recombinant CD89 was derived from human FcαRI/CD89 extracellular segment fused to a Flag tag (Figure 1C). When mixed with human plasma, followed by immunoprecipitation using anti-Flag agarose beads, recombinant CD89 was observed to coprecipitate with IgA in an approximately 1:1 molar ratio (Figure 1C). Under nonreducing conditions, the monomeric form of IgA, with two heavy and two light chains attached, appeared as an approximately 180-kD band. Interestingly, a higher molecular mass IgA band of approximately 600 kD was also visible (Figure 1D), suggesting some poly-IgA species were formed. There was concurrent IgG content coimmunoprecipitated with recombinant CD89 (Figure 1E), likely through binding to IgA. Having confirmed the binding of the recombinant CD89 probe to plasma IgA, we next examined plasma samples using recombinant CD89–directed immunoprecipitation. We were able to detect that IgG, IgM, and C3c coprecipitated with IgA (Supplemental Figure 1A). However, we were unable to see the difference between IgA nephropathy and healthy groups in terms of IgA galactose deficiency associated with its high-order complexes (Supplemental Figure 1, B and C).
Figure 1.
Recombinant CD89 binds plasma IgA. (A) A schematic illustration of canonic CD89’s function as the IgA Fc receptor on mononuclear phagocytes. Fc of IgA1 and IgA2 comprises Cα2 and Cα3 domains. After antibody-antigen binding between IgA and invading pathogens, the clustered presence of IgA Fc is specifically recognized by the Ig1 domain of CD89, leading to opsonization of the immune complex. Normal monomeric forms of IgA do not bind CD89. (B) By coupling recombinant CD89 (rCD89) to ELISA plates, the probe specifically captures poly-IgA in plasma samples. (C) Recombinant CD89 with Flag tag is linked to agarose beads conjugated with anti-Flag antibody. Plasma samples were incubated with either empty or recombinant CD89–coupled beads. SDS-PAGE showed recombinant CD89 captured IgA, as expected. IgA heavy (IgAH) and light (IgAL) were separated under reducing conditions (R). (D) Under nonreducing condition (NR), IgA formed two major bands on the gel, the approximately 180 kD mono-IgA and the approximately 600 kD poly-IgA. (E) Western blotting also detected the presence of IgG in recombinant CD89–precipitated IgA complex (IgAH and IgGH had distinct molecular masses of 65 versus 55 kD, as shown in composite image).
Recombinant CD89-Coated ELISA Selectively Detected Poly-IgA Complexes in Plasma Samples
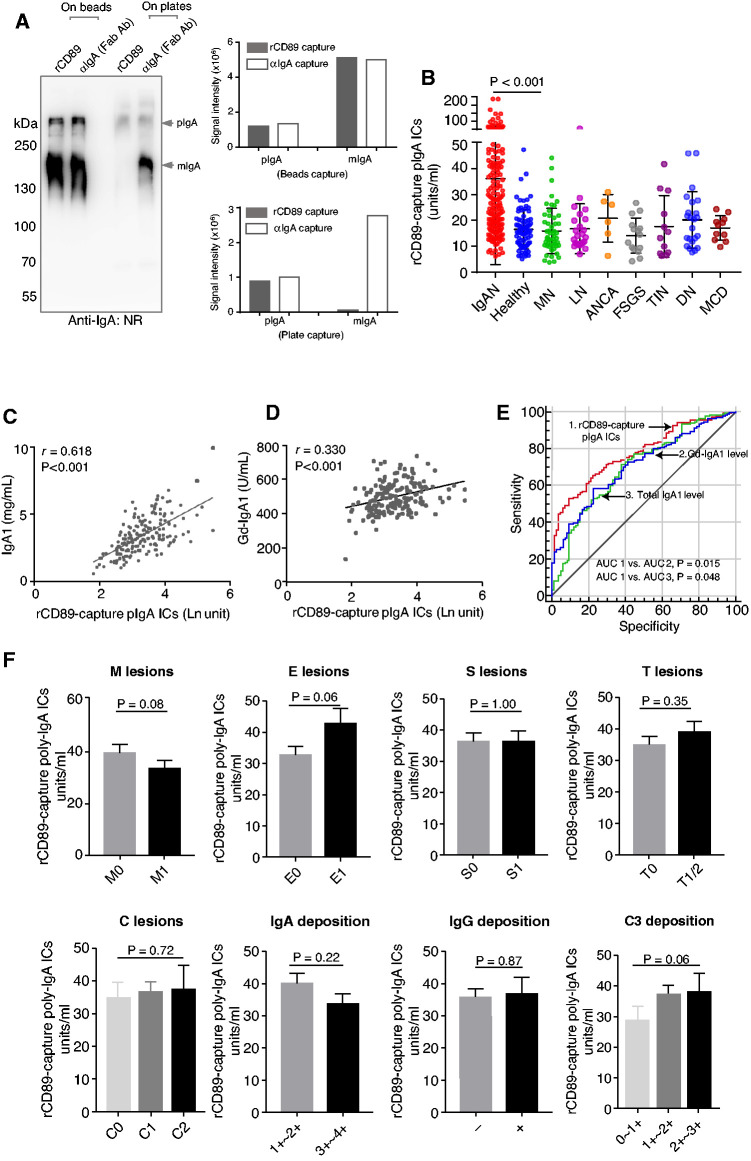
To increase throughput, we devised an ELISA-based assay for measuring poly-IgA levels in plasma samples of patients. The control wells coated with anti–IgA-Fab collected predominantly IgA monomers running at approximately 180 kD. Albeit at much lower intensity, the high molecular mass (approximately 600 kD) bands corresponding to the poly-IgA complex were visible in both recombinant CD89 and anti–IgA-Fab pull-down lanes. Strikingly, the recombinant CD89 lane completely lacked the mono-IgA band that occurred prominently in the anti–IgA-Fab control lane (Figure 2A). This result indicated the specificity of recombinant CD89 in capturing poly-IgA in plasma samples, as intended.
Figure 2.
