Abstract
Despite evidence of multiorgan tropism of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in patients with coronavirus disease 2019 (COVID-19), direct viral kidney invasion has been difficult to demonstrate. The question of whether SARS-CoV2 can directly infect the kidney is relevant to the understanding of pathogenesis of AKI and collapsing glomerulopathy in patients with COVID-19. Methodologies to document SARS-CoV-2 infection that have been used include immunohistochemistry, immunofluorescence, RT-PCR, in situ hybridization, and electron microscopy. In our review of studies to date, we found that SARS-CoV-2 in the kidneys of patients with COVID-19 was detected in 18 of 94 (19%) by immunohistochemistry, 71 of 144 (49%) by RT-PCR, and 11 of 84 (13%) by in situ hybridization. In a smaller number of patients with COVID-19 examined by immunofluorescence, SARS-CoV-2 was detected in 10 of 13 (77%). In total, in kidneys from 102 of 235 patients (43%), the presence of SARS-CoV-2 was suggested by at least one of the methods used. Despite these positive findings, caution is needed because many other studies have been negative for SARS-CoV-2 and it should be noted that when detected, it was only in kidneys obtained at autopsy. There is a clear need for studies from kidney biopsies, including those performed at early stages of the COVID-19–associated kidney disease. Development of tests to detect kidney viral infection in urine samples would be more practical as a noninvasive way to evaluate SARS-CoV-2 infection during the evolution of COVID-19–associated kidney disease.
Keywords: COVID-19, proximal tubule, podocyte, interstitial nephritis, pyelonephritis
Introduction
Although acute lung injury is the most prominent clinical manifestation in patients with severe coronavirus disease 2019 (COVID-19), AKI is also frequently observed. The reported incidence of AKI in COVID-19 ranges between 22% and 57% in patients who are hospitalized, and it is associated with high mortality (1–13). Angiotensin-converting enzyme 2 (ACE2), a protein that acts as the chief receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cell entry (14–17), is highly expressed in the kidney (18–20). However, it has been difficult to establish if the virus directly infects the kidney parenchyma, as recently pointed out by Khan et al. (21). The evidence in favor of, or against, direct kidney invasion by SARS-CoV-2 will be juxtaposed on Koch’s postulates, later revised for viral infections by Thomas Rivers in 1937, and more recently by Fredericks and Relman (22). We highlight the more relevant findings in support of or against kidney infectivity in lieu of a detailed point-by-point account of Koch’s postulates to establish whether SARS-CoV-2 can directly infect the kidney. This answer will be important in defining the pathophysiology of kidney injury seen in patients with severe COVID-19.
Although AKI is commonly identified in patients with COVID-19, more unique kidney manifestations, such as collapsing glomerulopathy, have also been described (23–38). AKI in patients with COVID-19 may be caused by factors common to a majority of cases of AKI in patients who are critically ill in the intensive care unit, including hypotension, sepsis, and exposure to nephrotoxins (39–42). There are, however, additional features that suggest a more complex pathophysiology (1,43). AKI in patients with COVID-19 could be mediated by overactivation of the innate immune system, cytokine release, complement activation, angiotensin II (Ang II) overactivity, the development of a hypercoagulable state, hypovolemia secondary to over diuresis, and/or increased central venous pressure secondary to high positive end-expiratory pressure (1,3,43–45). Analysis of 2600 patients admitted to the hospital with COVID-19 showed that after adjustment for demographics, comorbidities, vital signs, medications, and laboratory results, COVID-19 remained highly associated with AKI (46). Viral invasion of the kidney, if it occurs, could be an additional contributing factor to AKI and collapsing glomerulopathy. The prognosis may be worse than regular AKI, but further data are needed to understand the evolution of AKI and possible transition to CKD in some patients with COVID-19. This review examines the evidence in favor of and against SARS-CoV-2 kidney infection in patients with COVID-19 reported to date. Although most autopsy studies show no convincing evidence for SARS-CoV-2 in the kidney, the evidence in other studies is strong, perhaps because a more comprehensive analysis involving immunofluorescence (IF), RT-PCR, and in situ hybridization was performed.
Biology of Severe Acute Respiratory Syndrome Coronavirus 2 and Localization and Function of Full-Length Angiotensin-Converting Enzyme 2, its Main Receptor
SARS-CoV-2 belongs to the family of Coronaviridae, which are classified in α-, β-, γ, and δ virus (47). The positive-strand RNA genome of coronavirus is surrounded by a helical capsid, the nucleocapsid protein, which is surrounded by a lipid bilayer envelope (47). This envelope consists of membrane protein, envelope protein, and spike (S) protein (47). The S protein of SARS-CoV-2, a β coronavirus, has two components: S1, which contains the receptor-binding domain, and S2, which contains the fusion peptide (14,48). The S protein mediates cell entry and therefore is critical for the virus host range, but is also involved in inducing the host immune response (47). ACE2 in its full-length form is the main receptor that SARS-CoV-2 uses to enter host cells (2,14–17). In addition, ACE2 acts as monocarboxypeptidase cleaving phenylalanine from the C-terminus of its substrates, for example, causing the formation of Ang- (1–7) from Ang II (49–51). Other substrates of ACE2 include apelin-13, apelin-36, and the proinflammatory peptide des-arg9 bradykinin (52–54).
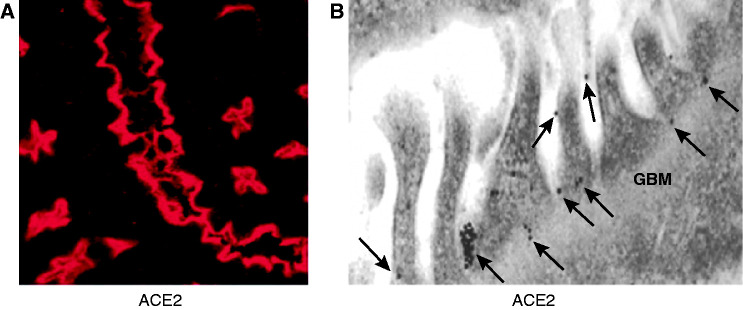
It is widely believed that surface membrane ACE2 decreases in COVID-19 as a result of ACE2 virus–protein complex internalization, and the deficit of ACE2 renders the infected organs more vulnerable to Ang II and des-arg9 bradykinin (2,55–57). Accumulation of these peptides can foster organ injury and adverse outcomes (2), especially in organs that express ACE2. In a study of ACE2 mRNA expression in 72 human tissues using real-time PCR, high levels were found in the kidney, testis, and cardiovascular tissues (58). In human lungs, mRNA expression of ACE2 can be detected on type II pneumocytes (59), and at the protein level by immunohistochemistry (IHC) on type I and type II pneumocytes (60). The latter cell type is considered the chief site of pathogenic infection by SARS-CoV-2. In the kidneys, ACE2 is abundantly expressed in the proximal tubule apical membrane (18–20) (Figure 1A). As demonstrated by IF and immunogold labeled electron microscopy (EM), mouse glomerular parietal and visceral epithelial cells (podocytes) also express full-length ACE2, but in much smaller amounts than the proximal tubule (Figure 1B) (18,19,61). In agreement, ACE2 has been detected in human proximal convoluted tubules and parietal epithelial cells of the Bowman’s capsule by IF (62).