Recombinant CD89 specifically purified poly-IgA immune complexes from plasma samples, showing elevated poly-IgA levels in IgA nephropathy. (A) Two recombinant CD89 (rCD89) immobilization methods, on beads versus on ELISA plates, were compared. A control anti-IgA antibody (Fab fragment) was also used for comparing the specificity of poly-IgA versus total IgA binding. Whereas recombinant CD89 on beads precipitated both poly-IgA (pIgA) and mono-IgA (mIgA), recombinant CD89 coated to ELISA plates precipitated only poly-IgA, with no presence of mono-IgA. The two bar graphs summarized the quantitative values of the gel results, showing the contrasting difference in the specificity of between bead- versus plate-captured poly-IgA with or without mono-IgA contamination, respectively. (B) The distributions of poly-IgA indices within IgA nephropathy and healthy and non-IgA nephropathy glomerular disease control groups. The median poly-IgA index for IgA nephropathy was 26.7 (IQR, 17.1–42.6) U/ml, as compared with 15.5 (IQR, 10.7–20.0) U/ml for healthy controls (P<0.001; the average index and SD values are marked). The combined poly-IgA index for all non-IgA nephropathy forms of GN, including membranous nephropathy (MN), lupus nephritis (LN), ANCA vasculitis, FSGS, tubulointerstitial nephritis (TIN), diabetic nephropathy (DN), and minimal change disease (MCD) was 14.8 (IQR, 10.5–21.9) U/ml. (C and D) Because levels of galactose-deficient IgA1 (Gd-IgA1) and total IgA1 of the IgA nephropathy cohort were also measured, positive correlations between Gd-IgA1 and poly-IgA (measured with recombinant CD89), and between total IgA1 and poly-IgA, were established (r=0.33 [P<0.001] and r=0.62 [P<0.001], respectively). (E) Receiver operating curves of poly-IgA, total IgA1, and Gd-IgA1 levels in patients with IgA nephropathy. (F) Poly-IgA indices were compared between low and high Oxford score groups of M, E, S, T, and C lesions, showing the general trend of higher poly-IgA indices associated with more severe E, T, and C lesions; however, the differences did not reach the level of statistical significance. Poly-IgA indices were compared between groups with low and high levels of IgA, IgG, and C3 deposition in the glomerulus. −, no deposition; +, with deposition; Ab, antibody; AUC, area under the curve; ICs, immune complexes; IQR, interquartile range; NR, nonreducing.
Measuring the Levels of Poly-IgA Immune Complexes in Patients Versus Healthy Controls
Next, we subjected a larger set of IgA nephropathy plasma samples, alongside non-IgA nephropathy controls, to the detection method. Here, we considered the detection of recombinant CD89–captured poly-IgA contents to be an index of the poly-IgA complex. We found levels of plasma poly-IgA indices (26.7 U/ml; IQR, 17.1–42.6 U/ml) were significantly higher among IgA nephropathy samples than those among healthy control samples (15.5 U/ml; IQR, 10.7–20.0 U/ml; P<0.001) (Figure 2B). The non-IgA nephropathy disease groups all had significantly lower poly-IgA indices than the IgA nephropathy group (combined average, 14.8 U/ml; IQR, 10.5–21.9 U/ml; P<0.001; Figure 2B). These results suggested that IgA nephropathy results in elevated poly-IgA levels in circulation, as expected, and that the new recombinant CD89–directed methodology can detect poly-IgA in plasma samples.
In parallel, we measured total IgA1 and galactose-deficient IgA1 levels in these samples. There were positive correlations between poly-IgA indices and plasma IgA1 levels (r=0.62; P<0.001) and, to a lesser degree, between poly-IgA indices and galactose-deficient IgA1 levels (r=0.33; P<0.001) (Figure 2, C and D). Nevertheless, there was insufficient evidence from the data to suggest causal relationships among CD89-captured poly-IgA complexes, galactose-deficient IgA1, and total IgA1.
To test the performance of this poly-IgA index as a new diagnostic biomarker, we applied a receiver operating curve that reflects the discrimination between patients and healthy controls, and this was compared with traditional indicators, such as total plasma IgA1 and galactose-deficient IgA1. Accuracy parameters and concordant statistics showed good discrimination between patients with IgA nephropathy and healthy controls for calculated poly-IgA indices (area under the curve [AUC], 0.78; 95% confidence interval [95% CI], 0.72 to 0.83; P<0.001), significantly better than total IgA1 levels (AUC, 0.71; 95% CI, 0.65 to 0.77; P=0.02) and galactose-deficient IgA1 levels (AUC, 0.70; 95% CI, 0.64 to 0.77; P=0.05) (Figure 2E).
Correlation between Poly-IgA Indices and Clinicopathologic Parameters in IgA Nephropathy
The baseline clinical and pathologic characteristics of 181 patients at the time of kidney biopsy are described in Table 1. We divided the cohort into low- and high-level groups on the basis of a cutoff value of poly-IgA index at the top 10% of the healthy controls. Compared with the patients with lower poly-IgA indices, the high-index group included patients with an older age, higher BP, lower eGFR, higher levels of proteinuria, and higher Oxford scores in E lesions and T lesions (Figure 2F and Table 1). We did not find the poly-IgA index to be associated with the intensity of IgA and IgG depositions in the glomerulus. Meanwhile, there was a weak correlation between plasma poly-IgA index and C3 deposition in the kidney (P=0.06). These results suggested that high levels of CD89-captured IgA immune complexes may affect the progression of IgA nephropathy, although larger future studies are needed to evaluate these findings.
Table 1.
The baseline clinical and pathologic characteristics of patients with IgA nephropathy stratified according to CD89-binding poly-IgA complex level
| Characteristics | Total | Low Level (<24.89 U/ml) | High Level (≥24.89 U/ml) | P Value |
|---|---|---|---|---|
| Patients, n | 181 | 85 | 96 | — |
| Sex, male, n (%) | 82 (45) | 42 (49) | 40 (42) | 0.30 |
| Age, yr, mean±SD | 37±12 | 35±11 | 39±13 | 0.03a |
| Mean arterial pressure, mm Hg, mean±SD | 93±13 | 92±14 | 95±12 | 0.08 |
| eGFR, ml/min per 1.73 m2, mean±SD | 76±33 | 82±34 | 71±32 | 0.02a |
| Proteinuria, g/d, median (IQR) | 1.10 (0.48–2.4) | 1.00 (0.45–1.92) | 1.26 (0.60–2.5) | 0.13 |
| Plasma IgA1, mg/ml, mean±SD | 3.44±1.41 | 2.69±1.08 | 4.10±1.34 | <0.001a |
| Plasma Gd-IgA1, U/ml, mean±SD | 497.4±89.00 | 473.72±91.66 | 518.37±81.45 | 0.001a |
| Oxford classification, n (%) | ||||
| M1 | 88 (49) | 45 (53) | 43 (45) | 0.27 |
| E1 | 61 (34) | 22 (26) | 39 (41) | 0.04a |
| S1 | 104 (58) | 50 (59) | 54 (56) | 0.73 |
| T1/2 | 69 (38) | 22 (26) | 47 (49) | 0.002a |
| C1/2 | 126 (70) | 57 (67) | 69 (72) | 0.52 |
IQR, interquartile range; M1, mesangial hypercellularity; E1, the presence of endocapillary proliferation; S1, segmental glomerulosclerosis/adhesion; T1/2, severity of tubular atrophy/interstitial fibrosis; C1/2, presence of crescent.