Figure 1.
Immunofluorescence and immunogold analysis of angiotensin-converting enzyme 2 (ACE2) in the kidney. (A) Immunofluorescence staining of ACE2 (red) in proximal tubules. (B) ACE2 immunogold labeling in glomeruli. ACE2 labeled with 15 nm of gold particles is distributed in podocyte foot processes and slit diaphragm (A, arrows). The glomerular basement membrane (GBM) does not have ACE2 immunogold particles (modified from ref. 19 with permission).
SARS-CoV-2 infection is also governed by specific proteases found in each cell type (14), chiefly TMPRSS2. Single-cell transcriptome analysis revealed that TMPRSS2 is highly expressed in the distal nephron, not in the proximal tubule (1). In kidney organoids of embryonic origin, ACE2 and TMPRSS2 colocalization can be seen by IF in proximal tubule–like structures (63). The areas of colocalization in these organoids are in the presumed apical border area, where ACE2 is abundantly expressed in the adult. Clearly, studies in human kidneys are needed to clarify the issue of ACE2 and TMPRSS2 localization within the kidney. It is possible that other proteases, similar to TMPRSS2, prime SARS-CoV-2 for internalization with ACE2 in the proximal tubule. Other proteases, such as furin and cathepsin L, which are necessary for SARS-CoV-2 processing (64), are more ubiquitously expressed, and are found in the proximal tubule (65,66).
How Could Severe Acute Respiratory Syndrome Coronavirus 2 Reach the Kidney?
Access of the virus to the kidney is obviously not as direct as it is in the lungs, where the route SARS-CoV-2 uses to reach these organs is inhalation. The mechanisms proposed here are theoretical considerations because our understanding of kidney infection by SARS-CoV-2 is still evolving. Viremia would be the expected route for SARS-CoV-2 to reach the kidney. Viremia, even transient, could lead to SARS-CoV-2 entry by binding to ACE2 in podocytes, providing an initial nidus for subsequent viral invasion of the kidney parenchyma. Most patients with COVID-19, however, do not have documented viremia detected by RNA levels (67,68). There is evidence that higher viral RNA loads in plasma are associated with increased disease severity and mortality in patients with COVID-19 (67,68). The possibility of viral exposure to podocytes needs to be considered. SARS-CoV-2 RNA and S protein have been documented in the glomerulus in autopsies of patients with COVID-19 (69,70). This could reflect viral RNA stuck in the glomerular filtration barrier, rather than viral infection. Evidence for SARS-CoV-2 RNA or protein in the glomerular cells nevertheless has been documented using both in situ hybridization and IF (69,70).
One next needs to consider how SARS-CoV-2 could reach the lumen of the proximal tubule to bind to the abundant ACE2 receptors in the apical membrane (1,21), instead of the basolateral membrane, which is in potential contact with the virus present in the blood stream. One possible circumferential route to the apical membrane of the proximal tubule is via the tubular fluid. After SARS-CoV-2 infects podocytes, access to the tubular fluid and subsequent binding to ACE2 in the apical membrane of the proximal tubule is a potential route. Alternatively, the virus could reach the apical membrane of the proximal tubule during cellular injury of the proximal tubule in patients with AKI. In this scenario, the virus could traverse the cell to interact with apical ACE2 protein. In primary human airway epithelia, ACE2 is expressed apically, and SARS-CoV-2 infection predominantly occurs on the apical surface, but infection can occur on the basolateral surface at low efficiency (71). This perhaps involves low level transcytosis of ACE2 to the basolateral membrane of the cell. Another possibility that lacks experimental support but is worthy of consideration is that when kidney damage occurs, there may be aberrant expression of ACE2 in the basolateral membrane. This is a possibility given the report of altered CD147 expression, another putative receptor for SARS-CoV-2, in patients with COVID-19. CD-147 is expressed basolateral on proximal and distal tubular epithelial cells (72) and, thus, could mediate SARS-CoV-2 cell entry.
How Can Severe Acute Respiratory Syndrome Coronavirus 2 Be Detected in the Kidney?
Different methods can be applied to detect the presence of SARS-CoV-2 in the kidney (Table 1). Strong evidence for the presence of viral RNA can be derived from in situ hybridization (73), RT-PCR (74), or viral growth in plaque assays (75,76). However, overinterpretation of these results is possible, especially with RT-PCR–based strategies on whole kidney samples because they will not distinguish parenchymal infection from the presence of virus within blood or urine. The nucleocapsid or S protein of SARS-CoV-2, moreover, can be detected by either IHC or IF. We found 14 reports using nine different antibodies to detect either spike protein or nucleocapsid protein of SARS-CoV-1 or -2 (Table 2). One important limitation of IHC is the potential of crossreactivity. Detection of SARS-CoV-2 proteins by IHC was less sensitive and specific than detection of SARS-CoV-2 RNA by RT-PCR or in situ hybridization in one report (77). Others found no difference between detection of SARS-CoV-2 by IHC and in situ hybridization (73). Other factors that can influence detection of proteins include tissue fixation, unmasking procedures, antibody dilution, and detection systems (78). Advanced techniques, such as protein mass spectrometry, which are more sensitive to detecting the presence of SARS-CoV-2 protein in nasopharynx epithelial swabs (79), could be used in studies to detect SARS-CoV-2 in kidney or the urine. EM is another common method of detection of viral-like particles, however, does not provide definitive evidence for SARS-CoV-2 virions (80).
Table 1.
Methods of viral detection to demonstrate presence of severe acute respiratory syndrome coronavirus 2
| Method | What Is Being Detected? |
|---|---|
| Electron microscopy | Virus-like particles |
| Immunohistochemistry | Viral protein |
| Immunofluorescence with confocal microscopy | Viral protein |
| In situ hybridization | Viral RNA |
| RT-PCR | Viral RNA |
| Plaque assay | Live virus |
| Protein mass spectrometry | Viral protein |
Table 2.
Findings of severe acute respiratory syndrome coronavirus 2 protein or RNA in kidney of patients with coronavirus disease 2019
| Author | Patients Stated to Have Severe Acute Respiratory Syndrome Coronavirus 2 Kidney Presence | Immunohistochemistry: Viral Protein |
Immunofluorescence: Viral Protein | RT-PCR: Viral RNA |
In situ Hybridization: Viral RNA | Postmortem Interval |
|---|---|---|---|---|---|---|
| Findings with evidence (postmortem) | ||||||
| Braun et al. (83) | 38/63 | Not done | Not done | 38/63 | Not done | Median: 5 days |
| Puelles et al. (69) | 16/27 | Not done | Exact number not given; spike glycoprotein antibody (3A2) (Abcam, ab272420), SARS-CoV SΔ10 within S2 domain protein (Genetex, GTX632604) |
16/26 | Exact number not given; RNA scopea |
Average: 2.8 days |
| Remmelink et al. (85) | 10/17 | Not done | Not done | 10/17 | Not done | 72–96 hrs |
| Bouquegneau (70) | 12/16 | 9/16; 2019-nCoV N-Protein (NP) rabbit polyclonal antibody (ABclonal #A20016) | Not done | 1/16 | 6/16; RNA scopea |
< 3 hrs |
| Su et al. (9) | 8/10 | Not done | 3/6; anti–SARS-CoV nucleo-protein antibody (40143T62; Sino Biologic, China) | Not done | Not done | 1–6 hrs |
| Bradley et al. (81) | 5/6 | 2/4; monoclonal antibody to the SARS-CoV-2 spike protein (GeneTex; Irvine, CA, USA) | Not done | 3/3 | Not done | <140 hrs |
| Hanley et al. (84) | 3/5 | Not done | Not done | 3/5 | Not done | <10 days |
| Schurink et al. (82) | 1/11 | 1/11; noncommercial monoclonal mouse antibody and polyclonal rabbit antibody for SARS-CoV-2 nucleocapsid protein (Sino Biologic & Nanommune, Irvine, CA, USA) | Not done | Not done | Not done | Median: 15 hrs |
| Ichimura et al. (unpublished observations) | 1/1 | Not done | 1/1; SARS-CoV nucleocapsid (rabbit, PA1–41098; Invitrogen, Waltham, MA, USA) | Not done | Not done | Not given |
| Diao et al. (13) | 6/6 | 6/6; anti–SARS-CoV-2 nucleocapsid protein antibody (Sino Biologic, Beijing, China or ab273434, Abcam) and anti-SARS spike glycoprotein antibody (ab273433, Abcam) | 6/6; anti–SARS-CoV-2 nucleocapsid protein antibody (Sino Biologic, Beijing, China or ab273434, Abcam) and anti-SARS spike glycoprotein antibody (ab273433, Abcam) | Not done | 3/3; RNA scopea |
< 24hrs |
| Findings with low evidence (biopsies) | ||||||
| Kudose et al. (97) | 2/16 | 0/16; mouse monoclonal IgG1 antibody against S2 subunit from SARS-CoV-2 spike protein from clone 1A9 (GeneTex, Irvine, CA, USA) and rabbit monoclonal antibody against nucleocapsid protein clone 001 (Sino Biologic, Beijing, China) (40143-R001) | Not done | Not done | 0/16 when performed automatically; 2/16 when performed manually; RNA scopea |
— |
| Findings with no evidence (biopsies) | ||||||
| Sharma et al. (98) | 0/10 | 0/10; antibody to SARS-CoV-2 nucleocapsid protein (Clone 1C7; Bioss, Woburn, MA) | Not done | Not done | Not done | — |
| Akilesh et al. (25) | 0/8 | 0/4; antibody to SARS-CoV nucleocapsid protein (40143-T62, Sinobiological, Wayne, PA, USA) | Not done | Not done | 0/4; RNA scopea |
— |
| Huang et al. (99) | 0/1 | 0/1; antibody to spike protein of SARS-CoV-2 (40150-R007), Sino Biologic, Beijing, China) | Not done | 0/1 | Not done | — |
| Wu et al. (24) | 0/6 | Not done | Not done | Not done | 0/6; RNA scopea | — |
| Sharma et al. (32) | 0/2 | Not done | Not done | Not done | 0/2; RNA scopea | — |
| Nasr et al. (33) | 0/1 | Not done | Not done | Not done | 0/1; RNA scopea | — |
| Larsen et al. (24) | 0/1 | Not done | Not done | Not done | 0/1; RNA scopea | — |
| Peleg et al. (28) | 0/1 | Not done | Not done | Not done | 0/1; not given | — |
| Couturier et al. (34) | 0/1 | Not done | Not done | 0/1 | Not done | — |
| Kissling et al. (36) | 0/1 | Not done | Not done | 0/1 | Not done | — |
| Lazareth et al. (27) | 0/1 | Not done | Not done | 0/1; Crystal Digital PCR-TM (Stilla Technologies, Villejuif, France) |
Not done | — |
| Findings with no evidence (postmortem) | ||||||
| Golmai et al. (100) | 0/12 | 0/12; primary mouse antibody for SARS-CoV-2 nucleocapsid protein (Clone 1C7, Bioss Woburn, MA, USA) | Not done | Not done | 0/4; RNA scopea |
Not given |
| Massoth et al. (77) | 0/7 | 0/3; SARS nucleocapsid antibody (NB100–56576; Novus Biologicals) | Not done | 0/5 | 0/7; RNA scopea |
Not given |
| Brook et al. (101) | 0/3 | 0/3; SARS Rabbit Polyclonal Nucleocapsid Protein Antibody (Novus NB100–56576) | Not done | 0/3 | 0/3; RNA scopea |
<3 hours |
| Sekulic et al. (102) | 0/2 | Not done | Not done | 0/2 | Not done | 29–39 hrs |
| Rocha et al. (73) | Not given | 0/8; antibodies to recombinant SARS-CoV-2 nucleocapsid protein/recombinant SARS nucleocapsid protein, Bioss, Woburn, MA, USA | Not done | Not done | 0/10 RNA scopea |
Not given |
| Santoriello et al. (87) | Not given | Not done | Not done | Not done | 0/10; RNA scopea |
Median: 21.8 hrs |
| Summary | Total number of patients with positive findingsb | IHC | IF | RT-PCR | In situ hybridization | |
| 102/235 patients (43%) | 18/94 patients (19%) |
10/13 patients (77%) |
71/144 patients (49%) |
11/84 patients (13%) |
||
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IHC, immunohistochemistry; IF, immunofluorescence.