P<0.05.
Independent Cohort Validation and Disease Progression Associated with Poly-IgA Index
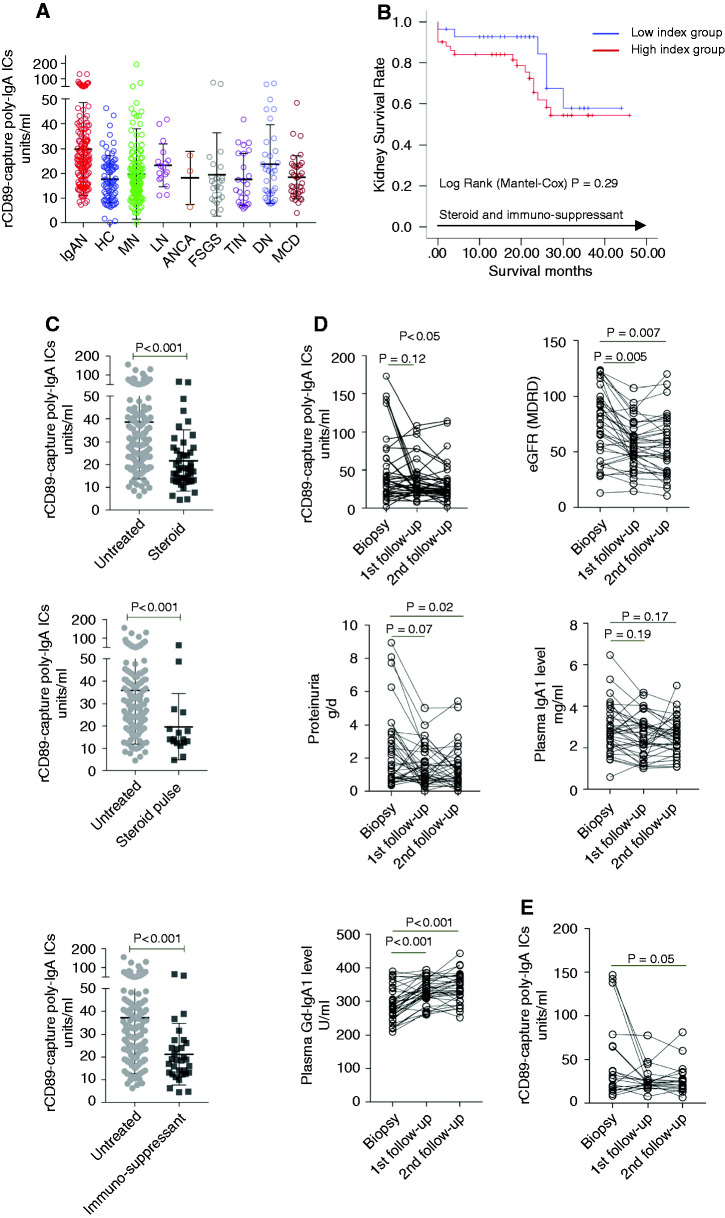
Next, we sought to further validate the results of the poly-IgA index using a different cohort, in which all patients were recruited prospectively, with their plasma samples collected at the time of kidney biopsy. To eliminate possible sample bias, in a 2-month period, we enrolled every patient who was waiting for kidney biopsy ahead of formal diagnoses of their kidney diseases. With the exceptions of a few dropouts due to low count of glomeruli, existence of comorbidities, or unavailability of reports on Oxford scores, a total of 428 patients were in this new cohort. These patients included 145 individuals who were subsequently diagnosed with IgA nephropathy, alongside a variety of other kidney disease types revealed by review of their biopsy specimens. We found poly-IgA indices in patients with IgA nephropathy were significantly higher than the levels seen in other glomerular or tubulointerstitial diseases (Figure 3A). These findings were consistent with those concluded from our original cohort of a selected variety of glomerular disease types (Figure 2B). To assess the prognostic value of the poly-IgA index, we performed an additional assay on a selected cohort of 79 patients with IgA nephropathy, all with high grades of pathologic parameters (crescents in >25% glomeruli) and who were undergoing either corticosteroid or immunosuppressant treatments. A total of 23 participants (29%) reached the end point, which was defined as kidney failure after a median follow-up of 21 months. We divided these patients into high- versus low-index groups on the basis of poly-IgA levels. We found that the high-index group had a faster disease progression (Figure 3B). However, the difference did not reach the level of statistical significance (P=0.29), possibly due to confounding of treatments that might have also lowered poly-IgA levels.
Figure 3.
New prospective cohort confirmed higher poly-IgA indices associated with IgA nephropathy, and high poly-IgA indices were associated with worse kidney outcome among patients undergoing steroid or immunosuppressant treatments. (A) A total of 428 patients were recruited during a 2-month period at Peking University First Hospital, and plasma samples were collected at the time of biopsy. Of these patients, 145 were subsequently diagnosed with IgA nephropathy (IgAN). Their poly-IgA indices were compared with the remaining patients with non-IgA nephropathy kidney disease. The IgA nephropathy group showed significantly higher levels of poly-IgA indices (median value, 26.3 U/ml) than any other kidney disease groups (combined median value, 16.4 U/ml; P<0.001), including MN, LN, ANCA nephritis, FSGS, TIN, DN, and MCD. (B) Kaplan–Meier analysis was performed to a new cohort of 79 patients with crescentic IgA nephropathy undergoing either steroid or immunosuppressant treatments (after biopsy diagnosis with a median follow-up time of 21 months). The high poly-IgA index group suffered from a higher kidney failure rate, although the difference did not reach statistical significance (P=0.29). (C) For assessing poly-IgA indices in association with treatments, we enrolled 174 patients whose biopsy specimens identified crescentic lesions at the time of plasma collection. Among these patients, 45, 17, and 32 individuals had been treated with corticosteroids, pulse corticosteroids, or immunosuppressants, respectively, and the 80 remaining patients were not treated. There were significant differences in the poly-IgA indices between untreated versus treatment groups (33.5 versus 17.9 U/ml between untreated and steroid groups, 29.8 versus 14.1 U/ml between untreated and pulse steroid groups, and 32.17 versus 18.59 U/ml between untreated and immunosuppressant groups; P<0.001, for all). (D) In addition, we studied 37 longitudinal samples from patients undergoing steroid or immunosuppressant treatments. Samples were collected at the time of biopsy, and during two subsequent follow-up visits with at least 3 months between the two visits. There was a significant reduction of poly-IgA index levels, together with lower levels of proteinuria (P=0.05 and P=0.02, respectively). In contrast, patients’ galactose-deficient IgA1 (Gd-IgA1) levels followed an upward trend. (E) Within this cohort, there were 19 patients who only started treatment after biopsy when first plasma samples were collected. Their poly-IgA indices were significantly reduced after treatment (P=0.05), particularly among those who had the highest levels of poly-IgA indices (approximately 150 U/ml) before treatments. ICs, immune complexes; rCD89, recombinant CD89.