By ACD, Newark, CA, USA.
The kidney of some patients was examined by more than one method, but for the total number, every patient was counted just once. Therefore, the total number of patients is lower than when adding up the number of patients examined by each individual method. Two reports (references 73,87) do not specify from how many patients they derived their sample and were excluded for the calculation of the total number of patients examined.
Evidence For and Against SARS-CoV-2 in the Kidney from Patients with COVID-19
In contrast to the lungs, it has been difficult to demonstrate the presence of SARS-CoV-2 in kidneys (Tables 2 and 3 and summarized in Figure 2). Particles that resemble the appearance of coronaviruses are clearly not sufficient to unequivocally document direct viral invasion (80). The family of Coronaviridae, moreover, is very large and common, and other members of the family, such as the common cold virus, could be mistaken for SARS-CoV-2. Nevertheless, it is worthwhile to mention studies that reported such particles in the early descriptions of patients with COVID-19. In total, seven of 21 studies that we found reported potential viral-like particles by EM. Of the 128 patients examined by EM, potential viral-like particles were found in the kidney of 16 patients (13%). In total, 11 samples were from autopsied kidneys (9,13,81) and only five from kidney biopsies performed in living patients; the latter were all in patients who were subsequently diagnosed with collapsing glomerulopathy (35–38).
Table 3.
Findings suggestive of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019 (extracted from Table 2)
| Finding | Viral Protein by Immunohistochemistry | Viral Protein by Immunofluorescence | Viral RNA by In situ Hybridization | Viral RNA by RT-PCR |
|---|---|---|---|---|
| Frequency of findings from patients examined | 18/94 (19%) |
10/13 patients (77%) |
11/84 patients (13%) |
71/144 (49%) |
| References showing detection | (13,70,81,82) | (9,13,69 and Ichimura et al., unpublished observations) | (13,69,70,97) | (69,70,81,83–85) |
| References showing no detection | (25,73,77,97–101) | – | (24,25,28,32,33,73, 77,87,100,101) | (27,34,36,77,99,101,102) |
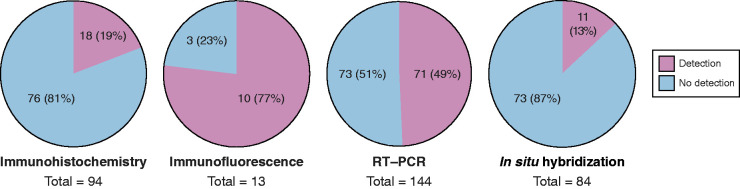
Figure 2.
Summary of data against and in favor of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in kidneys from patients with coronavirus disease 2019 (COVID-19). Number and percentage of patients in whom SARS-CoV-2 spike or nucleocapsid protein or RNA was detected (red) or not (blue). Each circle depicts immunohistochemistry, immunofluorescence, RT-PCR, or in situ hybridization. Data extracted from Tables 2 and 3.
Four of 12 IHC-based studies reported detection of viral protein in the kidney. Of the 94 patients with COVID-19 examined by IHC, 18 showed evidence for the presence of viral proteins in the kidney (19%) (Figure 2). Only postmortem samples stained positive for SARS-CoV-2 protein by IHC (13,70,81,82). Viral protein was accessed in four studies by immunofluorescent microscopy, all of them autopsy studies; viral protein was found in the kidney in 10 of 13 patients examined (77%) (9,13,69,and Ichimura et al., unpublished observations) (Figure 2). As detected by in situ hybridization, SARS-CoV-2 RNA was only found in four of 14 studies and 11 of 84 patients (13%) (Figure 2). One of these studies also used a probe specific for the antisense strand of the SARS-CoV-2 S gene and detected its presence in kidney tubules, strongly suggesting there is viral replication in the kidney (13). RT-PCR was used in 13 reports, and 71 of 144 patients (49%) showed presence of SARS-CoV-2 RNA in the kidney (Figure 2). Detection of SARS-CoV-2 RNA by RT-PCR was only observed in postmortem specimens (69,70,81,83–85). One of these studies also tested the kidney of one patient positive for subgenomic viral RNA transcripts by RT-PCR (84). Subgenomic viral RNA is only transcribed by infected cells and not packaged into virions (86). This can be taken as evidence of active replication of the virus in the kidney (84). However, it cannot be determined which cell type in the kidney was infected.
In total, in kidneys from 102 of 235 patients (43%), the presence of SARS-CoV-2 was suggested by at least one of the methods used (Table 2). Each patient was counted once, even if several methods were applied to the same patient. It must be noted that two studies gave the numbers of samples, but not the exact number of patients examined (73,87). These were excluded in the final calculation. The two methods that were more consistent with the presence of SARS-CoV-2 were IF and RT-PCR. IF was positive in 10 of 13 (77%) and RT-PCR in 71 of 144 (49%) samples examined (Figure 2). It is likely these methods are more sensitive and overestimate the virus presence, particularly because in situ hybridization yielded a much lower positivity rate for SARS-CoV-2 (only 11 of 84 samples; 13%). Unfortunately, many studies that performed RT-PCR did not concomitantly assay for in situ hybridization. Clearly, studies that use more than one method, preferably IF and RT-PCR for sensitivity and in situ hybridization to ensure specificity, are more likely to detect SARS-CoV-2 when present in the kidney.
Urine Viral Studies.
In some viral nephropathies, viruria can be easily demonstrated. For instance, high titers of BK virus in the urine are a frequent finding of patients with BK nephropathy (88–90). In a study of six kidney transplant recipients with BK nephropathy, Howell et al. found polyomavirus in the urine of all six patients by EM, in concordance with the presence of virus in biopsies (90). In contrast, the presence of SARS-CoV-2 in the urine has been difficult to document. A meta-analysis of 30 studies comparing SARS-CoV-2 RNA in urine, blood, and stool (91) found the incidence of detecting SARS-CoV-2 RNA in the urine was 8%, a much lower rate compared with presence in blood (21%) and stool (40%) (91). The presence of SARS-CoV-2 RNA in urine was associated with more severe disease in this meta-analysis (91). A more recent report that used an antigen-capture assay detected SARS-CoV-2 S1 protein in the urine of 25% of patients with COVID-19 (92).
SARS-CoV-2 in the urine has been reported to be infectious. Urine from two patients with COVID-19 was sufficient to transfer SARS-CoV-2 infection to ferrets (93,94), fulfilling one of the the main Kochs postulates. In these studies, SARS-CoV-2 RNA levels in nasal washes of the infected ferrets peaked 4 days after infection (94). In a case report, urine from a patient with COVID-19 was used to infect Vero E6 cells in vitro (95). A cytopathic effect was observed 3 days after infection, which was interpreted as presence of infectious/viable SARS-CoV-2 in the urine of this patient (95). Moreover, timing for successful detection of SARS-CoV-2 in urine might be critical. A study that collected urine of 67 patients with COVID-19 tested 13 of 231 (6%) urine samples positive for SARS-CoV-2 RNA (Tan et al., unpublished observations).
The overall relatively few cases with viruria in patients with COVID-19 are consistent with the low number of cases with virus present in kidney samples (see below). This may be because viruria of SARS-CoV-2 is rare, but could be in part due to the lack of an effective and sensitive detection method. Ultracentrifugation to concentrate viral particles and protein mass spectrometry have been proposed to detect the presence of SARS-CoV-2 in the urine (79,96). Ribonucleases in urine can also potentially degrade viral mRNA leading to false-negative results (91,96).
Discussion and Conclusions
It has been difficult to demonstrate the presence of SARS-CoV-2 in the kidneys of patients with COVID-19. Despite multiple negative studies, there are data that demonstrate kidney tropism of SARS-CoV-2 (Figure 2). Even then, there is no evidence that viral presence is directly the cause of AKI frequently seen in patients with COVID-19. When trying to detect viral presence in organs of autopsied patients who had COVID-19, one might not find viral RNA because the search for the virus was done too late after death when kidney autopsy tissue was available. It must be pointed out, therefore, that autopsy studies from patients who died from severe COVID-19 are far from ideal, yet represent the majority of patients with severe and lethal COVID-19 reported. To date, the presence of SARS-CoV-2 in the kidney has been described mainly post mortem. In kidney biopsies usually performed relatively late after the appearance of symptoms, it may be difficult to detect the virus in the kidney sample. Indeed, we could not find studies where kidney biopsies were done very early in the course of COVID-19–associated kidney disease.
Fulminant viremia and viruria are not typical features of COVID-19. Examining the urine early in the course of the disease with advanced techniques such as protein mass spectrometry or more practically with sensitive ELISA assays may provide additional evidence for SARS-CoV-2 kidney infectivity. The timing of the search for the virus may also be crucial. Availability of more information on viruria detected by sensitive methods as noted above could be used sequentially to attempt to assess possible early-stage viral invasion of the kidney in patients with COVID-19. The method of viral detection is an important consideration as well as the site of detection within the kidney. The danger to mistake internal vesicles or other physiologic parts of the cell for viral particles renders EM alone insufficient to search for SARS-CoV-2 presence in the kidneys. Detection of SARS-CoV-2 protein was more successful by IF (77%) than by IHC (19%). It must be noted, however, that the total number of patients tested by IF is very low (Figure 2). The numbers of patients in which SARS-CoV-2 RNA was detected are higher by RT-PCR than by in situ hybridization. However, in most studies, the two methods were not usually performed concurrently in the same patient. There are also important technical aspects. Spatial detection of SARS-CoV-2 by in situ hybridization, IHC, and IF requires a certain degree of tissue preservation, which can be limited due to autolysis. The overall sensitivity of these methods, therefore, can be lower than for RT-PCR. Limited autopsy material and the sampling bias can also lead to false-negative results. Good evidence for kidney tropism of SARS-CoV-2 in patients with COVID-19 should be provided by plaque assay using biopsy material. To our knowledge, there is only one study that successfully isolated SARS-CoV-2 from an autopsied kidney via plaque assay (83).
In summary, although many studies provide support against viral infectivity, there is also reasonable evidence from some comprehensive studies showing that kidney infectivity by SARS-CoV-2 may occur. These positive studies, so far, have been limited to kidney autopsy material. The timing of detection and the method used seem of critical importance for kidney detection. Correlating transcriptional analysis and viral presence in relatively large cohorts would be needed to gain further insight on SARS-CoV-2 infection in the kidney. In particular, studies involving early kidney biopsy tissue or autopsies done very soon after death would clearly improve our understanding of SARS-CoV-2 kidney infectivity. Assays in urine samples using ELISA would be the easiest way to monitor for SARS-CoV-2 in the kidney, and hopefully they will soon become available.
Disclosures
D.C. Batlle reports consultancy agreements with, and receiving honoraria from, AstraZeneca, Relypsa, and Tricida; reports receiving research funding from AstraZeneca, the Feinberg Foundation, and National Institute of Diabetes and Digestive and Kidney Diseases; reports serving as a scientific advisor or member of Relypsa and Tricida; reports being a founder and main owner of Angiotensin Therapeutics Inc., with no royalties or income at this time; reports being coinventor of an issued patent “Active Low Molecular Weight Variants of Angiotensin Converting Enzyme 2,” and provisional patents “Active low molecular weight variants of Angiotensin Converting Enzyme 2 (ACE2) for the treatment of diseases and conditions of the eye” and “Shorter soluble forms of Angiotensin Converting Enzyme 2 (ACE2) for treating and preventing coronavirus infection.” M.A. Sparks reports employment with Duke University and Durham Veterans Affairs Health Care System; reports receiving research funding from Renal Research Institute; and reports receiving honoraria from Elsevier for Nephrology Secrets. M.A. Sparks reports serving as a scientific advisor or member of American Board of Internal Medicine, Nephrology Board, Board of Director, NephJC, and the Editorial Boards of American Journal of Kidney Diseases, ASN Kidney News, Kidney360, and Kidney Medicine; Council for the Kidney in Cardiovascular Disease (KCVD) Membership & Communications Committee American Heart Association (AHA); KCVD Scientific & Clinical Education Lifelong Learning Committee AHA; and National Kidney Foundation (NKF) North Carolina Medical Advisory Board. P. Welling reports receiving research funding from LeDucq Foundation and National Institutes of Health; reports receiving honoraria from the American Physiological Society; reports serving on the Editorial Board of American Journal of Physiology-Renal Physiology, chair of the American Physiological Society Finance Committee, and Chair of Kidney Molecular Biology and Development, National Institutes of Health. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