Corticosteroid and Immunosuppressant Treatments Lowered Poly-IgA Levels in Blood
Next, we sought to examine whether steroids and immunosuppressants can lower poly-IgA levels. We enrolled 174 patients, all with pathologic crescentic lesions at the time of biopsy when plasma samples were collected. Among them, 45 patients had been treated with corticosteroids, an additional 17 patients were treated with pulse corticosteroid, and 32 patients were treated with immunosuppressants before the kidney biopsy. The remaining 80 patients were not treated before biopsy. We found that the levels of circulating poly-IgA complexes in patients who were treated with corticosteroid or immunosuppressants before biopsy were much lower than those who had not received these treatments (untreated versus steroid: 33.5 [IQR, 21.1–47.9] versus 17.9 [IQR, 12.9–26.8] U/ml, P<0.001; untreated versus pulse steroid: 29.8 [IQR, 18.9–43.8] versus 14.1 [IQR, 12.5–22.5] U/ml, P<0.001; untreated versus immunosuppressive drug: 32.2 [IQR, 19.0–45.0] versus 18.6 [IQR, 12.7–25.7] U/ml, P<0.001; Figure 3C). To further investigate whether treatments lead to reduced poly-IgA levels, we examined serial plasma samples covering three time points. A total of 37 patients were in this cohort, with their first time point being at the time of biopsy; this cohort included 19 patients who were not initially treated with steroids or immunosuppressants but were subsequently treated after their diagnoses. The remaining patients were treated before and after biopsy throughout the course of plasma collection. Overall, there was a significant reduction of poly-IgA levels during the course of treatments, accompanied with reduced proteinuria (Figure 3D). In contrast, total plasma IgA levels were not significantly changed during treatment; and galactose-deficient IgA1 levels even increased despite treatment with steroids or immunosuppressants. Interestingly, among the 19 patients who were not initially treated at the time of biopsy, a significant drop in their poly-IgA levels was observed (P=0.05; Figure 3E), particularly among those (three patients) with the highest initial poly-IgA levels. These new results suggest poly-IgA levels may have the potential to be useful as indicators of treatment response to steroids and immunosuppressants.
Proteomic Analysis of Recombinant CD89-Captured Poly-IgA Complexes in Patients and in Healthy Controls
It has been suggested that the molecular composition of IgA immune complexes could affect disease progression (1). Traditional methods for isolating poly-IgA relied on a two-step purification scheme (34,35), first with lectin Jacalin to extract total IgA, and then by size-exclusion chromatography to separate the high molecular mass poly-IgA fraction. However, the method has a number of caveats. Jacalin displays differential affinities to IgA’s galactose–N-acetylgalactosamine moieties, and there is a wide disparity among those with IgA nephropathy and healthy individuals with respect to the Jacalin-binding capacity of their serum IgA (36,37). In addition, Jacalin also binds non-IgA proteins, such as mucins, IgG3, IgD, and possibly Fab (38–40), making it challenging to distinguish proteins copurified directly from Jacalin versus indirectly via IgA. To circumvent these problems, we added an additional step to the workflow using an recombinant CD89 affinity trap after Jacalin and size-exclusion chromatography procedures to purify natural IgA complexes from plasma (Figure 4A). Nine male and six female patients with a range of disease severity were included (Table 2). We included another 15 samples from healthy individuals. Purified IgA complexes were subjected to liquid chromatography–tandem mass spectrometry to identify associated proteins. A combined total of 164 proteins were identified (Supplemental Tables 1–5). On the basis of label-free quantification values of individual proteins from patients with IgA nephropathy and healthy controls, the protein that distinguished the two groups the best was α1-microglobulin/bikunin (AMBP) (Figure 4B). AMBP was detected in complexes with IgA at approximately three- to four-fold higher levels in the IgA nephropathy group than in controls (label-free quantification intensity of 34.5±19.9 versus 9.1±40.0 for those with IgA nephropathy versus controls, respectively; P<0.001). To a lesser degree, complement C4b was detected in all samples, with generally higher levels in IgA nephropathy group than in controls (5.6±1.8 versus 3.7±2.1, respectively; P=0.01; Figure 4D). The only other protein that had statistically higher levels in the IgA nephropathy group was the fibrinogen α-chain (4.5 [IQR, 2.8–27.5] versus 2.3 [IQR 1.9–2.6], respectively; P=0.02; Figure 4B). In contrast, IgA-associated IgM and IgG2 was lower in the IgA nephropathy group as compared with controls (IgM: 64.9 [IQR, 43.5–91.1] versus 98.9 [IQR, 67.6–170.4], respectively; P=0.01; IgG2: 1.8 [IQR, 0–2.5] versus 2.7 [IQR, 1.9–3.6], respectively; P=0.01; Figure 4, C and D). Intriguingly, C1qα-, β- and γ-chains showed lower levels in the IgA nephropathy group (P=0.02; Figure 4D). IgA-bound C3 also showed lower levels in IgA nephropathy group (3.9 [IQR, 2.3–10.9] versus 11.5 [IQR, 9.7–18.2] for IgA nephropathy versus controls; P=0.04; Figure 4B). There were no statistical differences in IgA-associated IgG1/IgG3/IgG4, J-chain (IGJ), or secretory component (PIGR) levels between IgA nephropathy and control groups (Table 3).
Figure 4.