We wish to acknowledge productive discussions over the last several months on the topic of this paper with various members of the COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group composed of Matthew A. Sparks, Swapnil Hiremath, Daniel Batlle, Andrew South, Paul Welling, J. Matt Luther, Jordana Cohen, James Brian Byrd, Louise M. Burrell, Laurie Tomlinson, Vivek Bhalla, María José Soler, and Sundar Swaminathan.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, Swaminathan S; COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group : Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 31: 1380–1383, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson AM, Wysocki J, Batlle D: The interaction of SARS-CoV-2 and other coronavirus with ACE2 (Angiotensin Converting Enzyme 2) as their main receptor: Therapeutic implications. Hypertension 76: 1339–1349, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed MM, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF: Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney360 1: 614–622, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG: Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med 46: 1114–1116, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP; the Northwell COVID-19 Research Consortium : Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323: 2052–2059, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, Yi F, Yang HC, Fogo AB, Nie X, Zhang C: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou M, Zhang X, Qu J: Coronavirus disease 2019 (COVID-19): A clinical update. Front Med 14: 126–135, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, Abramowitz MK, Levy R, Kumar N, Mokrzycki MH, Coco M, Dominguez M, Prudhvi K, Golestaneh L: AKI in hospitalized patients with and without COVID-19: A comparison study. J Am Soc Nephrol 31: 2145–2157, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y: Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 8: 475–481, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, Tan Y, Wang H, Wang C, Liu L, Liu Y, Liu Y, Wang G, Yuan Z, Hou X, Ren L, Wu Y, Chen Y: Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun 12: 2506, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X: Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581: 215–220, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D: Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181: 281–292.e6, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, Graham BS, McLellan JS: Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D: Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: A renoprotective combination? Hypertension 43: 1120–1125, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D: Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Zou X, Chen K, Zou J, Han P, Hao J, Han Z: Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14: 185–192, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan S, Chen L, Yang C-R, Raghuram V, Khundmiri SJ, Knepper MA: Does SARS-CoV-2 infect the kidney? J Am Soc Nephrol 31: 2746–2748, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredricks DN, Relman DA: Sequence-based identification of microbial pathogens: A reconsideration of Koch’s postulates. Clin Microbiol Rev 9: 18–33, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velez JCQ, Caza T, Larsen CP: COVAN is the new HIVAN: The re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol 16: 565–567, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M, Chughtai A, Xie L, Gimenez JM, Sandow TA, Lusco MA, Yang H, Acheampong E, Rosales IA, Colvin RB, Fogo AB, Velez JCQ: AKI and collapsing glomerulopathy associated with COVID-19 and APOL1 high-risk genotype. J Am Soc Nephrol 31: 1688–1695, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akilesh S, Nast CC, Yamashita M, Henriksen K, Charu V, Troxell ML, Kambham N, Bracamonte E, Houghton D, Ahmed NI, Chong CC, Thajudeen B, Rehman S, Khoury F, Zuckerman JE, Gitomer J, Raguram PC, Mujeeb S, Schwarze U, Shannon MB, De Castro I, Alpers CE, Najafian B, Nicosia RF, Andeen NK, Smith KD: Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis 77: 82–93.e1, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaillard F, Ismael S, Sannier A, Tarhini H, Volpe T, Greze C, Verpont MC, Zouhry I, Rioux C, Lescure FX, Buob D, Daugas E: Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int 98: 241, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazareth H, Péré H, Binois Y, Chabannes M, Schurder J, Bruneau T, Karras A, Thervet E, Rabant M, Veyer D, Pallet N: COVID-19-related collapsing glomerulopathy in a kidney transplant recipient. Am J Kidney Dis 76: 590–594, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Nickolas T, Kisselev S, Gharavi A, Canetta P: Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep 5: 940–945, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shetty AA, Tawhari I, Safar-Boueri L, Seif N, Alahmadi A, Gargiulo R, Aggarwal V, Usman I, Kisselev S, Gharavi AG, Kanwar Y, Quaggin SE: COVID-19–associated glomerular disease. J Am Soc Nephrol 32: 33–40, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra V, Magoon S, Troyer DA, McCune TR: Collapsing focal segmental glomerulosclerosis and acute oxalate nephropathy in a patient with COVID-19: A double whammy. J Investig Med High Impact Case Rep 8: 2324709620963635, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S, Berhanu K: COVID-19-related glomerulopathy: A report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med 2: 488–492, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR: COVID-19-associated collapsing focal segmental glomerulosclerosis: A report of 2 cases. Kidney Med 2: 493–497, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nasr SH, Alexander MP, Cornell LD, Herrera LH, Fidler ME, Said SM, Zhang P, Larsen CP, Sethi S: Kidney biopsy findings in patients with COVID-19, kidney injury, and proteinuria. Am J Kidney Dis 77: 465–468, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couturier A, Ferlicot S, Chevalier K, Guillet M, Essig M, Jauréguiberry S, Collarino R, Dargelos M, Michaut A, Geri G, Roque-Afonso AM, Zaidan M, Massy ZA: Indirect effects of severe acute respiratory syndrome coronavirus 2 on the kidney in coronavirus disease patients. Clin Kidney J 13: 347–353, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noble R, Tan MY, McCulloch T, Shantier M, Byrne C, Hall M, Jesky M: Collapsing glomerulopathy affecting native and transplant kidneys in individuals with COVID-19. Nephron 144: 589–594, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, Lhopitallier L, Sadallah S, Fakhouri F: Collapsing glomerulopathy in a COVID-19 patient. Kidney Int 98: 228–231, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadosh BS, Pavone J, Wu M, Reyentovich A, Gidea C: Collapsing glomerulopathy associated with COVID-19 infection in a heart transplant recipient. J Heart Lung Transplant 39: 855–857, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshmukh S, Zhou XJ, Hiser W: Collapsing glomerulopathy in a patient of Indian descent in the setting of COVID-19. Ren Fail 42: 877–880, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fani F, Regolisti G, Delsante M, Cantaluppi V, Castellano G, Gesualdo L, Villa G, Fiaccadori E: Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J Nephrol 31: 351–359, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Zarbock A, Gomez H, Kellum JA: Sepsis-induced acute kidney injury revisited: Pathophysiology, prevention and future therapies. Curr Opin Crit Care 20: 588–595, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarjou A, Agarwal A: Sepsis and acute kidney injury. J Am Soc Nephrol 22: 999–1006, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Kellum JA, Prowle JR: Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol 14: 217–230, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Ng JH, Bijol V, Sparks MA, Sise ME, Izzedine H, Jhaveri KD: Pathophysiology and pathology of acute kidney injury in patients with COVID-19. Adv Chronic Kidney Dis 27: 365–376, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perlman S: COVID-19 poses a riddle for the immune system. Nature 584: 345–346, 2020 [DOI] [PubMed] [Google Scholar]