Proteomics identified IgA-associated proteins with their levels correlated with clinical parameters of IgA nephropathy. (A) Novel workflow with the incorporation of recombinant CD89–directed sample “clean-up” step for poly-IgA purification: recombinant CD89 (rCD89) binding, elution using Flag peptides, followed by SDS-PAGE separation of proteins and mass spectrometry (MS). (B–D) Constituent proteins of poly-IgA complexes with significantly different (P<0.05) levels between healthy control (HC) and IgA nephropathy (IgAN) groups. (E) Pair-wise comparisons of four IgA nephropathy parameters of disease severity (proteinuria, serum creatinine, glomerular crescent, and hematuria) in terms of correlation coefficients (r) of individual poly-IgA–associated proteins (color coded dots, see legend on the right of the panel). Average protein abundance is represented by the size of the dots. AMBP, α1-microglobulin/bikunin; CLU, clusterin; IGKV, immunoglobulin kappa variable cluster; HPR, haptoglobin-related protein; APOA1, apolipoprotein A-I; PIGR, polymeric immunoglobulin receptor; ALB, albumin; C3, complement 3; TGFB1, transforming growth factor beta-1; IGLV, immunoglobulin lambda variable cluster; NCL, ucleolin; IGLC, immunoglobulin lambda; IGHV, IgG heavy chain; IGHG, IgG heavy chain; APOH, apolipoprotein H; TF, transferrin; LFQ, label-free quantification.
Table 2.
The baseline clinical characteristics of patients with IgA nephropathy for mass spectrometry
| Patient | Age/Sex | Serum Creatinine (mg/dl) | eGFR (ml/min per 1.73 m2) | Initial Proteinuria (g/d) | Crescent (%) | Plasma IgA1 (mg/ml) | Plasma CD89-Binding Poly-IgA Complex (U/ml) |
|---|---|---|---|---|---|---|---|
| 1 | 14/M | 2.93 | 31 | 4.27 | 19 | 4.31 | 139.08 |
| 2 | 59/F | 0.77 | 82 | 4.23 | 17 | 2.96 | 26.09 |
| 3 | 38/F | 0.88 | 76 | 1.38 | 9 | 5.42 | 148.46 |
| 4 | 29/M | 4.15 | 18 | 4.65 | 67 | 4.04 | 69.25 |
| 5 | 41/M | 1.12 | 77 | 3.44 | 33 | 2.04 | 11.42 |
| 6 | 32/M | 1.10 | 82 | 2.74 | 3 | 4.27 | 28.86 |
| 7 | 53/M | 1.41 | 56 | 0.26 | 46 | 2.18 | 24.95 |
| 8 | 45/F | 4.11 | 12 | 8.99 | 72 | 6.40 | 33.71 |
| 9 | 25/F | 0.66 | 117 | 0.85 | 6 | 4.78 | 27.86 |
| 10 | 64/M | 3.64 | 18 | 9.79 | 81 | 5.65 | 37.49 |
| 11 | 35/F | 0.69 | 103 | 2.26 | 4 | 3.33 | 17.86 |
| 12 | 34/M | 3.51 | 21 | 5.53 | 100 | 4.21 | 22.59 |
| 13 | 22/F | 0.87 | 87 | 0.16 | 11 | 5.83 | 43.46 |
| 14 | 40/M | 1.20 | 71 | 0.11 | 0 | 4.01 | 32.08 |
| 15 | 25/M | 4.74 | 16 | 7.72 | 79 | 5.09 | 31.47 |
M, male; F, female.
Table 3.
The significant abundant proteins between the IgA nephropathy group and healthy control group
| Protein Names | Gene Names | Healthy Control (Label-Free Quantification Intensity/106) | IgA Nephropathy (Label-Free Quantification Intensity/106) | P Value |
|---|---|---|---|---|
| Fibrinogen α chain | FGA | 2.3 (1.9–2.6) | 4.5 (2.8–27.5) | 0.02 |
| Protein AMBP | AMBP | 9.1±4.0 | 34.5±19.9 | <0.001 |
| Complement C4-B | C4B | 3.7±2.1 | 5.6±1.8 | 0.01 |
| High mobility group protein B2 | HMGB2 | 1.4±0.5 | 1.0±0.5 | 0.03 |
| Immunoglobulin κ variable 2–29 | IGKV A18 | 68.5 (45.1–94.7) | 31.9 (19.6–60.6) | 0.02 |
| Igγ-2 chain C region | IGHG2 | 2.7 (1.9–3.6) | 1.8 (0–2.5) | 0.01 |
| Igμ chain C region | IGHM | 98.9 (67.6–170.4) | 64.9 (43.5–91.1) | 0.01 |
| Complement C3 | C3 | 11.5 (9.7–18.2) | 3.9 (2.3–10.9) | 0.04 |
| Complement C1q subcomponent subunit A | C1QA | 1.9 (1.0–2.7) | 0.9 (0.5–1.6) | 0.007 |
| Complement C1q subcomponent subunit B | C1QB | 1.9 (1.2–3.9) | 1.2 (0.9–2.3) | 0.02 |
| Complement C1q subcomponent subunit C | C1QC | 2.6 (1.8–4.1) | 1.6 (1.3–2.2) | 0.02 |
| TGF-β–induced protein ig-h3 | TGFBI | 0.3 (0.2–0.4) | 0.2 (0–0.3) | 0.02 |
| Serum albumin | ALB | 585.5±12 6.1 | 437.1±166.2 | 0.01 |
| Igγ-1 chain C region | IGHG1 | 32.5±9.8 | 27.3±9.8 | 0.15 |
| Igγ-3 chain C region | IGHG3 | 3.0 (2.6–3.8) | 3.7 (2.0–7.9) | 0.69 |
| Igγ-4 chain C region | IGHG4 | 1.4 (0.9–1.9) | 0.9 (0–1.4) | 0.16 |
| Polymeric Ig receptor; secretory component | PIGR | 4.3±1.9 | 6.2±4.2 | 0.11 |
| Ig J-chain | IGJ | 132.8±70.2 | 121.1±48.3 | 0.60 |
The values for individual proteins were analyzed by SPSS Data software to determine if they followed normal distributions (bell curve): see Methods for details. When they do, we subsequently calculated the standard deviations. Otherwise, we included the data ranges instead. AMBP, α1-microglobulin/bikunin.
Associations between Individual Protein Levels in Recombinant CD89-Captured IgA Complexes and Clinical Variables
Because the 15 patients with IgA nephropathy represented a diverse clinical spectrum of the disease, we performed a linear association study between clinical variables and individual protein levels in poly-IgA complexes. We focused on four clinical parameters of serum creatinine, proteinuria, percentage of glomerular crescents, and hematuria levels and performed a two-way clustering analysis (Figure 4E). A total of 16 proteins showed statistically significant correlation (P<0.05) to at least one of the clinical variables. IgA-bound AMBP, which showed elevated levels in IgA nephropathy, correlated with serum creatinine (Figure 4E). This is consistent with the recent observation of serum AMBP being elevated in CKD (41). The level of IgA-bound TGF-β–induced protein correlated with proteinuria, whereas serum albumin and complement C3 inversely correlated with proteinuria levels. We also found that some proteins, such as IgG3/IGHG3, β-2-glycoprotein 1/β2-GP1, and serotransferrin/TF in IgA complexes correlated with the severity of hematuria.
Discussion
IgA nephropathy is an immune deposition disease. On the basis of the multihit pathogenesis hypothesis, galactose-deficient IgA1 immune complexes are prone to mesangial IgA deposition, causing glomerular injury (4). However, diagnostic tools for measuring the level of poly-IgA complexes are not available in the clinic. Here, we developed a molecular probe for detecting poly- IgA complexes in plasma samples of IgA nephropathy. Using this recombinant CD89 probe, we demonstrated significantly higher levels of poly-IgA complexes in patients with IgA nephropathy. We showed that the circulating poly-IgA index has a better diagnostic efficiency than plasma IgA1 or galactose-deficient IgA1. Poly-IgA levels were associated with lower eGFR and higher-grade T lesions. In addition, poly-IgA levels were reduced with treatment with steroids and immunosuppressants. These findings suggest recombinant CD89–binding poly-IgA complexes might be used as a diagnostic or prognostic tool for IgA nephropathy and need further external validation.
Our methodology was based on the natural affinity of the IgA receptor CD89 toward poly-IgA complexes. The canonic function of the receptor is to capture IgA-opsonized particles (e.g., bacteria, viruses) to rid the body of the foreign invader through phagocytic activities (23,26,42). It has been speculated that CD89 selectively captures IgA-pathogen complexes, and largely spares normal IgA, simply through size recognition (43). CD89-positive Kupffer cells in the liver are responsible for phagocytic clearance of IgA-bacteria complexes in circulation, whereas free IgA does not initiate phagocytosis (44).
With respect to IgA nephropathy, it was well established that CD89 binds poly-IgA (45). Early studies investigated the possible role of soluble CD89 in complex with IgA in kidney deposition (29,46,47). However, this viewpoint was disputed by observation that IgA-CD89 circulating complexes were not being specific for primary IgA nephropathy, or disease progression (28,30,31). Our own study failed to detect soluble CD89 in the circulating poly-IgA complexes in IgA nephropathy by immunoblotting (Supplemental Figure 2). Furthermore, no CD89 protein was detected by staining the IgA nephropathy kidney sections with anti-CD89 antibody (Supplemental Figures 2 and 3). Instead, Launay et al. (29) suggested dysfunction of CD89-mediated clearance of IgA might partially contribute to the accumulation of circulatory poly-IgA levels in IgA nephropathy.
This study focused on the biophysical property of CD89 in our construction of an ex vivo diagnostic tool. The IgA-binding domain of CD89 was recombinantly produced as an affinity probe for specific detection of poly-IgA complexes. When recombinant CD89 was coated on the ELISA plate, only poly-IgA from plasma bound to the coated wells. Overall, the ELISA kit clearly distinguished poly- versus mono-IgA, outperforming total IgA1 or galactose-deficient IgA1 measurements in discriminating IgA nephropathy and control groups. There were weak correlations between poly-IgA and total IgA1, and poly-IgA and galactose-deficient IgA1 (r=0.62 and 0.33, respectively). However, our method does not distinguish antiglycan interactions between IgA and IgG (48) from IgA self-association. It is interesting to note that the SDS-PAGE–based detection of immune complexes were those linked through intermolecular disulfide bridges, which rarely occur in antibody-antigen interactions.
One of the future directions to further develop the assay involves multitarget measurements of the protein constituents in IgA complexes. This can be achieved through ELISA detection of a target protein that is cocaptured with IgA by recombinant CD89. To this end, we performed initial identification of all candidate proteins in the IgA complex by mass spectrometry. Due to the very small number of IgA nephropathy samples (15 in total), and the semiquantitative nature of liquid chromatography–tandem mass spectrometry detection of proteins, we have not been able to definitively pinpoint particular proteins that have added diagnostic and/or prognostic values. Our study identified candidate proteins in their association with the poly-IgA complex. Among these proteins, it is interesting to note that serum AMBP can covalently bind IgA and a variety of other plasma proteins (49). We found poly-IgA complexes isolated from patients with IgA nephropathy contained high levels of AMBP. Our recent study also found that plasma levels of AMBP-IgA complexes were associated with eGFR and Oxford T scores (50), suggesting the complex may negatively affect kidney function. Future studies in larger cohorts using recombinant CD89–directed ELISA detection of candidate proteins will provide further information on the diagnostic values of the new method.
In addition, this recombinant CD89–directed ELISA kit can be used in longitudinal follow-up of individual patients. This is particularly important because the clinical course of IgA nephropathy often involves dangerous flare-up episodes, frequently manifested as a short period of hematuria after an unrelated infection. It is plausible that the infection triggers whole-body immune reactivity that may include a spike of poly-IgA levels in the blood. The noninvasive serologic kit will be suitable to address this possibility, using either whole poly-IgA levels, its associated protein targets, or combined profiles, to advise treatment options.
Disclosures
J. Jin reports having ownership interest in Accubit Inc.; being an advisor to Enlighten Biotechnology Inc., who is developing technology that has a clinical application in diagnosis; having consultancy agreements with Enlighten Biotechnology Inc., Mannin Research Inc., and Qbio Med, Inc.; and serving as a scientific advisor for, or member of, Scientific Reports. J. Jin, J. Lv, and H. Zhang report applying for a patent related to the methodology described in this article for measuring the level of IgA complex in plasma samples. H. Zhang reports serving as a vice-director of the nephrology committee in the Beijing Society of Medicine, board committee member of nephrology in the Chinese Medical Doctor Association, board committee member of the Chinese Society of Nephrology, member of the International Society of Nephrology (ISN)–Advancing Clinical Trials committee and member of ISN Sister Renal Centers Global Outreach committee and having consultancy agreements with Calliditas, Janssen, Novartis, and OMEROS. All remaining authors have nothing to disclose.
Funding
This study was supported by National Natural Science Foundation of China grants 81670649 and 81925006, the Capital Health Development Research Project of China grant 2018-2-4073, Beijing Science and Technology Plan Project of China grant D181100000118003, and CAMS Innovation Fund for Medical Sciences grant 2019-I2M-5-046 (to J. Lv).
Supplementary Material
Acknowledgments
We are grateful to all patients and healthy control subjects for their participation in this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01300121/-/DCSupplemental.
Supplemental Appendix. Supplemental methods.