- 45.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR: Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 24: 100434, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moledina DG, Simonov M, Yamamoto Y, Alausa J, Arora T, Biswas A, Cantley LG, Ghazi L, Greenberg JH, Hinchcliff M, Huang C, Mansour SG, Martin M, Peixoto A, Schulz W, Subair L, Testani JM, Ugwuowo U, Young P, Wilson FP: The association of COVID-19 with acute kidney injury independent of severity of illness: A multicenter cohort study. Am J Kidney Dis 77: 490–499.e1, 1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F: Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol 3: 237–261, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W: Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 395: 565–574, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wysocki J, Ye M, Rodriguez E, González-Pacheco FR, Barrios C, Evora K, Schuster M, Loibner H, Brosnihan KB, Ferrario CM, Penninger JM, Batlle D: Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: Prevention of angiotensin II-dependent hypertension. Hypertension 55: 90–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Batlle D, Wysocki J, Soler MJ, Ranganath K: Angiotensin-converting enzyme 2: Enhancing the degradation of angiotensin II as a potential therapy for diabetic nephropathy. Kidney Int 81: 520–528, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ: A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Kuba K, Imai Y, Penninger JM: Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J 77: 301–308, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Kalea AZ, Batlle D: Apelin and ACE2 in cardiovascular disease. Curr Opin Investig Drugs 11: 273–282, 2010 [PubMed] [Google Scholar]
- 54.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, Prindle T, Fulton WB, Wang S, McCray PB Jr, Chappell M, Hackam DJ, Jia H: Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol 314: L17–L31, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sparks MA, South AM, Badley AD, Baker-Smith CM, Batlle D, Bozkurt B, Cattaneo R, Crowley SD, Dell’Italia LJ, Ford AL, Griendling K, Gurley SB, Kasner SE, Murray JA, Nath KA, Pfeffer MA, Rangaswami J, Taylor WR, Garovic VD: Severe acute respiratory syndrome coronavirus 2, COVID-19, and the renin-angiotensin system: Pressing needs and best research practices. Hypertension 76: 1350–1367, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verdecchia P, Cavallini C, Spanevello A, Angeli F: The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med 76: 14–20, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong J-C, Turner AJ, Raizada MK, Grant MB, Oudit GY: Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ Res 126: 1456–1474, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harmer D, Gilbert M, Borman R, Clark KL: Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532: 107–110, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J; HCA Lung Biological Network : SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 181: 1016–1035.e19, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamming I, Timens W, Bulthuis M, Lely A, Navis Gv, van Goor H.. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathology 203: 631–637, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lores E, Wysocki J, Batlle D: ACE2, the kidney and the emergence of COVID-19 two decades after ACE2 discovery. Clin Sci (Lond) 134: 2791–2805, 2020 [DOI] [PubMed] [Google Scholar]
- 62.Lely A, Hamming I, van Goor H, Navis G.. Renal ACE2 expression in human kidney disease. J Pathology 204: 587–593, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Wysocki J, Ye M, Hassler L, Gupta AK, Wang Y, Nicoleascu V, Randall G, Wertheim JA, Batlle D: A novel soluble ACE2 variant with prolonged duration of action neutralizes SARS-CoV-2 infection in human kidney organoids. J Am Soc Nephrol 32: 795–803, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F: Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 117: 11,727–11,734, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mayer G, Boileau G, Bendayan M: Sorting of furin in polarized epithelial and endothelial cells: Expression beyond the Golgi apparatus. J Histochem Cytochem 52: 567–579, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Cao Y, Liu X, Li Y, Lu Y, Zhong H, Jiang W, Chen AF, Billiar TR, Yuan H, Cai J: Cathepsin L activity correlates with proteinuria in chronic kidney disease in humans. Int Urol Nephrol 49: 1409–1417, 2017 [DOI] [PubMed] [Google Scholar]
- 67.Bermejo-Martin JF, Gonzalez-Rivera M, Almansa R, Micheloud D, Dominguez-Gil M, Resino S, Martín-Fernández M, Murua PR, Pérez-García F, Tamayo L: SARS-CoV-2 RNA viremia is associated with a sepsis-like host response and critical illness in COVID-19. Available at: https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03398-0. Accessed July 28, 2021
- 68.Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, Worrall D, Giguel F, Piechocka-Trocha A, Atyeo C, Fischinger S, Chan A, Flaherty KT, Hall K, Dougan M, Ryan ET, Gillespie E, Chishti R, Li Y, Jilg N, Hanidziar D, Baron RM, Baden L, Tsibris AM, Armstrong KA, Kuritzkes DR, Alter G, Walker BD, Yu X, Li JZ; Massachusetts Consortium for Pathogen Readiness : SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 11: 5493, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB: Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouquegneau A, Erpicum P, Grosch S, Habran L, Hougrand O, Huart J, Krzesinski JM, Misset B, Hayette MP, Delvenne P, Bovy C, Kylies D, Huber TB, Puelles VG, Delanaye P, Jouret F: COVID-19-associated nephropathy includes tubular necrosis and capillary congestion, with evidence of SARS-CoV-2 in the nephron. Kidney360 2: 639–652, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB Jr: ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79: 14614–14621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shimada M, Yamabe H, Osawa H, Nakamura N, Kumasaka R, Murakami R, Fujita T, Osanai T, Okumura K: Extracellular matrix metalloproteinase inducer is expressed in the proximal tubular epithelial cells of the human kidney. Nephrology (Carlton) 14: 171–178, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Best Rocha A, Stroberg E, Barton LM, Duval EJ, Mukhopadhyay S, Yarid N, Caza T, Wilson JD, Kenan DJ, Kuperman M, Sharma SG, Larsen CP: Detection of SARS-CoV-2 in formalin-fixed paraffin-embedded tissue sections using commercially available reagents. Lab Invest 100: 1485–1489, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C: Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25: 2000045, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keil SD, Ragan I, Yonemura S, Hartson L, Dart NK, Bowen R: Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light-based photochemical treatment. Vox Sang 115: 495–501, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, Chufang L, Jin Z, Zhenhua J, Haiming J, Kui Z, Shuxiang H, Jun D, Xiaobo L, Xiaotao H, Lin W, Nanshan Z, Zifeng Y: Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 156: 104761, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Massoth LR, Desai N, Szabolcs A, Harris CK, Neyaz A, Crotty R, Chebib I, Rivera MN, Sholl LM, Stone JR, Ting DT, Deshpande V: Comparison of RNA in situ hybridization and immunohistochemistry techniques for the detection and localization of SARS-CoV-2 in human tissues. Am J Surg Pathol 45: 14–24, 2021 [DOI] [PubMed] [Google Scholar]