Supplemental Figure 1. Purified monomeric IgA and polymeric IgA 6.
Supplemental Figure 2. Samples for mass spectrometry in SDS-PAGE gel and stained by GelCode Blue 7.
Supplemental Figure 3. Detection of soluble CD89 in polymeric IgA bound to CD89 from circulation of patients with IgAN.
Supplemental Table 1. List of proteins involved in immunoglobulin and immunoglobulin receptor.
Supplemental Table 2. List of proteins involved the complement system.
Supplemental Table 3. List of proteins involved lipoprotein and apolipoprotein.
Supplemental Table 4. List of proteins involved blood cell components and coagulation components.
Supplemental Table 5. List of proteins involved in inflammatory response.
References
- 1.Lai KN, Tang SC, Schena FP, Novak J, Tomino Y, Fogo AB, Glassock RJ: IgA nephropathy. Nat Rev Dis Primers 2: 16001, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Chen A, Yang SS, Lin TJ, Ka SM: IgA nephropathy: Clearance kinetics of IgA-containing immune complexes. Semin Immunopathol 40: 539–543, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Kiryluk K, Novak J: The genetics and immunobiology of IgA nephropathy. J Clin Invest 124: 2325–2332, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magistroni R, D’Agati VD, Appel GB, Kiryluk K: New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int 88: 974–989, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanfilippo F, Croker BP, Bollinger RR: Fate of four cadaveric donor renal allografts with mesangial IgA deposits. Transplantation 33: 370–376, 1982 [DOI] [PubMed] [Google Scholar]
- 6.van der Boog PJ, de Fijter JW, Bruijn JA, van Es LA: Recurrence of IgA nephropathy after renal transplantation. Ann Med Interne (Paris) 150: 137–142, 1999 [PubMed] [Google Scholar]
- 7.Kanatsu K, Doi T, Sekita K, Yoshida H, Nagai H, Hamashima Y: A comparative immunologic study of IgA nephropathy. Am J Kidney Dis 2: 618–625, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Coppo R, Basolo B, Martina G, Rollino C, De Marchi M, Giacchino F, Mazzucco G, Messina M, Piccoli G: Circulating immune complexes containing IgA, IgG and IgM in patients with primary IgA nephropathy and with Henoch-Schoenlein nephritis. Correlation with clinical and histologic signs of activity. Clin Nephrol 18: 230–239, 1982 [PubMed] [Google Scholar]
- 9.Coppo R, Basolo B, Piccoli G, Mazzucco G, Bulzomì MR, Roccatello D, De Marchi M, Carbonara AO, Barbiano di Belgiojoso G: IgA1 and IgA2 immune complexes in primary IgA nephropathy and Henoch-Schönlein nephritis. Clin Exp Immunol 57: 583–590, 1984 [PMC free article] [PubMed] [Google Scholar]
- 10.Czerkinsky C, Koopman WJ, Jackson S, Collins JE, Crago SS, Schrohenloher RE, Julian BA, Galla JH, Mestecky J: Circulating immune complexes and immunoglobulin A rheumatoid factor in patients with mesangial immunoglobulin A nephropathies. J Clin Invest 77: 1931–1938, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak J, Vu HL, Novak L, Julian BA, Mestecky J, Tomana M: Interactions of human mesangial cells with IgA and IgA-containing immune complexes. Kidney Int 62: 465–475, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J: IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 67: 504–513, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Tissandié E, Morelle W, Berthelot L, Vrtovsnik F, Daugas E, Walker F, Lebrec D, Trawalé JM, Francoz C, Durand F, Moura IC, Paradis V, Moreau R, Monteiro RC: Both IgA nephropathy and alcoholic cirrhosis feature abnormally glycosylated IgA1 and soluble CD89-IgA and IgG-IgA complexes: Common mechanisms for distinct diseases. Kidney Int 80: 1352–1363, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Obara T, Mizoguchi S, Shimozuru Y, Sato T, Hotta O: The complex of immunoglobulin A and uromodulin as a diagnostic marker for immunoglobulin A nephropathy. Clin Exp Nephrol 16: 713–721, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamouza H, Chemouny JM, Raskova Kafkova L, Berthelot L, Flamant M, Demion M, Mesnard L, Paubelle E, Walker F, Julian BA, Tissandié E, Tiwari MK, Camara NO, Vrtovsnik F, Benhamou M, Novak J, Monteiro RC, Moura IC: The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int 82: 1284–1296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki Y, Matsuzaki K, Suzuki H, Okazaki K, Yanagawa H, Ieiri N, Sato M, Sato T, Taguma Y, Matsuoka J, Horikoshi S, Novak J, Hotta O, Tomino Y: Serum levels of galactose-deficient immunoglobulin (Ig) A1 and related immune complex are associated with disease activity of IgA nephropathy. Clin Exp Nephrol 18: 770–777, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Lv J, Zhang X, Chen P, Zhao M, Zhang H: Secondary IgA nephropathy shares the same immune features with primary IgA nephropathy. Kidney Int Rep 5: 165–172, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bene MC, Faure G, Hurault de Ligny B, Kessler M, Duheille J: Immunoglobulin A nephropathy. Quantitative immunohistomorphometry of the tonsillar plasma cells evidences an inversion of the immunoglobulin A versus immunoglobulin G secreting cell balance. J Clin Invest 71: 1342–1347, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novak J, Raskova Kafkova L, Suzuki H, Tomana M, Matousovic K, Brown R, Hall S, Sanders JT, Eison TM, Moldoveanu Z, Novak L, Novak Z, Mayne R, Julian BA, Mestecky J, Wyatt RJ: IgA1 immune complexes from pediatric patients with IgA nephropathy activate cultured human mesangial cells. Nephrol Dial Transplant 26: 3451–3457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maliszewski CR, March CJ, Schoenborn MA, Gimpel S, Shen L: Expression cloning of a human Fc receptor for IgA. J Exp Med 172: 1665–1672, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro RC, Van De Winkel JG: IgA Fc receptors. Annu Rev Immunol 21: 177–204, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Bakema JE, van Egmond M: The human immunoglobulin A Fc receptor FcαRI: A multifaceted regulator of mucosal immunity. Mucosal Immunol 4: 612–624, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Herr AB, Ballister ER, Bjorkman PJ: Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature 423: 614–620, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Wines BD, Hulett MD, Jamieson GP, Trist HM, Spratt JM, Hogarth PM: Identification of residues in the first domain of human Fc alpha receptor essential for interaction with IgA. J Immunol 162: 2146–2153, 1999 [PubMed] [Google Scholar]
- 25.Morton HC, van Zandbergen G, van Kooten C, Howard CJ, van de Winkel JG, Brandtzaeg P: Immunoglobulin-binding sites of human FcalphaRI (CD89) and bovine Fcgamma2R are located in their membrane-distal extracellular domains. J Exp Med 189: 1715–1722, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carayannopoulos L, Hexham JM, Capra JD: Localization of the binding site for the monocyte immunoglobulin (Ig) A-Fc receptor (CD89) to the domain boundary between Calpha2 and Calpha3 in human IgA1. J Exp Med 183: 1579–1586, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berthelot L, Papista C, Maciel TT, Biarnes-Pelicot M, Tissandie E, Wang PH, Tamouza H, Jamin A, Bex-Coudrat J, Gestin A, Boumediene A, Arcos-Fajardo M, England P, Pillebout E, Walker F, Daugas E, Vrtosvnik F, Flamant M, Benhamou M, Cogné M, Moura IC, Monteiro RC: Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med 209: 793–806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyd JK, Barratt J: Immune complex formation in IgA nephropathy: CD89 a ‘saint’ or a ‘sinner’? Kidney Int 78: 1211–1213, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Launay P, Grossetête B, Arcos-Fajardo M, Gaudin E, Torres SP, Beaudoin L, Patey-Mariaud de Serre N, Lehuen A, Monteiro RC: Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med 191: 1999–2009, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Boog PJ, De Fijter JW, Van Kooten C, Van Der Holst R, Van Seggelen A, Van Es LA, Daha MR: Complexes of IgA with FcalphaRI/CD89 are not specific for primary IgA nephropathy. Kidney Int 63: 514–521, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Vuong MT, Hahn-Zoric M, Lundberg S, Gunnarsson I, van Kooten C, Wramner L, Seddighzadeh M, Fernström A, Hanson LÅ, Do LT, Jacobson SH, Padyukov L: Association of soluble CD89 levels with disease progression but not susceptibility in IgA nephropathy. Kidney Int 78: 1281–1287, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Jhee JH, Kang HY, Wu M, Nam BY, Chang TI, Jung SY, Park S, Kim H, Yun HR, Kee YK, Yoon CY, Park JT, Yoo TH, Kang SW, Han SH: Circulating CD89-IgA complex does not predict deterioration of kidney function in Korean patients with IgA nephropathy. Clin Chem Lab Med 56: 75–85, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJR, van de Winkel JGJ, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PWHI: Complement is activated by IgG hexamers assembled at the cell surface. Science 343: 1260–1263, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J: Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 104: 73–81, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC: Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 15: 622–634, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Andre PM, Le Pogamp P, Chevet D: Impairment of jacalin binding to serum IgA in IgA nephropathy. J Clin Lab Anal 4: 115–119, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Tomino Y, Ohmuro H, Takahashi Y, Suzuki Y, Saka S, Tashiro K, Shirato I, Koide H: Binding capacity of serum IgA to jacalin in patients with IgA nephropathy using jacalin-coated microplates. Nephron 70: 329–333, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Plomp R, Dekkers G, Rombouts Y, Visser R, Koeleman CA, Kammeijer GS, Jansen BC, Rispens T, Hensbergen PJ, Vidarsson G, Wuhrer M: Hinge-region O-glycosylation of human immunoglobulin G3 (IgG3). Mol Cell Proteomics 14: 1373–1384, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aucouturier P, Mihaesco E, Mihaesco C, Preud’homme JL: Characterization of jacalin, the human IgA and IgD binding lectin from jackfruit. Mol Immunol 24: 503–511, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Biewenga J, Hiemstra PS, Steneker I, Daha MR: Binding of human IgA1 and IgA1 fragments to jacalin. Mol Immunol 26: 275–281, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Makridakis M, Kontostathi G, Petra E, Stroggilos R, Lygirou V, Filip S, Duranton F, Mischak H, Argiles A, Zoidakis J, Vlahou A: Multiplexed MRM-based protein quantification of putative prognostic biomarkers for chronic kidney disease progression in plasma. Sci Rep 10: 4815, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morton HC, van Egmond M, van de Winkel JG: Structure and function of human IgA Fc receptors (Fc alpha R). Crit Rev Immunol 16: 423–440, 1996 [PubMed] [Google Scholar]
- 43.Reterink TJ, van Zandbergen G, van Egmond M, Klar-Mohamad N, Morton CH, van de Winkel JG, Daha MR: Size-dependent effect of IgA on the IgA Fc receptor (CD89). Eur J Immunol 27: 2219–2224, 1997 [DOI] [PubMed] [Google Scholar]
- 44.van Egmond M, van Garderen E, van Spriel AB, Damen CA, van Amersfoort ES, van Zandbergen G, van Hattum J, Kuiper J, van de Winkel JG: FcalphaRI-positive liver Kupffer cells: Reappraisal of the function of immunoglobulin A in immunity. Nat Med 6: 680–685, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Geissmann F, Launay P, Pasquier B, Lepelletier Y, Leborgne M, Lehuen A, Brousse N, Monteiro RC: A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J Immunol 166: 346–352, 2001 [DOI] [PubMed] [Google Scholar]
- 46.van der Boog PJ, van Zandbergen G, de Fijter JW, Klar-Mohamad N, van Seggelen A, Brandtzaeg P, Daha MR, van Kooten C: Fc alpha RI/CD89 circulates in human serum covalently linked to IgA in a polymeric state. J Immunol 168: 1252–1258, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Monteiro RC, Moura IC, Launay P, Tsuge T, Haddad E, Benhamou M, Cooper MD, Arcos-Fajardo M: Pathogenic significance of IgA receptor interactions in IgA nephropathy. Trends Mol Med 8: 464–468, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Suzuki H, Moldoveanu Z, Hall S, Brown R, Julian BA, Wyatt RJ, Tomana M, Tomino Y, Novak J, Mestecky J: IgA nephropathy: Characterization of IgG antibodies specific for galactose-deficient IgA1. Contrib Nephrol 157: 129–133, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Akerström B, Lögdberg L, Bergg˚ård T, Osmark P, Lindqvist A: Alpha(1)-microglobulin: A yellow-brown lipocalin. Biochim Biophys Acta 1482: 172–184, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Xu B, Zhu L, Wang Q, Zhao Y, Jia M, Shi S, Liu L, Lv J, Lai W, Ji J, Zhang H: Mass spectrometry-based screening identifies circulating immunoglobulinA-α1-microglobulin complex as potential biomarker in immunoglobulin A nephropathy. Nephrol Dial Transplant 36: 782–792, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.