- 78.Walker RA: Quantification of immunohistochemistry: Issues concerning methods, utility and semiquantitative assessment I. Histopathology 49: 406–410, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Indeykina M, Brzhozovskiy A, Bugrova A, Kononikhin A, Starodubtseva N, Petrotchenko E, Kovalev GI, Borchers CH, Sukhikh GT: Mass spectrometric detection of SARS-CoV-2 virus in scrapings of the epithelium of the nasopharynx of infected patients via nucleocapsid N protein. J Proteome Res 19: 4393–4397, 2020 [DOI] [PubMed] [Google Scholar]
- 80.Miller SE, Brealey JK: Visualization of putative coronavirus in kidney. Kidney Int 98: 231–232, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA: Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 396: 320–332, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH, de Bree GJ, Bulle EB, Aronica EM, Florquin S, Fronczek J, Heunks LMA, de Jong MD, Guo L, du Long R, Lutter R, Molenaar PCG, Neefjes-Borst EA, Niessen HWM, van Noesel CJM, Roelofs JJTH, Snijder EJ, Soer EC, Verheij J, Vlaar APJ, Vos W, van der Wel NN, van der Wal AC, van der Valk P, Bugiani M: Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe 1: e290–e299, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, Nörz D, Heinrich F, Meißner K, Wichmann D, Kluge S, Gross O, Pueschel K, Schröder AS, Edler C, Aepfelbacher M, Puelles VG, Huber TB: SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396: 597–598, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M: Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 1: e245–e253, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Remmelink M, De Mendoca R, D’Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, Rorive S, Goffard JC, De Witte O, Peluso L, Vincent JL, Decaestecker C, Taccone FS, Salmon I: Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Available at: https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03218-5. Accessed July 28, 2021 [DOI] [PMC free article] [PubMed]
- 86.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C: Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465–469, 2020 [DOI] [PubMed] [Google Scholar]
- 87.Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I, Barasch J, Radhakrishnan J, D’Agati V, Markowitz G: Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol 31: 2158–2167, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chon WJ, Aggarwal N, Kocherginsky M, Kane B, Sutor J, Josephson MA: High-level viruria as a screening tool for BK virus nephropathy in renal transplant recipients. Kidney Res Clin Pract 35: 176–181, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nickeleit V, Singh HK: Polyomaviruses and disease: Is there more to know than viremia and viruria? Curr Opin Organ Transplant 20: 348–358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Howell DN, Smith SR, Butterly DW, Klassen PS, Krigman HR, Burchette JL Jr, Miller SE: Diagnosis and management of BK polyomavirus interstitial nephritis in renal transplant recipients. Transplantation 68: 1279–1288, 1999 [DOI] [PubMed] [Google Scholar]
- 91.Roshandel MR, Nateqi M, Lak R, Aavani P, Sari Motlagh R, Shariat SF, Badr TA, Sfakianos J, Kaplan SA, Tewari AK: Diagnostic and methodological evaluation of studies on the urinary shedding of SARS-CoV-2, compared to stool and serum: A systematic review and meta-analysis. Cell Mol Biol 66: 148–156, 2020 [PubMed] [Google Scholar]
- 92.George S, Pal AC, Gagnon J, Timalsina S, Singh P, Vydyam P, Munshi M, Chiu JE, Renard I, Harden C, Ott IM, Watkins AE, Vogels D, Lu P, Tokuyama M, Venkataraman A, Casanovas-Massana A, Wyllie AL, Rao V, Campbell M, Farhadian SF, Grubaugh ND, Dela Cruz CS, Ko AI, Berna Perez AI, Akaho EH, Moledina DG, Testani J, John AR, Ledizet M, Mamoun CB; the Yale IMPACT Team: Evidence for SARS-CoV-2 spike protein in the urine of COVID-19 patients. Kidney360 2: 924–936, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim Y-I, Kim S-G, Kim S-M, Kim E-H, Park S-J, Yu K-M, Chang JH, Kim EJ, Lee S, Casel MAB, Um J, Song MS, Jeong HW, Lai VD, Kim Y, Chin BS, Park JS, Chung KH, Foo SS, Poo H, Mo IP, Lee OJ, Webby RJ, Jung JU, Choi YK: Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe 27: 704–709.e2, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeong HW, Kim S-M, Kim H-S, Kim Y-I, Kim JH, Cho JY, Kim SH, Kang H, Kim SG, Park SJ, Kim EH, Choi YK: Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin Microbiol Infect 26: 1520–1524, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun J, Zhu A, Li H, Zheng K, Zhuang Z, Chen Z, Shi Y, Zhang Z, Chen SB, Liu X, Dai J, Li X, Huang S, Huang X, Luo L, Wen L, Zhuo J, Li Y, Wang Y, Zhang L, Zhang Y, Li F, Feng L, Chen X, Zhong N, Yang Z, Huang J, Zhao J, Li YM: Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect 9: 991–993, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Briggs J, Knepper MA: JASN podcast: Does SARS-CoV-2 infect the kidney? Available at: http://www.asn-online.org/media/podcast/JASN/2020_11_24_JASN2020081229.mp3?&WT.MC_ID=ITL&utm_source=ITLd. Accessed July 18, 2021 [DOI] [PMC free article] [PubMed]
- 97.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, Canetta P, Ratner LE, Marasa M, Gharavi AG, Stokes MB, Markowitz GS, D’Agati VD: Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol 31: 1959–1968, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R, Khanin Y, Madireddy V, Larsen CP, Jhaveri KD, Bijol V; Northwell Nephrology COVID-19 Research Consortium : COVID-19–associated kidney injury: A case series of kidney biopsy findings. J Am Soc Nephrol 31: 1948–1958, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Y, Li X-J, Li Y-Q, Dai W, Shao T, Liu W-Y, Han M, Xu G, Liu L: Clinical and pathological findings of SARS-CoV-2 infection and concurrent IgA nephropathy: A case report. BMC Nephrol 21: 504, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Golmai P, Larsen CP, DeVita MV, Wahl SJ, Weins A, Rennke HG, Bijol V, Rosenstock JL: Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol 31: 1944–1947, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brook OR, Piper KG, Mercado NB, Gebre MS, Barouch DH, Busman-Sahay K, Starke CE, Estes JD, Martinot AJ, Wrijil L, Ducat S, Hecht JL: Feasibility and safety of ultrasound-guided minimally invasive autopsy in COVID-19 patients. Abdom Radiol (NY) 46: 1263–1271, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, Harding CV, Gilmore H, Sadri N: Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am J Clin Pathol 154: 190–200, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]